Abstract
Animal models refers to the animal experimental objects and related materials that can simulate human body established in medical research. As the second-largest disease in terms of morbidity and mortality after cardiovascular disease, cancer has always been the focus of human attention all over the world, which makes it a research hotspot in the medical field. At the same time, more and more animal models have been constructed and used in cancer research. With the deepening of research, the construction methods of cancer animal models are becoming more and more diverse, including chemical induction, xenotransplantation, gene programming, and so on. In recent years, patient-derived xenotransplantation (PDX) model has become a research hotspot because it can retain the microenvironment of the primary tumor and the basic characteristics of cells. Animal models can be used not only to study the biochemical and physiological processes of the occurrence and development of cancer in objects but also for the screening of cancer drugs and the exploration of gene therapy. In this paper, several main tumor animal models and the application progress of animal models in tumor research are systematically reviewed. Finally, combined with the latest progress and development trend in this field, the future research of tumor animal model was prospected.
Keywords: animal model, cancer, patient-derived xenotransplantation model, PDX model, tumor microenvironment
Introduction
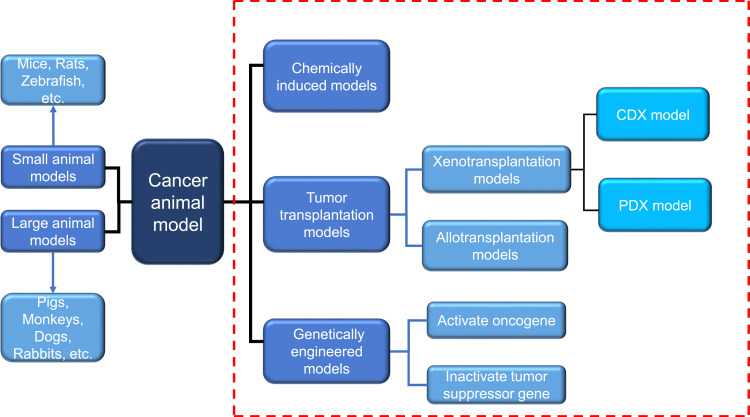
With the effective control of severe infectious diseases and the extension of human life expectancy, cancer has become one of the major diseases that seriously endanger human health. According to 2015 estimates by the World Health Organization (WHO), cancer is the first or second leading cause of death among people under the age of 70 in 91 of these countries.1 Under the combined influence of population aging and population growth, the number of new cancer cases each year is expected to rise from 18.1 million in 2018 to 29.4 million in 2040.2 Due to the late diagnosis of most cancers and inadequate prevention measures, cancer is becoming a heavy burden on residents in low-and middle-income countries. The development and research of new diagnostic methods and innovative treatment tools are essential to reduce the global incidence of cancer.The animal experiment is an important bridge between cell experiment and clinical experiment. Under certain conditions, the occurrence and development of animal diseases are similar to that of human beings, and animals have similar anatomy, physiology and heredity to human beings. Therefore, animal models are often used to study human diseases. In cancer research, the use of animal models can help us understand the genetic basis of cancer and the role of specific genes and gene mutations in the occurrence and development of cancer, which also facilitates the development and testing of antineoplastic drugs.3 With the continuous development of precision medicine and personalized medicine, researchers are looking for standardized and personalized tumor models that are more similar to human tumors.4 There are many animal types and construction methods used to construct cancer animal models, and the progress of each animal model in tumor research has its own characteristics, which will be described below (Figure 1).
Figure 1.
Two commonly used classification methods of cancer animal models. Dashed red box represents the classification according to different modeling methods. Another classification is carried out according to different species. Blue arrows indicate the species of animals included in this classification.
Abbreviations: PDX, patient-derived tumor xenograft; CDX, cell line-derived xenograft.
Mouse Model
The mouse genome is highly homologous to the human genome, which can simulate a series of biological characteristics such as the occurrence, development and metastasis of human cancer cells in vivo,5 and has the advantages of convenient feeding, low price and easy gene modification. It provides a good tool for cancer research and a valuable platform for drug discovery and verification. At present, there are four commonly used methods to construct mouse cancer model: chemically induced model, cell line-derived xenograft (CDX) model, patient-derived xenograft (PDX) model and genetically engineered mouse model (GEMM).6 The chemical induction model refers to the model of experimental tumor induced by chemical carcinogens, which has the advantage of imitating the occurrence of human cancer from the beginning of the carcinogenic process.7 But the main disadvantage of this method is that it takes 30–50 weeks to form a tumor after using carcinogens.8 The cell line-derived xenograft (CDX) model refers to the xenotransplantation model produced by subcutaneous injection of cancer cell lines into immunodeficient mice.9 The establishment of this model is simple and takes a short time to form a tumor, but after long-term culture in vitro, the biological behavior and tumor heterogeneity of human tumor cell lines are quite different from those of the original tumor tissue.10 The patient-derived xenograft (PDX) model is an animal model established by directly implanting tumor tissue samples from tumor patients into mice, which well maintains the characteristics of tumor histopathology and genetics.11 The genetically engineered mouse model (GEMM) is to induce tumorigenesis by promoting the expression of oncogenes (such as BRAF V600E in melanoma)12 or the deletion of tumor suppressor genes (such as PTEN in prostate cancer)13 by genetic engineering. Compared with the above two transplantation models, GEMM formed an orthotopic tumor in an innate immune maturation microenvironment (natural immune-proficient microenvironment), simulating the process of tumorigenesis.14 However, due to the species differences of the immune system among mammals, these existing models cannot accurately predict the interaction between the human immune system and tumor. Many antineoplastic drugs with a good therapeutic effect in preclinical animal models cannot play a corresponding role in tumor patients. Therefore, it is necessary to establish an animal model that cannot only replicate the tumor microenvironment, but also have a “humanized” immune system at the same time. The humanized mouse model of the human immune system is a mouse model that reconstructs the human immune system by implanting human hematopoietic cells, lymphocytes or tissues into immunodeficient mice.15 On this basis, the implantation of human tumor cells or tumor tissue can be used to study tumor growth in the environment of the human immune system and evaluate anti-tumor therapy, especially the effect of immunotherapy and related mechanism. At present, a variety of human tumor cell lines have been successfully established in humanized mice, such as lymphoma, glioma, breast cancer, colorectal cancer, kidney cancer and prostate cancer cell line.16–19 According to the method of human immune system reconstruction, the humanized mouse models of the immune system are divided into three categories: Hu-BLT (human bone marrow, liver and thymus) model, Hu-HSCs (human hematopoietic stem cell) model and Hu-PBL (human peripheral blood lymphocyte) model (Figure 2).
Figure 2.
Construction method of humanized mice of human immune system. The construction of humanized mice needs to use immunodeficient mice as a tool. By transplanting different human immune organs or cells into immunodeficient mice, three different humanized mice can be constructed. Among them, the Hu-HSC model also needs to destroy the hematopoietic function of bone marrow in mice.
Abbreviations: Hu-HSC, human hematopoietic stem cell; Hu-PBL, human peripheral blood lymphocyte; Hu-BLT, human bone marrow, liver and thymus.
Hu-BLT Model
The model is established by co-transplanting human embryonic liver and thymus into the renal capsule of immunodeficient mice and injecting liver hematopoietic stem cells from the same embryo into mice.20 It can reconstruct the human immune system most perfectly, and detect many kinds of human immune cells, such as T cells, B cells, macrophages and so on, in mice, thus producing human adaptive immune response.21,22 Therefore, the hu-BLT model is considered to be an important model for the study of cancer in the human immune system. For example, Vatakis et al23 used hu-BLT mice to build melanoma models and treated them with T-cell-based immunotherapy. Kaur et al implanted pancreatic tumor cells into hu-BLT mice and found that NK cells could block tumor growth through differentiation and cleavage.24 However, due to the complex and elaborate surgical procedures required in the establishment process and the limited sources of fetal liver and thymus, the application of the hu-BLT model is also limited.25
Hu-HSC Model
The construction of this model requires the destruction of bone marrow hematopoiesis in newborn or adult immunodeficient mice, and then the injection of human hematopoietic stem cell (HSC) into the body. Multi-line hu-HSC developed into immune cells, including B cells, T cells, NK cells and myeloid cells.26 These immune cells interact with transplanted tumor cells and can simulate the tumor microenvironment. Meraz et al27 constructed a hu-HSC model using fresh umbilical cord blood CD34+ HSCs to evaluate the immune response of lung cancer. Hu-HSC model can establish human innate immune system and lymphocytes, but it also has some limitations. For example, a small number of low-active human T cells could not be detected in the peripheral blood until 12 weeks after HSC was implanted into mice.28–30
Hu-PBL Model
Hu-PBL model was established by injecting human peripheral blood lymphocytes into immunodeficient mice. It is the simplest and most economical humanized mouse model at present. At present, the hu-PBL model has been used in many types of cancer research, such as lung cancer, thyroid cancer, cervical cancer, breast cancer, nasopharyngeal carcinoma and so on.31–35 Compared with the hu-HSC model, it can reconstruct high levels of T cells and is an ideal model for the study of mature effector T cells. However, due to the rejection of human T cells and mouse immune cells, this model is prone to graft-versus-host disease (GVHD), which shortens the life span of mice, which limits the research time.
Zebrafish Model
The zebrafish cancer model is a vertebrate model rising in recent years, and it is one of the most promising models at present. The genomes of zebrafish are homologous and conservative to humans, which provides a good basis for the study of the development of various cancers.36,37 Compared with the most commonly used mouse models, the zebrafish model has some unique advantages in cancer research:38 (1) small size, low cost and fast reproduction; (2) transparent embryos, it is convenient to observe and track the proliferation, spread and metastasis of cancer cells in real time; (3) transgenic zebrafish39 and immunodeficient zebrafish40 can remain transparent after adulthood. (3) because zebrafish is fertilized in vitro, the gene operation is relatively easy, and the transgenic animal model can be established quickly. At present, a variety of zebrafish cancer models have been established by means of transgenic, genome editing, xenotransplantation, drug-induced toxic damage and so on (Table 1).
Table 1.
Zebrafish Model
| Cancer Model | Modeling Mode | Finding | Reference |
|---|---|---|---|
| Acute lymphocytic leukemia | Transgenic model | They describe the first robust zebrafish pre-B ALL model. This model can reveal differences between MYC-driven pre-B vs T-ALL and be exploited to discover novel pre-B ALL therapies | [110] |
| Acute myeloid leukemia | Xenograft model | Bone marrow (BM) SOX4 expression could serve as an informative new biomarker for the clinical prognosis of AML patients. And the myeloid-specific expression of SOX4 can induce leukemic phenotype in zebrafish. | [111] |
| Breast cancer | Xenograft model | Administration of VEGFR inhibitors blocked tumor vascularization and a localized tumor growth but enhanced migration of neutrophils, which in turn promoted tumor invasion and formation of micrometastasis. | [112] |
| Breast cancer | Xenograft model | Grem1 is a pivotal factor in the reciprocal interplay between breast cancer cells and CAFs, which promotes cancer cell invasion. Targeting Grem1 could be beneficial in the treatment of breast cancer patients with high Grem1 expression. | [67] |
| Breast cancer | Xenograft model | When the tumor metastases, organ selectivity is driven by both vessel topography and cell-type-dependent extravasation. | [113] |
| Breast cancer | Xenograft model | SMYD3 is a pivotal SMAD3 cofactor that promotes TGFβ-dependent mesenchymal gene expression and cell migration in breast cancer. | [114] |
| Breast cancer | Xenograft model | PtPP-loaded and citrate-functionalized HA nanoparticles effectively decrease breast cancer cells survival both in vitro and in vivo in a similar manner to free PtPP. | [115] |
| Breast cancer and Liver cancer | Xenograft model | Furanodiene showed a markedly synergistic anti-cancer effect when used in combination with 5-FU (5-Fluorouracil) for both human breast cancer MDA-MB-231 cells and human liver cancer BEL-7402 cells xenotransplanted into zebrafish. | [68] |
| Colon Cancer | Xenograft model | After NR1D1 gene is knocked out, the cell viability is impaired and the formation of micrometastasis is reduced. | [116] |
| Colorectal cancer | Xenograft model | Endoglin-expressing fibroblasts enhanced colorectal tumor cell infiltration into the liver and decreased survival. And endoglin-expressing CAFs contribute to colorectal cancer progression and metastasis. | [117] |
| Colorectal cancer | Xenograft model | They found significantly less distant metastasis of ERB-041-treated cells compared to vehicle-treated cells. These results further support ERβ’s anti-tumor role in CRC and the possible use of its agonist in CRC patients. | [60] |
| Colorectal cancer | Transgenic model | They established a new transgenic zebrafish model with inducible expression of oncogenic krasV12 specifically in the intestine and observed high rates of intestinal tumors. | [61] |
| Colorectal cancer | Xenograft model | cGAMP inhibited migration through angiogenesis by up-regulating IL-2, TNF-α, and IFN-γ, whereas STAT3 down-regulation inhibited CXCL8, BCL-2, and VEGFA expression. | [118] |
| Colorectal carcinoma | Xenograft model | Zebrafish xenografts provide remarkable resolution to measure Cetuximab sensitivity. Zebrafish larvae xenografts constitute a promising fast assay for precision medicine, bridging the gap between genotype and phenotype in an in vivo setting. | [119] |
| Colorectal carcinoma | Xenograft model | L.sulphureus lectin (LSL) almost completely reduced growth, neovascularization and metastasis of human colorectal carcinoma and mouse melanoma. It could be used as safe adjuvant in chemotherapy against colorectal carcinoma and melanoma. | [120] |
| Colorectal carcinoma | Xenograft model | Crambescidine-816, −830, and −800 disrupt tumor cell adhesion and cytoskeletal integrity promoting the activation of the intrinsic apoptotic signaling, resulting in loss of mitochondrial membrane potential and concomitant caspase-3 cleavage and activation. | [121] |
| Gastric carcinoma | Xenograft model | The zebrafish xenograft study revealed that administration of Triphala inhibited the xenograft growth and metastasis of transplanted carcinoma cells in vivo. | [56] |
| Glioblastoma | Xenograft model | Treatment of GBM cells with compound 5 (CMP5) mirrored the effects of PRMT5 knockdown wherein it led to apoptosis of differentiated GBM cells and drove undifferentiated primary patient derived GBM cells into a nonreplicative senescent state. | [122] |
| Hepatocellular carcinoma | Transgenic model | Mifepristone-inducible and reversible krasV12 transgenic system offers a novel model for understanding hepatocarcinogenesis and a high-throughput screening platform for anti-cancer drugs. | [123] |
| Hepatocellular carcinoma | Transgenic model | A small Myc target gene set of 16 genes can be used to identify liver tumors due to Myc upregulation. And their zebrafish model demonstrated the conserved role of Myc in promoting hepatocarcinogenesis in all vertebrate species. | [124] |
| Hepatocellular carcinoma | Chemically-induced model | Triploid zebrafish demonstrated an overall increase in latency period in the development of both types of hepatic tumors (hepatocellular carcinomas and adenomas), a finding that can be interpreted as an increased resistance of triploid animals to the carcinogenic effect of N-nitrosodimethylamine. | [125] |
| Hepatocellular carcinoma | Transgenic model | Metformin can suppress NAFLD-associated HCC progression by decreasing the number of pro-inflammatory macrophages and increasing T cell infiltration. | [126] |
| Hepatocellular carcinoma | Transgenic model | For tumor-infiltrated neutrophils and macrophages, significantly higher densities in male liver tumors were observed in both xmrk and Myc models. And there was a higher rate of HSC activation accompanied with a higher level of serotonin in male liver tumors. | [127] |
| Hepatocellular carcinoma | Transgenic model | After krasV12 induction, fibrinogen was up-regulated in oncogenic hepatocytes. They reasoned that fibrinogen may bind to integrin αvβ5 on HSCs to activate HSCs. | [58] |
| Hepatocellular carcinoma | Transgenic model | Using the Tet-on system for liver-specific expression of fish oncogene xmrk, a hyperactive version of epidermal growth factor receptor homolog, they generated transgenic zebrafish with inducible development of liver cancer. | [128] |
| Hepatocellular carcinoma | Transgenic model | The distribution of neutrophils and macrophages in HCC was relatively uniform, whereas both types of immune cells were regionally clustered during tumor regression, especially with dominant blood vessel association of macrophage in late regression. | [129] |
| Hepatocellular carcinoma | Transgenic model | They used zebrafish model to screen for drugs that suppress β-catenin-induced liver growth, and identified two classes of hits, c-Jun N-terminal kinase (JNK) inhibitors and antidepressants, that suppressed this phenotype. | [130] |
| Hepatocellular carcinoma | Transgenic model | Their study provides an in vivo evidence of the relationship between chronic inflammation and tumorigenesis and reinforces the pivotal role of IL6 in the inflammation-associated hepatocarcinogenesis. | [59] |
| Hepatocellular carcinoma | Transgenic model | An inflammatory cue from oncogenic hepatocytes upon induction of krasV12 expression causes a rapid recruitment of neutrophils to oncogenic liver and the neutrophils play a promoting role in early hepatocarcinogenesis. | [131] |
| Leukemia | Transgenic model | Akt pathway activation is sufficient for tumor maintenance, even after loss of survival signals driven by the MYC oncogene. | [50] |
| Leukemia | Xenograft model | Xenotransplantation models of zebrafish can be used to screen non-teratogenic drugs for leukemia. | [132] |
| Leukemia | Transgenic model | After screening 26,400 molecules, they identified Lenaldekar (LDK), a compound that eliminates immature T cells in developing zebrafish without affecting the cell cycle in other cell types. | [54] |
| Leukemia | Xenograft model | Imaging-based LSC xenotransplant screening in zebrafish offers distinct advantages over other animal models and can greatly accelerate the phenotype-driven discovery of anti-LSC agents. | [133] |
| Liver cancer | Xenograft model | Most toxicants, namely chromium, bisphenol A, lindane, N-nitrosodiethylamine, and PCB126, resulted in increased inflammation and liver tumorigenesis, while arsenic and TCDD had opposite effects. | [134] |
| Liver cancer | Transgenic model | Halting RhoA signaling could augment Kras-mediated liver overgrowth and tumorigenesis. And activating Rho could be beneficial to suppress Kras-induced liver malignancies. | [135] |
| Lung cancer | Xenograft model | In the zebrafish xenograft model, knockdown of LINC00152 reduced the proliferation and migration of lung cancer cells and enhanced the inhibition effect of afatinib for lung cancer progression in cultured cells and the zebrafish xenograft model. | [69] |
| Lung cancer | Xenograft model | BPIQ-induced anti-lung cancer is involved in mitochondrial apoptosis. BPIQ could be a promising anti-lung cancer drug for further applications. | [136] |
| Lung cancer | Xenograft model | DFIQ exerts anticancer potential in vivo and in vitro and can induce apoptosis. DFIQ-induced apoptosis is associated with lysosome accumulation and the induction of the expression of apoptosis factors, such as Bax, Bad, and tBid. | [70] |
| Lung cancer | Xenograft model and Transgenic model | Bevacizumab, endostar and apatinib demonstrated remarkable angiogenesis and tumor inhibition effect in the zebrafish model, within the nonlethal dose range. Endostar and bevacizumab showed competitive anti-tumor efficacy. | [137] |
| Melanoma | Xenograft model | Nodal signaling has a key role in melanoma cell plasticity and tumorigenicity, thereby providing a previously unknown molecular target for regulating tumor progression. | [138] |
| Melanoma | Transgenic model | Although oncogenic NRAS expression alone was found to be insufficient to promote tumor formation, loss of functional p53 was found to collaborate with NRAS expression in the genesis of melanoma. | [44] |
| Melanoma | Transgenic model | BRAF activation is sufficient for f-nevus formation, that BRAF activation is among the primary events in melanoma development, and that the p53 and BRAF pathways interact genetically to produce melanoma. | [139] |
| Melanoma | Xenograft model | The zebrafish model reveals that Spint1a deficiency facilitates oncogenic transformation, regulates the tumor immune microenvironment crosstalk, accelerates the onset of SKCM and promotes metastatic invasion. | [46] |
| Melanoma | Transgenic model | In an adult model of chronic wounding in zebrafish, they show that repeated wounding with subsequent inflammation leads to a greater incidence of local melanoma formation. | [140] |
| Melanoma | Transgenic model | Transgenic THOR knockout produced fertilization defects in zebrafish and also conferred a resistance to melanoma onset. Likewise, ectopic expression of human THOR in zebrafish accelerated the onset of melanoma. | [141] |
| Melanoma | Xenograft model | Overexpression of bcl‐xL protein is able to enhance melanoma cell angiogenesis through increasing chemokine CXCL8 secretion. They demonstrate that this feature is associated with the increased ability of bcl‐xL overexpressing cells to enhance invasion in vivo. | [47] |
| Melanoma | Xenograft model | Employing in vivo imaging coupled with 3D reconstruction, they monitored the interactions between cancer cells and the external surface of zebrafish vessels. And they found that melanoma cells spread along the abluminal vascular surfaces. | [45] |
| Melanoma | Xenograft model | Combined MEK/autophagy inhibition reduced the invasive and metastatic potential of MEKi-resistant cells in an in vivo zebrafish xenograft. | [142] |
| Pancreatic cancer | Xenograft model | Xenografts of primary human tumors showed invasiveness and micrometastasis formation within 24 hours after transplantation, which was absent when non-tumor tissue was implanted. | [143] |
| Rhabdomyosarcoma | Transgenic model | Their novel zebrafish rhabdomyosarcoma model identifies a new PAX3-FOXO1 target, her3/HES3, that contributes to impaired myogenic differentiation and has prognostic significance in human disease. | [144] |
| T-cell acute lymphoblastic leukemia | Xenograft model | Using a focused chemical genomic approach, they demonstrate that xenografted cell lines harboring mutations in the NOTCH1 and PI3K/AKT pathways respond concordantly to their targeted therapies. | [145] |
| Thyroid carcinoma | Transgenic model | The expression of TWIST2 plays a role in an early step of BRAFV600E-mediated transformation. | [146] |
Zebrafish Melanoma Model
Melanoma is a highly malignant tumor derived from melanocytes, which is the most difficult to cure in skin cancer.41,42 With the deepening of the understanding of the molecular mechanism of melanoma invasion, proliferation and metastasis, remarkable achievements have been made in the treatment of melanin. Melanoma cells achieve the purpose of rapid growth and metastasis by interacting with the microenvironment.43 Therefore, the establishment of the zebrafish melanoma model is very important for the study of melanoma pathogenesis and anti-melanoma drugs.
Dovey et al44 constructed a zebrafish model expressing NRAS Q61K by transgenic technology, which proved that the loss of p53 and the expression of NRAS promote the occurrence of melanoma. After Fornabaio et al45 injected melanoma cells into zebrafish embryos, the interaction between cancer cells and the outer surface of zebrafish blood vessels was monitored, and the first zebrafish model of melanoma angiogenesis and extravascular migration was established. Gomez-Abenza et al46 in order to study the role of Spint1a in melanoma, zebrafish models were constructed by transgenic technology. The results show that the absence of Spint1a promotes the development of melanoma, which also provides a new direction for the treatment of melanoma. Gabellini et al47 demonstrated that the high expression of bcl-xL and pro-inflammatory chemokine interleukin-8 (CXCL8) in patients with melanoma led to a poor prognosis using a zebrafish model.
Zebrafish Leukemia Model
The similarity between zebrafish and human hematopoietic systems has led to the increasing use of zebrafish to simulate leukemia.48 The establishment of the zebrafish leukemia model plays an important role in understanding the occurrence, development and drug research of human leukemia. Langenau et al49 injected rag2, encoding a lymphocyte-specific promoter into zebrafish to drive the expression of the mouse-derived c-Myc gene. It was found that the fluorescence-labeled leukemia cells in zebrafish were implanted into the immunodeficient thymus, suggesting that the proto-oncogene c-Myc is involved in the formation of zebrafish tumors. Gutierrez et al50 found that 4-hydroxytamoxifen (4HT) can activate Myc, to induce acute T-lymphoblastic leukemia in zebrafish by constructing MYC-ER transgenic zebrafish. Corkery et al51 successfully established a leukemia zebrafish casper model by implanting K562 and NB-4 human leukemia cell lines into zebrafish casper embryos, and conducted targeted inhibitor intervention experiments on this model, which laid a good foundation for tumor research in this whole animal. Since the construction of the first leukemic zebrafish model in 2003, zebrafish has made a great contribution to the study of leukemia. Through these studies, we not only have a deeper understanding of the pathogenesis of leukemia, but also proved a variety of anti-leukemia drugs, including Nimesulide, Lenaldekar and Perphenazine.52–54
Zebrafish Digestive Tract Tumor Model
Due to the concealment of the disease, the difficulty of early diagnosis and the lack of effective treatment, the incidence and mortality of digestive tract tumors are at a high level. Understanding the molecular mechanism of digestive tract tumorigenesis and looking for new drug therapy targets is the focus of the current research. Zebrafish do not have stomach and genes that express specific gastric function, but the gastric cancer cells in the gastric cancer xenotransplantation model have high similarity with humans.55 Tsering et al56 induced zebrafish to establish a gastric cancer xenotransplantation model and found that Triphala could inhibit the growth and metastasis of transplanted gastric cancer cells. Zebrafish liver cancer gene is highly conservative with humans,57 which makes the zebrafish model widely used in liver cancer research. Yan et al58 found an increase in the level of fibrinogen in the zebrafish hepatocellular carcinoma model induced by krasV12. They also found that fibrinogen may bind to Integrin α v β 5 on HSC to activate hepatic stellate cells. Jung et al59 introduced the hIL6 gene into zebrafish to study the relationship between hIL6 expression and liver cancer. The results showed that the transgenic zebrafish liver developed into typical liver cancer, which indicated that the high level of hIL6 caused the occurrence of hepatocellular carcinoma. At present, zebrafish intestinal tumor models are mostly xenotransplantation models. With the development of genetic engineering technology, the application of transgenic models is increasing. Topi et al60 studied the effect of estrogen receptor activator ERB-041 on colon cancer cells in a zebrafish xenotransplantation model. Compared with the control group, it was found that distant metastasis of cancer cells decreased after ERB-041 treatment. Lu et al61 established a krasV12 transgenic zebrafish model induced by mifepristone, which provides a good in vivo model for the study of krasV12-induced colorectal cancer. Although zebrafish do not have a discrete pancreas, it has exocrine acinar cells and intestines similar to the functional and histological characteristics of mammalian pancreas.62 Guo et al63 established a model of pancreatic cancer xenotransplantation by injecting human pancreatic cancer cells into zebrafish and found that a small molecule U0126 can inhibit the proliferation and metastasis of human pancreatic cancer cells in zebrafish by inhibiting Ras/Raf/MEK/ERK pathway. This also shows the feasibility of the zebrafish model for screening and identifying new therapeutic drugs for pancreatic cancer.
Other Zebrafish Cancer Models
Breast cancer is the most common cancer among women,64 and the incidence is increasing and getting younger worldwide. The mouse is a traditional tool to study breast cancer. It has many disadvantages, such as long cycle, high cost, complex operation and so on.65,66 With the development of the zebrafish model, more and more breast cancer studies take zebrafish as the experimental object. Ren et al67 constructed xenograft zebrafish breast cancer (co-) injection models to study the role of BMP antagonists GREMLIN-1 (GREM1) in the infiltration and exudation of breast cancer cells. In this model, they found that GREM1 promotes fibrosis in breast cancer-associated fibroblast (CAF) and promotes intravasation and extravasation in breast cancer. Zhu et al68 established a breast cancer xenotransplantation model in zebrafish and used Furanodiene and 5-FU (5-Fluorouracil) to treat zebrafish. Compared with the results of furadiene alone, the results showed that the two drugs had an obvious synergistic anticancer effect. Lung cancer is a kind of cancer with the highest morbidity and mortality in the world. In order to improve the diagnosis and treatment of lung cancer, it is necessary to further understand the pathogenesis and related molecular mechanism of lung cancer. Shen et al69 established a xenotransplantation model after LINC00152 knockout lung cancer cells were implanted into zebrafish. The comparison between the control group and stereoscopic microscope showed that the silencing of LINC00152 could inhibit the proliferation and metastasis of lung cancer cells. The zebrafish model can also be used to evaluate the efficacy and safety of anti-lung cancer drugs to find a more suitable treatment. In order to evaluate the effect of DFIQ (a Novel Quinoline Derivative) on non-small-cell lung cancer (NSCLC) in vivo, Huang et al70 used the zebrafish lung cancer model to receive DFIQ treatment. By monitoring cell growth, migration and apoptosis, it was found that DFIQ could inhibit cancer cells to a certain extent. From the current research, with the in-depth study of the zebrafish tumor model, it will open a new way for the molecular research mechanism of various cancers.
Patient-Derived Tumor Xenograft (PDX) Model
The traditional xenotransplantation model is to establish a stable cell line by screening human tumor cells in vitro, subculturing them, and then injecting them into immunodeficient mice to establish model.71 This model is called the cell line-derived xenograft (CDX) model, which has the advantages of easy to obtain tumor cell lines and easy to repeat experiments. However, with the continuous passage of tumor cells in order to adapt to the external petri dish environment, the tumor microenvironment has changed, resulting in the formation of tumors in mice cannot accurately reflect the characteristics of the original tumor. PDX model is a tumor model established by transplanting fresh tumor tissue from patients into animals by surgery.72,73 At present, the animals used are mainly immunodeficient mice. With the exploration of researchers, zebrafish and other animals provide a new tool for the establishment of PDX models. Compared with the CDX model, the most important advantage of the PDX model is that it completely retains the tumor microenvironment, avoids the effect of repeated passage on tumor heterogeneity, and can better simulate the tumor growth process in patients.74 The researchers carried out various biological tests on the transplanted tumors in liver cancer, colorectal cancer, melanoma, esophageal cancer and borderline cancer. It was confirmed that the PDX model maintained the biological characteristics of primary tumors and provided an accurate animal model for the study of oncology.75–77 Because the tumor tissue comes from different patients and uses one model to correspond to the pattern of one patient, the PDX model can reflect the genetic diversity of patients.72 These advantages make the PDX model widely used in all kinds of cancer research (Table 2). With the development of tumor research, the shortcomings of the traditional PDX model are also exposed. The traditional PDX model generally uses immunodeficient mice, but the immune system of mice is different from that of humans, so it can reflect the interaction between immunity and tumor.78 At the same time, the life span of immunodeficient mice is short, At the same time, the life span of immunodeficient mice is short, and there is the possibility of spontaneous tumor.79 The success rate of PDX modeling was also different among different tumor types, and the success rate of colorectal cancer and lung cancer was significantly higher than that of prostate cancer.80 These shortcomings promote the emergence of some improved PDX models.
Table 2.
PDX Model
| Cancer Model | Animal Species | Finding | Reference |
|---|---|---|---|
| Adenoid cystic carcinoma | Zebrafish | The CR/zebrafish model mirrors the PDX mouse model and identifies regorafenib as a potential therapeutic drug to treat this cancer type that mimic the drug sensitivity profile in PDX model. | [147] |
| Breast cancer | Mouse | Through various experimental and computational approaches using human tumors within immunocompromised mice, the lung was found to be the most common site of relapse; lymph nodes and liver were the other most common metastatic sites. | [148] |
| Breast cancer | Zebrafish | They propose an original approach to study the metastatic process and cancer cell aggressiveness comprising the use of patient-derived primary cultures in the in vivo ZF model. | [149] |
| Breast cancer | Mouse | Growth of breast cancer PDX tumors was significantly enhanced by co-transplantation with ADSCs in vivo, and it was weakened when co-transplanted with the adipsin knocked-down ADSCs. | [150] |
| Breast cancer | Mouse | PARP inhibition can have activity beyond germline BRCA1/2 altered tumors, causing regression in a variety of molecular subtypes. | [151] |
| Breast cancer | Mouse | Concurrent inhibition of sfRon and PI3K in breast PDX tumors with wild-type PIK3CA provided durable tumor stasis after therapy cessation, whereas discontinuation of either monotherapy facilitated tumor recurrence. | [152] |
| Cervical cancer | Mouse | In vivo studies with PDXs revealed that TAO significantly decreased tumor weight for both primary squamous cell carcinoma and adenocarcinoma of the cervix. However, this anti-cancer effect was not seen in PDXs with recurrent cancers. | [153] |
| Colon Carcinoma | Mouse | HER2-specific CAR-T cells showed long-term persistence in vivo and effectively eliminated the freshly transplanted tumor tissues. | [154] |
| Colorectal cancer | Mouse | The gluconeogenic enzyme PCK1 enhanced liver metastatic growth by driving pyrimidine nucleotide biosynthesis under hypoxia. Therapeutic inhibition of the pyrimidine biosynthetic enzyme DHODH with leflunomide substantially impaired CRC liver metastatic colonization and hypoxic growth. | [155] |
| Colorectal cancer | Mouse | MAPK and EGFR pathway activations are two major molecular hallmarks of colorectal cancer. Concurrent EGFR and RAF inhibition demonstrated synergistic antitumor activity for colorectal cancer PDX models with a KRAS or BRAF mutation. | [156] |
| Colorectal cancer | Mouse | The combination of mefloquine with chemotherapeutic agents in the PDX model potentially disrupts the hierarchy of colorectal cancer cells and identify endolysosomal RAB5/7 and LAMP1/2 as promising therapeutic targets in CSCs. | [157] |
| Colorectal cancer | Mouse | HER2 activating mutations cause EGFR antibody resistance in colorectal cell lines, and PDXs with HER2 mutations show durable tumor regression when treated with dual HER2-targeted therapy. | [158] |
| Colorectal cancer | Mouse | Ex vivo culture of organoids generated from PDX demonstrates that metformin inhibits growth by executing metabolic changes to decrease oxygen consumption and activating AMPK-mediated pathways. | [159] |
| Colorectal cancer | Mouse | Anti-tumor activity of cabozantinib would be superior to regorafenib in CRC PDX models due to dual inhibition of MET and VEGFR2, as well as potentially other metabolic and autophagy mechanisms. | [160] |
| Esophageal cancer | Mouse | APIO-EE-9 significantly decreased the size of esophageal patient-derived xenograft (PDX) tumors implanted in SCID mice. It is a specific Aurora kinase inhibitor that could be developed as a therapeutic agent against esophageal cancer. | [161] |
| Gastric cancer | Zebrafish | They describe a new in vivo zPDX model of GC. This model can be used to study tumor angiogenesis, cell invasiveness and drug responses in a time-saving and cost-saving manner. | [162] |
| Gastric cancer | Mouse | They demonstrated that microRNA-133a-3p overexpression could block the activation of autophagy to ruin the abnormal glutaminolysis and further inhibit the growth and metastasis of gastric cancer cells | [163] |
| Gastric cancer | Mouse | Knockdown of circNRIP1 successfully blocked proliferation, migration, invasion and the expression level of AKT1 in GC cells. | [164] |
| Gastric cancer | Mouse | CAFs-derived LOX at liver metastatic niche of GC promotes niche formation and outgrowth thus predicts poor prognosis. Meanwhile tumor cells in niche secrete TGF-β1 to nourish CAFs and stimulate them to produce more LOX in turn. | [165] |
| Gastric cancer | Mouse | They explored the therapeutic potential of NNT inhibition in PDX models via in vivo siRNA treatment. Silencing NNT significantly suppressed tumor growth and induced cell apoptosis. | [166] |
| Gastric cancer | Mouse | ROS-activated ABL1 mediates inflammation through regulating NF-κB1 and STAT3, which subsequently leads to the development of GC and GC-related depression. | [167] |
| Gastric cancer | Mouse | Gastrin inhibited GC growth and enhanced the suppression of GC by cisplatin in mice or PGC cell culture models through activating the ERK-P65-miR23a/27a/24 axis or its components. | [168] |
| Gastric cancer | Mouse | LH inhibited tumorigenicity in gastric cancer through down-regulating the expression of MCL1. And LH combined with HA14–1 (inhibitor of BCL2) exhibited a more significant inhibitory effect than LH alone in vivo. | [169] |
| Gastric cancer | Mouse | In the PDX models with EGFR amplification, mRNA or protein overexpression, cetuximab treatment was associated with a better survival compared with that noted in the untreated group in the PDX models (P<0.05), while the survival was not statistically different in the other cases (P>0.05). | [170] |
| Gastric cancer | Mouse | The anti-tumor effect of trastuzumab was enhanced by its combination with anti-HER3 antibodies (1A5–3D4) in NCI-N87 xenograft and patient derived xenografts (PDX). Particularly in an HER2-negative whereas neuregulin1 (a ligand of HER3) positive PDX, the combination was also superior to monotherapy. | [171] |
| Gastric cancer | Mouse | 124I-trastuzumab was feasible to detect HER2-positive lesions in primary and metastatic gastric cancer patients and to differentiate HER2-positive and HER2-negative lesions quantitatively. | [172] |
| Hepatocellular carcinoma | Mouse | A CDK1 inhibitor (RO3306) in combination with sorafenib acts on hepatocellular carcinoma with a synergistic antitumor growth effect on PDX tumor models, which may be due to its effects on decreasing the liver cancer stem cell stemness via the CDK1/PDK1/β-Catenin signaling pathway. | [173] |
| High-grade serous ovarian cancer | Mouse | CUB-domain containing protein 1 (CDCP1) is over-expressed by the majority of HGSCs. CDCP1 has a role in HGSC and that it can be targeted to inhibit progression of this cancer. | [174] |
| Leukemia | Mouse | Zapadcine-1 drastically eliminates the xenografts in both CDX and PDX models of human acute leukemia. And it does not have notable toxic side effects on heart, liver, lung and kidney. | [175] |
| Lung cancer | Mouse | PG545 was highly effective in PDX that did not respond to conventional chemotherapy (cisplatin), while other PDX tumors responded well to cisplatin and to a lower extent to PG545. | [176] |
| Lung cancer | Rat | The severely immunodeficient SD‐RG rats support fast growth of PDX compared with mice, thus holding great potential to serve as a new model for oncology research. | [177] |
| Lung squamous cell carcinoma | Mouse | Combination therapy with a FGFR tyrosine kinase inhibitor and cisplatin reduced tumor growth by decreasing cell proliferation and increasing cell death. | [178] |
| Melanoma | Mouse | Genetic aberration of CDK4 pathway is a frequent event in acral melanoma. Acral melanoma cell lines and PDX containing CDK4 pathway aberrations are sensitive to CDK4/6 inhibitors. | [179] |
| Oesophageal adenocarcinoma | Mouse | APR-246 demonstrated potent antitumour activity in CLX and PDX models, and restored chemosensitivity to a cisplatin/5-fluorouracil-resistant xenograft model. | [180] |
| Ovarian cancer | Mouse | Treatment with an anti-CD20 monoclonal antibody, rituximab, not only inhibited the proliferation of established B-cell lymphoma in SCID mice but also prevented the occurrence of lymphomatous outgrowth in early-passage xenografts. | [181] |
| Ovarian cancer | Mouse | Both the activity of bevacizumab in combination with chemotherapy for the treatment of ovarian tumors and that this antitumor activity can be further improved by the addition of another targeted agents (MEK inhibitor). | [182] |
| Pancreatic cancer | Mouse | The VEGF pathway–mediated angiogenesis might influence tumor implantation as well as the growth in PDXs, thus affecting prognosis in patients or tumor‐bearing mice. | [183] |
| Pancreatic cancer | Mouse | miR-193a stimulated pancreatic cancer cell repopulation and metastasis through modulating TGF-β2/TGF-βRIII signalings, and miR-193a might be a potential therapeutic target for pancreatic cancer repopulation and metastasis. | [184] |
| Pancreatic cancer | Mouse | Adenosine induces apoptosis in pancreatic cancers, and GSK690693 can exert sensitizing effects when applied in combination with adenosine. | [185] |
| Pancreatic cancer | Mouse | They developed apratoxin S10 (Apra S10) as an anti-pancreatic cancer agent which potently inhibited the growth of both established and patient-derived primary pancreatic cancer cells. | [186] |
| Papillary renal cell carcinoma | Mouse | AZD6094 treatment resulted in tumor regressions, whereas sunitinib or crizotinib resulted in unsustained growth inhibition. | [187] |
| Prostate cancer | Mouse | MET in tumor cells is not a persistent therapeutic target for metastatic CRPC, but inhibition of VEGF-R2 and MET in endothelial cells and direct effects on osteoblasts are responsible for cabozantinib-induced tumor inhibition. | [188] |
| Prostatic carcinoma | Mouse | Cyclin D1 loss identifies prostate tumors with small cell differentiation and may identify a small subset of adenocarcinomas with poor prognosis. | [189] |
| Receptor-negative breast cancer | Mouse | Vandetanib treatment could be useful for patients with ER negative breast cancers overexpressing Vandetanib’s main targets. In a PDX model with no expression of RET nor EGFR, Vandetanib slowed tumor growth without inducing tumor regression. | [190] |
| Small cell lung cancer | Mouse | PARP trapping may play an important role in radiosensitization of SCLC cells, as talazoparib was a more effective radiosensitizer compared to veliparib at concentrations chosen to result in equivalent enzymatic inhibition. | [191] |
| Triple-negative breast cancer | Mouse | ATF4 expression inhibition reduced migration, invasiveness, mammosphere-forming efficiency, proliferation, epithelial-mesenchymal transition, and antiapoptotic and stemness marker levels. In PDX models, ATF4 silencing decreased metastases, tumor growth, and relapse after chemotherapy. | [192] |
| Triple-negative breast cancer | Mouse | Capecitabine was effective against 60% of TNBC PDX derived from tumors previously treated with anthracyclines and taxanes, and we identified TYMP and RB1 expression as putative biomarkers predictive of the response to capecitabine. | [193] |
| Triple-negative breast cancer | Mouse | Selinexor is a promising agent in the treatment of TNBC, with enhanced antitumor activity in combination with chemotherapy. | [194] |
Humanized Patient-Derived Xenograft (Hu-PDX) Model
Firstly, the immune system of severe immunodeficiency mice (NOG or NSG, etc.) was rebuilt into a state consistent with that of normal people or clinical patients, and then human tumor tissue blocks were orthotopically transplanted into immune system humanized mice. This model is called the hu-PDX model. This model can provide a growth environment more similar to that of the human body for tumors, and has important application value in tumor treatment and the study of tumor occurrence, development and metastasis, especially in tumor immunotherapy.81–83 Hu-PDX model has been applied to many types of tumor research. Lin et al28 established human immunodeficient mice by implanting peripheral blood lymphocytes and proved that this PBMCs-derived PDX model is an effective tool for studying PD-L1/PD-1 targeted cancer immunotherapy. Rosato et al84 demonstrated the availability of anti-programmed cell death-1 (PD-1) immunotherapy in the TNBC study after constructing a PDX model derived from triple-negative breast cancer patients. Sanmamed et al16 injected lymphocytes from gastric cancer patients into immunodeficient mice, then transplanted gastric cancer tissue from the same patient into mice and finally injected mice with nivolumab (a PD-1 inhibitor) and urelumab (an anti-CD137 agonist). The results show that the use of the above drugs can induce the attack of self-T cells and slow down the growth of tumors. However, there are some problems in the current Hu-PDX model, such as low success rate of modeling, short existence time of humanized immune system, incomplete immune function and so on. Future research should focus on improving the modeling technology of humanized mice and improving the efficiency and duration of immune system implantation.85
Patient-Derived Orthotropic Xenograft (PDOX) Model
Most of the transplantation sites of the PDX model are subcutaneous or renal capsule, lack of in situ environment for tumor growth. It has been found that orthotopic transplantation of tumor tissue into animal organs corresponding to the primary site can provide an in vivo environment suitable for tumor growth.86 Therefore, the PDOX model is established on the basis of the PDX model. Compared with the traditional PDX model, this model can simulate the evolution of human tumors in vivo more objectively and accurately. Hiroshima et al87 established 10 cases of subcutaneous injection of PDX model and 8 cases of PDOX model using cervical cancer tissue. The results showed that tumor metastasis was found in half of the PDOX model, but not in the PDX model. The results show that the PDOX model is more likely to show the biological characteristics of malignant tumor invasion and metastasis than the PDX model. And a number of studies have shown that swelling. The organ microenvironment in which the tumor grows can directly affect the biological characteristics of the tumor. PDOX model can also accurately predict the prognosis of cancer patients and select the most suitable individual treatment for patients. Hiroshima once again established the PDOX model and subcutaneous PDX model of human cervical cancer again and treated the two models with entinostat (a benzamide histone deacetylase inhibitor).88 Finally, it was found that only the tumor growth was inhibited in the PDOX model. Because most of the tumors in the PDOX model are located in vivo, it is difficult to observe the growth of tumors by traditional detection methods, and it is more difficult to find the location of metastatic focus.89 Making a PDOX model that is easy to measure has become a problem that needs to be solved in the future.
Mini Patient Derived Xenograft (Mini-PDX) Model
Mini-PDX model is a drug sensitivity test model established after human tumor tissue was transplanted into immunodeficient mice by a special method.90 This special method is to first inject the digested cell suspension of the patient’s tumor tissue into the microcapsule and then transplant the capsule into the mouse.91 Zhan et al92 established a Mini-PDX model using tumor tissues of patients with gallbladder cancer to detect the sensitivity of the five most commonly used chemotherapeutic drugs, (gemcitabine, oxaliplatin, 5-fluorouracil and nanoparticle albumin-bound (nab)-paclitaxel, and irinotecan). The results showed that the proliferation rate of gallbladder cancer cells in the model was relatively low after treatment with irinotecan and gemcitabine. The advantages of this model for drug sensitivity testing are short time, low cost, and high consistency with the results of the traditional PDX model. Zhang et al90 constructed Mini-PDX models of lung cancer, gastric cancer and pancreatic cancer, and used the PDX model as a reference to test the drug sensitivity of the Mini-PDX model. The results show that the consistency of the results of drug sensitivity testing using the Mini-PDX model and the traditional PDX model is 92%, but the time required to use Mini-PDX model is significantly shorter than that of the PDX model. This shows that this model can be used as a good substitute for the PDX model in evaluating cancer treatment. Because of these advantages, the Mini-PDX model is expected to become a tool to help cancer patients with personalized treatment.
Other Animal Models
With the deepening of cancer research, more and more animals are used to build animal models. At present, most of the animal models commonly used in cancer research are small animal models, such as mice, rats, zebrafish, fruit flies and so on. Among them, mice and zebrafish are the most widely used. Small animal models have many advantages, such as strong reproductive ability, low cost, easy maintenance and so on. However, because of its small size and limited blood supply, it is difficult to carry out interventions such as surgery and radiography on small animals.93 The emergence of some large animal cancer models has provided new directions for researchers, including dogs, non-human primates, tree shrews and pigs.
Compared with rodents, canine genomes are more similar to human genomes.94 Canines can spontaneously form some cancers, which are similar to human cancers in clinical, molecular and histological features.95 Uva et al96 analyzed gene expression in human and dog breast cancer and normal breast samples and found that uncontrolled genes in human breast cancer could also be observed in canine breast cancer samples. Ressel et al97 found that there is also a loss of expression of the PTEN gene in canine breast cancer, and the same phenomenon can be observed in human breast cancer. These findings support the possibility of canine cancer models as a study of human cancer. Non-human primates are closely related to human beings and highly similar to human beings in physiology, metabolism, immunity, heredity and many other aspects, so it is an excellent cancer research model. Puente et al98 found that almost all human cancer genes are highly conserved between chimpanzees and humans. However, due to the high cost of breeding and feeding, complex experimental techniques and ethical problems, the use of non-human primates has been limited.99 The tree shrew is a new experimental animal in recent years, its whole genome is very similar to primates, and its physiology and biochemistry, tissue anatomy and immunology are similar to humans.100 Tu et al101 constructed the pancreatic cancer model of tree shrew using lentivirus and analyzed the gene expression profile by RNA sequencing. The results showed that the molecular mechanism of the tree shrew pancreatic cancer model was more similar to that of the human pancreatic cancer model than that of the mouse pancreatic cancer model. Compared with non-human primates, it also has the advantages of small size, short reproductive cycle, easy feeding and so on. It also allows tree shrews to replace primates for cancer research. Ge et al102 successfully constructed a tree shrew breast cancer model with lentivirus expressing PyMT gene and found that chemotherapy drugs commonly used in human breast cancer (cisplatin and Ebramycin) can significantly inhibit tree shrew breast tumor. The pig genome has highly conserved epigenetic regulation and has high homology with the human genome.103,104 The anatomical, physiological and genetic characteristics of pigs are very similar to those of humans, and they are ideal animal models for cancer research. Mitchell et al105 induced hepatocellular carcinoma in pigs using diethylnitrosamine (DEN) and found that partial hepatic embolism could promote the construction of the model. And the emergence of gene editing pigs provides a new tool for the study of cancer-related genes. Wang et al106 established gene editing pigs expressing Cas9 under the induction of Cre enzyme, and established a pig model of lung cancer after activating one oncogene (KRAS) and five tumor suppressor genes (TP53, PTEN, APC, BRCA1 and BRCA2) at the same time. Importantly, studies have shown that young pigs can predict pharmacokinetics in children,107 which provides a basis for the use of piglet models to develop anticancer drugs.
Summary and Prospect
As an excellent tool to study the pathogenesis of human diseases and explore the principles of disease prevention and treatment, animal models are constantly providing new ideas for cancer research. However, there are some differences in physiology, heredity and immunity between animal models and human beings. Therefore, it is an urgent need for the current biomedical development to develop animal models that can better reflect human biological characteristics and replace human beings to carry out preclinical research. Because of the strong heterogeneity of cancer, the tumor tissue constructed by cell line-derived xenograft (CDX) model and traditional in vitro culture is different from humans, which cannot accurately reflect the biological process of human tumor tissue and reduce the efficiency of cancer research.108 The use of the patient-derived xenograft (PDX) model can better simulate the tumor growth process in patients, and the consistent rate of drug response is high.74 On this basis, in order to further simulate the physiological or pathological state of human beings, the humanized animal model came into being. In recent years, with the continuous development and improvement of gene-editing technology, it is possible to make a variety of cancer animal models, which has greatly promoted the related research of cancer. Researchers are working on a model that is more similar to the human genome.109 In the future, we can combine various construction methods with the help of gene-editing technology to construct a model that is more suitable for cancer research.
Acknowledgment
The following authors contributed equally to this work and are all first authors: Zhitao Li, Wubin Zheng, and Hanjin Wang.
Funding Statement
This work was supported by a grant from the Medical Science and Technology Development Foundation (ZKX18027) to Hanjin Wang.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Wild CP. The global cancer burden: necessity is the mother of prevention. Nat Rev Cancer. 2019;19(3):123–124. doi: 10.1038/s41568-019-0110-3 [DOI] [PubMed] [Google Scholar]
- 3.Schachtschneider KM, Schwind RM, Newson J, et al. The oncopig cancer model: an innovative large animal translational oncology platform. Front Oncol. 2017;7:190. doi: 10.3389/fonc.2017.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu C, Wu S, Schook LB, Schachtschneider KM. Translating human cancer sequences into personalized porcine cancer models. Front Oncol. 2019;9:105. doi: 10.3389/fonc.2019.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mural RJ, Adams MD, Myers EW, et al. A comparison of whole-genome shotgun-derived mouse chromosome 16 and the human genome. Science. 2002;296(5573):1661–1671. doi: 10.1126/science.1069193 [DOI] [PubMed] [Google Scholar]
- 6.Mendes N, Dias Carvalho P, Martins F, et al. Animal models to study cancer and its microenvironment. Adv Exp Med Biol. 2020;1219:389–401. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Yin T, Feng Y, et al. Mammalian models of chemically induced primary malignancies exploitable for imaging-based preclinical theragnostic research. Quant Imaging Med Surg. 2015;5(5):708–729. doi: 10.3978/j.issn.2223-4292.2015.06.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Minicis S, Kisseleva T, Francis H, et al. Liver carcinogenesis: rodent models of hepatocarcinoma and cholangiocarcinoma. Dig Liver Dis. 2013;45(6):450–459. doi: 10.1016/j.dld.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennecke P, Arlt MJ, Campanile C, et al. CXCR4 antibody treatment suppresses metastatic spread to the lung of intratibial human osteosarcoma xenografts in mice. Clin Exp Metastasis. 2014;31(3):339–349. doi: 10.1007/s10585-013-9632-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye F, Chen C, Qin J, Liu J, Zheng C. Genetic profiling reveals an alarming rate of cross-contamination among human cell lines used in China. FASEB J. 2015;29(10):4268–4272. doi: 10.1096/fj.14-266718 [DOI] [PubMed] [Google Scholar]
- 11.Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooijkaas AI, Gadiot J, van der Valk M, Mooi WJ, Blank CU. Targeting BRAFV600E in an inducible murine model of melanoma. Am J Pathol. 2012;181(3):785–794. doi: 10.1016/j.ajpath.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436(7051):725–730. doi: 10.1038/nature03918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kersten K, de Visser KE, van Miltenburg MH, Jonkers J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol Med. 2017;9(2):137–153. doi: 10.15252/emmm.201606857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786–798. doi: 10.1038/nri3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanmamed MF, Rodriguez I, Schalper KA, et al. Nivolumab and urelumab enhance antitumor activity of human T lymphocytes engrafted in Rag2-/-IL2Rgammanull immunodeficient mice. Cancer Res. 2015;75(17):3466–3478. doi: 10.1158/0008-5472.CAN-14-3510 [DOI] [PubMed] [Google Scholar]
- 17.Ashizawa T, Iizuka A, Nonomura C, et al. Antitumor effect of programmed death-1 (PD-1) blockade in humanized the NOG-MHC double knockout mouse. Clin Cancer Res. 2017;23(1):149–158. doi: 10.1158/1078-0432.CCR-16-0122 [DOI] [PubMed] [Google Scholar]
- 18.Roth MD, Harui A. Human tumor infiltrating lymphocytes cooperatively regulate prostate tumor growth in a humanized mouse model. J Immunother Cancer. 2015;3:12. doi: 10.1186/s40425-015-0056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Wen W, Yuan J, et al. Immunotherapy for human renal cell carcinoma by adoptive transfer of autologous transforming growth factor beta-insensitive CD8+ T cells. Clin Cancer Res. 2010;16(1):164–173. doi: 10.1158/1078-0432.CCR-09-1758 [DOI] [PubMed] [Google Scholar]
- 20.Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316–1322. doi: 10.1038/nm1431 [DOI] [PubMed] [Google Scholar]
- 21.Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol. 2008;324:149–165. doi: 10.1007/978-3-540-75647-7_10 [DOI] [PubMed] [Google Scholar]
- 22.Shimizu S, Hong P, Arumugam B, et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115(8):1534–1544. doi: 10.1182/blood-2009-04-215855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vatakis DN, Koya RC, Nixon CC, et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2011;108(51):E1408–E1416. doi: 10.1073/pnas.1115050108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur K, Kozlowska AK, Topchyan P, et al. Probiotic-treated super-charged NK cells efficiently clear poorly differentiated pancreatic tumors in Hu-BLT mice. Cancers (Basel). 2019;12(1):63. doi: 10.3390/cancers12010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reardon S. Trump administration halts fetal-tissue research by government scientists. Nature. 2019;570(7760):148. doi: 10.1038/d41586-019-01783-6 [DOI] [PubMed] [Google Scholar]
- 26.Rongvaux A, Takizawa H, Strowig T, et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meraz IM, Majidi M, Meng F, et al. An improved patient-derived xenograft humanized mouse model for evaluation of lung cancer immune responses. Cancer Immunol Res. 2019;7(8):1267–1279. doi: 10.1158/2326-6066.CIR-18-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin S, Huang G, Cheng L, et al. Establishment of peripheral blood mononuclear cell-derived humanized lung cancer mouse models for studying efficacy of PD-L1/PD-1 targeted immunotherapy. MAbs. 2018;10(8):1301–1311. doi: 10.1080/19420862.2018.1518948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G, Fan X, Cai Y, et al. Efficacy of dendritic cell-based immunotherapy produced from cord blood in vitro and in a humanized NSG mouse cancer model. Immunotherapy. 2019;11(7):599–616. doi: 10.2217/imt-2018-0103 [DOI] [PubMed] [Google Scholar]
- 30.Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J Immunol. 2006;176(4):2053–2058. doi: 10.4049/jimmunol.176.4.2053 [DOI] [PubMed] [Google Scholar]
- 31.Pyo KH, Kim JH, Lee JM, et al. Promising preclinical platform for evaluation of immuno-oncology drugs using Hu-PBL-NSG lung cancer models. Lung Cancer. 2019;127:112–121. doi: 10.1016/j.lungcan.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 32.Gyory F, Mezosi E, Szakall S, et al. Establishment of the hu-PBL-SCID mouse model for the investigation of thyroid cancer. Exp Clin Endocrinol Diabetes. 2005;113(7):359–364. doi: 10.1055/s-2005-865740 [DOI] [PubMed] [Google Scholar]
- 33.Liang ZX, Cheng Q, Chen HZ, Xie X, Ye DF. [Development of HPV16 positive cervical cancer model in the hu-PBL-SCID mouse and its immunological features]. Zhonghua Yi Xue Za Zhi. 2004;84(17):1465–1469. Chinese. [PubMed] [Google Scholar]
- 34.Chung MA, Luo Y, O’Donnell M, et al. Development and preclinical evaluation of a Bacillus Calmette-Guerin-MUC1-based novel breast cancer vaccine. Cancer Res. 2003;63(6):1280–1287. [PubMed] [Google Scholar]
- 35.Wang JJ, Liu YH, Li GC. Induction of protective and therapeutic anti-cancer immunity by using bispecific anti-idiotype antibody G22-I50 for nasopharyngeal carcinoma. Int Immunopharmacol. 2015;28(2):1026–1033. doi: 10.1016/j.intimp.2015.07.026 [DOI] [PubMed] [Google Scholar]
- 36.Teittinen KJ, Gronroos T, Parikka M, Ramet M, Lohi O. The zebrafish as a tool in leukemia research. Leuk Res. 2012;36(9):1082–1088. doi: 10.1016/j.leukres.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 37.Konantz M, Balci TB, Hartwig UF, et al. Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann N Y Acad Sci. 2012;1266:124–137. doi: 10.1111/j.1749-6632.2012.06575.x [DOI] [PubMed] [Google Scholar]
- 38.Veinotte CJ, Dellaire G, Berman JN. Hooking the big one: the potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Dis Model Mech. 2014;7(7):745–754. doi: 10.1242/dmm.015784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White RM, Sessa A, Burke C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2(2):183–189. doi: 10.1016/j.stem.2007.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Q, Moore JC, Ignatius MS, et al. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat Commun. 2016;7:10358. doi: 10.1038/ncomms10358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsao H, Fukunaga-Kalabis M, Herlyn M. Recent advances in melanoma and melanocyte biology. J Invest Dermatol. 2017;137(3):557–560. doi: 10.1016/j.jid.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 42.Gru AA, Becker N, Dehner LP, Pfeifer JD. Mucosal melanoma: correlation of clinicopathologic, prognostic, and molecular features. Melanoma Res. 2014;24(4):360–370. doi: 10.1097/CMR.0000000000000082 [DOI] [PubMed] [Google Scholar]
- 43.Olsen CM, Lane SW, Green AC. Increased risk of melanoma in patients with chronic lymphocytic leukaemia: systematic review and meta-analysis of cohort studies. Melanoma Res. 2016;26(2):188–194. doi: 10.1097/CMR.0000000000000219 [DOI] [PubMed] [Google Scholar]
- 44.Dovey M, White RM, Zon LI. Oncogenic NRAS cooperates with p53 loss to generate melanoma in zebrafish. Zebrafish. 2009;6(4):397–404. doi: 10.1089/zeb.2009.0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fornabaio G, Barnhill RL, Lugassy C, et al. Angiotropism and extravascular migratory metastasis in cutaneous and uveal melanoma progression in a zebrafish model. Sci Rep. 2018;8(1):10448. doi: 10.1038/s41598-018-28515-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez-Abenza E, Ibanez-Molero S, Garcia-Moreno D, et al. Zebrafish modeling reveals that SPINT1 regulates the aggressiveness of skin cutaneous melanoma and its crosstalk with tumor immune microenvironment. J Exp Clin Cancer Res. 2019;38(1):405. doi: 10.1186/s13046-019-1389-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabellini C, Gomez-Abenza E, Ibanez-Molero S, et al. Interleukin 8 mediates bcl-xL-induced enhancement of human melanoma cell dissemination and angiogenesis in a zebrafish xenograft model. Int J Cancer. 2018;142(3):584–596. doi: 10.1002/ijc.31075 [DOI] [PubMed] [Google Scholar]
- 48.Jing L, Zon LI. Zebrafish as a model for normal and malignant hematopoiesis. Dis Model Mech. 2011;4(4):433–438. doi: 10.1242/dmm.006791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langenau DM, Traver D, Ferrando AA, et al. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299(5608):887–890. doi: 10.1126/science.1080280 [DOI] [PubMed] [Google Scholar]
- 50.Gutierrez A, Grebliunaite R, Feng H, et al. Pten mediates Myc oncogene dependence in a conditional zebrafish model of T cell acute lymphoblastic leukemia. J Exp Med. 2011;208(8):1595–1603. doi: 10.1084/jem.20101691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corkery DP, Dellaire G, Berman JN. Leukaemia xenotransplantation in zebrafish–chemotherapy response assay in vivo. Br J Haematol. 2011;153(6):786–789. doi: 10.1111/j.1365-2141.2011.08661.x [DOI] [PubMed] [Google Scholar]
- 52.Yeh JR, Munson KM, Elagib KE, Goldfarb AN, Sweetser DA, Peterson RT. Discovering chemical modifiers of oncogene-regulated hematopoietic differentiation. Nat Chem Biol. 2009;5(4):236–243. doi: 10.1038/nchembio.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutierrez A, Pan L, Groen RW, et al. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J Clin Invest. 2014;124(2):644–655. doi: 10.1172/JCI65093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridges S, Heaton WL, Joshi D, et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood. 2012;119(24):5621–5631. doi: 10.1182/blood-2011-12-398818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255(1):12–29. doi: 10.1016/S0012-1606(02)00034-9 [DOI] [PubMed] [Google Scholar]
- 56.Tsering J, Hu X. Triphala suppresses growth and migration of human gastric carcinoma cells in vitro and in a zebrafish xenograft model. Biomed Res Int. 2018;2018:7046927. doi: 10.1155/2018/7046927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lam SH, Wu YL, Vega VB, et al. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol. 2006;24(1):73–75. doi: 10.1038/nbt1169 [DOI] [PubMed] [Google Scholar]
- 58.Yan C, Yang Q, Gong Z. Activation of hepatic stellate cells during liver carcinogenesis requires fibrinogen/integrin alphavbeta5 in zebrafish. Neoplasia. 2018;20(5):533–542. doi: 10.1016/j.neo.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Jung IH, Choi JH, Chung YY, Lim GL, Park YN, Park SW. Predominant activation of JAK/STAT3 pathway by interleukin-6 is implicated in hepatocarcinogenesis. Neoplasia. 2015;17(7):586–597. doi: 10.1016/j.neo.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Topi G, Satapathy SR, Dash P, et al. Tumour-suppressive effect of oestrogen receptor beta in colorectal cancer patients, colon cancer cells, and a zebrafish model. J Pathol. 2020;251(3):297–309. doi: 10.1002/path.5453 [DOI] [PubMed] [Google Scholar]
- 61.Lu JW, Raghuram D, Fong PA, Gong Z. Inducible intestine-specific expression of kras(V12) triggers intestinal tumorigenesis in transgenic zebrafish. Neoplasia. 2018;20(12):1187–1197. doi: 10.1016/j.neo.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menke AL, Spitsbergen JM, Wolterbeek AP, Woutersen RA. Normal anatomy and histology of the adult zebrafish. Toxicol Pathol. 2011;39(5):759–775. doi: 10.1177/0192623311409597 [DOI] [PubMed] [Google Scholar]
- 63.Guo M, Wei H, Hu J, Sun S, Long J, Wang X. U0126 inhibits pancreatic cancer progression via the KRAS signaling pathway in a zebrafish xenotransplantation model. Oncol Rep. 2015;34(2):699–706. doi: 10.3892/or.2015.4019 [DOI] [PubMed] [Google Scholar]
- 64.Fahad Ullah M. Breast cancer: current perspectives on the disease status. Adv Exp Med Biol. 2019;1152:51–64. [DOI] [PubMed] [Google Scholar]
- 65.McCarthy A, Savage K, Gabriel A, Naceur C, Reis-Filho JS, Ashworth A. A mouse model of basal-like breast carcinoma with metaplastic elements. J Pathol. 2007;211(4):389–398. [DOI] [PubMed] [Google Scholar]
- 66.Fantozzi A, Christofori G. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006;8(4):212. doi: 10.1186/bcr1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ren J, Smid M, Iaria J, et al. Cancer-associated fibroblast-derived Gremlin 1 promotes breast cancer progression. Breast Cancer Res. 2019;21(1):109. doi: 10.1186/s13058-019-1194-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu XY, Guo DW, Lao QC, et al. Sensitization and synergistic anti-cancer effects of Furanodiene identified in zebrafish models. Sci Rep. 2019;9(1):4541. doi: 10.1038/s41598-019-40866-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen W, Pu J, Sun J, et al. Zebrafish xenograft model of human lung cancer for studying the function of LINC00152 in cell proliferation and invasion. Cancer Cell Int. 2020;20:376. doi: 10.1186/s12935-020-01460-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang HW, Bow YD, Wang CY, et al. DFIQ, a novel quinoline derivative, shows anticancer potential by inducing apoptosis and autophagy in NSCLC cell and in vivo zebrafish xenograft models. Cancers (Basel). 2020;12(5):1348. doi: 10.3390/cancers12051348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keysar SB, Astling DP, Anderson RT, et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol Oncol. 2013;7(4):776–790. doi: 10.1016/j.molonc.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007;2(2):247–250. doi: 10.1038/nprot.2007.25 [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Sudilovsky D, Zhang B, et al. A human prostatic epithelial model of hormonal carcinogenesis. Cancer Res. 2001;61(16):6064–6072. [PubMed] [Google Scholar]
- 74.Owonikoko TK, Zhang G, Kim HS, et al. Patient-derived xenografts faithfully replicated clinical outcome in a Phase II co-clinical trial of arsenic trioxide in relapsed small cell lung cancer. J Transl Med. 2016;14(1):111. doi: 10.1186/s12967-016-0861-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marangoni E, Vincent-Salomon A, Auger N, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13(13):3989–3998. doi: 10.1158/1078-0432.CCR-07-0078 [DOI] [PubMed] [Google Scholar]
- 76.Dodbiba L, Teichman J, Fleet A, et al. Primary esophageal and gastro-esophageal junction cancer xenograft models: clinicopathological features and engraftment. Lab Invest. 2013;93(4):397–407. doi: 10.1038/labinvest.2013.8 [DOI] [PubMed] [Google Scholar]
- 77.El-Rifai W, Harper JC, Cummings OW, et al. Consistent genetic alterations in xenografts of proximal stomach and gastro-esophageal junction adenocarcinomas. Cancer Res. 1998;58(1):34–37. [PubMed] [Google Scholar]
- 78.Tanaskovic O, Verga Falzacappa MV, Pelicci PG, Kim CH. Human cord blood (hCB)-CD34+ humanized mice fail to reject human acute myeloid leukemia cells. PLoS One. 2019;14(9):e0217345. doi: 10.1371/journal.pone.0217345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moyer AM, Yu J, Sinnwell JP, et al. Spontaneous murine tumors in the development of patient-derived xenografts: a potential pitfall. Oncotarget. 2019;10(39):3924–3930. doi: 10.18632/oncotarget.27001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giraud J, Bouriez D, Seeneevassen L, et al. Orthotopic patient-derived xenografts of gastric cancer to decipher drugs effects on cancer stem cells and metastatic dissemination. Cancers (Basel). 2019;11(4):560. doi: 10.3390/cancers11040560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morton JJ, Bird G, Keysar SB, et al. XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene. 2016;35(3):290–300. doi: 10.1038/onc.2015.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rongvaux A, Willinger T, Martinek J, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32(4):364–372. doi: 10.1038/nbt.2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X, Qi Z, Wei H, Tian Z, Sun R. Characterization of human B cells in umbilical cord blood-transplanted NOD/SCID mice. Transpl Immunol. 2012;26(2–3):156–162. doi: 10.1016/j.trim.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 84.Rosato RR, Davila-Gonzalez D, Choi DS, et al. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018;20(1):108. doi: 10.1186/s13058-018-1037-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Danisch S, Slabik C, Cornelius A, et al. Spatiotemporally skewed activation of programmed cell death receptor 1-positive T cells after epstein-barr virus infection and tumor development in long-term fully humanized mice. Am J Pathol. 2019;189(3):521–539. doi: 10.1016/j.ajpath.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sicklick JK, Leonard SY, Babicky ML, et al. Generation of orthotopic patient-derived xenografts from gastrointestinal stromal tumor. J Transl Med. 2014;12:41. doi: 10.1186/1479-5876-12-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hiroshima Y, Zhang Y, Zhang N, et al. Establishment of a patient-derived orthotopic Xenograft (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS One. 2015;10(2):e0117417. doi: 10.1371/journal.pone.0117417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hiroshima Y, Maawy A, Zhang Y, et al. Patient-derived mouse models of cancer need to be orthotopic in order to evaluate targeted anti-metastatic therapy. Oncotarget. 2016;7(44):71696–71702. doi: 10.18632/oncotarget.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Igarashi K, Kawaguchi K, Kiyuna T, et al. Patient-derived orthotopic xenograft (PDOX) mouse model of adult rhabdomyosarcoma invades and recurs after resection in contrast to the subcutaneous ectopic model. Cell Cycle. 2017;16(1):91–94. doi: 10.1080/15384101.2016.1252885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang F, Wang W, Long Y, et al. Characterization of drug responses of mini patient-derived xenografts in mice for predicting cancer patient clinical therapeutic response. Cancer Commun (Lond). 2018;38(1):60. doi: 10.1186/s40880-018-0329-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frank ND, Jones ME, Vang B, et al. Evaluation of reagents used to coat the hollow-fiber bioreactor membrane of the Quantum(R) Cell Expansion System for the culture of human mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2019;96:77–85. doi: 10.1016/j.msec.2018.10.081 [DOI] [PubMed] [Google Scholar]
- 92.Zhan M, Yang RM, Wang H, et al. Guided chemotherapy based on patient-derived mini-xenograft models improves survival of gallbladder carcinoma patients. Cancer Commun (Lond). 2018;38(1):48. doi: 10.1186/s40880-018-0318-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mei LJ, Yang XJ, Tang L, Hassan AH, Yonemura Y, Li Y. Establishment and identification of a rabbit model of peritoneal carcinomatosis from gastric cancer. BMC Cancer. 2010;10:124. doi: 10.1186/1471-2407-10-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pinho SS, Carvalho S, Cabral J, Reis CA, Gartner F. Canine tumors: a spontaneous animal model of human carcinogenesis. Transl Res. 2012;159(3):165–172. doi: 10.1016/j.trsl.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 95.Gardner HL, Fenger JM, London CA. Dogs as a model for cancer. Annu Rev Anim Biosci. 2016;4:199–222. doi: 10.1146/annurev-animal-022114-110911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uva P, Aurisicchio L, Watters J, et al. Comparative expression pathway analysis of human and canine mammary tumors. BMC Genomics. 2009;10:135. doi: 10.1186/1471-2164-10-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ressel L, Millanta F, Caleri E, Innocenti VM, Poli A. Reduced PTEN protein expression and its prognostic implications in canine and feline mammary tumors. Vet Pathol. 2009;46(5):860–868. doi: 10.1354/vp.08-VP-0273-P-FL [DOI] [PubMed] [Google Scholar]
- 98.Puente XS, Velasco G, Gutierrez-Fernandez A, Bertranpetit J, King MC, Lopez-Otin C. Comparative analysis of cancer genes in the human and chimpanzee genomes. BMC Genomics. 2006;7:15. doi: 10.1186/1471-2164-7-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pouladi MA, Morton AJ, Hayden MR. Choosing an animal model for the study of Huntington’s disease. Nat Rev Neurosci. 2013;14(10):708–721. doi: 10.1038/nrn3570 [DOI] [PubMed] [Google Scholar]
- 100.Fan Y, Huang ZY, Cao CC, et al. Genome of the Chinese tree shrew. Nat Commun. 2013;4:1426. doi: 10.1038/ncomms2416 [DOI] [PubMed] [Google Scholar]
- 101.Tu Q, Yang D, Zhang X, et al. A novel pancreatic cancer model originated from transformation of acinar cells in adult tree shrew, a primate-like animal. Dis Model Mech. 2019;12(4):dmm038703. doi: 10.1242/dmm.038703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ge GZ, Xia HJ, He BL, et al. Generation and characterization of a breast carcinoma model by PyMT overexpression in mammary epithelial cells of tree shrew, an animal close to primates in evolution. Int J Cancer. 2016;138(3):642–651. doi: 10.1002/ijc.29814 [DOI] [PubMed] [Google Scholar]
- 103.Schachtschneider KM, Madsen O, Park C, Rund LA, Groenen MA, Schook LB. Adult porcine genome-wide DNA methylation patterns support pigs as a biomedical model. BMC Genomics. 2015;16:743. doi: 10.1186/s12864-015-1938-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Groenen MA, Archibald AL, Uenishi H, et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature. 2012;491(7424):393–398. doi: 10.1038/nature11622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mitchell J, Tinkey PT, Avritscher R, et al. Validation of a preclinical model of diethylnitrosamine-induced hepatic neoplasia in yucatan miniature pigs. Oncology. 2016;91(2):90–100. doi: 10.1159/000446074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang K, Jin Q, Ruan D, et al. Cre-dependent Cas9-expressing pigs enable efficient in vivo genome editing. Genome Res. 2017;27(12):2061–2071. doi: 10.1101/gr.222521.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roth WJ, Kissinger CB, McCain RR, et al. Assessment of juvenile pigs to serve as human pediatric surrogates for preclinical formulation pharmacokinetic testing. AAPS J. 2013;15(3):763–774. doi: 10.1208/s12248-013-9482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kucharavy A, Rubinstein B, Zhu J, Li R, Mogilner A. Robustness and evolvability of heterogeneous cell populations. Mol Biol Cell. 2018;29(11):1400–1409. doi: 10.1091/mbc.E18-01-0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leidy-Davis T, Cheng K, Goodwin LO, et al. Viable mice with extensive gene humanization (25-kbp) created using embryonic stem cell/blastocyst and CRISPR/zygote injection approaches. Sci Rep. 2018;8(1):15028. doi: 10.1038/s41598-018-33408-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Borga C, Park G, Foster C, et al. Simultaneous B and T cell acute lymphoblastic leukemias in zebrafish driven by transgenic MYC: implications for oncogenesis and lymphopoiesis. Leukemia. 2019;33(2):333–347. doi: 10.1038/s41375-018-0226-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu JW, Hsieh MS, Hou HA, Chen CY, Tien HF, Lin LI. Overexpression of SOX4 correlates with poor prognosis of acute myeloid leukemia and is leukemogenic in zebrafish. Blood Cancer J. 2017;7(8):e593. doi: 10.1038/bcj.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He S, Lamers GE, Beenakker JW, et al. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J Pathol. 2012;227(4):431–445. doi: 10.1002/path.4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paul CD, Bishop K, Devine A, et al. Tissue architectural cues drive organ targeting of tumor cells in zebrafish. Cell Syst. 2019;9(2):187–206 e116. doi: 10.1016/j.cels.2019.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fenizia C, Bottino C, Corbetta S, et al. SMYD3 promotes the epithelial-mesenchymal transition in breast cancer. Nucleic Acids Res. 2019;47(3):1278–1293. doi: 10.1093/nar/gky1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nadar RA, Asokan N, Degli Esposti L, et al. Preclinical evaluation of platinum-loaded hydroxyapatite nanoparticles in an embryonic zebrafish xenograft model. Nanoscale. 2020;12(25):13582–13594. doi: 10.1039/D0NR04064A [DOI] [PubMed] [Google Scholar]
- 116.Basti A, Fior R, Yalin M, et al. The core-clock gene NR1D1 impacts cell motility in vitro and invasiveness in a zebrafish xenograft colon cancer model. Cancers (Basel). 2020;12(4):853. doi: 10.3390/cancers12040853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paauwe M, Schoonderwoerd MJA, Helderman R, et al. Endoglin expression on cancer-associated fibroblasts regulates invasion and stimulates colorectal cancer metastasis. Clin Cancer Res. 2018;24(24):6331–6344. doi: 10.1158/1078-0432.CCR-18-0329 [DOI] [PubMed] [Google Scholar]
- 118.Jiang X, Liu G, Hu Z, Chen G, Chen J, Lv Z. cGAMP inhibits tumor growth in colorectal cancer metastasis through the STING/STAT3 axis in a zebrafish xenograft model. Fish Shellfish Immunol. 2019;95:220–226. doi: 10.1016/j.fsi.2019.09.075 [DOI] [PubMed] [Google Scholar]
- 119.Fior R, Povoa V, Mendes RV, et al. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc Natl Acad Sci U S A. 2017;114(39):E8234–E8243. doi: 10.1073/pnas.1618389114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petrovic J, Glamoclija J, Ilic-Tomic T, et al. Lectin from Laetiporus sulphureus effectively inhibits angiogenesis and tumor development in the zebrafish xenograft models of colorectal carcinoma and melanoma. Int J Biol Macromol. 2020;148:129–139. doi: 10.1016/j.ijbiomac.2020.01.033 [DOI] [PubMed] [Google Scholar]
- 121.Roel M, Rubiolo JA, Guerra-Varela J, et al. Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget. 2016;7(50):83071–83087. doi: 10.18632/oncotarget.13068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Banasavadi-Siddegowda YK, Welker AM, An M, et al. PRMT5 as a druggable target for glioblastoma therapy. Neuro Oncol. 2018;20(6):753–763. doi: 10.1093/neuonc/nox206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nguyen AT, Emelyanov A, Koh CH, Spitsbergen JM, Parinov S, Gong Z. An inducible kras(V12) transgenic zebrafish model for liver tumorigenesis and chemical drug screening. Dis Model Mech. 2012;5(1):63–72. doi: 10.1242/dmm.008367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Z, Zheng W, Wang Z, et al. A transgenic zebrafish liver tumor model with inducible Myc expression reveals conserved Myc signatures with mammalian liver tumors. Dis Model Mech. 2013;6(2):414–423. doi: 10.1242/dmm.010462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mizgireuv IV, Majorova IG, Gorodinskaya VM, Khudoley VV, Revskoy SY. Carcinogenic effect of N-nitrosodimethylamine on diploid and triploid zebrafish (Danio rerio). Toxicol Pathol. 2004;32(5):514–518. doi: 10.1080/01926230490496311 [DOI] [PubMed] [Google Scholar]
- 126.Shwartz A, Goessling W, Yin C. Macrophages in zebrafish models of liver diseases. Front Immunol. 2019;10:2840. doi: 10.3389/fimmu.2019.02840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang Q, Yan C, Gong Z. Activation of liver stromal cells is associated with male-biased liver tumor initiation in xmrk and Myc transgenic zebrafish. Sci Rep. 2017;7(1):10315. doi: 10.1038/s41598-017-10529-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Z, Huang X, Zhan H, et al. Inducible and repressable oncogene-addicted hepatocellular carcinoma in Tet-on xmrk transgenic zebrafish. J Hepatol. 2012;56(2):419–425. doi: 10.1016/j.jhep.2011.07.025 [DOI] [PubMed] [Google Scholar]
- 129.Li Z, Luo H, Li C, et al. Transcriptomic analysis of a transgenic zebrafish hepatocellular carcinoma model reveals a prominent role of immune responses in tumour progression and regression. Int J Cancer. 2014;135(7):1564–1573. doi: 10.1002/ijc.28794 [DOI] [PubMed] [Google Scholar]
- 130.Evason KJ, Francisco MT, Juric V, et al. Identification of chemical inhibitors of beta-catenin-driven liver tumorigenesis in zebrafish. PLoS Genet. 2015;11(7):e1005305. doi: 10.1371/journal.pgen.1005305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yan C, Huo X, Wang S, Feng Y, Gong Z. Stimulation of hepatocarcinogenesis by neutrophils upon induction of oncogenic kras expression in transgenic zebrafish. J Hepatol. 2015;63(2):420–428. doi: 10.1016/j.jhep.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pruvot B, Jacquel A, Droin N, et al. Leukemic cell xenograft in zebrafish embryo for investigating drug efficacy. Haematologica. 2011;96(4):612–616. doi: 10.3324/haematol.2010.031401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang B, Shimada Y, Kuroyanagi J, Umemoto N, Nishimura Y, Tanaka T. Quantitative phenotyping-based in vivo chemical screening in a zebrafish model of leukemia stem cell xenotransplantation. PLoS One. 2014;9(1):e85439. doi: 10.1371/journal.pone.0085439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang Q, Salim L, Yan C, Gong Z. Rapid analysis of effects of environmental toxicants on tumorigenesis and inflammation using a transgenic zebrafish model for liver cancer. Mar Biotechnol (NY). 2019;21(3):396–405. doi: 10.1007/s10126-019-09889-8 [DOI] [PubMed] [Google Scholar]
- 135.Chew TW, Liu XJ, Liu L, Spitsbergen JM, Gong Z, Low BC. Crosstalk of Ras and Rho: activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafish models. Oncogene. 2014;33(21):2717–2727. doi: 10.1038/onc.2013.240 [DOI] [PubMed] [Google Scholar]
- 136.Chiu CC, Chou HL, Chen BH, et al. BPIQ, a novel synthetic quinoline derivative, inhibits growth and induces mitochondrial apoptosis of lung cancer cells in vitro and in zebrafish xenograft model. BMC Cancer. 2015;15:962. doi: 10.1186/s12885-015-1970-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jin Y, Wei L, Jiang Q, et al. Comparison of efficacy and toxicity of bevacizumab, endostar and apatinib in transgenic and human lung cancer xenograftzebrafish model. Sci Rep. 2018;8(1):15837. doi: 10.1038/s41598-018-34030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Topczewska JM, Postovit LM, Margaryan NV, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12(8):925–932. doi: 10.1038/nm1448 [DOI] [PubMed] [Google Scholar]
- 139.Patton EE, Widlund HR, Kutok JL, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15(3):249–254. doi: 10.1016/j.cub.2005.01.031 [DOI] [PubMed] [Google Scholar]
- 140.Antonio N, Bonnelykke-Behrndtz ML, Ward LC, et al. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015;34(17):2219–2236. doi: 10.15252/embj.201490147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hosono Y, Niknafs YS, Prensner JR, et al. Oncogenic role of THOR, a conserved cancer/testis long non-coding RNA. Cell. 2017;171(7):1559–1572 e1520. doi: 10.1016/j.cell.2017.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Verykiou S, Alexander M, Edwards N, et al. Harnessing autophagy to overcome mitogen-activated protein kinase kinase inhibitor-induced resistance in metastatic melanoma. Br J Dermatol. 2019;180(2):346–356. doi: 10.1111/bjd.17333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marques IJ, Weiss FU, Vlecken DH, et al. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer. 2009;9:128. doi: 10.1186/1471-2407-9-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kendall GC, Watson S, Xu L, et al. PAX3-FOXO1 transgenic zebrafish models identify HES3 as a mediator of rhabdomyosarcoma tumorigenesis. Elife. 2018;7. doi: 10.7554/eLife.33800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bentley VL, Veinotte CJ, Corkery DP, et al. Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia. Haematologica. 2015;100(1):70–76. doi: 10.3324/haematol.2014.110742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Anelli V, Villefranc JA, Chhangawala S, et al. Oncogenic BRAF disrupts thyroid morphogenesis and function via twist expression. Elife. 2017;6. doi: 10.7554/eLife.20728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen C, Choudhury S, Wangsa D, et al. A multiplex preclinical model for adenoid cystic carcinoma of the salivary gland identifies regorafenib as a potential therapeutic drug. Sci Rep. 2017;7(1):11410. doi: 10.1038/s41598-017-11764-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alzubi MA, Turner TH, Olex AL, et al. Separation of breast cancer and organ microenvironment transcriptomes in metastases. Breast Cancer Res. 2019;21(1):36. doi: 10.1186/s13058-019-1123-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mercatali L, La Manna F, Groenewoud A, et al. Development of a patient-derived xenograft (PDX) of breast cancer bone metastasis in a zebrafish model. Int J Mol Sci. 2016;17(8):1375. doi: 10.3390/ijms17081375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Goto H, Shimono Y, Funakoshi Y, et al. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene. 2019;38(6):767–779. doi: 10.1038/s41388-018-0477-8 [DOI] [PubMed] [Google Scholar]
- 151.Evans KW, Yuca E, Akcakanat A, et al. A population of heterogeneous breast cancer patient-derived xenografts demonstrate broad activity of PARP inhibitor in BRCA1/2 wild-type tumors. Clin Cancer Res. 2017;23(21):6468–6477. doi: 10.1158/1078-0432.CCR-17-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bieniasz M, Radhakrishnan P, Faham N, De La OJ, Welm AL. Preclinical efficacy of ron kinase inhibitors alone and in combination with PI3K inhibitors for treatment of sfRon-expressing breast cancer patient-derived xenografts. Clin Cancer Res. 2015;21(24):5588–5600. doi: 10.1158/1078-0432.CCR-14-3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Noh JJ, Kim MS, Cho YJ, et al. Anti-cancer activity of As4O6 and its efficacy in a series of patient-derived xenografts for human cervical cancer. Pharmaceutics. 2020;12(10):987. doi: 10.3390/pharmaceutics12100987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Teng R, Zhao J, Zhao Y, et al. Chimeric antigen receptor-modified T cells repressed solid tumors and their relapse in an established patient-derived colon carcinoma xenograft model. J Immunother. 2019;42(2):33–42. doi: 10.1097/CJI.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamaguchi N, Weinberg EM, Nguyen A, et al. PCK1 and DHODH drive colorectal cancer liver metastatic colonization and hypoxic growth by promoting nucleotide synthesis. Elife. 2019;8. doi: 10.7554/eLife.52135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yao YM, Donoho GP, Iversen PW, et al. Mouse PDX trial suggests synergy of concurrent inhibition of RAF and EGFR in colorectal cancer with BRAF or KRAS mutations. Clin Cancer Res. 2017;23(18):5547–5560. doi: 10.1158/1078-0432.CCR-16-3250 [DOI] [PubMed] [Google Scholar]
- 157.Takeda M, Koseki J, Takahashi H, et al. Disruption of endolysosomal RAB5/7 efficiently eliminates colorectal cancer stem cells. Cancer Res. 2019;79(7):1426–1437. doi: 10.1158/0008-5472.CAN-18-2192 [DOI] [PubMed] [Google Scholar]
- 158.Kavuri SM, Jain N, Galimi F, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5(8):832–841. doi: 10.1158/2159-8290.CD-14-1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mohamed Suhaimi NA, Phyo WM, Yap HY, et al. Metformin inhibits cellular proliferation and bioenergetics in colorectal cancer patient-derived xenografts. Mol Cancer Ther. 2017;16(9):2035–2044. doi: 10.1158/1535-7163.MCT-16-0793 [DOI] [PubMed] [Google Scholar]
- 160.Scott AJ, Arcaroli JJ, Bagby SM, et al. Cabozantinib exhibits potent antitumor activity in colorectal cancer patient-derived tumor xenograft models via autophagy and signaling mechanisms. Mol Cancer Ther. 2018;17(10):2112–2122. doi: 10.1158/1535-7163.MCT-17-0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jin G, Yao K, Guo Z, et al. APIO-EE-9 is a novel Aurora A and B antagonist that suppresses esophageal cancer growth in a PDX mouse model. Oncotarget. 2017;8(32):53387–53404. doi: 10.18632/oncotarget.18508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu JQ, Zhai J, Li CY, et al. Patient-derived xenograft in zebrafish embryos: a new platform for translational research in gastric cancer. J Exp Clin Cancer Res. 2017;36(1):160. doi: 10.1186/s13046-017-0631-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang X, Li Z, Xuan Z, et al. Novel role of miR-133a-3p in repressing gastric cancer growth and metastasis via blocking autophagy-mediated glutaminolysis. J Exp Clin Cancer Res. 2018;37(1):320. doi: 10.1186/s13046-018-0993-y [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 164.Zhang X, Wang S, Wang H, et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18(1):20. doi: 10.1186/s12943-018-0935-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li Q, Zhu CC, Ni B, et al. Lysyl oxidase promotes liver metastasis of gastric cancer via facilitating the reciprocal interactions between tumor cells and cancer associated fibroblasts. EBioMedicine. 2019;49:157–171. doi: 10.1016/j.ebiom.2019.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li S, Zhuang Z, Wu T, et al. Nicotinamide nucleotide transhydrogenase-mediated redox homeostasis promotes tumor growth and metastasis in gastric cancer. Redox Biol. 2018;18:246–255. doi: 10.1016/j.redox.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang T, Zhou F, Yuan X, et al. Reactive oxygen species are involved in the development of gastric cancer and gastric cancer-related depression through ABL1-mediated inflammation signaling pathway. Oxid Med Cell Longev. 2019;2019:5813985. doi: 10.1155/2019/5813985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zu LD, Peng XC, Zeng Z, et al. Gastrin inhibits gastric cancer progression through activating the ERK-P65-miR23a/27a/24 axis. J Exp Clin Cancer Res. 2018;37(1):115. doi: 10.1186/s13046-018-0782-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li C, Deng C, Pan G, et al. Lycorine hydrochloride inhibits cell proliferation and induces apoptosis through promoting FBXW7-MCL1 axis in gastric cancer. J Exp Clin Cancer Res. 2020;39(1):230. doi: 10.1186/s13046-020-01743-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang X, Fu R, Hu Y, et al. EGFR gene status predicts response and survival benefit in a preclinical gastric cancer trial treating patientderived xenografts with cetuximab. Oncol Rep. 2017;38(4):2387–2393. doi: 10.3892/or.2017.5907 [DOI] [PubMed] [Google Scholar]
- 171.Wang Q, Zhang X, Shen E, et al. The anti-HER3 antibody in combination with trastuzumab exerts synergistic antitumor activity in HER2-positive gastric cancer. Cancer Lett. 2016;380(1):20–30. [DOI] [PubMed] [Google Scholar]
- 172.Guo X, Zhou N, Chen Z, et al. Construction of (124)I-trastuzumab for noninvasive PET imaging of HER2 expression: from patient-derived xenograft models to gastric cancer patients. Gastric Cancer. 2020;23(4):614–626. doi: 10.1007/s10120-019-01035-6 [DOI] [PubMed] [Google Scholar]
- 173.Wu CX, Wang XQ, Chok SH, et al. Blocking CDK1/PDK1/beta-Catenin signaling by CDK1 inhibitor RO3306 increased the efficacy of sorafenib treatment by targeting cancer stem cells in a preclinical model of hepatocellular carcinoma. Theranostics. 2018;8(14):3737–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Harrington BS, He Y, Davies CM, et al. Cell line and patient-derived xenograft models reveal elevated CDCP1 as a target in high-grade serous ovarian cancer. Br J Cancer. 2016;114(4):417–426. doi: 10.1038/bjc.2015.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang S, Zheng C, Zhu W, et al. A novel anti-DR5 antibody-drug conjugate possesses a high-potential therapeutic efficacy for leukemia and solid tumors. Theranostics. 2019;9(18):5412–5423. doi: 10.7150/thno.33598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Katz A, Barash U, Boyango I, et al. Patient derived xenografts (PDX) predict an effective heparanase-based therapy for lung cancer. Oncotarget. 2018;9(27):19294–19306. doi: 10.18632/oncotarget.25022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.He D, Zhang J, Wu W, et al. A novel immunodeficient rat model supports human lung cancer xenografts. FASEB J. 2019;33(1):140–150. doi: 10.1096/fj.201800102RR [DOI] [PubMed] [Google Scholar]
- 178.Weeden CE, Holik AZ, Young RJ, et al. Cisplatin increases sensitivity to FGFR inhibition in patient-derived xenograft models of lung squamous cell carcinoma. Mol Cancer Ther. 2017;16(8):1610–1622. doi: 10.1158/1535-7163.MCT-17-0174 [DOI] [PubMed] [Google Scholar]
- 179.Kong Y, Sheng X, Wu X, et al. Frequent genetic aberrations in the CDK4 pathway in acral melanoma indicate the potential for CDK4/6 inhibitors in targeted therapy. Clin Cancer Res. 2017;23(22):6946–6957. doi: 10.1158/1078-0432.CCR-17-0070 [DOI] [PubMed] [Google Scholar]
- 180.Liu DS, Read M, Cullinane C, et al. APR-246 potently inhibits tumour growth and overcomes chemoresistance in preclinical models of oesophageal adenocarcinoma. Gut. 2015;64(10):1506–1516. doi: 10.1136/gutjnl-2015-309770 [DOI] [PubMed] [Google Scholar]
- 181.Butler KA, Hou X, Becker MA, et al. Prevention of human lymphoproliferative tumor formation in ovarian cancer patient-derived xenografts. Neoplasia. 2017;19(8):628–636. doi: 10.1016/j.neo.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ricci F, Guffanti F, Damia G, Broggini M. Combination of paclitaxel, bevacizumab and MEK162 in second line treatment in platinum-relapsing patient derived ovarian cancer xenografts. Mol Cancer. 2017;16(1):97. doi: 10.1186/s12943-017-0662-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Guo S, Gao S, Liu R, et al. Oncological and genetic factors impacting PDX model construction with NSG mice in pancreatic cancer. FASEB J. 2019;33(1):873–884. doi: 10.1096/fj.201800617R [DOI] [PubMed] [Google Scholar]
- 184.Fang C, Dai CY, Mei Z, et al. microRNA-193a stimulates pancreatic cancer cell repopulation and metastasis through modulating TGF-beta2/TGF-betaRIII signalings. J Exp Clin Cancer Res. 2018;37(1):25. doi: 10.1186/s13046-018-0697-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang D, Zhang Q, Ma Y, et al. Augmenting the therapeutic efficacy of adenosine against pancreatic cancer by switching the Akt/p21-dependent senescence to apoptosis. EBioMedicine. 2019;47:114–127. doi: 10.1016/j.ebiom.2019.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cai W, Ratnayake R, Gerber MH, et al. Development of apratoxin S10 (Apra S10) as an anti-pancreatic cancer agent and its preliminary evaluation in an orthotopic patient-derived xenograft (PDX) model. Invest New Drugs. 2019;37(2):364–374. doi: 10.1007/s10637-018-0647-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schuller AG, Barry ER, Jones RD, et al. The MET inhibitor AZD6094 (Savolitinib, HMPL-504) induces regression in papillary renal cell carcinoma patient-derived xenograft models. Clin Cancer Res. 2015;21(12):2811–2819. doi: 10.1158/1078-0432.CCR-14-2685 [DOI] [PubMed] [Google Scholar]
- 188.Varkaris A, Corn PG, Parikh NU, et al. Integrating murine and clinical trials with cabozantinib to understand roles of MET and VEGFR2 as targets for growth inhibition of prostate cancer. Clin Cancer Res. 2016;22(1):107–121. doi: 10.1158/1078-0432.CCR-15-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tsai H, Morais CL, Alshalalfa M, et al. Cyclin D1 loss distinguishes prostatic small-cell carcinoma from most prostatic adenocarcinomas. Clin Cancer Res. 2015;21(24):5619–5629. doi: 10.1158/1078-0432.CCR-15-0744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hatem R, Labiod D, Chateau-Joubert S, et al. Vandetanib as a potential new treatment for estrogen receptor-negative breast cancers. Int J Cancer. 2016;138(10):2510–2521. doi: 10.1002/ijc.29974 [DOI] [PubMed] [Google Scholar]
- 191.Laird JH, Lok BH, Ma J, et al. Talazoparib is a potent radiosensitizer in small cell lung cancer cell lines and xenografts. Clin Cancer Res. 2018;24(20):5143–5152. doi: 10.1158/1078-0432.CCR-18-0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gonzalez-Gonzalez A, Munoz-Muela E, Marchal JA, et al. Activating transcription factor 4 modulates TGFbeta-induced aggressiveness in triple-negative breast cancer via SMAD2/3/4 and mTORC2 signaling. Clin Cancer Res. 2018;24(22):5697–5709. doi: 10.1158/1078-0432.CCR-17-3125 [DOI] [PubMed] [Google Scholar]
- 193.Marangoni E, Laurent C, Coussy F, et al. Capecitabine efficacy is correlated with TYMP and RB1 expression in PDX established from triple-negative breast cancers. Clin Cancer Res. 2018;24(11):2605–2615. doi: 10.1158/1078-0432.CCR-17-3490 [DOI] [PubMed] [Google Scholar]
- 194.Arango NP, Yuca E, Zhao M, et al. Selinexor (KPT-330) demonstrates anti-tumor efficacy in preclinical models of triple-negative breast cancer. Breast Cancer Res. 2017;19(1):93. doi: 10.1186/s13058-017-0878-6 [DOI] [PMC free article] [PubMed] [Google Scholar]