Abstract
Hepatitis C virus (HCV) remains a global public health problem even though more than 95% of infections can be cured by treatment with direct-acting antiviral agents. Resolution of viremia post antiviral therapy does not lead to protective immunity and therefore reinfections can occur. Immune cell detection of HCV activates signaling pathways that produce interferons and trigger the innate immune response against the virus, preventing HCV replication and spread. Cells in the innate immune system, including natural killer, dendritic, and Kupffer cells, interact with infected hepatocytes and present viral antigens to T and B cells where their effector responses contribute to infection outcome. Despite the immune activation, HCV can evade the host response and establish persistent infection. Plans to understand the correlates of protection and strategies to activate proper innate and adaptive immune responses are needed for development of an effective prophylactic vaccine that stimulates protective immunity and limits HCV transmission.
Keywords: Hepatitis C virus, DAA, IFN, ISGs, Innate cells, T cells, B cells, viral persistence, vaccine
Worldwide Effects of HCV Infection
Most chronic liver diseases have been attributed to hepatitis viruses [1]. An estimated 71 million persons worldwide are chronically infected with hepatitis C virus (HCV). HCV is spontaneously cleared by approximately 20% of infected adults. The remaining 80% progress to chronic hepatitis and are at risk for developing cirrhosis, hepatocellular carcinoma, or end-stage liver disease [2]. The time course between initial HCV infection and activation of innate and adaptive immune responses is critical for HCV control and clearance. Increasing our understanding of the immune response in individuals who spontaneously resolve their infection would provide invaluable guidance for vaccine development. Effective vaccination strategies are required to limit transmission and reinfection among vulnerable populations. We review the innate, humoral, and cellular adaptive immune responses to HCV infection and HCV vaccine strategies.
Molecular Virology
HCV is a member of the Hepacivirus genus in the family Flaviviridae; its only known hosts are humans and chimpanzees. Virus particles comprise a core (capsid), encapsulated by the viral envelope with associated E1 and E2 glycoproteins [3]. The 10 kb positive-sense single-strand RNA genome contains an internal ribosome entry site (IRES) at the 5’ UTR, enabling cap-independent translation of a single polyprotein [4]. Post-translational modifications by cellular and viral proteases generates 3 structural (capsid, E1, E2) and 7 non-structural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B). HCV RNA replicase is error prone and lacks proofreading activity, resulting in high mutation rates that yield millions of viral quasispecies from a single infection [4]. There are 7 major HCV genotypes, which are further classified into multiple subtypes [5]. HCV RNA acquires mutations that allow the virus to escape the immune response, which contributes to the diversity and evolution of HCV genome.
Innate Immune Response
RNA pathogen recognition receptors
Interactions between HCV and cells of the innate immune system determine viral clearance or progression to chronic infection. HCV infection of hepatocytes activates intracellular pathogen recognition receptors, including toll-like receptor 3 (TLR3), eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2, also called PKR), DExD/H-box helicase 58 (DDX58, also called RIGI), DExH-box helicase 58 (DHX58, also called LGP2), and interferon induced with helicase C domain 1 (IFIH1, also called MDA5) (Figure 1) [6–10]. Inside hepatocytes, HCV RNA exists as a double-stranded RNA (dsRNA) replication intermediate or single-stranded RNA with a free 5’ triphosphate end and 3’ poly U/UC tract [7]. RIGI binds to the 5’ triphosphate and 3’ poly U motif configuration of HCV RNA to recruit mitochondrial antiviral signaling protein (MAVS), which induces phosphorylation and activation of transcription factors IRF3 and NF-κb, resulting in expression of genes that encode interferons (IFNs) [10]. RIGI is also activated through non-canonical pathways including Riplet signaling, which is targeted by NS3–4A [11]. MDA5 is activated by HCV dsRNA replicative intermediates and the virally induced, long noncoding RNA (CFAP58-DT or lnclTPRIP-1) to induce IFNs, via a MAVS-dependent pathway [7, 12]. LGP2 functions upstream of MDA5 and positively regulates HCV-induced IFN signaling in infected hepatocytes [6].
Figure 1. Induction and Evasion of Innate Antiviral Responses by HCV.
HCV RNA structures are detected by cellular RNA sensors. In hepatocytes (left), RIGI and MDA5 recognize 5’ triphosphate RNA, dsRNA, and the long noncoding RNA lncITPRIP-1, respectively, to activate adaptor protein MAVS. MAVS recruits and activates TBK1-regulated phosphorylation of transcription factors IRF3 and NF-κb, resulting in NAP1L1-mediated nuclear translocation and production of type I interferons (IFNB) and type III interferons (IFNL). RIGI and MDA5 signaling are antagonized by HCV proteins (shown in red). HCV NS3 sequesters TBK1; HCV NS5A promotes degradation of NAP1L1; and HCV NS3/4 cleaves ring finger protein 135 (RNF135 or Riplet) and MAVS to prevent IFN expression. Endosomal TLR3 recognizes HCV dsRNA and signals through the adaptor protein TRIF to activate IRF3 hepatoma cell culture experiments. It is not clear whether endocytic uptake of cytoplasmic dsRNA occurs in hepatocytes of HCV-infected livers. HCV NS3/4A cleaves TRIF, impeding IFN signaling. HCV IRES and dsRNA bind PKR to promote or reduce its activity. HCV NS5A stimulates PKR to inhibit translation in immune cells, reducing expression of IFNs. Secreted IFNB and IFNL bind to their respective receptors IFNAR1, IFNAR2, and IFNLR1, IL10RB, expressed on hepatocytes and immune cells (right) to activate JAKs to phosphorylate STAT proteins. STATs form complexes with IRF9 and translocate to the nucleus, where they induce expression of ISGs, which amplify the IFN-mediated, antiviral immune response. HCV core upregulates SOCS3 (an inhibitor of JAK2) and the HCV NS5A protein suppresses STAT1 phosphorylation to impede expression of ISGs. Abbreviations are as follows: TBK1, TANK binding kinase 1; NAP1L1, nucleosome assembly protein 1 like 1; JAK1, Janus kinase 1; STAT, signal transducer and activator of transcription 1; SOCS3, suppressor of cytokine signaling 3; IRF9, interferon regulatory factor 9.
The regulation and activation of PKR and TLR3 during HCV infection is not clear. Although some studies found that PKR activation and host translational shutoff inhibit HCV replication in human hepatoma cells, others found that activation does not affect expression HCV genes or replication [9]. The role of PKR during HCV infection is complex because different domains of the HCV IRES activate and inhibit PKR. Additional, the HCV protein, NS5A and NS5B, binds PKR during late stages of replication to regulate its activity [9, 13]. Further research in needed to understand how interaction of PKR and NS5A affects the regulation of HCV replication. TLR3 detects HCV and signals via the adaptor complex that contains toll like receptor adaptor molecule 1 (TICAM1, also called TRIF) and TNF receptor associated factor (TRAF), resulting in activation of IRF3 and NF-κb [8]. Given that HCV dsRNA is localized to the cytosol, interaction with endosomal TLR3 is intriguing, as it implies a transport mechanism for HCV RNA into the endocytic vesicle. Further research is required to understand the effects of HCV-induced TLR3 activation on HCV replication in infected hepatocytes.
New Roles for IFNs
IFN production is an important part of the innate immune response against HCV infection. Type I IFNs (IFNA or IFNB; expressed ubiquitously) and type III IFNs (IFNL1–IFNL4; secreted by lymphocytes, macrophages, dendritic cells, epithelial cells) mediate antiviral responses [14]. Secretion of IFNB stimulates production of IFNA, which before the development of direct-acting antiviral (DAA) agents was used in treatment of chronic HCV infection [15]. A recent study found that the frameshift product of HCV core protein, termed the F protein, induce expression of IFNs in an HCV genotype-dependent manner [16]. Type III IFNs has been associated with spontaneous clearance of HCV and resolution of infection following treatment with pegylated IFNA and ribavirin. Genome-wide research studies have identified single-nucleotide polymorphisms (SNPs) in the IFNL3 and IFNL4 genes that associate with clearance of HCV [17]. However, more detailed analyses into the correlation between SNPs in IFNL3 and IFNL4 and their effects on protein expression and function would be worthy of further exploration.
IFN-stimulated genes
Detection of HCV by pathogen recognition receptors induce expression and secretion of IFNs, as described above. IFNs bind interferon-receptors on hepatocytes and immune cells to activate signaling pathways that lead to expression of interferon-stimulated genes (ISGs). HCV-infected liver tissues have upregulated expression of the ISGs 2’−5’-oligoadenylate synthetase 1 (OAS1), ribonuclease L, RIGI, DDX60, ADAR, PKR, ISG20, IRF1, IRF7, RSAD2, IFIT, IFITM, ISG15, USP18, CH25H, and RSAD2 [14]. ISGs are expressed in liver tissues from patients with chronic HCV infection, but there is an inverse correlation between response to pegylated IFNA/ribavirin combination therapy and low levels of ISG expression [18]. This is likely due to sustained ISG-mediated production of IFNs and cytokines by infected hepatocytes and innate immune cells, causing significant inflammation and liver injury. Chimpanzees and humans with high expression of ISGs in the liver prior to therapy are less likely to clear HCV infection and had poor IFNA treatment outcomes following treatment with pegylated IFNA and ribavirin [19, 20]. Moreover, liver tissues from patients with SNPs in IFNL3 associated with HCV clearance expressed lower levels of ISGs than patients without these polymorphisms [21]. High levels of ISG expression in liver therefore reduce the odds of a sustained response to anti-HCV therapy. Elevated resistance to ISG such as IFITMs enhances neutralizing antibody responses and promote HCV variant escape due to selection pressures [22].
Innate immunity
Progression of liver inflammation during HCV infection involves interactions between hepatocytes and innate immune cells, including natural killer cells (NK), dendritic cells (DCs), and liver-resident macrophages called Kupffer cells. Persistent HCV infection results in expansion and differentiation of NK cells, which varies among patients [23]. Characterization of NK cell phenotype and receptor expression have revealed modifications in NK cell function during HCV acute and chronic infection [24]. Response to IFN-based therapy has been associated with SNPs in KIR2DL3, which encodes and NK receptor, and homozygosity for a polymorphism in the gene encoding its ligand, HLA-C (the C1/C1 genotype) [25]. These findings indicate that polymorphisms that alter NK cell function can affect HCV clearance, but more studies are needed.
Plasmacytoid DCs (pDCs) produce high levels of IFNA, which amplifies the innate immune response, whereas myeloid DCs (classical BDCA1+ and cross-presenting BDCA3+) produce IFNL to activate the adaptive immune response [26]. The frequency of circulating pDC is reduced in patients with chronic HCV infection [27] Nevertheless, pDCs from these patients, when stimulated with agonists of TLR7 and TLR8, produce high levels of cytokines and chemokines, indicating that they remain responsive to HCV during persistent infection [26]. Frequencies of pDC from patients with chronic HCV infection scheduled for liver transplantation are similar in liver and blood. However, frequencies of BDCA1+ myeloid DCs are higher in blood, whereas frequencies BDCA3+ myeloid DCs are higher in liver [28]. These results suggest that DC function during end-stage liver disease contribute to the inflammation associated with persistent HCV infection.
HCV components or progeny virions can be phagocytosed by Kupffer cells, resulting in production of IL1B and IL18 [29]. IL1B is a marker of inflammation in patients with chronic hepatitis. Patients with cirrhosis have increased levels of IL18 in blood and increased levels of IL1B in liver [30]. HCV proteins, specifically core and p7, activate the NLR family pyrin domain containing 3 (NLRP3) inflammasome to stimulate release of IL1B from Kupffer cells, primary human macrophages, and transformed macrophages [31, 32]. Findings from a meta-analysis of polymorphisms in IL18 and in the solute carrier family 44 member 4 gene (SLC44A4, also called CTL4) revealed that the SNPs CTLA4 rs231775, CTLA4 rs5742909, IL18 rs1946518, and IL18 rs187238, increase susceptibility to HCV infection [33]. Future studies to characterize the effects of these SNPs on gene expression and production function, and to elucidate the role of Kupffer cells in HCV infection would be of interest.
Adaptive Immune Responses
CD8+ T cells
CD8+ T cells are important in the clearance of acute HCV infection. Spontaneous clearance of primary, acute infection is associated with broad, HCV-specific responses of CD8+ T cells, which appear 8–12 weeks after infection in peripheral blood and liver of humans and chimpanzees [34]. HCV-specific CD8+ T cells from spontaneous resolvers display CD127 and PD-1 surface markers, produce robust levels of IFNG, TNFA, and perforin, and react with multiple HCV epitopes [35–38]. In contrast, circulating CD8+ T cells from patients with persistent infection have impaired proliferation and cytotoxic functions and react with fewer HCV epitopes, which might select for expansion of mutant viruses that escape detection by T cells [35–38]. Furthermore, most of these cells are functionally exhausted as evident by their high expression of inhibitory markers PD-1, 2B4, CD160, TIM3, and KLRG1 [39, 40]. Exhaustion of CD8+ T cells during HCV infection might result from dysregulation of metabolic pathways that control cell respiration or altered expression of nucleosome genes, (i.e., histone proteins), which regulate T cell differentiation [41].
CD8+ T cells are required for long-term protection against HCV reinfection. Following HCV clearance, HCV-specific CD8+ T cells differentiate into T-effector (TEM) or T central memory (TCM) cells, and long-lived memory CD8+ cells express high levels of CD127 [35, 37]. Reinfection of chimpanzees with homologous HCV induces rapid IFNG-expressing, memory CD8+ T cell infiltration into the liver [42]. Importantly, antibody-mediated depletion of memory CD8+ T cells in chimpanzees that resolved primary HCV infection delays resolution of a secondary infection until the reappearance of antigen-specific CD8+ T cells [43]. Successful resolution of secondary HCV infection is associated with increased specificity of CD8+ T-cell receptor repertoire and improved polyfunctionality [44]. In contrast, during chronic infection, HCV-specific CD8+ T cells have a terminally exhausted (PD1hiEomeshiTCF1−CD127−) or memory-like phenotype (CD127+PD-1+TCF1+), are less polyfunctional, and have less-specific TCR repertoires [44–46]. Exhausted HCV-specific CD8+ T cells might be maintained epigenetically by expression of thymocyte selection associated high mobility group box (TOX) [47], and are incapable of mounting strong recall responses upon re-exposure to antigen [46]. In contrast, TCF1 and BCL2 enable survival of memory-like CD8+ T cells in the absence of antigen, facilitating proliferation upon re-exposure to antigen [45, 46]. Interferon-free therapies partially restore proliferation of CD8+ T cells during chronic HCV infection but do not prevent exhaustion of intrahepatic CD8+ T cells or viral escape [48].
CD4+ T cells
CD4+ T cells are critical for clearance of primary HCV infection. Spontaneous clearance of HCV is associated with broad, long-lived, HCV-specific CD4+ T cells. However, in patients with chronic infection, HCV-specific CD4+ T cells are either short-lived or absent [39]. Most HCV-specific CD4+ T cells from spontaneous resolvers are T-helper 1 (Th1) cells that express T-box transcription factor 21 (TBX21, also called TBET), IFNG, IL2, and TNF as well as Th17 cells that express IL17A and IL21 [39, 49, 50]. Th1 cells expand following HCV vaccination regimens and during treatment with DAAs, concurrent with a reduction in viremia [51, 52].
Although HCV-specific CD4+ T cells are reduced in chronically infected HCV individuals, compared to spontaneous resolvers, frequencies and specificities of CD4+ T cells are similar during the early acute phase of infection in patients with either outcome [39]. There have been many studies demonstrating the failure of CD4+ T cells to control HCV in patients who develop chronic infection. T-cell exhaustion, marked by an elevated expression of PD-1, CTLA-4, TIGIT, and TIM-3 among other inhibitory markers, might contribute to the loss of HCV-specific CD4+ T cells in chronically infected individuals [39, 49]. However, a fraction of these exhausted cells may retain effector function, as blockade of PD-1 and other inhibitory molecules restores their function and proliferation [49, 53]. These findings suggest that agents that block PD-1 or other T-cell inhibitory molecules might be developed for treatment of chronic HCV infection.
Similar to CD8+ T cells, CD4+ T cells are required for protection against HCV reinfection. Depletion of memory CD4+ T cells from chimpanzees resulted in chronic re-infection with homologous virus even after the return of this population in peripheral blood [54]. Patients with resolved HCV infections have a greater frequency of HCV-specific CD4+ memory T cells, with downregulated inhibitory surface molecules [39]. Moreover, these cells have increased proliferative capacity and cytokine production compared with cells from resolvers who develop a secondary persistent infection [55]. In patients with chronic HCV infection, increased proliferation of memory Th1 cells before initiation of DAA or interferon-based therapy correlates with a sustained virologic response (SVR) [56]. Specificity of CD4+ T cells to evolving HCV antigens is also required to protect against reinfection. Relapse of HCV infection in vaccinated chimpanzees is associated with emergence of mutated viral epitopes that are not recognized by CD4+ T cells [57].
T follicular helper (Tfh) cells have been correlated with spontaneous clearance of acute HCV infection. Tfh cells mediate germinal center reactions that promote affinity maturation of antigen-specific B cells. Circulating and intrahepatic HCV-specific Tfh cells expand during early stages of acute HCV infection in spontaneous resolvers but not in patients with chronic infections, and their presence correlates with HCV-specific neutralizing antibodies [58, 59]. Moreover, HCV-specific Tfh cells rapidly increase as viremia subsides in persistently infected individuals who achieve SVR following DAA treatment, whereas HCV-specific Th1 cells contract [60]. Given the role of Tfh cells in the clearance of HCV infection, new vaccine approaches should aim to elicit robust HCV-specific Tfh-cell responses in addition to neutralizing antibodies and other cell responses.
Although strong responses by Th1 and Tfh cells promote HCV clearance, other CD4+ T cell subsets reportedly hinder effective control of HCV infection. High frequencies of T-regulatory cells (Treg cells, CD4+CD25+Foxp3+) are found in peripheral blood and livers of patients with chronic HCV infection, where they suppress HCV-specific CD8+ and CD4+ T cells [61]. Thus, Tregs that linger after successful DAAand/or interferon therapy may still facilitate secondary HCV infection following re-exposure[62].
B cells
There has been controversy over the role of B cells in clearance of acute HCV infection. Initially, it was thought that B cells made little contribution to HCV clearance, as humans and chimpanzees are capable of resolving acute HCV infection without producing appreciable levels of neutralizing antibodies (nAbs) against HCV. However, over the last decade, multiple studies report a possible correlation between early appearance of nAbs during acute infection with spontaneous clearance [63, 64]. In fact, rapid nAb production may favor clearance of primary HCV acute infection more than enhanced nAb cross-reactivity, since chronically infected individuals also produce nAbs against multiple HCV genotypes during persistent infection [65]. However, the mechanisms of early nAb production in individuals that resolve acute HCV infection are undefined. Intriguingly, PBMCs from spontaneous resolvers have gene expression patterns associated with B-cell receptor signaling, despite an overall reduction in B-cell numbers [66]. Also, increased numbers of Tfh cells correlate with early production of antibodies in patients who spontaneously resolve their infections, suggesting that early development of Tfh cells promote a faster expansion of HCV-specific B cells that produce nAbs [58, 59]. However, more research is needed to determine whether these Tfh cells interact directly with HCV-specific B cells to promote spontaneous resolution of infection.
NAbs might also help protect against HCV reinfection. Passive transfer of nAbs protected chimpanzees and mice with humanized livers from HCV challenge [67]. Vaccination of chimpanzees with recombinant HCV E1E2 glycoproteins induced production of nAbs and strong CD4+ T cell responses that increased resolution of infection after HCV challenge [68]. Moreover, a fraction of injection drug users who resolved more than 1 HCV infection retained nAbs to conserved domains on the E2 glycoprotein [69, 70]. However, not all cases of HCV spontaneous clearance are associated with long-lived nAbs. Paradoxically, HCV-specific antibodies decrease over time in serum of some individuals who spontaneously resolve acute HCV infection, as well as in patients who achieved SVR to treatment with interferon or DAAs [71]. Maintenance of antibodies against HCV might therefore require continual stimulation of antigen-specific B cells. Indeed, HCV E2-specific B cell frequencies increase as acute HCV infection progresses to persistent infection [72].
HCV Vaccine Strategies and Development
Although 70% of individuals exposed to HCV develop persistent infection, pan-genotypic DAAs are effective in more than 95% of patients [73]. However, an estimated 95% of HCV cases worldwide remain undiagnosed and HCV transmission continues to increase among high-risk populations [74]. These groups are particularly disengaged from routine healthcare or are disproportionately affected by the expensive costs of DAAs. More importantly, treatment with pan-genotypic DAAs does not protect against HCV reinfection, so there is still a great need for a widely available and effective vaccine [75].
There are several vaccine strategies that are currently in progress, including viral vectors, DNA–plasmid, recombinant proteins, and prime-boost vaccination strategies. An earlier phase 1 trial study (Okairos/GSK vaccine), primed healthy participants with an attenuated chimpanzee adenovirus (ChAd3) viral vector then boosted with modified vaccinia virus Ankara (MVA) vector that encoded HCV nonstructural proteins NS3-NS5B. After MVA boost, CD4+ and CD8+ T cell memory and effector responses were sustained, indicating the potential for success of T-cell based vaccine strategy [76]. However, a later study by the same group revealed that vaccination of chronically HCV infected patients did not reconstitute T-cell immunity as was seen in healthy subjects [77]. The Okairos/GSK vaccine was the first to enter phase 1 and 2 trials for prevention of chronic HCV infection (see ClinicalTrials.gov NCT01436357 for details). The goal was to test a vaccine designed to prime T cells for reduction in rate of HCV persistence, but not necessarily prevent infection. Unfortunately, this prophylactic T cell-based vaccine did not reduce the overall frequency of chronic infections in study participants. This perhaps implies that generation of neutralizing antibodies should be a vital component of an effective vaccine against HCV, coupled with stimulation of robust T cell responses [78]. In any case, preventing reinfection after a sustained response to DAA therapy might be the most feasible application for a preventive HCV vaccine and is perhaps more attainable than a vaccine that induces sterilizing immunity in HCV-naïve individuals.
Future Directions
There have been tremendous advances in HCV research and in treatment of chronic infection, including our ability to cure most patients with DAA therapy. Despite this important accomplishment, the global incidence of HCV transmission remains high, because DAAs are unable to confer protective immunity to cured patients and cannot prevent new infections. Moreover, resistance-associated substitutions in the HCV genome can reduce the effectiveness of DAAs. A vaccine that induces protective immunity against HCV infection is therefore needed - especially for patients who received DAA therapy but are at risk for re-exposure. A vaccine that induces responses of T and B cells against multiple HCV genotypes and prevents selection of virus escape mutants would be a laudable achievement. Thus, investigating mechanisms of immune control of HCV infection and virus escape, especially focusing on the protective, memory immune responses of patients who have cleared acute infections more than once, may guide us towards the design and implementation of more effective vaccination strategies needed to combat the spread of this elusive human pathogen.
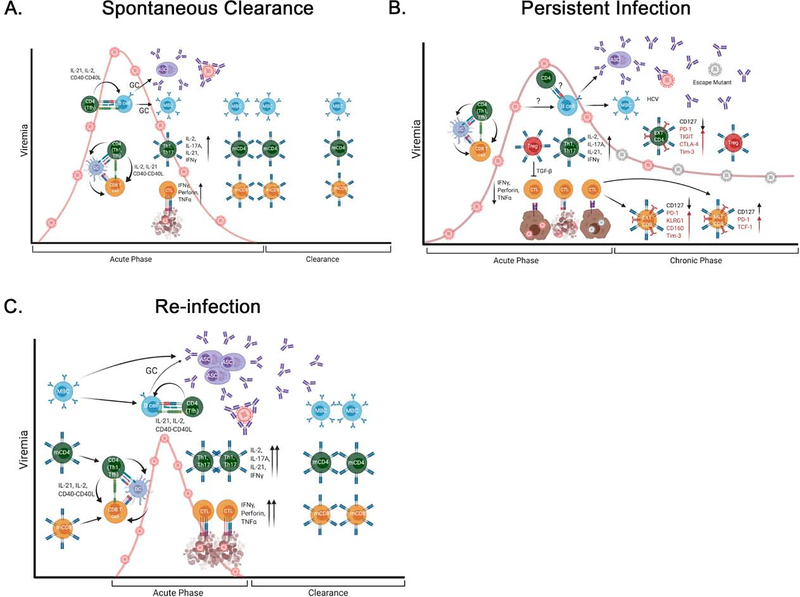
Figure 2. Adaptive immune responses in spontaneous clearance, persistence, and re-infection of HCV.
(A) Spontaneous clearance: during acute HCV infection (0–6 months), CD4+ T cells (Th1 and Tfh cells) activate HCV-specific CD8+ T and B cells indirectly (via DCs) or directly (via interactions between CD40 and CD40L). Activated CD8+ T cells and B cells enter germinal centers, where they proliferate and differentiate into effector T cells with cytotoxic function or antibody-secreting cells (ASCs), respectively. Cytolytic T cells (CTLs) kill infected hepatocytes, whereas Th1 and Th17 cells release cytokines that promote localized inflammation and potentiate the activities of CTLs. ASCs release antibodies that neutralize autologous HCV. (B) Persistence: activation of B cells and CD8+ T cells by CD4+ T cells (Th1 and Tfh cells) is limited. Treg cells reduce the activity of CTLs, and mutant viruses escape antibody neutralization and killing by T cells. As viremia persists, constant expression of inhibitory molecules (red) results in T-cell exhaustion (EXT), but some CD8+ T cells acquire a memory-like phenotype (TCF1+PD-1+). (C) Re-infection: existing memory CD4+ and CD8+ T cells and memory B cells (MBCs) are rapidly activated and proliferate upon re-exposure to HCV. Activated, HCV-specific T and B cells can undergo further rounds of affinity maturation in germinal centers and/or rapidly differentiate into effector T cells or ASCs, respectively. Cytotoxic activity and nAbs are increased during secondary HCV infection, leading to faster clearance and expansion of memory T and B cells. (GC = germinal center; mCD4+ = memory CD4+ + T cell; mCD8+ = memory CD8+ + T cell; MTL CD8+ = memory-like CD8+ T cell).
Acknowledgments
Funding
This project was supported by National Institutes of Health (NIH) grants R01AI136533, R01AI124680, R01AI096882 and R01AI126890 to A.G.; Office of Research Infrastructure Programs/Office of the Director (ORIP/OD) P51OD011132 (formerly National Center For Research Resources (NCRR) P51RR000165) to the Yerkes National Primate Research Center (A.G.) and NIH Institutional Research and Academic Career Development Award (IRACDA) Fellowships in Research and Science Teaching (FIRST) at Emory 5K12GM000680-19 (J.D.S). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- NS
nonstructural
- poly U/UC
poly uridine/uridine-cytidine
- TBK1
TANK Binding Kinase 1
- IKKε
inhibitor of nuclear factor-κB kinase
- IRF1/3/7
interferon regulatory factor 1/3/7
- NF-κb
nuclear factor-κB kinase
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- TRAF
TNF receptor associated factors
- IFNL3/4
interferon lambda3/4
- OAS1
2’−5’-oligoadenylate synthetase 1
- RIGI
retinoic-acid inducible gene I
- DDX60
DExD/H-Box Helicase 60
- ADAR
adenosine deaminase acting on RNA
- PKR
eukaryotic translation initiation factor 2 alpha kinase 2
- ISG20
interferon stimulated exonuclease gene 20
- RSAD2
radical S-adenosyl methionine domain containing 2
- IFIT
IFN- induced protein with tetratricopeptide repeats
- IFITM
interferon-induced transmembrane
- USP18
ubiquitin-specific protease 18
- CH25H
cholesterol 25-hydroxylase
- KIR2DL3
killer cell immunoglobulin like receptor two Ig domains and long cytoplasmic tail 3
- HLA-C1
human leukocyte antigen major histocompatibility complex class I
- BDCA1/3
blood dendritic cell antigens
- IL
interleukin
- NAP1L1
nucleosome assembly protein 1 like 1
- JAK1
Janus kinase 1
- STAT
signal transducer and activator of transcription 1
- SOCS3
suppressor of cytokine signaling 3
Footnotes
Potential conflict of interest: Nothing to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collaborators G.B.D.C.o.D., Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 2018. 392(10159): p. 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sebastiani G, Gkouvatsos K, and Pantopoulos K, Chronic hepatitis C and liver fibrosis. World J Gastroenterol, 2014. 20(32): p. 11033–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukh J, The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol, 2016. 65(1 Suppl): p. S2–S21. [DOI] [PubMed] [Google Scholar]
- 4.Tsukiyama-Kohara K and Kohara M, Hepatitis C Virus: Viral Quasispecies and Genotypes. Int J Mol Sci, 2017. 19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Ngoc C, et al. , Differential prevalence and geographic distribution of hepatitis C virus genotypes in acute and chronic hepatitis C patients in Vietnam . PLoS One, 2019. 14(3): p. e0212734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hei L and Zhong J, Laboratory of genetics and physiology 2 (LGP2) plays an essential role in hepatitis C virus infection-induced interferon responses. Hepatology, 2017. 65(5): p. 1478–1491. [DOI] [PubMed] [Google Scholar]
- 7.Du X, et al. , Hepatitis C virus replicative double-stranded RNA is a potent interferon inducer that triggers interferon production through MDA5. J Gen Virol, 2016. 97(11): p. 2868–2882. [DOI] [PubMed] [Google Scholar]
- 8.Li K, et al. , Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology, 2012. 55(3): p. 666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki R, et al. , Activation of protein kinase R by hepatitis C virus RNA-dependent RNA polymerase. Virology, 2019. 529: p. 226–233. [DOI] [PubMed] [Google Scholar]
- 10.Brisse M and Ly H, Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5. Front Immunol, 2019. 10: p. 1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vazquez C, Tan CY, and Horner SM, Hepatitis C Virus Infection Is Inhibited by a Noncanonical Antiviral Signaling Pathway Targeted by NS3-NS4 A. J Virol, 2019. 93(23).* Studies in human liver cells (Huh7) the HCV NS3-NS4A protein complex cleaves Riplet to regulate the TBK1-IRF3 signaling pathway to prevent innate immune activation. This study identifies a antiviral role for Riplet during HCV infection and HCV innate immune evasion mechanism.
- 12.Xie Q, et al. , Long Noncoding RNA ITPRIP-1 Positively Regulates the Innate Immune Response through Promotion of Oligomerization and Activation of MDA5. J Virol, 2018. 92(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toroney R, et al. , Regulation of PKR by HCV IRES RNA: importance of domain II and NS5A. J Mol Biol, 2010. 400(3): p. 393–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong MT and Chen SS, Emerging roles of interferon-stimulated genes in the innate immune response to hepatitis C virus infection. Cell Mol Immunol, 2016. 13(1): p. 11–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padmanabhan P, Garaigorta U, and Dixit NM, Emergent properties of the interferon-signalling network may underlie the success of hepatitis C treatment. Nat Commun, 2014. 5: p. 3872. [DOI] [PubMed] [Google Scholar]
- 16.Lai YT, et al. , Genotypic Regulations of Type I Interferon Induction Pathways by the Frame-Shift (F) Proteins of Hepatitis C Virus. J Virol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg BR, et al. , Genetic Variation at IFNL4 Influences Extrahepatic Interferon-Stimulated Gene Expression in Chronic HCV Patients . Journal of Infectious Diseases, 2018. 217(4): p. 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dustin LB, Cashman SB, and Laidlaw SM, Immune control and failure in HCV infection--tipping the balance. J Leukoc Biol, 2014. 96(4): p. 535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung PS and Shin EC, Interferon Response in Hepatitis C Virus-Infected Hepatocytes: Issues to Consider in the Era of Direct-Acting Antivirals. Int J Mol Sci, 2020. 21(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramamurthy N, et al. , Impact of Interferon Lambda 4 Genotype on Interferon-Stimulated Gene Expression During Direct-Acting Antiviral Therapy for Hepatitis C. Hepatology, 2018. 68(3): p. 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swiatek-Koscielna B, et al. , Prevalence of IFNL3 rs4803217 single nucleotide polymorphism and clinical course of chronic hepatitis C. World J Gastroenterol, 2017. 23(21): p. 3815–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wrensch F, et al. , Interferon-Induced Transmembrane Proteins Mediate Viral Evasion in Acute and Chronic Hepatitis C Virus Infection. Hepatology, 2019. 70(5): p. 1506–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strunz B, et al. , Chronic hepatitis C virus infection irreversibly impacts human natural killer cell repertoire diversity. Nat Commun, 2018. 9(1): p. 2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Njiomegnie GF, et al. , Immunomodulation of the Natural Killer Cell Phenotype and Response during HCV Infection. J Clin Med, 2020. 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehermann B, Natural Killer Cells in Viral Hepatitis. Cell Mol Gastroenterol Hepatol, 2015. 1(6): p. 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle EH, et al. , Individual liver plasmacytoid dendritic cells are capable of producing IFNalpha and multiple additional cytokines during chronic HCV infection. PLoS Pathog, 2019. 15(7): p. e1007935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolganiuc A, et al. , Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol, 2006. 177(10): p. 6758–68. [DOI] [PubMed] [Google Scholar]
- 28.Yoshio S, et al. , Human blood dendritic cell antigen 3 (BDCA3)(+) dendritic cells are a potent producer of interferon-lambda in response to hepatitis C virus. Hepatology, 2013. 57(5): p. 1705–15. [DOI] [PubMed] [Google Scholar]
- 29.Li P, et al. , The role of Kupffer cells in hepatic diseases. Mol Immunol, 2017. 85: p. 222–229. [DOI] [PubMed] [Google Scholar]
- 30.Estevez J, et al. , Differential Serum Cytokine Profiles in Patients with Chronic Hepatitis B, C, and Hepatocellular Carcinoma. Sci Rep, 2017. 7(1): p. 11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farag NS, et al. , The p7 viroporin of the hepatitis C virus contributes to liver inflammation by stimulating production of Interleukin-1beta. Biochim Biophys Acta Mol Basis Dis, 2017. 1863(3): p. 712–720. [DOI] [PubMed] [Google Scholar]
- 32.Negash AA, et al. , Modulation of calcium signaling pathway by hepatitis C virus core protein stimulates NLRP3 inflammasome activation. PLoS Pathog, 2019. 15(2): p. e1007593.* Using primary human macrophages and THP-1 cells, the authors identified the core protein of HCV as an inducer of NLRP3 inflammason activation and IL-1b production. This study demonstrates that the HCV core protein stimulates release of IL-1b leading to hepatic inflammation.
- 33.Yu Y, et al. , Relationship of genetic polymorphisms in CTLA-4 and IL-18 with viral hepatitis: evidence from a meta-analysis. Epidemiol Infect, 2019. 147: p. e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung PS, Racanelli V, and Shin EC, CD8(+) T-Cell Responses in Acute Hepatitis C Virus Infection. Front Immunol, 2014. 5: p. 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seigel B, et al. , Factors that determine the antiviral efficacy of HCV-specific CD8(+) T cells ex vivo. Gastroenterology, 2013. 144(2): p. 426–36. [DOI] [PubMed] [Google Scholar]
- 36.Jo J, et al. , Low perforin expression of early differentiated HCV-specific CD8+ T cells limits their hepatotoxic potential. J Hepatol, 2012. 57(1): p. 9–16. [DOI] [PubMed] [Google Scholar]
- 37.Lauer GM, et al. , High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology, 2004. 127(3): p. 924–36. [DOI] [PubMed] [Google Scholar]
- 38.Walker A, et al. , Distinct Escape Pathway by Hepatitis C Virus Genotype 1a from a Dominant CD8+ T Cell Response by Selection of Altered Epitope Processing. J Virol, 2016. 90(1): p. 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen DY, et al. , Hepatitis C virus-specific CD4+ T cell phenotype and function in different infection outcomes. J Clin Invest, 2020. 130(2): p. 768–773.* A longitudinal study of HCV-specific CD4+T cells demonstrated that CD4+ Tcells express high levels of inhibitory markers, PD-1 and CTLA-4, which are downregulated upon HCV clearance and subsequent memory cell differentiatoin. During chronic infection, expression of PD-1 and CTLA-4 is maintained and CD4+ Tcells are short-lived. This study demonstrate the importance of inhbitory regulation of CD4+ memory cell development in HCV infection.
- 40.Aregay A, et al. , Elimination of hepatitis C virus has limited impact on the functional and mitochondrial impairment of HCV-specific CD8+ T cell responses. J Hepatol, 2019. 71(5): p. 889–899. [DOI] [PubMed] [Google Scholar]
- 41.Wolski D, et al. , Early Transcriptional Divergence Marks Virus-Specific Primary Human CD8(+) T Cells in Chronic versus Acute Infection. Immunity, 2017. 47(4): p. 648–663 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanford RE, Walker CM, and Lemon SM, The Chimpanzee Model of Viral Hepatitis: Advances in Understanding the Immune Response and Treatment of Viral Hepatitis. ILAR J, 2017. 58(2): p. 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoukry NH, et al. , Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med, 2003. 197(12): p. 1645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdel-Hakeem MS, et al. , Selective expansion of high functional avidity memory CD8 T cell clonotypes during hepatitis C virus reinfection and clearance. PLoS Pathog, 2017. 13(2): p. e1006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utzschneider DT, et al. , T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity, 2016. 45(2): p. 415–27. [DOI] [PubMed] [Google Scholar]
- 46.Wieland D, et al. , TCF1(+) hepatitis C virus-specific CD8(+) T cells are maintained after cessation of chronic antigen stimulation. Nat Commun, 2017. 8: p. 15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alfei F, et al. , TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature, 2019. 571(7764): p. 265–269.** CD8+ T cells exhaustion during chronic HCV infection is regulated by expression of thymocyte selection associated high mobility group box (TOX). This study demonstrates that TOX plays an important role in suppression of CD8+ T cell effector function during persistent HCV infection.
- 48.Callendret B, et al. , Persistent hepatitis C viral replication despite priming of functional CD8+ T cells by combined therapy with a vaccine and a direct-acting antiviral. Hepatology, 2016. 63(5): p. 1442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kared H, et al. , Galectin-9 and IL-21 mediate cross-regulation between Th17 and Treg cells during acute hepatitis C. PLoS Pathog, 2013. 9(6): p. e1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang P, et al. , The Transcription Factor T-Bet Is Required for Optimal Type I Follicular Helper T Cell Maintenance During Acute Viral Infection. Front Immunol, 2019. 10: p. 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chmielewska AM, et al. , Combined adenovirus vector and hepatitis C virus envelope protein prime-boost regimen elicits T cell and neutralizing antibody immune responses. J Virol, 2014. 88(10): p. 5502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burchill MA, et al. , Memory re-differentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J Viral Hepat, 2015. 22(12): p. 983–91. [DOI] [PubMed] [Google Scholar]
- 53.Raziorrouh B, et al. , Inhibitory molecules that regulate expansion and restoration of HCV-specific CD4+ T cells in patients with chronic infection. Gastroenterology, 2011. 141(4): p. 1422–31, 1431 e1–6. [DOI] [PubMed] [Google Scholar]
- 54.Grakoui A, et al. , HCV persistence and immune evasion in the absence of memory T cell help. Science, 2003. 302(5645): p. 659–62. [DOI] [PubMed] [Google Scholar]
- 55.Abdel-Hakeem MS, et al. , Signatures of protective memory immune responses during hepatitis C virus reinfection. Gastroenterology, 2014. 147(4): p. 870–881 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendez-Lagares G, et al. , Memory T Cell Proliferation before Hepatitis C Virus Therapy Predicts Antiviral Immune Responses and Treatment Success. J Immunol, 2018. 200(3): p. 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puig M, et al. , CD4+ immune escape and subsequent T-cell failure following chimpanzee immunization against hepatitis C virus. Hepatology, 2006. 44(3): p. 736–45. [DOI] [PubMed] [Google Scholar]
- 58.Raziorrouh B, et al. , Virus-Specific CD4+ T Cells Have Functional and Phenotypic Characteristics of Follicular T-Helper Cells in Patients With Acute and Chronic HCV Infections. Gastroenterology, 2016. 150(3): p. 696–706 e3. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, et al. , Circulating CXCR3(+) Tfh cells positively correlate with neutralizing antibody responses in HCV-infected patients. Sci Rep, 2019. 9(1): p. 10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smits M, et al. , Follicular T helper cells shape the HCV-specific CD4+ T cell repertoire after virus elimination. J Clin Invest, 2020. 130(2): p. 998–1009.* CD4+ T cell population analyzed from patients with chronic HCV infection revealed that cells with Tfh signature prolong after HCV elimination with DAA therapy. This study implicates the importance of a Tfh population that may contribute to HCV immunity following DAA treatment.
- 61.Barjon C, et al. , Role of regulatory T-cells during hepatitis C infection: From the acute phase to post-transplantation recurrence. Dig Liver Dis, 2015. 47(11): p. 913–7. [DOI] [PubMed] [Google Scholar]
- 62.Langhans B, et al. , Increased peripheral CD4(+) regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J Hepatol, 2017. 66(5): p. 888–896. [DOI] [PubMed] [Google Scholar]
- 63.Kinchen VJ, et al. , Broadly Neutralizing Antibody Mediated Clearance of Human Hepatitis C Virus Infection. Cell Host Microbe, 2018. 24(5): p. 717–730 e5.** Generation of broadly neutalizing antibodies (bNAbs) coincided with spontaneous clearance of HCV during acute infection. Longitudinal analysis of bNAbs isolated from spontaneous resolvers showed that these bNAbs targeted E2 to neutralize autologous HCV variants, which drove them to acquire mutations on E2 that resulted in a loss of viral fitness.
- 64.Walker MR, et al. , Clearance of hepatitis C virus is associated with early and potent but narrowly-directed, Envelope-specific antibodies. Sci Rep, 2019. 9(1): p. 13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swann RE, et al. , Broad Anti-Hepatitis C Virus (HCV) Antibody Responses Are Associated with Improved Clinical Disease Parameters in Chronic HCV Infection. J Virol, 2016. 90(9): p. 4530–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg BR, et al. , Longitudinal transcriptomic characterization of the immune response to acute hepatitis C virus infection in patients with spontaneous viral clearance. PLoS Pathog, 2018. 14(9): p. e1007290.* Longitudinal trancriptomic analysis of PBMCs collected from high risk exposure patients before and after acute HCV infection revealed elevated innate immune expression signatures but reduced B cell transcriptional makers during infection. This study identifies tranciptomic signatures in patients following natural exposure due to risky drug injections.
- 67.Bukh J, et al. , Immunoglobulin with High-Titer In Vitro Cross-Neutralizing Hepatitis C Virus Antibodies Passively Protects Chimpanzees from Homologous, but Not Heterologous, Challenge. J Virol, 2015. 89(17): p. 9128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meunier JC, et al. , Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J Infect Dis, 2011. 204(8): p. 1186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merat SJ, et al. , Cross-genotype AR3-specific neutralizing antibodies confer long-term protection in injecting drug users after HCV clearance. J Hepatol, 2019. 71(1): p. 14–24. [DOI] [PubMed] [Google Scholar]
- 70.Keck ZY, et al. , Broadly neutralizing antibodies from an individual that naturally cleared multiple hepatitis C virus infections uncover molecular determinants for E2 targeting and vaccine design. PLoS Pathog, 2019. 15(5): p. e1007772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kee KM, et al. , Decreased anti-hepatitis C virus titer and associated factors in chronic hepatitis C patients after sustained virological response: a prospective study. J Gastroenterol Hepatol, 2012. 27(6): p. 1106–11. [DOI] [PubMed] [Google Scholar]
- 72.Boisvert M, et al. , Novel E2 Glycoprotein Tetramer Detects Hepatitis C Virus-Specific Memory B Cells. J Immunol, 2016. 197(12): p. 4848–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collins LF, et al. , Direct-Acting Antivirals Improve Access to Care and Cure for Patients With HIV and Chronic HCV Infection. Open Forum Infect Dis, 2018. 5(1): p. ofx264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lyons MS, et al. , Prevalence of Diagnosed and Undiagnosed Hepatitis C in a Midwestern Urban Emergency Department. Clinical Infectious Diseases, 2016. 62(9): p. 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shoukry NH, Hepatitis C Vaccines, Antibodies, and T Cells. Front Immunol, 2018. 9: p. 1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swadling L, et al. , A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med, 2014. 6(261): p. 261ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swadling L, et al. , Highly-Immunogenic Virally-Vectored T-cell Vaccines Cannot Overcome Subversion of the T-cell Response by HCV during Chronic Infection. Vaccines (Basel), 2016. 4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen F, et al. , Antibody Responses to Immunization With HCV Envelope Glycoproteins as a Baseline for B-Cell-Based Vaccine Development. Gastroenterology, 2020. 158(4): p. 1058–1071 e6.** Analysis of blood samples from mice, non-human primates (NHPs), and volunteers in a phase 1 vaccine trial demonstrated that NHPs are prmosing models for studying the effectiveness of HCV immunogens to elicit neutralizing antibody responses. This study provides guideline efforts for development of a vaccine that elcicts broad neutralization towards HCV genotypes.