Abstract
HIV stigma is a persistent barrier to curbing the spread of HIV and improving quality of life for people living with HIV. We developed and pilot tested Maisha, an HIV stigma reduction intervention in antenatal care (ANC) with two objectives: 1) among individuals living with HIV, reduce internalized and anticipated HIV stigma, with subsequent improvements in HIV care engagement, and 2) among individuals who are HIV-seronegative, reduce HIV stigmatizing attitudes. We enrolled and baselined 1039 women and 492 male partners presenting to a first ANC appointment and randomized them to standard of care or the Maisha intervention. All women living with HIV (WLHIV) and a subset of HIV-negative participants completed a 3-month follow-up assessment. Participation in the three Maisha sessions was high (99.6%, 92.8%, 89.3%), and nearly all participants noted satisfaction with the intervention content (99.8%) and counselor (99.8%). Among 55 WLHIV, care engagement outcomes did not differ by condition. Among 293 HIV-negative participants, Maisha participants had significantly greater reductions in the moral judgment sub-scale of the stigma attitudes measure (p<.001), but not the social distancing subscale. The ANC setting, where women and their partners are routinely tested for HIV, is an ideal venue for addressing HIV stigma. The Maisha intervention was feasible and acceptable, and had an impact on HIV stigma attitudes. A full trial is needed to examine impacts on HIV outcomes; modifications to the intervention should be considered to reduce social alienation of PLWH.
Keywords: Tanzania, HIV, Stigma, Pregnancy, Prevention of Mother to Child Transmission, Antenatal care
INTRODUCTION
Services for the prevention of mother-to-child transmission (PMTCT) of HIV reduce the incidence of vertical mother-to-child transmission and improve long-term health outcomes for women living with HIV (WLHIV) (1,2). Additionally, PMTCT is an important entry point for HIV testing and care engagement, addressing gaps in the HIV prevention and treatment continuum (3). Under PMTCT guidelines, clinics conduct universal HIV testing during antenatal care (ANC), which is followed by the initiation of lifelong antiretroviral therapy (ART) for pregnant and breastfeeding WLHIV (3). PMTCT programs have been incredibly successful in achieving universal testing for pregnant women, identifying people of childbearing age who are living with HIV, and reducing infant HIV infections; however, concerns remain regarding sustained care engagement throughout the pregnancy and postpartum periods (4,5).
Overall retention in HIV care during the pregnancy and postpartum periods has been suboptimal (6,7). Our systematic review of PMTCT programs across Africa reported retention rates of 72.9% in the first 6 months of care initiation among pregnant and postpartum women, rates substantially lower than other groups of people living with HIV (PLWH) (6). Data from Tanzania echoes these challenges of PMTCT care engagement. In prior studies of pregnant WLHIV who were enrolled in PMTCT in Moshi, Tanzania, medical record data revealed that 23% of women diagnosed with HIV during their first ANC visit never returned to PMTCT care at the clinic where they were diagnosed. Among those who returned after the first appointment, 21% had indications of poor engagement at six months postpartum (7). This falls short of overall goals for HIV care engagement (8), and has implications for perinatal transmission and forward transmission to sexual partners.
Multiple studies have noted the impact of HIV-related stigma on care linkage and retention in PMTCT programs (7,9-11). For PLWH, stigma serves as a barrier to diagnosis, ART uptake and adherence, and engagement in clinical appointments (9,10). Stigma also impedes disclosure of one’s HIV status to others to elicit social support, while contributing to emotional distress and social alienation (9,10,12). In the general population, HIV stigmatizing attitudes contribute to both subtle and explicit discrimination against PLWH (13).
Existing HIV stigma reduction interventions for PLWH have shown significant and positive outcomes among PLWH and their communities (14-17). Interventions aimed at increasing knowledge about HIV stigma and stigma coping mechanisms have been particularly promising, as several studies have found that these interventions decreased participants’ experience of fear, increased self-esteem, and fostered feelings of hope, acceptance and trust (14,15,17). Further, interventions that have targeted the social networks of PLWH found that family and community members became more aware of their enacted stigma and showed more compassion towards PLWH after the interventions (14,15). Stigma reduction interventions have also been successful at increasing the interaction between PLWH and community members while reducing HIV stigma (18). Although several HIV stigma reduction interventions have been successful, none have utilized the ANC setting to address stigma among a general population of pregnant and postpartum women and their male partners who present to care. However, stigma reduction in a PMTCT setting (for WLHIV) shows promise. In South Africa, an intervention in the PMTCT setting was able to decrease HIV-related stigma, including personalized (social) stigma, concerns about disclosing one’s status to others, and concerns about public attitudes toward HIV (19).
The ANC setting offers a unique opportunity to address HIV stigma, in order to have an early and meaningful impact on linkage and retention to HIV services, and to address misinformation and prejudicial attitudes about HIV among the general population (13,20). In Tanzania, women are tested for HIV during their first ANC appointment, undergo post-test counseling, and initiate ART immediately after diagnosis (21). Guidelines also encourage women to present to their first ANC appointment with their male partners, providing a key entry point for HIV testing and linkage to HIV services for men. In a setting of universal HIV testing, the heightened emotions around HIV testing offers a teachable moment in which attitudes and behaviors about HIV can be changed (22-24). This setting can provide a window of opportunity to develop empathy toward PLWH, and to prepare themselves for the possibility of receiving an HIV diagnosis.
Our team developed Maisha (Swahili for “Life”), a counseling intervention to address HIV stigma among pregnant women and their male partners presenting to a first ANC appointment (24,25). The Maisha intervention aimed to address HIV stigma in order to: 1) improve early PMTCT care engagement among WLHIV, and 2) reduce HIV stigmatizing attitudes among individuals who are HIV seronegative. In this study, we examined the feasibility and acceptability of Maisha in an ANC setting in Tanzania, and the potential impacts of the intervention in achieving the identified objectives.
METHODS
The study was a pilot randomized control trial aimed at determining the feasibility, acceptability, and potential efficacy of Maisha, an HIV stigma counseling intervention for women and their partners at entry into ANC. The study had two parallel groups: the Maisha intervention and standard of care comparison, with a 1:1 allocation ratio. Study enrollment occurred between April and November 2019, with follow-up occurring through February 2020.
The study is registered at ClinicalTrials.gov (NCT03600142). Previously published manuscripts provide additional details about the study design, methods (25) and development of the intervention (24). The study was approved by the ethical review committees at the Tanzanian National Institute for Medical Research and the Kilimanjaro Christian Medical Center, as well as the ethical review boards at Duke University and University of Utah.
Setting
The study was conducted in two government health centers in the Moshi urban municipality, Tanzania. Together, the two facilities provide care for approximately 2,500 pregnant women per year; an estimated 4.8% of pregnant women seen at the clinics are living with HIV, including both those known to be living with HIV at the time of entry into ANC and those who test positive during their first ANC appointment. The clinics follow the National guidelines, whereby all patients are required to have an HIV test at entry to ANC, unless they present a clinic card confirming that they have been previously diagnosed with HIV (21). Pregnant patients are strongly encouraged to bring their male partner to the first ANC visit for pregnancy education and partner HIV testing.
Participants
Women were eligible to enroll in the study if they were at least 18 years of age, pregnant, attending a first ANC appointment at one of the two study clinics, and able to understand and speak Swahili. Accompanying male partners of enrolled women were also eligible to enroll. Potential participants were identified by the clinic nurses in the waiting room, prior to any ANC appointment procedures.
The clinic nurses, in cooperation with research staff, provided a brief description of the research activities, including the time commitment. Individuals who expressed interest were referred to meet with a research staff in a private research office to confirm eligibility and learn more about the study. The research staff read the informed consent form aloud, provided a paper copy, and answered any questions before written informed consent was obtained. Contact information and preferred methods of contact were gathered from participants to schedule follow-up assessments.
Procedures
After providing consent, participants completed a structured survey using audio computer-assisted self-interview (ACASI) technology on tablets running Questionnaire Development System (QDS) software (Nova Research Company, Version 5.0). ACASI modality increases participant privacy in providing their responses and thereby improves data validity by minimizing social desirability bias (27). The ACASI included a series of 4 quality check questions throughout the survey (e.g., “Please select ‘Disagree’ in response to this item”), where participants were instructed to choose a specific answer in order to confirm their understanding of the ACASI system.
Following completion of the baseline survey, we randomized participants to receive either the standard of care HIV testing and counseling, or the standard of care plus the Maisha intervention. Female participants were randomized at a 1:1 ratio using a block randomization method (10 per block) and prepared ahead of time using an online randomization program; male participants were assigned to the same condition as their partners.
All individuals who were living with HIV were contacted to return for a 3-month follow-up assessment, and data from their medical records were abstracted to classify care engagement outcomes. A sub-set of individuals who were HIV negative were also invited to return for a 3-month follow-up assessment, in order to observe changes in stigmatizing attitude scores. Individuals with stigmatizing attitude scores greater than 14 were eligible for follow-up; of those who met criteria, a random 60% were invited for follow-up, in order to reach a minimum sample powered to examine differences in HIV stigma attitudes outcomes.
Study conditions
Control condition
Participants randomized to the control condition received the standard of care HIV testing and counseling protocol in the clinic, which was administered by clinic nurses. According to Tanzanian national PMTCT guidelines, HIV pre-test counseling provides education about HIV and prepares a woman (and her partner, if present) for HIV testing (21). If an individual tests positive for HIV, counseling should help the woman/couple accept the HIV test result, educate them about HIV, and discuss their treatment. The guidelines do not include any stigma-specific content in pre- and post-test counseling. Women living with HIV should be registered for PMTCT care and immediately initiated on ART, which is provided during the clinic appointment. Male partners who test positive for HIV should be referred to the HIV Care and Treatment Clinic (CTC) located in the same health center for same-day initiation of ART.
Intervention condition
Participants randomized to the intervention condition received the standard of care plus the Maisha intervention. The development and content of the Maisha intervention has been described in greater detail elsewhere (24). Maisha was developed based on the HIV Stigma Framework (28,29), which emphasizes the unique experiences of stigma among PLWH and describes the various stigmatizing attitudes and behaviors that contribute to the experience of stigma. Among PLWH, the Framework identifies three components of HIV stigma: internalized (negative beliefs and feelings about oneself), enacted (actual prejudice and discrimination from others), and anticipated stigma (fear of mistreatment that may occur if one’s status becomes known) (Earnshaw & Chaudoir, 2009). The therapeutic approach of Maisha is based on principles of Cognitive-Behavioral Therapy (CBT) (29,30). Consistent with CBT, the intervention content focuses on addressing automatic negative thoughts about the self, future, and world, tracking these onto HIV stigma in its multiple manifestations. Maisha involves up to three sessions, which are delivered by the facilitator (a Tanzanian with university-level education who received training and supervision from the study team) to either the individual woman or the woman and her partner. All sessions were conducted in a private research room located in a building next to the study clinic.
Maisha session one was delivered immediately following the baseline assessment. If the woman enrolled in the intervention together with her male partner, they received this session together. The session included a video and a brief counseling session (approximately 20 minutes in total). The 8-minute video depicts a couple who test positive for HIV during a first ANC visit, and follows them as they learn to accept their status, navigate disclosing their status to her mother-in-law, and commit to taking daily therapy. The counseling curriculum then builds on the video to provide psycho-education on the various components of HIV stigma (enacted stigma, internalized stigma, and anticipated stigma), lead participants to reflect on HIV stigmatizing attitudes, and encourage empathy and inclusion toward PLWH in the community.
Maisha session two was delivered immediately following the first ANC appointment to all women identified as living with HIV, along with their male partners, if enrolled. The goal of session two is to link back to the video content, address immediate stigma-related concerns, and foster commitment to initiate and/or continue treatment. The facilitator returns to content from session one to discuss how issues of internalized, anticipated and enacted stigma might relate to their situation of living with HIV, and to try and reduce these as barriers of HIV care engagement.
Maisha session three was delivered two weeks after session two. WLHIV are asked to come to session three alone, without their partners, so that they are free to discuss any relationship concerns. The goal of the third and final session is to provide WLHIV continued assistance in managing stigma, foster a longer-term commitment to PMTCT care, and develop a plan for overcoming barriers to care. Additional Third Wave cognitive behavioral concepts are introduced in this session, including the acceptance of worry as a normal response to life’s challenges. The facilitator links back to prior content on stigma expressions, and emphasizes that one’s feelings (internalized stigma) and thoughts (anticipated stigma) are connected. Finally, the counselor and participant consolidate the components of Maisha into an individualized action plan, which includes goals for HIV care engagement and ART adherence, strengthening social support, and considering selective HIV disclosures. Like session 2, session 3 lasted between 60 and 90 minutes.
Feasibility and acceptability measures
All intervention sessions were recorded (with participant consent), and each week, one session from each counselor was reviewed during a group supervision session. Supervision was led by a Tanzanian physician with a Masters in Public Mental Health and also attended by the study coordinator (a Tanzanian with a Masters in Public Health). A US counseling psychologist reviewed the supervision notes and provided feedback in weekly videoconference meetings. Sessions for review were typically chosen at random from those conducted in the previous week, but occasionally, counselors requested review of specific sessions where they wished to receive additional feedback. Using a structured form (31), the counselors and supervising staff assessed the recorded sessions for intervention fidelity (i.e., core components of the intervention manual were covered in the session) and presence of key counseling skills (i.e., being non-judgmental, eliciting hope, empathy, active listening, acknowledging patient experience, allowing expression of emotion, and avoiding advice-giving). Both session coverage and counseling skills were evaluated on a scale of 0 to 4 (“not done” to “excellent with a pre-established threshold score of 3 established for session fidelity. During the group supervision meetings, the team provided in-person feedback to the counselors, including discussions of successes and challenges, to aid in the counselor’s skill development. Over the implementation period, a total of 48 sessions (5.9%) were assessed for fidelity.
At the 3-month follow-up assessment, participants in the intervention condition were asked in the ACASI assessment to provide feedback on specific aspects of the intervention, including time spent in the intervention, and the perceived helpfulness of various aspects of the intervention (e.g., the video, counseling, visuals and breathing exercise). Participants were asked to state their agreement on a scale of 1 to 4 (strongly disagree to strongly agree) on a variety of statements. All intervention participants were provided two statements about the counselor (i.e., “I liked the counselor” and “I felt the counselor listened to me and responded to my concerns”) and four statements about the intervention content (i.e., the intervention was “useful,” “helped me feel more prepared to take an HIV test and receive my results,” “helped me learn about different types of stigma,” and “helped me learn how I can support people living with HIV”). Participants living with HIV were additionally asked their level of agreement with the impact of the intervention content on the acceptance of their HIV status, motivation to engage with HIV care, HIV status disclosure, hopefulness about the future, and coping with HIV stigma. Following the ACASI assessment, the research staff asked Maisha participants a series of open-ended questions to capture more detailed thoughts about the intervention content (video and counseling), their feelings about the counselor, and suggestions for improvements. Participants living with HIV were asked how the intervention impacted their feelings about their HIV status and their motivation for HIV care engagement. The open-ended responses were audio recorded and later translated and transcribed from Swahili to English by a trained translator.
Outcome measures
Survey measures were selected based on previous HIV and stigma research in East Africa. All measures were translated from English into Swahili, back-translated, and discussed as a team to reach consensus on the best translation, establish face validity, and confirm the cultural context of the constructs.
Measured outcomes for HIV-positive participants
For participants living with HIV, the primary outcome was HIV care retention, and the secondary outcomes were internalized HIV stigma, anticipated stigma, HIV acceptance and depression.
HIV care retention.
Among WLHIV, retention in HIV care at 3-month follow-up was assessed via medical record review, with retention defined as having no more than a 60 day gap between any two PMTCT visits at the study clinic, or having record of an official transfer to another clinic (32). Viral load testing was not routinely done in that time period and was therefore not assessed.
Internalized HIV stigma.
Internalized HIV stigma reflects the degree to which PLWH endorse the negative beliefs about themselves due to their HIV status. This construct was measured with Scale A of the HIV and Abuse Related Shame Inventory (HARSI) (33). Participants rated their agreement with 13 statements (e.g., “I struggle with feelings of worthlessness because I have HIV”) in the past month, on a scale of 0-4 (“not at all” to “very much”). The score ranged from a possible 0 to 52 (α=0.877).
Anticipated stigma.
Participants listened to a series of statements about the reaction they expected if people knew their HIV status (e.g., “If people knew my HIV status, I would be shunned at social gatherings.”). The 12 items were developed based on local experience and previous work in East Africa (34). For each statement, participants responded from 0 (“strongly disagree”) to 4 (“strongly agree”). The score ranged from a possible 0 to 48 (α=0.914).
HIV acceptance.
Studies have argued that positive aspects of adaptation, such as resilience, coping, and acceptance, are important indicators of illness outcomes. To examine HIV acceptance, we adapted the Acceptance subscale of the Illness Cognition Questionnaire (35). The measure includes six items (e.g., “I can have a complete life with my HIV status”) and participants were asked to respond from 0 (“not at all”) to 3 (“completely”). The score ranged from a possible 0 to 18 (α=0.835).
Depression.
Depression was measured by the Edinburgh Postnatal Depression Scale (EPDS) (36). The measure includes 10 items that ask about depression symptomatology in the previous ten days. Response options were standardized for ease of administration in Swahili, ranging from 0 (“not at all”) to 4 (“very often”). The score ranged from a possible 0 to 40 (α=0.814).
Measured outcomes for HIV-negative participants
For participants who were HIV negative, the primary outcome was attitudes toward PLWH, and the secondary outcome was anticipated HIV stigma.
Attitudes toward PLWH.
HIV stigmatizing attitudes were measured using a modified version of Personal and Attributed Stigma Scale (PASS) (37,38). The scale was adapted to the local context based on formative qualitative data collection and revised after a pilot of the measure with 88 individuals. The final measure included 18 items, and participants responded from 0 (“strongly disagree”) to 4 (“strongly agree”). Exploratory factor analysis suggested a two-factor model: 6 items reflected the construct of blame/judgement (e.g., “I would be ashamed if someone in my family has HIV”; α=0.84) and 12 items reflected the construct of interpersonal distancing (e.g., “I would not eat together with someone I knew had HIV”; α=0.92).
Anticipated stigma.
Participants listened to a series of statements about the reaction they expected from others if they had HIV (e.g., “If I had HIV, I would be shunned at social gatherings.”). The 12 items were developed based on local experience and previous work in East Africa (34). For each statement, participants were asked to respond from 0 (“strongly disagree”) to 4 (“strongly agree”). The score ranged from a possible 0 to 48 (α=0.874).
Sample size
Prior to commencing the study, we considered our ability to detect significant differences by intervention conditions in our two groups: WLHIV and HIV-negative individuals. For WLHIV, we were aware that our resources of time and funding would not be sufficient to detect a significant difference in the HIV care outcome, and that we would only be able to detect observational signals of impact. We aimed for a minimum of 50 WLHIV, which is considered an adequate sample to assess feasibility and acceptability in a pilot intervention study (26). Given an estimated 5% HIV prevalence among women presenting for ANC, this required us to enroll 1000 women presenting to ANC, in order to enroll 50 WLHIV. For HIV-negative individuals, a sample size of 352 participants (176 participants per condition) with both baseline and follow-up data was identified as sufficient to detect significant differences in overall stigma scores in the HIV negative population. This number was estimated to detect a small intervention effect (d=0.3), with two-sided significance level of 5% and 80% power.
Data analysis
Feasibility and acceptability of the intervention were described by recruitment and retention patterns. Acceptability of intervention delivery was based on the session rating scales and participant satisfaction. For recruitment and retention, we described the proportion of eligible participants who attended each of the three Maisha sessions, as well as the proportion of participants who completed the 3-month assessment. The fidelity to the intervention was assessed by examining the percentage of components from the Maisha intervention manual that were covered in the reviewed sessions, as recorded in the session rating scales. Survey data at the 3-month follow-up was used to describe acceptability of the intervention, with >80% satisfaction used as a metric of acceptability. Open-ended questions were thematically coded to summarize participants’ perceptions of the intervention, suggestions for changes, and feasibility moving forward. A coding structure for the qualitative data was proposed by GK based on a careful reading of the data, and confirmed through consultation with the first author. Coding was conducted by a single individual (GK), a Tanzanian national who had previously served as the study coordinator and was familiar with the intervention content and structure.
Prior to initiating analysis of efficacy, we examined whether differences existed between the intervention and control conditions, using chi-square tests (for categorical variables) and t-tests (for continuous variables). Potential efficacy of the intervention was examined using an intent-to-treat approach. Analysis was conducted separately among WLHIV, and individuals (women and men) who were identified as HIV-negative. For WLHIV (n=55), chi-square tests were used to assess differences between conditions in the proportion of participants who were retained in HIV care at 3 months post enrollment. For women who presented to care with an established HIV diagnosis and completed follow-up (n=29), we used profile plots to display changes from baseline to 3 months in internalized stigma, anticipated stigma, depression and HIV acceptance.
For participants who were identified as HIV negative and completed the 3-month follow-up survey (n=378), we used one-way analysis of covariance (ANCOVA) to model stigma attitudes scores at 3 months, controlling for baseline scores and with enrollment arm as an indicator variable. Analysis was conducted separately for each of the two stigma sub-scales (i.e. moral judgment and social distancing). In the secondary analysis, subgroup differences between men and women were explored using stratified analysis. The same procedures were conducted for the anticipated stigma outcome.
The outcome analysis was repeated excluding participants who answered at least two of the four ACASI validity checks incorrectly (11.0% of the sample). Both approaches yielded statistically similar results; therefore, results with the full sample were reported in the paper.
RESULTS
Sample Characteristics
Characteristics of the study sample are described in Table 1. The study enrolled 1039 pregnant women and 492 male partners. The median (Q1, Q3) age of participants was 26 years (23, 30). The majority of participants (61.7%) were married, less than half (41.5%) had any secondary school education, and less than one-fifth (19.7%) reported regular employment. Of the sample, 32 women (3.1%) and 5 men (1.0%) reported at the baseline that they had previously been diagnosed with HIV, and an additional 23 women (2.3%) and 6 men (1.2%) were newly diagnosed with HIV during their first ANC appointment (i.e., after baseline). In total, 5.3% of women (55/1039) and 2.2% men (11/492) in our sample were living with HIV. Demographic characteristics and HIV status did not differ significantly across the intervention and control conditions.
Table 1.
Baseline characteristics of participants (n=1532)
| Control (n = 773) |
Intervention (n = 758) |
Total | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Women | 519 (67.1%) | 520 (68.6%) | 1039 (67.9%) | 0.541 |
| Men | 254 (32.9%) | 238 (31.4%) | 492 (32.1) | |
| Age [median (Q1, Q3)] | 26 (23, 31) | 26 (22, 30) | 26 (23, 30) | 0.468 |
| Had secondary education | 319 (41.3%) | 316 (41.7%) | 635 (41.5%) | 0.659 |
| Married | 484 (62.7%) | 458 (60.6%) | 942 (61.7%) | 0.778 |
| Employed | 141 (18.2%) | 161 (21.3%) | 302 (19.7%) | 0.155 |
| HIV positive | 31 (4.0%) | 35 (4.6%) | 66 (4.3%) | 0.508 |
| Stigmatizing attitudes | ||||
| Moral judgement [median (Q1, Q3)] | 3 (1, 7) | 4 (1, 7) | 3 (1, 7) | 0.684 |
| Social distancing [median (Q1, Q3)] | 7 (1, 14) | 8 (2, 15) | 7 (2, 14) | 0.214 |
| Anticipated stigma [median (Q1, Q3)] | 7 (3, 13) | 8 (3, 13) | 8 (3, 13) | 0.625 |
Feasibility and acceptability
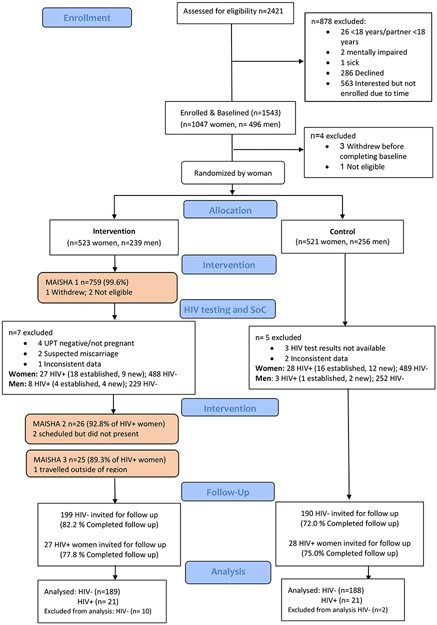
Among the 762 participants assigned to the intervention condition, 759 (99.6%) completed session one; of the 28 WLHIV assigned to the intervention condition, 26 (92.8%) completed session two, and 25 (89.3%) completed session three. Of the three WLHIV who missed any counseling sessions, one had travelled, and two scheduled but did not present for the session (Figure 1).
Fig 1.
Study flow chart
In the 48 sessions that were independently evaluated for quality control, fidelity to intervention content and presence of core counseling components were high. Counsellors were rated as effectively completing 93%, 92% and 94% of all intervention components for sessions one, two and three, respectively, and received an average rating of 3.7 out of 4 across the seven core counselling competencies.
Feedback on the intervention was positive. Among intervention participants who completed the 3-month follow-up survey, 99.5% (185/186) reported that they liked the counselor and 99.5% (185/186) found the content to be useful. Regarding intervention length, 76.3% (142/186) felt the length and number of sessions were sufficient, while 14.5% reported the intervention was too short and 8.1% reported it was too long. Among WLHIV who completed the 3-month follow up, all the women allocated to the intervention condition (n=22) agreed that the intervention helped them deal with stigma and made them more hopeful for the future.
In the open-ended questions, Maisha participants discussed their experiences with and perspectives of the Maisha intervention. Participants commonly noted the rapport they developed with the Maisha counselors, and appreciated the respect and empathy that they conveyed. Five primary themes emerged in their responses: gaining new knowledge/ perspectives on HIV, feeling empowered to take an HIV test, feeling empathy for PLWH, hope that you can live a normal life with HIV, and support and motivation to engage in HIV care and disclose an HIV status (Table 2).
Table 2.
Themes identified related to participants’ experiences with the Maisha intervention (n=221)
| Theme 1: New knowledge/perspectives on HIV |
| “I felt so good to interact with the counselor. The kindness and respectful communication of the counselor was essential to me. I was willing to receive and learn from his advice because he managed to be an excellent counselor to me.” (Male, 31 years old, HIV negative) |
| “The video was good, and I learned from a new perspective that was included in the video. Also, I liked the use of images because it helps to reflect on different scenarios.” (Female, 38 years old, HIV negative) |
| Theme 2: Feeling empowered to take an HIV test |
| “The counselor helped me to overcome the fear I had before testing. I would like to get the same type of support if I had to retake the counselling session.” (Male, 35 years old, HIV negative) |
| “The video and counselor prepared me well to receive HIV test results. That is why I did not think of harming myself or something else, which is terrible.” (Female, 22 years old, living with HIV) |
| Theme 3: Empathy for PLWH |
| “From the video, I learned that you could live with people who are living with HIV. Thus, I should not discriminate and stigmatize people with HIV/AIDS. Instead, I should love and take good care of them.” (Female, 37 years old, HIV negative) |
| “In case my partner is found with HIV, I learned the appropriate way of accepting HIV status as a family. I think it is essential to remain positive and maintain family solidarity.” (Male, 27 years old, HIV negative) |
| Theme 4: Hope that you can live a normal life with HIV |
| “Watching that video gave me hope and speaking with the counselor made me feel happy and encouraged to live.” (Female, 28 years old, living with HIV) |
| “The video was good and thoughtful. The lesson I got from the video is that getting HIV is not the end of life.” (Female, 34 years old, HIV negative) |
| Theme 5: Support and motivation to engage in HIV care and disclose an HIV status |
| “The video and counseling sessions boosted my confidence. I am still in care, and I am doing well with adherence.” (Female, 39 years old, living with HIV) |
| “By watching the video, I was encouraged to know that I could disclose my HIV status to close people and get the support I need.” (Female, 22 years old, living with HIV) |
Potential for longitudinal effects on HIV care engagement and other outcomes among WLHIV
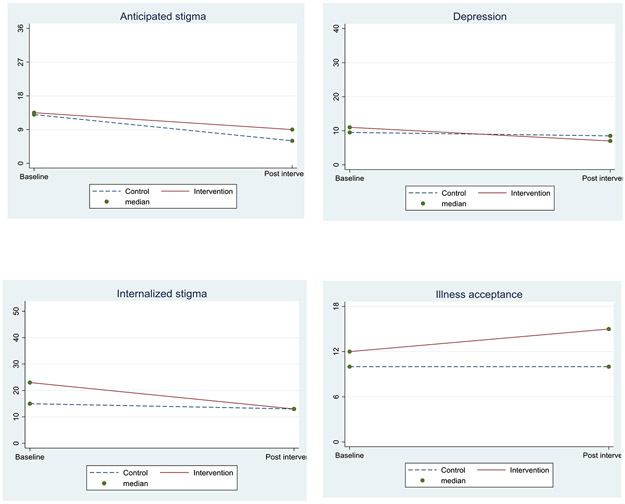
Of the 53 WLHIV with complete data, HIV care engagement at 3 months was similar between participants in the intervention condition and participants in the control condition (88.9% vs 88.5%). Profile plots in Figure 2 provide a visual presentation of change in anticipated stigma, depression, internalized stigma and illness acceptance from baseline to post intervention among the 29 women who knew their HIV status before enrollment in the study and completed follow-up. Although not powered to detect significant subgroup differences, the intervention had an effect in the direction we expected, except for anticipated stigma. Participants in the intervention condition had greater reductions in internalized stigma (β= −3.5; 95% CI: −9.4, 2.4) and depression (β= −1.1; 95% CI: −4.2, 1.9), and were more accepting of their HIV status (β= 2.6; 95% CI: −0.2, 5.3) than the control group. Intervention participants had lesser reductions in anticipated stigma than the control group (β= 2.0; 95% CI: −3.3, 7.3).
Fig 2.
Profile plots showing outcomes by condition among women with known HIV diagnosis prior to first ANC (n=29)
Longitudinal effects on HIV stigma attitudes
Table 3 presents the changes in stigmatizing attitude scores, by sub-scale, among the 378 individuals who were HIV-negative and completed follow-up. There was a significantly greater decrease in moral judgement scores in the intervention group compared to the control group (β= −1.5; 95% CI: −2.3, −0.7) from baseline to post intervention. There was also a larger decrease in the social distancing scores in the intervention group compared to the control group (β= −1.2; 95% CI: −3.3, 0.7), but it was not statistically significant. Similarly, we observed a larger decrease in anticipated stigma in the intervention group compared to the control group (β= −1.1; 95% CI: −3.3, 0.7), but it was not significant. Subgroup analysis by gender (Table 4) showed that women in the intervention condition had significantly lower moral judgment scores (β= −1.8; 95% CI: −2.8, −0.8) compared to their control counterparts, but there were no significant changes in social distancing (β= −1.5; 95% CI: −4.0, 0.9) or anticipated stigma (β= −0.7; 95% CI: −2.6, 1.2). Among men, there was no significant difference between conditions in any of the outcomes.
Table 3.
Regression estimates of intervention effects from ANCOVA model, including sensitivity analysis
| ITT (Multiple imputation) (n=378) | Complete cases only (n=293) | |||
|---|---|---|---|---|
| Regression coefficient (95%CI) | p-value | Regression coefficient (95%CI) | p-value | |
| Moral Judgment | −1.5(−2.3, −0.7) | <0.001 | −1.5(−2.3, −0.7) | <0.001 |
| Social distancing | −1.2(−3.3, 0.7) | 0.216 | −1.2(−3.2, 0.9) | 0.272 |
| Anticipated stigma | −1.1(−3.3, 0.7) | 0.150 | −1.2(−2.7, 0.3) | 0.114 |
Table 4.
Regression estimates of intervention effects from ANCOVA model, stratified by gender
| Men (n=126) |
Women (n=252) |
|||
|---|---|---|---|---|
| Regression coefficient (95%CI) | p-value | Regression coefficient (95%CI) | p-value | |
| Moral Judgment | −1.0(−2.5, 0.4) | 0.166 | −1.8(−2.8, −0.8) | <0.001 |
| Social distancing | −0.04(−3.6, 3.6) | 0.982 | −1.5(−4.0, 0.9) | 0.216 |
| Anticipated stigma | −2.0 (−4.4, 0.4) | 0.103 | −0.7(−2.6, 1.2) | 0.462 |
DISCUSSION
Stigma has a persistent, negative impact on a variety of outcomes along the HIV care continuum. However, evidence-based interventions to reduce community-level stigma and address the impact of stigma on PLWH are scarce (39-42), and study design and reporting of such interventions often lack rigor (41). A systematic review of stigma reduction interventions in low- and middle-income countries revealed notable limitations in theoretical frameworks, use of validated measures, and descriptions of the intervention content and delivery processes (40). In this manuscript, we presented the results of a pilot study of Maisha, a video and counseling intervention delivered in the ANC setting to reduce HIV stigmatizing attitudes and improve HIV care engagement. The intervention was based on a strong theoretical framework, and the randomized control trial measured both implementation and outcome data. The study found that the intervention was highly feasible (evaluated by recruitment patterns and intervention attendance) and acceptable (evaluated by participants’ assessment). We were not able to observe a difference in HIV care engagement among the 55 WLHIV in our sample. Among HIV negative individuals, the intervention had a positive impact on participants’ attitudes of moral judgment toward PLWH, but did not impact attitudes endorsing social distancing toward PLWH. The Maisha intervention shows promise in addressing HIV stigma in a setting of routine HIV testing, and would benefit from further evaluation, particularly considering HIV clinical outcomes.
HIV testing is a moment of heightened emotion and reflection, which may create a “window of opportunity” to address HIV stigmatizing attitudes. In providing qualitative feedback, participants in the Maisha intervention noted that they developed greater empathy for PLWH. This was further reflected in the outcomes on moral judgment, with women in the Maisha condition having significantly greater reductions in moral judgment attitudes toward PLWH. Participants noted that the intervention made them more ready to accept an HIV diagnosis and that it helped them to see HIV as a manageable illness, which may explain the impacts on the moral judgment sub-scale. It is notable, however, that the intervention did not have impacts on the social distancing sub-scale of the HIV stigma attitudes measure. While knowledge about HIV, including modes of transmission, has increased over time (43), this may not translate into behavior change. A study of HIV stigma across contexts found that individuals were often fearful of casual interactions with PLWH because of “preoccupation with unlikely modes of transmission” (44). To impact behaviors that isolate PLWH, future iterations of the Maisha interventions should provide clear and direct messaging about how HIV is transmitted and how it is not transmitted.
We observed that the effect of Maisha on HIV stigmatizing attitudes differed by gender, with the intervention having more positive outcomes for women than for men. Prior research has suggested that men have higher levels of HIV stigmatizing attitudes (45,46). There are several social and structural factors (47) contributing to this observation; however, male gender norms seem to heighten the propensity of men towards stigma and prevent men from developing and expressing empathy towards PLWH (48,49). Often, men are expected to demonstrate traditionally masculine qualities such as caretaking and harsh protectiveness that do not coexist with perceived weakness, including notions of illness. Thus, accepting an HIV positive status, whether in oneself or in a loved one, may threaten men’s perceived ability to meet their designated roles in the society. Concurrently, men tend to distance themselves from PLWH due to fear of anticipated stigma as result of associating themselves with a person living with HIV (50). To attain a significant reduction of stigma among men, it is essential to develop interventions that are tailored to address the intersection of HIV stigma and notions of masculinity (51,52). In future iterations, it is also important to think about how to reach men who don’t attend the first ANC visit with their partners, as these men are likely to have more HIV stigmatizing attitudes and to be less willing to engage in HIV testing.
We found that 11% of women in our study had poor care engagement at the 3-month follow-up; this is similar to our previous medical record review, where we found that 11.2% WLHIV were identified as LTFU to HIV care from the first ANC visit (53). Our observational cohort of pregnant WLHIV found that HIV care engagement dropped off over time into the postpartum period; by 6 months postpartum, 21% of WLHIV had indicators of poor HIV care engagement (7). In our qualitative work, fear of HIV stigma was a major driver of poor care engagement (54-57), echoing other work that has found the same (58-61). Among WLHIV, efforts to address HIV stigma should ideally begin at the time of diagnosis, in order to forestall internalized and anticipated HIV stigma, navigate the complex process of HIV disclosure, and foster the skills needed to navigate experiences of enacted stigma. In order to maximize and sustain impacts, a multi-level intervention that integrates the Maisha intervention with stigma reduction training for health care providers and HIV stigma reduction messages that are integrated across the entire health system should be considered.
The pilot study of Maisha was not powered to show impacts on HIV care engagement outcomes, based on both the small number of participants living with HIV and the short follow up period. However, the absence of any trend in difference by conditions does beg questions about how the intervention may be modified for greater impact. It is possible that the short intervention duration for PLWH (just three sessions) may be insufficient to reduce the internalized and anticipated stigma constructs that drive poor care engagement, and future iterations of the intervention should consider additional contact for WLWH, in order to have a more sustained and intensive intervention. In order to assess the capacity for Maisha to be embedded into clinical care, future studies should assess delivery of Maisha by existing clinic staff (e.g., nurses or community health workers), the acceptability of the intervention among those workers, and any resulting impacts on participant satisfaction. Studies have shown that pregnant women may switch clinics after diagnosis if they perceive the clinical setting as stigmatizing or if they don’t trust that their status will remain confidential (11,62). Creating a culture that is centered around a stigma reduction intervention such as Maisha may motivate women to enter PMTCT at the clinic where they present for presentation.
The results of this pilot feasibility trial must be interpreted in the context of its limitations. Although the study enrolled over 1000 pregnant women during the recruitment period, only 55 of those women were identified as living with HIV, making it substantially underpowered to observe statistically significant differences in HIV care engagement outcomes. Additionally, because HIV testing only occurred after baseline, we did not have baseline data on HIV-associated outcomes (e.g., HIV acceptance, internalized HIV stigma) among individuals newly diagnosed with HIV, precluding our ability to examine longitudinal intervention effects in this group. We are also aware that our outcome measures may be subject to reporting bias. The care engagement outcome was assessed via medical record review and is dependent on both the quality of documentation and the information available to the clinic. In particular, women who silently transfer care to another clinic may be misclassified as out of care. The ACASI data also introduces potential bias. While ACASI can minimize social desirability bias (27), it also reduces the opportunity for real-time quality checks that come with an interviewer-administered survey. Finally, fidelity data were only assessed in a sub-set of sessions. While the supervisor ratings of interventionist performance on select sessions were likely more reliable than self-ratings, we recognize that this may not capture fidelity across all intervention sessions.
Conclusion
Four decades after the emergence of HIV, stigma remains a persistent barrier for outcomes across the HIV care cascade. Interventions to reduce HIV stigma in the community and mitigate the impact of stigma on care engagement are an urgent public health challenge. This study demonstrates the potential of intervening on HIV stigma in the setting of routine HIV testing in ANC. In particular, the outcomes show the potential for harnessing an ANC environment to reduce HIV stigmatizing attitudes, which may be a novel way to reduce HIV stigma in the general population. The intervention has potential for scalability in Tanzania and more broadly in sub-Saharan Africa; the video could be shown in clinic waiting room and the structured intervention content could be integrated into existing pre- and post- HIV testing sessions. While Maisha shows promise, it should be further evaluated for impacts on HIV stigmatizing attitudes and PMTCT care engagement in a range of clinical settings.
Acknowledgments:
This study was funded grants from the Fogarty International Center (R21 TW 011053) and Duke Center for AIDS Research (P30 AI064518). We also acknowledge fellowship support received from the NIH Office of Behavioral and Social Science Research (OBSSR) and the Fogarty International Center (D43 TW009337). The team is grateful for the support of the Tanzanian Ministry of Health, our fantastic research staff (Hashim Mdetele, Ismail Amiri, Betina Licky, Eva Olomi, and Joseph Charles), and the personnel in the study clinic.
Contributor Information
Melissa H. Watt, University of Utah, Department of Population Health Sciences, 295 Chipeta Way, Williams Building, Room 1N410, Salt Lake City, UT 84108
Linda Minja, Kilimanjaro Clinical Research Institute, Moshi, Tanzania.
Brandon A. Knettel, Duke University, Duke Global Health Institute, Box 90519, Durham, NC 27701
Rimel N. Mwamba, Duke University, Duke Global Health Institute, Box 90519, Durham, NC 27701
Haika Osaki, Kilimanjaro Clinical Research Institute, Moshi, Tanzania.
James S. Ngocho, Kilimanjaro Christian Medical University College, Moshi, Tanzania
Godfrey A. Kisigo, Duke University, Duke Global Health Institute, Box 90519, Durham, NC 27701
Jenny Renju, London School of Hygiene and Tropical Medicine, Department of Population Health, London, UK; Kilimanjaro Christian Medical University College, Department of Epidemiology and Biostatistics, Moshi, Tanzania.
Joao R.N. Vissoci, Duke University, Duke Global Health Institute, Box 90519, Durham, NC 27701
Saumya Sao, Duke University, Duke Global Health Institute, Box 90519, Durham, NC 27701.
Blandina T. Mmbaga, Kilimanjaro Clinical Research Institute, Moshi, Tanzania
REFERENCES
- 1.Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. Aids. 2012. October 23;26(16):2039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. On the Fast-Track to an AIDS-free generation [Internet]. UNAIDS; 2016. [cited 2017 Nov 14]. Available from: http://www.unaids.org/sites/default/files/media_asset/GlobalPlan2016_en.pdf [Google Scholar]
- 3.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach (Second edition) [Internet]. Geneva: WHO; 2016. Available from: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1 [PubMed] [Google Scholar]
- 4.World Health Organization. Global guidance on criteria and processes for validation: Elimination of Mother-to-Child Transmission of HIV and Syphilis [Internet]. 2017. [cited 2018 Feb 13]. Available from: http://www.who.int/reproductivehealth/publications/emtct-hiv-syphilis/en/
- 5.Psaros C, Remmert JE, Bangsberg DR, Safren SA, Smit JA. Adherence to HIV care after pregnancy among women in sub-Saharan Africa: falling off the cliff of the treatment cascade. Current HIV/AIDS reports. 2015. March;12(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knettel BA, Cichowitz C, Ngocho JS, Knippler ET, Chumba LN, Mmbaga BT, et al. Retention in HIV Care During Pregnancy and the Postpartum Period in the Option B+ Era: A Systematic Review and Meta-Analysis of Studies in Africa. J Acquir Immune Defic Syndr. 2018. April;77(5):427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watt MH, Cichowitz C, Kisigo G, Minja L, Knettel BA, Knippler E, et al. Predictors of postpartum HIV care engagement for women enrolled in prevention of mother-to-child transmission (PMTCT) programs in Tanzania. AIDS Care. 2019. June 3;31(6):687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNAIDS. 90–90-90: An ambitious treatment target to help end the AIDS epidemic [Internet]. Geneva: UNAIDS; 2014. Available from: www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf [Google Scholar]
- 9.Gesesew HA, Gebremedhin Tesfay A, Demissie TD, Kerie MW, Sudhakar M, Mwanri L. Significant association between perceived HIV related stigma and late presentation for HIV/AIDS care in low and middle-income countries: A systematic review and meta-analysis. PLoS ONE. 2017;12(3):e0173928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buregyeya E, Naigino R, Mukose A, Makumbi F, Esiru G, Arinaitwe J, et al. Facilitators and barriers to uptake and adherence to lifelong antiretroviral therapy among HIV infected pregnant women in Uganda: a qualitative study. BMC Pregnancy Childbirth [Internet]. 2017. March 21;17. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5360052/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon SA, Kennedy CE, Winch PJ, Kombe M, Killewo J, Kilewo C. Stigma, Facility Constraints, and Personal Disbelief: Why Women Disengage from HIV Care During and After Pregnancy in Morogoro Region, Tanzania. AIDS Behav. 2017. January 1;21(1):317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onono M, Kwena Z, Turan J, Bukusi EA, Cohen CR, Gray GE. “You Know You Are Sick, Why Do You Carry A Pregnancy Again?” Applying the Socio-Ecological Model to Understand Barriers to PMTCT Service Utilization in Western Kenya. J AIDS Clin Res [Internet]. 2015. June [cited 2017 Oct 10];6(6). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4596237/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young SD, Hlavka Z, Modiba P, Gray G, Rooyen HV, Richter L, et al. HIV-Related Stigma, Social Norms, and HIV Testing in Soweto and Vulindlela, South Africa: National Institutes of Mental Health Project Accept (HPTN 043): JAIDS Journal of Acquired Immune Deficiency Syndromes. 2010. December;55(5):620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pretorius JB, Greeff M, Freeks FE, Kruger A. A HIV stigma reduction intervention for people living with HIV and their families. Health SA Gesondheid. 2016. December 1;21:187–95. [Google Scholar]
- 15.French H, Greeff M, Watson MJ, Doak CM. A Comprehensive HIV Stigma-reduction and Wellness-enhancement Community Intervention: A Case Study. Journal of the Association of Nurses in AIDS Care. 2015. January;26(1):81–96. [DOI] [PubMed] [Google Scholar]
- 16.Rao D, Desmond M, Andrasik M, Rasberry T, Lambert N, Cohn SE, et al. Feasibility, Acceptability, and Preliminary Efficacy of the Unity Workshop: An Internalized Stigma Reduction Intervention for African American Women Living with HIV. AIDS Patient Care and STDs. 2012. September 17;26(10):614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uys L, Chirwa M, Kohi T, Greeff M, Naidoo J, Makoae L, et al. Evaluation of a Health Setting-Based Stigma Intervention in Five African Countries. AIDS Patient Care and STDs. 2009. December;23(12):1059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apinundecha C, Laohasiriwong W, Cameron MP, Lim S. A community participation intervention to reduce HIV/AIDS stigma, Nakhon Ratchasima province, northeast Thailand. AIDS Care. 2007. October;19(9):1157–65. [DOI] [PubMed] [Google Scholar]
- 19.Peltzer K, Babayigit S, Rodriguez VJ, Jean J, Sifunda S, Jones DL. Effect of a multicomponent behavioural PMTCT cluster randomised controlled trial on HIV stigma reduction among perinatal HIV positive women in Mpumalanga province, South Africa. SAHARA J. 2018;15(1):80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossman CI, Stangl AL. Editorial: Global action to reduce HIV stigma and discrimination. J Int AIDS Soc. 2013. November 13;16(3 Suppl 2):18881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanzania Ministry of Health and Social Welfare. National guidelines for comprehensive care services for prevention of mother-to-child transmission of HIV and keeping mothers alive. 2013. [Google Scholar]
- 22.Eaton LA, Cherry C, Cain D, Pope H. A Novel Approach to Prevention for At-Risk HIV-Negative Men Who Have Sex With Men: Creating a Teachable Moment to Promote Informed Sexual Decision-Making. Am J Public Health. 2011. March;101(3):539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawson PJ, Flocke SA. Teachable moments for health behavior change: a concept analysis. Patient Educ Couns. 2009. July;76(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watt M, Knettel B, Knippler E, Kisigo G, Ngocho J, Renju J, et al. The development of Maisha, a video-assisted counseling intervention to address HIV stigma at entry into antenatal care in Tanzania. Evaluation and Program Planning. 2020. Online ahead of print. Doi: 10.1016/j.evalprogplan.2020.101859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watt MH, Knippler ET, Minja L, Kisigo G, Knettel BA, Ngocho JS, et al. A counseling intervention to address HIV stigma at entry into antenatal care in Tanzania (Maisha): study protocol for a pilot randomized controlled trial. Trials [Internet]. 2019. 20(1): 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining Feasibility and Pilot Studies in Preparation for Randomised Controlled Trials: Development of a Conceptual Framework. PLoS ONE. 2016;11(3):e0150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van de Wijgert J, Padian N, Shiboski S, Turner C. Is audio computer-assisted self-interviewing a feasible method of surveying in Zimbabwe? Int J Epidemiol. 2000. October;29(5):885–90. [DOI] [PubMed] [Google Scholar]
- 28.Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV Stigma Mechanisms and Well-Being among PLWH: A Test of the HIV Stigma Framework. AIDS Behav. 2013. June;17(5):1785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Earnshaw VA, Chaudoir SR. From conceptualizing to measuring HIV stigma: a review of HIV stigma mechanism measures. AIDS Behav. 2009. December;13(6):1160–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tshabalala J, Visser M. Developing a Cognitive Behavioural Therapy Model to Assist Women to Deal with HIV and Stigma. South African Journal of Psychology. 2011. March 1;41(1):17–28. [Google Scholar]
- 31.Kohrt BA, Jordans MJD, Rai S, Shrestha P, Luitel NP, Ramaiya MK, et al. Therapist competence in global mental health: Development of the ENhancing Assessment of Common Therapeutic factors (ENACT) rating scale. Behav Res Ther. 2015. June;69:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni M-L, Gardner LI, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012. December 15;61(5):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neufeld S, Sikkema K, Lee R, Kochman A, Hansen N. The development and psychometric properties of the HIV and Abuse Related Shame Inventory (HARSI). AIDS and Behavior. 2012;16(4):1063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turan JM, Bukusi EA, Onono M, Holzemer WL, Miller S, Cohen CR. HIV/AIDS Stigma and Refusal of HIV Testing Among Pregnant Women in Rural Kenya: Results from the MAMAS Study. AIDS and Behavior; New York. 2011. August;15(6):1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evers AW, Kraaimaat FW, van Lankveld W, Jongen PJ, Jacobs JW, Bijlsma JW. Beyond unfavorable thinking: the illness cognition questionnaire for chronic diseases. J Consult Clin Psychol. 2001. December;69(6):1026–36. [PubMed] [Google Scholar]
- 36.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British journal of psychiatry : the journal of mental science. 1987. June;150:782–6. [DOI] [PubMed] [Google Scholar]
- 37.Visser MJ, Kershaw T, Makin JD, Forsyth BW. Development of parallel scales to measure HIV-related stigma. AIDS Behav. 2008. September;12(5):759–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visser MJ, Makin JD, Vandormael A, Sikkema KJ, Forsyth BWC. HIV/AIDS stigma in a South African community. AIDS Care. 2009. February;21(2):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersson GZ, Reinius M, Eriksson LE, Svedhem V, Esfahani FM, Deuba K, et al. Stigma reduction interventions in people living with HIV to improve health-related quality of life. The Lancet HIV. 2020. February;7(2):e129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kemp CG, Jarrett BA, Kwon C-S, Song L, Jetté N, Sapag JC, et al. Implementation science and stigma reduction interventions in low- and middle-income countries: a systematic review. BMC Med. 2019. December;17(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengupta S, Banks B, Jonas D, Miles MS, Smith GC. HIV interventions to reduce HIV/AIDS stigma: a systematic review. AIDS Behav. 2011. August;15(6):1075–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stangl AL, Lloyd JK, Brady LM, Holland CE, Baral S. A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come? J Int AIDS Soc [Internet]. 2013. November 13;16(3Suppl 2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3833106/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan BT, Tsai AC. HIV knowledge trends during an era of rapid antiretroviral therapy scale-up: an analysis of 33 sub-Saharan African countries. Journal of the International AIDS Society. 2018;21(7):e25169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogden J, Nyblade L. Common at its Core: HIV-Related Stigma Across Contexts. International Center for Research on Women. 2005; [Google Scholar]
- 45.Visser MJ, Makin JD, Lehobye K. Stigmatizing attitudes of the community towards people living with HIV/AIDS. J Community Appl Soc Psychol. 2006. January;16(1):42–58. [Google Scholar]
- 46.Herek GM, Capitanio JP. Public reactions to AIDS in the United States: a second decade of stigma. Am J Public Health. 1993. April;83(4):574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Treves-Kagan S, El Ayadi AM, Pettifor A, MacPhail C, Twine R, Maman S, et al. Gender, HIV Testing and Stigma: The Association of HIV Testing Behaviors and Community-Level and Individual-Level Stigma in Rural South Africa Differ for Men and Women. AIDS Behav. 2017. September;21(9):2579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dageid W, Govender K, Gordon SF. Masculinity and HIV disclosure among heterosexual South African men: implications for HIV/AIDS intervention. Culture, Health & Sexuality. 2012. September;14(8):925–40. [DOI] [PubMed] [Google Scholar]
- 49.Mfecane S Narratives of HIV disclosure and masculinity in a South African village. Culture, Health & Sexuality. 2012. November;14(sup1):S109–21. [DOI] [PubMed] [Google Scholar]
- 50.Mburu G, Ram M, Siu G, Bitira D, Skovdal M, Holland P. Intersectionality of HIV stigma and masculinity in eastern Uganda: implications for involving men in HIV programmes. BMC Public Health [Internet]. 2014. December [cited 2018 Dec 4];14(1). Available from: http://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-14-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhead R, Skovdal M, Takaruza A, Maswera R, Nyamukapa C, Gregson S. The multidimensionality of masculine norms in east Zimbabwe: implications for HIV prevention, testing and treatment. AIDS. 2019. March;33(3):537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skovdal M, Ssekubugu R, Nyamukapa C, Seeley J, Renju J, Wamoyi J, et al. The rebellious man: Next-of-kin accounts of the death of a male relative on antiretroviral therapy in sub-Saharan Africa. Global Public Health. 2019. September 2;14(9):1252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cichowitz C, Mazuguni F, Minja L, Njau P, Antelman G, Ngocho J, et al. Vulnerable at Each Step in the PMTCT Care Cascade: High Loss to Follow Up During Pregnancy and the Postpartum Period in Tanzania. AIDS and Behavior. 2019. 23(7): 1824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonnington O, Wamoyi J, Ddaaki W, Bukenya D, Ondenge K, Skovdal M, et al. Changing forms of HIV-related stigma along the HIV care and treatment continuum in sub-Saharan Africa: a temporal analysis. Sex Transm Infect. 2017. July 1;93(Suppl 3):e052975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knettel BA, Minja L, Chumba L, Oshosen M, Cichowitz C, Mmbaga BT, et al. Serostatus disclosure among a cohort of HIV-infected pregnant women enrolled in HIV care in Moshi, Tanzania: A mixed-methods study. SSM - Population Health. 2018. November 15; 7:007–7. doi: 10.1016/j.ssmph.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oshosen M, Knettel B, Knippler E, Relf M, Mmbaga B, Watt M. “She just told me not to cry”: The experiences of HIV Testing and Counseling (HTC) among pregnant women living with HIV in Tanzania. AIDS and Behavior. 2020. Online ahead of print doi: 10.1007/s10461-020-02946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kisigo G, Ngocho J, Knettel B, Oshosen M, Mmbaga B, Watt M. “At home, no one knows”: A qualitative study of retention challenges among women living with HIV in Tanzania. PlosOne. 2020; 15(8): e0238232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eaton LA, Earnshaw VA, Maksut JL, Thorson KR, Watson RJ, Bauermeister JA. Experiences of stigma and health care engagement among Black MSM newly diagnosed with HIV/STI. Journal of Behavioral Medicine. 2018. August;41(4):458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Treves-Kagan S, Steward WT, Ntswane L, Haller R, Gilvydis JM, Gulati H, et al. Why increasing availability of ART is not enough: a rapid, community-based study on how HIV-related stigma impacts engagement to care in rural South Africa. BMC Public Health [Internet]. 2016. January 28 [cited 2020 May 8];16(83). Available from: http://link.gale.com/apps/doc/A447597827/AONE?u=duke_perkins&sid=zotero&xid=4489aec3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayieko J, Brown L, Anthierens S, Van Rie A, Getahun M, Charlebois ED, et al. “Hurdles on the path to 90–90-90 and beyond”: Qualitative analysis of barriers to engagement in HIV care among individuals in rural East Africa in the context of test-and-treat. PLoS ONE. 2018. August 30;13(8):e0202990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCoy K, Lipira L, Kemp CG, Nevin PE, Huh D, M Turan J, et al. Exploring HIV-Related Stigma as a Determinant of Engagement in HIV Care by African American Women. J Assoc Nurses AIDS Care. 2020. April;31(2):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nyblade L, Stockton MA, Giger K, Bond V, Ekstrand ML, Lean RM, et al. Stigma in health facilities: why it matters and how we can change it. BMC Med. 2019. December;17(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]