Abstract
Background
Despite the benefits of breast milk and colostrum for the health and survival of children, early prelacteal feeding is commonly practiced worldwide, particularly in low- and middle-income countries. The aim of this study was to evaluate the pooled prevalence and determinants of prelacteal feeding in Eastern Africa.
Methods
This study was carried out within 11 East African countries from 2010 to 2018, a pooled study of prelacteal feeding was performed. For assessing model fitness and contrast, intra-class correlation coefficient, median odds ratio, proportional change in variance, and deviance were used. In order to identify possible covariates associated with prelacteal feeding in the study area, the multilevel multivariable logistic regression model was adapted. Adjusted Odds Ratio was used with 95% confidence interval to declare major prelacteal factors.
Results
The pooled prevalence of prelacteal feeding in Eastern Africa was 12% (95% CI: 11.42–12.53%), with the highest prevalence of prelacteal feeding in the Comoros (39%) and the lowest in Malawi (3%). Multilevel multivariable logistic regression model; wealth index (AOR = 1.22; 95% CI 1.03–1.34), ANC visit (AOR = 1.42; 95% CI: 1.12–1.79), institutional delivery (AOR = 0.58; 95% CI: 0.58–0.64), small birth size (AOR = 1.14; 95% CI: 1.30–1.26), delivery type (AOR = 2.61; 95% CI: 2.30–2.96), and high community ANC visit (AOR = 0.90; 95% CI: 0.84–0.97) were significantly associated with prelacteal feeding in Eastern Africa.
Conclusion
In East Africa, the magnitude of prelacteal feeding was still high. The possible determinants of prelacteal feeding in Eastern Africa were wealth index, birth interval, delivery mode, place of delivery, ANC visit, and community ANC visit. Structural improvements are required for women with caesarean births to achieve optimal breastfeeding practice in Eastern Africa.
Keywords: prelacteal feeding, optimal breastfeeding, multilevel, Eastern Africa
Introduction
One of the most successful types of action to ensure the health and survival of children is breastfeeding.1–3 Early breastfeeding helps protect against child infection and prevents the long-term onset of adult obesity, cardiovascular disease and diabetes.2,4,5 Despite the benefits of breast milk and colostrum for child health and survival, early prelacteal feeding is commonly practiced worldwide, especially in lower and middle income countries.4,6 Early initiation of breastfeeding within 1 hour of birth is recommended by the World Health Organization (WHO) and the United Nation Children’s Fund (UNICEF); exclusive breastfeeding for the first 6 months of life and the introduction of nutritionally sufficient and healthy supplementary (solid) foods at 6 months, along with continued breastfeeding up to or after 2 years of age.7,8 However, many infants do not receive optimal feeding ie only 44% of infants aged less than 6 months worldwide were exclusively breastfeed over the period of 2015–2020.8 Evidence shows that only 47–57% of infants under 2 months and 25–31% of infants 2–5 months were exclusively breastfed in Africa, Asia, Latin America, and the Caribbean countries alone.9 Prelacteal is defined as the administration of any food except mother’s milk to a newborn before beginning breastfeeding within three days, one of the major barriers to exclusive breastfeeding.10,11 Prelacteal feeding is the primary barrier to exclusive breastfeeding (EBF) and is an important measure of infant mortality and morbidity.6,12,13 Forty-five percent of infectious neonatal deaths, 30% of diarrheal deaths and 18% of acute respiratory infections are associated with prelacteal feeding in children younger than 5 years of age.1,14 In addition, prelacteal feeding is considered as the major cause of low physical development, poor intelligence, and poorer resistance to disease.15,16 Long-term wellbeing and expressive advantages for infants with inhibition of child mortality and morbidity are strongly correlated with optimal breastfeeding practice.17–19 The availability of optimum breastfeeding has the ability to prevent 1.4 million deaths annually among children under five years of age.20 Approximately 96% of all child deaths occur within the first 6 months of life are due to non-exclusive breastfeeding, which is significantly higher in Asian and African countries.21 Some of the most important methods recommended for reducing the risk of neonatal and infant mortality in developing and developed countries are antenatal care (ANC) and health facility delivery.22–24 Since early ANC services are accessed, multiple health care programs are given to improve maternal and child well-being, including the risk of prelacteal feeding.25–27 Therefore, during ANC visits, various nutritional and other health-related education is delivered by healthcare professionals, which could make a significant contribution to the timely initiation of breastfeeding and exclusive breastfeeding in resource-limited countries for infants.28,29 Similarly, institutional delivery allows babies to get skin-to-skin contact with their mothers, which would increase the probability of initiating early breastfeeding, decrease the provision of prelacteal feeding, and prolonged the duration of breastfeeding.30–32 Some studies have been performed in eastern Africa to assess the incidence of prelacteal feeding and related factors.33–41 The results of these scattered studies showed that the prevalence of prelacteal feeding across Eastern African countries varied widely. Given that countries are similar to a certain Eastern African situation, better intervention and up-to-date knowledge on the prevalence of prelacteal feeding is crucial. However, owing to these limited and fragmented studies, no studies have been performed to establish the pooled prevalence of prelacteal feeding practice and its determinants in Eastern Africa. The outcome variable coded as 1 = provided prelacteal feeds, and 0 = did not provide prelacteal feeds. Evidence showed that breastfeeding practices could be affected by interaction of individual, family, socio-economic, and community factors.42 This study aimed to estimate the pooled prevalence of prelacteal feeding practice and its determinants in Eastern Africa. The results of this study will provide guidance to policymakers and program managers working in the field of nutrition and breastfeeding.
Methods
Source of Data and Study Population
After registering and specifying the purpose of the analysis, the data set was obtained from the demographic and health survey (DHS) website (http://www.dhsprogram.com). In the demographic and health survey (DHS) in Eastern Africa, we used pooled data. With individuals nested within nations, the pooled data has a hierarchical structure. The analysis of this study was limited to children born in the five years preceding the survey. The 11 East African countries in which data extracted include Burundi, Ethiopia, Kenya, Comoros, Madagascar, Malawi, Mozambique, Rwanda, Tanzania, Uganda, Zambia, and Zimbabwe (Table 1).
Table 1.
Eastern African Demographic and Health Survey Characteristics of Children
| Country | Survey Year | Frequency | Percentage |
|---|---|---|---|
| Burundi | 2016–17 | 13,192 | 10.98 |
| Comoros | 2012 | 3149 | 2.62 |
| Ethiopia | 2016 | 10,641 | 8.86 |
| Kenya | 2013 | 20,964 | 17.45 |
| Malawi | 2015–16 | 17,286 | 14.39 |
| Mozambique | 2011 | 5178 | 4.31 |
| Ruanda | 2014–15 | 7856 | 6.54 |
| Tanzania | 2014–15 | 10,233 | 8.52 |
| Uganda | 2016 | 15,522 | 12.92 |
| Zambia | 2018 | 9959 | 8.29 |
| Zimbabwe | 2015 | 6132 | 5.11 |
| All | 120,112 | 100.00 |
There 20 countries in WHO regions of East Africa among those only 14 countries had DHS data. For this study 11 countries were included (Figure 1).
Figure 1.
Schematic diagram for the collection of countries surveyed by East African countries.
Study Variables
The outcome variable was prelacteal feeding that was added for all last-born past five years prior to the survey.5 Two sets of explanatory variables were included in this analysis (individual and community-level). The community’s characteristics can directly influence the decision of women to feed prelacteal or alter the relationship between individual characteristics and the decision to prelacteal feeding. Similarly, the impact of community-level factors may also be modified by individual-level factors.
Individual-Level Variables
Both maternal (socio-demographic and maternal health service-related features) and child-related variables have been included at this stage.
Religion, household wealth index, mother’s age at birth, maternal education, maternal occupation, ANC visit, type of delivery assistant, mode of delivery, place of delivery, and media exposure are all maternal variables.
Child-Related Variables
The child’s sex was reported as being both male and female. The birth order tells about the birth order of the child and has been registered in the EDHS data set as a continuous variable. It was graded as first, second or third, and fourth or more, respectively. Baby size at birth: based on the understanding of the mother and reported as small (very small, less than average), medium (average), big (larger than average and very large).
Community-Level Variables
Non-aggregate community-level variables were place of residence and area. The place of residence has been registered as rural and urban. The area was described as the province from which a child comes from. By aggregation from an individual level, another group of community-level variables was developed using average approaches to conceptualize the neighbourhood effect on the implementation of prelacteal. Education for women in the neighbourhood, community poverty, community visit to the ANC, community place of delivery.
Data Management and Analysis
The research for this thesis was performed using version 15 of STATA (STATA Corporation. IC., TX, USA). For the calculation of descriptive statistics such as proportions, sampling weights were used to account for non-proportional distribution of the sample to strata. In the case of standard regression models, the research participants are considered to be independent of the outcome variable. Nevertheless, units in the same category are rarely independent when data are ordered in hierarchies.43 Units from the same setting (cluster) are more similar to each other in relation to other units, or in relation to the outcome of interest than units from another setting. This may then lead to a breach of the assumption of independence which could have the effect of underestimating standard errors and increasing Type I error rates (increases rate of false positivity of our results). In such circumstances, multilevel modelling can simultaneously account for person and community-level variables and provide a more comprehensive understanding of prelacteal feeding factors.44 Multilevel models are therefore developed to overcome the analytical problems that arise when data are hierarchically organized, and sampled data are a sample of several stages of this hierarchical population, such as DHS, in which children are nested in households, and households are nested in clusters, and there is an intra-group correlation. In order to estimate both independent (fixed) effects of explanatory variables and community-level random effects on the initiation of prelacteal feeding, a two-level mixed-effect logistic regression model was fitted. The person (children) is the first level and the cluster is the second level (community). Therefore, using the two-level multilevel model, the record of the likelihood of implementing prelacteal feeding was modelled as follows:
 |
where i and j are the units of level 1 (individual) and level 2 (population) respectively; X and Z apply to variables of the individual and community level, respectively; 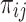 is the likelihood of having prelacteal feeds in the jth community for the ith mother; the βs are the fixed coefficients-therefore, there is a corresponding efficiency for each one-unit increase in X/Z (a set of predictor variables). Whereas in the absence of control of predictors,
is the likelihood of having prelacteal feeds in the jth community for the ith mother; the βs are the fixed coefficients-therefore, there is a corresponding efficiency for each one-unit increase in X/Z (a set of predictor variables). Whereas in the absence of control of predictors,  is the intercept-the effect on the likelihood of mother on the provision of prelacteal feed; and μj indicates the random effect for the jth community (effect of the community on the decision of mother to provide prelacteal feed). The clustered data existence and the within and between community variations were taken into account by assuming that each community has a different intercept (
is the intercept-the effect on the likelihood of mother on the provision of prelacteal feed; and μj indicates the random effect for the jth community (effect of the community on the decision of mother to provide prelacteal feed). The clustered data existence and the within and between community variations were taken into account by assuming that each community has a different intercept (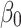 ) and fixed coefficient (β).
) and fixed coefficient (β).
In the bi-variable multilevel logistic regression model, the individual and community level variables associated with prelacteal feeding were independently tested and variables that were statistically significant at p-value 0.20 were considered for the final individual and community level adjustments. In the multivariable multilevel analysis, variables with p-value ≤0.05 were declared as significant determinants of prelacteal feeding practice.
Model Building
Four models were fitted. The first was the null model containing no exposure variables which was used to check variation in community and provide evidence to assess random effects at the community level. Then, model I was the multivariable model adjustment for individual-level variables and model II was adjusted for community-level factors. In model III, possible candidate variables from both individual and community-level variables were fitted with the outcome variable.
Parameter Estimation Method
The intra-class correlation (ICC) is approximated to calculate the group effect (variation) as; ICC = 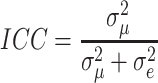 where
where 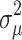 denotes the variance of the group level; and
denotes the variance of the group level; and  denotes the variance of the individual level that is set for the distribution of logs to
denotes the variance of the individual level that is set for the distribution of logs to 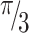 (equal to 3.29). In addition, the Community Variance (PCV) proportional shift and the Median Odds Ratio (MOR) were used to calculate community variance. The aim of MOR is to convert the variance of the area level into the commonly used scale of the odds ratio (OR), which has a coherent and intuitive meaning. When randomly selecting two areas, the MOR is defined as the median value of the odds ratio between the area at highest risk and the area at lowest risk. If going to another region with a higher risk, the MOR can be conceptualized as the increased risk that (in median) will have. It is determined by
(equal to 3.29). In addition, the Community Variance (PCV) proportional shift and the Median Odds Ratio (MOR) were used to calculate community variance. The aim of MOR is to convert the variance of the area level into the commonly used scale of the odds ratio (OR), which has a coherent and intuitive meaning. When randomly selecting two areas, the MOR is defined as the median value of the odds ratio between the area at highest risk and the area at lowest risk. If going to another region with a higher risk, the MOR can be conceptualized as the increased risk that (in median) will have. It is determined by  .45 Where; VA is the variance of the region standard, and 0.6745 is the 75th percentile of the normal distribution’s cumulative distribution function with mean 0 and variance 1, see the detailed definition.46 Whereas the proportional variance shift is determined as43
.45 Where; VA is the variance of the region standard, and 0.6745 is the 75th percentile of the normal distribution’s cumulative distribution function with mean 0 and variance 1, see the detailed definition.46 Whereas the proportional variance shift is determined as43
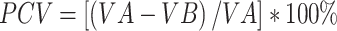 , where; VA = original model variance and VB = model variance with more terms.
, where; VA = original model variance and VB = model variance with more terms.
Result
Socio-Demographic Features of Study Participants in Eastern Africa
A total of 120, 112 children born in the last five years prior to the DHS survey of each nation were included in this report. For girls, the average age was 28 months, with a standard deviation of 17 months. The majority of women aged (60%) are less than 20 years of age. Kenya had (17.45%) more children and Comoros 3 had the smallest number of children (2.62%) (Table 1). The majority of women (52.60%) had primary education, while 26,161 (23.7%) were uneducated. Regarding marital status, large number of women (70.13%) were married. In terms of place delivery, 73.2% of women were delivered at the health facility and a large number of women attended (65%) ANC visits at the second trimester (Table 2).
Table 2.
Socio-Demographic Features in Eastern Africa of Study Participants
| Variables | Frequency | Percentage |
|---|---|---|
| Women education | ||
| No education | 26,161 | 23.70 |
| Primary | 58,033 | 52.60 |
| Secondary | 26,239 | 23.80 |
| Wealth index | ||
| Poor | 52,309 | 47.37 |
| Middle | 20,080 | 18.18 |
| Rich | 38,044 | 34.45 |
| Woman’s age | ||
| <20 | 61,441 | 57.92 |
| 20–34 | 44,409 | 41.87 |
| 34+ | 221 | 0.21 |
| Marital status | ||
| Single | 22,184 | 20.09 |
| Married | 77,448 | 70.13 |
| Divorced | 10,801 | 9.78 |
| Husband/partner educational status | ||
| No education | 16,400 | 19.71 |
| Primary | 41,278 | 49.61 |
| Secondary | 25,528 | 30.68 |
| Birth order | ||
| 1 | 26,002 | 23.55 |
| 2–3 | 39,336 | 35.62 |
| 4–5 | 24,187 | 21.90 |
| 6+ | 20,908 | 18.93 |
| Time of ANC visit | ||
| 1st trimester | 23,447 | 33.03 |
| 2nd trimester | 46,125 | 64.98 |
| 3rd trimester | 1414 | 1.99 |
| No of ANC visit | ||
| No ANC visit | 4655 | 5.93 |
| 1–3 | 31,672 | 40.34 |
| 4+ | 42,178 | 53.73 |
| Place of delivery | ||
| Home | 29,603 | 26.81 |
| Health Institution | 80,830 | 73.19 |
| Residence | ||
| Rural | 83,960 | 76.03 |
| Urban | 26,473 | 23.97 |
| Early initiation of breastfeeding | ||
| Yes | 47,902 | 72.55 |
| No | 18,124 | 27.45 |
| Assistant delivery | ||
| Yes | 23,666 | 21.85 |
| No | 84,643 | 78.15 |
| Size of child at birth | ||
| Large | 28,369 | 29.76 |
| Average | 50,715 | 53.20 |
| Small | 16,243 | 17.04 |
| Skin to skin contact | ||
| Yes | 36,658 | 49.95 |
| No | 36,727 | 50.05 |
| Media exposure | ||
| Yes | 69,963 | 63.40 |
| No | 40,384 | 36.60 |
| Mode of delivery | ||
| Vaginal | 99,839 | 94.33 |
| Caesarean section | 6000 | 5.67 |
| Sex of child | ||
| Male | 55,690 | 50.43 |
| Female | 54,743 | 49.57 |
| Parity | ||
| 1 | 17,486 | 15.83 |
| 2–3 | 42,179 | 38.19 |
| 4–5 | 26,433 | 23.94 |
| 6+ | 24,335 | 22.04 |
Abbreviation: PLAF, prelacteal feeding.
The Pooled Prevalence of Prelacteal Feeding in Eastern Africa
The pooled prevalence of prelacteal feeding in East African countries was 12% (95% CI: 11.42–12.51%), with the highest prelacteal feeding in Comoros (39%) and the lowest prelacteal feeding in Malawi (3%) (Figure 2).
Figure 2.
Percentage of prelacteal feeding in each Eastern Africa countries.
Determinants of Prelacteal Feeding in Eastern Africa
The Fixed Effect Analysis Result
The model with smaller deviance and the greater probability (MODEL IV) was better suited to the data and based on this model, the interpretation of the fixed results was performed. Both the individual and community-level variables, model IV was modified. As a result, the quantity of household income, birth order, time of ANC visit, place of delivery, presence of delivery assistance, size of child at birth, immediate skin-to-skin touch, delivery mode, birth interval, community ANC visit, and community media exposure significantly associated with prelacteal feeding in eastern Africa.
Among the rich, the likelihood of having prelacteal feeding was 1.22 times higher than that of the poor (AOR 1.22; 95% CI = 1.10–1.34). Compared to the first trimester, the likelihood of having prelacteal feeding was 1.42 times higher for women attending ANC visits in the third trimester (AOR = 1.42; 95% CI = 1.12–1.79). Compared with home delivery, the odds of receiving prelacteal feeding for women who delivered in a health facility were 58% lower (AOR = 0.58; 95% CI = 0.52–0.64). The likelihood of providing prelacteal feeding among women whose infant size was on average was 16% lower compared to large birth size, while the odds of providing prelacteal feeding among small birth size was 1.14 times higher compared to large birth size (AOR = 0.84; 95% CI = 0.78–0.91) and (AOR = 1.14; 95% CI = 1.03–1.26). In women who did not have immediate skin-to-skin contact with their newborn infant, the likelihood of having prelacteal feeding was 10% higher compared to immediate skin-to-skin contact (AOR= 1.10; 95% CI= 1.01–1.18). Compared to vaginal delivery, the likelihood of receiving prelacteal feeding was 2.61 times higher for women delivered by caesarean section (AOR = 2.61; 95% CI = 2.30–2.96). The likelihood of providing prelacteal feeding for individuals with a birth space greater than 2 years was 86% lower than those less than 2 years (AOR = 0.86; 95% CI = 0.78–0.94). Among those communities with high ANC visits in Eastern Africa (AOR = 0.90; 95% CI = 0.84–0.97), the odds of having prelacteal feeding was reduced by 90%. The odds of practicing prelacteal feeding have also been increased by 11% among populations who are highly unaware of media attention (AOR = 1.11; 95% CI = 103–1.20) in Eastern Africa (Table 3).
Table 3.
A Multivariable Multilevel Study of Prelacteal Feeding Factors from 2010 to 2018 in Eastern Africa
| Variables | Model II AOR (95% CI) | Model III | Model IV AOR (95% CI) |
|---|---|---|---|
| Women education | |||
| No education | 1.00 | 1.00 | |
| Primary | 0.98 (0.89–1.09) | 0.98 (0.88–1.08) | |
| Secondary+ | 1.01 (0.88–1.15) | 1.00 (0.87–1.14) | |
| Wealth index | |||
| Poor | 1.00 | 1.00 | |
| Middle | 1.01 (0.91–1.11) | 0.99 (0.90–1.09) | |
| Rich | 1.24 (1.12–1.36) | 1.22 (1.10–1.34)*** | |
| Woman’s age | |||
| <20 | 1.00 | 1.00 | |
| 20–34 | 1.01 (0.94–1.09) | 1.01 (0.94–1.09) | |
| 34+ | 1.09 (0.51–2.33) | 1.07 (0.50–2.29) | |
| Marital status | |||
| Single | 1.00 | 1.00 | |
| Married | 0.68 (0.63–0.74) | 0.68 (0.63–0.74)*** | |
| Husband/partner education | |||
| No education | 1.00 | 1.00 | |
| Primary | 0.92 (0.82–1.02) | 0.92 (0.82–1.02) | |
| Secondary+ | 0.97 (0.85–1.12) | 0.97 (0.85–1.20) | |
| Birth order of child | |||
| 1 | 1.00 | 1.00 | |
| 2–3 | 0.85 (0.77–0.93) | 0.85 (0.77–0.93)*** | |
| 4–5 | 0.86 (0.77–0.96) | 0.86 (0.77–0.96)** | |
| 6+ | 0.97 (0.86–1.08) | 0.96 (0.86–1.08) | |
| Trimester at ANC visit | |||
| 1st trimester | 1.00 | 1.00 | |
| 2nd trimester | 0.94 (0.87–1.01) | 0.94 (0.87–1.02) | |
| 3rd trimester | 1.41 (1.12–1.79) | 1.42 (1.12–1.79)** | |
| ANC visit | |||
| No | 1.00 | 1.00 | |
| Yes | 0.99 (0.92–1.07) | 0.99 (0.92–1.07) | |
| Place of delivery | |||
| Home | 1.00 | ||
| Health Institution | 0.58 (0.52–0.63) | 0.58 (0.52–0.64)*** | |
| Assistant delivery | |||
| No | 1.00 | 1.00 | |
| Yes | 0.90 (0.82–0.98) | 0.90 (0.83–0.98)** | |
| Size of a child at birth | |||
| Large | 1.00 | 1.00 | |
| Average | 0.84 (0.78–0.91) | 0.84 (0.78–0.91)*** | |
| Small | 1.14 (1.03–1.26) | 1.14 (1.03–1.26)** | |
| Skin to skin contact | |||
| Yes | 1.00 | 1.00 | |
| No | 1.10 (1.01–1.20) | 1.10 (1.01–1.18)** | |
| Media exposure | |||
| Yes | 1.00 | 1.00 | |
| No | 1.12 (1.03–1.22) | 1.11 (1.02–1.21)** | |
| Mode of delivery | |||
| Vaginal | 1.00 | 1.00 | |
| Cesarian section | 2.61 (2.31–2.96) | 2.61 (2.30–2.96)*** | |
| Sex of child | |||
| Male | 1.00 | 1.00 | |
| Female | 0.92 (0.86 0.98) | 0.92 (0.86–0.98)** | |
| Birth Interval | |||
| < 24 | 1.00 | 1.00 | |
| ≥ 24 | 0.86 (0.78–0.94) | 0.86 (0.78–0.94)*** | |
| Residence | |||
| Urban | 1.00 | 1.00 | |
| Rural | 0.92 (0.84–1.02) | 0.93 (0.84–1.02) | |
| Community poverty | |||
| Low | 1.00 | 1.00 | |
| High | 0.97 (0.91–1.04) | 0.96 (0.89–1.04) | |
| Community ANC visit | |||
| Low | 1.00 | 1.00 | |
| High | 0.85 (0.80–0.90) | 0.90 (0.84–0.97)** | |
| Community education | |||
| Low | 1.00 | 1.00 | |
| High | 1.00 (0.94–1.06) | 1.04 (0.96–1.12) | |
| Community media exposure | |||
| Low | 1.00 | 1.00 | |
| High | 1.14 (1.07–1.22) | 1.11 (1.03–1.20)** | |
Notes: ***Significant at 0.01. **Significant at 0.05.
Abbreviation: AOR, adjusted odds ratio.
The Outcome of a Random Effect Study
Table 4 presents the fixed effects (a test of association) and the random intercept for prelacteal feeding. The empty model’s outcome showed that there was considerable variability in the odds of having population variation with prelacteal feeding (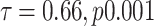 ). The MOR was also 2.17 (95% CI 1.14–4.15), indicating that when respondents switched from low to high-risk categories, the likelihood of having prelacteal feeding was 2.17 times higher. This demonstrated the presence of substantial variability across various populations in the provision of prelacteal feeding. Group variance (community variance = 0.48; p-value < 0.001) remained important but decreased in the full model (model adjusted for both individual and community-level factors). Approximately 48% of the overall variance of prelacteal feeding practice can be attributed to contextual level factors that have remained important even after certain contextual risk factors have been considered. In this model, the proportional change in variance (PCV) was 27.3%, suggesting that the population and person level variables explained 27.3% of the community variance found in the null model (Table 4).
). The MOR was also 2.17 (95% CI 1.14–4.15), indicating that when respondents switched from low to high-risk categories, the likelihood of having prelacteal feeding was 2.17 times higher. This demonstrated the presence of substantial variability across various populations in the provision of prelacteal feeding. Group variance (community variance = 0.48; p-value < 0.001) remained important but decreased in the full model (model adjusted for both individual and community-level factors). Approximately 48% of the overall variance of prelacteal feeding practice can be attributed to contextual level factors that have remained important even after certain contextual risk factors have been considered. In this model, the proportional change in variance (PCV) was 27.3%, suggesting that the population and person level variables explained 27.3% of the community variance found in the null model (Table 4).
Table 4.
Variability at Community Level and Model Fitness for Prelacteal Feeding Assessment Among Women of Reproductive Age in Eastern Africa
| Parameter | Null Model | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Community-level variance | 0.66 | 0.47 | 0.42 | 0.48 |
| ICC | 0.17 | 0.13 | 0.17 | 0.13 |
| MOR (95% CI) | 2.17 (1.14–4.15) | 1.92 (1.20–3.08) | 2.25 (1.15–4.37) | 2.66 (1.66 −4.26) |
| PCV (%) | Reference | 28.80% | 36.4% | 27.3% |
| Model comparison | ||||
| Log-likelihood ratio | −16, 912.14 | −11, 763.54 | −16, 885.61 | −11, 755.44 |
| Deviance (−2LL) | 33, 824.28 | 23, 527.08 | 33, 771.22 | 23, 510.88 |
Discussion
The overall objective of this study was to investigate the pooled prevalence and determinants of prelacteal feeding practice among mothers who have children under 5 years in eastern Africa from 2010 to 2018 using Demographic and Health data set. In Eastern Africa, the pooled prevalence of prelacteal feeding was 12% (95% CI = 11.42–12.51%) with the highest prelacteal feeding practice in Comoros (39%) and the lowest prelacteal feeding practice in Malawi (3%). This was lower than a study conducted in Kenya at 26%,47 India at 47%,48 Nepal at 30%,49 Nigeria at 50%,32 and South Sudan at 53%.33 This disparity in prelacteal feeding may be due to a variation in the nature of the study, so our study was focused on pooled data in the DHS data set covering a wide number of populations, whereas the other studies were carried out in a small region of the study population in a single population.
In this study, we obtained that institutional delivery and delayed ANC attendance were significantly associated with prelacteal feeding practice in Eastern Africa. In Eastern Africa, those mothers who deliver in health facilities decreased the practice of prelacteal feeding by 58%, in line with studies conducted in Ethiopia50,51 systematic review and meta-analysis.52 Since the mother who delivers in a health facility has a strong opportunity to obtain postnatal therapy from healthcare practitioners on the impact of prelacteal feeding, good position, and an attachment to breastfeeding. There is positive evidence that postnatal therapy on prelacteal feeding has made a substantial contribution to avoiding prelacteal feeding.31,41 However, mothers who deliver at home are less likely to realize the importance of exclusive breastfeeding and the harms of pre-lacteal feeding as they are supported by a conventional birth attendant who promotes the practice of prelacteal feeding. Even, in line with a study conducted in Eastern Ethiopia,53 Nigeria,54 and Pakistan.6 In this study, delayed initiation of ANC visits was more likely to practice prelacteal feeding in Eastern Africa in line with other studies.30,50,53,55 Hence, the mothers who attend ANC visit at the third trimester are more likely to provide prelacteal feeding to their newborn, since those mothers who have delayed ANC visit may come to health facility for the purpose of taking treatments and most of pregnant mothers attend ANC visit after the onset of complications, due to this, mothers less likely to receive different nutritional and other health-related educations from health professionals.
The results of this study indicated that mothers delivered by caesarean section were more likely to provide prelacteal feeding in agreement with a study conducted in Ethiopia,56 a systematic review and meta-analysis in lower and middle-income countries,25 Mexico,27 and Australia.24 Hence, pregnant women delivered by the caesarean section are subject to delayed initiation of breastfeeding since the mother may take a long time to recover from anaesthesia and additional discomfort may postpone the start of lactation, opening up an opportunity for their newborn child to provide prelacteal feeding.
This study evidenced that the likelihood of practicing prelacteal feeding was higher among those individuals who had high economic status in Eastern Africa countries consistent with a study conducted in Vietnam and Nepal.17,55 Hence, these women from high socio-economic status can have the ability to pay and feed their children even if butter and plain water are traditionally practiced in most low-income countries. Even though butter and plain water are typically practiced in most low-income countries, those women of high socioeconomic status may have the ability to pay and feed extra foods to their children.
The findings of this study revealed that the initiation of prelacteal feeding in Eastern African countries was encouraged by those infants who had no skin touch. Since infants without skin contact may delay the onset of lactation among mothers and the introduction of breast milk to their infants may be limited, similar results are recorded in the Democratic Republic of Congo.12
In addition, birth spacing is substantially correlated with Eastern African prelacteal feeding, in line with a Northern Ethiopia study.42 Mothers with a pre-birth period of more than 24 months decrease prelacteal feeding by 86%. Therefore, wide birth spaces can allow their child to gain knowledge about the relevance of breast milk as well as mothers have the ability to deliver healthy infants because they have an appropriate birth period that is ideal for the fetus to develop in the uterus. The result of this study also revealed that small birth size promotes the mother to provide prelacteal feeding in accordance with a study conducted in Egypt.57 This may be attributed to the misperception of the tiny birth babies of the mother benefiting from extra feeding because they perceive that only breast milk did not fulfill their infants’ nutritional needs of their newborns. In this study, we found that group ANC visits significantly reduce the practice of prelacteal feeding by 90%, in line with other studies.13,17,32,56 This may be due to the fact that health practitioners will provide different nutritional and other health-related education during the follow-up period among those mothers who have high-community ANC visits that may have a major impact on reducing the practice of prelacteal feeding. The rise in awareness and attitude towards the nutritional qualities of their infant’s breast milk also has an effect on the provision of prelacteal feeding.
Strengths and Limitation of the Study
In terms of strengths, the data used in this analysis were derived from nationally representative data and the covariates were the same and comparable across all countries in the 11 East Africa DHS dataset. The research was population-based with a response rate of >90% and the data were pooled to establish a broad sample size that enhances the generalizability of prelacteal feeding recorded from 2010 to 2018 within 5 years prior to each country survey.
In order to educate program planners and nutrition policymakers on prioritization and relevant strategies, the research could also define the significant determinants of prelacteal feeding across 11 East African countries. Due to the cross-sectional nature of the research design, the result of this study does not create a true causal association between the outcome variable in the event of limitation. Within 5 years prior to the study, the data were obtained on the basis of self-report from mothers and this may be a possible recall bias.
Conclusions
The overall prevalence of prelacteal feeding in Eastern Africa is high, and adequate breastfeeding remains a challenge. Both individual and community-level variables were associated with the high prevalence of breastfeeding. Late initiation of ANC visits, rich wealth quantile, small birth size, caesarean delivery, and lack of immediate skin-to-skin contact were significantly associated with the provision of prelacteal feeding. Low community ANC visits and low community media exposure play a major role in supporting prelacteal feeding practice in Eastern Africa. However, assistant delivery, institutional delivery, and low birth order are significantly associated with the avoidance of prelacteal feeding in Eastern Africa. More enlightenment is required to minimize the practice of pre-lacteal feeding in Eastern Africa. We strongly recommended that health care workers give special emphasis to promote mothers to attend early ANC visit to reduce prelacteal feeding practice and improve exclusive breastfeeding. In addition, encourage institutional delivery service utilization to minimize prelacteal feeding practice in Eastern Africa. Moreover, in order to experience skin-to-skin contact and early initiation of breastfeeding, structural improvements are required for women with caesarean births to achieve optimal breastfeeding practice in Eastern Africa.
Acknowledgments
We highly recognize the DHS measure program for granting access to DHS data sets from East Africa.
Funding Statement
There is no funding received for this study.
Abbreviation
ANC, antenatal care; AOR, adjusted odds ratio; CI, confidence interval; DHS, demographic and health survey; ICC, intra-class correlation coefficient; LLR, log-likelihood ratio; LR, likelihood ratio; MOR, median odds ratio; SSA, Sub-Saharan Africa; WHO, World Health Organization.
Data Sharing Statement
Online information is available and can be accessed from www.measuredhs.com.
Ethical Consideration
Data access permission was obtained from the demographic and health survey measure by an online request from https://dhsprogram.com/Data/terms-of use.cfm after all respondents were given a description of the study purpose and its procedures. Regarding ethical concerns, this research procedure was carried out in compliance with the Helsinki Declaration of the World Medical Association (WMA).
Consent for Publication
Not applicable because secondary data analysis was used for this study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Victora CG, Bahl R, Barros AJD, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. doi: 10.1016/S0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- 2.Pérez‐Escamilla R, Martinez JL, Segura‐Pérez S. Impact of the Baby‐friendly Hospital Initiative on breastfeeding and child health outcomes: a systematic review. Matern Child Nutr. 2016;12(3):402–417. doi: 10.1111/mcn.12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardlaw T, You D, Hug L, et al. UNICEF Report: enormous progress in child survival but greater focus on newborns urgently needed. Reprod Health. 2014;11(1):1–4. doi: 10.1186/1742-4755-11-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pem D. Factors affecting early childhood growth and development: golden 1000 days. Adv Practice Nurs. 2015;1(101):0347–2573. [Google Scholar]
- 5.Horta BL, Victora CG. Long-Term Effects of Breastfeeding. Geneva: World Health Organization; 2013:74. [Google Scholar]
- 6.Asim M, Ahmed ZH, Hayward MD, et al. Prelacteal feeding practices in Pakistan: a mixed-methods study. Int Breastfeed J. 2020;15(1):1–11. doi: 10.1186/s13006-020-00295-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marriott BP, White A, Hadden L, et al. World Health Organization (WHO) infant and young child feeding indicators: associations with growth measures in 14 low‐income countries. Matern Child Nutr. 2012;8(3):354–370. doi: 10.1111/j.1740-8709.2011.00380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unicef. From the First Hour of Life: Making the Case for Improved Infant and Young Child Feeding Everywhere. New York: UNICEF; 2016. [Google Scholar]
- 9.Lamberti LM, Walker CL, Noiman A, et al. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health. 2011;11(3):1–12. doi: 10.1186/1471-2458-11-S3-S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen P, Binns CW, Ha AVV, et al. Prelacteal and early formula feeding increase risk of infant hospitalisation: a prospective cohort study. Arch Dis Child. 2020;105(2):122–126. doi: 10.1136/archdischild-2019-316937 [DOI] [PubMed] [Google Scholar]
- 11.Demographic E. Addis Ababa, Ethiopia, and Rockville. Maryland, MA, USA: CSA and ICF; 2016. [Google Scholar]
- 12.Sanghvi T. Delayed initiation of breastfeeding in Bukavu, South Kivu, eastern Democratic Republic of the Congo: a cross-sectional study. PLoS One. 2018;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takele WW, Tariku A, Wagnew F, et al. Magnitude of prelacteal feeding practice and its association with place of birth in Ethiopia: a systematic review and meta-analysis, 2017. Arch Public Health. 2018;76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kambale RM, Buliga JB, Isia NF, et al. Delayed initiation of breastfeeding in Bukavu, south Kivu, eastern Democratic Republic of the Congo: a cross-sectional study. Int Breastfeed J. 2018;13(1):6. doi: 10.1186/s13006-018-0150-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amele EA, Demissie BW, Desta KW, et al. Prelacteal feeding practice and its associated factors among mothers of children age less than 24 months old in Southern Ethiopia. Ital J Pediatr. 2019;45(1):1–8. doi: 10.1186/s13052-019-0604-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijayalakshmi S, Patil R, Datta SS, et al. Feeding practices and morbidity pattern of infants in a rural area of puducherry-a follow up study. J Community Med Health Educ. 2014;4(304):2161–0711.1000304. doi: 10.4172/2161-0711.1000304 [DOI] [Google Scholar]
- 17.Nguyen PH, Keithly SC, Nguyen NT, et al. Prelacteal feeding practices in Vietnam: challenges and associated factors. BMC Public Health. 2013;13(1):932. doi: 10.1186/1471-2458-13-932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nkoka O, Ntenda PAM, Kanje V, et al. Determinants of timely initiation of breast milk and exclusive breastfeeding in Malawi: a population-based cross-sectional study. Int Breastfeed J. 2019;14(1):37. doi: 10.1186/s13006-019-0232-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agho KE, Ogeleka P, Ogbo F, et al. Trends and predictors of prelacteal feeding practices in Nigeria (2003–2013). Nutrients. 2016;8(8):462. doi: 10.3390/nu8080462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0 [DOI] [PubMed] [Google Scholar]
- 21.Watkins K. The state of the world’s children 2016: a fair chance for every child. ERIC; 2016. [Google Scholar]
- 22.Habtewold TD, Sharew NT, Alemu SM. Evidence on the effect of gender of newborn, antenatal care and postnatal care on breastfeeding practices in Ethiopia: a meta-analysis and meta-regression analysis of observational studies. BMJ Open. 2019;9(5):e023956. doi: 10.1136/bmjopen-2018-023956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dashti M, Scott JA, Edwards CA, et al. Determinants of breastfeeding initiation among mothers in Kuwait. Int Breastfeed J. 2010;5(1):7. doi: 10.1186/1746-4358-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora A, Manohar N, Hayen A, et al. Determinants of breastfeeding initiation among mothers in Sydney, Australia: findings from a birth cohort study. Int Breastfeed J. 2017;12(1):39. doi: 10.1186/s13006-017-0130-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavle JA, LaCroix E, Dau H, et al. Addressing barriers to exclusive breast-feeding in low-and middle-income countries: a systematic review and programmatic implications. Public Health Nutr. 2017;20(17):3120–3134. doi: 10.1017/S1368980017002531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tekaly G, Kassa M, Belete T, et al. Pre-lacteal feeding practice and associated factors among mothers having children less than two years of age in Aksum town, Tigray, Ethiopia, 2017: a cross-sectional study. BMC Pediatr. 2018;18(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernández-Cordero S, Lozada-Tequeanes AL, Fernández-Gaxiola AC, et al. Barriers and facilitators to breastfeeding during the immediate and one month postpartum periods, among Mexican women: a mixed methods approach. Int Breastfeed J. 2020;15(1):1–12. doi: 10.1186/s13006-020-00327-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zakar R, Zakar MZ, Zaheer L, et al. Exploring parental perceptions and knowledge regarding breastfeeding practices in Rajanpur, Punjab Province, Pakistan. Int Breastfeed J. 2018;13(1):24. doi: 10.1186/s13006-018-0171-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Argaw MD, Asfaw MM, Ayalew MB, et al. Factors associated with prelacteal feeding practices in Debre Berhan district, North Shoa, Central Ethiopia: a cross-sectional, community-based study. BMC Nutr. 2019;5(1):14. doi: 10.1186/s40795-019-0277-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chea N, Asefa A. Prelacteal feeding and associated factors among newborns in rural Sidama, South Ethiopia: a community based cross-sectional survey. Int Breastfeed J. 2018;13(1):7. doi: 10.1186/s13006-018-0149-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibadin O, Ofili NA, Monday P, et al. Prelacteal feeding practices among lactating mothers in Benin City, Nigeria. Niger J Paediatr. 2013;40(2):139–144. [Google Scholar]
- 32.Berde AS, Yalcin SS, Ozcebe H, et al. Determinants of pre-lacteal feeding practices in urban and rural Nigeria; a population-based cross-sectional study using the 2013 Nigeria demographic and health survey data. Afr Health Sci. 2017;17(3):690–699. doi: 10.4314/ahs.v17i3.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tongun JB, Sebit MB, Ndeezi G, et al. Prevalence and determinants of pre-lacteal feeding in South Sudan: a community-based survey. Glob Health Action. 2018;11(1):1523304. doi: 10.1080/16549716.2018.1523304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanjohi M, Griffiths P, Wekesah F, et al. Sociocultural factors influencing breastfeeding practices in two slums in Nairobi, Kenya. Int Breastfeed J. 2016;12(1):5. doi: 10.1186/s13006-016-0092-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyanga NM, Musita C, Otieno A, et al. Factors influencing knowledge and practice of exclusive breastfeeding in Nyando district, Kenya. Af j Food Agric Nutr Develop. 2012;12(6). [Google Scholar]
- 36.Kamudoni P, Maleta K, Shi Z, et al. Infant feeding practices in the first 6 months and associated factors in a rural and semiurban community in Mangochi District, Malawi. J Human Lactation. 2007;23(4):325–332. doi: 10.1177/0890334407307567 [DOI] [PubMed] [Google Scholar]
- 37.Chisenga M, Kasonka L, Makasa M, et al. Factors affecting the duration of exclusive breastfeeding among HIV-infected and -uninfected women in Lusaka, Zambia. J Human Lactation. 2005;21(3):266–275. doi: 10.1177/0890334405279251 [DOI] [PubMed] [Google Scholar]
- 38.Mundagowa PT, Chadambuka EM, Chimberengwa PT, et al. Determinants of exclusive breastfeeding among mothers of infants aged 6 to 12 months in Gwanda District, Zimbabwe. Int Breastfeed J. 2019;14(1):30. doi: 10.1186/s13006-019-0225-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engebretsen IMS, Wamani H, Karamagi C, et al. Low adherence to exclusive breastfeeding in Eastern Uganda: a community-based cross-sectional study comparing dietary recall since birth with 24-hour recall. BMC Pediatr. 2007;7(1):10. doi: 10.1186/1471-2431-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogah A, Ajayi AM, Akib S, et al. A cross-sectional study of pre-lacteal feeding practice among women attending Kampala International University teaching hospital maternal and child health clinic, Bushenyi, Western Uganda. Asian J Med Sci. 2012;4(3):79–85. [Google Scholar]
- 41.Maonga AR, Mahande MJ, Damian DJ, et al. Factors affecting exclusive breastfeeding among women in Muheza District Tanga northeastern Tanzania: a mixed method community based study. Matern Child Health J. 2016;20(1):77–87. doi: 10.1007/s10995-015-1805-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tekaly G, Kassa M, Belete T, et al. Pre-lacteal feeding practice and associated factors among mothers having children less than two years of age in Aksum town, Tigray, Ethiopia, 2017: a cross-sectional study. BMC Pediatr. 2018;18:18. doi: 10.1186/s12887-018-1284-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein H. Multilevel Statistical Models. Vol. 922. John Wiley & Sons; 2011. [Google Scholar]
- 44.Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Public Health. 2000;21(1):171–192. doi: 10.1146/annurev.publhealth.21.1.171 [DOI] [PubMed] [Google Scholar]
- 45.Goldstein H, Browne W, Rasbash J. Partitioning variation in multilevel models. Understanding Stat Stat Issues Psychol Edu Soc Sci. 2002;1(4):223–231. doi: 10.1207/S15328031US0104_02 [DOI] [Google Scholar]
- 46.Demographic T. Program HS. Guide to DHS Statistics. [Google Scholar]
- 47.Lakati A, Makokha OA, Binns CW, et al. The effect of pre-lacteal feeding on full breastfeeding in Nairobi, Kenya. East Afr J Public Health. 2011;7(3):258–262. doi: 10.4314/eajph.v7i3.64737 [DOI] [PubMed] [Google Scholar]
- 48.Gupta A, Chhabra P. Infant and young child feeding practices and its determinants in an urbanized village of Delhi. Int J Med Public Health. 2015;5(3). [Google Scholar]
- 49.Khanal V, Lee AH, Karkee R, et al. Prevalence and factors associated with prelacteal feeding in Western Nepal. Women Birth. 2016;29(1):12–17. doi: 10.1016/j.wombi.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 50.Bayih WA, Mekonen DK, Kebede SD. Prevalence and associated factors of prelacteal feeding among neonates admitted to neonatal intensive care units, North central Ethiopia, 2019. BMC Public Health. 2020;20(1):1–11. doi: 10.1186/s12889-020-09578-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorrie MB, Amaje E, Gebremeskel F. Pre-lacteal feeding practices and associated factors among mothers of children aged less than 12 months in Jinka Town, South Ethiopia, 2018/19. PLoS One. 2020;15(10):e0240583. doi: 10.1371/journal.pone.0240583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Temesgen H, Negesse A, Woyraw W, et al. Prelacteal feeding and associated factors in Ethiopia: systematic review and meta-analysis. Int Breastfeed J. 2018;13:13. doi: 10.1186/s13006-018-0193-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bekele Y, Mengistie B, Mesfine F. Prelacteal feeding practice and associated factors among mothers attending immunization clinic in Harari region public health facilities, Eastern Ethiopia. Open J Prev Med. 2014;2014. [Google Scholar]
- 54.Benova L, Siddiqi M, Abejirinde I-OO, et al. Time trends and determinants of breastfeeding practices among adolescents and young women in Nigeria, 2003–2018. BMJ Global Health. 2020;5(8):e002516. doi: 10.1136/bmjgh-2020-002516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khanal V, Adhikari M, Sauer K, et al. Factors associated with the introduction of prelacteal feeds in Nepal: findings from the Nepal demographic and health survey 2011. Int Breastfeed J. 2013;8(1):9. doi: 10.1186/1746-4358-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belachew AB, Kahsay AB, Abebe YG. Individual and community-level factors associated with introduction of prelacteal feeding in Ethiopia. Arch Public Health. 2016;74(1):6. doi: 10.1186/s13690-016-0117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El-Gilany A-H, Abdel-Hady DM. Newborn first feed and prelacteal feeds in Mansoura, Egypt. BioMed Res Int. 2014;2014:1–7. doi: 10.1155/2014/258470 [DOI] [PMC free article] [PubMed] [Google Scholar]




