Abstract
Objectives
Talaromycosis is an invasive mycosis endemic to Southeast Asia. This study aimed to investigate the epidemiology, clinical features and prognostic factors of HIV-associated talaromycosis in Guangdong, China.
Methods
We retrospectively evaluated HIV patients hospitalized with histopathology- or culture-confirmed talaromycosis between 2011 and 2017. Factors associated with poor prognosis were identified using logistic regression.
Results
Overall, 1079 patients with HIV-associated talaromycosis were evaluated. Both the number and prevalence of talaromycosis among HIV admissions increased from 125 and 15.7% in 2011 to 253 and 18.8% in 2017, respectively, reflecting the increase in HIV admissions. Annual admissions peaked during the rainy season between March and August. Common clinical manifestations included fever (85.6%), peripheral lymphadenopathy (72.3%), respiratory symptoms (60.8%), weight loss (49.8%), skin lesions (44.5%) and gastrointestinal symptoms (44.3%). Common laboratory abnormalities were hypoalbuminaemia (98.6%), anaemia (95.6%), elevated aspartate aminotransferase level (AST) (76.9%), elevated alkaline phosphatase level (55.8%) and thrombocytopenia (53.7%). The median CD4 count was 9 cells/μL. Talaromyces marneffei was isolated from blood and bone marrow cultures of 66.6% and 74.5% of patients, respectively. The rate increased to 86.6% when both cultures were performed concurrently. At discharge, 14% of patients showed worsening conditions or died. Leucocytosis, thrombocytopenia, elevated AST, total bilirubin, creatinine and azole monotherapy independently predicted poor prognosis.
Conclusions
The incidence of HIV-associated talaromycosis has increased in Guangdong with the high HIV burden in China. Skin lesions were seen in less than half of patients. Induction therapy with azole alone is associated with higher mortality. Findings from this study should help to improve treatment of the disease.
Keywords: China, HIV, penicilliosis, Talaromyces marneffei, talaromycosis
Introduction
Talaromycosis, previously called penicilliosis, is a systemic fungal infection caused by the dimorphic fungus Talaromyces marneffei. It mainly affects immunodeficient individuals such as those infected with HIV-AIDS [1]. Talaromyces marneffei is endemic to Southeast Asia. In highly endemic regions in northern Thailand, Vietnam and southern China (Guangdong, Guangxi and Hong Kong) [2–6], the prevalence of talaromycosis as an HIV-associated opportunistic infection (OI) is only lower than that of tuberculosis and cryptococcosis [2,3]. The incidence of talaromycosis is also increasing in the HIV-negative population due to the expanding use of immunosuppressive drugs and chemotherapy and the rising number of solid and bone marrow transplantations [7–9]. Evidence suggests that the endemic areas of talaromycosis are increasing due to improved mass transportation and global warming [10–12]. Without timely diagnosis and effective antifungal treatment, the risk of mortality increases by up to 50% [2–6].
Guangdong is the most populous province of China, with a population of 113 million people. Guangdong is a crucial endemic area of talaromycosis because its warm and humid climate favours the growth of T. marneffei. Further, it has the highest HIV burden in China, with over 50 000 people living with HIV/AIDS in 2019[13]. The Guangzhou Eighth People’s Hospital (GZEPH) is the primary referral centre for HIV care in the capital city Guangzhou, with more than 15 000 HIV-infected patients registered for care. Talaromycosis is a common OI being treated at our hospital. Despite improved access to antiretroviral therapy (ART) in China, the number of both AIDS and talaromycosis admissions has increased over the past 10 years. However, this increasing trend has not been documented. In a review of 668 reported cases in mainland China from 1984 to 2009, 84% of infections were from Guangxi and Guangdong [14]. Despite being a hotspot for talaromycosis, there have only been a few studies on talaromycosis from Guangdong, and knowledge of the clinical epidemiology of talaromycosis in China is lacking [4,15].
This study aimed to investigate the epidemiology, clinical characteristics, antifungal treatments and prognostic predictors of talaromycosis outcomes in HIV patients in order to obtain evidence to improve the diagnosis and treatment of this emerging infection. Towards this goal, we conducted a systematic chart review of all HIV-infected patients hospitalized at GZEPH with talaromycosis over a 6-year period from 2011 to 2017. It is hoped that our findings will fill the gap in this area.
Methods
Study design and population
This retrospective cohort study was conducted at GZEPH, which admits more than 3000 HIV-infected patients annually. The study was approved by the Ethics Committee of GZEPH (approval no: 201908121). Informed consent was obtained.
We evaluated talaromycosis patients who were admitted to GZEPH between 1 January 2011 and 31 December 2017. The inclusion criteria were as follows: (1) HIV infection, (2) culture- or histopathologically confirmed diagnosis of talaromycosis, and (3) age ≥ 18 years. Patients with incomplete clinical data, laboratory results and therapy information were excluded from the analyses.
Diagnosis of talaromycosis
Talaromycosis was defined as any illness in which T. marneffei was identified via histopathology or was isolated from specimens including blood, bone marrow, bronchoalveolar lavage (BAL), pleural and/or other body fluid samples. Talaromyces marneffei was isolated according to standard culture techniques on Sabouraud dextrose agar [1]. Talaromyces marneffei was cultured as a mould at 25°C and as a yeast at 37°C. Identification was based upon the morphology of the colonies, demonstration of mould-to-yeast conversion, presence of a red pigment production at 25°C and microscopic morphology. Histopathological diagnosis was based on the identification of characteristic yeast cells with a midline septum in tissue sections of the lymph node, skin, lung, liver and bone marrow [1].
Data collection
The data were collected from the hospital’s electronic medical records and microbiology records. All patients were cross-checked for duplication in the database by name, date of birth and address. Only data from the first talaromycosis admission were collected. Data were independently double-entered into a Microsoft Access 2010 database to ensure data accuracy. They included demographics, modes of transmission, admission date, concurrent OIs, ART history, clinical symptoms and signs, laboratory results including a CD4 T-cell count, CD4/CD8 ratio, culture data, pathological examination, initial antifungal therapy regimen and patient outcomes.
Leucocytosis was defined as a white blood cell (WBC) count of > 9.5 × 109 cells/L; leucopenia, WBC count < 3.5 × 109 cells/L; anaemia, haemoglobin (Hb) < 130 g/L; thrombocytopenia, platelet count < 125×109 cells/L; hypoalbuminaemia, serum albumin level < 40 g/L. The thresholds for elevation of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin (TBIL) and creatinine levels were 50 U/L, 40 U/L, 125 U/L, 26 μmol/L and 133 μmol/L, respectively.
Antifungal treatment and outcome evaluation
Antifungal therapy was initiated in all patients within 12 h after the diagnosis of talaromycosis. Amphotericin B deoxycholate (AMB) was administered intravenously at a dose of 0.5–0.7 mg/kg per day for 2 weeks, followed by oral itraconazole capsule, 200 mg twice daily for a subsequent duration of 10 weeks. Patients who were intolerant or had contraindications to AMB were treated with intravenous voriconazole (400 mg every 12 h for the first day and then 200 mg every 12 h for 14 days) or intravenous itraconazole (200 mg twice daily for 2 days, then 200 mg once daily for 14 days). Thereafter, all patients were switched to oral itraconazole capsule, 200 mg twice daily for a subsequent duration of 10 weeks, followed by 200 mg once daily to prevent relapse until the patients regained a CD4 T-cell count of > 100 cells/μL for > 6 months [16]. Combination therapy with AMB and azole drugs was administered when the blood cultures at day 7 were still positive, even with AMB or azole alone. AMB was switched to voriconazole or itraconazole in cases of intolerable side effects. Voriconazole or itraconazole was switched to AMB when side effects to azoles occurred. ART was initiated in ART-naïve patients around 2 weeks after the initiation of antifungal therapy according to the national guidelines [16].
The outcomes at the time of discharge were evaluated by two physician investigators in consultation with a third physician in case of doubts. The outcomes were categorized according to a previous publication from Vietnam [16], as follows: (1) good outcome, defined as cure or improvement (negative repeated blood cultures, temperature returning to normal, and disappearance or reduction of skin lesions); (2) poor outcome, defined as death in the hospital, death at home, or worsening disease and expected to die at home (i.e. patients who were moribund or deteriorated clinically and were taken from hospital to die at home as per local custom); (3) outcome non-assessable (i.e. patients who left hospital early against medical advice or were transferred to other hospitals). The survival status was determined at least 12 weeks after discharge from a combination of medical records at GZEPH, the national report network of infectious diseases, and telephone surveys.
Statistical analysis
All statistical analyses were conducted using SPSS (v.25.0). Variables were described as the mean ± standard deviation or median and interquartile range (IQR). Student’s t-test and χ2 test were used for pairwise comparison of groups for continuous and categorical variables, respectively. The Wilcoxon rank-sum test was used for non-normally distributed data. Univariate and multivariate logistic regression analyses were performed using forward stepwise selection of predictor variables. Statistically significant variables (P < 0.05) in the univariate analysis were entered into a multivariate logistic regression model where continuous variables were dichotomized on the basis of the mean values and after exclusion of correlated variables.
Results
Incidence of talaromycosis amongst hospitalized HIV patients
Of the 7575 HIV-infected patients identified, 1214 (16.0%) had histopathologically and/or culture-confirmed talaromycosis. Both the number of talaromycosis admissions and the prevalence of talaromycosis among HIV admissions decreased between 2011 and 2012. However, they then steadily increased from 125 and 15.7% in 2011 to 253 and 18.8% in 2017, respectively, with a mean talaromycosis prevalence of 15.7% ± 2.4%. During the same study period, the number of admissions for HIV-related OI or malignancy consistently increased from 794 in 2011 to 1344 in 2017 (Fig. 1).
Fig. 1.
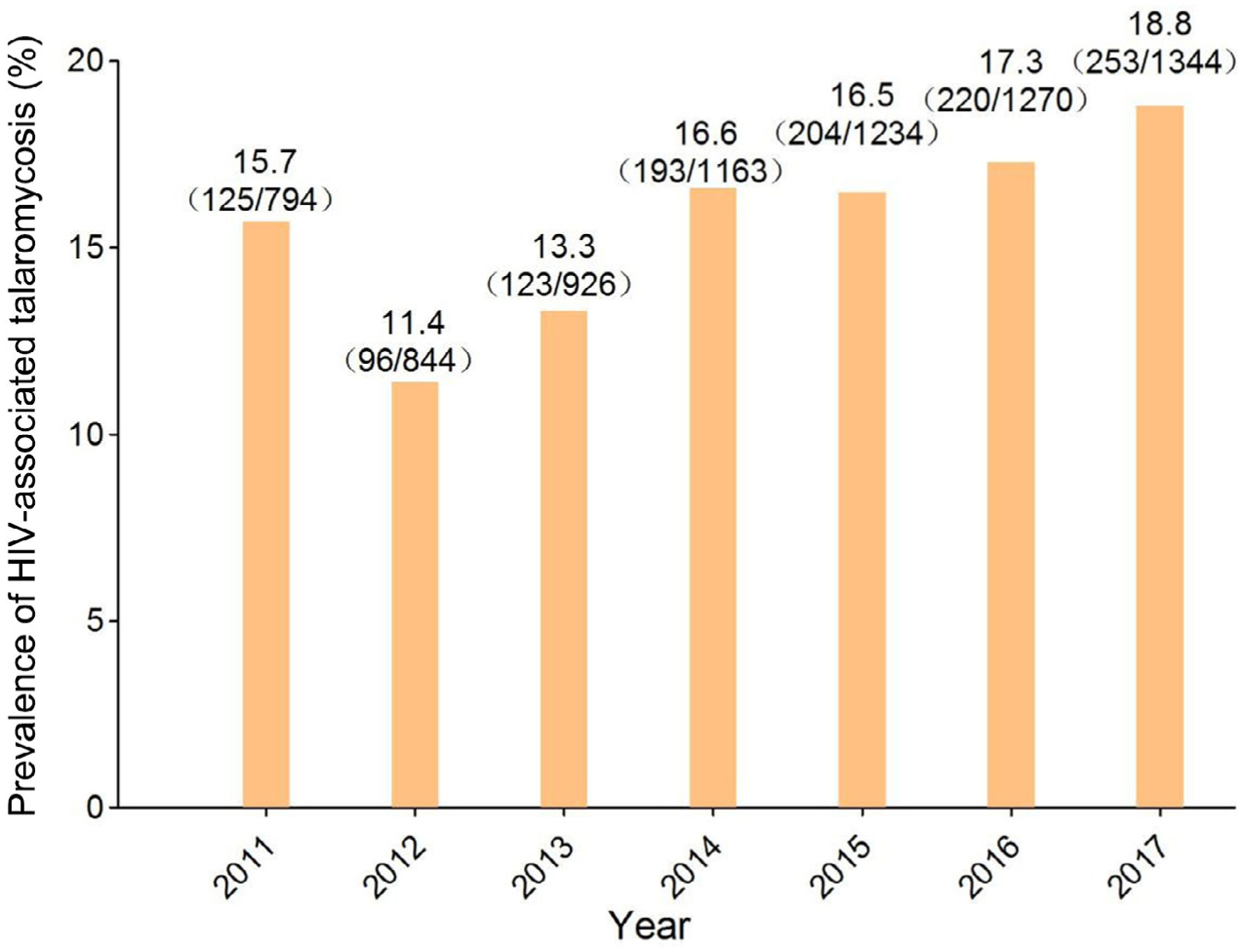
Prevalence of HIV-associated talaromycosis among HIV admissions from 2011 to 2017.
Talaromycosis seasonality
The monthly case distribution by number and percentage over the 6-year study period is shown in Fig. 2. The number of talaromycosis admissions was substantially higher during the rainy season from March to August [monthly mean: 128 (range: 106–148) cases] than during the dry season [monthly mean: 75 (range: 51–91) cases]. Compared with the dry season, the number of admissions during the rainy season increased by an average of 73%. The lowest numbers of admission were in November and December, which are the driest and coldest months of the year.
Fig. 2.
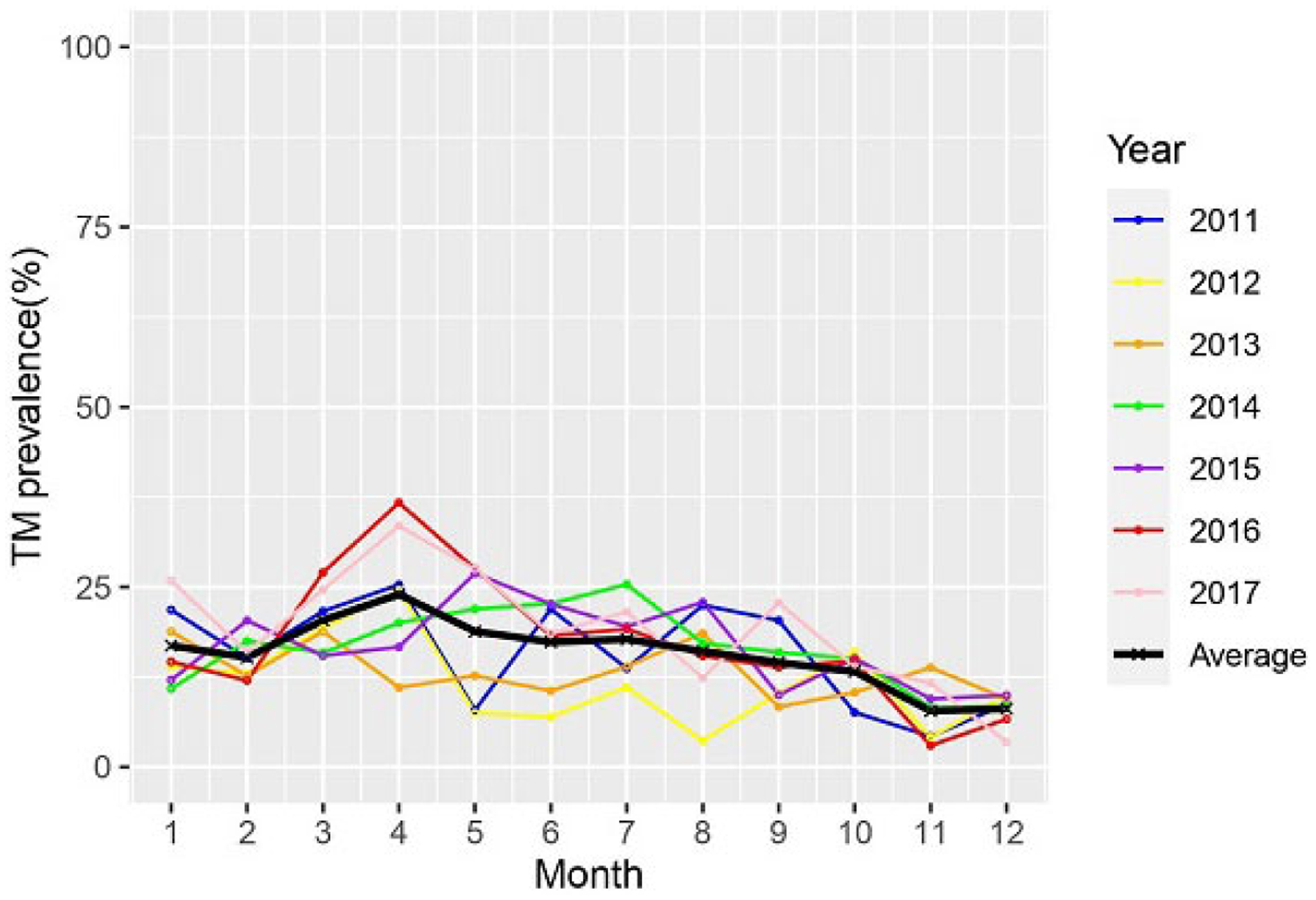
Total talaromycosis (TM) case distribution by month over a 6-year period from 2011 to 2017.
Epidemiological characteristics
A total of 1079 patients had complete data. The mean age was 38.8 ± 11.9 years (range: 18–78). The majority of the patients were male, with a male-to-female ratio of 3.9:1. Overall, 819 (75.9%) cases were from Guangdong province, and 260 (24.1%) were from the surrounding provinces [Hunan, 85 (7.9%); Guangxi, 63 (5.9%); Jiangxi, 35 (3.2%); Sichuan, 33 (3.1%); and Hubei, 19 (1.8%)]. The most common route of HIV transmission was sex (85.8%), followed by intravenous drug use (10.3%, 111/1079), blood transfusion (1.8%, 19/1079), vertical transmission (0.7%, 8/1079); and unknown (1.4%, 15/1079; see Table 1).
Table 1.
Clinical, laboratory and radiographic characteristics and initial antifungal therapy outcomes in the 1079 patients with HIV-associated talaromycosis
| Outcome [n (%)] | |||||
|---|---|---|---|---|---|
| Characters | Total (n = 1079) | Good (n = 928) | Poor (n = 151) | Statistics | P-value |
| Age [mean (SD)] | 38.8 (11.9) | 38.5 (11.9) | 40.0 (11.7) | − 1.48a | 0.14 |
| Sex | |||||
| Male | 861 (79.8) | 741 (79.8) | 120 (79.5) | 0.01b | 0.91 |
| Female | 218 (20.2) | 187 (20.2) | 31 (20.5) | ||
| Intravenous drug | 111 (10.3) | 98 (10.6) | 13 (8.6) | 0.54b | 0.48 |
| 968 (89.7) | 830 (89.4) | 138 (91.4) | |||
| ART history before hospitalization | 109 (10.1) | 97 (10.5) | 12 (7.9) | 0.85b | 0.36 |
| Fever | 924 (85.6) | 790 (85.1) | 134 (88.7) | 1.38b | 0.24 |
| Respiratory symptoms | 734 (68.0) | 630 (67.9) | 104 (68.9) | 0.02b | 0.88 |
| Digestive symptoms | 478 (44.3) | 397 (42.8) | 81 (53.6) | 6.21b | 0.01 |
| Weight loss | 537 (49.8) | 462 (49.8) | 75 (49.7) | 0.00b | 0.98 |
| Skin lesions | 480 (44.5) | 404 (43.5) | 76 (50.3) | 2.43b | 0.12 |
| Chest imaging abnormality | 896/1047 (85.6) | 785/912 (86.1) | 111/135 (82.2) | 1.41b | 0.23 |
| Splenomegaly | 538/985 (54.6) | 489/876 (55.8) | 49/109 (45.0) | 4.62b | 0.032 |
| Hepatomegaly | 510/986 (51.7) | 466/877 (53.1) | 44/109 (40.4) | 6.33b | 0.012 |
| CD4 [median (IQR)] | 9.0 (4.0–20.0) | 10.0 (5.0–21.0) | 7.5 (3.0–14.8) | 63 636c | 0.007 |
| CD4/CD8 [median (IQR)] | 0.04 (0.02–0.08) | 0.04 (0.02–0.09) | 0.04 (0.02–0.07) | 58 822c | 0.25 |
| Anaemia | 1031 (95.6) | 888 (95.7) | 143 (94.7) | 0.30b | 0.59 |
| Thrombocytopenia | 579 (53.7) | 465 (50.1) | 114 (75.5) | 33.7b | < 0.001 |
| WBC countd | |||||
| Normal | 572 (53.0) | 503 (54.2) | 69 (45.7) | 64.1b | < 0.001 |
| Leucocytosis | 91 (8.4) | 53 (5.7) | 38 (25.2) | ||
| Leucopenia | 416 (38.6) | 372 (40.1) | 44 (29.1) | ||
| Hypoalbuminaemia | 1043/1058 (98.6) | 906/921 (98.4) | 137/137 (100.0) | 1.25b | 0.26 |
| Elevated ALT | 398/1060 (37.5) | 335/921 (36.4) | 63/139 (45.3) | 4.13b | 0.04 |
| Elevated AST | 822/1069 (76.9) | 689/924 (74.6) | 133/145 (91.7) | 20.8b | < 0.001 |
| Elevated TBIL | 138/1057 (13.1) | 95/920 (10.3) | 43/137 (31.4) | 46.60b | < 0.001 |
| Elevated ALP | 585/1049 (55.8) | 493/913 (54.0) | 92/136 (67.6) | 8.9b | 0.002 |
| Elevated creatinine | 84/1068 (7.9) | 40/923 (4.3) | 44/145 (30.3) | 117.0b | < 0.001 |
| Co-infected with TB | 233 (21.6) | 213 (23.0) | 20 (13.2) | 7.35b | 0.006 |
| Co-infected with CMV | 187 (17.3) | 152 (16.4) | 35 (23.2) | 4.19b | 0.04 |
| Positive Talaromyces marneffei in blood culture | 696/1045 (66.6) | 585/905 (64.6) | 111/140 (79.3) | 11.7b | < 0.001 |
| Positive T. marneffei in bone marrow | 685/919 (74.5) | 616/821 (75.0) | 69/98 (70.4) | 0.986b | 0.321 |
| Initial antifungal therapy | |||||
| AMB + azole | 451 (41.8) | 412 (44.4) | 39 (25.8) | 37.8b | < 0.001 |
| AMB alone | 147 (13.6) | 137 (14.8) | 10 (6.6) | ||
| Azole alone | 481 (44.6) | 379 (40.8)d | 102 (67.5)d | ||
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMB, amphotericin B; ART, antiretroviral therapy; AST, aspartate aminotransferase; CMV, cytomegalovirus; IQR, interquartile range; SD, standard deviation; TB, tuberculosis; TBIL, total bilirubin; WBC, white blood cell.
Reference ranges: WBC, 3.5–9.5 × 109 cells/L; Hb, 130–175 g/L; platelet, 125–350 × 109; albumin, 40–55 g/L; ALT, 9–50 U/L; AST, 15–40 U/L; elevated TBIL, > 26 μmol/L; elevated ALP, > 125 U/L; elevated creatinine, > 133 μmol/L.
t-test;
χ2 test;
Wilcoxon rank-sum test;
Significant difference with other two groups.
Clinical characteristics
The median (IQR) duration from symptom onset to hospital admission was 30 (15–60) days, and the median duration of hospitalization was 27 (17–36) days. The clinical characteristics of the patients are summarized in Table 1. The most common symptoms and signs were fever (85.6%), peripheral lymphadenopathy (72.3%), respiratory symptoms (60.8%), weight loss > 5 kg within the last 3 months (49.8%), skin lesions (44.5%) and gastrointestinal symptoms (44.3%).
Concurrent OIs were diagnosed in 457 patients (42.4%), including presumed or microbiology-confirmed tuberculosis (n = 233, 20.1%), non-tuberculous mycobacterial infections (n = 15, 1.4%), Pneumocystis pneumonia (n = 197, 18.3%), cytomegalovirus (CMV) infections (n = 187, 17.3%), other bacterial pneumonia (n = 259, 24.0%), and cryptococcosis (n = 33, 3.1%). Kaposi sarcoma and lymphoma were diagnosed in four and five patients, respectively. Coinfections with hepatitis B and hepatitis C were present in 159 (14.7%) and 90 (8.3%) patients, respectively.
Laboratory and radiographic characteristics
The most common laboratory abnormality was hypoalbuminaemia (98.6%; severe hypoalbuminaemia, 34.7%), followed by anaemia (95.6%; severe anaemia, 20.0%), leucopenia (38.6%), thrombocytopenia (53.7%), and elevated levels of AST (76.9%), ALT (37.5%) and ALP (55.8%). For immune indexes, the CD4 T-cell count ranged between 0 and 168 cells/μL, with a median (IQR) of 9.0 (4.0–20.0) cells/μL. Overall, 91.9% (992/1079) of the patients had a CD4 T-cell count < 50 cells/μL. The CD4/CD8 ratio ranged between 0 and 0.14, with a median (IQR) of 0.04 (0.02–0.08) (Table 1).
A total of 1047 patients underwent chest radiography or computed tomography (CT). Of these, 896 (85.6%) had abnormalities, including patchy infiltration, diffuse military pattern, pleural effusion or lung cavity. A total of 986 patients had abdominal ultrasound or CT results; of these, 595 (60.3%) showed splenomegaly and 549 (55.7%) showed hepatomegaly (Table 1).
Diagnostic investigations
A total of 1045 (97.2%) and 919 (85.2%) patients underwent blood and bone marrow cultures, respectively. Talaromyces marneffei was isolated in 66.6% (696/1045) and 74.5% (685/919) of patients who underwent blood and bone marrow cultures, respectively. The positive rate was significantly higher in bone marrow than in blood culture (χ2 = 14.7, P < 0.001). The positive rate increased to 86.6% (n = 789) in the 911 patients who underwent concurrent blood and bone marrow cultures. This positive rate in the combined culture was significantly higher than that in the bone marrow or blood culture alone (χ2 = 42.5, P < 0.001; χ2 = 106.5, P < 0.001, respectively). Of the 427 patients who underwent fibreoptic bronchoscopy, the BAL cultures were positive for T. marneffei in 28.1% (120/427). Meanwhile, there were 339 patients who underwent lung biopsy, and the lung tissue pathology of 71 (20.9%) patients showed yeast cells with central septation characteristic of T. marneffei. A total of 1030 patients (95.5%) underwent serum galactomannan testing, and 747 (72.5%) tested positive.
Treatment and outcomes
All patients received antifungal therapy within 12 h after the diagnosis of talaromycosis. There were 147 (13.6%) patients who received AMB alone; 451 (41.8%) patients who received a combination of AMB and azoles; and 481 (44.6%) patients who received azole alone. We found that 10.1% (109/1079) of the patients had initiated ART at least 2 weeks before hospitalization. The remaining patients started ART within 2 weeks after antifungal therapy.
At discharge, 86.0% (928/1079) of the patients were cured or improved, whereas 14.0% (151/1079) of the patients died or were discharged in a deteriorated condition. Among the 86 (8.0%, 86/1079) patients who died in hospital, the median (IQR) time from admission to death was 5.5 (2–17) days (range: 1–123). Of these, 34 (40.0%, 34/86) had concurrent OIs. At the end of the 90-day follow-up, the known number of deaths increased from 86 (8.0%) to 128 (11.9%), and the median (IQR) time from discharge to death was 3 (2–8) days (range: 1–14).
Prognostic factors
The patients were classified into two groups according to outcomes as the good outcome (improvement or cure, N = 928) and bad outcome (deterioration or death, N = 151) groups. Twenty-seven demographic, clinical, laboratory and other treatment factors were investigated for their impact on patient outcomes. The results of the comparison of these factors between the two outcome groups are shown in Table 1. The poor outcome group had: significantly higher digestive tract symptoms; lower CD4 T-cell count; higher rate of thrombocytopenia; elevated serum ALT, AST, TBIL, ALP, and creatinine levels; higher prevalence of CMV coinfection; higher prevalence of positive T. marneffei blood cultures; and higher rate of azole monotherapy (all P < 0.05).
The results of the multivariate logistic regression analysis showed that leucocytosis, thrombocytopenia, elevated serum AST, TBIL and creatinine levels, and antifungal therapy with azole alone were independent risk factors for poor outcome (all adjusted P < 0.05; Table 2).
Table 2.
Multivariate logistic regression analysis of the prognostic factors of HIV-associated talaromycosis
| 95% CI for OR | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables (factors) | B | SE | Wald | df | P value | OR | Lower | Upper |
| Thrombocytopenia | 0.552 | 0.260 | 4.508 | 1 | 0.034 | 1.736 | 1.043 | 2.890 |
| WBC count | 27.079 | 2 | 0.000 | |||||
| Leucocytosis | 1.417 | 0.336 | 17.755 | 1 | 0.000 | 4.126 | 2.134 | 7.977 |
| Leucopenia | −0.468 | .263 | 3.161 | 1 | 0.075 | 0.626 | 0.374 | 1.049 |
| Elevated creatine | 0.761 | 0.234 | 10.605 | 1 | 0.001 | 2.141 | 1.354 | 3.384 |
| Elevated AST | 0.933 | 0.369 | 6.384 | 1 | 0.012 | 2.542 | 1.233 | 5.240 |
| Elevated TBIL | 0.708 | 0.286 | 6.128 | 1 | 0.013 | 2.030 | 1.159 | 3.554 |
| Initial antifungal therapy | 18.614 | 0.000 | ||||||
| AMB ± azole | −0.220 | 0.418 | 0.278 | 1 | 0.598 | 0.802 | 0.353 | 1.821 |
| Azole alone | 0.863 | 0.392 | 4.857 | 1 | 0.028 | 2.371 | 1.100 | 5.108 |
| Constant | −3.697 | 0.509 | 52.799 | 1 | 0.000 | 0.025 | ||
AMB, amphotericin B; AST, aspartate aminotransferase; B, beta; df, degree of freedom; OR, odds ratio; SE, standard error; TBIL, total bilirubin; WBC, white blood cell.
Discussion
Guangdong is the largest province with the highest HIV burden in China and is also an important endemic area for talaromycosis. As the largest specialized hospital for HIV care in southern China, GZEPH admits more than 3000 HIV in-patients and follows up more than 15 000 HIV outpatients on ART annually. Although most of our patients are residents of Guangdong, a significant proportion are from the neighbouring provinces of Guangxi, Hunan, Jiangxi and Yunan, making the patient population at GZEPH fairly representative of those from southern China. In this retrospective study, we found a high prevalence of talaromycosis among HIV admissions, at 16.0%. Although a similarly high prevalence has been reported in other highly endemic areas in northern Thailand and Vietnam, such high prevalence was usually seen before or during the early ART era [17–19]. In addition to identifying a high prevalence of talaromycosis in the contemporary ART era, we also found a steady upward trend of HIV-associated talaromycosis in Guangdong, with a peak of 19% in 2017. The increase in incidence was not due to a reduction in the number of HIV admissions. By contrast, it reflected a consistent upward trend in HIV admissions during the same study period. This is a concerning trend which highlights the need for further research into the epidemiology and management of both talaromycosis and HIV care in China.
We also found that the incidence of talaromycosis increased by 73% during the rainy season in Guangdong. Although infections occur throughout the year, there was a clear peak period from March to August each year. Our data are consistent with the documented seasonality of talaromycosis in northern Thailand and Vietnam [17,20,21]. In these studies, the incidence of talaromycosis increased by 30% in Vietnam and by 50% in northern Thailand. By contrast, the incidence of cryptococcosis, another common HIV-associated mycosis, did not show any seasonality during the same study period. High humidity, rather than precipitation, was found to be the driving factor of talaromycosis seasonality. This is probably by creating a favourable condition for T. marneffei growth and for the release of spores into the environment [20]. Our study provides further support for this hypothesis and calls for further epidemiology studies into the environmental reservoir of T. marneffei and risks of exposure and acute infections in susceptible populations.
Although the majority of the cases were from Guangdong, 24.1% were from neighbouring provinces, some of which (e.g. Hunan, Sichuan and Jiangxi) are not traditionally considered to be endemic areas for talaromycosis. However, some studies have shown that the geographic range of talaromycosis is expanding, with autochthonous cases being reported in previously non-endemic areas such as Beijing and the eastern provinces of China [22,23], and in Assam in India [24]. Clinicians should be aware of this changing epidemiology when assessing the patient’s travel history and considering talaromycosis in the differential diagnoses.
Consistent with the findings of previous studies [5–8,17–20], we found that infection is indolent with nonspecific symptoms. The median (IQR) time from symptom onset to admission was 30 (15–60) days, reflecting both the lack of awareness and a challenge of early diagnosis. The most specific manifestations of talaromycosis are the classic, central umbilicated skin nodules. However, only 44.5% of the patients in this study had skin lesions, demonstrating the importance of blood cultures for diagnosis. The prevalence of skin lesions in our study is similar to that reported in northern Thailand (40.7%) and in eastern China (43.8%) [18,19,25]. However, it is lower than the prevalence reported in Vietnam (72–83%) [18]. The reason for this discrepancy is not clear.
Prominent laboratory abnormalities in our patients included liver inflammation and myelosuppression, supporting fungal dissemination in the reticuloendothelial system. Notably, the CD4 T-cell count was < 50 cells/μL in 91.9% of patients, indicating a profound level of immune dysfunction in susceptible individuals. Radiographic abnormalities in the lung and findings of hepatomegaly and splenomegaly were common, but these findings lacked specificity. A significant proportion (42.4%) of the patients had concurrent OIs, with tuberculosis being the most common, followed by pneumocystosis, other bacterial pneumonia and CMV infection. Coinfection with tuberculosis in those with HIV/AIDS and talaromycosis is common and complicates treatment due to polypharmacy and drug–drug interactions between azoles, rifampicin and antiretroviral drugs.
Culture remains the golden standard for talaromycosis diagnosis, but blood culture was positive in only 66.6% of the patients in our study. Bone marrow culture provided significantly higher diagnostic yield (74.5%), and when combined with blood culture, the yield increased further to 86.6%. We routinely conduct bone marrow aspiration in AIDS patients presenting with fever and evidence of bone marrow suppression at our hospital. The procedure is safe and well tolerated; therefore, we highly recommend this procedure to improve the diagnosis of talaromycosis. We also routinely use the commercially available GM (Galactomannan) assay to assist with the rapid diagnosis of talaromycosis. This assay has been shown to highly cross react with T. marneffei, with a sensitivity of 96% and a specificity of 91% [26]. The GM assay yielded a higher diagnostic rate than did blood culture (72.5% vs. 66%), consistent with a previous study [25]. A recent study using the Mp1p immunoassay showed that it has superior sensitivity to blood culture in the diagnosis of talaromycosis [27]. These antigen-detection assays are promising methods for an early diagnosis and treatment of talaromycosis, which have been shown to reduce mortality. In addition, the matrix-assisted laserdesorption/ionization time-of-flight and real-time polymerase chain reaction is being developed for rapid detection of T. marneffei [28–30].
The overall rates of poor outcome and mortality at discharge in our cohort were 14% and 8.0%, respectively, lower than the mortality rates of 17–28% reported in previous studies [10,17,25]. This lower mortality can be attributed to our more aggressive diagnostic strategy with the routine use of bone marrow culture and GM antigen testing, which improves diagnostic yield and facilitates earlier diagnosis and treatment, respectively.
With respect to prognostic factors of talaromycosis outcomes, we identified both novel and established factors. While thrombocytopenia has consistently been shown to predict mortality [10,17,30], we also established elevated levels of serum AST, total bilirubin and creatine as novel prognostic factors. To the best of our knowledge, this is also the first retrospective study to determine that induction therapy with azoles (voriconazole or itraconazole) was associated with a higher mortality rate than AMB. Our real-life patient outcome data confirmed the findings of a recent randomized controlled trial in Vietnam showing the superiority of AMB over itraconazole for mortality outcomes [2]. Moreover, it also further supports the 2019 guidelines from the US recommending against the use of itraconazole as induction therapy for talaromycosis [31].
This study has some limitations owing to its retrospective design. However, to our best knowledge, this study is the largest retrospective analysis of the epidemiology, clinical features, and prognostic factors of HIV-associated talaromycosis, thus enabling a robust analysis. Further research into the epidemiology, diagnosis and treatment of this emerging fungal infection in China and in Southeast Asia is needed.
In conclusion, the incidence of HIV-associated talaromycosis has increased in Guangdong with the high HIV burden in China. Our findings also confirmed the seasonality of talaromycosis in southern China during the rainy season. Skin lesions were present in only 44.5% of patients. Induction therapy with azole alone was associated with higher mortality, supporting the use of AMB induction therapy. These study findings would probably have a broader impact on treating the disease.
Acknowledgements
Conflict of interest: T Le has received investigator-initiated research funding from Gilead Sciences outside of the submitted work. The remaining authors declare no competing interests.
Financial disclosure: This study was supported by the Chinese 13th Five-Year National Science and Technology Major Project (2018ZX10302103-002, 2018ZX10302104-002-004, 2018ZX10302104-001-007), Guangzhou Basic Research Program on People’s Livelihood Science and Technology (202002020005), the National Institute of Health (R01AI143409 to TL), the Duke University Center for AIDS Research (CFAR), and an NIH-funded programme (P30 AI064518 to TL).
References
- 1.Cao C, Xi L, Chaturvedi V. Talaromycosis (penicilliosis) due to Talaromyces (Penicillium) marneffei: insights into the clinical trends of a major fungal disease 60 years after the discovery of the pathogen. Mycopathologia 2019; 184: 709–720. [DOI] [PubMed] [Google Scholar]
- 2.Le T, Kinh NV, Cuc NTK et al. A trial of itraconazole or amphotericin b for hiv-associated talaromycosis. N Engl J Med 2017; 376: 2329–2340. [DOI] [PubMed] [Google Scholar]
- 3.Pruksaphon K, Intaramat A, Ratanabanangkoon K et al. Development and characterization of an immunochromatographic test for the rapid diagnosis of Talaromyces (Penicillium) marneffei. PLoS One 2018; 13: e0195596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei HL, Li LH, Chen WS et al. Susceptibility profile of echinocandins, azoles and amphotericin B against yeast phase of Talaromyces marneffei isolated from HIV-infected patients in Guangdong, Chian. Eur J Clin Microbiol Infect Dis 2018; 37: 1099–1102. [DOI] [PubMed] [Google Scholar]
- 5.Jiang J, Meng S, Huang S et al. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: a retrospective cohort study. Clin Microbiol Infect 2019; 25: 233–241. [DOI] [PubMed] [Google Scholar]
- 6.Wu TC, Chan JW, Ng CK et al. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Hong Kong Med J 2008; 14: 103–109. [PubMed] [Google Scholar]
- 7.Wipasa J, Chaiwarith R, Chawansuntati K et al. Characterization of anti-interferon-γ antibodies in HIV-negative immunodeficient patients infected with unusual intracellular microorganisms. Exp Biol Med 2018; 243: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Lin Z, Shi X et al. Retrospective analysis of 15 cases of Penicillium marneffei infection in HIV-positive and HIV-negative patients. Microb Pathog 2017; 105: 321–325. [DOI] [PubMed] [Google Scholar]
- 9.Lee PP, Lao-Araya M, Yang J et al. Application of flow cytometry in the diagnostics pipeline of primary immunodeficiencies underlying disseminated Talaromyces marneffei infection in HIV-negative children. Front Immunol 2019; 10: 2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng J, Gui X, Cao Q et al. A clinical study of acquired immunodeficiency syndrome associated Penicillium marneffei infection from a non-endemic area in China. PLoS One 2015; 10: e0130376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Chen K, Dhungana N et al. Characterization of clinical isolates of Talaromyces marneffei and related species, California, USA. Emerg Infect Dis 2019; 25: 1765–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matos AC, Alves D, Saraiva S et al. Isolation of Talaromyces marneffei from the skin of an Egyptian mongoose (Herpestes ichneumon) in Portugal. J Wildl Dis 2019; 55: 238–241. [DOI] [PubMed] [Google Scholar]
- 13.Bureau of Disease Control and Prevention, National Health Commission. New progress in AIDS prevention and control in China in 2019. AIDS STD China 2019; 25: 1205. [Google Scholar]
- 14.Hu Y, Zhang J, Li X et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia 2013; 175: 57–67. [DOI] [PubMed] [Google Scholar]
- 15.Wang YF, Xu HF, Han ZG et al. Serological surveillance for Penicillium marneffei infection in HIV-infected patients during 2004–2011 in Guangzhou, Chian. Clin Microbiol Infect 2015; 21: 484–489. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Available at http://aidsinfo.nih.gov/guidelines (accessed 01 January 2011).
- 17.Le T, Wolbers M, Chi NH et al. Epidemiology, seasonality, and predictors of outcome of AID-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis 2011; 52: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson M, Nguyen LHT, Wertheim HF et al. Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in northern Vietnam. AIDS Res Ther 2012; 9: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis 2013; 13: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philip LB, Thuy L, Vo Minh Q et al. Environmental predictors and incubation period of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Vietnam. Clin Infect Dis 2013; 56: 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chariyalertsak S, Sirisanthana T, Supparatpinyo K et al. Seasonal variation of disseminated Penicillium marneffei infections in northern Thailand: a clue to the reservoir? J Infect Dis 1996; 173: 1490–1493. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Sang J, Li R et al. Disseminated Penicillium marneffei infection with verrucoid lesions in an AIDS patient in Beijing, a non-endemic region. Eur J Dermatol 2010; 20: 378–380. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Zhang RF, Shen YZ et al. Clinical characteristics and prognosis of penicilliosis among human immunodeficiency virus-infected patients in Eastern China. Am J Trop Med Hyg 2017; 96: 1350–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saikia L, Nath R, Mahanta J. Penicillium marneffei infection in Assam. Indian J Dermatol Venereol Leprol 2010; 76: 75–76. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Zheng Y, Wu F et al. Evaluation of quantitative real-time PCR and Platelia Galactomannan assays for the diagnosis of disseminated Talaromyces marneffei infection. Med Mycol 2020; 58: 181–186. [DOI] [PubMed] [Google Scholar]
- 26.Huang YT, Hung CC, Liao CH et al. Detection of circulating galactomannan in serum samples for diagnosis of Penicillium marneffei infection and cryptococcosis among patients infected with human immunodeficiency virus. J Clin Microbiol 2007; 45: 2858–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borman AM, Fraser M, Szekely A et al. Rapid and robust identification of clinical isolates of Talaromyces marneffei based on MALDI-TOF mass spectrometry or dimorphism in Galleria mellonella. Med Mycol 2019; 57: 969–975. [DOI] [PubMed] [Google Scholar]
- 28.Hien HTA, Thanh TT, Thu NTM et al. Development and evaluation of a real-time polymerase chain reaction assay for the rapid detection of Talaromyces marneffei MP1 gene in human plasma. Mycoses 2016; 59: 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Zhang R, Shen Y et al. Clinical characteristics and prognosis of Penicilliosis among human immunodeficiency virus-infected patients in Eastern China. Am J Trop Med Hyg 2017; 96: 1350–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thu NTM, Chan JFW, Ly VT et al. Superiority of a novel Mp1p antigen detection enzyme immunoassay compared to standard BACTEC blood culture in the diagnosis of talaromycosis. Clin Infect Dis 2020; 21: ciaa826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf (accessed 29 June 2020). [Google Scholar]


