Abstract
Purpose.
Our goal is to assess the ability of physicians to detect coronary calcifications in dual energy chest x-rays processed by a previously developed advanced algorithm. Because the chest x-ray is the most common imaging procedure, because the presence of coronary calcium provides proof of coronary artery disease, and because adherence to therapy can improve health, successful detection could positively impact healthcare for a large number of patients.
Methods.
Both dual energy chest and corroborative CT calcium score images were acquired. Dual energy images were processed with the advanced techniques, including sliding organ registration, so as to enhance coronary calcifications in two-shot dual energy acquisitions. We performed ROC to determine physicians’ ability to detect coronary calcifications. Since detection might be easier with heavier calcifications, we used various Agatston score cut-points for determining cases actually positive with calcification in the ROC analysis.
Results.
In many cases, coronary calcifications were made more visible with the advanced processing as compared to conventional processing. At an Agatston cut-point of 300, coronary calcifications were detected with AUC = 0.85. There were marginal effects on detection performance found with increased x-ray exposure, nearby Agatston cut-point values, and coronary artery territory.
Conclusions.
Coronary calcifications can be detected in dual energy chest x-rays. The ability to detect disease compares very favorably to other accepted screening methods (e.g., x-ray mammography). As the chest x-ray is an already ordered procedure, there is an opportunity to detect a very large number of persons with coronary artery disease at zero or low cost.
Keywords: coronary calcification, dual energy, ROC, image processing, image registration, screening exam, Agatston
Introduction
Advanced processing has been created to enable detection of coronary calcium from low-cost, low radiation, dual energy chest x-rays [1-3]. Because the chest x-ray is the most common imaging procedure with 100s of millions of exams per year, because the presence of coronary calcium provides proof of coronary artery disease, and because providing patients proof of immediate disease improves adherence to therapy, successful detection could positively impact healthcare for a very large number of patients. As this would be an already ordered exam, cost to healthcare would be minimal. This is significant, as a recent AHA report [4] indicates a new, alarming increase in cardiovascular disease, probably due to obesity and diabetes. It is America’s costliest disease and costs are predicted to double to $1.1T by 2035 [5],
Our research builds on very strong evidence from well-established CT coronary artery calcium (CAC) imaging. A large number of studies have shown that CT calcium score (Agatston) aids risk prediction [6-10] and is more predictive than any other single biomarker, including lipids [8, 11]. The 2018 ACC/AHA Cholesterol Guidelines [12], states that CAC scoring can be used to reclassify risk identification of patients who will potentially benefit from statin therapy. As a result, there has been renewed interest in CT calcium score as a test to guide therapeutic decision making and “derisk,” individuals so that they may not need to take treatments (e.g., aspirin and statin therapy) that may have side effects. With the advent of emerging, sometimes expensive cardiovascular therapies, there is a need for better ways to characterize risk, phenotype patients, and personalize treatments.
There have been previous, related reports. Gilkeson et al. determined the ability to detect coronary calcifications in standard dual energy and in standard, single energy chest x-rays [13]. They found significant improvement with dual energy. Mafi et al. evaluated the ability to detect and assess coronary calcium from conventional “bone” images from 2-shot dual energy chest x-rays and found good agreement with CT coronary calcium score imaging on a limited patient dataset [3]. Wen et al. developed a specialized registration and calcification enhancement algorithm, which in preliminary studies was found to be superior to commercial software for imaging coronary calcifications [1].
In this report, we compare image results obtained with specialized software, CorCalDx-viz to an existing clinical product, and perform a ROC study to determine the ability of Radiologists to detect coronary calcifications in images processed by CorCalDx-viz. Since conventional dual energy chest x-rays are optimized for imaging the lungs, there is sometimes insufficient penetration in the heart. To accommodate for this, we evaluate a specialized dose control protocol which will improve x-ray penetration in the heart region. Understanding that detection will depend upon the amount of calcification present (e.g., Agatston score), we analyze ROCs with different Agatston score cut points for calcification (+) cases.
Materials and Methods
Image acquisition.
CT and dual-energy images were acquired under two protocols, both under IRB approval. First, retrospective images from 21 patients were acquired, when there was a conventional dual-energy acquisition and a CT exam taken within 1-year of each other. Second, in a prospective study, we recruited 44 participants from a group normally getting a cardiac calcium score (dates:03-2016 to 02-2017). In the prospective study, we obtained conventional and “high” exposure, PA and oblique views on a DE scanner (Discovery XR656, GE Healthcare) plus a calcium score CT. In high exposure mode, we ensured adequate x-ray exposure in the heart by using exposure sensors over the spine and right lung in an averaging mode. For conventional exposures, images were acquired using a sensor over the right lung. Although there were paired conventional and high dose acquisitions from the same patient, subtracted images looked different as high and low kVp acquisitions acquired without ECG gating. Hence, to gather more measurements, in some analyses we combined images into a single PA group of 109 patient image acquisitions. Raw DE images for processing, as well as standard, bone, and soft tissue images from the GE scanner were captured. Dual energy x-ray gives much improved conspicuity of some lung conditions and of calcifications in the heart at the expense of about twice the dose of a conventional single-shot chest x-ray. CT calcium imaging was done using a standard clinical protocol and vessel-tree-specific Agatston CT calcium scores were obtained. Volumetric CT images were processed to visualize coronary calcium in views corresponding to DE views using 3D-to-2D registration [1, 14].
Image processing to enhance calcifications (CorCalDx-viz).
Described in detail elsewhere [1], we used CorCalDx-viz software to enhance coronary calcifications in two-shot dual energy x-ray acquisitions. Briefly, for both high and low kVp images, we processed to reduce noise, estimated a scatter image and subtracted it. We registered high and low kVp pairs using non-rigid, sliding organ registration with the low kVp image as the reference image. A subtracted image was created using parameters optimized for coronary calcification visualization.
ROC analysis.
In the ROC study, we assessed the ability to detect coronary calcium in images processed with CorCalDx-viz. Typically, two territories prone to coronary calcifications are visible in PA chest x-rays. The left ventricle triangular region (LVR), covers the left main, left anterior descending and left circumflex arteries. The right atrial region (RAR) covers the right coronary artery (Fig 5). From CT images, we obtained calcium Agatston scores for each region. Three radiology resident readers were trained by Dr. Gilkeson to read coronary calcium images, with examination of a variety of dual energy case studies with corresponding CT exams. Readers were also trained in the software used for ROC scoring. Readers examined PA views and gave a calcification likelihood score (1-5) for the LVR and RAR. We scored corresponding CT images in each of the two regions using semi-automated, clinical software.
Fig 5.
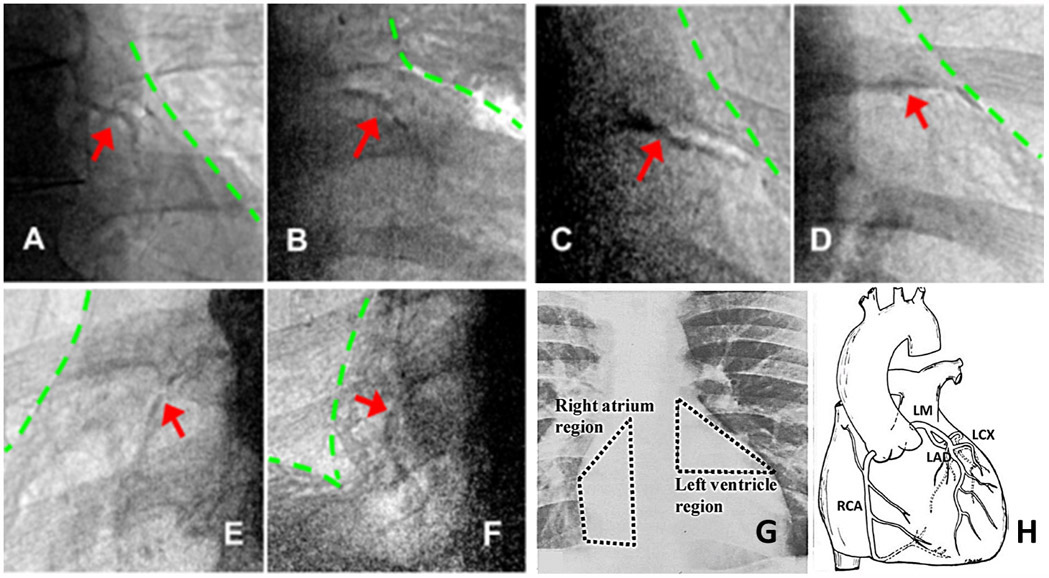
Examples of coronary calcium found with CorCalDx-Viz. The red arrows indicate locations of coronary calcium plaques and the green lines indicate heart and diaphragm boundaries. (A)-(D) LM/LAD calcifications in left ventricle, the coronary artery calcium triangle. (E)-(F) RCA calcifications on the right atrium. (G)-(H) Anatomical illustration of regions important for coronary calcium, provided courtesy of reference [26].
Since larger, denser calcifications were presumed to be easier to detect in dual energy, we performed ROC analyses with different CT Agatston score cut-points for labeling cases as calcification (+) (actual disease present). For example, for CT Agatston score > 300, we collected arterial segments having 0-50 (deemed disease absent) and score >300 (disease present). We then computed an ROC in the normal way by sweeping the likelihood to create true positives (TPs), FPs, TNs, and FNs. Note that this was only meaningful if we left out calcifications between 50 and 300 because it would not make sense to count these as FP errors when there was calcification present. We lumped 0-50 scores as disease absent because this is likely at the threshold for DE detection. ROC data were analyzed using a modification of the “parametric ROC” MATLAB software as implemented by Bantis 1. We used both empirical and parametric analysis, but report parametric analyses. The parametric analysis uses maximum likelihood to do the estimation. The function provided ROC curves averaged across readers by averaging parameters of the binormal model. It also provided Fs, confidence intervals, and p values for testing between treatment groups (e.g., x-ray dose).
Results
Back ground data for the 65 participants follow. For the prospective study, there were 22 men and 22 women with age 57.1 ± 13.9 (sd). Age and gender were not available for the retrospective study, as data were de-identified in the IRB protocol. Subjects included a range of territory CT calcium Agatston scores (Fig 1).
Fig 1.
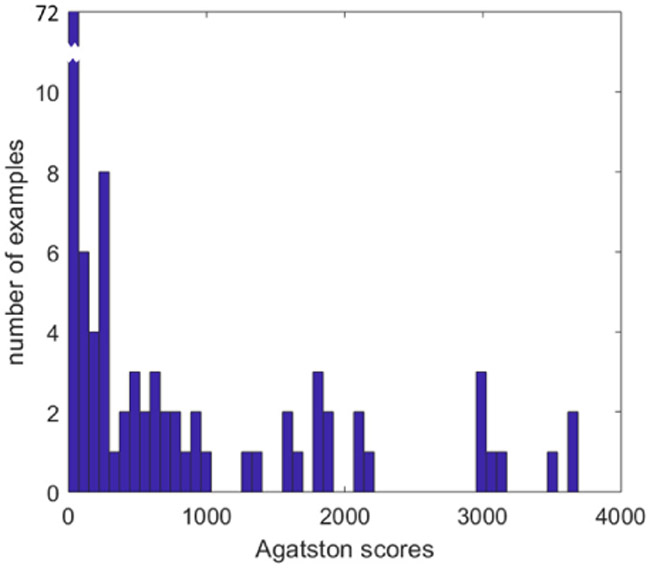
Histogram of territory Agatston scores. The total number of samples is 130, with 72 falling into the calcification (−) bin corresponding to Agatston 0 to 50.
Some figures illustrate our methodology. Both dual energy x-rays and corroborative CT calcium score images were acquired (Fig 2). The CorCalDx-viz software significantly reduces motion artifacts and enhances calcifications as compared to conventional commercial “bone image” processing (Fig 3). For the prospective data set, images were acquired at a conventional dose and a higher dose acquired using exposure sensors in both the right lung and spine combined (Fig 4). Images obtained at the higher dose had considerably reduced noise in the heart region.
Fig 2.
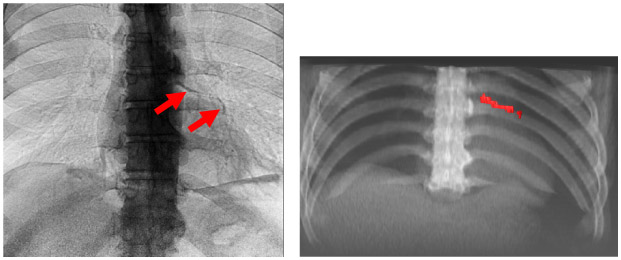
Dual energy x-ray showing coronary calcification with corresponding corroborative CT (left and right, respectively). The calcification in the LVR has an Agatston score of 632. It is clearly present in dual energy chest x-ray image after processing with CorCalDx-viz. The CT image volume was registered with DE data to help determine correspondence. This is a high dose image acquisition.
Fig 3.
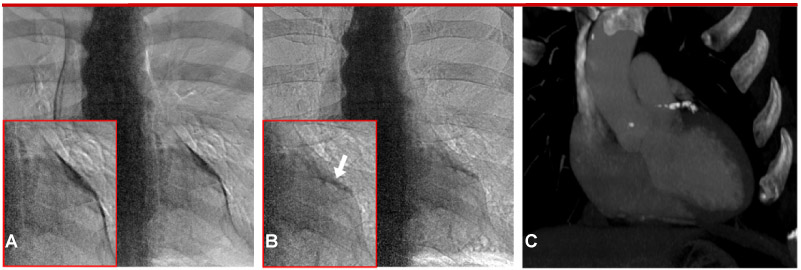
CorCalDx-viz (B) compared to commercial “bone image” processing (A). The motion artifact present in A obscures the calcification and leads to confounding structures due to mismatched pulmonary arteries. Artifacts are greatly reduced in B. The corresponding CT calcium score image is shown in C with an Agatston score of 341 in the LAD. In this case, the CT image was not registered to the dual energy images in order to better show the extent of the calcification.
Fig 4.
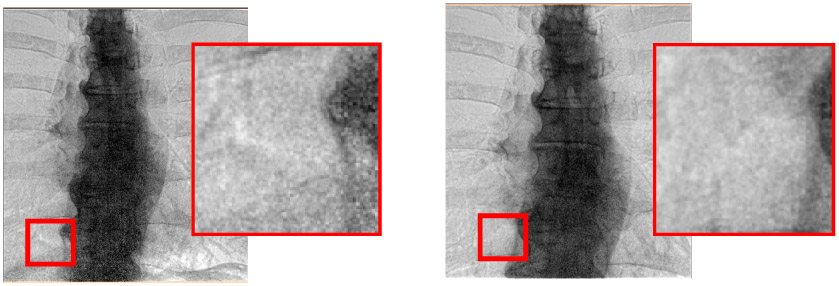
Effect of conventional and high exposure acquisition (left and right, respectively). Images are acquired with conventional “right lung” and “spine + right lung” dose sensing chamber acquisitions, respectively. Clearly, inset images show that the higher dose processed images have much lower noise in the spine and lower portions of the image. The acquisition (kVp, mAs, ms) values for low and high voltage acquisitions are [(60 kVp, 250 mA, 14ms), (120 kVp, 200mA, 5ms)] and [(60 kVp, 630 mA, 9ms), (120kVp, 400mA, 3ms)], respectively. Averaging across patients, we determined that the conventional and high exposure acquisitions gave dose areas products of 2 dGy·cm2 and 3.5 dGy·cm2, respectively, as determined from the DICOM header. Nominally, we increases the dose area product by a factor of 1.75, from an average of 2 dGy·cm2 to 3.5 dGy·cm2. It was necessary to increase mA to maintain short exposures needed to minimize blurring.
CorCalDx-viz enabled detection of coronary calcium. Fig 5 shows multiple examples of coronary calcifications seen following processing with CorCalDx-viz. It also shows the location in chest x-rays of the coronary artery territories present in the left ventricular region (LVR) and right atrial region (RAR).
Various ROC curves were analyzed. With a cut-point of 300 and data combined across LVR and RAR and across conventional and high dose, all three readers had similar performance for detection of coronary calcifications in processed dual energy images (Fig 6A). As a result, we collapsed data across readers in subsequent analyses. In Fig 6B, we analyzed detection performance as a function of the cut-point for calcification (+). A cut point of 300 Agatston score gave the highest ROC value of 0.85 (CI: 0.74 – 0.86). Calcification detection was similar in LVR and RAR (Fig 6C and 6D). There was a small, but consistent, improvement in detection performance with the high dose versus the conventional dose acquisitions (Figs 6C and 6D). When LVR and RAR data were combined, AUCs for high and conventional dose acquisitions were 0.85 (CI: 0.79 – 0.88) and 0.82 (CI: 0.78 – 0.86), respectively. However, there was a statistically insignificant difference in AUC between doses (p = 0.3139) with ANOVA, when we treated readers and cases as random samples [15, 16].
Fig 6.
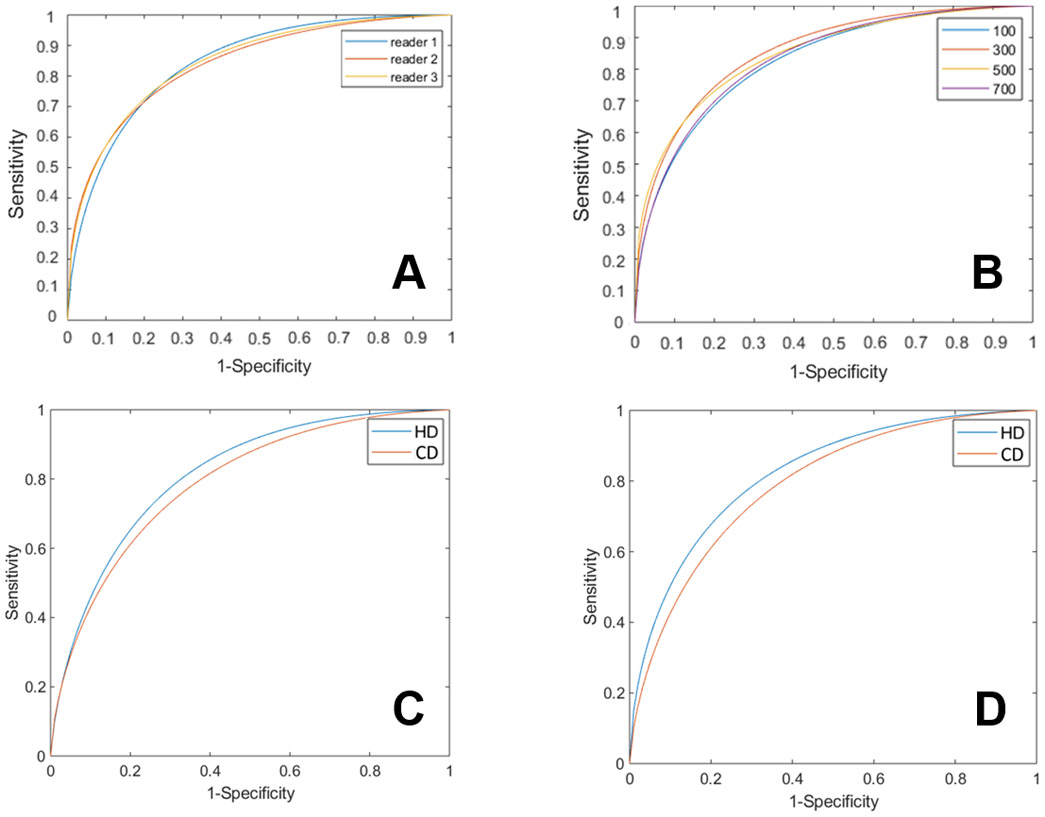
ROC analysis. A: Individual reader ROC for Agatston cut point of 300 for calcification (+). Individual readers’ curves with AUCs for readers 1-3, 0.83, 0.81, and 0.82, respectively. Average ROC curve of three readers were obtained by averaging the parameters of the bi-normal ROC model as suggested by Metz et al.[15] and used subsequent panels. B: Average ROCs of three readers as a function of the Agatston cut point for calcification (+). Cut-points are Agatston 100, 300, 500, and 700, as shown (see text for analysis method). Conventional and high exposure cases, and LVR and AVR territories are combined to get better statistics. Average AUCs are 0.78 (CI: 0.72-0.83), 0.85 (CI: 0.74-0.86), 0.83 (CI: 0.7-0.84) and 0.82 (CI: 0.71-0.83), respectively. C: Average ROC curve of three readers at LVR, high and low dose acquisitions gave AUCs of 0.85 (CI: 0.79-0.88) and 0.82 (CI: 0.78-0.86), respectively. D: Average ROC curve of three readers at AVR, high and low dose acquisitions gave AUCs of 0.86 (CI: 0.78-0.88) and 0.82 (CI: 0.76-0.84), respectively.
Discussion
With CorCalDx-viz processing of dual energy chest x-rays, we have shown that it is possible to detect coronary artery calcifications. The CorCalDx-viz processing method gives improved visualizations and better detection performance, as demonstrated in a previous study with a small number of cases [1]. In addition, in the previous report, we identified individual calcifications in CT which very closely matched sizes of calcifications seen in registered dual energy, providing direct proof of correspondence and the ability to visualize coronary calcifications in dual energy. In digital phantom studies, we identified that effects from beam hardening and mismatched scatter correction gave manageable errors [2]. Simulations demonstrated that pulsations and movements of pulmonary arteries could create significant confounders. The current clinical evaluation report supports these observations. The AUC for detection was 0.85 with a cut-point of 300 Agatston score for calcification (+). Anecdotally, overlapping pulmonary arteries were deemed confounders, which likely reduced performance. There was insignificant difference in detection between the LVR and RAR regions. There was a consistent improvement with high versus conventional dose, but differences were insignificant. If appropriate processing was added to all dual energy chest x-rays, this would enable inadvertent calcification detection on an already ordered chest x-ray.
Although we have chosen a cut-off of Agatston 300 for most analyses, in Figure 6B, we also analyzed Agatston 100. In this case, the AUC was degraded from 0.85 to 0.78, respectively. For the case of coronary calcifications CT calcium score exams, it is desirable to detect any calcifications for risk prediction. Nevertheless, for a front line, opportunistic identification of calcification from a chest x-ray, a cut-off of Agatston 300 seems reasonable. Please note that AHA suggested Agatston >300 patients would have higher chance of cardiovascular disease [17]. Of course if any calcium is detected in a dual energy chest x-ray, that patient can be referred to a CT calcium score exam if desired.
Detection performance for coronary artery calcification (AUC ~ 0.85) tends to be in the range of other screening modalities. For comparison, reports which include x-ray mammography for detection of cancer report values in the range from 0.7 to 0.83 [18-21]. One of the largest studies for breast mammography (with 49,528 cases) compared film and digital imaging. They reported AUCs from 0.54 to 0.84 for various sub-groups of women. The poorest performance (AUC = 0.54) was reported for women aged younger than 50 years with dense breasts. Chest x-ray detection of lung cancer is relatively poor (AUC = 0.52-0.69) [22]. Even lung cancer detection with low dose CT is poor (AUC = 0.66-0.68), tending to provide many false positive detections [23]. So, although we would like to improve coronary calcium detection from dual energy chest x-rays, results are very competitive with other medical imaging screening studies. Moreover, readers in this study really had minimal training (a few minutes), unlike readers in a study such as breast mammography, where readers will have had multiple years of experience.
Using ROC results, we can estimate how dual energy imaging of coronary calcium could contribute to screening for coronary calcium. We can obtain true positive rate (TPR) and false positive rate (FPR) from an operating point on an ROC curve. We assume a population of 1000 persons with a calcification prevalence, P, at a given calcification (+) cut point. The number of calcifications correctly detected is given by NCD = 1000*P*TPR and the number of false detections is NFD = 1000*(1-P)*FPR. Assuming an older population with P=0.15 at an Agatston calcification (+) cut point of 300 and TPR = 0.83 and FPR=0.3, we get NCD = 124, NFD = 255, and 26 calcifications missed. Among the 255, many of these will have some level of calcium. Assuming that the ratio of persons having scores >10 to >300 in our study applies, we get a prevalence of 0.28/0.15(1.87), giving ~70 additional calcification cases. Hence, the additional cost would be that for 379 CT calcium score exams with roughly 50% of them positive for a calcification > 10 Agatston score. Although that would be a significant healthcare cost, the cost savings would be 62% as compared to that for ordering CT calcium score exams for everyone, a step which has been proposed by some. Alternatively, as methods for coronary calcium from DE become more reliable, physicians could act on the result from a dual energy exam without consideration of a CT calcium score. After all, if the intervention is a recommendation to change life style and take statins, there is little downside to a false positive. Our calculations are only a rough estimate. A more thorough analysis would include population prevalence numbers and would account for the increased probability of detecting calcifications under 300 Agatston as compared to random chance. From numbers above, 50% of those persons identified as having a calcification would have a true calcification. Compare this to x-ray mammography where only about 5% of patients asked to have a follow up exam, have breast cancer [24].
It is likely that coronary calcium detection from dual energy x-rays can be improved. Readers in our study are in training, as compared to established screening studies where participants often have many years of experience. With additional training, AUCs might be improved. Pulmonary arteries pulsation during acquisition contribute to confounding structures [2]. Since pulmonary arteries do not necessarily move with the heart, it is difficult to register both overlaying structures. In general, images which confounded readers tended to be those with significant misregistration between high and low kV images, suggesting that EKG triggering might much improve results [25]. Although there appeared to be calcifications in some images, oblique views were deemed not useful and were not analyzed in the ROC study. In addition to EKG gated acquisitions, it is possible that continued R&D in image registration and image processing might lead to improved detection performance.
Acknowledgements.
This project was supported by the Case-Coulter Translational Research Partnership (PY15-P410) and by a sponsored research award from General Electric Healthcare. This research is a collaboration between Case Western Reserve University and University Hospitals of Cleveland, Cleveland Medical Center. Special thanks go out to the team at University Hospitals of Cleveland who collected the images used in this work. We thank Katelyn Nye and John Sabol of GE Healthcare for their support on this project. The veracity guarantor, Hao Wu, affirms that to the best of his knowledge that all aspects of this paper are accurate. Software described herein was developed for investigational use. It is not available for clinical usage, nor is it FDA approved.
Funding. This project was supported by the Case-Coulter Translational Research Partnership (PY15-P410) and by a sponsored research award from General Electric Healthcare.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest/competing interests. none
Availability of data and material. Data are owned by the University Hospitals of Cleveland under a grant from GE Healthcare. Data are not publically available.
Code availability. CorCalDx-viz software was developed in house. It is not publically available.
References
- 1.Wen D, Nye K, Zhou B, et al. (2018) Enhanced coronary calcium visualization and detection from dual energy chest x-rays with sliding organ registration. Computerized Medical Imaging and Graphics 64:12–21. 10.1016/j.compmedimag.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 2.Zhou B, Wen D, Nye K, et al. (2017) Detection and quantification of coronary calcium from dual energy chest x-rays: Phantom feasibility study. Medical Physics 44:5106–5119. 10.1002/mp.12474 [DOI] [PubMed] [Google Scholar]
- 3.Mafi JN, Fei B, Roble S, et al. (2012) Assessment of Coronary Artery Calcium Using Dual-Energy Subtraction Digital Radiography. Journal of Digital Imaging 25:129–136. 10.1007/s10278-011-9385-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardiovascular Disease Costs Will Exceed $1 Trillion by 2035, Warns the American Heart Association ∣ American Heart Association. http://newsroom.heart.org/news/cardiovascular-disease-costs-will-exceed-1-trillion-by-2035-warns-the-american-heart-association. Accessed 17 Feb 2017
- 5.(2017) Cardiovascular disease: a costly burden for America - Projections through 2035. http://www.heart.org/idc/groups/heart-public/@wcm/@adv/documents/downloadable/ucm_491543.pdf. Accessed 27 Feb 2017
- 6.Budoff MJ, Gul KM (2008) Expert review on coronary calcium. Vase Health Risk Manag 4:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenland P, Bonow RO, Brundage BH, et al. (2007) ACCF/AHA 2007 Clinical Expert Consensus Document on Coronary Artery Calcium Scoring By Computed Tomography in Global Cardiovascular Risk Assessment and in Evaluation of Patients With Chest Pain: A Report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Developed in Collaboration With the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. Journal of the American College of Cardiology 49:378–402. 10.1016/j.jacc.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 8.Hecht HS (2014) Coronary Artery Calcium Scanning: The Key to the Primary Prevention of Coronary Artery Disease. Endocrinology and Metabolism Clinics of North America 43:893–911. 10.1016/j.ecl.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 9.Blaha MJ, Yeboah J, A1 Rifai M, et al. (2016) Providing Evidence for Subclinical CVD in Risk Assessment. Global Heart 11:275–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugliese G, Iacobini C, Fantauzzi CB, Menini S (2015) The dark and bright side of atherosclerotic calcification. Atherosclerosis 238:220–230. 10.1016/j.atherosclerosis.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 11.Martin SS, Blaha MJ, Blankstein R, et al. (2014) Dyslipidemia, Coronary Artery Calcium, and Incident Atherosclerotic Cardiovascular Disease. Circulation 129:77–86. 10.1161/CIRCULATIONAHA.113.003625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundy Scott M, Stone Neil J, Bailey Alison L, et al. (2019) 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139:el082–ell43. 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilkeson RC, Sachs PB (2006) Dual Energy Subtraction Digital Radiography: Technical Considerations, Clinical Applications, and Imaging Pitfalls. Journal of Thoracic Imaging 21:303–313. 10.1097/01.rti.0000213646.34417.be [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Gilkeson RC, Fei B (2007) Automatic 3D-to-2D registration for CT and dualenergy digital radiography for calcification detection. Medical Physics 34:4934. 10.1118/1.2805994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillis SL (2007) A Comparison of Denominator Degrees of Freedom Methods for Multiple Observer ROC Analysis. Stat Med 26:596–619. 10.1002/sim.2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillis SF, Berbaum KS, Metz CE (2008) Recent Developments in the Dorfman-Berbaum-Metz Procedure for Multireader ROC Study Analysis. Acad Radiol 15:647–661. 10.1016/j.acra.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeboah J, Young R, McClelland RL, et al. (2016) Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. Journal of the American College of Cardiology 67:139–147. 10.1016/j.jacc.2015.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly KM, Dean J, Lee S-J, Comulada WS (2010) Breast cancer detection: radiologists’ performance using mammography with and without automated whole-breast ultrasound. Eur Radiol 20:2557–2564. 10.1007/s00330-010-1844-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg WA, Zhang Z, Lehrer D, et al. (2012) Detection of Breast Cancer with Addition of Annual Screening Ultrasound or a Single Screening MRI to Mammography in Women with Elevated Breast Cancer Risk. JAMA 307:1394–1404. 10.1001/jama.2012.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gennaro G, Toledano A, di Maggio C, et al. (2010) Digital breast tomosynthesis versus digital mammography: a clinical performance study. Eur Radiol 20:1545–1553. 10.1007/s00330-009-1699-5 [DOI] [PubMed] [Google Scholar]
- 21.Pisano ED, Hendrick RE, Yaffe MJ, et al. (2008) Diagnostic Accuracy of Digital versus Film Mammography: Exploratory Analysis of Selected Population Subgroups in DMIST. Radiology 246:376–383. 10.1148/radiol.2461070200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Hoop B, Schaefer-Prokop C, Gietema HA, et al. (2010) Screening for Lung Cancer with Digital Chest Radiography: Sensitivity and Number of Secondary Work-up CT Examinations 1. Radiology 255:629–637 [DOI] [PubMed] [Google Scholar]
- 23.Awai K, Murao K, Ozawa A, et al. (2004) Pulmonary Nodules at Chest CT: Effect of Computer-aided Diagnosis on Radiologists’ Detection Performance. Radiology 230:347–352. 10.1148/radiol.2302030049 [DOI] [PubMed] [Google Scholar]
- 24.Evidence Summary: False-Positive and False-Negative Rates of Digital Mammography Screening: Breast Cancer: Screening - US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/evidence-summary-false-positive-and-false-negative-rates-of-/breast-cancer-screening1. Accessed 18 Dec 2018
- 25.Sabol JM, Liu R, Saunders R, et al. (2006) The impact of cardiac gating on the detection of coronary calcifications in dual-energy chest radiography: a phantom study. In: Medical Imaging 2006: Physics of Medical Imaging. International Society for Optics and Photonics, p 61421F [Google Scholar]
- 26.Souza AS, Bream PR, Elliott LP (1978) Chest Film Detection of Coronary Artery Calcification. The Value of the CAC Triangle. Radiology 129:7–10. 10.1148/129.17 [DOI] [PubMed] [Google Scholar]


