Abstract
Background
Despite severe outbreaks of COVID-19 among colleges and universities across the USA during the Fall 2020 semester, the majority of institutions did not routinely test students. While high-frequency repeated testing is considered the most effective strategy for disease mitigation, most institutions do not have the necessary infrastructure or funding for implementation. Therefore, alternative strategies for testing the student population are needed. Our study detailed the implementation and results of testing strategies to mitigate SARS-CoV-2 spread on a university campus, and we aimed to assess the relative effectiveness of the different testing strategies.
Methods
For this retrospective cohort study, we included 6273 on-campus students arriving to a large public university in the rural USA (Clemson, SC, USA) for in-person instruction in the Fall 2020 semester (Sept 21 to Nov 25). Individuals arriving after Sept 23, those who tested positive for SARS-CoV-2 before Aug 19, and student athletes and band members were not included in this study. We implemented two testing strategies to mitigate SARS-CoV-2 spread during this period: a novel surveillance-based informative testing (SBIT) strategy, consisting of random surveillance testing to identify outbreaks in residence hall buildings or floors and target them for follow-up testing (Sept 23 to Oct 5); followed by a repeated weekly surveillance testing (Oct 6 to Nov 22). Relative changes in estimated weekly prevalence were examined. We developed SARS-CoV-2 transmission models to compare the relative effectiveness of weekly testing (900 daily surveillance tests), SBIT (450 daily surveillance tests), random surveillance testing (450 daily surveillance tests), and voluntary testing (0 daily surveillance tests) on disease mitigation. Model parameters were based on our empirical surveillance data in conjunction with published sources.
Findings
SBIT was implemented from Sept 23 to Oct 5, and identified outbreaks in eight residence hall buildings and 45 residence hall floors. Targeted testing of residence halls was 2·03 times more likely to detect a positive case than random testing (95% CI 1·67–2·46). Weekly prevalence was reduced from a peak of 8·7% to 5·6% during this 2-week period, a relative reduction of 36% (95% CI 27–44). Prevalence continued to decrease after implementation of weekly testing, reaching 0·8% at the end of in-person instruction (week 9). SARS-CoV-2 transmission models concluded that, in the absence of SBIT (ie, voluntary testing only), the total number of COVID-19 cases would have increased by 154% throughout the semester. Compared with SBIT, random surveillance testing alone would have resulted in a 24% increase in COVID-19 cases. Implementation of weekly testing at the start of the semester would have resulted in 36% fewer COVID-19 cases throughout the semester compared with SBIT, but it would require twice the number of daily tests.
Interpretation
It is imperative that institutions rigorously test students during the 2021 academic year. When high-frequency testing (eg, weekly) is not possible, SBIT is an effective strategy to mitigate disease spread among the student population that can be feasibly implemented across colleges and universities.
Funding
Clemson University, USA.
Introduction
Institutions of higher education had severe outbreaks of COVID-19 after reopening in the Fall 2020 semester (beginning in August),1 forcing some institutions to shut down campus facilities and shift to online learning.1, 2 As of Dec 15, 2020, at least 85 colleges and universities across the USA had reported over 1000 cases of COVID-19.3 The testing and isolation of confirmed COVID-19 cases are considered the most effective strategies for mitigating the spread of SARS-CoV-2 among the student population.4, 5, 6 Institutions have implemented a wide range of testing strategies for SARS-CoV-2 detection, including on-demand testing (eg, for students with symptoms), random surveillance testing, and high-frequency surveillance testing (ie, testing repeated weekly or twice per week).4, 7 Although existing evidence suggests that the most successful strategies at mitigating the spread of SARS-CoV-2 involve frequent, repeated testing of all students,8, 9 many institutions do not have the infrastructure to support such testing.
Research in context.
Evidence before this study
Colleges and universities across the USA had large SARS-CoV-2 outbreaks during the Fall 2020 semester. Institutions of higher education implemented various testing strategies to mitigate disease spread, from voluntary testing alone to high-frequency repeated testing of all students. We searched Google Scholar with the terms “(“Covid-19” or “coronavirus” or “SARS-CoV-2”) AND (“university” or “college” or “campus”) AND (“tests” or “testing” or “screening” or “mitigation”)” for articles published in any language from Jan 1, 2020, to Jan 15, 2021. We found only one study detailing the implementation and results of testing strategies for student populations. This study showed that screening all students twice per week throughout the semester by use of pooled SARS-CoV-2 testing was effective in preventing outbreaks on college campuses. However, many institutions have insufficient resources to do such high-frequency testing; it is estimated that only 6% of large colleges and universities in the USA did so during the Fall 2020 semester. We found no studies documenting the effectiveness of alternative student testing strategies using empirical data.
Added value of this study
We implemented a novel surveillance-based informative testing (SBIT) strategy for SARS-CoV-2 detection, followed by repeated weekly testing, in a large rural public university campus in the USA during the Fall 2020 semester. To our knowledge, our study was the first to conceptualise an SBIT approach for outbreak detection and containment and the first to document the implementation, results, and relative effectiveness of surveillance testing strategies for SARS-CoV-2 mitigation. SBIT was effective in detecting and containing COVID-19 outbreaks and mitigating SARS-CoV-2 spread on university campuses. By focusing testing resources on potential hotspots (ie, residence hall outbreaks), SBIT detected more SARS-COV-2 positive cases among the student population than random surveillance testing alone, despite using the same number of daily tests. Empirical findings and SARS-CoV-2 transmission models confirmed that repeated high-frequency surveillance testing (ie, weekly or twice per week testing) are optimal for disease mitigation.
Implications of all the available evidence
Most institutions of higher education in the USA provided only voluntary testing for students; this strategy leaves many COVID-19 cases undetected and contributes to an increase in disease spread on campus and in surrounding communities. It is imperative that institutions routinely test students throughout the 2021 academic year. In the absence of sufficient testing capacity for high-frequency testing, SBIT is a viable intervention for disease mitigation. Additionally, it is estimated that many institutions of higher education in the USA have the necessary testing infrastructure to implement an SBIT approach.
Compared with high-frequency surveillance testing, random surveillance testing strategies require a lower number of daily tests. Although surveillance testing of the entire student population is important for monitoring disease spread on university campuses, this might not be sufficient for mitigating disease spread if the number of weekly tests are small compared with the number of students.8 However, random surveillance testing can be useful for estimating disease prevalence and monitoring local outbreaks on and off campus. Furthermore, upon outbreak detection, additional testing can be used to target high-risk populations. We refer to this strategy as surveillance-based informative testing (SBIT). In this setting, random testing is done to detect potential cluster outbreaks (eg, residence hall outbreaks). Upon detection of an outbreak, a portion of available tests the following day are allocated to the entire cluster. Residence halls are prone to such outbreaks because the spread of SARS-CoV-2 can occur quickly in these close-quarter environments.2, 10 Through early detection of residence hall outbreaks followed by testing and removal of all infected students, SBIT has the potential to mitigate disease spread on college and university campuses.
Few studies have documented the effectiveness of surveillance testing strategies for COVID-19 mitigation in institutions of higher education. In this study, we detail the implementation and results of two testing strategies aimed to mitigate the spread of SARS-CoV-2 in a large rural public university campus in the USA: a novel SBIT approach, and repeated weekly testing. We aimed to develop SARS-CoV-2 transmission models to compare the relative effectiveness of several testing strategies on the reduction of SARS-CoV-2 prevalence.
Methods
Study design and participants
In this retrospective cohort study, we evaluated testing strategies implemented at Clemson University (Clemson, SC, USA) during the Fall 2020 semester. The university began its semester online on Aug 19, and shifted to in-person instruction between Sept 21 and Nov 25. The staggered arrival of on-campus students to 35 residence halls occurred between Sept 11 and 20. Mandatory surveillance testing was done between Sept 23 and Nov 22. All students with access to main campus facilities were subjected to mandatory testing.
The eligible population for this study consisted of 6273 on-campus students arriving to residence halls before implementation of surveillance testing (Sept 23). To maintain a consistent surveillance population, individuals arriving after this date were not included in this study. Additionally, individuals testing positive for SARS-CoV-2 before online instruction (Aug 19) were not included, because these individuals could theoretically re-enter the surveillance testing pool throughout the semester. As advised by the Atlantic Coast Conference Medical Advisory Group, student athletes and band members took part in their own testing protocols and were thus not included in this study. Deidentified testing outcomes for all students in this study were collected through case files reported by Rymedi (Greenville, SC, USA), Clemson University's Student Health Center, and test upload files. Ethical review for this study was obtained by the Institutional Review Board of Clemson University. No consent was needed for this study; students consented to being tested, and we used de-identified data for these analyses.
Procedures
Testing was mandated during online instruction for all students; an initial negative SARS-CoV-2 test was required before receiving clearance for main campus facility access. Students had the option of taking a SARS-COV-2 test on their own and uploading the result to an online portal or to use a university testing facility. On-campus students were required to provide a negative SARS-CoV-2 test taken within 10 days of campus arrival and were tested again on arrival. Students who tested positive were placed in isolation for 10 days. For pre-arrival testing, accepted methods of testing included a negative PCR test with nasal, throat, or saliva swabs taken within 10 days before reporting to campus; or positive serologic antibody tests obtained within 40 days.11 All tests administered on campus were PCR-based tests.
Surveillance testing began for all eligible students on Sept 23, 2 days after the start of in-person instruction. On-campus students were subjected to two surveillance testing strategies: SBIT and repeated weekly testing. Off-campus students were subjected to random surveillance testing alone. All students were contacted by email (at least 24 h in advance) and directed to the on-campus surveillance testing centre. This testing site also provided voluntary testing for symptomatic students, students directed to testing by contact tracers, or students tested for other reasons. Anterior nasal swabs were collected by health professionals on site. Before Oct 6, surveillance testing was done by use of anterior nasal swabs with an estimated test sensitivity of 97%.12 Beginning on Oct 6, testing capacity increased due to the implementation of an in-house saliva-based test (test sensitivity ≥95%). Additional details on testing protocols and procedures are described in the appendix (p 1). Students were notified of test results by text message and email. The student health centre also did testing for students; these students were notified of test results by university medical staff. Students were required to isolate for 10 days upon notification of a positive test result. Students testing positive were excluded from surveillance testing for 90 days from the test collection date.13
Implementation of SBIT occurred between Sept 23 and Oct 5. SBIT consisted of randomly sampling a portion of the on-campus student population using the available tests on a given day. We defined an outbreak in a residence hall building as at least two students from the same building testing positive for SARS-CoV-2 in a 7-day period.14 If an outbreak was detected in a residence hall building, then a portion of available tests the following day were used to test the entire building. To improve the precision of the SBIT strategy, we transitioned to targeting outbreaks in residence hall floors between Sept 29 and Oct 5, where we defined an outbreak as at least one student testing positive for SARS-CoV-2 in one floor. If the number of students in targeted residence hall buildings (or floors) exceeded the available number of tests, residence hall buildings (or floors) were prioritised on the basis of the proportion of tests that were positive for SARS-CoV-2. Residence hall buildings (threshold of two or more SARS-COV-2 positive cases) or floors (threshold of one or more SARS-COV-2 positive cases) that exceeded the threshold but were not tested were ranked again the following testing cycle. Any residual tests on a given day were used for random testing. Students out of town on assigned targeted testing date were instructed to get tested upon campus return.
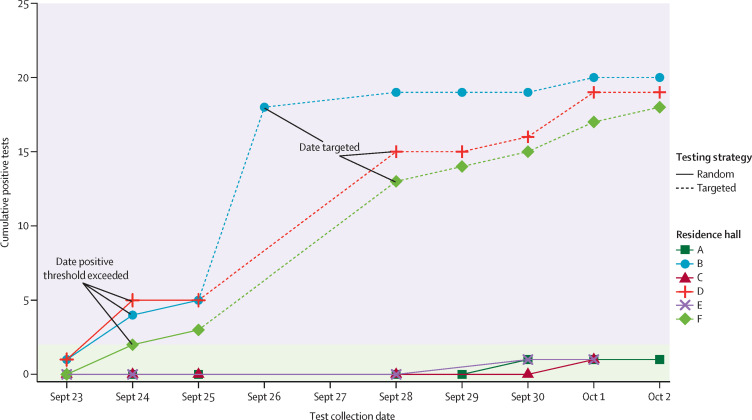
The typical random surveillance test turnaround time was 24–48 h. Because students were notified for targeted testing 24 h in advance, the period between the date that the threshold was exceeded and the date of targeted testing was typically 3 days. However, due to insufficient testing capacity and reduced weekend testing, the lag between the test collection date of the random surveillance tests and that of the targeted tests typically exceeded 3 days in practice. Figure 1 illustrates the SBIT implementation using a sample of residence halls. Priority was given for tests collected from students identified for targeted testing and symptomatic students, with a typical test turnaround time lower than 24 h.
Figure 1.
Illustration of the SBIT strategy
Sample of six residence halls during the first 10 days of SBIT implementation, three of which were targeted for testing. Points indicate date at test collection. Residence halls with at least two students testing positive for SARS-COV-2 (outbreak threshold) were selected for targeted testing the following available day. Date targeted is the scheduled date of mandatory targeted testing for all students in the residence hall. Because of a lag between the date of test collection and results received, and limits on testing capacity and reduced weekend testing, there was a delay of at least 48 h between when the threshold was exceeded and the targeted testing. SBIT=surveillance-based informative testing.
Beginning on Oct 6, all eligible on-campus students were required to get tested on a weekly basis up to Nov 20. On-campus students were directed for testing on a particular day on the basis of their residence hall. Targeted testing was not done during this period.
In addition to the testing strategies described, the following strategies were implemented to migitate the spread of SARS-CoV-2 on campus: mask mandates, social distancing, hybrid learning (consisting of both in-person and online classes), and limitation of in-person classes to one-third capacity. Contact tracing was implemented throughout the semester. At the initial notification of a positive test, the clinical team called the individual to give instructions on isolation and facilitated moving (if necessary). The team also asked about household contacts and immediately contacted those individuals. Contact tracers from both Clemson University and South Carolina's Department of Health and Environmental Control (SCDHEC) followed up with students to complete a full case investigation and gather information on additional contacts.
Several factors led to the decision to allocate a disproportionately higher number of surveillance tests for on-campus students than for those off campus. On the basis of models indicating that early spikes in COVID-19 cases would occur within the first several weeks of students returning to campus,15 the university phased the return of students to Clemson by delaying both the arrival of on-campus students to residence halls and in-person instruction by 1 month. On one hand, because on-campus students were set to arrive to congregated residential housing over the course of a week, there was an increased risk of outbreaks in this population.10 On the other, the majority of off-campus students were already living in Clemson during the summer of 2020. Additionally, surveillance testing during the start of in-person instruction revealed a much lower disease prevalence in the off-campus population than in the on-campus population.
Statistical analysis
For each time interval corresponding to online instruction, in-person instruction, and different stages of surveillance testing, we assessed the total number of SARS-COV-2 tests done (Teststotal), the total number of positive tests (Testspositive), and calculated the percentage of positive SARS-COV-2 tests (Testspositive/Teststotal). We compare differences in percentage of positive tests using risk ratios with Wald CIs. We also assessed the number of unique individuals tested (Ntested) and the number of unique individuals testing positive (Npositive) in each time interval and calculated the proportion of individuals testing positive (Npositive/Ntested). We estimated the number of COVID-19 cases and the prevalence of COVID-19 among the susceptible population at week t as a weighted average of the number of unique individuals testing positive from the voluntary and surveillance groups (additional details in appendix p 2). We compared changes in weekly prevalence estimates using risk ratios; 95% CIs were computed with percentile bootstrap with 200 resamples.16 We used R, version 4.0.2, for the statistical analysis.
SARS-CoV-2 transmission modelling
We developed a metapopulation model using a cross-coupling matrix for SARS-CoV-2 transmission17 to assess the relative effect of multiple testing strategies on reduction of COVID-19 prevalence. The following compartments were included for each cluster (ie, residence hall buildings and off-campus population): S, E, IA, IS, TE, TI, H, and R. Here, S is the number of susceptible students who have not been infected, E is the number of students who have been exposed to the disease but are not yet infectious (ie, cannot transmit the disease to others), IA is the number of students with asymptomatic infection, IS is the number of students with symptomatic infection, TE is the number of exposed students testing positive, TI is the number of infectious students testing positive, H is the number of students requiring isolation housing, and R is the number of recovered students and are no longer infectious. Additional model details are provided in the appendix (pp 3, 5, 8).
We used two compartments to allow for a lower test sensitivity among individuals in the exposed (latent) compartment than in the infectious compartments.18, 19 After a lag of 24 h after test collection, positive cases were transferred to isolation. We assume that students with symptoms were voluntarily tested and isolated 3 days after becoming infectious (accounting for a 2-day presymptomatic period20), with the exception of those detected through surveillance testing beforehand. We set the reproductive number (Rt) to 3.21, 22 Model input parameters are provided in the appendix (p 6). These parameters were based on our empirical surveillance data in conjunction with published sources.8, 13, 17, 18, 19, 20, 21, 22, 23
The model assumes that 6273 on-campus students were distributed across 35 residence hall buildings. We also incorporated an off-campus population of 12 275 students to account for the effect of on-campus and off-campus student interactions on the effectiveness of on-campus testing strategies. Initial model states were based on our empirical testing data (appendix p 6). Under voluntary testing, only individuals in the symptomatic infectious compartment (IS) are tested. Under SBIT and random surveillance testing, 450 daily surveillance tests are done (equivalent to testing 7·2% of the student population daily). All surveillance testing strategies include voluntary testing for symptomatic students. The protocol for SBIT was set to emulate that of Clemson University during the first week of in-person instruction (appendix p 3). We compared the relative effectiveness of these strategies with high-frequency repeated testing, which is considered the gold standard. Additionally, we assessed the effectiveness of these strategies under a hypothetical scenario in which implementation occurs upon student arrival on campus (resulting in a reduced number of initial infections at implementation).
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of this manuscript.
Results
6273 students arriving to on-campus residence halls before Sept 23, 2020, were included in our study. In the month preceding in-person instruction, 326 (5·2%) on-campus students tested positive for SARS-COV-2, and 179 (3·0%) tested positive on mandatory pre-arrival or arrival testing (table ). During in-person instruction, 1234 (21·0%) on-campus students tested positive for SARS-COV-2. Demographic information was available for all on-campus students tested at the university's surveillance testing centre (6100 individuals): median age was 18 years (IQR 18–19), and 3297 (54·0%) were women. An average of 5·63 (SD 2·78) tests were done per eligible on-campus student during in-person instruction. Of the 5909 on-campus students eligible for surveillance testing, 5870 (99·3%) were tested by university-provided vendors by the end of in-person instruction. Timely compliance among those instructed to get tested, defined as testing between 1 day before to 3 days after the mandated date, was 91·2%. Compliance within 7 days was 96·2%.
Table.
Test results for on-campus students (n=6273)
| Teststotal | Testspositive(% of Teststotal) | Ntested | Npositive(% of Ntested) | |||
|---|---|---|---|---|---|---|
| Online instruction (Aug 19 to Sept 20) | 12 040 | 349 (2·9%) | 6254 | 326 (5·2%) | ||
| Pre-arrival and arrival testing (Sept 1–20)* | 11 028 | 190 (1·7%) | 5963 | 179 (3·0%) | ||
| Voluntary testing post arrival (Sept 12–20)† | 605 | 19 (3·1%) | 574 | 19 (3·3%) | ||
| In-person instruction (Sept 21 to Nov 25) | 33 510 | 1277 (3·8%) | 5870 | 1234 (21·0%) | ||
| Surveillance testing‡ | 29 658 | 788 (2·7%) | 5552 | 770 (13·9%) | ||
| Voluntary testing† | 3852 | 489 (12·7%) | 2554 | 480 (18·8%) | ||
| SBIT (Sept 23 to Oct 5) | 6353 | 664 (10·5%) | 4741 | 645 (13·6%) | ||
| Surveillance testing | 5379 | 387 (7·2%) | 4296 | 379 (8·8%) | ||
| Random testing | 3420 | 179 (5·2%) | 2911 | 176 (6·0%) | ||
| Targeted testing | 1959 | 208 (10·6%) | 1872 | 203 (10·8%) | ||
| Voluntary testing† | 974 | 277 (28·4%) | 867 | 273 (31·5%) | ||
| Weekly testing (Oct 6 to Nov 22) | 25 937 | 567 (2·2%) | 5183 | 551 (10·6%) | ||
| Surveillance testing | 24 278 | 401 (1·7%) | 4958 | 391 (7·9%) | ||
| Voluntary testing† | 1659 | 166 (10·0%) | 1094 | 165 (15·1%) | ||
Data are n or n (%), and all dates are for 2020. We define Teststotal as the number of COVID-19 tests and Testspositive as the number of COVID-19 positive tests. NTested is the number of unique individuals tested for COVID-19 and NPositive is the number of unique individuals with a positive test result. Because of the potential of multiple testing categories per student, the total number of students tested and those testing positive will not necessarily add up to the N within each period. SBIT=surveillance-based informative testing.
Test completed within 10 days before arrival or within 2 days after arrival.
Voluntary tests are defined as tests not mandated by the university surveillance strategy and include testing of symptomatic students, students directed to testing by contact tracers, or students tested for other reasons.
Random surveillance testing done between Sept 23 and Nov 22.
On the basis of random testing during the first 3 days of SBIT (Sept 23–25), we estimated that COVID-19 prevalence was 6·7% (during this period). Outbreaks were identified in eight residence hall buildings. Targeted testing between Sept 26–28 detected a positivity rate of at least 10% in five of these eight residence hall buildings. A total of 45 floors were identified for targeted testing between Sept 29 and Oct 5; 13 had a positivity rate of at least 10%. For residence hall buildings or floors exceeding the threshold, the average time between the collection date of random surveillance tests and the date of targeted testing was 3·71 days (SD 1·69). Of the 5379 surveillance tests done during implementation of SBIT, 1959 (36·4%) were targeted surveillance tests and 3420 (63·6%) were random surveillance tests. Compared with random tests, targeted tests were 2·03 times more likely to detect a SARS-COV-2 positive case (95% CI 1·67–2·46; table). Between Sept 23 and Oct 5, voluntary testing detected 273 (42·3%) of 645 SARS-COV-2 positive cases, and SBIT identified the remaining 57·7% of cases (table).
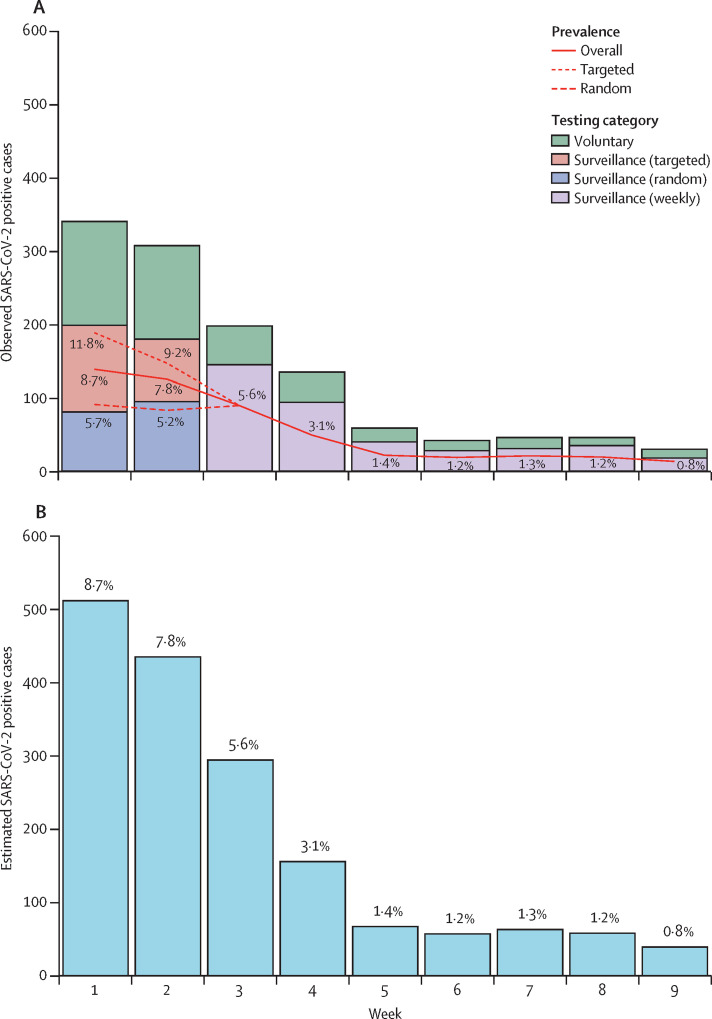
Aggressive targeted testing implemented during the first 2 weeks of in-person instruction contributed to the reduction in on-campus prevalence (figure 2A ). Prevalence was reduced during this period from a peak of 8·7% (week 1) to 5·6% (week 3), a relative reduction of 36% (95% CI 27–44). After implementation of weekly testing in week 3, (relative) prevalence decreased by 75% (71–81) over the following 2 weeks (week 5 prevalence 1·4%). Estimated prevalence during the final week of surveillance testing (week 9) was 0·8%. Estimated weekly cases (on the basis of prevalence estimates) are presented in figure 2B. The total number of estimated infections throughout surveillance testing was 1619.
Figure 2.
Weekly SARS-CoV-2 positive cases and prevalence estimates among on-campus students during in-person instruction
(A) Observed SARS-CoV-2 positive cases and prevalence estimates for entire population (solid line) and from surveillance tests done during implementation of SBIT (targeted: dotted line; random: dashed line); surveillance testing began on Sept 23, 2020; targeted testing was done by residence hall building (Sept 26–28) or floor (Sept 29 to Oct 5), and repeated weekly surveillance testing was done between Oct 6 and Nov 22. (B) Estimated weekly SARS-COV-2 cases (percentages represent estimated overall prevalence); week 1 (beginning Sept 23) and week 3 (beginning Oct 6) consist of 6 days to account for timing of surveillance testing implementation (Sept 23), transition to targeted testing of residence hall floors (Sept 29), and implementation of weekly testing (Oct 6).
12 275 eligible off-campus students were granted access to campus facilities before in-person instruction (appendix p 4). 1347 (11·5%) of 11 715 off-campus students tested during online instruction and 632 (6·7%) of 9384 tested during in-person instruction were positive for SARS-COV-2 (appendix p 7). Weekly off-campus prevalence fluctuated between 0·5% and 3·4% throughout in-person instruction (appendix p 11).
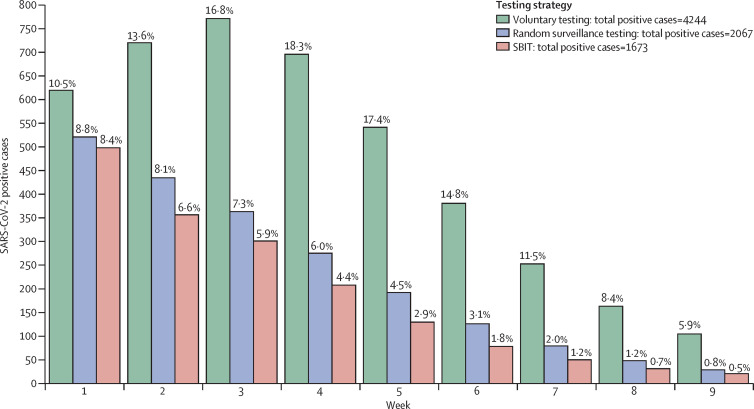
The results of the SARS-CoV-2 transmission models are displayed in figure 3 . The high proportion of infections at the start of testing (6·7%) led to early and large outbreaks, regardless of testing strategy. The number of infections detected during the first week was 619 with voluntary testing, 518 with random surveillance testing, and 495 with SBIT. Under voluntary testing, prevalence increased from 10·5% in week 1 to 16·8% in week 3, a relative increase of 60%. During this period, prevalence decreased from 8·8% to 7·3% under random surveillance testing (a relative decrease of 17%) and decreased from 8·4% to 5·9% under SBIT (relative decrease of 30%).
Figure 3.
Expected number and prevalence of weekly on-campus SARS-COV-2 positive cases based on SARS-CoV-2 transmission models
Three testing strategies are compared here: voluntary testing for symptomatic individuals, random surveillance testing, and SBIT. Both surveillance testing strategies include 450 daily surveillance tests and voluntary testing for symptomatic individuals. The proportion of individuals infected at the semester start was 6·7%. Percentages represent the prevalence of new weekly SARS-COV-2 positive cases relative to the susceptible population at the beginning of each week. Total infections represent the number of infections over the course of 9 weeks. SBIT=surveillance-based informative testing.
Compared with SBIT (1673 total infections), random surveillance testing alone (2067 total infections) would have resulted in 24% more infections and voluntary testing alone (4244 total infections) would have resulted in 154% more infections throughout the semester. Weekly testing (1068 total infections) would have resulted in 36% fewer infections and twice-weekly testing (468 total infections) would have resulted in 72% fewer infections throughout the semester compared with SBIT (appendix p 9), but weekly testing would require two times the number of daily tests, and twice-weekly would require four times the number of daily tests. The performance of all testing strategies improved when implemented upon student arrival to campus (assuming a baseline COVID-19 prevalence of 1%; appendix p 10).22
Discussion
Testing strategies for mitigating SARS-CoV-2 spread on college and university campuses have not been extensively studied. Our study found evidence that SBIT can detect and contain on-campus outbreaks. Targeted testing of residence halls during a 2-week implementation period, which was twice as likely to detect a positive SARS-COV-2 case than random testing, led to a substantial reduction in prevalence. Our modelling study concluded that in the absence of SBIT, COVID-19 cases would have increased by 154%. Although this study and others provide strong evidence that frequent repeated testing of the entire student population is optimal for outbreak prevention,8, 9 many institutions do not have the required testing capacity for implementation. Among US institutions with at least 5000 undergraduates that had in-person classes in Fall 2020, only 6% did high-frequency repeated testing throughout the semester.9, 24 However, 25% of these institutions are estimated to have implemented random surveillance testing during the Fall 2020 semester,24 and thus have the necessary infrastructure to easily transition to an SBIT approach. Despite using the same number of daily tests, our modelling study concluded that random surveillance testing alone would have led to a 24% increase in the number of COVID-19 cases throughout the semester compared with SBIT, further indicating that an SBIT approach is more resource efficient.
Our empirical data underscore the usefulness of optimising testing resources through an SBIT approach, as nearly 60% of SARS-COV-2 positive cases were detected through SBIT during its implementation period. The high rate of compliance with mandatory testing among the student population, despite few consequences for noncompliance, indicates that such testing on a public university campus is feasible and acceptable to the student population. In the absence of such surveillance testing strategies and isolation of positive cases, these students might have gone undetected, and thus contributed to an increase in disease spread on campus and in surrounding communities, potentially forcing the university to shut down operations.
Effective surveillance testing strategies are economical when the alternative is university closure. External vendors charged the university approximately US$80 per test. These tests were gradually phased out with the development of an in-house saliva-based alternative, which cost $7·38 per test. On one hand, depending on whether in-house tests or external tests were used, we estimate that testing an on-campus population of 6000 students would cost a university $22 000–255 000 per week with SBIT and $44 000–510 000 per week with weekly testing. On the other, we estimate that Clemson University generated approximately $1·65 million per week in housing and dining costs that would have been lost if the university was forced to shut down.
We continue to recommend that institutions of higher education test all students before campus arrival. Testing mandated by Clemson University within 1 month before in-person instruction detected over 1700 students who were positive for SARS-COV-2 (9·4% of the student population tested), providing additional empirical evidence that pre-arrival testing can prevent hundreds to thousands of infected students from returning to campus.22 Contact tracing, and other preventive strategies to limit the spread of SARS-CoV-2, are less effective if implemented after such large outbreaks occur.15, 22, 25, 26
In addition to pre-arrival testing, SBIT can reduce the reliance on contact tracers because all contacts that live in the same residence hall floor (or building) are immediately tested. The benefit of SBIT was evident during the initial surge in cases between late September and early October, when university medical staff required 24 h to notify close contacts because of the high number of positive cases. As cases began to decrease in mid October, this process was reduced to a few hours. Similar trends were seen with contact tracers from SCDHEC, who also experienced delays during the early semester surge.
The approaches discussed in this paper are subject to several limitations. First, due to logistical challenges, surveillance testing was not mandatory in the 14-day period between the first wave of student arrival to residence halls and the start of in-person classes, probably contributing to the rise in on-campus disease prevalence early in the semester. Second, because repeated weekly testing was implemented after the removal of many positive cases detected through SBIT, the relative effectiveness of the two strategies could not be directly assessed with use of empirical data. Third, the associations between the implementation of testing strategies and outcomes are not causal; other factors might have contributed to the drop in disease prevalence throughout the semester.
Therefore, we implemented mathematical models to compare the effect of these strategies in mitigating disease spread. However, certain simplistic assumptions were made (eg, homogeneous mixing).27 Additionally, we did not examine the effect of exogenous infections from other populations (eg, community residents). Although the parameters of these models were governed by published literature, and the initial states of our models were based on our empirical data, they might vary across institutions. Our results show that the effectiveness of testing strategies is sensitive to these initial states. Therefore, we integrated our models into a web-based application that allows for adjustment of initial conditions and parameters (eg, test sensitivity, test turnaround time, and so on).
For example, lengthening the test turnaround time reduces the effectiveness of testing strategies by allowing continued contact between students who are infectious and susceptible individuals. At Clemson, tests collected from symptomatic students and other high-risk students (ie, students selected for targeted testing or referred by contact tracers) were expedited and results were returned in under 24 h. For institutions that are unable to achieve these quick turnaround times because of logistical constraints, rapid testing could be used for these high-risk individuals. These tests typically have a turnaround time of several hours and can help expedite the isolation of confirmed cases and quarantine of suspected contacts.28
Finally, this study did not examine the effect of testing on mitigating community spread. Mandatory surveillance testing for university employees and contractors using campus facilities indicated a stable prevalence in this population throughout in-person instruction (fluctuating between 0·6% and 1·7%),29 suggesting that student infections did not spread to this population. However, institutional mask mandates and social distancing on campus probably created a barrier between potentially infectious students and susceptible employees. Residents in local communities might not be subjected to such interventions. Research on student–community transmission is therefore needed to better understand the effect of student–community spread.
Given that college and university students tend to socialise in clusters, the results of our study are generalisable to targeted testing of residence halls across campus environments. However, compared with city campuses, Clemson's campus is in a rural university town that had reduced interactions with non-university affiliated residents during the Fall 2020 semester. Therefore, an increase in testing frequency might be required for other settings where a high number of interactions occur between students and local communities with high COVID-19 prevalence.
SBIT can also be extended to at-risk environments beyond university residence halls, such as off-campus student organisations2, 7 (eg, fraternal or athletic organisations) or apartment residencies. Sufficient testing is also necessary to mitigate disease spread in local communities or schools.7, 30 SBIT might be useful for detecting and containing outbreaks in these settings through random and targeted testing of potential hotspots, such as nursing homes or classrooms. If surveillance testing of the population is not practical, community or individual self-reported data could be used to detect outbreaks.31
Even with the dissemination of the recently approved vaccines, public health mitigation strategies will need to continue into the foreseeable future.32, 33 Between the start and end of the Fall 2020 semester, COVID-19 prevalence increased nearly five-fold nationwide.34 Therefore, it is imperative that institutions rigorously test students before arrival and during the 2021 academic year. As SARS-CoV-2 testing becomes more widely available among institutions, careful consideration should be given to the use of testing resources. When institutional testing capacity cannot support high-frequency repeated testing to mitigate SARS-CoV-2 spread, SBIT is an effective and economical alternative that can be feasibly implemented across colleges and universities.
Data sharing
Data collected for this study have not received institutional approval to be shared at this time.
Declaration of interests
DD reports support from the State of South Carolina (CARES act) and US National Institutes of Health (R01 MH111366) during the conduct of this study, and is the founder of Accessible Diagnostics. CCC reports personal fees from Christopher Colenda for public health consulting to Clemson University during the conduct of this study. All other authors declare no competing interests. Clemson University did not influence the work or findings of this report.
Acknowledgments
Acknowledgments
We thank the Clemson's administration, medical staff, and all other testing providers who helped implement and manage SARS-CoV-2 testing at Clemson University. We thank Clemson's Computing & Information Technology department for their role in collecting, managing, and distributing test results. We thank L Banu Sivaraj for her assistance in the creation of figures for this study. LR, CM, and CAK acknowledge salary support from Clemson University for consulting and modelling work pertaining to development and implementation of strategies (project #1502934). YY and BL report financial support (graduate assistantship) from Clemson University during the conduct of this study. CCC is an independent paid advisor to Clemson University on COVID-19 matters.
Contributors
LR, CM, CAK, and CCC contributed to the conceptualisation of the study. LR, LP, and CCC did the literature search. LR and BL did the figures and visualisations. LR, CM, and YY contributed to the methods. LR, CM, CAK, LP, and DD contributed to project administration. LR, CM, and LP verified the data. LR wrote the first draft, and all other authors reviewed the manuscript. The corresponding author had full access to all the data in this study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Ojo A, Forman HP, Gross CP. Colleges and COVID-19 data dashboards—not just an academic exercise. JAMA Health Forum. 2020;1 doi: 10.1001/jamahealthforum.2020.1272. [DOI] [PubMed] [Google Scholar]
- 2.Wilson E, Donovan CV, Campbell M, et al. Multiple COVID-19 clusters on a university campus—North Carolina, August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1416–1418. doi: 10.15585/mmwr.mm6939e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The New York Times Tracking the coronavirus at U.S. colleges and universities. The New York Times. 2020 https://www.nytimes.com/interactive/2020/us/covid-college-cases-tracker.html [Google Scholar]
- 4.Walke HT, Honein MA, Redfield RR. Preventing and responding to COVID-19 on college campuses. JAMA. 2020;324 doi: 10.1001/jama.2020.20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis M. COVID-19 outbreak among college students after a spring break trip to Mexico—Austin, Texas, March 26-April 5, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:830–835. doi: 10.15585/mmwr.mm6926e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gressman PT, Peck JR. Simulating COVID-19 in a university environment. Math Biosci. 2020;328 doi: 10.1016/j.mbs.2020.108436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrios LC, Green RF, Honein MA. The role of testing in reducing SARS-CoV-2 transmission on college campuses. J Adolesc Health. 2021;68:1–2. doi: 10.1016/j.jadohealth.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paltiel AD, Zheng A, Walensky RP. Assessment of SARS-CoV-2 screening strategies to permit the safe reopening of college campuses in the United States. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denny TN, Andrews L, Bonsignori M, et al. Implementation of a pooled surveillance testing program for asymptomatic SARS-CoV-2 infections on a college campus—Duke University, Durham, North Carolina, Aug 2–Oct 11, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1743–1747. doi: 10.15585/mmwr.mm6946e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasper MR, Geibe JR, Sears CL, et al. An outbreak of COVID-19 on an aircraft carrier. N Engl J Med. 2020;383:2417–2426. doi: 10.1056/NEJMoa2019375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemson News COVID-19 testing plans finalized for employees. 2020. https://news.clemson.edu/covid-19-testing-plans-finalized-for-employees/
- 12.Afzal A. Molecular diagnostic technologies for COVID-19: limitations and challenges. J Adv Res. 2020;26:149–159. doi: 10.1016/j.jare.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Centers for Disease Control and Prevention Duration of isolation and precautions for adults with COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html
- 14.US Centers for Disease Control and Prevention Interim Guidance for influenza outbreak management in long-term care and post-acute care facilities. 2020. https://www.cdc.gov/flu/professionals/infectioncontrol/ltc-facility-guidance.htm
- 15.Rennert L, Kalbaugh C, McMahan C, Shi L, Colenda CC. The urgent need for phased university reopenings to mitigate the spread of COVID-19 and conserve institutional resources: a modeling study. medRxiv. 2020 doi: 10.1101/2020.08.25.20182030. published online Aug 31. (preprint). [DOI] [Google Scholar]
- 16.Efron B, Tibshirani R. Chapman & Hall; New York, NY: 1993. An introduction to the bootstrap. [Google Scholar]
- 17.Lloyd AL, Jansen VAA. Spatiotemporal dynamics of epidemics: synchrony in metapopulation models. Math Biosci. 2004;188:1–16. doi: 10.1016/j.mbs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Surkova E, Nikolayevskyy V, Drobniewski F. False-positive COVID-19 results: hidden problems and costs. Lancet Respir Med. 2020;8:1167–1168. doi: 10.1016/S2213-2600(20)30453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 21.US Centers for Disease Control and Prevention COVID-19 pandemic planning scenarios. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html
- 22.Rennert L, Kalbaugh CA, Shi L, McMahan C. Modelling the impact of presemester testing on COVID-19 outbreaks in university campuses. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-042578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjørnstad ON. Springer International Publishing; Cham: 2018. Epidemics: models and data using R. [Google Scholar]
- 24.Nadworny E, McMinn S. Even in COVID-19 hot spots, many colleges aren't aggressively testing students. NPR. 2020 https://www.npr.org/2020/10/06/919159473/even-in-covid-hot-spots-many-colleges-arent-aggressively-testing-students [Google Scholar]
- 25.Hellewell J, Abbott S, Gimma A, et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kretzschmar ME, Rozhnova G, Bootsma MCJ, van Boven M, van de Wijgert JHHM, Bonten MJM. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health. 2020;5:e452–e459. doi: 10.1016/S2468-2667(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowd JB, Andriano L, Brazel DM, et al. Demographic science aids in understanding the spread and fatality rates of COVID-19. Proc Natl Acad Sci USA. 2020;117:9696–9698. doi: 10.1073/pnas.2004911117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brendish NJ, Poole S, Naidu VV, et al. Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): a prospective, interventional, non-randomised, controlled study. Lancet Respir Med. 2020;8:1192–1200. doi: 10.1016/S2213-2600(20)30454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemson University COVID-19 dashboard. 2021. https://www.clemson.edu/covid-19/testing/dashboard.html
- 30.Panovska-Griffiths J, Kerr CC, Stuart RM, et al. Determining the optimal strategy for reopening schools, the impact of test and trace interventions, and the risk of occurrence of a second COVID-19 epidemic wave in the UK: a modelling study. Lancet Child Adolesc Health. 2020;4:817–827. doi: 10.1016/S2352-4642(20)30250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varsavsky T, Graham MS, Canas LS, et al. Detecting COVID-19 infection hotspots in England using large-scale self-reported data from a mobile application: a prospective, observational study. Lancet Public Health. 2021;6:e21–e29. doi: 10.1016/S2468-2667(20)30269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpp KG, Loewenstein G, Buttenheim AM. Behaviorally informed strategies for a national COVID-19 vaccine promotion program. JAMA. 2020;325:125–126. doi: 10.1001/jama.2020.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanne JH. Covid-19: US cases surge but vaccine distribution is slow. BMJ. 2021;372:n42. doi: 10.1136/bmj.n42. [DOI] [PubMed] [Google Scholar]
- 34.US Centers for Disease Control and Prevention COVID-19 cases, deaths, and trends in the US | CDC COVID data tracker. 2020. https://covid.cdc.gov/covid-data-tracker
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for this study have not received institutional approval to be shared at this time.