Abstract
This study evaluated the relationship among nausea, anxiety, and orthostatic symptoms in pediatric patients with chronic unexplained nausea. We enrolled 48 patients (36 females) aged 15 ± 2 years. Patients completed the Nausea Profile, State-Trait Anxiety Inventory for Children and underwent 70° head upright tilt testing (HUT) to assess for orthostatic intolerance (OI) and measure heart rate variability (HRV). We found nausea to be significantly associated with trait anxiety, including total nausea score (r = 0.71, p < 0.01) and 3 subscales: somatic (r = 0.64, p < 0.01), gastrointestinal (r = 0.48, p = 0.01), and emotional (r = 0.74, p < 0.01). Nausea was positively associated with state anxiety, total nausea (r = 0.55, p < 0.01), somatic (r = 0.48, p < .01), gastrointestinal (r = .30, p < .05), and emotional (r = .64, p < .01) subscales. Within 10 min of HUT, 27 patients tested normal and 21 demonstrated OI. After 45 min of HUT, only 13 patients (27 %) remained normal. Nausea reported on the Nausea Profile before HUT was associated with OI measured at 10 min of tilt (nausea total r = 0.35, p < 0.05; nausea emotional subscale r = 0.40, p < 0.01) and lower HRV at 10 min of HUT (F = 6.39, p = 0.01). We conclude that nausea is associated with both anxiety symptoms and OI. The finding of decreased HRV suggests an underlying problem in autonomic nervous system function in children and adolescents with chronic unexplained nausea.
Keywords: Anxiety, Nausea, Orthostatic intolerance, Heart rate variability, Functional gastrointestinal disorders, Autonomic dysfunction
Introduction
Chronic nausea is a debilitating and under-recognized symptom in children and adolescents with precipitating triggers and mechanisms that are not well understood. With prolonged symptoms, the child’s and family’s daily life can be profoundly disrupted with extended school absences and reduced quality of life (Sagawa et al. 2013; Tarbell and Li 2013) that impacts not only the child, but also the family as a whole. When nausea is persistent and unexplained by routine diagnostic tests, it is often labeled as “functional” by default. As a result, treatment is empiric and constrained by a lack of an evidence-based strategy.
Recently, studies have reported evidence of an association between autonomic dysfunction in children and functional gastrointestinal disorders (FGIDs), including chronic nausea (Antiel et al. 2008), cyclic vomiting syndrome (Rashed et al. 1999; Chelimsky and Chelimsky 2007), and functional abdominal pain (Chelimsky et al. 2001; Axelrod et al. 2006; Jarrett et al. 2012). Specifically, patients with these functional symptoms have been shown to have both increased sympathetic activity and reduced parasympathetic vagal modulation of the heart as demonstrated by lower heart rate variability (HRV) (To et al. 1999; Sowder et al. 2010). The association between the autonomic nervous system and FGIDs has also been demonstrated in pediatric patients with nausea and orthostatic intolerance, characterized by symptoms such as lightheadedness, blood pressure instability, and syncope in pediatric patients presenting with nausea, including a subset with postural orthostatic tachycardia syndrome (POTS) (Stewart 2000; Sullivan et al. 2005). Further, it has been shown that treatment of the orthostatic intolerance may improve gastrointestinal symptoms including nausea (Fortunato et al. in press).
Due to their chronic nature and limited and often only modestly effective treatment options, these conditions are often associated with psychological sequelae. For example, anxiety is a common comorbidity in children with FGIDs, including functional abdominal pain (Dorn et al. 2003; Campo et al. 2004; Dufton et al. 2009; Shelby et al. 2013) and cyclic vomiting syndrome (Tarbell and Li 2008; Coskun and Alyanak 2011). While there is a substantial literature documenting an association between anxiety and gastrointestinal symptoms such as nausea, vomiting, and abdominal pain, the mechanisms underlying this association have not been determined. It is not clear whether these associations represent independent comorbidities or whether there are similar underlying etiologies. One commonality in both anxiety and nausea is the finding of altered sympathovagal balance, as measured by low HRV (Boyce et al. 2001; Yeragani et al. 2001; Sharma et al. 2011), raising the possibility that autonomic dysfunction may play a role in both anxiety and nausea symptoms.
The objective of this exploratory study was to evaluate the relationship among nausea, anxiety, and orthostatic symptoms in pediatric patients with chronic unexplained nausea and to determine the potential role of the autonomic nervous system by measuring HRV.
Methods
Participants
A research associate identified participants with chronic unexplained nausea from a pediatric gastroenterology clinic at Wake Forest Baptist Medical Center. Patients aged 10–19 were recruited from the clinic if they meet Rome III criteria for childhood functional dyspepsia with nausea as the predominant symptom. These criteria included: persistent or recurrent pain or discomfort (including nausea) in the upper abdomen not relieved with defecation and not associated with an inflammatory, anatomic, metabolic, or neoplastic process with symptoms at least once per week for at least 2 months prior to diagnosis (Foundation 2006). Patients were excluded if a metabolic, mechanical, or mucosal inflammatory cause had been defined to explain their gastrointestinal symptoms. Patients with significant cardiac or cardiovascular disease, malignancy, or other comorbid conditions precluding successful completion of a 45-min tilt test were excluded. Subjects who were unwilling to discontinue medications affecting autonomic function were excluded. Only English speaking participants were enrolled. The Wake Forest Baptist Medical Center’s Institutional Review Board approved this study. Parents and children provided written consent/assent prior to study participation.
Measures
Pediatric participants completed the Nausea Profile (NP) (Muth et al. 1996) within 2 weeks of head upright tilt testing (HUT), a 17-item questionnaire validated in adults, that assesses the subjective experience of nausea symptoms and provides a Total score and 3 subscale scores: Somatic, Gastrointestinal Distress, and Emotional Distress. Nausea scores may range from 0 to 100, with higher scores indicative of more intense nausea. The State-Trait Anxiety Inventory for Children (STAI-C) (Speilberger et al. 1973) was used to assess state anxiety (SA), transient anxiety symptoms, and Trait anxiety (TA), enduring anxiety symptoms, with higher scores indexing more anxiety. Scores may range from 20 to 60. The STAI-C was administered on the same day and prior to HUT.
Head upright tilt (HUT): The HUT was used to assess orthostatic intolerance (OI). Participants were asked to discontinue all medications that affect autonomic function 7 days before testing. The tilt test was performed from 0° to 70° for 45 min to objectively define cardiovascular OI. OI was diagnosed if any of the following conditions was present on the HUT test: 1. postural orthostatic tachycardia syndrome (POTS): defined as a HR ≥120 beats per minute or a 40 beats per minute increase from baseline sustained for 2 min during the first 10 min of tilt; 2. orthostatic hypotension (OH): defined as a 25 mmHg decrease in systolic blood pressure from baseline sustained for a minimum of 2 min; and 3. neurocardiogenic syncope: defined by the presence of hypotension and syncope; 4. postural orthostatic tachycardia syndrome associated with syncope: defined by the presence of POTS followed by syncope during the 45-min HUT. Continuous blood pressure and heart rate readings were acquired from noninvasive finger arterial pressure measurements for a minimum of 5 min in subjects in the supine position, prior to the tilt, and continuously throughout the 70° tilt. Systolic blood pressure and RR interval (RRI) files were acquired (BIOPAC acquisition software, Santa Barbara, CA) at 1,000 HZ and analyzed using Nevrokard BRS software (Nevrokard BRS, Medistar, Ljubljana, Slovenia). Heart rate variability was measured by the root mean square of successive beat-to-beat differences in R–R interval duration (rMSSD) (Shaltout et al. 2011).
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics for the Macintosh, version 21.0 (2013). Cronbach’s alpha was used to evaluate the internal reliability of the NP in the pediatric sample. Pearson’s correlation coefficients were used to evaluate associations among anxiety, nausea, OI, and HRV. ANOVA was used to evaluate differences in HRV between participants with and without OI at 10 and 45 min of HUT. Missing data for any dependent variable were excluded from analyses. Given the exploratory nature of this research results with p < 0.05 were considered statistically significant.
Results
Forty-eight patients, 36 females and 12 males were enrolled in the study (mean age: 15 years, SD = 2). In addition to chronic unexplained nausea of at least 3 months duration, patients also had comorbid medical disorders, including tension or migraine headaches (n = 18, 37 %), asthma (n = 4, 8 %), gastroparesis (n = 4, 8 %), gastroesophageal reflux disease (n = 3, 6 %), seizure disorder (n = 1, 2 %), Chiari malformation (n = 1, 2 %), and inflammatory bowel disease (n = 1, 2 %). Psychiatric diagnoses included mood disorders (n = 9, 19 %), attention disorders (n = 4, 8 %), anxiety disorders (n = 3, 6 %), and autistic spectrum disorder (n = 1, 2 %). Patient comorbid symptoms included lightheadedness, dizziness (n = 38, 79 %), abdominal pain (n = 25, 52 %), vomiting (n = 22, 46 %), fatigue (n = 15, 31 %), and other gastrointestinal symptoms such as dysphagia, early satiety, constipation, and diarrhea (n = 22, 46 %). Patient gastrointestinal symptoms were managed with acid-suppressing agents (n = 20, 42 %), antiemetics (n = 14, 29 %), laxatives (n = 10, 9 %), analgesics (n = 7, 15 %), low-dose tricyclic antidepressants (n = 7, 15 %), and promotility agents (n = 4, 8 %). Psychiatric disorders were managed with selective serotonin reuptake inhibitors (n = 5, 10 %), atypical antidepressants (n = 3, 6 %), stimulant medications (n = 4, 8 %), and atypical antipsychotic agents (n = 2, 4 %).
The NP, originally developed to assess nausea in adults, demonstrated excellent internal reliability in the pediatric participants (Total Score: Cronbach’s α = 0.941; subscale scores: Cronbach’s α range = 0.86–0.92). Nausea Scores (mean, SD) were as follows: Total Nausea: 43 (21); Emotional Distress: 29 (24); Gastrointestinal Distress: 56 (25); Somatic Distress: 46 (22). Mean State Anxiety was 35 (SD = 7), and mean Trait Anxiety was 37 (SD = 8). Anxiety was significantly associated with the Total Nausea score and all 3 subscales (Table 1).
Table 1.
Associations among anxiety and nausea symptoms
| Emotional distress | Gastrointestinal distress | Somatic distress | Total nausea | |
|---|---|---|---|---|
| State anxiety | 0.64** | 0.30* | 0.48** | 0.55** |
| Trait anxiety | 0.74** | 0.48** | 0.64** | 0.71** |
Values are Pearson’s correlation coefficients
p < 0.05;
p ≤ 0.01
The response to tilt was defined in 2 groups: response within the first 10 min of tilt and the response throughout the entire 45 min of tilt. Within the first 10 min of tilt, 21 patients (44 %) were categorized as demonstrating OI, including POTS (n = 17), OH (n = 2), syncope (n = 2), and normal (n = 27). By the end of the 45-min tilt, 35 patients (73 %) demonstrated OI, including POTS (n = 12), OH (n = 5), syncope alone (n = 8), POTS with syncope (n = 10), and normal (n = 13) (Fig. 1).
Fig. 1.
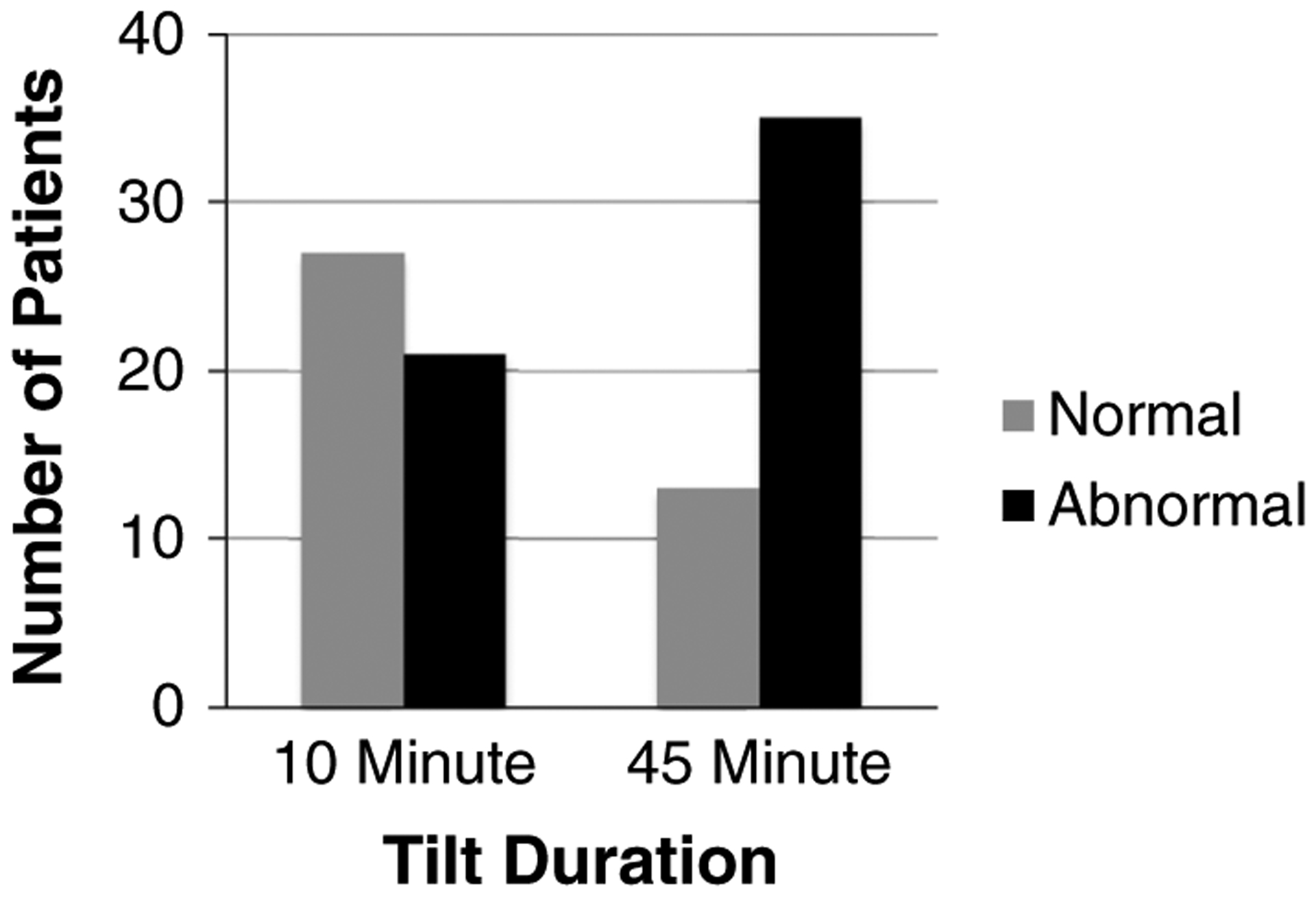
Number of patients with symptoms of orthostatic intolerance (abnormal) at 10 and 45 min of head upright tilt
Nausea as assessed by NP collected prior to HUT was associated with OI (defined at 10 min of tilt) and lower HRV at 10 min (F = 6.39, p = 0.01) (Table 2). OI (defined at 45 min of tilt) was not significantly associated with HRV (F = 0.185, ns). Anxiety scores were not significantly associated with OI or HRV.
Table 2.
Association of nausea with orthostatic intolerance and heart rate variability (HRV)
| Nausea Profile | 10-min tilt | 45-min tilt | HRV: rMssD |
|---|---|---|---|
| Emotional distress | 0.40** | ns | ns |
| Gastrointestinal distress | 0.27 | ns | −0.32* |
| Somatic distress | 0.28 | ns | −0.35* |
| Total nausea | 0.35* | ns | −0.35* |
Values are Pearson’s correlation coefficients
p < 0.05;
p < 0.01
Discussion
Chronic nausea is a very common symptom in children and is often associated with serious comorbid symptoms making it difficult to define objectively. The cause of chronic nausea is complex, with precipitating triggers and mechanisms not well described. Children with persistent nausea unexplained by routine diagnostic testing often have evidence of psychiatric symptoms such as anxiety and depression (Tarbell and Li 2008). The origin of these symptoms is likely multifactorial often overlapping with other physical symptoms such as abdominal discomfort. We chose to use the State anxiety scale for this study specifically as it does not tap into the physical manifestations of anxiety (e.g., racing heart rate and abdominal discomfort). Our data showed that anxiety strongly correlated with nausea in pediatric patients with chronic symptoms. These findings are consistent with previous studies which demonstrated a high prevalence of anxiety symptoms in pediatric patients with functional gastrointestinal symptoms such as nausea, vomiting, and abdominal pain (Campo et al. 2004; Tarbell and Li 2008; Dufton et al. 2009) and underscores the need for a multidisciplinary approach in treating these patients.
To better understand this patient population, we used the tilt table test as a provocative stimulus to determine potential triggers for their nausea. We previously identified a group of children between the ages of 10–18 years whose diagnostic workup for chronic unexplained nausea had unexpectedly revealed the presence of orthostatic intolerance, primarily defined as POTS (Fortunato et al. 2011). In our subjects, nausea was positively associated with OI. This association between OI and gastrointestinal symptoms has been described previously. For instance, posturally mediated heart rate changes resulting in abnormal gastric electrical activity in patients with functional abdominal pain have recently been shown in children (Friesen et al. 2007; Safder et al. 2010). Several studies have also shown that abnormal gastric myoelectrical activity in pediatric patients may have a role in the pathophysiology of functional dyspepsia, nausea, and feeding problems (Cucchiara et al. 1992; Koch et al. 1993; Riezzo et al. 2000; Levy et al. 2001; Diamanti et al. 2003; Friesen et al. 2006).
Because of the high incidence of anxiety symptoms in our population, we also compared the relationship between anxiety and OI. We found that anxiety was not associated with OI. While we did not demonstrate a specific link between anxiety and OI, this may in part be due to sampling methods or limitations of the instrument used to assess anxiety. Improvements in the assessment of anxiety, through the use of methods such as ecological momentary assessment (Shiffman et al. 2008), which allows for “in the moment” tracking of symptoms could improve our understanding of the relationships among anxiety, nausea, and orthostatic symptoms. The use of technology, such as a smart phone application (Runyan et al. 2013), to gather these data could contribute improved ecological validity to our understanding of these relationships. Further, the use of standardized diagnostic anxiety interviews, such as the Anxiety Disorder Interview Schedule (Silverman and Albano 2004) could better characterize the anxiety symptoms in the participants, allowing for a more detailed understanding of the relationship between categorical anxiety disorders, nausea, and orthostatic symptoms, which could assist in the development of phenotypes to assist in individualized approaches to the management of chronic nausea.
To further explore the potential role of the autonomic nervous system particularly in light of the high incidence of OI in patients with chronic nausea, we studied the change in HRV during the tilt test. Nausea was associated with reduced HRV during HUT, which is consistent with other studies that have found autonomic dysfunction, including OI and lower cardiovagal tone and enhanced sympathovagal balance in pediatric patients with chronic or intermittent nausea and vomiting (To et al. 1999; Sullivan et al. 2005; Chelimsky and Chelimsky 2007; Antiel et al. 2008). Further, the finding that children with functional abdominal pain also had evidence of reduced HRV and that the use of biofeedback to improve cardiovagal tone is associated with symptom improvement (Sowder et al. 2010) holds promise as a potential treatment for the children in this study. Anxiety symptoms were not associated with HRV in this study. A more thorough assessment of anxiety as discussed above may improve the ability to better evaluate the relationship between anxiety and reduced HRV as has been documented in prior research (Mussgay and Rüddel 2004). We used the Nausea Profile (Muth et al. 1996), a validated method to assess nausea in adults in this study as there are few validated scales for assessing nausea in children and adolescents (Baxter et al. 2011). Our finding that the Nausea Profile is a reliable scale for the assessment of nausea in older children and adolescents can help with more standardized assessment of multiple dimensions of nausea in this population, allowing for comparisons across studies.
Our study has several limitations. The sample was derived from a pediatric gastroenterology subspecialty clinic, which may have biased the generalizability of the findings to the general pediatric population. Further, 75 % of the participants were female, and thus these findings may not be applicable to male pediatric patients with chronic nausea. The measurement of anxiety and nausea symptoms with validated measures was performed prior to the tilt testing and thus the relationship among anxiety symptoms and nausea was not assessed in real time. Further, the use of methods such as ecological momentary assessment could allow for the characterization of intra-individual patterns in the association between nausea, orthostatic symptoms, and anxiety that could inform the development of phenotypes of children with chronic unexplained nausea that is not possible with the cross-sectional design used in the current study.
The mechanism for nausea in the subjects in this study remains unclear, but is likely multifactorial. The high prevalence of OI does raise the possibility of an underlying autonomic etiology as the primary cause for nausea in our subjects. This may in fact represent a new phenotype of chronic nausea previously deemed idiopathic in nature. While the pathophysiology of this potential association is not well defined, we have recently observed an exaggerated increase in vasopressin during tilt in subjects with OI and tilt induced nausea as one possible contributing factor (Wagoner et al. 2013). It is imperative that future research and study designs employ a multidisciplinary approach, including disciplines such as gastroenterology, cardiology, psychology, endocrinology, and neurology. This will allow for a more thorough approach to elucidate the mechanisms underlying chronic nausea in children and adolescents. Further, the use of a comparison group of children with a FGID such as irritable bowel syndrome without nausea is needed to further our understanding of the specific relationship among nausea, orthostatic intolerance, and anxiety symptoms. This comparison is essential to determine whether the relationships among these symptoms identified in this study are more broadly generalizable.
In conclusion, the observation that nausea, anxiety, orthostatic symptoms, and reduced cardiovagal tone coexist in children presenting with chronic unexplained nausea offers the potential to increase our understanding of the underlying pathophysiological mechanisms. While the mechanism(s) explaining these associations have not been fully established, the early data suggest the presence of an underlying problem in autonomic nervous system function in children and adolescents with unexplained nausea. The need for further research is critical to development of more rational and focused treatment strategies.
Acknowledgments
This work was supported by a grant from the American Heart Association, National Center Clinical Research Program, AHA12CRP9420029 (John Fortunato, MD PI). We also acknowledge the contributions of Anya Brown, study coordinator, for her participation in the collection and management of the data for this study.
Abbreviations
- OI
Orthostatic intolerance
- FGIDs
Functional gastrointestinal disorders
- STAI-C
State-Trait Anxiety Scale for Children
- NP
Nausea Profile
- HRV
Heart rate variability
- HUT
Head upright tilt
- POTS
Postural orthostatic tachycardia syndrome
- OH
Orthostatic hypotension
Footnotes
Conflict of interest The authors declare that they have no conflict of interest relevant to this article to disclose.
Contributor Information
Sally E. Tarbell, Department of Child Psychiatry and Behavioral Sciences, Children’s Hospital Colorado, University of Colorado Anschutz Medical Campus, B130, 13123 E. 16th Ave., Aurora, CO 80045, USA Department of Pediatrics, Digestive Health Institute, Children’s Hospital Colorado, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Hossam A. Shaltout, Hypertension and Vascular Research Center, Wake Forest University, Winston-Salem, NC, USA Departments of Obstetrics and Gynecology and General Surgery, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Ashley L. Wagoner, Neuroscience Graduate Program, Wake Forest Graduate School of Arts and Sciences, Wake Forest School of Medicine, Winston-Salem, NC, USA
Debra I. Diz, Hypertension and Vascular Research Center, Wake Forest University, Winston-Salem, NC, USA Neuroscience Graduate Program, Wake Forest Graduate School of Arts and Sciences, Wake Forest School of Medicine, Winston-Salem, NC, USA.
John E. Fortunato, Department of Pediatrics, Digestive Health Institute, Children’s Hospital Colorado, University of Colorado Anschutz Medical Campus, Aurora, CO, USA Hypertension and Vascular Research Center, Wake Forest University, Winston-Salem, NC, USA.
References
- Antiel R, Risma J, Grothe R, Brands C, Fischer P (2008) Orthostatic intolerance and gastrointestinal motility in adolescents with nausea and abdominal pain. J Pediatr Gastroenterol Nutr 46:285–288 [DOI] [PubMed] [Google Scholar]
- Axelrod F, Chelimsky GC, Weese-Mayer D (2006) Pediatric autonomic disorders. Pediatrics 118:309–321 [DOI] [PubMed] [Google Scholar]
- Baxter A, Watcha M, Baxter W, Leong T, Wyatt M (2011) Development and validation of a pictorial nausea rating scale for children. Pediatrics 127:e1542–e1549 [DOI] [PubMed] [Google Scholar]
- Boyce W, Quas J, Alkon A, Smider N, Essex M, Kupfer D (2001) Autonomic reactivity and psychopathology in middle childhood. Br J Psychiatry 179:144–150 [DOI] [PubMed] [Google Scholar]
- Campo J, Bridge J, Ehrmann M et al. (2004) Recurrent abdominal pain, anxiety and depression in primary care. Pediatrics 113:817–824 [DOI] [PubMed] [Google Scholar]
- Chelimsky TC, Chelimsky GC (2007) Autonomic abnormalities in cyclic vomiting syndrome. J Pediatr Gastroenterol Nutr 44:300–326 [DOI] [PubMed] [Google Scholar]
- Chelimsky GC, Boyle J, Tusing L, Chelimsky TC (2001) Autonomic abnormalities in children with functional abdominal pain: coincidence or etiology? J Pediatr Gastroenterol Nutr 33:47–53 [DOI] [PubMed] [Google Scholar]
- Coskun M, Alyanak B (2011) Psychiatric co-morbidity and efficacy of mirtazapine treatment in young subjects with chronic or cyclic vomiting syndrome: a case series. J Neurogastroenterol Motil 17:305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiara S, Minella R, Riezzo G, Vallone G, Vallone P, Castellone F, Auricchio S (1992) Reversal of gastric electrical dysrhythmias by cisapride in children with functional dyspepsia. Report of three cases. Dig Dis Sci 37:1136–1140 [DOI] [PubMed] [Google Scholar]
- Diamanti A, Bracci F, Gambarara M et al. (2003) Gastric electric activity assessed by electrogastrography and gastric emptying scintigraphy in adolescents with eating disorders. J Pediatr Gastroenterol Nutr 37:35–41 [DOI] [PubMed] [Google Scholar]
- Dorn L, Campo J, Thato S, Dahl R, Lewin D, Chandra R, DiLorenzo C (2003) Psychological comorbidity and stress reactivity in children and adolescents with recurrent abdominal pain and anxiety disorders. J Am Acad Child Adolesc Psychiatry 42:66–75 [DOI] [PubMed] [Google Scholar]
- Dufton L, Dunn M, Compas B (2009) Anxiety and somatic complaints in children with recurrent abdominal pain and anxiety disorders. J Pediatr Psychol 34:176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato J, Shaltout H, Larkin M, Rowe P, Diz D, Koch K (2011) Fludrocortisone improves nausea in children with orthostatic intolerance (OI). Clin Auton Res 21:419–423 [DOI] [PubMed] [Google Scholar]
- Fortunato J, Wagoner A, Harbinson R, D’Agostino R Jr, Shaltout H, Diz D (in press) Effect of fludrocortisone acetate on chronic unexplained nausea and abdominal pain in children with orthostatic intolerance. J Pediatr Gastroenterol Nutr. doi: 10.1097/MPG.0000000000000305 [DOI] [PubMed] [Google Scholar]
- Foundation R (2006) Rome III: functional gastrointestinal disorders, 3rd edn. Allen Press, Lawrence [Google Scholar]
- Friesen C, Lin Z, Hyman P et al. (2006) Electrogastrography in pediatric functional dyspepsia: relationship to gastric emptying and symptom severity. J Pediatr Gastroenterol Nutr 42:265–269 [DOI] [PubMed] [Google Scholar]
- Friesen C, Lin Z, Schurman J, Andre L, McCallum R (2007) Autonomic nervous system response to a solid meal and water loading in healthy children: its relatioon to gastric myoelectrical activity. Neurogastroenterol Motil 19:376–382 [DOI] [PubMed] [Google Scholar]
- IBM SPSS Statistics for the Mac (2013) IBM SPSS Statistics for the Mac. IBM Corporation, Armonk [Google Scholar]
- Jarrett M, Heitkemper M, Czyzewski D, Zeltzer L, Shulman R (2012) Autonomic nervous system function in young children with functional abdominal pain or irritable bowel syndrome. J Pain 13:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K, Tran T, Stern R, Bingaman S, Sperry N (1993) Gastric myoelectrical activity in premature and term infants. Neurogastroenterol Motil 5:41–47 [DOI] [PubMed] [Google Scholar]
- Levy J, Harris J, Chen J, Sapoznikov D, Riley B, De La Nuez W, Khaskelberg A (2001) Electrogastrographic norms in children: toward the development of standard methods, reproducible results, and reliable normative data. J Pediatr Gastroenterol Nutr 33:455–461 [DOI] [PubMed] [Google Scholar]
- Mussgay L, Rüddel H (2004) Autonomic dysfunctions in patients with anxiety throughout therapy. J Psychophysiol 18:27–37 [Google Scholar]
- Muth E, Stern R, Thayer J, Koch K (1996) Assessment of the multiple dimensions of nausea: the Nausea Profile (NP). J Psychosom Res 40:511–520 [DOI] [PubMed] [Google Scholar]
- Rashed H, Abell T, Familoni B, Cardoso S (1999) Autonomic function in cyclic vomiting syndrome and classic migraine. Dig Dis Sci 44(8 suppl):74S–78S [PubMed] [Google Scholar]
- Riezzo G, Chiloiro M, Guerra V, Borrelli O, Salvia G, Cucchiara S (2000) Comparison of gastric electrical activity and gastric emptying in healthy and dyspeptic children. Dig Dis Sci 45:517–524 [DOI] [PubMed] [Google Scholar]
- Runyan J, Steenbergh T, Bainbridge C, Daugherty D, Oke L, Fry B (2013) A smartphone ecological momentary assessment intervention “App” for collecting real-time data and promoting self awareness. PLOS/one. doi: 10.1371/journal.pone.0071325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safder S, Chelimsky TC, O’Riordan M, Chelimsky GC (2010) Gastric electrical activity becomes abnormal in the upright position in patients with postural orthostatic tachycardia syndrome. J Pediatr Gastroenterol Nutr 51:314–318 [DOI] [PubMed] [Google Scholar]
- Sagawa T, Okamura S, Kakizaki S, Zhang Y, Morita K, Mori M (2013) Functional gastrointestinal disorders in adolescents and quality of school life. J Gastroenterol Hepatol 28:285–290 [DOI] [PubMed] [Google Scholar]
- Shaltout H, Diz D, Fortunato J (2011) Fludrocortisone acetate improves nausea and autonomic function during tilt in children with postural orthostatic tachycardia syndrome. In: Joint Meeting of the International Society for Autonomic Neuroscience (ISAN)/22nd Symposium of the American Autonomic Society (AAS), Buzios, Rio de Janeiro, Brazil [Google Scholar]
- Sharma R, Balhara Y, Sagar R, Deepak K, Mehta M (2011) Heart rate variability study of childhood anxiety disorders. J Cardiovasc Dis Res 2:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby G, Shirkey K, Sherman A et al. (2013) Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics 132:475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone A, Hufford M (2008) Ecological momentary assessment. Annu Rev Clin Psychol 4:1–32 [DOI] [PubMed] [Google Scholar]
- Silverman W, Albano A (2004) Anxiety disorder interview schedule (ADIS-IV). Child and parent schedules. Oxford University Press, New York [Google Scholar]
- Sowder E, Gevirtz R, Shapiro W, Ebert C (2010) Restoration of vagal tone: a possible mechanism of functional abdominal pain. Appl Psychophysiol Biofeedback 35:199–206 [DOI] [PubMed] [Google Scholar]
- Speilberger C, Edwards CD, Lushene J, Montouri J, Platzek D (1973) The State-Trait Anxiety Inventory for Children (STAIC). Consulting Psychologists Press, Palo Alto, CA [Google Scholar]
- Stewart J (2000) Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attentuated vagal baroflex and potentiated sympathetic vasomotion. Pediatr Res 48:218–226 [DOI] [PubMed] [Google Scholar]
- Sullivan S, Hanauer P, Rowe P, Barron D, Darbari A, Oliva-Hemker M (2005) Gastrointestinal symptoms associated with orthostatic intolerance. J Pediatr Gastroenterol Nutr 40:425–428 [DOI] [PubMed] [Google Scholar]
- Tarbell S, Li BUK (2008) Psychiatric symptoms in children and adolescents with cyclic vomiting syndrome and their parents. Headache 48:259–266 [DOI] [PubMed] [Google Scholar]
- Tarbell S, Li BU (2013) Health-related quality of life in children and adolescents with cyclic vomiting syndrome: a comparison with published data on youth with irritable bowel syndrome and organic gastrointestinal disorders. J Pediatr 163:493–497 [DOI] [PubMed] [Google Scholar]
- To J, Issenman RM, Kamath MV (1999) Evaluation of neurocardiac signals in pediatric patients with cyclic vomiting syndrome through power spectral analysis of heart rate variability. J Pediatr 135:363–366 [DOI] [PubMed] [Google Scholar]
- Wagoner A, Shaltout H, D’Agostino J, R B, Diz D, Fortunato J (2013) Increase in arginine vasopressin is associated with the reduction in blood pressure in patients with chronic nausea and orthostatic intolerance. In: North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN), Chicago, IL [Google Scholar]
- Yeragani V, Radhakrishna R, Pohl R, Jampala V, Balon R (2001) Heart rate and QT variability in children with anxiety disorders: a preliminary report. Depress Anxiety 13:72–77 [DOI] [PubMed] [Google Scholar]


