Abstract
Purpose
Anterior vertebral body tethering (VBT) is a non-fusion, minimally invasive, growth-modulating procedure with some early positive clinical outcomes reported in pediatric patients with idiopathic scoliosis (IS). VBT offers potential health-related quality of life (HRQoL) benefits over spinal fusion in allowing patients to retain a greater range of motion after surgery. We conducted an early cost-utility analysis (CUA) to compare VBT with fusion as a first-choice surgical treatment for skeletally immature patients (age >10 years) with moderate to severe IS, who have failed nonoperative management, from a US integrated healthcare delivery system perspective.
Patients and Methods
The CUA uses a Markov state transition model, capturing a 15-year period following index surgery. Transition probabilities, including revision risk and subsequent fusion, were based on published surgical outcomes and an ongoing VBT observational study (NCT02897453). Patients were assigned utilities derived from published patient-reported outcomes (PROs; SRS-22r mapped to EQ-5D) following fusion and the above VBT study. Index and revision procedure costs were included. Probabilistic (PSA) and deterministic sensitivity analyses (DSA) were performed.
Results
VBT was associated with higher costs but also higher quality-adjusted life years (QALYs) than fusion (incremental costs: $45,546; QALYs gained: 0.54). The subsequent incremental cost-effectiveness ratio for VBT vs fusion was $84,391/QALY gained. Mean PSA results were similar to the base case, indicating that results were generally robust to uncertainty. The DSA indicated that results were most sensitive to variations in utility values.
Conclusion
This is the first CUA comparing VBT with fusion in pediatric patients with IS and suggests that VBT may be a cost-effective alternative to fusion in the US, given recommended willingness-to-pay thresholds ($100,000–$150,000). The results rely on HRQoL benefits for VBT compared with fusion. For improved model accuracy, further analyses with longer-term PROs for VBT, and comparative effectiveness studies, would be needed.
Keywords: idiopathic scoliosis, cost-effective analysis, spinal fusion, vertebral body tethering, pediatric
Introduction
Idiopathic scoliosis (IS) is the most common form of structural spine deformity in pediatric patients, with reported prevalence estimates ranging between 0.47–5.2% of children.1–3 Pediatric patients with IS present with chest deformities and shoulder or waist asymmetry and may experience pain.4–6 Curves with Cobb angles of greater than 50° at skeletal maturity result in lasting deformity, impaired pulmonary function and reduced health-related quality of life (HRQoL) if left untreated.7,8 Despite this, there are limited definitive treatment options available for patients who are skeletally immature other than bracing or spinal fusion.9
When bracing has failed, or if the spinal curve is rapidly progressing, surgical treatment is usually recommended for Cobb angles greater than 45–50° to prevent subsequent progression.7,10,11 The goal of surgical intervention is to correct the deformity and stabilize the spinal curve to prevent further curve progression; with spinal fusion, this is typically achieved with posterior instrumentation with rods anchored to the spine with pedicle screws (Figure 1A).5,12 Spinal fusion results in decreased spinal mobility and range of motion over the instrumented levels, with loss of motion increasing with each additional lower instrumented vertebra in pediatric patients with IS.13–16 There is a need for an intervention that can correct spinal curvature deformities in pediatric patients aged >10 years, while allowing for continued growth and preserved range of motion. Several growth-friendly devices are available, such as the growth guidance system (GGS), magnetically controlled growing rods (MCGR) and traditional growing rods (TGR). However, the use of these technologies in recent studies has been limited to younger patient populations,17–31 with current indications in the US limited to patients with early-onset scoliosis (<10 years of age),32,33 and while these technologies allow fusion to be delayed until skeletal maturity is reached, a final fusion procedure is still required.
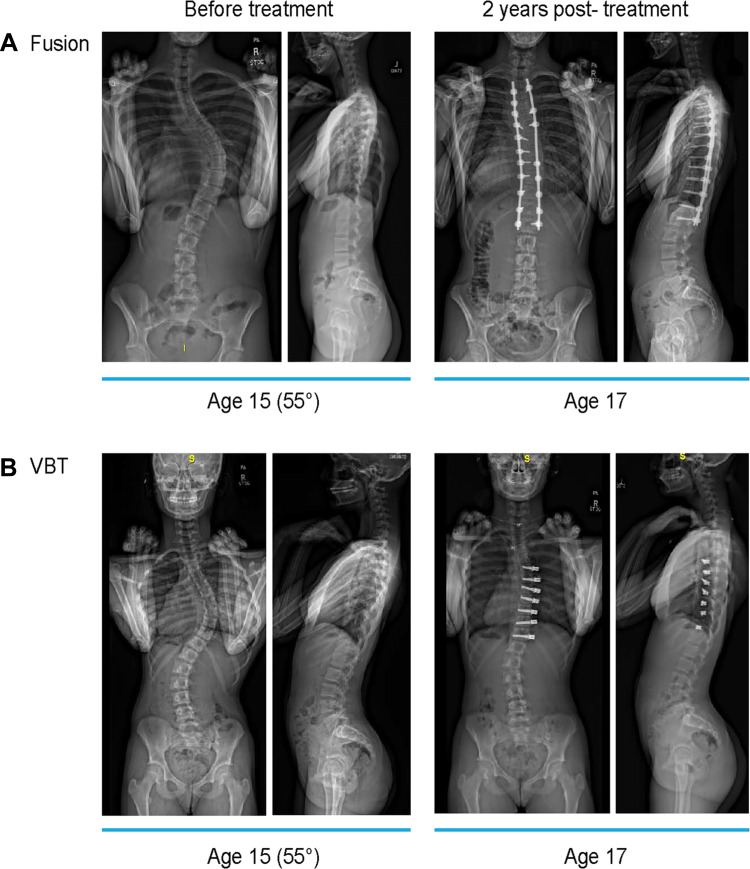
Figure 1.
Examples of pediatric patients with idiopathic scoliosis before and 2-years after surgical intervention with (A) spinal fusion or (B) VBT. (A) 15-year-old patient with 55° right thoracic curve treated with spinal fusion at 2 years postoperative follow-up. (B) 15-year-old patient with 55° right thoracic curve treated with anterior vertebral body tethering at 2 years postoperative follow-up.
Abbreviation: VBT, anterior vertebral body tethering.
Anterior vertebral body tethering (VBT) is a non-fusion, minimally invasive growth-modulating procedure that can provide treatment without the need for fusion (Figure 1B); early reports of the clinical efficacy and safety of VBT indicate some positive outcomes for skeletally immature patients aged >10 years with IS.34–38 Spinal tethering offers an alternative treatment to spinal fusion for pediatric patients aged >10 years with IS where significant continued growth is expected. Compared with spinal fusion, VBT may minimize the impact on growth while offering improved range of motion and faster return to normal activities.14 Of note, the mechanism of action of VBT relies on continued spinal growth, thus, current indications are limited to skeletally immature patients.34,39
While costs associated with spinal fusion as a treatment for pediatric patients with IS have been reported in the literature, few cost-effectiveness analyses investigating spinal fusion in IS have been performed.40–43 Additionally, to the best of our knowledge, there are no previous costing or cost-effectiveness analyses investigating VBT for the treatment of IS. VBT is associated with higher device costs compared with spinal fusion.44–47 However, given that VBT may provide benefits in terms of improved range of motion and faster return to normal activities, which may lead to gains in HRQoL for patients who receive VBT as compared with spinal fusion, there is a need for studies investigating the cost-effectiveness of these two procedures.
The objective of this research was to perform a cost-utility analysis (CUA) of spinal tethering as compared with spinal fusion to estimate the incremental differences in costs and utilities in terms of quality-adjusted life-years (QALYs) associated with treating pediatric patients with IS. Our hypothesis is that, over time, utility gains associated with spinal tethering as compared with spinal fusion would sufficiently offset the initial higher cost of the procedure to consider the technology a cost-effective use of healthcare resources from the perspective of an integrated US health care delivery system.
Patients and Methods
The Consolidated Health Economics Evaluation Reporting Standards (CHEERS) statement was followed for reporting the findings of this analysis.48
Decision Problem
The aim of the analysis was to investigate whether VBT is cost-effective as a first-choice surgical treatment option for pediatric patients with moderate to severe IS who have failed nonoperative management, from a US perspective. To address this decision problem, a CUA was undertaken to compare VBT to spinal fusion from the perspective of the US integrated healthcare delivery system (IDS). A CUA is appropriate since differences in both costs and HRQoL may be expected between VBT and spinal fusion.
A willingness-to-pay threshold (WTP) of $100,000 was chosen, corresponding to the lower end of the range ($100,000–$150,000) used by the Institute for Clinical and Economic Review in presenting health-benefit price benchmarks, and in line with WHO-CHOICE guidelines (advocating for thresholds of 2–3 times the gross domestic product per capita).49,50
Population
The patient population chosen for the CUA are those represented by the current FDA indications for VBT which include skeletally immature patients (age >10 years), with Sanders stage ≤5, moderate to severe IS, and who have failed nonoperative management. This is in line with the eligibility criteria for the single-center, non-randomized clinical study that supported FDA approval of a first-generation VBT device (The Tether™, Zimmer Biomet, Westminster, Colorado) under an Investigational Device Exemption (IDE) application (NCT02897453).39 These eligibility criteria included Cobb angles of 30–65°, which is reflected in the current US FDA indications. However, in our clinical practice surgery is typically offered for patients with Cobb angles greater than 40°. In NCT02897453, 56% of patients had a Cobb angle >40° and 84% had a Cobb angle >35°; the mean preoperative Cobb angle was 40°.
Approval was obtained for NCT02897453 from the Western Institutional Review Board, and all patients provided written consent prior to study enrollment. The study was conducted according to the principles of good clinical practices as defined under the US FDA regulations and the International Conference on Harmonisation Guidance for Good Clinical Practice.
In our clinical experience, there are more spinal levels instrumented in spinal fusion surgery than VBT. With the lack of published comparative data, we conservatively assumed that patients would require operation over a mean of 7.625 vertebral levels, whether they received spinal fusion or VBT procedure. This was based on the number of instrumented levels reported for patients across the ongoing VBT observational study (NCT02897453), and another independent study in which patients received VBT.35,39
Comparator(s)
Spinal fusion was chosen as the relevant comparator for the CUA as it represents the current standard of care in our clinical practice in the US for pediatric patients aged >10 years with moderate to severe IS who have failed nonoperative management.
Model Structure
A Markov cohort state transition model with a quarterly cycle length was used to perform the CUA. The model was developed in Microsoft Excel version 1908. A Markov model was considered to be an appropriate and transparent choice of structure, given the potential for patients to experience, and recover from, multiple revision events.
A 15-year time horizon was chosen for the base case of the analysis, beginning with the index procedures. A long-term time horizon (lifetime) was not considered appropriate due to the immaturity of utility data available for the analysis; extensive extrapolation over such a time horizon would be associated with considerable uncertainty. However, differences in HRQoL outcomes for patients treated with VBT and spinal fusion (eg due to improved range of motion), if present upon reaching skeletal maturity, are anticipated to persist into the long term. Therefore, it was important to choose a time horizon of sufficient length to capture plausible mid- to long-term differences in HRQoL outcomes. Additional time horizons (5, 10, and 20 years) were explored in scenario analyses.
Patients entered the model in the spinal fusion or VBT index procedure health states, in which they incurred index procedure costs and were assigned preoperative utility. Patients could then transition to the postoperative health states, where no further costs were incurred, and patients were assigned mean postoperative utilities for the corresponding index procedure. Most patients remained in this state for the remainder of the time horizon. However, patients were modeled to be at risk of requiring revision procedures throughout the model time horizon and could transition to revision health states from any other health state, with the exception of a final absorbing health state where patients were not eligible for additional fusion revision procedures (“Ineligible (Fusion)” health state). In the revision health states, patients incurred revision procedure costs and were assigned preoperative utility. Patients in the VBT group were also modeled to be at risk of requiring a subsequent spinal fusion procedure.
Patients who received two spinal fusion revisions could transition to the absorbing “Ineligible (Fusion)” health state, to reflect the fact that clinicians would be unlikely to recommend more than two spinal fusion revisions in practice, even if some patients were not deriving utility benefit from their fusion procedure relative to preoperative HRQoL. This assumption thus avoids overestimating the costs of spinal fusion revisions in the long term. No such cap was placed on the number of VBT revisions that patients may receive, given that patients could “escape” to the spinal fusion health states. No death state was included in the model given that patients were young, and the procedures are not associated with a significant risk of death.43,51 Also, there is unlikely to be a difference in mortality between the procedures.52
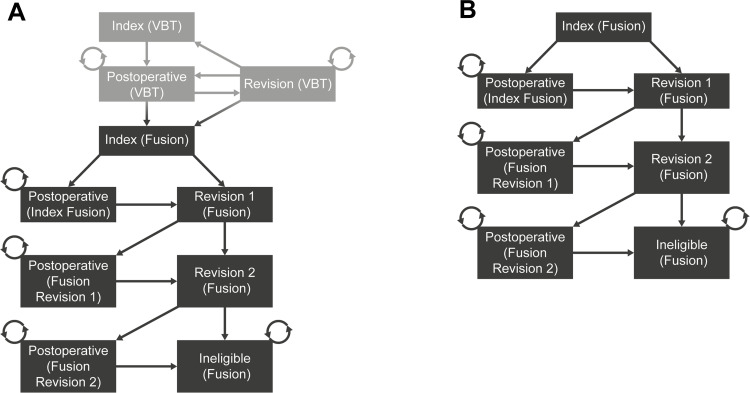
Diagrams of the model structure for patients in the VBT and spinal fusion treatment groups are provided in Figure 2. Follow-up and monitoring costs were not included based on the assumption that resource use would be similar across the VBT and spinal fusion treatment groups. Costs and disutilities associated with complications of the procedures (outside of those that necessitate revisions) were not included in the analysis, given that it was assumed that resource-use relating to the management of complications would be similar across the VBT and spinal fusion treatment groups, and the HRQoL impact of complications was already captured in the mean postoperative utilities. Costs were presented in 2020 US dollars, and costs and benefits were discounted at a rate of 3% per year, as per the Institute for Clinical and Economic Review’s 2020–2023 Value Assessment Framework.49
Figure 2.
Structure diagram. (A) VBT treatment group (B) fusion treatment group. The fusion treatment arm structure is same across both groups (dark grey boxes).
Abbreviation: VBT, anterior vertebral body tethering.
Model Inputs
Model inputs were derived, where possible, from the most relevant values identified in the published literature. Three categories of inputs were estimated: transition probabilities, health state utility weightings, and cost and resource use inputs. Model input values can be found in Table 1 (see also Supplementary Tables 1–4).
Table 1.
Model Parameter Estimates
| Parameter | Estimate | Source |
|---|---|---|
| Quarterly probability of VBT revision | 0.77%a (equivalent to a cumulative 2-year revision probability of 6.02%) | NCT02897453, Hoernschemeyer et al35,39 |
| Quarterly probability of spinal fusion revision (patients with spinal fusion index or revision procedure in previous quarter), Quarterly probability of spinal fusion revision (patients without spinal fusion index or revision procedure in previous quarter) | 0.22%b, 1.69%b (equivalent to a 2-year cumulative revision probability of 3.2%) | Ahmed et al40 |
| Quarterly probability of requiring spinal fusion index procedure for patients in the VBT treatment group (with VBT revision in previous quarter), Quarterly probability of requiring spinal fusion index procedure for patients in the VBT treatment group (without VBT revision in previous quarter) | 1.18%a, 0.19%a (equivalent to a 2-year cumulative probability of spinal fusion following VBT index procedure of 2.5%) | NCT02897453, Hoernschemeyer et al35,39 |
| Preoperative utilities (VBT and spinal fusion) | 0.783 | Aghdasi et al 2020, using SRS-22r to EQ-5D mapping algorithm from Wong et al43,51 |
| Postoperative VBT utility | 0.925 | NCT02897453, using SRS-22r to EQ-5D mapping algorithm from Wong et al39,51 |
| Postoperative spinal fusion utility | 0.875 | Aghdasi et al 2020, using SRS-22r to EQ-5D mapping algorithm from Wong et al43,51 |
| Index VBT cost | $79,231c | See Supplementary Table 3 VBT index procedure cost calculations |
| Index spinal fusion, fusion revision cost (assumed equal in base case) | $45,816d | See Supplementary Table 1 fusion index procedure cost calculations |
| Non-device costs, all procedures | $28,616e | See Supplementary Table 3 VBT index procedure cost calculations |
| Fusion device costs | $17,200 | An independent survey of spinal surgeons in the US; value also aligns with existing fusion costing studies44–47 |
| Index VBT device costs | $50,615c | See Supplementary Table 3 VBT index procedure cost calculations |
| VBT revision device costs | $8,804f | See Supplementary Table 3 VBT index procedure cost calculations |
Notes: aSee Supplementary Table 4. bSee Supplementary Table 2. cSee Supplementary Table 3. dSee Supplementary Table 1. eCalculated by subtracting spinal fusion device costs from index spinal fusion procedure costs, non-device costs assumed same for all procedures. fCalculated assuming requirement of 1 cord in 50% of revisions, and 2 anchors in 50% of revisions.
Abbreviations: EQ-5D, EuroQol 5-Dimension; SRS-22r, Scoliosis Research Society Outcomes 22-Item Questionnaire; VBT, anterior vertebral body tethering.
Transition Probabilities
To derive VBT revision rates, revision event data were pooled from the 88 patients included across NCT02897453 and Hoernschemeyer et al35,39 Calculations used to derive these rates are presented in Supplementary Table 1 A constant rate of VBT revision was assumed, allowing for the calculation of quarterly revision rates.
To derive spinal fusion revision rates, data were used from Ahmed et al, a large prospective study of fusion outcomes.40 Ahmed et al reported actuarial fusion survival at time points of 3 months, and 1, 2, 5, and 10 years after index surgery, where any spine re-operation was defined as a terminal event. The population of the study consisted of 1435 pediatric and young adult patients, with an average age of 15 years at surgery, who had spinal fusion to treat IS.40 Cobb angle and Sanders stage were not reported for this population. Calculations used to derive the spinal fusion revision rates are presented in Supplementary Table 2. The rate of spinal fusion revision by the 3-month time point was used to inform quarterly revision probabilities in the model for patients who had an index or revision procedure in the previous quarter. A constant rate of spinal fusion revision was assumed for 3 months to 10 years postoperatively and was used to inform quarterly revision probabilities in the model for patients who had not had an index or revision procedure in the previous quarter.
For both the VBT and spinal fusion revision probabilities, it was necessary to extrapolate revision probabilities, by assuming that they could be applied, without adjustment, across the full 15-year model time horizon. As specified, Ahmed et al had a maximum follow-up of 10 years, whereas NCT02897453 had a mean follow-up of 4.8 years (range: 2–7 years), and Hoernschemeyer et al 3.2 years (range: 2.2–5.2 years).35,39 Since re-revision rates were not identified in the literature, it was assumed that the rate of VBT or spinal fusion revision was independent of the number of prior revisions.
Transition probabilities from VBT health states to the spinal fusion index procedure health state were calculated using the rate at which patients with VBT underwent subsequent fusion in the pooled population of NCT02897453 and Hoernschemeyer et al.35,39 Based on our clinical experience, we assumed that patients could not have a spinal fusion index procedure within one-quarter of a VBT index procedure. Separate probabilities were calculated for patients who had just had an operation (eg revision VBT procedure in the previous quarter), and those who had not, based on follow-up for patients with and without prior VBT revisions in the pooled population of NCT02897453 and Hoernschemeyer et al, respectively.35,39 Due to a lack of data on HRQoL and revision rates experienced by patients that received spinal fusion following VBT, these patients were modeled identically to patients in the spinal fusion treatment group.
Heath State Utility Weighting
Three utility weightings were applied to health states in the model; preoperative utility, a mean postoperative utility following VBT and a mean postoperative utility following spinal fusion. Data from NCT02897453 were used to derive the mean postoperative VBT utility, and data from Aghdasi et al to derive the mean postoperative spinal fusion and preoperative utilities.39,43 This was a large meta-analysis that reported preference-based outcomes for 1494 pediatric and young adult patients that underwent spinal fusion for the treatment of IS, with a mean age of 14.6 years.43 Cobb angle and Sanders stage was not reported for this population.
Scoliosis Research Society Outcomes Questionnaire (SRS-22r) scores were reported for both the NCT02897453 and Aghdasi et al cohorts.39,43 For VBT, scores from the NCT02897453 cohort were reported over a range of time points, from 27–89 months after index surgery.39 For spinal fusion, Aghdasi et al reported three sets of mean scores from their cohort, the first at 24 months prior to index surgery, the second at 24 months after index surgery, and the third over the range >60 months after index surgery.43
An algorithm from Wong et al was used to convert these scores to EuroQol 5-Dimension (EQ-5D) index scores.51 “Model 2” from this publication was chosen based on available data and goodness-of-fit statistics. In the base case of the model, the algorithm was applied to the mean SRS-22r outcome scores calculated from all reports in the NCT02897453 cohort to generate postoperative VBT utility.39 To generate the postoperative spinal fusion utility, the algorithm was applied to a weighted average (based on number of reports) of the mean SRS-22r outcome scores from Aghdasi et al 24 months after index surgery and >60 months after index surgery.43 Given that patients eligible for VBT would otherwise receive spinal fusion in clinical practice and that preoperative utility data were not available from NCT02897453, we assumed that preoperative utility for patients eligible for spinal fusion would be applicable to the modelled population. Therefore, we applied the algorithm to the mean SRS-22r outcome scores at 24 months prior to index surgery from Aghdasi et al to generate preoperative utility.43
Since the latest reports from NCT02897453 and the majority of reports from Aghdasi et al were recorded at <8 years after index surgery, we assumed that the postoperative utilities derived from these sources would persist across the 15-year base case time-horizon of the model.39,43 However, scenario analyses were performed in which time-dependent postoperative utilities were applied, based on variation in the mean SRS-22r scores across the two postoperative timepoints in Aghdasi et al.43
Costs and Resource Use Inputs
The CUA was conducted from the perspective of the US IDS, and as described above, costs due to follow-up and monitoring, and the treatment of complications (other than those necessitating revisions), were not considered. Costs were included for VBT and spinal fusion index and revision procedures.
Medicare payments were used as a proxy for provider costs, as per the cost analysis by Luhmann et al.42 The cost of an index spinal fusion procedure was calculated as the sum of hospital inpatient facility costs and physician professional fees associated with the procedure. Hospital inpatient facility costs were based on Medicare diagnosis-related group (DRG) data, and physician fees on current procedural terminology (CPT) data.53,54 The calculations used to derive the total cost of the index spinal fusion procedure are detailed in Supplementary Table 1.
Due to a lack of available data, revision of spinal fusion was assumed to have the same cost as the index procedure. In practice, some revision spinal fusion procedures may have lower costs if constructs are not replaced. A scenario analysis was performed in which revision spinal fusion procedure costs were reduced to address this possibility.
The cost of an index VBT procedure was calculated based on the assumption that the non-device costs of the index VBT procedure are equal to the non-device costs of the index spinal fusion procedure. The calculations used to derive the cost of the index VBT procedure are detailed in Supplementary Table 3.
Since the inpatient DRG payments for an index spinal fusion procedure are bundled to include the spinal fusion device cost, the non-device costs for the spinal fusion procedure were calculated by subtracting an estimated spinal fusion device cost from the index fusion procedure cost. The estimate of $17,200 was based on an independent survey of spinal surgeons in the US and is in line with existing fusion costing studies.44–47 The VBT device cost was calculated based on unit costs for VBT device components and mean component usage recorded across NCT02897453 and Hoernschemeyer et al.35,39 Unit costs for VBT device components were based on list prices given by the manufacturer of a first-generation VBT device.
The cost of revision of VBT was calculated based on the following assumptions: non-device costs are identical to the index procedure; new VBT cords are required in 50% of revisions, and 2 additional levels are operated on in 50% of revisions. This resulted in a reduced cost relative to the VBT index procedure. These assumptions were made based on NCT02897453 and the Hoernschemeyer et al study, in which roughly half of VBT revision procedures required only a cord cut and no additional implant costs.35,39
Sensitivity Analyses
A probabilistic and a deterministic sensitivity analysis (PSA and DSA, respectively) were conducted to test the robustness of the model results. For the DSA, inputs were varied by the standard deviation when available. Standard deviation was chosen to reflect a suitable degree of variation in these inputs; standard error was judged to result in too little variation to demonstrate the sensitivity of the results to the inputs. In the absence of data on standard deviation for inputs, inputs were varied by ±20%. The standard deviation for all utility weightings was set at 7% of the mean, based on the standard deviation calculated for the postoperative VBT utility.43 VBT-to-revision and VBT-to-fusion transition probabilities that could not be varied in the DSA (as they formed sets of co-dependent probabilities) were varied manually. Standard distributions for health economic methodology were used in the PSA, and 1000 simulations were run.55
Scenario Analyses
Scenario analyses were performed to investigate the impact of alternative assumptions and input values. The results of the DSA were used to inform the selection of scenario analyses, with priority given to those inputs and assumptions to which the model results were most sensitive. These included the postoperative utility weightings and index procedure costs.
The preoperative utility weightings and revision procedure costs were shown to have little impact on results when varied in the DSA. Also, data were not available to support alternative assumptions or inputs for these parameters. Therefore, scenario analyses were not performed focusing on these parameters. Since the preferences of healthcare providers and payers for more immediate returns on investment may vary, both the time horizon and discounting rates (benefits and costs) were varied in scenario analyses. The time horizon was varied to 5, 10, and 20 years, and discounting rates were varied to 0 and 5%. Two scenario analyses were performed investigating alternative assumptions for utility weightings, where data were available to support alternative assumptions and inputs. Firstly, an alternative SRS-22r to EQ-5D mapping algorithm with a similar goodness of fit, “Model 1” from Wong et al, was utilized to calculate the utility inputs.51 Secondly, the mean SRS-22r outcome scores over the range >60 months after index surgery from Aghdasi et al were used to calculate a postoperative spinal fusion utility that was applied after 5 years in the model, rather than applying a utility based on the weighted average of scores at 24 months and >60 months throughout the model time-horizon.43
A costing scenario was performed in which non-device costs for VBT procedures were assumed to be 80% of non-device costs for spinal fusion procedures, as opposed to 100%. This scenario was performed because VBT procedures may have lower non-device costs than spinal fusion procedures. An additional costing scenario was performed in which revision of spinal fusion costs was reduced by 50% to reflect the fact that some fusion revision procedures may have reduced costs if constructs are not replaced. Finally, a set of scenarios were performed in which the VBT revision rates were changed. Quarterly VBT revision rates were varied to produce cumulative 2-year VBT revision probabilities of 10%, 20%, and 40% per patient. The upper limit of these probabilities, 40%, was based on a retrospective study from Newton et al where 7 of 17 patients underwent revisions over a mean follow-up of 2.5 years (range: 2–4 years).56
Results
Base Case Analyses
In the base case analysis, over the 15-year time horizon, VBT was associated with an estimated 11.30 total discounted QALYs as compared with 10.76 for spinal fusion, an incremental gain of 0.54 (Table 2). The total discounted costs were estimated to be $96,897 for VBT whereas the total costs considered for spinal fusion were estimated to be $51,351, a difference of $45,546. The resulting incremental cost-effectiveness ratio (ICER) for VBT versus spinal fusion was $84,391. At a WTP threshold of $100,000 per QALY gained, the higher costs associated with VBT were offset by the higher QALYs gained, yielding a net monetary benefit (NMB) of $8424.
Table 2.
Cost-Effectiveness in the Base Case Analysis
| VBT | Spinal Fusion | |
|---|---|---|
| Total costs ($) | $96,897 | $51,351 |
| Total QALYs | 11.30 | 10.76 |
| Incremental costs ($) | $45,546 | – |
| Incremental QALYs | 0.54 | – |
| ICER versus fusion ($/QALY gained) | $84,391 | – |
| NMBa | $8424 | – |
Note: aNMB calculated at a willingness-to-pay threshold of $100,000.
Abbreviations: ICER, incremental cost-effectiveness ratio; NMB, net monetary benefit; QALYs, quality-adjusted life years; VBT, anterior vertebral body tethering.
Sensitivity Analyses
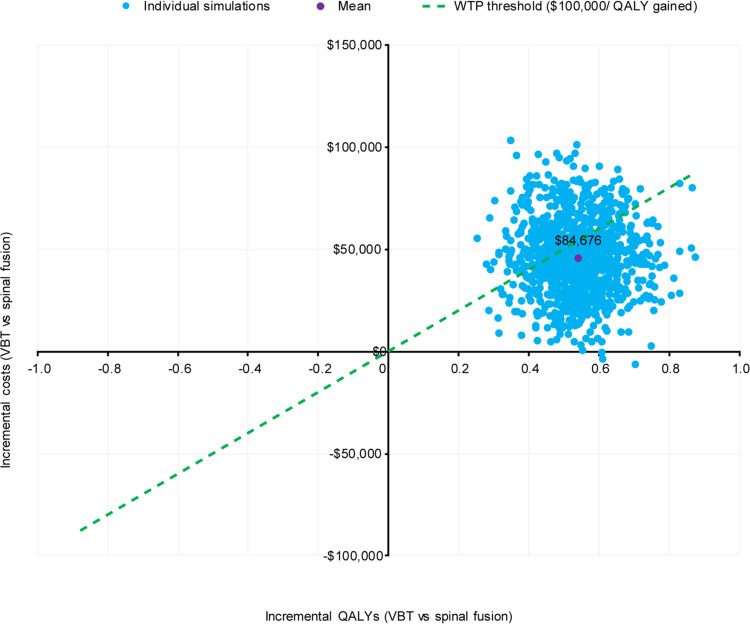
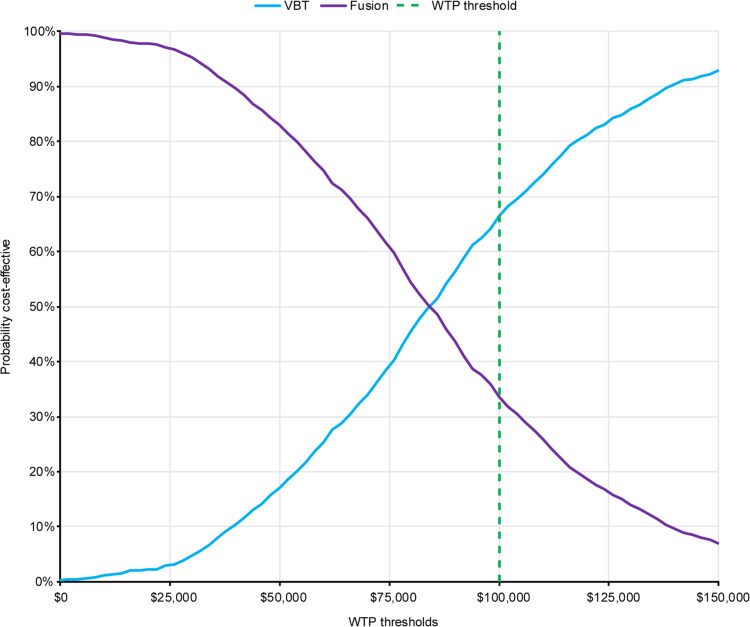
A scatterplot of 1000 probabilistic simulations is presented in Figure 3, revealing a low level of variability in the results. At a WTP threshold of $100,000 per QALY gained, VBT was cost-effective in 66.4% of simulations (Figure 4). The mean probabilistic results were similar to the base case results, with incremental costs and QALYs of $45,700 and 0.54, respectively, resulting in an incremental cost per QALY gained of $84,676 for VBT as compared with spinal fusion.
Figure 3.
PSA Scatterplot of 1000 simulations on an incremental cost-effectiveness plane. Dashed line indicates WTP threshold used in this analysis, corresponding to the lower end of the range recommended by the WHO-CHOICE guidelines.49,50
Abbreviations: PSA, probabilistic sensitivity analysis; QALYs, quality-adjusted life years; VBT, anterior vertebral body tethering; WHO, World Health Organization; WTP, willingness-to-pay.
Figure 4.
Cost-effectiveness acceptability curve. Dashed line indicates WTP threshold used in this analysis, corresponding to the lower end of the range recommended by the WHO-CHOICE guidelines.49,50
Abbreviations: VBT, anterior vertebral body tethering; WHO, World Health Organization; WTP, willingness-to-pay.
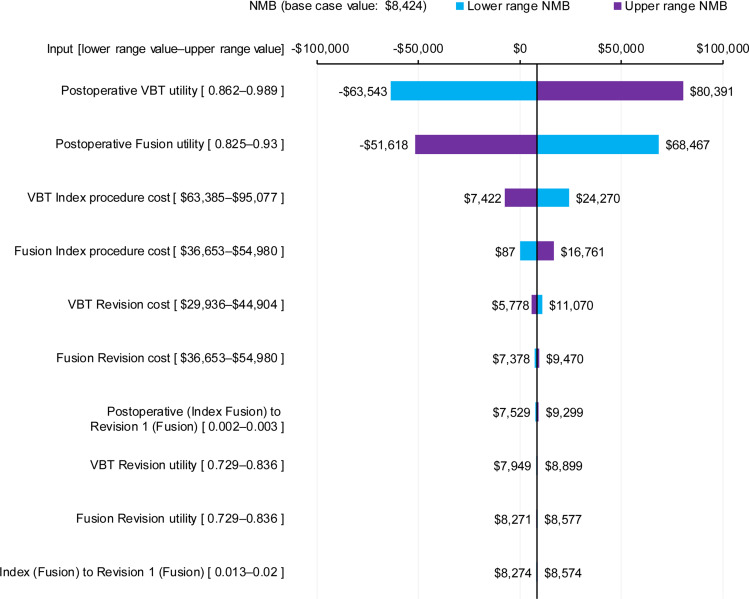
The DSA indicated that cost-effectiveness results were most sensitive to variation in the postoperative utility weightings (Figure 5), followed by index procedure costs for VBT and spinal fusion. A threshold analysis found that a minimum VBT postoperative utility of 0.918 (base case value 0.925) was required for an ICER below $100,000 (provided the base case fusion postoperative utility of 0.877 is used), equating to a difference of 0.041 between the postoperative VBT and fusion utility weightings. The results were less sensitive to variations in the transition probabilities, including revision probabilities, the preoperative utility weightings, and the cost of revision procedures. Manual variations in the VBT revision probability and spinal fusion probabilities for patients in the VBT group were found to have little effect on model results. Varying the VBT revision probability by ±20%, while keeping the spinal fusion probabilities constant, produced ICERs of $89,801 and $79,032, respectively. Varying the aforementioned spinal fusion probabilities by ±20%, while keeping VBT revision probabilities constant, produced ICERs of $86,667 and $82,124, respectively.
Figure 5.
One-way sensitivity analysis. Sensitivity of NMB (based on a WTP threshold of $100,000/QALY) to changes in top 10 model parameters; lowering the parameter indicated in blue, increasing the parameter indicated in purple. Postoperative (Index Fusion) to Revision 1 (Fusion) is the probability of revision without prior spinal fusion in the last three months; Index (Fusion) to Revision 1 (Fusion) is the probability of revision with prior spinal fusion in the last three months. Please note that the VBT revision probabilities were not varied in the DSA, as they could not be varied in isolation of other independent parameters; these probabilities were instead varied manually, and the results are reported in the sensitivity analyses section.
Abbreviations: NMB, net monetary benefit; DSA, deterministic sensitivity analysis; QALYs, quality-adjusted life years; VBT, anterior vertebral body tethering; WTP, willingness-to-pay.
Scenario Analyses
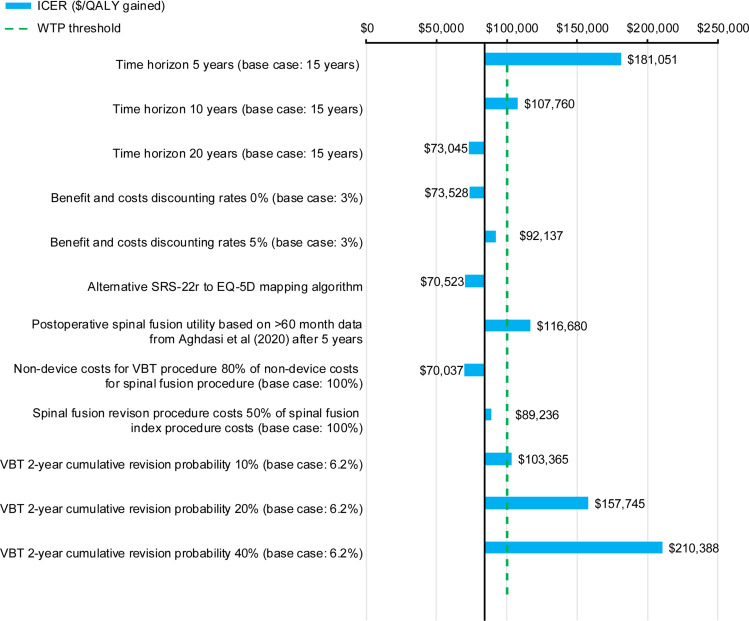
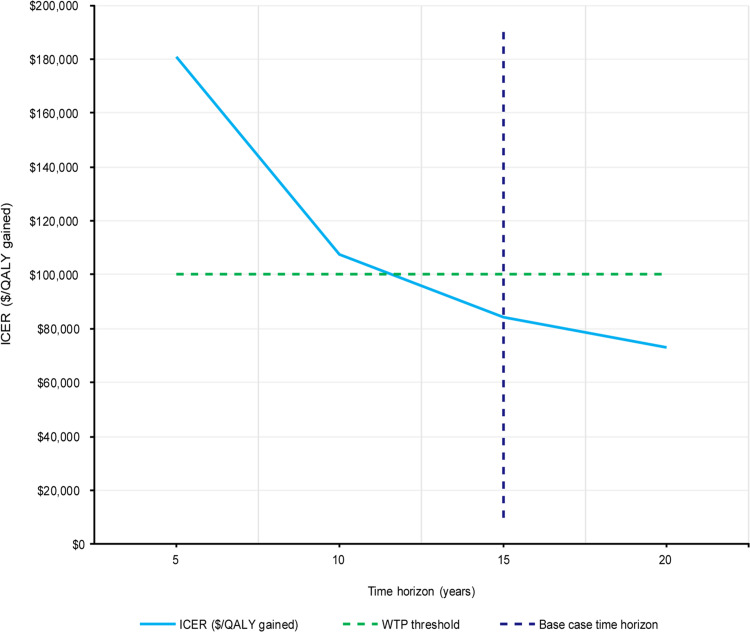
The results of scenario analyses that were performed are presented in Figure 6. Varying the 15-year time horizon over which costs and QALYs were accrued had a large effect on the ICER. Time horizons of 5 years, 10 years, and 20 years produced ICERs of $181,051, $107,760 and $73,045, respectively. VBT becomes cost-effective as compared with spinal fusion within the 12th year after index procedure. The dependency of the ICER on the length of time horizon is presented in Figure 7; extending the time horizon was shown to lower the ICER. Varying the discounting rates for costs and benefits from 3% to 0% was favorable for VBT, while increasing these rates to 5% had the opposite effect, producing ICERs of $73,528 and $92,131, respectively. Using the alternative SRS-22r to EQ-5D mapping algorithm from Wong et al produced an ICER of $70,523.51 Applying 5-year postoperative spinal fusion utility after the 5-year time point produced an ICER of $116,680. The costing scenario in which non-device costs for VBT procedures were set equal to 80% of non-device costs for spinal fusion procedures produced an ICER of $70,037. The costing scenario in which fusion revision procedure costs were reduced by 50% produced an ICER of $89,236. The scenarios in which the quarterly VBT revision rates were varied to produce cumulative 2-year VBT revision probabilities of 10%, 20%, and 40% produced ICERs of $103,366, $157,745 and $310,388, respectively. A threshold analysis was performed indicating that a cumulative 2-year VBT revision probability of 9.3% or below was required to produce an ICER below $100,000, exceeding the base case value of 6.02%.
Figure 6.
Scenario analysis ICERS. Dashed line indicates WTP threshold used in this analysis, corresponding to the lower end of the range recommended by the WHO-CHOICE guidelines.49,50
Abbreviations: EQ-5D, EuroQol 5-Dimension; ICERs, incremental cost-effectiveness ratios; QALYs, quality-adjusted life years; SRS-22r, Scoliosis Research Society Outcomes 22-Item Questionnaire; VBT, anterior vertebral body tethering; WHO, World Health Organization; WTP, willingness-to-pay.
Figure 7.
ICER versus time horizon. Vertical dashed line indicates the 15-year time horizon used in the base case; horizontal dashed line indicates WTP threshold used in this analysis, corresponding to the lower end of the range recommended by the WHO-CHOICE guidelines49,50
Abbreviations: ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life years; WHO, World Health Organization; WTP, willingness-to-pay.
Discussion
The results of this analysis suggest that, from the perspective of the US IDS, VBT may be a cost-effective treatment compared with fusion for pediatric patients with IS. The deterministic and probabilistic ICERs of $84,391 and $84,676 per QALY gained, respectively, are below the recommended US WTP threshold of $100,000.57 At this WTP threshold, VBT was cost-effective in 66.4% of probabilistic iterations, suggesting that the results are robust with respect to combined parameter uncertainty, although the limitations associated with studies reporting clinical outcomes for the VBT procedure should be considered in the interpretation of these findings.
At the time of these analyses, studies reporting clinical outcomes for the VBT procedure include relatively small patient numbers,34–38 with a small subset of those who reported key patient-reported outcomes34,35 needed for a cost-effectiveness analysis as compared to the extensive reporting of studies describing spinal fusion for pediatric patients with IS spanning decades and large sample sizes.7,43 Also, studies reporting clinical outcomes for the VBT procedure provide a maximum length of follow-up of 7 years, whereas the base case time horizon for the CUA was 15 years.
The mean postoperative utility weightings following VBT and spinal fusion are the key sources of uncertainty in the cost-effectiveness results. Clinical plausibility for the differences in postoperative utility weightings includes the difference in mechanism of action between the two surgical interventions, with spinal fusion inherently limiting mobility and VBT’s tethering mechanism relying on (and therefore not hindering) continued growth and range of motion. VBT has the potential to increase mobility and range of motion as compared to spinal fusion,14 which may result in improved HRQoL in the long term, underpinning the differences in postoperative utility weightings.
The VBT revision rate scenarios produced ICERs that were considerably larger than the base case ICER. However, these scenarios modeled much higher VBT revision rates compared with those observed from the 88 patients included across NCT02897453 and Hoernschemeyer et al.35,39 The retrospective study from Newton et al which informed these scenarios involved a much smaller sample size (17 versus 88), and thus the revision rate observed in this study is subject to greater uncertainty.56 Insufficient data were available from this study to pool alongside NCT02897453 and Hoernschemeyer et al.
This analysis differs in several ways from previous analyses of surgical correction for IS. Most notably, to the best of our knowledge no previous cost-effectiveness analyses have been published for VBT. Additionally, there exist very few cost-effectiveness analyses for surgical treatment of IS across the literature; previous analyses of surgical interventions are generally limited to costing analyses and do not consider differences in utility, and thus there is a lack of precedence for what constitutes appropriate methodology for cost-effectiveness analysis in this setting.41,42,58,59
Finally, our analysis evaluated costs and benefits over a 15-year time horizon in the base case, whereas other recent cost analyses for pediatric scoliosis report on 6-year time horizons,41,42 or shorter.58,59 A longer time horizon was appropriate for the present study because although the majority of costs of surgical interventions in IS are likely to be accrued in a short period following the index surgery, clinical benefits, such as range of motion and return to normal activities, may plausibly be much longer lasting. Also, the time horizon was selected to align with the consensus-based recommendations from the US panel on Cost-effectiveness in Health and Medicine.57,60
Strengths
This was the first CUA conducted for VBT. Where assumptions were required, consideration was given to their clinical plausibility based on our clinical experience in the US, and conservative assumptions were made where necessary. For example, our results suggest that VBT may be cost-effective as compared to spinal fusion even though the WTP threshold chosen represents the conservative lower range of recommended thresholds, as per ICER and WHO guidelines.49,50
Uncertainty in the model was explored through DSA and PSA. Additionally, the impacts of key assumptions and inputs to which the model was particularly sensitive were tested with scenario analyses. Scenario analyses in general revealed a low amount of variability. Although the results were very sensitive to the length of time horizon selected, this was expected as VBT has larger upfront costs and a long-term utility benefit compared to spinal fusion.
Limitations
This CUA would benefit from further research into postoperative utilities, particularly VBT postoperative utility, as there were uncertainties in their estimation, and they were shown to have a large impact on model results when varied in the DSA. Uncertainties derive from the small sample size of NCT02897453 (N=57) that was used to determine the VBT postoperative utility. Additional uncertainties arise from differences between the patient populations used to derive the spinal fusion and VBT postoperative utilities.39,43
While fusion represents the current standard of care in the US for pediatric patients aged >10 years of age who have failed non-operative management, fusion is not limited to skeletally immature patients and is therefore used in a broader patient population than VBT. As this is the first CUA for VBT and no head-to-head prospective studies have been conducted for VBT versus fusion, data for the target patient population are limited. Thus, the inputs for VBT and fusion used in this model are derived from populations that differ in several respects. For example, the mean age at the time of surgery for patients in NCT02897453 was 12.4 years, and the mean age at the time of surgery for patients undergoing spinal fusion in the Aghdasi et al cohort was 14.6 years. It is unclear whether these populations can be considered fully comparable. No adjustments were made to account for factors such as age difference between the cohorts or curve magnitude, which is typically greater in cohorts undergoing spinal fusion. It is worth noting that utilities, as measured by SRS-22r, have been reported to reduce as children progress through adolescence.61 The patient population was defined as per current US FDA indications based on Sanders stage and Cobb angle, which were not reported for the published populations used to derive revision rate and utility inputs.40,43 We were therefore not able to confirm a Sanders stage or Cobb angle match between our patient population and the populations used to derive these model inputs. Despite these limitations, we feel we have used the most appropriate inputs available to derive a plausible estimate and hope that our model serves as a framework for future work once more data become available.
On the other hand, revision rates of spinal fusion are higher in skeletally immature patients, and we reported fusion revision rates based on patients who were older than those undergoing VBT. Thus, revision rates for spinal fusion may be under-estimated by this model. Additionally, the current model uses a 2-year cumulative revision rate for VBT of 6.02%; however, there is a wide range of published tethering revision rates from 0–40%.35,36,56 The uncertainty in VBT revision rates may be due to small sample sizes of existing studies, follow-up timeframe variation or variability in surgeon experience; further investigation is warranted.
In our experience, the efficacy of VBT procedures may differ between thoracic/lumbar tethering, so differences in patient populations in this respect could result in differing postoperative utility independent of the intervention received, although there are limited data available to confirm. Similarly, we considered postoperative care to be equivalent between VBT and fusion as there are limited data evaluating any differences between the two procedures. Any reduction in VBT procedural costs over time would result in improvements in the cost-effectiveness estimates. Additionally, the aggregate data used to inform the model comprised all Lenke curve types, and VBT may only be appropriate for select flexible curve types such as Lenke 1 and 5 patterns.
The scenario analyses presented here highlight the need for longer-term VBT postoperative utility data. SRS-22r scores reported >60 months after index surgery in Aghdasi et al had improved relative to those reported at 24 months. Therefore, the scenario analysis where a postoperative spinal fusion utility derived from the outcome scores >60 months after index surgery was applied after 5 years in the model produced an ICER over the $100,000 WTP threshold.43 Utility data at time points >60 months were available for VBT patients in NCT02897453, however were not considered given the comparatively low number of patients being followed up beyond this time point, which would likely introduce further uncertainty around postoperative utilities.
In addition to the above, some assumptions were made in the face of uncertainty and could not be explored in scenario analyses, as there were not the data to suggest plausible alternative scenarios. These assumptions included that patients treated with VBT and subsequent fusion could be treated identically to patients treated with fusion alone, that a patient could undergo a maximum of two fusion revisions, and that revision rates were independent of the number of prior revisions. It is uncertain how well these assumptions reflect real-world practice due to a lack of available data. However, the first two assumptions were unlikely to have had a large effect because only a small proportion of patients received both index VBT and index fusion procedures or reached the Ineligible (Fusion) absorbing health state, due to the low probabilities involved. In the VBT group, 6% of patient time was spent in fusion health states, and the percentage of total patient time spent in the Ineligible (Fusion) state was less than 0.1% in either treatment group. The third assumption is unlikely to have a large effect due to the low impact that variations in revision probabilities had on model results (as demonstrated in the DSA).
Conclusion
This is the first cost-effectiveness analysis comparing VBT with spinal fusion in pediatric patients with IS. The results of the analysis suggest that VBT may be considered cost-effective compared with spinal fusion from the US IDS perspective if a decision-maker is willing to pay at least $84,391 per QALY gained, as VBT provides additional health gains, which represent efficient resource use despite higher initial costs. On threshold analysis of revision surgery rates, VBT remained cost-effective up to a 2-year cumulative revision probability of 9.3%. With base case assumptions, VBT became cost-effective in the 12th year after index surgery, with a cumulative 2-year revision rate of 6.02% for VBT and 3.2% for spinal fusion. Further analyses with larger VBT sample sizes, longer-term VBT data, and head-to-head studies are warranted to help address remaining uncertainties and inform decision-making for the most appropriate treatment for pediatric patients.
Acknowledgments
The authors thank the patients and their caregivers, as well as the investigators and their teams who took part in the NCT02897453 study. This study was funded by Zimmer Biomet. This work was presented at the 14th International Congress on Early Onset Scoliosis (virtual, November 14, 2020).
Data Sharing Statement
The authors are not able to share the data from the IDE study (NCT02897453) for a number of reasons. These include that the information is part of an ongoing FDA IDE study in support of a product approval; these data are proprietary and solely owned by Zimmer Biomet and Shriners Hospital.
Disclosure
DWP is consultant for SI-Bone, Globus; reports royalties from Springer; royalties to institution from Globus and Medtronic; research support from Medtronic, Mizuho OSI, and AO Spine. ANL is consultant with research support from Globus, Medtronic, DePuy Synthes and Orthopediatrics. In addition, ANL has a patent US10667845 B2 issued. AFS is a paid consultant for DePuy, Ethicon, Globus Medical, Misonix, Picara, Mirius, and Stryker; paid consultant and IP royalties from NuVasive, Zimmer Biomet; Board or committee member of Pediatric Spine Study Group and Setting Scoliosis Straight Foundation. WR, HB, AP, WM are employees of Costello Medical Consulting Ltd; report consultancy fees from Zimmer Biomet for development of the model, editorial support and publication coordination. RD is an employee and shareholder of Zimmer Biomet. The authors report no other conflicts of interest in this work.
References
- 1.Cheng JC, Castelein RM, Chu WC, et al. Adolescent idiopathic scoliosis. Nat Rev Dis Primers. 2015;1(1):15030. doi: 10.1038/nrdp.2015.30 [DOI] [PubMed] [Google Scholar]
- 2.Dunn J, Henrikson NB, Morrison CC, et al. Screening for adolescent idiopathic scoliosis: evidence report and systematic review for the US preventive services task force. JAMA. 2018;319(2):173–187. doi: 10.1001/jama.2017.11669 [DOI] [PubMed] [Google Scholar]
- 3.Konieczny MR, Senyurt H, Krauspe R. Epidemiology of adolescent idiopathic scoliosis. J Child Orthop. 2013;7(1):3–9. doi: 10.1007/s11832-012-0457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20(2):274–279. doi: 10.1007/s00586-010-1657-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helenius L, Diarbakerli E, Grauers A, et al. Back pain and quality of life after surgical treatment for adolescent idiopathic scoliosis at 5-year follow-up: comparison with healthy controls and patients with untreated idiopathic scoliosis. J Bone Joint Surg Am. 2019;101(16):1460–1466. doi: 10.2106/JBJS.18.01370 [DOI] [PubMed] [Google Scholar]
- 6.Ramirez N, Johnston CE, Browne RH. The prevalence of back pain in children who have idiopathic scoliosis. J Bone Joint Surg Am. 1997;79(3):364–368. doi: 10.2106/00004623-199703000-00007 [DOI] [PubMed] [Google Scholar]
- 7.Larson AN, Baky F, Ashraf A, et al. Minimum 20-year health-related quality of life and surgical rates after the treatment of adolescent idiopathic scoliosis. Spine Deform. 2019;7(3):417–427. doi: 10.1016/j.jspd.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 8.Weinstein SL, Zavala DC, Ponseti IV. Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Joint Surg Am. 1981;63(5):702–712. doi: 10.2106/00004623-198163050-00003 [DOI] [PubMed] [Google Scholar]
- 9.Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1(1):2. doi: 10.1186/1748-7161-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridwell KH. Surgical treatment of idiopathic adolescent scoliosis. Spine (Phila Pa 1976). 1999;24(24):2607–2616. doi: 10.1097/00007632-199912150-00008 [DOI] [PubMed] [Google Scholar]
- 11.Weiss HR, Goodall D. Rate of complications in scoliosis surgery - a systematic review of the PubMed literature. Scoliosis. 2008;3(1):9. doi: 10.1186/1748-7161-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YJ, Lenke LG, Cho SK, et al. Comparative analysis of pedicle screw versus hook instrumentation in posterior spinal fusion of adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2004;29(18):2040–2048. doi: 10.1097/01.brs.0000138268.12324.1a [DOI] [PubMed] [Google Scholar]
- 13.Danielsson AJ, Romberg K, Nachemson AL. Spinal range of motion, muscle endurance, and back pain and function at least 20 years after fusion or brace treatment for adolescent idiopathic scoliosis: a case-control study. Spine. 2006;31(3):275–283. doi: 10.1097/01.brs.0000197652.52890.71 [DOI] [PubMed] [Google Scholar]
- 14.Pahys JM, Samdani AF, Hwang SW, et al. Anterior vertebral body tethering for adolescent idiopathic scoliosis preserves trunk motion compared to posterior spinal fusion at two year follow-up. Scoliosis Research Society Annual Meeting. Virtual, Spine Deformity; 2020. [Google Scholar]
- 15.Helenius I, Remes V, Yrjönen T, et al. Comparison of long-term functional and radiologic outcomes after Harrington instrumentation and spondylodesis in adolescent idiopathic scoliosis: a review of 78 patients. Spine. 2002;27(2):176–180. doi: 10.1097/00007632-200201150-00010 [DOI] [PubMed] [Google Scholar]
- 16.Padua R, Padua S, Aulisa L, et al. Patient outcomes after Harrington instrumentation for idiopathic scoliosis: a 15- to 28-year evaluation. Spine. 2001;26(11):1268–1273. doi: 10.1097/00007632-200106010-00019 [DOI] [PubMed] [Google Scholar]
- 17.Haapala H, Saarinen AJ, Salonen A, et al. Shilla growth guidance compared with magnetically controlled growing rods in the treatment of neuromuscular and syndromic early-onset scoliosis. Spine. 2020;45(23):E1604–E1614. doi: 10.1097/BRS.0000000000003654 [DOI] [PubMed] [Google Scholar]
- 18.Luhmann SJ, McCarthy RE. A comparison of shilla growth guidance system and growing rods in the treatment of spinal deformity in children less than 10 years of Age. J Pediatr Orthop. 2017;37(8):e567–e574. doi: 10.1097/BPO.0000000000000751 [DOI] [PubMed] [Google Scholar]
- 19.Nazareth A, Skaggs DL, Illingworth KD, et al. Growth guidance constructs with apical fusion and sliding pedicle screws (SHILLA) results in approximately 1/3rd of normal T1-S1 growth. Spine Deform. 2020;8(3):531–535. doi: 10.1007/s43390-020-00076-7 [DOI] [PubMed] [Google Scholar]
- 20.Bess S, Akbarnia BA, Thompson GH, et al. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. JBJS. 2010;92(15):2533–2543. doi: 10.2106/JBJS.I.01471 [DOI] [PubMed] [Google Scholar]
- 21.Myung KS, Skaggs DL, Thompson GH, et al. Nutritional improvement following growing rod surgery in children with early onset scoliosis. J Child Orthop. 2014;8(3):251–256. doi: 10.1007/s11832-014-0586-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe K, Uno K, Suzuki T, et al. Risk factors for complications associated with growing-rod surgery for early-onset scoliosis. Spine. 2013;38(8):E464–8. doi: 10.1097/BRS.0b013e318288671a [DOI] [PubMed] [Google Scholar]
- 23.Abdelaal A, Munigangaiah S, Trivedi J, et al. Magnetically controlled growing rods in the treatment of early onset scoliosis: a single centre experience of 44 patients with mean follow-up of 4.1 years. Bone Jt Open. 2020;1(7):405–414. doi: 10.1302/2633-1462.17.BJO-2020-0099.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akbarnia BA, Pawelek JB, Cheung KM, et al. Traditional growing rods versus magnetically controlled growing rods for the surgical treatment of early-onset scoliosis: a case-matched 2-year study. Spine Deform. 2014;2(6):493–497. doi: 10.1016/j.jspd.2014.09.050 [DOI] [PubMed] [Google Scholar]
- 25.Arhewoh RE, Mo M, Luhmann SJ. Analysis of 280 magnetically controlled growing rod lengthenings comparing external remote control readouts and radiographic measurements: impact of patient and deformity factors. J Pediatr Orthop. 2021;41(2):e105–e110. doi: 10.1097/BPO.0000000000001678 [DOI] [PubMed] [Google Scholar]
- 26.Calderaro C, Labianca L, Dolan LA, et al. Early-onset scoliosis treated with magnetically controlled growing rods. Orthopedics. 2020;43(6):e601–e608. doi: 10.3928/01477447-20200910-04 [DOI] [PubMed] [Google Scholar]
- 27.Dragsted C, Fruergaard S, Jain MJ, et al. Distraction-to-stall versus targeted distraction in magnetically controlled growing rods. J Pediatr Orthop. 2020;40(9):e811–e817. doi: 10.1097/BPO.0000000000001585 [DOI] [PubMed] [Google Scholar]
- 28.Gomez JA, Mackey C, Hanstein R et al.14th International congress on early onset scoliosis: November 14, 2020. Spine Deform. 2020;8(6):1389–1422. doi: 10.1007/s43390-020-00234-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klyce W, Mitchell SL, Pawelek J, et al. Characterizing use of growth-friendly implants for early-onset scoliosis: a 10-year update. J Pediatr Orthop. 2020;40(8):e740–e746. doi: 10.1097/BPO.0000000000001594 [DOI] [PubMed] [Google Scholar]
- 30.Lorenz HM, Hecker MM, Braunschweig L, et al. Continuous lengthening potential after four years of magnetically controlled spinal deformity correction in children with spinal muscular atrophy. Sci Rep. 2020;10(1):22420. doi: 10.1038/s41598-020-79821-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obid P, Yiu K, Cheung K, et al. Magnetically controlled growing rods in early onset scoliosis: radiological results, outcome, and complications in a series of 22 patients. Arch Orthop Trauma Surg. 2020. doi: 10.1007/s00402-020-03518-z [DOI] [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration, Office of Device Evaluation. SHILLA™ Growth guidance system approval letter; July 17, 2014. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf14/K140750.pdf. Accessed January14, 2021.
- 33.U.S. Food and Drug Administration, Office of Device Evaluation. MAGEC® Spinal bracing and distraction system approval letter; February 27, 2014. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf14/k140178.pdf. Accessed January14, 2021.
- 34.Samdani AF, Ames RJ, Kimball JS, et al. Anterior vertebral body tethering for immature adolescent idiopathic scoliosis: one-year results on the first 32 patients. Eur Spine J. 2015;24(7):1533–1539. doi: 10.1007/s00586-014-3706-z [DOI] [PubMed] [Google Scholar]
- 35.Hoernschemeyer DG, Boeyer ME, Robertson ME, et al. Anterior vertebral body tethering for adolescent scoliosis with growth remaining: a retrospective review of 2 to 5-year postoperative results. J Bone Joint Surg Am. 2020;102(13):1169–1176. doi: 10.2106/JBJS.19.00980 [DOI] [PubMed] [Google Scholar]
- 36.Newton PO, Bartley CE, Bastrom TP, et al. Anterior spinal growth modulation in skeletally immature patients with idiopathic scoliosis: a comparison with posterior spinal fusion at 2 to 5 years postoperatively. J Bone Joint Surg Am. 2020;102(9):769–777. doi: 10.2106/JBJS.19.01176 [DOI] [PubMed] [Google Scholar]
- 37.Wong HK, Ruiz JNM, Newton PO, et al. Non-fusion surgical correction of thoracic idiopathic scoliosis using a novel, braided vertebral body tethering device: minimum follow-up of 4 years. JB JS Open Access. 2019;4(4):e0026. doi: 10.2106/JBJS.OA.19.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pehlivanoglu T, Oltulu I, Ofluoglu E, et al. Thoracoscopic vertebral body tethering for adolescent idiopathic scoliosis: a minimum of 2 years’ results of 21 patients. J Pediatr Orthop. 2020;40(10):575–580. doi: 10.1097/BPO.0000000000001590 [DOI] [PubMed] [Google Scholar]
- 39.Zimmer Biomet. The Tether™ Vertebral body tethering system [Instructions for Use]. U.S. Food and Drug Administration website; June 2019. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf19/H190005D.pdf. Accessed October26, 2020.. [Google Scholar]
- 40.Ahmed SI, Bastrom TP, Yaszay B, et al. 5-year reoperation risk and causes for revision after idiopathic scoliosis surgery. Spine. 2017;42(13):999–1005. doi: 10.1097/BRS.0000000000001968 [DOI] [PubMed] [Google Scholar]
- 41.Polly D Jr, Ackerman SJ, Schneider K, et al. Cost analysis of magnetically controlled growing rods compared with traditional growing rods for early-onset scoliosis in the US: an integrated health care delivery system perspective. Clinicoecon Outcomes Res. 2016;8:457–465. doi: 10.2147/CEOR.S113633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luhmann SJ, McAughey EM, Ackerman SJ, et al. Cost analysis of a growth guidance system compared with traditional and magnetically controlled growing rods for early-onset scoliosis: a US-based integrated health care delivery system perspective. Clinicoecon Outcomes Res. 2018;10:179–187. doi: 10.2147/CEOR.S152892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aghdasi B, Bachmann KR, Clark D, et al. Patient-reported outcomes following surgical intervention for adolescent idiopathic scoliosis: a systematic review and meta-analysis. Clin Spine Surg. 2020;33(1):24–34. doi: 10.1097/BSD.0000000000000822 [DOI] [PubMed] [Google Scholar]
- 44.Baky FJ, Echternacht SR, Milbrandt TA, et al. Predictors of cost for posterior spinal fusion in adolescent idiopathic scoliosis. Spine Deform. 2020;8(3):421–426. doi: 10.1007/s43390-020-00053-0 [DOI] [PubMed] [Google Scholar]
- 45.Jaquith BP, Chase A, Flinn P, et al. Screws versus hooks: implant cost and deformity correction in adolescent idiopathic scoliosis. J Child Orthop. 2012;6(2):137–143. doi: 10.1007/s11832-012-0400-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malik AT, Yu E, Kim J, et al. Understanding costs in a 90-day episode of care following posterior spinal fusions for adolescent idiopathic scoliosis. World Neurosurg. 2019;130:e535–e541. doi: 10.1016/j.wneu.2019.06.149 [DOI] [PubMed] [Google Scholar]
- 47.Workman JK, Wilkes J, Presson AP, et al. Variation in adolescent idiopathic scoliosis surgery: implications for improving healthcare value. J Pediatr. 2018;195:213–219.e3. doi: 10.1016/j.jpeds.2017.12.031 [DOI] [PubMed] [Google Scholar]
- 48.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 49.The Institute For Clinical And Economic Review. 2020–2023 value assessment framework. Available from: https://icer-review.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf. Accessed October 6, 2020.
- 50.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed]
- 51.Wong CKH, Cheung PWH, Samartzis D, et al. Mapping the SRS-22r questionnaire onto the EQ-5D-5L utility score in patients with adolescent idiopathic scoliosis. PLoS One. 2017;12(4):e0175847. doi: 10.1371/journal.pone.0175847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwan KYH, Koh HY, Blanke KM, et al. Complications following surgery for adolescent idiopathic scoliosis over a 13-year period. Bone Joint J. 2020;102B(4):519–523. doi: 10.1302/0301-620X.102B4.BJJ-2019-1371.R1 [DOI] [PubMed] [Google Scholar]
- 53.Fiscal Year 2020 Medicare Inpatient Prospective Payment System, Final rule (CMS–1716-F) and correction notice (CMS-1716-CN2). Federal Register, August 16, 2019 and October 8, 2019.
- 54.Calendar Year 2020 Medicare Physician Fee Schedule, Final rule (CMS-1715-F) and correction notice (CMS-1715-CN). Federal Register, November 15, 2019 and January 2, 2020.
- 55.Andrew H. Briggs KC, Mark J. Sculpher. Decision Modelling for Health Economic Evaluation. 2nd ed. Oxford: Oxford University Press; 2006..
- 56.Newton PO, Kluck DG, Saito W, et al. Anterior spinal growth tethering for skeletally immature patients with scoliosis: a retrospective look two to four years postoperatively. J Bone Joint Surg Am. 2018;100(19):1691–1697. doi: 10.2106/JBJS.18.00287 [DOI] [PubMed] [Google Scholar]
- 57.Kepler CK, Wilkinson SM, Radcliff KE, et al. Cost-utility analysis in spine care: a systematic review. Spine J. 2012;12(8):676–690. doi: 10.1016/j.spinee.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 58.Charroin C, Abelin-Genevois K, Cunin V, et al. Direct costs associated with the management of progressive early onset scoliosis: estimations based on gold standard technique or with magnetically controlled growing rods. Orthop Traumatol Surg Res. 2014;100(5):469–474. doi: 10.1016/j.otsr.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 59.Su AW, Milbrandt TA, Larson AN. Magnetic expansion control system achieves cost savings compared to traditional growth rods: an economic analysis model. Spine. 2015;40(23):1851–1856. doi: 10.1097/BRS.0000000000001077 [DOI] [PubMed] [Google Scholar]
- 60.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 61.Daubs MD, Hung M, Neese A, et al. Scoliosis research society-22 results in 3052 healthy adolescents aged 10 to 19 years. Spine. 2014;39(10):826–832. doi: 10.1097/BRS.0000000000000280 [DOI] [PubMed] [Google Scholar]