Abstract
Objective
To compare risk factors, clinical presentation, and outcomes after posterior circulation arterial ischemic stroke (PCAIS) and anterior circulation arterial ischemic stroke (ACAIS) in neonates and children.
Methods
In this international multicenter observational study including neonates and children up to 18 years of age with arterial ischemic stroke (AIS), we compared clinical and radiologic features according to stroke location.
Results
Of 2,768 AIS cases, 507 (18%) were located in the posterior circulation, 1,931 (70%) in the anterior circulation, and 330 (12%) involved both. PCAIS was less frequent in neonates compared to children (8.8% vs 22%, p < 0.001). Children with PCAIS were older than children with ACAIS (median age 7.8 [interquartile range (IQR) 3.1–14] vs 5.1 [IQR 1.5–12] years, p < 0.001), and more often presented with headache (54% vs 32%, p < 0.001) and a lower Pediatric NIH Stroke Scale score (4 [IQR 2–8] vs 8 [IQR 3–13], p = 0.001). Cervicocephalic artery dissections (CCAD) were more frequent (20% vs 8.5%, p < 0.001), while cardioembolic strokes were less frequent (19% vs 32%, p < 0.001) in PCAIS. Case fatality rates were equal in both groups (2.9%). PCAIS survivors had a better outcome (normal neurologic examination at hospital discharge in 29% vs 21%, p = 0.002) than ACAIS survivors, although this trend was only observed in children and not in neonates.
Conclusion
PCAIS is less common than ACAIS in both neonates and children. Children with PCAIS are older and have a higher rate of CCAD, lower clinical stroke severity, and better outcome than children with ACAIS.
Current knowledge about pediatric posterior circulation arterial ischemic stroke (PCAIS) is largely based on a few, mostly single-center retrospective studies that report PCAIS in 10%–43% of cases of childhood arterial ischemic stroke (AIS), and rarely in neonates.1–7 Cerebral vasculopathies are the most commonly identified etiologies of childhood PCAIS, and recurrence rates are reportedly higher when compared to anterior circulation arterial ischemic stroke (ACAIS).1,2,4,8 Clinical outcomes for children with PCAIS are available through a limited number of studies and range from a good outcome in 55% of children with no case fatalities to fatality rates as high as 26% and poor outcome in 45% of survivors.1–4,8
These discrepant reports in the literature and the fact that PCAIS has not been directly compared to ACAIS except for stroke recurrence led us to review data from a large prospective multicenter study, the International Pediatric Stroke Study (IPSS), over a 10-year period to provide comparative information on risk factors, clinical presenting features, and outcomes following ACAIS and PCAIS in neonates and children. We hypothesized that children with PCAIS frequently have nonspecific symptoms such as headache or altered level of consciousness, have different risk factors, and differ in terms of stroke outcomes and recurrence compared to children with ACAIS.
Methods
The methods of the IPSS have been described in detail.9,10 In brief, the IPSS is an international multicenter observational study prospectively including children and neonates with arterial ischemic stroke or cerebral sinovenous thrombosis since 2003. All participating centers are listed in appendix 1. Figure e-1 (doi.org/10.5061/dryad.1250k52) illustrates the IPSS enrollment.
For this analysis, we included all patients (neonates aged 0 to 28 days of life and children aged 29 to <19 years at onset) with AIS registered between 2003 and 2014 for whom stroke location was known. Children with a stroke involving both the anterior and the posterior circulation were excluded.
Variable definitions
AIS was defined as an acute event with clinical and radiologic evidence of cerebral ischemia, including (1) a focal neurologic deficit of acute onset and (2) CT or MRI showing infarct in location consistent with neurologic signs and symptoms. Patients with diffuse or bilateral infarction related to hypoxic ischemic events were included only if a definite focal, single arterial infarct in a specified vascular territory was also present. Stroke locations were reported by local site investigators as isolated involvement of the posterior circulation (PCAIS), isolated involvement of the anterior circulation (ACAIS), or involvement of both the anterior and posterior circulation (BCAIS). The anterior circulation was defined as the territory of the internal carotid, middle, or anterior cerebral artery; the posterior circulation as the territory of the vertebral, basilar, cerebellar, or posterior cerebral arteries. Clinical stroke severity at diagnosis was assessed with the Pediatric NIH Stroke Scale (PedNIHSS).11 Outcome was assessed with an examination by a pediatric neurologist and the Pediatric Stroke Outcome Measure, a standardized neurologic outcome measure validated for pediatric ischemic stroke.12 In addition, parents or guardians answered 2 outcome questions at follow-up: (1) “Has your child recovered completely from the stroke?” (2) “Does your child need extra help with day-to-day activities compared with other children of the same age?” Hemorrhagic transformation of AIS was defined according to the European–Australasian Cooperative Acute Stroke Study (ECASS II).13 Recurrent ischemic events were defined as any clinical or subclinical ischemic event after the index stroke.
Statistical analyses
Statistical analyses were performed using Stata version 15 (StataCorp LP, College Station, TX). Data are reported descriptively as numbers and frequencies. For variables with missing data, the term valid n indicates the number of patients for whom information was available. Continuous data are expressed as median and interquartile ranges (IQR). Groups were compared according to stroke location (ACAIS vs PCAIS) using the Mann-Whitney U test for continuous variables, and Fisher exact or Pearson χ2 test for categorical variables, as appropriate. A 2-sided p value ≤0.05 was considered statistically significant. Multivariable logistic regression analysis was performed to identify predictors of recurrent ischemic events and neurologic deficits at hospital discharge, including variables with a p value ≤0.1 in the univariable logistic regression analysis.
Standard protocol approvals, registrations, and patient consents
The study protocol was approved by relevant local authorities in all participating centers and conducted according to national rules concerning ethics committee approval and informed consents. The IPSS is registered at ClinicalTrials.gov (NCT00084292).
Data availability
All data used for analysis are presented in the tables and figure in this article. Data will be shared after ethics approval if requested by other investigators for purposes of replicating the results.
Results
Stroke location was recorded in 2,768 (87%) patients with AIS (88% of neonates, 86% of children), with the majority being ACAIS (n = 1,931%, 70%), followed by PCAIS (n = 507, 18%) and BCAIS (n = 330, 12%). After excluding the 330 patients with BCAIS, 2,438 participants were included in this study (703 neonates and 1,735 children). PCAIS was less frequent in neonates than children (8.8% vs 22%, p < 0.001).
Childhood stroke
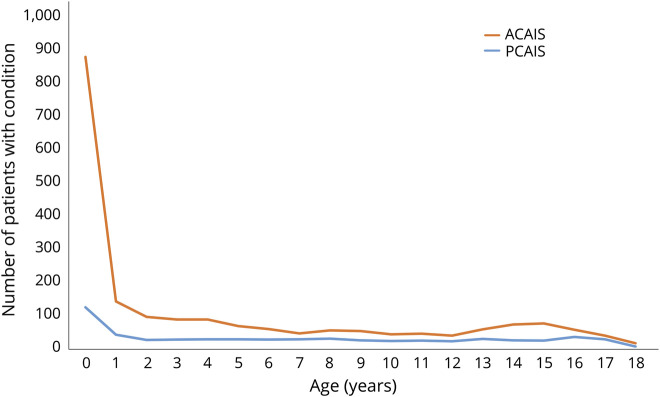
Children with PCAIS were older (median age 7.8 years, IQR 3.1–14 years) than children with ACAIS (median age 5.1 years, IQR 1.5–12 years, p < 0.001). Age distribution according to stroke territory is displayed in the figure.
Figure. Age distribution according to stroke territory.
ACAIS = anterior circulation arterial ischemic stroke; PCAIS = posterior circulation arterial ischemic stroke.
The male preponderance was more marked in PCAIS (64%) than ACAIS (56%) (p = 0.002). This difference persisted after adjusting for preceding head trauma (p = 0.014), but was no longer present when adjusting for cervicocephalic artery dissection (CCAD, p = 0.3).
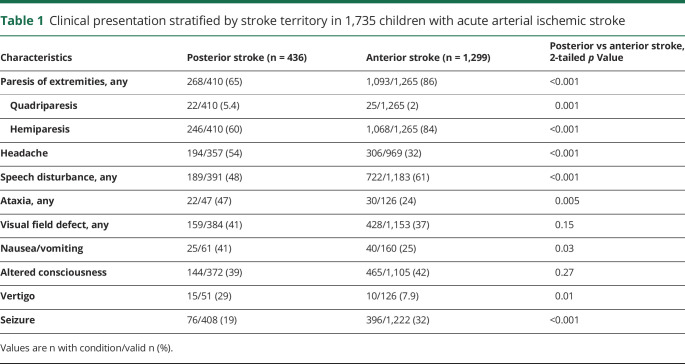
The most common presenting signs and symptoms in PCAIS were limb paresis (65%), headache (54%), speech disturbance (48%), and ataxia (47%). Apart from obvious differences due to stroke location, displayed in detail in table 1, children with PCAIS more often had headache at stroke onset (p < 0.001), while seizures were less frequent than in ACAIS (p < 0.001). Altered level of consciousness (p = 0.27) and visual field defects (p = 0.15) did not differ.
Table 1.
Clinical presentation stratified by stroke territory in 1,735 children with acute arterial ischemic stroke
Stroke severity at presentation was reported in 273 patients (16% of ACAIS, 15% of PCAIS) and was lower in PCAIS with a median PedNIHSS of 4 (IQR 2–8) compared to ACAIS (median PedNIHSS 8 [IQR 3–13], p = 0.001).
Time from symptom onset to radiologic confirmation of diagnosis was available in 449 children (25% of ACAIS, 28% of PCAIS), and was similar in both groups, with a median of 19.8 (IQR 6.8–48) hours in PCAIS compared to 13 (IQR 5–28) hours in ACAIS (p = 0.25). MRI was the imaging modality most frequently required to diagnose PCAIS (MRI 68%, CT 32%), while a CT scan was sufficient for diagnosis in nearly half of ACAIS cases (MRI 56%, CT 44%, p < 0.001).
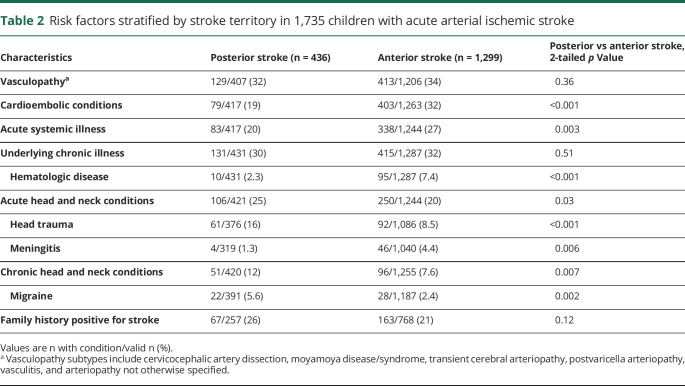
Vasculopathy and chronic illness were the 2 most common risk factors for both PCAIS and ACAIS. However, the distribution of subtypes within these groups differed, with CCAD being more common in PCAIS (20%) than in ACAIS (8.5%, p < 0.001), while moyamoya and transient cerebral arteriopathy (TCA) were more frequent in ACAIS than in PCAIS (moyamoya 14% vs 2.8%, p < 0.001, TCA 5% vs 2%, p = 0.033). Hematologic disease, including iron deficiency anemia, sickle cell disease, or any kind of prothrombotic state, were less frequent in PCAIS (2%) than ACAIS (7%, p = 0.001). Cardioembolic conditions and acute systemic illness were less common in PCAIS than in ACAIS, while acute and chronic diseases affecting the head or neck including migraine were more frequent in PCAIS (table 2).
Table 2.
Risk factors stratified by stroke territory in 1,735 children with acute arterial ischemic stroke
Children with PCAIS more often received an antithrombotic agent during the acute phase than did children with ACAIS (84% in PCAIS, 77.1% in ACAIS, p < 0.003). Thrombolysis was rarely performed for stroke in any location (5.1% in ACAIS, 2% in PCAIS, p = 0.07); 5 thrombectomies were performed, all in ACAIS. Hemorrhagic transformation of stroke, occurring in 5.5% of PCAIS and 6.6% of ACAIS cases (p = 0.53), and neurosurgical procedures, such as decompressive craniotomy or CSF drainage, performed in 14% of ACAIS and 9.1% of PCAIS cases (p = 0.1), were similar.
The fatality rate was identical for PCAIS and ACAIS (both 2.9%). In survivors, outcome data were obtained in 92% of patients with PCAIS and 97% of patients with ACAIS at discharge and in 52% of patients with PCAIS and 46% of patients with ACAIS after a median follow-up duration of 1.5 (IQR 0.5–3.1) years, which did not differ between the groups (p = 0.5).
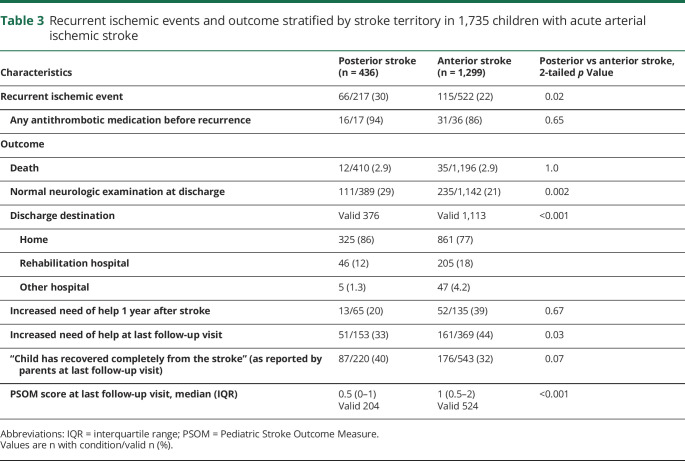
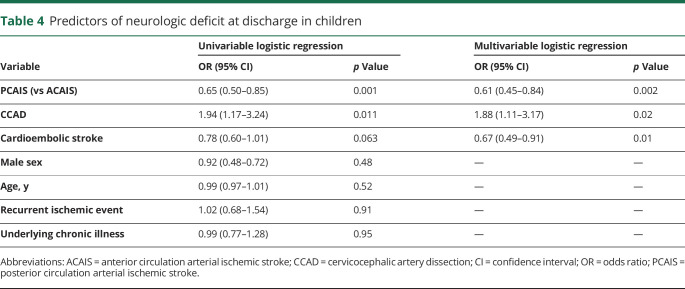
Survivors of PCAIS were more frequently reported to have a normal neurologic examination at hospital discharge than survivors of ACAIS (table 3). In the multivariable logistic regression analysis, the risk for a neurologic deficit at discharge was decreased in children with PCAIS (odds ratio [OR] 0.61, 95% confidence interval [CI] 0.45–0.84; p = 0.002) and cardioembolic stroke (OR 0.67; 95% CI 0.49–0.91; p = 0.01), and increased with CCAD (OR 1.88, 95% CI 1.11–3.17; p = 0.02) (table 4).
Table 3.
Recurrent ischemic events and outcome stratified by stroke territory in 1,735 children with acute arterial ischemic stroke
Table 4.
Predictors of neurologic deficit at discharge in children
Full recovery was reported by parents of roughly a third of children after PCAIS and ACAIS at the last follow-up visit (table 3).
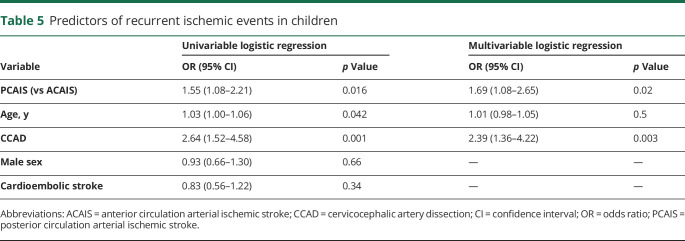
Recurrent ischemic events were more frequent in PCAIS than ACAIS despite similar rates of secondary preventive antithrombotic treatment. The multivariable logistic regression analysis revealed PCAIS (OR 1.69, 95% CI 1.08–2.65; p = 0.02) and CCAD (OR 2.39, 95% CI 1.36–4.22; p = 0.003) as risk factors for recurrent ischemic events (table 5).
Table 5.
Predictors of recurrent ischemic events in children
Neonatal stroke
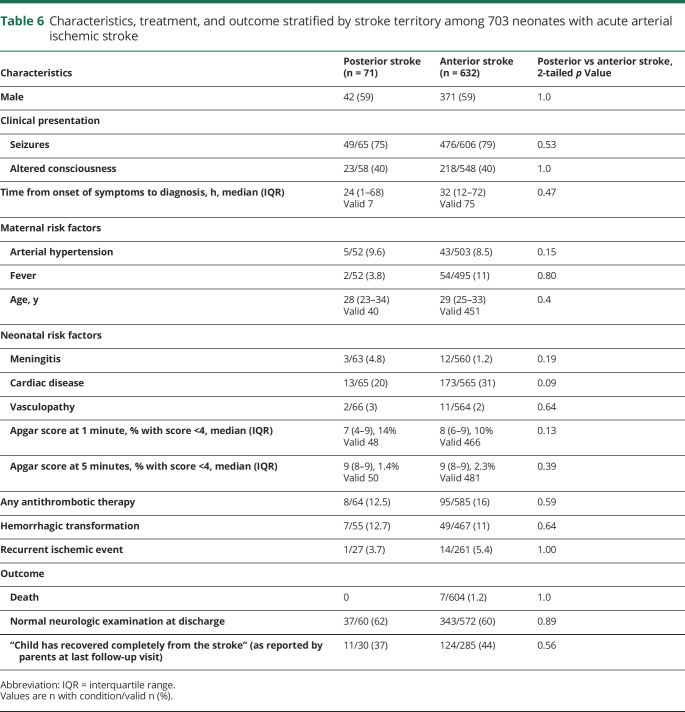
Neonates with PCAIS did not differ from neonates with ACAIS in terms of clinical presentation, sex distribution, risk factors, diagnostic imaging modality, complications such as hemorrhagic transformation, recurrence, and outcome (table 6). Cardiac disease was present in the only patient with recurrent ischemic event after PCAIS, and more frequent in recurrences after ACAIS (13% vs 2% in noncardiac patients, p = 0.001). Complete recovery from stroke at last follow-up visit (median follow-up 2 years) was reported in more than one third of neonates with PCAIS.
Table 6.
Characteristics, treatment, and outcome stratified by stroke territory among 703 neonates with acute arterial ischemic stroke
Discussion
This large international observational study comprehensively compares PCAIS with ACAIS throughout the pediatric age groups, demonstrating that pediatric PCAIS is less frequent than ACAIS in both neonates and children, and confirming our hypothesis of a distinct clinical presentation, risk factor profile, and outcome in children, but not in neonates with PCAIS compared with ACAIS.
PCAIS presenting beyond the newborn period is associated with older age at stroke onset and different risk factor profiles, with higher rates of CCAD, acute/chronic head and neck conditions, and migraine, and lower rates of cardioembolic conditions, transient cerebral arteriopathy, moyamoya arteriopathy, acute systemic illnesses, and hematologic disease, when compared to ACAIS. In a systematic review of CCAD and stroke in childhood, patients with CCAD leading to PCAIS were younger than those with ACAIS,14 while CCAD occurred at any age throughout childhood in a more recent prospective study.15 Lower rates of cardioembolic events in PCAIS are not unexpected as the anterior circulation carries the majority of the cerebral blood supply, and as a consequence emboli from the heart are more likely to travel to the middle or anterior cerebral artery.16 In children, cardioembolic events often occur in the perioperative period in younger children with complex congenital heart disease.17 Similarly, involvement of the posterior circulation occurs later in children with moyamoya syndrome.18
A marked male preponderance has been reported in PCAIS.1–4,8 These previous studies did not correct for potential confounding factors such as preceding trauma and CCAD. In the current study, the association between PCAIS and male sex persisted even when correcting for trauma, but not for CCAD. The reason for this finding remains to be determined. Higher testosterone levels have been reported in some pediatric stroke patients compared to healthy controls, but these findings were not analyzed further according to stroke location or etiology.19 A male preponderance has also been noted in adult patients with CCAD,20,21 but was more marked in the anterior than in the posterior circulation,22 and sex differences in putative risk factors, such as hypertension, hypercholesterolemia, or past smoking, may explain the higher frequency of CCAD in men.
Clinical presentation did differ, as hypothesized, with nonspecific symptoms such as headache, vertigo, nausea, and vomiting being more common in PCAIS. However, seizures that may also mislead and delay the diagnosis were less common in PCAIS than in ACAIS, while altered consciousness was reported equally in both groups. In contrast to a previous report, these differences in clinical presentation did not seem to lead to a longer time to diagnosis.23
MRI remains the imaging modality of choice to radiologically confirm diagnosis in PCAIS, as our data and previous studies show. CT has lower sensitivity for detection of acute infarction, particularly for smaller brainstem or cerebellar lesions, due to bony artifacts from the base of the skull.23–25 This study also highlights the importance of vascular imaging, including sequences for vessel wall pathologies, such as CCAD, which are highly prevalent in PCAIS.26,27
Fatality rates were identical for PCAIS and ACAIS. In survivors, outcome was better and the need for inpatient rehabilitation was lower in PCAIS compared with ACAIS at discharge. Similar differences were observed at a median of 1.5 years after the stroke, even though children with PCAIS more often had recurrent ischemic events. Cervicocephalic artery dissections were associated with increased odds of neurologic deficits but outcomes differed for children with PCAIS vs ACAIS. While neurologic deficits occurred more often in CCAD compared with non-CCAD (93% vs 76%, p < 0.001) in ACAIS, this was not the case in PCAIS. This difference is no longer present in the multivariable analysis because ACAIS cases outnumber PCAIS cases and because only a minority of strokes occurred as a consequence of CCAD (20% in PCAIS and 9% in ACAIS).
Relatively good outcome in children compared with adults has already been described after basilar artery stroke.28 However, recent reports comparing ACAIS and PCAIS in adults show more similarities than differences, not only in initial stroke severity,29 but also in terms of clinical outcome, which was favorable (modified Rankin Scale score 0–2) in a majority of patients with ACAIS and patients with PCAIS.30
Previously reported as a rare occurrence in neonates,5,6 this study shows that PCAIS accounts for almost 10% of AIS in this age group. No significant differences between neonates with PCAIS and ACAIS were identified. A prior case series described 18 neonates with posterior cerebral artery stroke, 5 of whom did not have concomitant hypoxic-ischemic encephalopathy or hypoglycemia.7 We did not have serum glucose measurements available for cases recruited to the current study but Apgar scores were similar in all stroke territories; 14% of patients with PCAIS and 10% of patients with ACAIS had an Apgar score <4 at 1 minute and 1.4% of patients with PCAIS and 2.3% of patients with ACAIS had an Apgar score <4 at 5 minutes.
The main strengths of this study were the prospective international multicenter design, allowing enrollment of a large number of children, data collection according to a predefined standardized protocol, and comparison of neonates and children with PCAIS and ACAIS. However, information on some variables of interest, such as the PedNIHSS, which indicates stroke severity, recurrent ischemic events, and outcome data after discharge from hospital were missing in a substantial part of the study participants. Furthermore, selection bias towards more severe cases is a possibility because most enrolling sites were tertiary pediatric care centers. This may also explain the higher recurrence rates after neonatal stroke observed in the current study, compared to the previous literature. The IPSS does not provide detailed information on imaging modalities such as the rate of cervical angiographies performed or the MRI sequences used; thus diagnosis of certain conditions such as vasculopathies may be underrecognized or overestimated.
PCAIS is less common than ACAIS in the pediatric population. Children with PCAIS are older, have higher rates of CCAD and other head and neck conditions, lower stroke symptom severity at presentation, increased stroke recurrence risk, and better outcome than children with ACAIS.
Acknowledgment
The authors thank the children, their caregivers, and staff of all participating centers for their contributions to this study.
Glossary
- ACAIS
anterior circulation arterial ischemic stroke
- AIS
arterial ischemic stroke
- BCAIS
arterial ischemic stroke of both the anterior and posterior circulation
- CCAD
cervicocephalic artery dissection
- CI
confidence interval
- IPSS
International Pediatric Stroke Study
- IQR
interquartile range
- OR
odds ratio
- PCAIS
posterior circulation arterial ischemic stroke
- PedNIHSS
Pediatric NIH Stroke Scale
- TCA
transient cerebral arteriopathy
Footnotes
Editorial, page 149
CME Course: NPub.org/cmelist
Author contributions
B.G.S. designed/conceptualized the study, analyzed/interpreted the data, drafted the manuscript, and collected data. M.F.R. designed/conceptualized the study, interpreted the data, collected data, and reviewed and edited the manuscript for content. M.C., W.D.L., L.A.B., L.L.B., C.K.F.: data collection, critical review of the manuscript, editing manuscript for content. A.P. analyzed/interpreted the data and reviewed the manuscript, editing manuscript for content. M.T.M., M.S.: initiated, designed, conceptualized, and supervised the study, analyzed/interpreted the data, revised the manuscript, and collected data. All authors agreed on submission of the present version of the manuscript.
Study funding
This research was supported by The Auxilium Foundation.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Ganesan V, Chong WK, Cox TC, Chawda SJ, Prengler M, Kirkham FJ. Posterior circulation stroke in childhood: risk factors and recurrence. Neurology 2002;59:1552–1556. [DOI] [PubMed] [Google Scholar]
- 2.Mackay MT, Prabhu SP, Coleman L. Childhood posterior circulation arterial ischemic stroke. Stroke 2010;41:2201–2209. [DOI] [PubMed] [Google Scholar]
- 3.McCrea N, Saunders D, Bagkeris E, Chitre M, Ganesan V. Diagnosis of vertebral artery dissection in childhood posterior circulation arterial ischaemic stroke. Dev Med Child Neurol 2016;58:63–69. [DOI] [PubMed] [Google Scholar]
- 4.Carey S, Wrogemann J, Booth FA, Rafay MF. Epidemiology, clinical presentation, and prognosis of posterior circulation ischemic stroke in children. Pediatr Neurol 2017;74:41–50. [DOI] [PubMed] [Google Scholar]
- 5.Grunt S, Mazenauer L, Buerki SE, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics 2015;135:e1220–1228. [DOI] [PubMed] [Google Scholar]
- 6.Laugesaar R, Kolk A, Tomberg T, et al. Acutely and retrospectively diagnosed perinatal stroke: a population-based study. Stroke 2007;38:2234–2240. [DOI] [PubMed] [Google Scholar]
- 7.van der Aa NE, Dudink J, Benders MJ, et al. Neonatal posterior cerebral artery stroke: clinical presentation, MRI findings, and outcome. Dev Med Child Neurol 2013;55:283–290. [DOI] [PubMed] [Google Scholar]
- 8.Uohara MY, Beslow LA, Billinghurst L, et al. Incidence of recurrence in posterior circulation childhood arterial ischemic stroke. JAMA Neurol 2017;74:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golomb MR, Fullerton HJ, Nowak-Gottl U, Deveber G, International Pediatric Stroke Study Group. Male predominance in childhood ischemic stroke: findings from the International Pediatric Stroke Study. Stroke 2009;40:52–57. [DOI] [PubMed] [Google Scholar]
- 10.Mackay MT, Wiznitzer M, Benedict SL, et al. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol 2011;69:130–140. [DOI] [PubMed] [Google Scholar]
- 11.Ichord RN, Bastian R, Abraham L, et al. Interrater reliability of the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) in a multicenter study. Stroke 2011;42:613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitchen L, Westmacott R, Friefeld S, et al. The pediatric stroke outcome measure: a validation and reliability study. Stroke 2012;43:1602–1608. [DOI] [PubMed] [Google Scholar]
- 13.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–1251. [DOI] [PubMed] [Google Scholar]
- 14.Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology 2001;57:1155–1160. [DOI] [PubMed] [Google Scholar]
- 15.Wintermark M, Hills NK, DeVeber GA, et al. Clinical and imaging characteristics of arteriopathy subtypes in children with arterial ischemic stroke: results of the VIPS study. AJNR Am J Neuroradiol 2017;38:2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyajian RA, Schwend RB, Wolfe MM, Bickerton RE, Otis SM. Measurement of anterior and posterior circulation flow contributions to cerebral blood flow: an ultrasound-derived volumetric flow analysis. J Neuroimaging 1995;5:1–3. [DOI] [PubMed] [Google Scholar]
- 17.Dowling MM, Hynan LS, Lo W, et al. International Paediatric Stroke Study: stroke associated with cardiac disorders. Int J Stroke 2013;8(suppl A100):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JY, Kim SK, Cheon JE, et al. Posterior cerebral artery involvement in moyamoya disease: initial infarction and angle between PCA and basilar artery. Childs Nerv Syst 2013;29:2263–2269. [DOI] [PubMed] [Google Scholar]
- 19.Normann S, de Veber G, Fobker M, et al. Role of endogenous testosterone concentration in pediatric stroke. Ann Neurol 2009;66:754–758. [DOI] [PubMed] [Google Scholar]
- 20.Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology 2006;67:1050–1052. [DOI] [PubMed] [Google Scholar]
- 21.Metso AJ, Metso TM, Debette S, et al. Gender and cervical artery dissection. Eur J Neurol 2012;19:594–602. [DOI] [PubMed] [Google Scholar]
- 22.von Babo M, De Marchis GM, Sarikaya H, et al. Differences and similarities between spontaneous dissections of the internal carotid artery and the vertebral artery. Stroke 2013;44:1537–1542. [DOI] [PubMed] [Google Scholar]
- 23.Rafay MF, Pontigon AM, Chiang J, et al. Delay to diagnosis in acute pediatric arterial ischemic stroke. Stroke 2009;40:58–64. [DOI] [PubMed] [Google Scholar]
- 24.McGlennan C, Ganesan V. Delays in investigation and management of acute arterial ischaemic stroke in children. Dev Med Child Neurol 2008;50:537–540. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan J, Miller SP, Phan TG, Mackay MT. Delayed recognition of initial stroke in children: need for increased awareness. Pediatrics 2009;124:e227–234. [DOI] [PubMed] [Google Scholar]
- 26.Dlamini N, Yau I, Muthusami P, et al. Arterial wall imaging in pediatric stroke. Stroke 2018;49:891–898. [DOI] [PubMed] [Google Scholar]
- 27.Tan MA, DeVeber G, Kirton A, Vidarsson L, MacGregor D, Shroff M. Low detection rate of craniocervical arterial dissection in children using time-of-flight magnetic resonance angiography: causes and strategies to improve diagnosis. J Child Neurol 2009;24:1250–1257. [DOI] [PubMed] [Google Scholar]
- 28.Goeggel Simonetti B, Ritter B, Gautschi M, et al. Basilar artery stroke in childhood. Dev Med Child Neurol 2013;55:65–70. [DOI] [PubMed] [Google Scholar]
- 29.von Sarnowski B, Schminke U, Grittner U, et al. Posterior versus anterior circulation stroke in young adults: a comparative study of stroke aetiologies and risk factors in stroke among young Fabry patients (sifap1). Cerebrovasc Dis 2017;43:152–160. [DOI] [PubMed] [Google Scholar]
- 30.De Marchis GM, Kohler A, Renz N, et al. Posterior versus anterior circulation strokes: comparison of clinical, radiological and outcome characteristics. J Neurol Neurosurg Psychiatry 2011;82:33–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for analysis are presented in the tables and figure in this article. Data will be shared after ethics approval if requested by other investigators for purposes of replicating the results.