Abstract
It is still unclear if the ability of key regulators of actin cytoskeletal remodeling to influence lipid metabolism contributes to kidney injury. In this issue of Cell Metabolism, Fu et al. (2020) show that junctional adhesion molecule-like (JAML) is a novel mediator of glomerular disease progression while suggesting a direct link between defects in cell adhesion and lipotoxicity.
The glomerulus acts as a selective-size filter within the kidney to remove toxic compounds from the blood. Glomerular diseases, whether primary or secondary to systemic disorders such as diabetes, account for a large number of cases of chronic kidney disease, a condition that affects millions of patients worldwide. In addition to several hemodynamic and metabolic mediators of glomerular injury, recent emphasis has been placed on the role of glomerular lipid dysmetabolism in these conditions. In particular, podocytes, which are functionally specialized and terminally differentiated cells of the glomerular filtration barrier, are highly sensitive to lipotoxicity. Podocyte loss is an irreversible phenomenon that has been found to predict disease progression across a spectrum of clinical and experimental models of glomerular disorders.
The maintenance of proper podocyte actin cytoskeletal dynamics has long been recognized as a key to proper podocyte function and as a potential therapeutic target across several models of kidney disease (Schiffer et al., 2015). Under physiological conditions, podocytes rely primarily on anaerobic glucose metabolism for energy homeostasis and to a lesser extent on β-oxidation of lipids. Dys-regulated lipid metabolism leading to the accumulation of cholesterol or to elevated levels of intracellular free fatty acids has been shown to contribute to lipotoxicity-induced podocyte injury, and to be associated with mitochondrial dysfunction (Ducasa et al., 2019). Interestingly, there seems to be a vicious cycle between mitochondrial dysfunction, inflammation, and altered lipid metabolism in podocytes (Figure 1).
Figure 1. A Vicious Cycle between Mitochondrial Dysfunction, Inflammation, the Cytoskeleton, and Lipid Dysmetabolism in Podocytes.
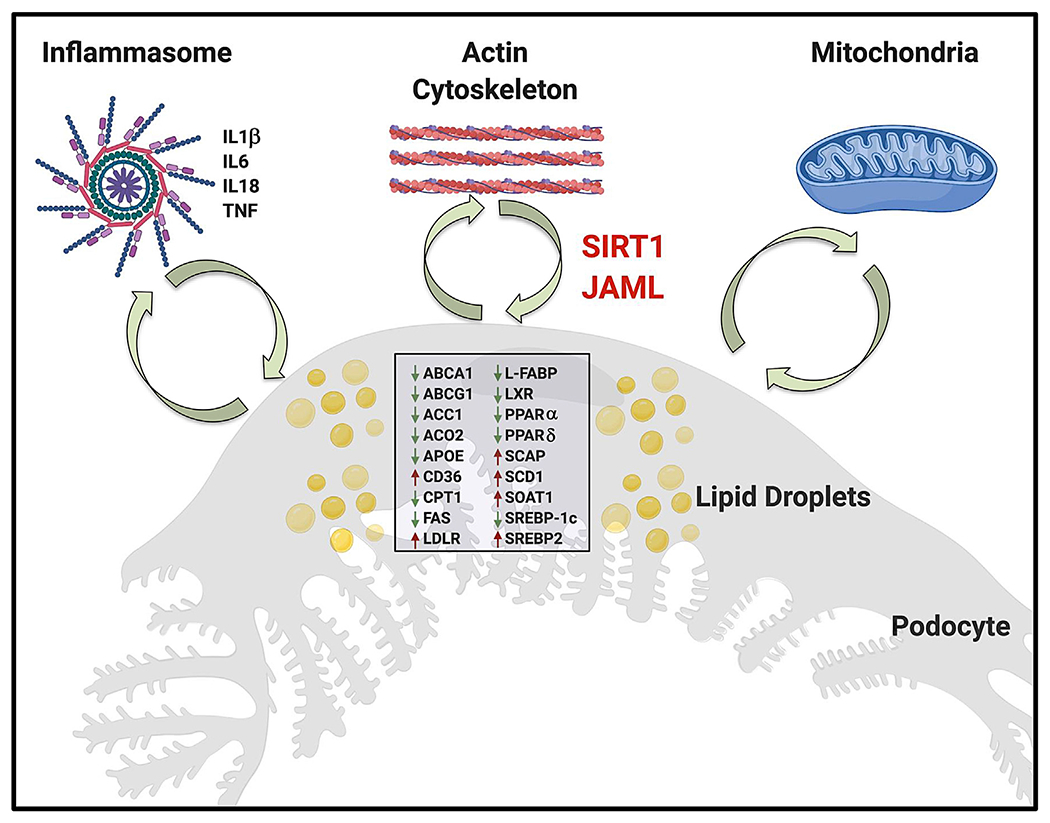
In a vicious cycle, the accumulation of lipids in the form of lipid droplets in podocytes, due to altered expression of a variety of genes important in regulating lipid metabolism, leads to mitochondrial dysfunction and inflammation. Vice versa, increased mitochondrial dysfunction and inflammation can directly affect lipid metabolism. This study identifies JAML, a junction adhesion molecule (JAM)-like protein, as an important mediator regulating podocyte lipid metabolism through Sirt1-mediated SREBP1 signaling. ABCA1, ATP-binding cassette subfamily A member 1; ABCG1, ATP-binding cassette subfamily G member 1; ACC1, acetyl-CoA carboxylase alpha; ACO2, aconitase 2; APOE, apolipoprotein E; CD36, cluster of differentiation 36; CPT1, carnitine palmitoyltransferase 1A; FAS, FAS cell surface death receptor; L-FABP, fatty acid-binding protein; IL6, interleukin 6; IL18, interleukin 18; LDLR, low-density lipoprotein receptor; LXR, liver X receptor α; NLRP3, NLR family pyrin-domain-containing 3; PPARα, peroxisome proliferator-activated receptor alpha; PPARδ, peroxisome proliferator-activated receptor delta; SCAP, SREBF chaperone; SCD1, stearoyl-CoA desaturase; SREBP1c, sterol regulatory element-binding transcription factor 1; SREBP2, sterol regulatory element-binding transcription factor 2; SOAT1, sterol O-acyltransferase; TNF, tumor necrosis factor alpha.
Altered cellular lipid metabolism and accumulation of lipid droplets in association with mitochondrial dysfunction and/or inflammation occurs in a variety of tissues, including, but not limited to, the kidney, adipose tissue, liver, and brain, thus contributing to many age-related metabolic and non-metabolic diseases such as kidney disease, atherosclerosis, Alzheimer’s disease, Parkinson’s disease, cardiovascular diseases, age-related macular degeneration, and others. Recently, mammalian lipid droplets have been demonstrated to be innate immune hubs that integrate cell metabolism and host defense, highlighting once more an active role of intracellular lipids as key modulators of cell function and survival (Bosch et al., 2020).
While the interplay between renal lipids, mitochondrial dysfunction, and inflammation has been studied, the interplay between lipids and the actin cytoskeleton is less well understood. Recent work demonstrated a link between RhoA-dependent acto-myosin contraction and SREPBP1 activation (Bertolio et al., 2019), and decreased expression of phosphatase and tensin homolog (PTEN) was shown to inactivate cofilin-1 leading to F-actin formation and promoting the excessive lipid uptake by podocytes (Shi et al., 2020), suggesting that a similar viscious cycle between renal lipids and actin cytoskeleton remodeling might exist.
In this issue of Cell Metabolism, a study by Fu et al. (2020) demonstrates that expression of junctional adhesion molecule-like (JAML), a protein shown to be localized at the plasma membrane in the areas of cell-cell contacts where it enhances cell adhesion of leukocytes to endothelial cells, is significantly induced in podocytes treated with sera obtained from patients with diabetic kidney disease (DKD) and by other stimuli present in diabetic milieu such as high glucose, palmitic acid, cholesterol, and oxidized low-density lipoprotein (ox-LDL). Additionally, JAML expression was found increased in the glomeruli of experimental models of DKD, and in glomeruli and sera of patients with DKD as well as of patients with focal and segmental glomerulosclerosis (FSGS) and membranous nephropathy (MN). More importantly, JAML expression positively correlated with serum creatinine and lipid accumulation and negatively correlated with the estimated glomerular filtration rate (a clinical readout of kidney function) in patients with DKD, while podocyte-specific genetic JAML deficiency attenuated lipid accumulation and renal dysfunction in two diabetic mouse models of DKD (namely, streptozocin high-fat-diet-fed and db/db mice). Finally, Sirt1 was identified as a key mediator linking JAML to AMP activated protein kinase/sterol regulatory element-binding protein 1 (AMPK/SREBP1) signaling, an interesting observation as a role for JAM family members in lipid metabolism was only recently discovered (Rahman et al., 2016).
The primary effect of JAML on lipid species such as cholesteryl esters and free fatty acids accompanied by an increase in lipid droplets challenges the concept that lipid droplets are a cellular defense against lipotoxicity. It suggests instead that lipid droplets are very active scavenging organelles that can orchestrate the function of other organelles in response to stress by modulating storage and lipolysis across different subcellular compartments. While this study demonstrates a link between JAML and lipid metabolism, it does not address whether JAML directly affects actin dynamics via the AMPK pathway, similarly to what was recently described for TRPC6 in mediating insulin-dependent activation of AMPK, actin reorganization, and glucose uptake in podocytes (Rachubik et al., 2018). JAML may also indirectly influence the ability of lipid droplets to supply free cholesterol to plasma membranes, where key constituents of the glomerular filtration barrier such as podocin rely on cholesterol binding to function and transactivate other proteins, such as TRPC6. Sodium-glucose co-transporter 2 (SGLT2) inhibitors were recently found to be beneficial in patients with diabetes and kidney disease. While the renoprotective effects of these agents are thought to be related to glycosuria and to protection from glomerular hyperfiltration, the observation that they are also clinically beneficial in non-metabolic kidney diseases (Heerspink et al., 2020) and that they induce AMPK and SIRT1 in adipocytes (Yang et al., 2020) strongly suggests that renoprotection by SGLT2 inhibitors may be mediated through improved lipid metabolism and fatty acid oxidation.
Understanding and identifying the key pathways contributing to podocyte injury is fundamental to the development of new therapeutic strategies for the treatment of patients with DKD and other glomerular diseases. The identification of JAML as a novel mediator contributing to glomerular disease progression and of its role in regulating lipid metabolism in podocytes further strengthens the concept that targeting cellular lipid metabolism may represent a novel therapeutic strategy for patients with glomerular diseases of metabolic and non-metabolic origin. Whether targeting JAML or lipid metabolism is sufficient to prevent glomerular disease progression in patients with glomerular diseases remains to be established.
ACKNOWLEDGMENTS
A.F. and S.M. are supported by NIH grants R01DK117599, R01DK104753, and R01CA227493, and by Boehringer Ingelheim. A.F. is supported by NIH grants U54DK083912, UM1DK100846, U01DK116101, and UL1TR000460 (Miami Clinical Translational Science Institute, Translational Science Institute, from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities). We give a special thanks to the Katz family for their continuous support. Figure was created with https://biorender.com/.
DECLARATION OF INTERESTS
A.F. and S.M. are inventors on pending or issued patents (PCT/US11/56272, PCT/US12/62594, PCT/US2019/041730, PCT/US2019/032215, PCT/US13/36484, and PCT 62/674,897) aimed at diagnosing or treating proteinuric kidney diseases. They stand to gain royalties from their future commercialization of these patents. A.F. is Vice President of L&F Health LLC and is a consultant for ZyVersa Therapeutics, Inc. ZyVersa Therapeutics, Inc. has licensed worldwide rights to develop and commercialize hydroxypropyl-beta-cyclodextrin from L&F Research for the treatment of kidney disease. S.M. holds indirect equity interest in, and potential royalty from, ZyVersa Therapeutics, Inc. by virtue of assignment and licensure of a patent estate.
REFERENCES
- Bertolio R, Napoletano F, Mano M, Maurer-Stroh S, Fantuz M, Zannini A, Bicciato S, Sorrentino G, and Del Sal G (2019). Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nat. Commun. 10, 1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Sánchez-Álvarez M, Fajardo A, Kapetanovic R, Steiner B, Dutra F, Moreira L, López JA, Campo R, Marí M, et al. (2020). Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science 370, eaay8085. [DOI] [PubMed] [Google Scholar]
- Ducasa GM, Mitrofanova A, Mallela SK, Liu X, Molina J, Sloan A, Pedigo CE, Ge M, Santos JV, Hernandez Y, et al. (2019). ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J. Clin. Invest. 129, 3387–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Sun Y, Wang M, Hou Y, Huang W, Zhou D, Wang Z, Yang S, Tang W, Zhen J, et al. (2020). Elevation of JAML promotes diabetic kidney disease by modulating podocyte lipid metabolism. Cell Metab. 32, this issue, 1052–1062. [DOI] [PubMed] [Google Scholar]
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. ; DAPA-CKD Trial Committees and Investigators (2020). Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446. [DOI] [PubMed] [Google Scholar]
- Rachubik P, Szrejder M, Rogacka D, Audzeyenka I, Rychłowski M, Angielski S, and Piwkowska A (2018). The TRPC6-AMPK pathway is involved in insulin-dependent cytoskeleton reorganization and glucose uptake in cultured rat podocytes. Cell. Physiol. Biochem. 51, 393–410. [DOI] [PubMed] [Google Scholar]
- Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, Neish AS, Li H, Tan S, et al. (2016). Loss of junctional adhesion molecule a promotes severe steatohepatitis in mice on a diet high in saturated fat, fructose, and cholesterol. Gastroenterology 151, 733–746.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M, Teng B, Gu C, Shchedrina VA, Kasaikina M, Pham VA, Hanke N, Rong S, Gueler F, Schroder P, et al. (2015). Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models. Nat. Med. 21, 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Wang C, Zhou X, Li Y, Ma Y, Zhang R, and Li R (2020). Downregulation of PTEN promotes podocyte endocytosis of lipids aggravating obesity-related glomerulopathy. Am. J. Physiol. Renal Physiol. 318, F589–F599. [DOI] [PubMed] [Google Scholar]
- Yang X, Liu Q, Li Y, Tang Q, Wu T, Chen L, Pu S, Zhao Y, Zhang G, Huang C, et al. (2020). The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte 9, 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]


