Abstract
Phospholipid transfer protein, ~80 kDa, transfers phospholipids from micelles to lipid binding proteins. The acceptor protein in plasma is apolipoprotein-A1, 28 kDa. Previously, phospholipid transfer protein was found in tears but an acceptor protein was not identified. To search for the acceptor protein(s) in tears a fluorescent phospholipid transfer assay was altered to omit the extrinsic acceptor. Human tears were incubated with fluorescent micelles and showed marked transfer activity verifying a native acceptor protein must be present. Reconstituted tears without tear lipocalin (lipocalin-1) eliminated the transfer of phospholipids. To determine if phospholipid transfer protein is involved in carrying phospholipid to the surface of tears from tear lipocalin, a fraction enriched in phospholipid transfer protein was injected into the subphase of a tear mimicking buffer in which tear lipocalin was present. The addition of phospholipid transfer protein did not increase the thickness of the surface layer regardless of the presence of lipid bearing tear lipocalin. The data show that phospholipid transfer protein transfers phospholipid from micelles to tear lipocalin. Phospholipid transfer protein does not transport the phospholipid. While tear lipocalin has no intrinsic transfer activity from micelles, it is the acceptor protein for phospholipid transfer protein in tears.
Keywords: tear lipocalin, lipocalin-1, phospholipid transport protein, human tears, micelles, ellipsometry
Graphical Abstract
1. Introduction
Phospholipid transfer protein in blood transfers phospholipids from micelles to an acceptor protein, apolipoprotein A1 [1]. Jauhiainen et al. discovered phospholipid transfer protein in human tears [2]. Modeling studies have shown that normal phase and inverse micelles may form in tears with theoretical compression [3]. The concentration of phospholipid in tears has been estimated to be about 34 μM exceeding the critical micellar concentration in buffer 1.36 μM [4-6]. Tears from normal subjects contain neither micelles nor apolipoprotein A1 [7]. The lack of an obvious acceptor protein was posited as the explanation for an apparent lower specific activity of phospholipid transfer protein in tears [2]. Jauhainen et al.[2] hypothesized that phospholipid transfer protein could scavenge and carry phospholipid directly to the superficial layer of the tear fluid. Alternatively, phospholipid transfer protein was posited to bind, transport and eliminate phospholipids from tears through the nasolacrimal duct [2]. However, nearly all phospholipids in tears are bound to tear lipocalin, also referred to as lipocalin-1, rather than phospholipid transfer protein [8]. The failure to detect a fluorescently labeled phospholipid intermediate in either the transfer from fluorescent labeled vesicles or in tears that were spiked with fluorescently labeled phospholipids are strong evidence against a transport function [9,10]. Rather, tear lipocalin is known to bind and remove phospholipid from the corneal surface [10]. The possibility that tear lipocalin is the acceptor protein in human tears deserves consideration. Recent evidence suggests phospholipids reside both in the bulk of tears bound to tear lipocalin and at the surface of the lipid layer [6]. The precise mechanism how phospholipids arrive at the tear-air interface is not understood. A modified phospholipid fluorescence transfer assay was used to determine if there is an acceptor protein in tears. The possibility that phospholipid transfer protein transports phospholipid directly to the tear film surface was tested with ellipsometry.
2. Materials and Methods
1,2 dipalmitoyl-sn-glycero 3-phosphatidyl choline (DPPC) was purchased from Avanti Polar lipids, Inc. (Alabaster, AL). Buffer mimicking tear electrolyte concentration, (26 mM NaHC03, 140mM NaCl, 24 mM KCl, 0.8 mM CaCl2 0.61 mM MgCl2)[11] was made from chemicals purchased from Millipore-Sigma, St. Louis, MO.
2.1. Collection of human tears
Human tears were collected and pooled from 15 healthy volunteers, ages 19-21, in accordance with the tenets of the Declaration of Helsinki and the project was approved by the institutional review board. Informed consent was obtained from donors. The subjects were screened to exclude symptoms of dry eye disease as previously published [12]. Tear samples were collected with polished glass tips and glass transfer pipettes, then placed in polytetrafluoroethylene-lined glass vials and stored under nitrogen at −80C° until use.
2.2. Centrifugal concentration of high molecular weight proteins in tears
Tears, were applied to a pre-rinsed Amicon Ultracell 50kDa cutoff membrane (Millipore-Sigma, St Louis, MO) and centrifuged at 4500g for 10 minutes. The retentate containing high molecular weight proteins were collected by inversion of the device followed by centrifugation at 800 g for 3 min.
2.3. Reconstitution of tears
Tear proteins were separated from pooled human tears by gel filtration (Sephadex G-100; Sigma-Aldrich, St. Louis, MO), as described [13]. Fractions that passed through a dextran column (DEAE-Sephadex; Sigma-Aldrich, St. Louis, MO) (tear lipocalin binds with the resin) were collected and combined with the remaining G-100 fractions depleted of tear lipocalin and reconstituted in buffer to the original volume. Tricine polyacrylamide gel electrophoresis was performed to confirm that the resultant mixture was depleted of tear lipocalin, by detailed methods previously indicated [14].
2.4. Phospholipid Transfer Assay
The phospholipid transfer assay was performed by the 2 phase method of Masson [15]. Phospholipid transfer activity was determined as the rate of transfer of 4-chloro7-nitrobenzofurazan (NBD) labeled phospholipid in donor micelles (PLTP Activity Assay Kit , Millipore-Sigma, St. Louis, MO) to a potential acceptor after selective precipitation of micelles and liposomes. A standard curve was created starting with donor particles mixed as four serial dilutions in 2-propanol in concentrations from 60-960 nM including a blank without donor particles. Liposomes were precipitated by the addition of 350 μL of tris buffered saline, followed by 300 μL of a solution that contained 500 mM NaCl, 215 mM MnCl2 and 445 U/ml of heparin. After vortex and centrifugation (9000g at 10 minutes), the supernatant was measured for steady state fluorescence intensity λex=465 nm. Peak heights in the emission spectra were compared at λem=535 nm with a Chirascan spectrometer (Applied Photophysics, Beverly, MA) integrated with a QEDPro charged coupling device as the detector (Ocean Optics, Orlando, Fla). Samples were tested using mediated transfer of proprietary fluorescent substrates by phospholipid transfer protein to a known acceptor protein, high density lipoprotein (HDL) using the same protocol as above. The concentration and amounts of fluorescent phospholipids transferred was determined from the relative fluorescence on the calibration curve after subtraction of background fluorescence from that of the supernatant. In order to find the acceptor for phospholipid transfer in tears, the phospholipid transfer assay above was modified to omit the addition of the acceptor protein. The other steps were unchanged.
2.5. Ellipsometry
Ellipsometry was performed with an imaging nulling ellipsometer nanofilm-EP4 from Accurion Gmbh (Gottingen, Germany) as previously described in nulling mode with a 658 nm 50 mW laser [16]. Additionally, the ellipsometer and active vibration isolation device were isolated in a customized cabinet to exclude stray light and air currents. A 5x microscope objective was used to increase the field of the scan. Angle of incidence scans were collected at 0.5° intervals from 50 and 57°. The data were modeled for a phospholipid surface layer (index of refraction 1.453-1.463), under air and over an aqueous substrate (index of refraction, n = 1.333), all considered transparent films in the model. Polarization and phase shift are referred to as psi (Ψ) and delta (Δ). Ψ and Δ values are derived from angular measurements of the polarizer and analyzer and were measured at each pixel in the scanned area and averaged by the instrumentation software. The lateral resolution approximates 1 μm as given by the overall resolution provided by the optics in the reflected light beam. The reflectance (r) matrix is described by the ratio ρ = Rp /Rs = tan (Ψ) eiΔ where the amplitude of the parallel (p) and orthogonal (s) components of the reflected light are normalized to initial amplitude of the incoming light with the ellipsometric angles Ψ and Δ, respectively. To obtain thickness, measured ellipsometric angles Ψ and Δ were parameters input into the Accurion proprietary modeling software (EP4Model software). The software uses a Berreman matrix algorithm for multilayered films fits and a Levenberg-Marquardt multivariate regression algorithm to Fresnel equations for multilayered films. An adequate fit was taken as a root mean square error of less than 15. Solutions containing analytes including fractions centrifugally enriched in phospholipid transfer protein and purified tear lipocalin were injected into the subphase. Phospholipid, gravimetrically prepared and dissolved in chloroform, was layered on the substrates by adding 1 μl to cover the surface area of the sample chamber as described. A minimum of 3 measurement scans were made from each of a minimum of three samples. The scans were done in different regions that encompassed the thinnest and thickest measurements. Scans of each region were performed in duplicate. The thicknesses are reported as a range of change in mean thickness from successive addition of tear components. Mean thicknesses are the average of sample thicknesses at each pixel over 3 different regions for each sample. The range is shown for 3 different samples. The system was initially calibrated with a silicon dioxide wafer of known surface layer thickness.
3. Results and Discussion
3.1. Phospholipid transfer activity
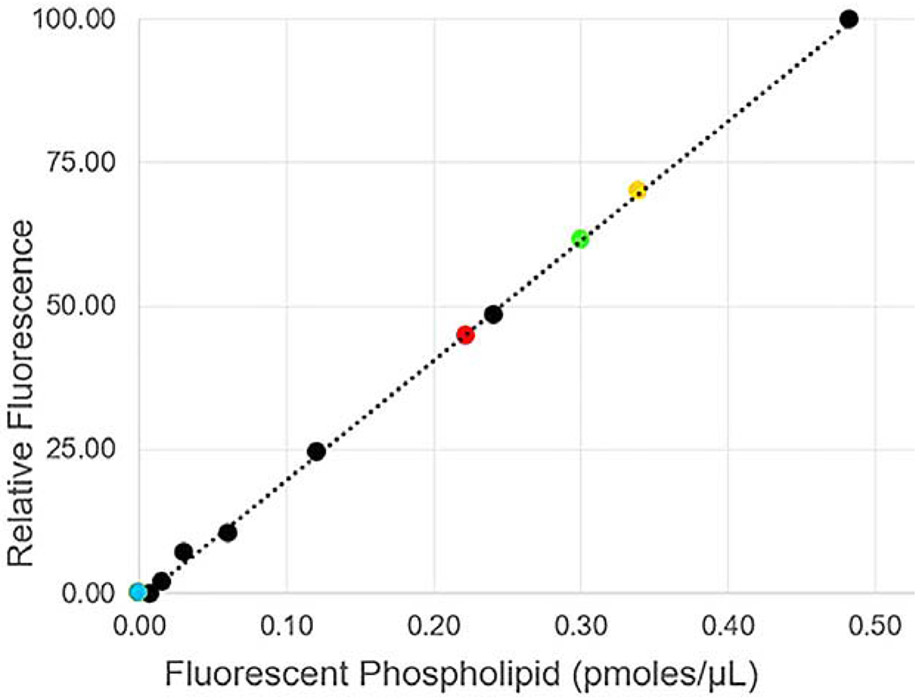
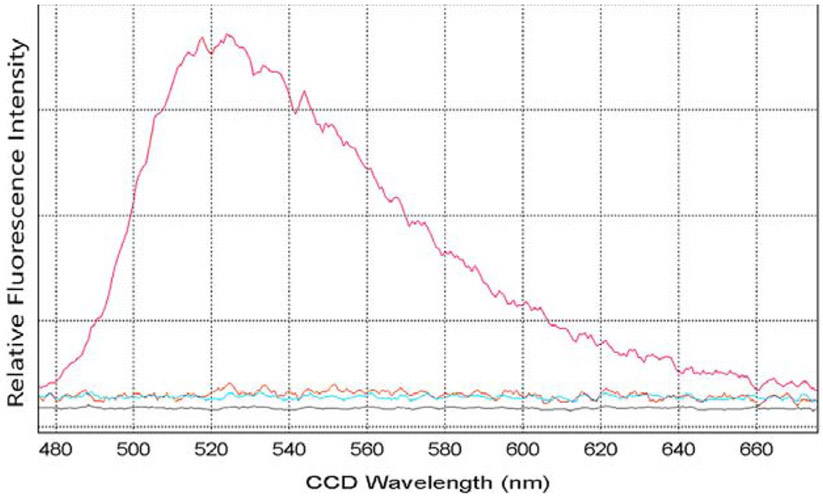
Data from the transfer of fluorescent phospholipid from tears and tear components are shown on the graph with the standard curve used for calibration of assays (Figure 1). The tear components included the centrifugally separated components (high and low molecular weight (55 kDa cutoff), tears without an added acceptor and reconstituted tears without lipocalin. The amount of fluorescently labeled phospholipid transferred can be compared for tears and the high molecular weight fraction from the same lot. For both tears and the high molecular weight fraction the amounts of fluorescent phospholipid transferred were similar, 0.33 and 0.34 pmoles, respectively. This is expected as the source of the enriched protein in centrifugal fractions came from the same pooled lot and volume of tears (5 μl). In this lot, tears showed slightly less activity than the high molecular weight fractions alone, excluding an additive effect from additional enzymes present in the lower molecular fractions of tears. Similar to that described by Jauhiainen et al. [17] the low molecular weight protein fraction had no discernible intrinsic activity to remove phospholipids from micelles (Figure 1). This fraction contains all of the lipid binding proteins of tears including tear lipocalin, lipophilin and apolipoprotein D with masses of 17.7kDa, 16.4kDa and ~28kDa [18-20]. Although tear lipocalin has no transfer activity, the data indicate that it is the major acceptor protein for phospholipid transfer protein in tears. Tears were fractionated by liquid chromatography and reconstituted without tear lipocalin to search for the acceptor protein(s) in tears. This lot of tears was stored longer than the first lot, which may explain the slightly lower amount of transferred phospholipid, 0.22 pmoles versus 0.33 pmoles transferred. The emission fluorescence spectra from the modified phospholipid transfer assays (omitting the extrinsic acceptor protein) of tears versus reconstituted tears without lipocalin are shown in Figure 2. Phospholipid transfer was detected in tears but not in tears without tear lipocalin. In the presence of the extrinsic acceptor, alone, no phospholipid transfer was identified. Phospholipid transfer activity from micelles in tears requires the presence of both phospholipid transfer protein and its acceptor, tear lipocalin. In addition the published hypothesis that phospholipid transfer protein could transfer phospholipid from tear lipocalin in the bulk of tears to the surface lipid film [2,6] was tested with ellipsometry.
Figure 1.
Standard curve of the phospholipid protein transfer assay. Black points and dashed line show standard curve for serial diluted amounts of phospholipid measured in 2-propanol. The amounts of fluorescent phospholipid transferred with tears and tear fractions are transposed on the standard curve. Yellow point, high molecular weight centrifugal fraction from 5 μl of tears lot 1, Green point, tears 5 μl; Red point, tears lot 2 normalized to 5 μl , Blue point, tears without tear lipocalin, and low molecular weight fraction (both with no activity).
Figure 2.
Fluorescence spectra from modified assay without an extrinsic acceptor to identify the acceptor for phospholipid transfer activity in tears.
Fluorescent labeled micelles 6 μl, were incubated for 30 minutes at 37 degrees. Samples from various tear components and control solutions, were made from 40 μl of tears. Fluorescence spectra, λex= 465 nm were taken at after precipitation and centrifugal removal of the remaining micelles. The solutions included whole tears (red), tears without tear lipocalin (orange), purified tear lipocalin (blue) and high density lipoprotein (negative control).
3.2. Ellipsometry to monitor the surface of tears for transfer of phospholipids
Ellipsometry measurements (Table 1) test the contribution of added tear components to surface film formation. In these studies tear mimic buffer was used rather than tears to avoid interactions with the complex lipid mixture in tears [16]. Both tear lipocalin and the components of the high molecular weight centrifugal concentrate increase the thickness of the surface film. The main protein in the high molecular weight fraction is lactoferrin and exists in tears at a concentration 1.7 mg/ml [21]. The concentration of PLTP in tears is only 10.9 ± 2.4 μg/mL [2]. Both tear lipocalin and lactoferrin are known to unfold at the surface of aqueous solution so increased surface film thickness is expected when either are instilled in the subphase [14]. However, the data here show that the thickness increased by only about 1 nm when the high molecular weight fraction was added to the subphase already containing tear lipocalin. And the high molecular weight fraction added without tear lipocalin in the subphase also contributed about 1 nm in thickness. Therefore, the addition of phospholipid transfer protein to a solution containing tear lipocalin does not result in additional phospholipid on the surface of tears. The increase seen with purified tear lipocalin alone is greater than with the high molecular weight fraction, so phospholipid delivery to the surface by tear lipocalin cannot be excluded. Since tear lipocalin unfolds at the surface probably most of the thickness is due to protein and little thickness is contributed by lipid. In addition, tear lipocalin is capable of adsorbing and penetrating a lipid layer comprised of Meibomian lipids [22]. However, the known monolayer thickness for phospholipids is about 2.6 to 3.2 nm [16]. The addition of phospholipid to the surface of buffer that contained both the high molecular weight fractions of tears and tear lipocalin did increase the thickness to exceed that of a monolayer. Polarization-modulated Fourier transform infrared reflective absorption spectroscopy studies suggest that absorbance attributable to phospholipid in tears may be less than that observed with a monolayer of DPPC [6]. Therefore, the smaller increase in surface thickness by tear lipocalin alone indicates that if some phospholipid transport occurs at the surface, a complete monolayer is not formed.
Table 1. Ellipsometry of Tear Components.
Tear components were added in concentrations to approximate the final concentration of the major component in tears. Final concentration of tear lipocalin was 74μM. HMWF (high molecular weight fraction from tears), was added to approximate the final concentration of lactoferrin in tears 19 μM. The range of increased thickness is reported from a minimum 3 repeat samples in which average thicknesses were calculated from all pixels of a scan, with minimum of at least 3 separate regions for each sample.
| Sample | Mean increase in thickness (range in nm) |
|---|---|
| Tear buffer | 0.3-0.4 |
| Successive addition of HMWF to tear buffer | 0.9-1.0 |
| Successive addition of tear lipocalin to tear buffer #1 | 1.4-1.7 |
| Successive addition of HMWF to tear lipocalin/tear buffer | 0.8-.9 |
| Successive addition DPPC added to above | 4.0-4.9 |
3.3. Functional significance
The apparent absence of transport of phospholipids to the surface in tears raises the question as to the function of the phospholipid transfer protein in tears. One obvious hypothesis is that phospholipid transfer protein and tear lipocalin work in concert to disrupt micelles. Micelles have a higher index of refraction (~1.46) than aqueous (1.33). Micelles range in size from 2-20 nm depending on their composition [23]. Since Raleigh light scattering is a function of the square of the index of refraction, and inversely proportional to the fourth power of the wavelength, even small micelles would increase isotropic scattering particularly in the shorter wavelengths [24]. Reduction of scattered light in the tear film may be advantageous to maintain clear vision without chromatic distortion.
Highlights.
Lipocalin-1 or tear lipocalin is the acceptor for phospholipid transfer protein in human tears.
Phospholipid transfer protein does not carry or transfer phospholipid to the surface of tears.
Lipocalin-1 and phospholipid transport protein work together to remove light scattering micelles from tears.
Acknowledgements
Funding: This work was supported by National Institute of Health [EY 11224 (BG), and EY 00331 (Institute Core)] as well as the Edith and Lew Wasserman Endowed Professorship (BG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Helmkamp GM, Phospholipid transfer proteins: Mechanism of action, J. Bioenerg. Biomembr 18 (1986) 71–91. 10.1007/BF00743477. [DOI] [PubMed] [Google Scholar]
- [2].Jauhiainen M, Setälä NL, Ehnholm C, Metso J, Tervo TMT, Eriksson O, Holopainen JM, Setala NL, Ehnholm C, Metso J, Tervo TMT, Eriksson O, Holopainen JM, Phospholipid transfer protein is present in human tear fluid, Biochemistry. 44 (2005) 8111–8116. 10.1021/bi050151k. [DOI] [PubMed] [Google Scholar]
- [3].Wizert A, Iskander DR, Cwiklik L, Organization of Lipids in the Tear Film: A Molecular-Level View, PLoS One. 9 (2014) e92461. 10.1371/journal.pone.0092461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang H, Dudley EG, Harte F, Critical synergistic concentration of lecithin phospholipids improves the antimicrobial activity of eugenol against Escherichia coli, Appl. Environ. Microbiol 83 (2017). 10.1128/AEM.01583-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rohit A, Stapleton F, Brown SHJ, Mitchell TW, Willcox MDP, Comparison of tear lipid profile among basal, reflex, and flush tear samples., Optom. Vis. Sci 91 (2014) 1391–5. 10.1097/OPX.0000000000000411. [DOI] [PubMed] [Google Scholar]
- [6].Glasgow BJ, Evidence for Phospholipids on the Surface of Human Tears, Invest. Ophthalmol. Vis. Sci 61 (2020) 19. 10.1167/iovs.61.14.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kawai S, Nakajima T, Hokari S, Komoda T, Kawai K, Apolipoprotein A-I concentration in tears in diabetic retinopathy, Ann. Clin. Biochem 39 (2002) 56–61. 10.1258/0004563021901748. [DOI] [PubMed] [Google Scholar]
- [8].Dean AW, Glasgow BJ, Mass Spectrometric Identification of Phospholipids in Human Tears and Tear Lipocalin, Investig. Opthalmology Vis. Sci 53 (2012) 1773. 10.1167/iovs.11-9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huuskonen J, Olkkonen VM, Jauhiainen M, Metso J, Somerharju P, Ehnholm C, Acyl chain and headgroup specificity of human plasma phospholipid transfer protein, Biochim. Biophys. Acta - Lipids Lipid Metab 1303 (1996) 207–214. 10.1016/0005-2760(96)00103-8. [DOI] [PubMed] [Google Scholar]
- [10].Gasymov OK, Abduragimov AR, Prasher P, Yusifov TN, Glasgow BJ, Tear lipocalin: evidence for a scavenging function to remove lipids from the human corneal surface., Invest. Ophthalmol. Vis. Sci 46 (2005) 3589–96. 10.1167/iovs.05-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Van Haeringen NJ, Clinical biochemistry of tears, Surv. Ophthalmol 26 (1981) 84–96. 10.1016/0039-6257(81)90145-4. [DOI] [PubMed] [Google Scholar]
- [12].Mokhtarzadeh M, Casey R, Glasgow BJ, Fluorescein punctate staining traced to superficial corneal epithelial cells by impression cytology and confocal microscopy., Investig. Ophthalmol. Vis. Sci 52 (2011) 2127–35. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3080172&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Glasgow BJ, Abduragimov AR, Farahbakhsh ZT, Faull KF, Hubbell WL, Tear lipocalins bind a broad array of lipid ligands., Curr. Eye Res. 14 (1995) 363–72. http://www.ncbi.nlm.nih.gov/pubmed/7648862 (accessed March 1, 2016). [DOI] [PubMed] [Google Scholar]
- [14].Glasgow BJ, Marshall G, Gasymov OK, Abduragimov AR, Yusifov TN, Knobler CM, Tear lipocalins: potential lipid scavengers for the corneal surface., Invest. Ophthalmol. Vis. Sci 40 (1999) 3100–7. http://www.ncbi.nlm.nih.gov/pubmed/10586930 (accessed June 20, 2016). [PubMed] [Google Scholar]
- [15].Masson D, Drouineaud V, Moiroux P, Gautier T, Dautin G, Schneider M, Fruchart-Najib J, Jauhiainen M, Ehnholm C, Sagot P, Gambert P, Jimenez C, Lagrost L, Human seminal plasma displays significant phospholipid transfer activity due to presence of active phospholipid transfer protein, Mol. Hum. Reprod 9 (2003) 457–464. 10.1093/molehr/gag062. [DOI] [PubMed] [Google Scholar]
- [16].Glasgow BJ, Ellipsometry of human tears, Ocul. Surf 17 (2019) 341–346. 10.1016/j.jtos.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Saaren-Seppälä H, Jauhiainen M, Tervo TMT, Redl B, Kinnunen PKJ, Holopainen JM, Interaction of purified tear lipocalin with lipid membranes, Investig. Ophthalmol. Vis. Sci 46 (2005) 3649–3656. 10.1167/iovs.05-0176. [DOI] [PubMed] [Google Scholar]
- [18].Glasgow BJ, Abduragimov AR, Yusifov TN, Gasymov OK, Horwitz J, Hubbell WL, Faull KF, A conserved disulfide motif in human tear lipocalins influences ligand binding., Biochemistry. 37 (1998) 2215–25. 10.1021/bi9720888. [DOI] [PubMed] [Google Scholar]
- [19].Lehrer RI, Xu G, Abduragimov A, Dinh NN, Qu XD, Martin D, Glasgow BJ, Lipophilin, a novel heterodimeric protein of human tears., FEBS Lett. 432 (1998) 163–7. http://www.ncbi.nlm.nih.gov/pubmed/9720917 (accessed March 1, 2016). [DOI] [PubMed] [Google Scholar]
- [20].Soria J, Acera A, Merayo-Lloves J, Durán JA, González N, Rodriguez S, Bistolas N, Schumacher S, Bier FF, Peter H, Stöcklein W, Suárez T, Tear proteome analysis in ocular surface diseases using label-free LC-MS/MS and multiplexed-microarray biomarker validation, Sci. Rep 7 (2017). 10.1038/s41598-017-17536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fullard RJ, Snyder C, Protein levels in nonstimulated and stimulated tears of normal human subjects, Investig. Ophthalmol. Vis. Sci 31 (1990) 1119–1126. [PubMed] [Google Scholar]
- [22].Millar TJ, Mudgil P, Butovich IA, Palaniappan CK, Adsorption of human tear lipocalin to human Meibomian lipid films, Investig. Ophthalmol. Vis. Sci 50 (2009) 140–151. 10.1167/iovs.08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Johnsson M, Edwards K, Liposomes, Disks, and Spherical Micelles: Aggregate Structure in Mixtures of Gel Phase Phosphatidylcholines and Poly(Ethylene Glycol)-Phospholipids, 2003. [DOI] [PMC free article] [PubMed]
- [24].Rayleigh Lord, XXXIV. On the transmission of light through an atmosphere containing small particles in suspension, and on the origin of the blue of the sky , London, Edinburgh, Dublin Philos. Mag. J. Sci 47 (1899) 375–384. 10.1080/14786449908621276. [DOI] [Google Scholar]