Abstract
Background:
Stress during pregnancy and maternal inflammation are two common prenatal factors that impact offspring development. Asthma is the leading chronic condition complicating pregnancy and a common source of prenatal stress and inflammation.
Objective:
The goal of this study was to characterize the developmental impact of repeated allergic asthma inflammation during pregnancy on offspring behavioral outcomes and brain inflammation.
Methods:
Pregnant female C57BL/6 mice were sensitized with ovalbumin (OVA) or PBS vehicle control and then randomly assigned to receive daily aerosol exposures to the same OVA or PBS treatment during early, gestational days (GD)2-GD9, or late pregnancy, GD10-GD17. Maternal sera were collected after the first and last aerosol induction regimen and measured for concentrations of corticosterone, anti-OVA IgE, and cytokine profiles. Juvenile male and female offspring were assessed for locomotor and social behaviors, and later as adults assessed for anxiety-like, and marble burying behaviors using a series of behavioral tasks and their brains were evaluated for region-specific differences in cytokine concentrations.
Results:
In early gestation, both PBS and OVA-exposed dams had similar serum corticosterone concentration at the start (GD2) and end (GD9) of daily aerosol inductions. Only OVA-exposed dams showed elevations in cytokines that imply a diverse and robust T helper cell-mediated immune response. Male offspring of early OVA-exposed dams showed decreases in open-arm exploration in the elevated plus maze and increased marble burying without concomitant changes in locomotor activity or social interactions. These behavioral deficits in early OVA-exposed male offspring were associated with lower concentrations of G-CSF, IL-4, IL-7, IFNγ, and TNFα in the hypothalamus. In late gestation, both PBS and OVA-exposed dams had increased corticosterone levels at the end of daily aerosol inductions (GD17) compared to at the start of inductions (GD10). Male offspring from both PBS and OVA-exposed dams in late gestation showed similar decreases in open arm exploration on the elevated plus maze compared to OVA male offspring exposed in early gestation. No behavioral differences were present in female offspring across all treatment groups; however, females of dams exposed to OVA during early gestation displayed similar reductions as males in hypothalamic G-CSF, IL-7, IL-4, and IFNγ.
Discussion:
The inflammatory responses from maternal allergic asthma in early gestation and resulting increases in anxiety-like behavior in males support a link between the timing of prenatal insults and sex-specific developmental outcomes. Moreover, the heightened stress responses in late gestation and concomitant dampened inflammatory response to allergic asthma suggest that interactions between the maternal immune and stress-response systems shape early life fetal programming.
Keywords: Pregnancy, Allergic Asthma, Autism, Cytokines, Corticosterone, Mouse, Behavior
1.0. INTRODUCTION
Asthma is among the leading chronic conditions that result in complications during pregnancy (Shebl and Chakraborty 2019), and the prevalence of allergies and asthma have been increasing steadily for several decades (Lundback et al. 2016). Allergies and asthma are estimated to affect 8% of individuals in the US (Shebl and Chakraborty 2019), and one-third of women with asthma report more severe symptoms during pregnancy (Belanger et al. 2010; Schatz et al. 1988). This is significant given that several clinical studies have associated maternal allergies and asthma during pregnancy with adverse outcomes in offspring, including respiratory illness (Spiegel et al. 2018), low birth weight (Robijn et al. 2019), and increased risk for neurodevelopmental deficits (Auger et al. 2019). In a recent clinical case-control study, children born to mothers with asthma had a higher likelihood of later having a diagnosis of autism spectrum disorder (Gong et al. 2019), and similar links were shown between maternal asthma and attention deficit/hyperactivity disorder (ADHD) (Instanes et al. 2017; Liu et al. 2019). In mice, episodes of allergic asthma during pregnancy resulted in changes in circulating serum cytokines, including IL-6 and IL-17A, accompanied by behavioral alterations in offspring (Schwartzer et al. 2017). This is important as maternal cytokines including IL-6 and IL-17A have been shown to impact fetal brain development in rodents (Choi et al. 2016; Smith et al. 2007), and elevated maternal serum cytokine levels in humans are associated with greater risk of mental illness in the offspring (Ratnayake et al. 2013). While preclinical rodent studies support the clinical associations between maternal inflammation, allergies, and asthma with neurodevelopmental risks in offspring, these animal models are limited in that they are often based on a single acute, or series of transient, inflammatory responses (Meyer 2019; Weber-Stadlbauer and Meyer 2019). However in humans, allergies and asthma often present as chronic inflammatory responses rather than acute inflammatory episodes (Bousquet et al. 2000). This discrepancy between rodent studies of acute allergic asthma in pregnancy and human clinical studies of chronic maternal inflammation underscores a need to better model the developmental impact of persistent maternal inflammation on offspring outcomes. To address this, we compared the impact of repeated daily allergic asthma inductions during the first versus second half of pregnancy on offspring neurobehavioral outcomes in mice.
The timing and duration of prenatal environmental insults, including maternal immune activation, affect different developmental processes that rely on typical neuroimmune signaling, resulting in unique behavioral outcomes based on when in gestation the perturbation occurs. For example in mice, maternal exposure to the viral mimic polyI:C impaired sensorimotor gating and reduced exploratory behavior in offspring when exposure occurred in early pregnancy (prenatal day 9) while maternal polyI:C in late gestation (prenatal day 17) impaired working memory and increased perseverative behaviors (Meyer et al. 2006; Meyer et al. 2008). In humans, a case-control study of mothers and children revealed a 7-fold increase in schizophrenia risk for individuals born to mothers exposed to influenza during the first trimester of pregnancy (Brown et al. 2004), while bacterial infection and asthma in the second trimester were both associated with an increased risk for having a child with autism spectrum disorder (Atladottir et al. 2010; Croen et al. 2005). Furthermore, maternal fever during the first two trimesters has also been reported with ADHD (Gustavson et al. 2019). Overall, maternal immune activation during specific windows during pregnancy is linked to increased risk in offspring of neurodevelopmental and neurodegenerative disorders (Knuesel et al. 2014), highlighting the importance of timing as a modulating factor in the link between maternal inflammation and offspring development.
Allergies and asthma are mediated by a heterogeneous T-helper (Th) cell response, further modulated by stress related signaling and hypothalamic-pituitary-adrenal (HPA) axis hormones (Chen and Miller 2007). While Th-2 cells and associated cytokines are commonly credited as the primary signals of allergic asthma, increased severity or recruitment of other immune cells can be coordinated by Th-1, Th-17 and regulatory T cells to further mediate the immune response (Lloyd and Hessel 2010). Moreover, elevations in HPA axis glucocorticoids, such as cortisol and corticosterone, in response to environmental stressors can modulate allergic asthma immune reactivity (Besedovsky and del Rey 2006) and these hormones directly impact fetal development. More specifically, prenatal stress is linked to myriad neurodevelopmental and neuropsychiatric disorders across the lifespan that include susceptibility to anxiety, depression, autism spectrum disorder, and ADHD (Hodes and Epperson 2019). Evidence from rodent studies further support this link between prenatal stress and behavioral abnormalities, with prenatally stressed offspring exhibiting increased anxiety, hyperactivity, and social behavior deficits (for review see (Silberman et al. 2016)). Given the physiological interactions between immune and neuroendocrine function, it is important to assess the combined neurodevelopmental impact of prenatal stress with persistent maternal allergic asthma during pregnancy (Hantsoo et al. 2019).
Both the immune system and HPA axis undergo modifications during pregnancy to support fetal development. The immune system adapts in early pregnancy by shifting towards a Th-2 phenotype to help prevent fetal rejection, followed by subsequent immunological changes as pregnancy progresses (Mor and Cardenas 2010). In addition, the HPA axis is hyperactive at key timepoints in pregnancy marked by elevations in circulating cortisol/corticosterone in the third trimester in humans (Duthie and Reynolds 2013). Considering these different temporal shifts in immune system signaling as well as the changes in HPA activity during early compared to late phases of gestation and their known consequences (individually) on prenatal neurodevelopment, we hypothesized that offspring born to mothers exposed to daily allergic asthma inductions in late gestation would exhibit a distinct behavioral phenotype and concomitant changes in neuroimmune signaling compared to offspring born to dams exposed in early gestation. To test this, pregnant mice were exposed daily through early (GD2-GD9) or late (GD10-GD17) pregnancy and offspring were assessed for social, anxiety, and locomotor behaviors in late juvenile/early adulthood followed by an evaluation of the levels of cytokines in multiple brain regions associated with these behaviors.
Given that prolonged alterations in adult central nervous system immune signaling are reported in several neuropsychiatric and neurodevelopmental disorders with developmental origins linked to maternal inflammation during pregnancy (Khandaker et al. 2015; Siniscalco et al. 2018), we sought to directly test whether repeated allergic asthma throughout gestation imparts lasting neuroimmune changes in the adult brain, specifically the prefrontal cortex, thalamus, hypothalamus, and amygdala. These regions were selected because of their well-established roles in modulating social hierarchy (Wang et al. 2014), stress reactivity (Liu et al. 2020), and repetitive motor functions (Kim et al. 2016). In addition, we also evaluated the immunological and endocrine adaptations to repeated allergic asthma exposures in maternal serum throughout pregnancy. Considering that stress hormones can enhance or suppress immune signaling, and the documented developmental deficits caused by maternal allergic asthma and prenatal stress individually, we hypothesized that pregnant mice exposed to daily inductions would show a rise in corticosterone over pregnancy, with distinct peripheral cytokine and antibody responses in early versus late gestation in response to varying corticosterone levels.
2.0. METHODS
2.1. Animals
Male and female C57BL/6J mice generated from breeding pairs purchased from Jackson Laboratory (Bar Harbor, MA, USA) were bred and maintained at Mount Holyoke College, South Hadley, MA and maintained at ambient room temperature on a 12 h light/dark cycle (lights on at 0800 h). Mice were group housed in individually ventilated cages with same-sex littermates until breeding at 8-weeks of age. Cages were maintained in a temperature-controlled (23°C) vivarium with food and water provided ad libitum. All procedures were performed with approval by the Mount Holyoke College Institutional Animal Care and Use Committee and in accordance with the guidelines provided by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Maternal Allergic Asthma (gestational OVA exposure)
Sexually naive female C57BL/6J mice were sensitized with 10μg ovalbumin (OVA, Sigma, St Louis, MO, USA) and 1mg (Al)OH3 (Invitrogen, San Diego, CA, USA) dissolved in 200μl of phosphate buffered saline (PBS) injected intraperitoneally at 6 and 7 weeks of age. Beginning at 8 weeks of age, females were mated overnight, presence of seminal plug was checked daily, noted as gestational day (GD)0.5, and single-housed. Pregnant mice were randomly assigned to receive either an aerosolized solution of 1% (wt/vl) OVA in PBS or PBS alone for eight daily 45-minute induction sessions on either GD2-GD9 (Early) or GD10-GD17 (Late) always occurring between 0800 h and 1000 h (Figure 1A). Only dams with 3 or more pups were included in the study. Based on this criterion, only 2 dams were excluded from the study, each from the PBS Late group.
Figure 1: Diagram of experimental procedures and sample size.

(A) Female mice were sensitized to OVA-Alum (↓) prior to pregnancy and exposed to OVA or PBS aerosolized inductions (■) in early (GD2-9) or late (GD10-17) gestation. Whole blood was collected (⇣) after the first and final induction. (B) A total of 51 dams were treated with PBS or OVA and were either sacrificed at the end of treatment or serum cytokine analysis or left undisturbed for behavioral assessments of offspring.
2.3. Experimental Design
A total of 51 dams were used to generate four treatment groups: Early-PBS (n=14), Early-OVA (n=12), Late-PBS (n=13), Late-OVA (n=12). A subset of these dams (n=27) were designated for maternal sera collection (Figure 1B) and the remaining mice (n=24) were left undisturbed after the final induction, except for cage changes, through parturition and until weaning at postnatal day P(21) for subsequent behavioral analyses on offspring. In order to minimize the impact of blood draws on offspring development, dams used for offspring behavior did not undergo blood collection. Maternal sera were collected from the lateral saphenous vein in awake, restrained dams immediately after the first induction (GD2 or GD10) and last induction (GD9 or GD17). Four hours after the final induction, dams were anesthetized using 3% isoflurane and blood collected from cardiac puncture. Blood was allowed to clot at room temperature for 30 minutes, centrifuged at 10000 x g for 10 minutes at 4°C, and then serum collected and stored at −80°C. An additional 8 pregnant female mice were sensitized with PBS or OVA containing 1mg (Al)OH3 but were left undisturbed (i.e. no induction) control group, and blood was collected concurrently with experimental mice on GD2 and GD9 (n=4) or GD10 and GD17 (n=4).
2.4. Corticosterone and Anti-OVA IgE ELISA
Maternal serum was evaluated for concentrations of corticosterone and Anti-OVA IgE using commercially available colorimetric ELISA kits. Sera from both first (GD2 or GD10) and final (GD9 or GD17) inductions and equivalent gestational days in untreated dams were assessed using a competitive corticosterone ELISA kit (Invitrogen, EIACORT, and Arbor Assays, K014-H1) per manufacturer specifications. Sensitivity of kits were 18.6 pg/mL with an interassay CV of 7.9%. Anti-OVA IgE was evaluated in sera collected on the last induction (GD9 or GD17) using a sandwich ELISA (Cayman Chemicals) with a sensitivity of 3.12 ng/mL. All samples were processed in triplicate and unknown quantities were extrapolated from a standard curve using a 4-parameter logistic regression. Anti-OVA IgE antibodies were undetectable in one OVA dam; thus it was excluded from serum analyses.
2.5. Multiplex Bead-Based Immunoassay – Maternal Sera
A 17-plex Th1/Th2/Th9/Th17/Th22/Treg Procarta Mouse Cytokine kit (Invitrogen, EPX170-26087-901) was used according to manufacturer’s instructions to quantify the following cytokines: IFNγ, IL-12(p70), IL-13, IL-1β, IL-2, IL-4, IL-5, IL-6, TNFα, GM-CSF, IL-18, IL-10, IL-17A, IL-22, IL-23, IL-27, and IL-9. Bead sets were analyzed on a MAGPix system (Luminex) using xPONENT 4.1 software. Unknown sample cytokine concentrations were estimated using a 5-parameter logistic regression curve derived from the known reference cytokine concentrations supplied by the manufacturer. Supernatant aliquots did not undergo multiple freeze thaw cycles. The sensitivity of this assay allowed for the detection of cytokine concentration with the following limit of detection: IFNγ: 0.09 pg/mL; IL-12(p70): 0.21 pg/mL; IL-13: 0.16 pg/mL; IL-1β: 0.14 pg/mL; IL-2: 0.10 pg/mL; IL-4: 0.03 pg/mL; IL-5: 0.32 pg/mL; IL-6: 0.21 pg/mL; TNFα: 0.39 pg/mL; GM-CSF: 0.19 pg/mL; IL-18: 9.95 pg/mL; IL-10: 0.69 pg/mL; IL-17A: 0.08 pg/mL; IL-22: 0.24 pg/mL; IL-23: 2.21 pg/mL; IL-27: 0.34 pg/mL; IL-9: 0.28 pg/mL. Analytes from all samples were above the minimum detectable limits.
2.6. Offspring Behavior Assessments
A total of 24 dams were used to generate 136 offspring (male = 68, female = 68) for behavioral testing (Figure 1B). Behavioral assessments began at P21 immediately after weaning with the juvenile social interaction task. At 8 weeks of age mice were evaluated for anxiety-like behavior using the elevated plus maze and perseverative digging in the marble burying task. All behavioral assessments were performed between 0800 h and 1200 h by individuals blind to treatment conditions. All offspring from each litter completed each behavioral task in the same order according to the timeline outlined above, beginning with the social interaction task, followed by the elevated plus maze and then the marble burying task.
2.6.1. Locomotor activity and social interaction
On the morning of weaning, experimental offspring were evaluated for changes in interactive social behavior using the reciprocal social interaction (SI) task. Experimental mice were placed individually into clean plastic cages (25 cm × 14 cm × 12 cm) and allowed to habituate for 20 minutes while being video recorded to monitor locomotor activity. Experimental mice were then quickly returned to their home cage and marked with blue (experimental mice) or pink (sex- and weight-matched (±5 g) C57BL/6J-stimulus mice) hair chalk (OPAWZ, Ontario, CAN). Experimental and stimulus mice were returned to the arena at opposite ends and allowed to interact for 20 min. Mice were recorded during this time and later scored for the total distance traveled, amount of social sniffing, body contact, approach, and avoidance behaviors using EthoVision XT 15 three-point body and social interaction modules. Each stimulus mouse was used 2-4 times throughout the study and never more than once per day.
2.6.2. Elevated plus maze
To evaluate changes in general anxiety, 8-week-old mice were assessed using an elevated plus maze constructed of white Plexiglas in full light plus a 500W portable halogen work light to serve as an aversive stimulus. The apparatus consisted of two open arms (30 cm × 5 cm × 0.5 cm) and two perpendicular closed arms (30 cm × 5 cm × 15 cm) extending from a central platform. The entire maze was elevated approximately 1 m from the floor. Mice were video recorded and placed in the central platform and allowed to freely explore the maze for 5 min. The videos were later scored using EthoVision XT 15 for the number of entries into each arm, time spent exploring the edges of the open arm, time spent peeking into open arms from the center, and the total time spent in each arm. Reductions in the percent of open arm exploration (time in the open arm divided by the total time in both the open and closed arms) were interpreted as increased anxiety.
2.6.3. Marble burying
One week after the elevated plus maze task, male and female offspring were habituated individually for 10 min to opaque plastic mouse cages (25 x 14 x 12 cm) filled with a 4-cm thick layer of clean corncob bedding. Following habituation, animals were returned to their home cage and 15 glass marbles were laid out in five rows of three marbles placed equidistance apart. Mice were then returned to their testing cages and allowed to explore under full lighting for 10 min. At the end of the 10-min period, animals were gently removed from the testing cages and the number of marbles buried was recorded by an observer blind to treatment condition. Only marbles covered by 75% or more bedding were counted as buried.
2.7. Brain punch and tissue prep
One week following the final behavior assessment, 3-4 offspring from each group and sex (n = 31, lmale and 1 female per litter) were anesthetized with 3% isoflurane and whole brains were dissected, flash frozen in liquid nitrogen, and stored at −80°C. Prior to sectioning, brains were warmed to −20°C and all tools, including stainless-steel microdissection block and razor blade used to section, were chilled prior to use. 1.0 mm cryostat sections were taken from each brain and the entire prefrontal cortex was collected using a razor blade. For the thalamus, hypothalamus, and amygdala isolations, a chilled 1.6mm bore blunted syringe was used to punch holes in the tissue for removal. All microdissections were taken while referencing the Allen Institute’s Mouse Brain Atlas. After removal, tissue was stored at −80°C until further use. Prior to cytokine analysis, Cell Signaling Technologies cell lysis buffer (Cell Signaling Technologies, Danvers, MA), containing protease and phosphatase inhibitors was used for tissue lysis. Brain regions were weighed and the appropriate amount of protein lysis buffer added (10x dry weight of tissue). Briefly, the tissue sections were incubated in lysis buffer with agitation for 20 min on ice followed by sonication for 30 seconds. Cell lysate was then vortexed at top speed for 30 seconds and centrifuged at 20,000 x g for 10 minutes at 4°C. Supernatant was aliquoted and stored at −80°C until needed. Protein concentrations were measured using Bio-Rad Benchmark Plus Spectrophotometer system.
2.8. Multiplex Bead-Based Immunoassay – Brain Homogenates
Analysis of brain cytokines was performed using multiplex mouse 25-plex bead immunoassay (Milliplex mouse cytokine/chemokine magnetic bead panel #MCYTMAG70PMX25BK). The following cytokines were quantified: granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon γ (IFN-γ), interleukin-1α (IL-1α), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, interferon gamma-induced protein 10 (IP-10), keratinocyte-derived cytokine (KC), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, MIP-2, chemokine (C-C motif) ligand 5 (RANTES), tumor necrosis factor α (TNF-α). Standards and reagents were all prepared according to manufacturer’s recommendations. Briefly, each brain sample was diluted to reflect 70μg/mL of protein concentration per sample, and samples were run in duplicate. Twenty-five microliters of sample, standards, and buffer blanks were loaded to the 96-well plate with appropriate amounts of assay buffer and matrix solution. The plate was then incubated overnight with antibody-coupled magnetic beads. The following day, after a series of washes, the plate was incubated with a biotinylated detection antibody on a shaker for 1 hour. Streptavidin-phycoerythrin was added and incubation while shaking continued for 30 min. Washes were done using Bio-Plex handheld magnet (Bio-Rad Laboratories, Hercules, CA, USA). After the final wash, the plate was analyzed using plate reader Bio-Rad Bio-Plex 200 (Bio-Rad Laboratories, Hercules, CA, USA) and analyzed using Bio-Plex Manager software (Bio-Rad Laboratories). The following were the minimal amounts of detectable cytokine concentrations: G-CSF: 1.7 pg/mL; GM-CSF: 10.9 pg/mL; IFNγ: 1.1 pg/mL; IL-1α: 10.3 pg/mL; IL-1β: 5.4 pg/mL; IL-2: 1.0 pg/mL; IL-4: 0.4 pg/mL; IL-5: 1.0 pg/mL; IL-6: 1.1 pg/mL; IL-7: 1.4 pg/mL; IL-9: 17.3 pg/mL; IL-10: 2.0 pg/mL; IL-12 (p40): 3.9 pg/mL; IL-12 (p70): 4.8 pg/mL; IL-13: 7.8 pg/mL; IL-15: 7.4 pg/mL; IL-17: 0.5 pg/mL; IP-10: 0.8 pg/mL; KC: 2.3 pg/mL; MCP-1: 6.7 pg/mL; MIP-1α: 7.7 pg/mL; MIP-1β: 11.9 pg/mL; MIP-2: 30.6 pg/mL; RANTES: 2.7 pg/mL; TNF-α:2.3 pg/mL. Sample concentration that fell below minimal detection value were given a proxy value of half the limit of detection for statistical comparisons.
2.9. Statistical Analyses
Data were analyzed using RStudio version 1.1.1335 (2019). Corticosterone concentrations were log-transformed to meet assumptions of normality, confirmed using quantile-quantile plots, and assessed using a treatment by timing mixed-model ANOVA followed by Tukey post hoc comparisons from the “nlme” and “stats” packages. Anti-OVA IgE was evaluated using two-way (treatment by timing) ANOVA followed by Tukey post hoc corrections from the “car” package. Correlations between maternal corticosterone and IgE concentrations in OVA-treated dams were assessed using pearson’s r correlation coefficient. Maternal sera cytokines were assessed using nonparametric Kruskall-Wallis followed by pairwise comparisons using Dunn’s test using “dunn.test” package.
To control for pseudoreplication and litter-to-litter variations, offspring measures were evaluated using multilevel modeling to control for Type I error (Lazic and Essioux 2013; Lazic et al. 2018). All behavioral measures were assessed using separate linear mixed-effects models for male and female offspring with maximum likelihood estimates and Type III sums of squares using the “nlme” package. First, a basic 2-level random effects model was constructed with Yij representing each behavioral observation for the ith animal of level 1 nested in the jth litter at level 2. Yij was equal to the level 1 intercept β0j plus the residual, noted as rij. Then each animal was nested in litter at level 2 represented by a fixed intercept for litter as γ 00, plus a litter-specific random intercept that varied by j litter, u0j. The random intercept was assumed to be normally distributed with a mean of 0 and variance equal to τ00. The random effects model is represented by the following equation:
Next, fixed effects were added to the model and tested for model fit using the log likelihood ratio test. In this mixed-effects model, which included both fixed and random components, Yij was estimated from the level one intercept β0j and the main effect of β1j for OVA treatment and β2j, for timing (Late). A third model was then constructed that included the addition of β3j for the two-way interaction of treatment by timing (OVA:Late) and tested for model fit compared to the random effects and fixed main effects models. The full model included fixed main effects and interaction as well as variance components for both level 1 intercept, represented by γ00, and random level 2 error (litter), u0j, plus the remaining residual, rij.
To account for overdispersion in the marble burying counts, data were fitted with a negative binomial model that included random effects for litter. All model variations for behavioral tasks were assessed using the likelihood ratios test and the best model was selected based on the AIC criteria. The final model was assessed graphically to verify the data met the assumptions of linearity, normality or poisson (marble burying), and homogeneity of variance. Models which included a significant interaction component in the fixed effect were confirmed with Wald’s Test using F- values estimated using Kenward-Rogers degrees of freedom and Type III sums of squares followed by post hoc analysis using Tukey corrections.
Adult brain cytokine concentrations were assessed using three-way Analysis of Variance (treatment x timing x sex) followed by simple main effects analysis for significant interactions and Tukey post hoc corrections when applicable. Brain cytokines were correlated with behavioral measures using Pearson’s r correlation coefficients.
3.0. RESULTS
3.1. Maternal Corticosterone
To quantify the natural rise in maternal corticosterone throughout gestation, sera were collected from untreated pregnant dams concurrently with dams exposed to PBS and OVA inductions (Figure 2A). In untreated control dams, levels of corticosterone differed across gestational days, F(3,9) = 25.59, p < 0.001. At the start of pregnancy (GD2), corticosterone concentration was estimated at 29 ng/mL, with levels rising by GD9, 60.5ng/mL (CI: 0.14 – 120.9), t(9) = −0.92, p = 0.80. On GD10, corticosterone levels increased to 99.3 ng/mL (CI: 48.25-150.4), t(9) = 2.96, p = 0.02, with a more substantial rise observed on GD17 to 206.8 ng/nL (CI: 160.69-252.90), t(9) = 6.11, p < 0.001. Pairwise comparisons with Tukey correction confirmed that corticosterone levels at GD17 were higher than GD9, t(9) = 7.79, p < 0.001, and GD10, t(9) = 3.69, p = 0.02.
Figure 2: Corticosterone and anti-OVA IgE antibody concentrations in maternal serum of undisturbed dams and dams exposed to aerosolized PBS or OVA in early or late gestation.
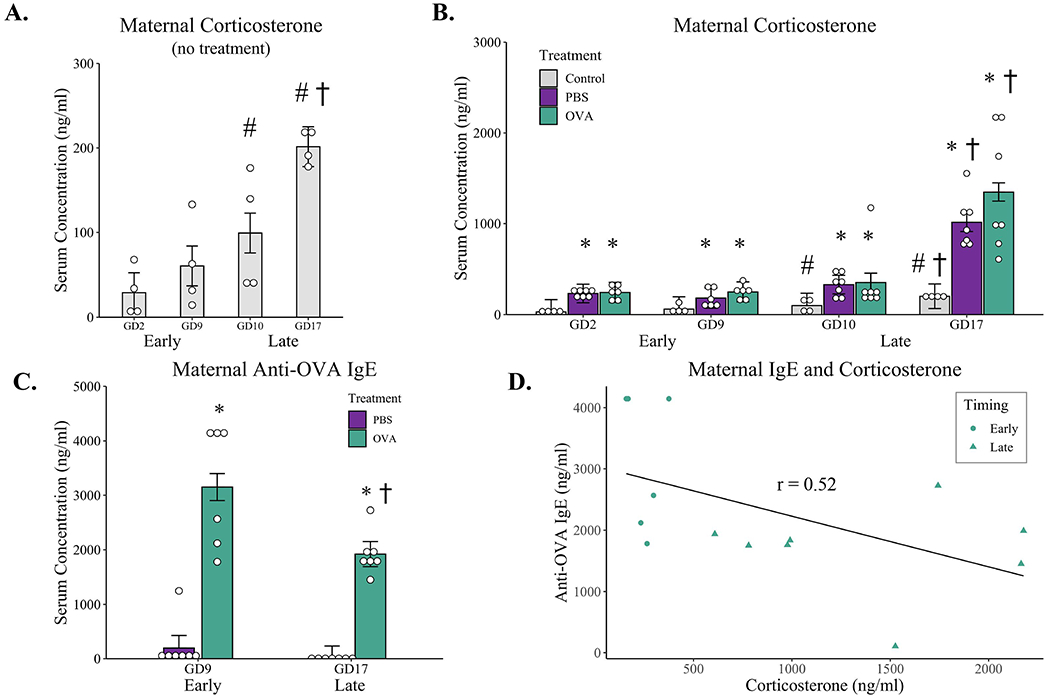
(A) Corticosterone concentrations in maternal sera throughout pregnancy from undisturbed dams: n = 4 for all timepoints. (B) Serum concentration of corticosterone taken immediately after the first (GD2 or GD10) and last (GD9 or GD17) inductions from dams exposed to aerosolized PBS or OVA daily in early or late pregnancy compared to untreated controls. (C) Anti-OVA IgE concentrations in sera from PBS and OVA-exposed dams taken four hours after the final induction in early (G9) or late (G17) gestation. (D) Negative correlation between maternal anti-OVA IgE antibodies and corticosterone concentrations in OVA-treated dams taken at GD10 (Early) and GD17 (Late). * p < 0.05 compared to gestational day-matched untreated control, † p < 0.05 compared to treatment-matched GD9 and GD10, # p < 0.05 compared to GD2 control; all determined by mixed-model ANOVA with Turkey post hoc corrections. Correlation assessed using pearson’s r correlation coefficient. Open dots represent individual mice; bars represent marginal means +/− SE. Early-PBS n = 6-7 (serum was not available from one mouse on GD9 due to technical mistakes), Early-OVA n = 6, Late-PBS n = 7, Late-OVA n = 7.
A separate repeated-measures model was fitted which included maternal sera collected after repeated PBS and OVA aerosol inductions, and corticosterone concentrations were compared to undisturbed control dams (Figure 2B). The model confirmed an effect of time over gestation, F(3,29) = 49.43, p < 0.001, with corticosterone levels averaging 168 ng/mL (CI: 34.6-302) across all dams at GD2 and increasing beginning at GD10, t(29) = 3.74, p = 0.004, to an average of 260 ng/mL (CI: 129.3-392). This rise in corticosterone continued through GD17, t(29) = 9.88, p < 0.001, with a mean concentration of 857 ng/mL (CI: 733.2-980) across all three conditions. There was also a main effect of aerosolized exposure treatment, F(2, 32) = 48.42, p < 0.001, with higher levels of corticosterone observed in both PBS, t(29) = 8.58, p < 0.001, and OVA dams, t(29) = 9.12, p < 0.001, compared to undisturbed controls. Specifically, undisturbed control dams averaged 97.8ng/mL over the four timepoints measured, while PBS and OVA dams had a 4 to 5-fold higher average concentration of corticosterone: PBS, 439.1ng/mL (CI: 324.2-554); OVA 548.6ng/mL (CI: 430.8-666). These elevations in corticosterone in response to aerosol inductions were similar between PBS and OVA dams, t(29) = 0.71, p = 0.76.
There was a treatment by timing interaction, F(6, 29) = 2.66, p = 0.04, with elevations in corticosterone in response to aerosolized inductions dependent on gestational timing. Specifically, maternal corticosterone increased from GD10 to GD17 in both PBS, t(29) 4.64, p = 0.007, and OVA dams, t(29) = 6.25, p < 0.001, with concentrations of corticosterone peaking on GD17 to a concentration of 1014.5 ng/ml (CI: 811.4-1218) in PBS-treated dams and 1349.0 ng/ml (CI: 1145.9-1552) in OVA-treated dams. These differences in corticosterone between the first and last induction dates were not present in early gestation (between GD2 to GD9) for either PBS-treated, t(29) = 1.39, p = 0.96, or OVA-treated dams, t(29) = 0.97, p = 0.99.
3.2. Anti-OVA IgE
Four hours after the final PBS or OVA induction (G9 for Early, G17 for Late), maternal serum was collected and measured for the presence of Anti-OVA IgE using ELISA (Figure 2C). A treatment-by-timing ANOVA revealed an effect of treatment, F(1, 23) = 76.45, p < 0.001, with an average of 2535.5 ng/mL (CI: 2186-2885) of anti-OVA IgE present in OVA-exposed dams compared to PBS 98.5 ng/mL in PBS-treated mice. While there was no difference in anti-OVA IgE concentrations in PBS sera between Early and Late treatment groups, F(1,23) = 0.35, p = 0.56, there was a treatment by timing interaction, F(1,23) = 4.89, p = 0.04, with higher levels of anti-OVA IgE present in OVA-exposed dams in the Early condition, 3149.91 ng/mL (CI: 2637-3663), compared to treatment-matched OVA dams exposed in late gestation, 1921.06 ng/mL (CI: 1446-2396), t(23) = 3.64, p = 0.007. There was a moderate negative correlation between maternal IgE concentrations and serum corticosterone levels in OVA-treated dams, r = −0.52, t(12) = −2.14, p = 0.054, with higher stress hormone levels associated with a lower concentration of anti-OVA IgE antibodies (Figure 2D).
3.3. Serum Cytokines
Maternal serum was collected 4 hours after the final induction at either GD9 (Early) or GD17 (Late). In the Early group, several cytokines were elevated in maternal sera of OVA-treated dams compared to PBS-exposed mice including, IFNγ, IL-18, IL-5, IL-17A, IL-22, IL-23, TNFα, IL-1β, IL-6, and IL-10. Conversely in the late exposure groups, no differences were found between PBS and OVA-treated dams for any of the cytokines measured (Table 1). Serum cytokine levels were similar for PBS-exposed dams at both early and late timepoints. Correlation analysis between maternal serum corticosterone and cytokines did not yield any significant findings.
Table 1:
Maternal Serum Cytokines
| Cytokines | Early |
Late |
||
|---|---|---|---|---|
| PBS | OVA | PBS | OVA | |
| GM-CSF | 2.76 (0.64) | 4.02 (3.05) | 2.76 (2.15) | 3.31 (2.41) |
| IFNγ | 2.13 (1.17) | 58.16 (158.46)* | 2.01 (2.38) | 2.37 (7.53) |
| IL-1β | 1.18 (1.46) | 10.91 (8.35)* | 2.94 (2.95) | 5.21 (3.29) |
| IL-2 | 2.68 (1.36) | 5.64 (5.72) | 3.83 (4.53) | 3.67 (3.12) |
| IL-4 | 1.01 (0.65) | 0.93 (0.97) | 0.63 (0.61) | 1.17 (0.47) |
| IL-5 | 23.12 (19.76) | 112.14 (348.99)* | 13.87 (18.30) | 24.67 (19.81) |
| IL-6 | 13.06 (6.78) | 93.12 (259.01)* | 13.74 (21.03) | 25.92 (10.88) |
| IL-9 | 18.12 (5.51) | 35.14 (29.55) | 18.12 (15.42) | 27.52 (21.09) |
| IL-10 | 5.95 (17.11) | 126.63 (605.22)* | 5.95 (26.23) | 22.39 (24.41) |
| IL-12(p70) | 1.19 (0.99) | 1.10 (6.53) | 1.13 (0.80) | 0.83 (0.25) |
| IL-13 | 2.51 (0.27) | 2.98 (1.94) | 2.51 (1.92) | 2.15 (0.68) |
| IL-17A | 1.84 (1.19) | 6.42 (26.70)* | 1.91 (3.26) | 3.22 (2.13) |
| IL-18 | 91.85 (113.60) | 637.73 (686.39)* | 242.58 (299.44) | 310.52 (104.53) |
| IL-22 | 39.62 (78.51) | 922.12 (13960.94)* | 42.03 (115.26) | 155.66 (246.32) |
| IL-23 | 23.91 (20.76) | 488.19 (4243.98)* | 26.16 (88.87) | 75.75 (141.77) |
| IL-27 | 4.44 (2.44) | 6.21 (8.93) | 5.32 (4.15) | 9.29 (5.72) |
| TNFα | 5.40 (3.67) | 18.84 (28.19)* | 6.38 (9.60) | 13.74 (9.75) |
Cytokines concentrations (pg/ml) in maternal serum collected 4 hours after inductions in either early (GD9) or late (GD17) gestation. Data represent median (IQR).
p < 0.05 compared to PBS-Early as determined by Dunn’s Test
3.4. Locomotor Activity
Male and female offspring had similar locomotor activity in an open field at 21 days of age: males 3760.67 cm (CI: 3514.09 - 4007.26); females 3787.26 cm (CI: 3490.86 – 4083.67). A random effects-only model was used for both male and female offspring (Table S1) and no differences were observed in total distance traveled for male or female offspring across treatment groups or gestational timing (Figure 3A).
Figure 3: Behavioral outcomes of juvenile male and female offspring of dams exposed to aerosolized PBS or OVA in early or late gestation.

Pregnant female mice were exposed to either aerosolized PBS or OVA during early (GD2-GD9) or late (GD10-GD17) gestation and offspring were tested at postnatal day 21 for locomotor activity and social interaction. Reported is (A) the total distance traveled during 20 minutes of habituation to a novel open field arena prior to the social interaction task; (B) social sniffing of a novel age- and sex-matched stimulus mouse; (C) total time spent in close proximity to a novel stimulus mouse; (D) cumulative time moving towards the novel stimulus mouse in the social interaction task. Open dots represent individual mice; bars represent marginal means +/− SE. Early-PBS (7 litters) males n = 17, females n = 21; Early-OVA (6 litters) males n = 16, females n = 17; Late-PBS (6 litters) males n = 18, females n = 14. Late-OVA (5 litters) males n = 17, females n = 16.
3.5. Social Interactions
No treatment or timing effects were present in the social interaction task. For all behaviors measured, the random effect-only models were best suited for both male and female offspring (Table S2–5). For social sniffing, male offspring spent an average of 48.02 seconds (CI: 40.3 - 55.74) sniffing the novel sex- and weight-matched stimulus mice, with similar social sniffing times observed in female offspring 47.76 (CI: 39.93 - 55.6) (Table S2, Figure 3B). In addition, total time in physical contact with the sex- and weight-matched stimulus mouse (i.e. body contact) was similar in both male, 231.84 seconds (CI: 206.5- 257.21) and female offspring, 218.31 seconds (CI: 196.47-240.14), for all treatment groups (Table S3, Figure 3C). No differences in approach times were observed in male, 187.57 seconds (CI: 171.12 - 204.03) and female offspring, 188.36 seconds (CI: 171.20- 205.52) (Table S4, Figure 3D). Finally, avoidance behavior was no different between all treatment groups for both male (CI: 158.10 – 190.22) and female (CI: 153.86 – 174.99) offspring (Table S5).
3.6. Elevated Plus Maze
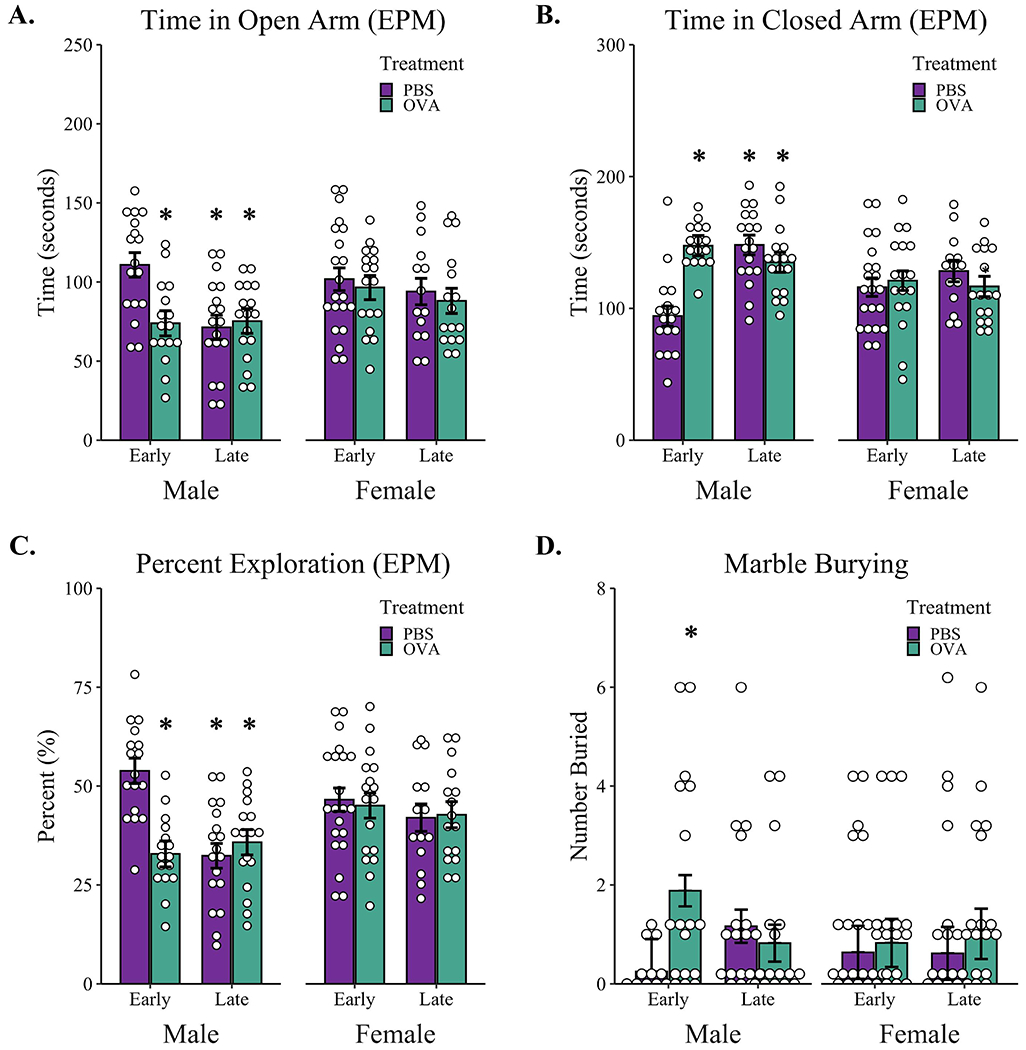
To assess the long-term stress response of offspring to gestational allergic asthma exposure, adult offspring were evaluated for anxiety-like behaviors using the elevated plus maze. For female offspring, a random-effects only model was used for time spent in the open arm with an estimated average of 95.31 seconds (CI: 87.25-103.36) exploring the open arm, and no differences observed between treatment groups or timing of exposure (Table S6). For male offspring, a mixed-effects model was chosen that included a treatment-by-time interaction as a fixed effect and litter as random effects (Table S6). Male Early-PBS offspring spent an estimated 108 seconds (CI: 90.93-125.52) exploring the open arms of the maze. Conversely, male Early-OVA offspring had an approximately 36-second reduction in open arm exploration, β = −36.38 (CI: −62.01 - −10.75), t(18) = −2.89, p = 0.001. A similar decrease in open arm exploration was present in male offspring of both Late-PBS and Late-OVA dams: Late-PBS, β = −39.73 (CI: −65.57 - −23.89), t(18) = −3.13, p = 0.001; Late-OVA, β = 43.91 (CI: 7.27-80.56), t(18) = 2.44, p = 0.03, with no difference in open arm exploration time between PBS and OVA-exposed male offspring in late gestation, t(18) = −0.59, p = 0.94 (Figure 4A).
Figure 4: Behavioral outcomes of adult male and female offspring of dams exposed to aerosolized PBS or OVA in early or late gestation.
Pregnant female mice were exposed to either aerosolized PBS or OVA during early (GD2-GD9) or late (GD10-GD17) gestation and offspring were tested in adulthood with the elevated plus maze and marble burying tasks (A-D). Reported is (A) total time adult offspring spent in the open arm of the elevated plus maze; (B) total time in the Closed arms of the elevated plus maze; (C) percent of time offspring spent in the open arm of the elevated plus maze as a percentage of the total time spent in both open and Closed arms; (D) number of marbles buried in the marble burying task. * p < 0.05 compared to Early-PBS as determined by linear mixed effects modeling with treatment and timing as fixed effects and litters as random effects. Open dots represent individual mice; bars represent marginal means +/− SE. Early-PBS (7 litters) males n = 17, females n = 21; Early-OVA (6 litters) males n = 16, females n = 17; Late-PBS (6 litters) males n = 18, females n = 14. Late-OVA (5 litters) males n = 17, females n = 16. EPM - elevated plus maze.
For time spent in the Closed arms, female offspring had similar Closed arm exploration times in both treatment conditions and gestational exposure times. A random-effects model (Table S7) estimated an average of 120.52 seconds (CI: 112.54-128.51) of Closed arm exploration for female offspring during the five-minute elevated plus maze task. For male offspring, a mixed-effects model with a treatment-by-time interaction estimated that Early-PBS offspring spent an average of 94.97 seconds (CI: 80.59-108.99) in the Closed arm. Male Early-OVA mice spent an average of 53 seconds longer in the Closed arm compared to Early-PBS male offspring, β = 53.08 (CI 31.95-74.20), t(18) = 5.12, p < 0.001. Similarly, male offspring from dams exposed during late gestation spent more time in the closed arm compared to sex-matched Early-PBS offspring, β = 54.06 (CI: 33.11-75.0), t(18) = 5.26, p < 0.001, with no difference in Closed arm exploration between male Late-PBS and Late-OVA mice, t(18) = 1.38, p = 0.53 (Figure 4B).
Percent open arm exploration was calculated as the time spent in the open arms divided by the total time spent in the open and Closed arms. There were no differences in percent open arm exploration in female mice across all treatment and timing conditions (Table S8). A random-effects only model confirmed female offspring spent 44% of their exploration time in the open arms, CI: 40.80-47.48. For male offspring, a mixed-effects model with treatment-by-timing interaction as a fixed effect (Table S8) estimated male Early-PBS offspring spent 53% (CI: 46.35-59.98) of their time exploring the open arms of the elevated plus maze. Conversely, male Early-OVA mice spent only 32.2% (CI: 24.8-39.6) of their time exploring the open arms, β = −20.95, t(18) = −4.23, p < 0.001. This lower percent open arm exploration was also present in male Late-PBS, β = −21.80, t(18) = −4.39, p < 0.001, and Late-OVA offspring, β = 25.73, t(18) = −3.65, p = 0.002. Specifically, male offspring born to mothers exposed to PBS or OVA aerosol in late gestation had similar percent open arm exploration times, t(18) = −0.95, p = 0.78, with mean values of 31.4% (CI: 23.9-38.8) for PBS-treated and 36.1% (CI: 28.7-43.6) for OVA-treated dams (Figure 4C).
Despite differences in exploration times in the elevated plus maze resulting from prenatal aerosol inductions, male and female offspring from all treatment groups showed similar levels of locomotor activity in this task. A random-effects only model estimated that male offspring traveled an average of 1810 cm (CI: 1714.64-1904.72) and female offspring traveled an average distance of 1887cm (CI: 1798.16-1975.96) during the 5-minute elevated plus maze task. No differences in distance traveled were present between PBS and OVA treatment conditions or gestational timing (Table S9).
3.7. Marble Burying
Male offspring from Early-PBS dams buried no more than 1 marble out of 15 possible during the 10-minute task. In comparison, male Early-OVA mice buried upwards of 4 to 6 marbles compared to sex- and time-matched offspring from PBS-exposed dams, β = 2.02, z = 2.77, p = 0.028 (Table S10, Figure 4D). Male offspring from dams exposed in late gestation also buried more marbles compared to PBS-exposed offspring in early gestation, β = 1.54, z = 2.09, p = 0.037, although this mean increase of one marble was no longer significant when correcting for multiple comparisons (p 0.16). Similarly, there was no difference in the number of marbles buried between Late-PBS and Late-OVA offspring, z = 0.69, p = 0.90. For female offspring, no differences were observed in marble burying behavior between treatment groups or timing (Table S10, Figure 4D). On average, female offspring buried only 1 marble during the 10-minute task.
3.8. Region-Specific Brain Cytokine Concentrations
Following behavioral assays, whole brains were taken from one random male and female adult offspring per litter and region-specific cytokine analysis was performed on the thalamus, hypothalamus, prefrontal cortex, and amygdala. While there were no differences in inflammatory cytokines observed in the thalamus, prefrontal cortex, or amygdala in any treatment groups (Tables S11–13), there were several sex- and timing-dependent differences present in the hypothalamus (Table S14). A 3-way ANOVA revealed a treatment by timing interaction for the levels of G-CSF in the hypothalamus, F(1, 23) = 30.22, p < 0.001. A simple main effects analysis confirmed treatment differences between PBS and OVA-exposed offspring in the early, F(1, 13) = 30.86, p < 0.001, but not late exposure conditions, F(1, 14) = 2.97, p = 0.11. Specifically, male and female offspring of Early OVA-exposed dams had a 4-fold lower concentration of G-CSF in the hypothalamus compared to sex and timing-matched offspring of PBS-exposed dams (Figure 5A). A similar treatment by timing interaction was also present for IL-7, F(1, 23) = 14.31, p < 0.001, IL-4 F(1, 23) = 4.60, p = 0.043, and IFNγ concentrations, F(1, 23) = 13.30, p < 0.01 with lower cytokine levels present in male and female OVA-Early offspring compared to sex and timing-matched PBS offspring for IL-7, F(1, 13) = 26.63, p < 0.001, IL-4, F(1, 13) = 11.8, p = 0.004, and IFNγ, F(1, 13) 22.10, p < 0.001. These treatment effects in male and female offspring of Early-OVA dams represent more than a 2-fold reduction for both IL-7 and IL-4 levels in the hypothalamus (Figure 5B, 5C) and a 1.5-fold decrease in IFNγ levels compared to PBS-Early offspring (Figure 5D). While OVA exposure in late gestation did not impact concentrations of IL-7, IL-4, or IFNγ in the hypothalamus, there was a timing by sex interaction for IL-7, F(1, 23) = 6.60, p = 0.02, with female offspring of late-exposed dams showing lower concentrations compared to male late-exposed offspring of both treatment groups, F(1, 14) = 9.78, p = 0.007 (Figure 5B). Interestingly, there was a treatment by sex interaction for TNFα in the early-exposure condition, F(1, 11)= 7.75, p = 0.02, that was not present across late-exposure groups, F(1, 12) = 0.0005, p = 0.98. Specifically, male Early-OVA offspring had a two-fold reduction in TNFα compared to sex-matched PBS-Early offspring and these lower concentrations of TNFα were not present in female offspring exposed in early gestation (Figure 5E). Finally, there were no significant correlations between hypothalamic cytokine concentrations and any behaviors measured.
Figure 5: Cytokine concentrations in the hypothalamus of male and female adult offspring of OVA or PBS-exposed dams.
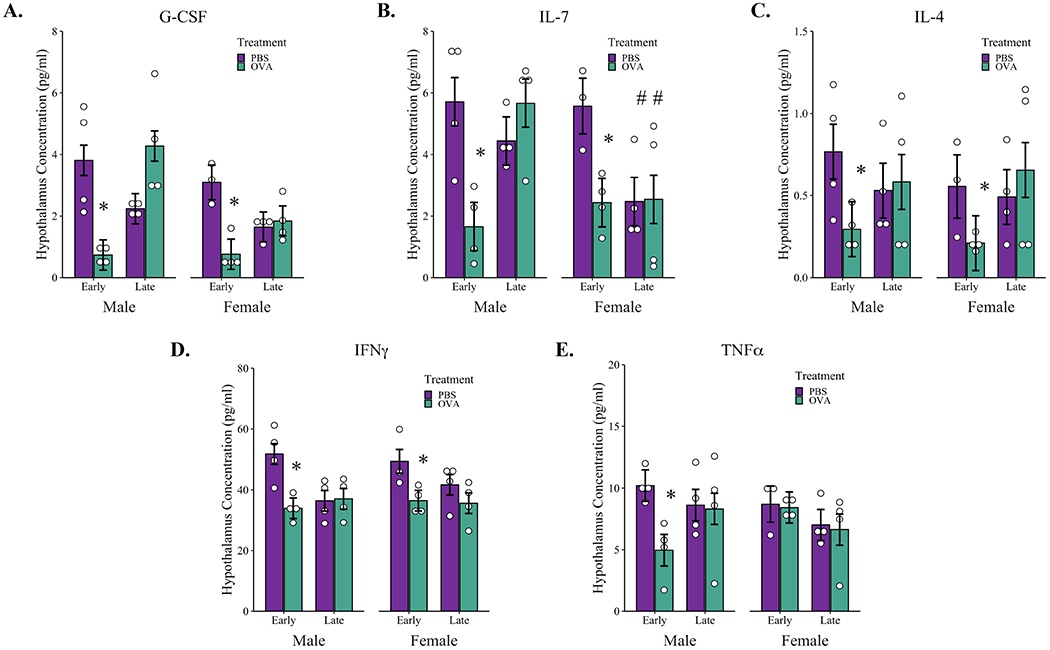
The hypothalamus was dissected from adult brains following completion of behavioral testing and the concentration of hypothalamic cytokine levels were assessed using multiplex bead-based immunoassays. The concentrations of (A) G-CSF, (B) IL-7, (C) IL4, (D) IFNγ and (E) TNFα are represented as pg/mL after being normalized to total protein content. * p < 0.05 compared to sex and timing-matched control, # p < 0.05 compared to timing-matched male offspring as determined by factorial ANOVA followed by simple main effects. Open dots represent individual mice; boxplots represent median and IQR. Early-PBS males n = 4, females n = 3; Early-OVA males n = 4, females n = 4; Late-PBS males n = 4, females n = 4. Late-OVA males n = 4, females n = 4.
4.0. DISCUSSION
Adverse experiences during pregnancy including prenatal stress, elevated maternal glucocorticoids, and chronic inflammation from allergies and asthma can have lasting impacts on offspring brain and behavior development. We hypothesized that the timing of allergic asthma exposure during pregnancy would result in distinct behavioral and neuroinflammatory outcomes in offspring based on whether the exposure occurred in early or late gestation. Our examination of maternal serum corticosterone and cytokine concentrations across pregnancy revealed marked changes in stress and immune responses between early and late gestation. In support of our hypothesis, dams in late gestation had the highest circulating corticosterone levels and, anti-OVA IgE antibodies were dampened in late vs. early-exposed OVA dams. Subsequently, dams exposed to OVA in late gestation did not show increased levels of any of the 25 T helper cell associated cytokines. This heightened corticosterone response to an environmental stressor in late gestation coincided with a dampening of immune activation in response to allergic asthma, determined by a reduction in anti-OVA IgE antibodies produced and an absence of serum cytokine elevations following the eight-day treatment. Behavioral assessments of offspring revealed sex-specific differences, with male offspring from Early-OVA dams displaying decreases in open-arm exploration in the elevated plus maze and increased repetitive marble burying behavior compared to sex and time-matched offspring of Early-PBS dams. When aerosol inductions occurred in late gestation, male offspring of both PBS and OVA-exposed dams showed similar heightened anxiety-like states comparable to levels observed in offspring of Early-OVA dams. In female offspring, no differences in behavioral measures were observed between all treatment and timing groups, suggesting a sex-specific impact of daily maternal allergic asthma episodes on offspring behavior. Finally, early gestational allergic asthma reduced expression of several cytokines in the hypothalamus of adult male and female offspring, including G-CSF, IL-7, IL-4, IFNγ, and TNFα (males only). Together, the behavioral changes observed in male offspring and reduced hypothalamic cytokines in males and females, along with the unique stress and maternal immune responses measured between late and early gestation suggest that the timing of adverse experiences during pregnancy determine the developmental impact on offspring through changes in maternal physiology.
Corticosterone and IgE-mediated Inflammation
Over the course of pregnancy in humans and rodents there is an increase in HPA axis signaling and a resulting rise in serum cortisol and corticosterone levels. At the start of pregnancy in humans, salivary cortisol levels are low with concentrations increasing through late pregnancy (Obel et al. 2005). Similarly in rodents, corticosterone levels remain relatively low in the first half of pregnancy and begin to rise after GD10, with a peak concentration by GD16-17 (Barlow et al. 1974; Montano et al. 1991). This pattern of increased maternal corticosterone in late pregnancy was evident in our undisturbed control and aerosol exposed animals on GD17. Compared to the undisturbed control dams, corticosterone levels were higher in mice undergoing both OVA and PBS aerosol inductions at all timepoints measured during pregnancy, suggesting that the stimulus of the aerosol exposure apparatus and the associated regular handling by researchers induces a stress response. Despite these elevations in stress hormone following aerosol inductions, no differences were observed in serum corticosterone levels between OVA and PBS groups in either early or late gestation, indicating that allergic asthma does not further impact peripheral corticosterone levels. This elevation in serum corticosterone in response to aerosol exposure but not allergic asthma was also reported in a previous mouse study using a similar OVA allergic asthma paradigm (Sutherland et al. 2009). While serum corticosterone levels were similar between OVA and PBS-treated dams, the relative increases in corticosterone from the stress of aerosol inductions differed between early and late pregnancy, with dams exposed in late pregnancy showing larger elevations in serum corticosterone. This phenomenon has been reported in previous mouse studies as well, with pregnant mice exposed to an acute stressor on GD14 and GD17 showing a much greater stress response compared to non-pregnant mice (Barlow et al. 1974) and unstressed pregnant mice (Jafari et al. 2017). The dynamic HPA axis signaling during pregnancy that promotes fetal development (Brunton et al. 2008) is likely to influence concurrent environmental immune stimuli in ways that alter the natural course of fetal neurodevelopment.
Increases in maternal corticosterone following repeated OVA-inductions during late gestation were associated with no detectible serum cytokine response and attenuated anti-OVA IgE concentrations compared to early OVA-exposed dams. Antigen-specific IgE is released in response to an acute allergic response, and the reductions in anti-OVA IgE coupled with unaltered serum cytokines following aerosolized OVA inductions in late gestation suggest that stress-induced elevations in corticosterone suppressed the allergic immune response. Indeed, this is directly supported by our finding that serum corticosterone levels negatively correlated with circulating anti-OVA IgE antibodies in OVA-exposed dams. In fact, glucocorticoid receptor activation through corticosterone and dexamethasone was shown to inhibit IgE-mediated allergic responses in vitro (Nakamura et al. 2016). Similarly, stress-induced increases in corticosterone levels in mice following an acute forced swim task was shown to dampen cytokine levels in bronchoalveolar lavage fluid (BALF) following OVA-aerosol challenge (Sutherland et al. 2009). This observed elevation in stress reactivity through increases in circulating maternal corticosterone in late gestation helps explain the concomitant suppression of peripheral inflammation in the serum of dams following OVA-induced allergic asthma in the present study. Specifically, we postulate that the natural rise in serum corticosterone was exacerbated by repeated aerosol exposure which inhibited the inflammatory signaling and suppressed OVA-induced allergic immune activation in late gestation.
Impact of Maternal Cytokine Changes
During early gestation, repeated OVA inductions increased circulating serum cytokines compared to PBS-exposed dams reflecting a heterogeneous T cell response. In this study we saw a marked increase in IL-5 in maternal serum following early gestational OVA-inductions. This is not surprising given that allergic asthma is characterized by a Th-2-dominant immune response marked by elevations in IL-4, IL-5, and IL-13. However, maternal serum cytokine elevations in response to Early-OVA inductions also favored a Th-17 phenotype, characterized by elevations in IL-17A, IL-22, and IL-23, along with canonical pro-inflammatory innate markers including IL-1β, IL-6, IL-18, and TNFα which are associated with multiple types of T helper cell responses. Th-17 polarization has been shown to accompany OVA-mediated allergic asthma in mice (Manni et al. 2016) and there is strong evidence that antigen-specific allergic asthma requires reciprocal interactions between Th-2 and Th-17 pathways (Choy et al. 2015; Hasegawa et al. 2017). Our data further support this interaction and provide evidence for Th-1 and innate immune signaling as well. Concomitant increases in anti-OVA specific IgE antibody concentrations with Th-2, Th-17, and other T helper cell-associated cytokines suggest that allergic asthma responses of the lungs through inhalation of aerosolized OVA inductions can alter peripheral maternal inflammation and recruit a heterogeneous T cell response.
Glucocorticoid signaling can also impact maternal cytokine signaling given that glucocorticoid receptor activation dampens T helper cell responses to allergic asthma. Indeed, glucocorticoids are often prescribed for autoimmune diseases due to their anti-inflammatory properties (Goodin 2014). In the current study, early gestational exposure to maternal allergic asthma increased circulating cytokines while late gestational exposure did not have a similar effect. Importantly, serum from OVA-exposed dams at G17 showed significantly elevated corticosterone levels above all earlier timepoints, making it plausible that glucocorticoids played a role in dampening the immune response to MAA during late gestation. In fact, lower levels of anti-OVA IgE, a proxy for allergic inflammation, was correlated with higher concentrations of corticosterone. These differences in cytokine and IgE concentrations in OVA-exposed dams between late and early gestation, along with the relatively low cytokine concentrations observed in late gestation, highlight an important interaction between gestational timing and corticosterone in mediating the allergic asthma response throughout pregnancy.
Behavioral Impacts of Maternal Allergic Asthma
Maternal immune activation through Th-17 signaling has been shown to impact offspring neurodevelopment and behavior in rodents (Choi et al. 2016). In mice, maternal immune activation from a viral mimic, polyI:C, at G12.5 resulted in social behavior deficits and increased marble burying behavior driven by increases in maternal IL-17A cytokine levels (Choi et al. 2016). In the present study, maternal inflammation from repeated aerosolized OVA-inductions during the first half of gestation increased marble burying and anxiety-like behaviors. This is in contrast to previous maternal allergic asthma studies where inductions throughout gestation, elicited reductions in social interactions in addition to increased marble burying (Schwartzer et al. 2015; Schwartzer et al. 2017). Importantly, these earlier maternal allergic asthma paradigms used acute OVA-inductions at three distinct gestational days, GD9, GD12, and GD17, each of which correspond with unique and important neuroimmunological processes that impact brain development and behavioral outcomes (Estes and McAllister 2016; Mac Giollabhui et al. 2019; Madhusudan et al. 2013). Though speculative, it is possible that offspring social behavior deficits are only present in response to maternal allergic asthma inflammation occurring during a single or combination of inflammatory episodes during specific gestational windows. Additionally, some unknown maternal response to repeated aerosol-inductions versus a single exposure may even have more severe developmental consequences that mask social behavior deficits.
Male Early-OVA offspring showed increases in anxiety-related behaviors in the elevated plus maze while exploration times for offspring of Late-OVA and Late-PBS dams were similar to those seen in male offspring from Early-OVA dams. This lower level of open arm exploration in late-exposed offspring may be a result of sex-specific fetal programming from a combination of the elevated corticosterone levels resulting from induction-induced stress and the natural rise of corticosterone during late gestation. Indeed maternal stress has been well documented to impact fetal brain and behavior development in both humans and animal models (Glover 2015; Manzari et al. 2019; Walsh et al. 2019), including increased risk of anxiety (McLean et al. 2018). Importantly, offspring behavioral changes in response to maternal OVA-exposure were only present in male offspring. These sex-dependent behavioral effects mirror studies in humans that demonstrate a sex difference in nervous system development and disease (for review see (Pearse and Young-Pearse 2019), as well as differences in fetal sensitivity to maternal endocrine function. Specifically, elevations in maternal corticosterone during pregnancy and in response to acute stressors are mirrored by sex-dependent changes in fetal corticosterone levels. For example, Montano et al (1991) showed that in mice, female fetuses have higher baseline corticosterone levels compared to male offspring on GD17. In the same study, an examination of fetal corticosterone levels 1 hour after an acute stressor to pregnant dams at GD17 revealed elevations in male fetal serum corticosterone but not female fetuses (Montano et al. 1991). Similarly, male and female rat fetuses showed elevations in corticosterone in response to repeated stress-induced corticosterone increases to the dam in pregnancy, but only male fetus had accompanying increases in plasma ACTH. These differences in male and female HPA hormones during gestation underscore sex-specific responses to gestational stress and complement our behavioral findings that were only present in male offspring despite similar changes in neuroinflammation between males and females.
Hypothalamic Cytokine Levels in Adult Offspring
It is now well established that changes in cytokines in the CNS can influence social behavior, anxiety, and stereotypies or repetitive behavior (Eisenberger et al. 2017; Kirsten and Bernardi 2017). Given the behavioral changes associated with maternal allergic asthma in both mice (Schwartzer et al. 2015; Schwartzer et al. 2017) and humans (Gong et al. 2019; Instanes et al. 2017; Liu et al. 2019), we investigated whether the anxiety and repetitive marble burying behaviors observed in our OVA-exposed offspring would be met with region-specific changes in cytokines of the brain. Upon analysis, we found that cytokine levels of G-CSF, IFNγ, IL-4, IL-7, and TNFα were all reduced in the hypothalamus of Early-OVA adult offspring, while all late exposure groups, which also showed increased anxiety behavior, showed no differences in the brain cytokine levels. Importantly, these differences in cytokine levels among Early-OVA treatment groups were not present in any other brain region investigated. The hypothalamus acts as the control center of the endocrine system within the CNS, and some of its functions include regulating temperature, hunger, sleep, and stress responses through modulation of the HPA axis (Spangelo et al. 1995). In addition, the hypothalamus participates in regulating inflammatory responses to infection and behavioral responses including anxiety and attachment behaviors, and changes in hypothalamic cytokine signaling, including IL-1, IL-2, IL-6, and TNFα, can directly modulate hormone activity by impacting the release of anterior pituitary hormones (Jones and Kennedy 1993; McCann et al. 2000).
In the present study, Early-OVA exposure resulted in both behavioral changes in males and corresponding reductions in several hypothalamic cytokines, including IL-4 and IFNγ, suggesting a potential link between hypothalamic inflammatory signaling and behavioral disposition that may be sex-specific. Several investigators have looked at how altered levels of cytokines in the hypothalamus can impact behavior. For example, reductions in IL-4 have been associated with increased anxiety behavior (Moon et al. 2015), and depletion of IFNγ through genetic knockout models alters social interactions (Filiano et al. 2016) and stress reactivity (Litteljohn et al. 2010), findings that parallel the behavioral and hypothalamic cytokine analyses we observed in our Early-OVA group. In addition, IL-7 and TNFα have both been implicated to have regulatory roles on the hypothalamic activity resulting in both neuropeptide release and behavioral alterations (Dietrich et al. 2017; Hao et al. 2016; Kirsten and Bernardi 2017; Macia et al. 2010). Despite these links between hypothalamic function and behavior, we were unable to observe any correlations between brain cytokine levels and any behavioral measures recorded. A power analysis of our correlation indicates very low statistical power, 38%, given that only a subset of mice were used for brain cytokine measures. Indeed, an estimated 85 mice would be needed to detect a moderate correlation (r = 0.3) between brain cytokines and behavioral outcomes at 80% power. Nonetheless, findings across multiple studies support the notion that decreases in cytokines found in the Early-OVA mice may contribute to the behavioral phenotypes observed in adult male offspring, and underscore the need to investigate why similar changes in hypothalamic cytokines were not associated with any behavioral changes in females.
For both behavioral and brain cytokine assessments, the timing of the gestational exposure directly impacts offspring outcomes. Importantly, the early exposure window (G2.5-9.5) coincides with the development and seeding of the brain with microglial progenitors. During this window, microglia are susceptible to epigenetic changes that can impact their function for the remainder of life (Vogel Ciernia et al. 2018). Microglia are the brain resident macrophage, which are long-lived cells that participate in many functions within the CNS. They not only participate in immune functions such as inflammatory responses and phagocytosis but also participate in shaping the synaptic network during development and can impact neuronal survival throughout the life of an organism (Hashimoto et al. 2013; Paolicelli et al. 2011; Schafer et al. 2012; Tremblay et al. 2010; Zhan et al. 2014). Early in development, microglial myeloid progenitors originate from the yolk sac and migrate to the brain, where they will remain and undergo very little replacement from peripheral monocytes (Squarzoni et al. 2015). This allows for the potential for lifelong epigenetic changes to take place during initial seeding of the fetal brain.
Of special importance to the current study, microglia number and function vary between males and females during perinatal development(Nelson and Lenz 2017; Schwarz et al. 2012), so perturbations to the fetal immune environment can differentially impact male and female offspring. These sexually dimorphic responses could contribute to the sex-specific behavioral and brain outcomes we observed in offspring of Early OVA treated dams. That is, elevations in maternal serum cytokines in response to Early OVA inductions could impart sex-specific differences to the developing offspring because of the sexually dimorphic nature of microglia colonization. This notion is further supported by the recent finding that T helper cells in the brain around the time of birth are essential for microglial maturation in mice(Pasciuto et al. 2020). Importantly, the cytokines we chose to examine in our work are essential to T cell differentiation, activation, and signaling. Though speculative, it is plausible that elevated regulatory cytokines in maternal serum of Early OVA dams could alter the translocation and phenotype of T cells in the developing brains of their offspring, resulting in sex-specific alteration in microglial maturation. In fact, Vogel Ciernia et al. (2018) showed that transcriptomic data from offspring from dams exposed to allergic asthma showed developmental genes associated with synaptic function were dysregulated. This suggests a potential for microglial priming and altered functional response. As such, it may be useful to further investigate microglial function in maternal induced allergic asthma models as they are a likely sources of, and responders to, IL-7, TNFα, IFNγ, IL-4, and G-CSF (Arbelaez et al. 2015; Basso et al. 2017; Gadani et al. 2012; Kawanokuchi et al. 2006). To investigate this further, future studies will require isolation of microglia and challenge with an inflammatory stimulus, such as LPS or OVA, to elucidate how changes in hypothalamic cytokine expression impacts brain-immune function in adulthood.
Limitations
While the combination of maternal corticosterone and immune measures complement the offspring behavioral and brain cytokine findings to support a link between the timing of prenatal insults and developmental outcomes, our ability to conclude a causal link is limited given that maternal serum was not assessed in dams used for offspring behavioral measures. As a result, the heightened corticosterone response in both PBS and OVA-treated dams in late pregnancy and elevated cytokine concentrations of Early-OVA dams cannot be correlated with the offspring behavioral measures or hypothalamic cytokine levels. The repeated handling and restraint needed for repeated maternal blood collection likely serves as an additional stressor that could impact fetal development and potentially confound offspring behavioral measures, which is why we used separate cohorts of dams for maternal and offspring outcomes. Therefore, careful consideration of experimental design is needed to effectively measure maternal signaling factors without disrupting offspring development in order to accurately link maternal markers of stress and inflammation with changes in offspring behavioral phenotypes. A related limitation of our study design was the lack of serum anti-OVA IgE antibody collection from the subset of dams used for offspring outcomes. The absences of this analysis to confirm all OVA-exposed dams elicited an allergic response prevents confirmation of MAA treatment and adds an additional source of variability to offspring outcome measures. Despite this limitation, the observed behavioral changes in the current study indicate the OVA treatment was effective. Moreover, our study did not include behavioral outcomes from undisturbed control dams that were not exposed to the induction nebulizer and, therefore, it remains unknown the extent to which offspring behavioral changes are impacted by the stressor of daily nebulizer exposures alone.
Conclusions
Our data suggest an immune-mediated impact of maternal allergic asthma in early gestation to increase anxiety-like and repetitive digging behaviors in male offspring and decrease expression of select cytokines in the hypothalamus of males and females. Expected elevations in maternal cytokine levels following allergic asthma in late gestation were suppressed in parallel with rising corticosterone, preventing an allergic asthma-induced change in offspring behavior and brain cytokine response. This evidence of early life programming through changes in maternal HPA and immune signaling raise important questions surrounding the role of routes of communication at the maternal-fetal interface, in particular the placenta. Moreover, it remains unknown whether these changes in offspring behavior result from epigenetic changes in peripheral immune regulatory states or disruptions to physiological and neuroimmunological activities during brain development. Nonetheless, understanding the physiological consequences of prenatal stress and inflammation on offspring development will be useful in the future for effectively managing allergies, asthma, and other environmental stressors during pregnancy to promote offspring behavioral and mental health.
Supplementary Material
HIGHLIGHTS.
Maternal asthma induced a peripheral T helper cell-mediated immune response in dams
Chronic allergic asthma in early gestation increased anxiety-like behavior in male offspring
Aerosol exposures in late pregnancy elevated maternal stress with no change in serum cytokines
Early maternal asthma exposure decreased cytokines in adult offspring hypothalamus
ACKNOWLEDGEMENTS
The authors would like to thank Kathleen Byrne, Maddy Berkowitz-Cerasano, Felicity Emerson, Megan Johnson, Kerry Ouimette, Rebecca Goodier, Fiona Chace-Donahue, Arina Kazakova, Ann Osborn, and Maliha Rahman for their technical expertise. Funding was provided by NIH R15HD082638, NIH R15MH119500, RO1MH118209, RO1HD090214, R21MH116383, R21ES025560 and the CTSC Pilot Translational and Clinical Studies Program, A Child/Lifespan Health Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arbelaez CA, Glatigny S, Duhen R, Eberl G, Oukka M, Bettelli E. 2015. Il-7/il-7 receptor signaling differentially affects effector cd4+ t cell subsets involved in experimental autoimmune encephalomyelitis. J Immunol 195:1974–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, et al. 2010. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40:1423–1430. [DOI] [PubMed] [Google Scholar]
- Auger N, Arbour L, Kabageni A, Healy-Profitos J, Ayoub A, Fraser WD. 2019. Prepregnancy asthma and the subsequent risk of central nervous system defects in offspring. Birth Defects Res 111:254–260. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Morrison PJ, Sullivan FM. 1974. Plasma corticosterone levels during pregnancy in the mouse: The relative contributions of the adrenal glands and foeto-placental units. J Endocrinol 60:473–483. [DOI] [PubMed] [Google Scholar]
- Basso L, Lapointe TK, Iftinca M, Marsters C, Hollenberg MD, Kurrasch DM, et al. 2017. Granulocyte-colony-stimulating factor (g-csf) signaling in spinal microglia drives visceral sensitization following colitis. Proc Natl Acad Sci U S A 114:11235–11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger K, Hellenbrand ME, Holford TR, Bracken M. 2010. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol 115:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A. 2006. Regulating inflammation by glucocorticoids. Nat Immunol 7:537. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. 2000. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161:1720–1745. [DOI] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. 2004. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry 61:774–780. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA, Douglas AJ. 2008. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol 20:764–776. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. 2007. Stress and inflammation in exacerbations of asthma. Brain Behav Immun 21:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. 2016. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. 2015. Th2 and th17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med 7:301ra129. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. 2005. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: A case-control study. Arch Pediatr Adolesc Med 159:151–157. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Zimmer MR, Bober J, Horvath TL. 2017. Hypothalamic agrp neurons drive stereotypic behaviors beyond feeding. Cell 169:559. [DOI] [PubMed] [Google Scholar]
- Duthie L, Reynolds RM. 2013. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology 98:106–115. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, Irwin MR. 2017. In sickness and in health: The co-regulation of inflammation and social behavior. Neuropsychopharmacology 42:242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, McAllister AK. 2016. Maternal immune activation: Implications for neuropsychiatric disorders. Science 353:772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, et al. 2016. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535:425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. 2012. Il-4 in the brain: A cytokine to remember. J Immunol 189:4213–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V 2015. Prenatal stress and its effects on the fetus and the child: Possible underlying biological mechanisms. Adv Neurobiol 10:269–283. [DOI] [PubMed] [Google Scholar]
- Gong T, Lundholm C, Rejno G, Bolte S, Larsson H, D’Onofrio BM, et al. 2019. Parental asthma and risk of autism spectrum disorder in offspring: A population and family-based case-control study. Clin Exp Allergy 49:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin DS. 2014. Glucocorticoid treatment of multiple sclerosis. Handb Clin Neurol 122:455–464. [DOI] [PubMed] [Google Scholar]
- Gustavson K, Ask H, Ystrom E, Stoltenberg C, Lipkin WI, Suren P, et al. 2019. Maternal fever during pregnancy and offspring attention deficit hyperactivity disorder. Sci Rep 9:9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantsoo L, Kornfield S, Anguera MC, Epperson CN. 2019. Inflammation: A proposed intermediary between maternal stress and offspring neuropsychiatric risk. Biol Psychiatry 85:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Sheng Z, Potian J, Deak A, Rohowsky-Kochan C, Routh VH. 2016. Lipopolysaccharide (lps) and tumor necrosis factor alpha (tnfalpha) blunt the response of neuropeptide y/agouti-related peptide (npy/agrp) glucose inhibited (gi) neurons to decreased glucose. Brain Res 1648:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Uga H, Mori A, Kurata H. 2017. Increased serum il-17a and th2 cytokine levels in patients with severe uncontrolled asthma. Eur Cytokine Netw 28:8–18. [DOI] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. 2013. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Epperson CN. 2019. Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry 86:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instanes JT, Halmoy A, Engeland A, Haavik J, Furu K, Klungsoyr K. 2017. Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol Psychiatry 81:452–459. [DOI] [PubMed] [Google Scholar]
- Jafari Z, Mehla J, Afrashteh N, Kolb BE, Mohajerani MH. 2017. Corticosterone response to gestational stress and postpartum memory function in mice. PLoS One 12:e0180306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TH, Kennedy RL. 1993. Cytokines and hypothalamic-pituitary function. Cytokine 5:531–538. [DOI] [PubMed] [Google Scholar]
- Kawanokuchi J, Mizuno T, Takeuchi H, Kato H, Wang J, Mitsuma N, et al. 2006. Production of interferon-gamma by microglia. Mult Scler 12:558–564. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. 2015. Inflammation and immunity in schizophrenia: Implications for pathophysiology and treatment. Lancet Psychiatry 2:258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lim CS, Kaang BK. 2016. Neuronal mechanisms and circuits underlying repetitive behaviors in mouse models of autism spectrum disorder. Behav Brain Funct 12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten TB, Bernardi MM. 2017. Prenatal lipopolysaccharide induces hypothalamic dopaminergic hypoactivity and autistic-like behaviors: Repetitive self-grooming and stereotypies. Behav Brain Res 331:25–29. [DOI] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, et al. 2014. Maternal immune activation and abnormal brain development across cns disorders. Nat Rev Neurol 10:643–660. [DOI] [PubMed] [Google Scholar]
- Lazic SE, Essioux L. 2013. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci 14:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic SE, Clarke-Williams CJ, Munafo MR. 2018. What exactly is ‘n’ in cell culture and animal experiments? PLoS Biol 16:e2005282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litteljohn D, Cummings A, Brennan A, Gill A, Chunduri S, Anisman H, et al. 2010. Interferon-gamma deficiency modifies the effects of a chronic stressor in mice: Implications for psychological pathology. Brain Behav Immun 24:462–473. [DOI] [PubMed] [Google Scholar]
- Liu WZ, Zhang WH, Zheng ZH, Zou JX, Liu XX, Huang SH, et al. 2020. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat Commun 11:2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Dalsgaard S, Munk-Olsen T, Li J, Wright RJ, Momen NC. 2019. Parental asthma occurrence, exacerbations and risk of attention-deficit/hyperactivity disorder. Brain Behav Immun 82:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Hessel EM. 2010. Functions of t cells in asthma: More than just t(h)2 cells. Nat Rev Immunol 10:838–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundback B, Backman H, Lotvall J, Ronmark E. 2016. Is asthma prevalence still increasing? Expert Rev Respir Med 10:39–51. [DOI] [PubMed] [Google Scholar]
- Mac Giollabhui N, Breen EC, Murphy SK, Maxwell SD, Cohn BA, Krigbaum NY, et al. 2019. Maternal inflammation during pregnancy and offspring psychiatric symptoms in childhood: Timing and sex matter. J Psychiatr Res 111:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia L, Viltart O, Delacre M, Sachot C, Heliot L, Di Santo JP, et al. 2010. Interleukin-7, a new cytokine targeting the mouse hypothalamic arcuate nucleus: Role in body weight and food intake regulation. PLoS One 5:e9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudan A, Vogel P, Knuesel I. 2013. Impact of prenatal immune system disturbances on brain development. J Neuroimmune Pharmacol 8:79–86. [DOI] [PubMed] [Google Scholar]
- Manni ML, Mandalapu S, McHugh KJ, Elloso MM, Dudas PL, Alcorn JF. 2016. Molecular mechanisms of airway hyperresponsiveness in a murine model of steroid-resistant airway inflammation. J Immunol 196:963–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzari N, Matvienko-Sikar K, Baldoni F, O’Keeffe GW, Khashan AS. 2019. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: A systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol 54:1299–1309. [DOI] [PubMed] [Google Scholar]
- McCann SM, Kimura M, Karanth S, Yu WH, Mastronardi CA, Rettori V. 2000. The mechanism of action of cytokines to control the release of hypothalamic and pituitary hormones in infection. Ann N Y Acad Sci 917:4–18. [DOI] [PubMed] [Google Scholar]
- McLean MA, Cobham VE, Simcock G. 2018. Prenatal maternal distress: A risk factor for child anxiety? Clin Child Fam Psychol Rev 21:203–223. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. 2006. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci 26:4752–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. 2008. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun 22:469–486. [DOI] [PubMed] [Google Scholar]
- Meyer U 2019. Neurodevelopmental resilience and susceptibility to maternal immune activation. Trends Neurosci 42:793–806. [DOI] [PubMed] [Google Scholar]
- Montano MM, Wang MH, Even MD, vom Saal FS. 1991. Serum corticosterone in fetal mice: Sex differences, circadian changes, and effect of maternal stress. Physiol Behav 50:323–329. [DOI] [PubMed] [Google Scholar]
- Moon ML, Joesting JJ, Blevins NA, Lawson MA, Gainey SJ, Towers AE, et al. 2015. Il-4 knock out mice display anxiety-like behavior. Behav Genet 45:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Cardenas I. 2010. The immune system in pregnancy: A unique complexity. Am J Reprod Immunol 63:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakano N, Ishimaru K, Ando N, Katoh R, Suzuki-Inoue K, et al. 2016. Inhibition of ige-mediated allergic reactions by pharmacologically targeting the circadian clock. J Allergy Clin Immunol 137:1226–1235. [DOI] [PubMed] [Google Scholar]
- Nelson LH, Lenz KM. 2017. The immune system as a novel regulator of sex differences in brain and behavioral development. J Neurosci Res 95:447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel C, Hedegaard M, Henriksen TB, Secher NJ, Olsen J, Levine S. 2005. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology 30:647–656. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. 2011. Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458. [DOI] [PubMed] [Google Scholar]
- Pasciuto E, Burton OT, Roca CP, Lagou V, Rajan WD, Theys T, et al. 2020. Microglia require cd4 t cells to complete the fetal-to-adult transition. Cell 182:625–640 e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse RV 2nd, Young-Pearse TL. 2019. Lost in translational biology: Understanding sex differences to inform studies of diseases of the nervous system. Brain Res 1722:146352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnayake U, Quinn T, Walker DW, Dickinson H. 2013. Cytokines and the neurodevelopmental basis of mental illness. Front Neurosci 7:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robijn AL, Murphy VE, Gibson PG. 2019. Recent developments in asthma in pregnancy. Curr Opin Pulm Med 25:11–17. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz M, Harden K, Forsythe A, Chilingar L, Hoffman C, Sperling W, et al. 1988. The course of asthma during pregnancy, post partum, and with successive pregnancies: A prospective analysis. J Allergy Clin Immunol 81:509–517. [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Chang C, Onore CE, Ashwood P. 2015. Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice. Transl Psychiatry 5:e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Coburn MA, Rose DR, Hughes HK, Ashwood P. 2017. Behavioral impact of maternal allergic-asthma in two genetically distinct mouse strains. Brain Behav Immun 63:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. 2012. Sex differences in microglial colonization of the developing rat brain. J Neurochem 120:948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shebl E, Chakraborty RK. 2019. Asthma in pregnancy. In: Statpearls. Treasure Island (FL). [PubMed] [Google Scholar]
- Silberman DM, Acosta GB, Zorrilla Zubilete MA. 2016. Long-term effects of early life stress exposure: Role of epigenetic mechanisms. Pharmacol Res 109:64–73. [DOI] [PubMed] [Google Scholar]
- Siniscalco D, Schultz S, Brigida AL, Antonucci N. 2018. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. 2007. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27:10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangelo BL, Judd AM, Call GB, Zumwalt J, Gorospe WC. 1995. Role of the cytokines in the hypothalamic-pituitary-adrenal and gonadal axes. Neuroimmunomodulation 2:299–312. [DOI] [PubMed] [Google Scholar]
- Spiegel E, Shoham-Vardi I, Goldbart A, Sergienko R, Sheiner E. 2018. Maternal asthma is an independent risk factor for long-term respiratory morbidity of the offspring. Am J Perinatol 35:1065–1070. [DOI] [PubMed] [Google Scholar]
- Squarzoni P, Thion MS, Garel S. 2015. Neuronal and microglial regulators of cortical wiring: Usual and novel guideposts. Front Neurosci 9:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MA, Shome GP, Hulbert LE, Krebs N, Wachtel M, McGlone JJ. 2009. Acute stress affects the physiology and behavior of allergic mice. Physiol Behav 98:281–287. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. 2010. Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8:e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Ciernia A, Careaga M, LaSalle JM, Ashwood P. 2018. Microglia from offspring of dams with allergic asthma exhibit epigenomic alterations in genes dysregulated in autism. Glia 66:505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K, McCormack CA, Webster R, Pinto A, Lee S, Feng T, et al. 2019. Maternal prenatal stress phenotypes associate with fetal neurodevelopment and birth outcomes. Proc Natl Acad Sci U S A 116:23996–24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kessels HW, Hu H. 2014. The mouse that roared: Neural mechanisms of social hierarchy. Trends Neurosci 37:674–682. [DOI] [PubMed] [Google Scholar]
- Weber-Stadlbauer U, Meyer U. 2019. Challenges and opportunities of a-priori and a-posteriori variability in maternal immune activation models. Current Opinion in Behavioral Sciences 28:119–128. [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, et al. 2014. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17:400–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


