Abstract
Purpose:
To improve the signal-to-noise ratio (SNR) and image sharpness for whole brain isotropic 0.5mm three-dimensional (3D) T1 weighted (T1w) turbo spin echo (TSE) intracranial vessel wall imaging (IVWI) at 3T.
Methods:
The variable flip angle (VFA) method enables useful optimization across scan efficiency, SNR and relaxation induced point spread function (PSF) for TSE imaging. A convolutional neural network (CNN) was developed to retrospectively enhance the acquired TSE image with PSF blurring. The previously developed VFA method to increase SNR at the expense of blur can be combined with the presented PSF correction to yield long echo train length (ETL) scan while the acquired image remains high SNR and sharp. The overall approach can enable an optimized solution for accelerated whole brain high-resolution 3D T1w TSE IVWI. Its performance was evaluated on healthy volunteers and patients.
Results:
The PSF blurred image acquired by a long ETL scan can be enhanced by CNN to restore similar sharpness as a short ETL scan, which outperforms the traditional linear PSF enhancement approach. For accelerated whole brain IVWI on volunteers, the optimized isotropic 0.5mm 3D T1w TSE sequence with CNN based PSF enhancement provides sufficient flow suppression and improved image quality. Preliminary results on patients further demonstrated its improved delineation for intracranial vessel wall and plaque morphology.
Conclusion:
The CNN enhanced VFA TSE imaging enables an overall image quality improvement for high-resolution 3D T1w IVWI, and may provide a better tradeoff across scan efficiency, SNR and PSF for 3D TSE acquisitions.
Keywords: turbo spin echo, variable flip angle, point spread function, deep learning, generative adversarial network, intracranial vessel wall imaging
1. Introduction
Stroke is one of the leading cause of mortality and disability worldwide [1]. MR intracranial vessel wall imaging (IVWI) provides an important clinical solution for stroke diagnosis. Turbo spin echo (TSE) imaging allows sufficient signal-to-noise (SNR) and proper signal contrast between the vessel wall and its surrounding tissues. Therefore, it has been widely used for high-resolution three-dimensional (3D) Cartesian IVWI at 3T [2–8]. The intrinsic black blood properties of TSE sequence caused by effects of intravoxel dephasing and incoherent echo formation can achieve good vessel wall and lumen signal contrast. T1 weighted (T1w) TSE sequences with anti-drive equilibrium [5,6] can further provide effective cerebrospinal fluid (CSF) suppression and have demonstrated their ability in detecting intraplaque hemorrhage [9] and thrombosis [10,11] as well as their essential value for contrast-enhanced imaging [7,12].
The variable flip angle (VFA) refocusing pulse train can be designed for 3D TSE imaging by using the extended phase graph (EPG) [13–15]. This VFA design strategy can provide a tradeoff across signal-to-noise ratio (SNR), point spread function (PSF), and scan efficiency for one specific tissue type. However, for isotropic high-resolution (e.g. ≤0.5mm) IVWI at 3T, it might be difficult to achieve the ideal PSF response for the targeted tissue while compensating for the losses on SNR and/or scan efficiency. In this case, the acquired PSF response is typically imperfect resulting in image blurring and resolution loss when SNR and scan efficiency are prioritized for optimization. Furthermore, the signal evolutions for different tissues will be driven differently by a chosen TSE refocusing pulse train due to their distinct relaxation properties. There are theoretical limitations that the ideal PSF performance cannot be simultaneously achieved across various tissue properties by a linear based PSF correction designed for one tissue type. Therefore, alternate correction methods for such relaxation induced PSF blurring are required to restore the desired acquisition resolution for TSE imaging, particularly for demanding high-resolution applications.
Deep convolutional neural network (CNN) has emerged as a flexible and powerful supervised learning technique to establish a nonlinear low dimensional manifold between the input source and output target domains given a large amount of qualified and labeled training datasets [16–20]. Recent studies [21–23] have demonstrated its superior performance for image super resolution that can retrospectively improve the acquisition resolution. This also implies the potential of CNN to enhance the PSF blurred image for TSE acquisition. In addition, the trained CNN at the inference phase can be implemented efficiently with the advanced deep learning hardware, which enables a straightforward deployment in the clinical environment. Such CNN based PSF enhancement can also create additional opportunities to improve SNR and scan efficiency for TSE imaging by optimizing the VFA refocusing pulse train design and the echo train length (ETL).
In this work, a deep CNN based PSF enhancement approach is presented to retrospectively reduce the relaxation induced PSF blurring for 3D TSE scans. This presented approach can be combined with the previously developed VFA method to produce a long ETL TSE acquisition with high SNR and sharp image. The overall approach allows further optimization for whole brain isotropic 0.5mm 3D T1w TSE IVWI in terms of the image quality and scan efficiency. The performance of this approach was evaluated by in vivo intracranial imaging experiments on healthy subjects and patients.
2. Material and Methods
2.1. SNR priority VFA design for TSE Refocusing Pulse Train
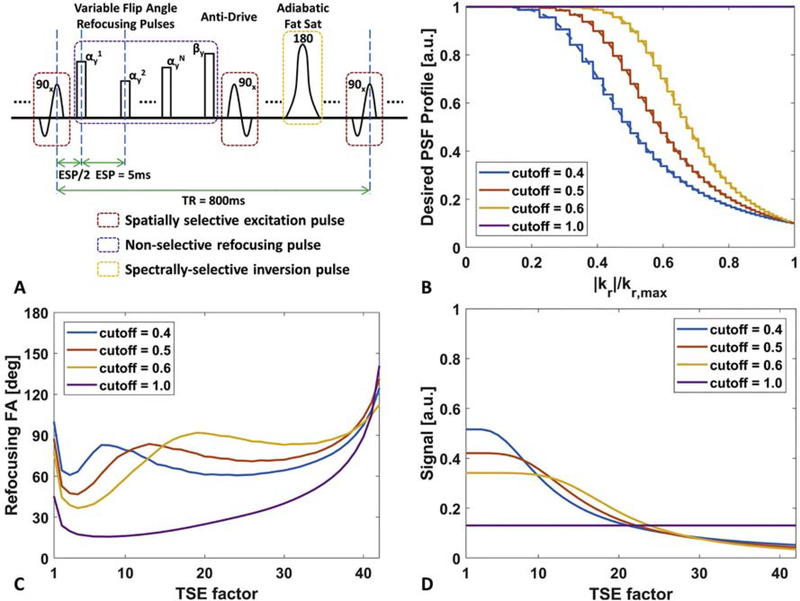
By exploiting a variable echo signal evolution, the SNR priority VFA design strategy attempts to improve the echo signal acquired for the central k-space region by reducing the echo signal for peripheral k-space acquisition. For the 3D Cartesian T1w TSE with a centric k-space acquisition ordering, this results in a two-dimensional (2D) low-pass filtering PSF profile in the phase- and slice-encoding plane. In this work, a group of normalized Butterworth low-pass filtering PSF profiles with different cutoff values was selected as the desired TSE signal evolution curves to design the corresponding VFA refocusing pulse train. The low-pass filtering PSF profile was scaled to derive the corresponding VFA based on the EPG theory [13,14], where the scaling factor was determined by an iterative search to meet the maximum refocusing flip angle constraint. The SNR priority VFA design procedure and several examples are illustrated in Fig. 1.
Figure 1:
The diagram of 3D T1 weighted (T1w) turbo spin echo (TSE) sequence with a variable flip angle (VFA) refocusing pulse train design. (A) 3D T1w TSE sequence with VFA refocusing pulse trains, anti-drive and adiabatic spectral fat suppression. (B) Desired lowpass filtering profiles with different cutoff frequencies to be used as signal evolution curves of the reference tissue in extended phase graph (EPG) algorithm. The cutoff value of 1.0 refers to the point spread function (PSF) priority VFA design method, and the others are for signal-to-noise ratio (SNR) priority VFA design method. (C) The corresponding VFA refocusing pulse trains converted by EPG algorithm. (D) The TSE steady state echo signal curves for different VFA pulse trains generated by Bloch simulation after 30 repetition cycles. Note that SNR priority VFA design offer much higher echo signals during the first half echo train acquisition compared to PSF priority VFA design.
2.2. Spatial Temporal Subspace Representation for TSE Imaging
To construct the relation between the ideal PSF image and the acquired image with PSF blurring, the spatial temporal subspace representation for the multi-echo images [24–26] is used. This helps to understand the underlying mechanisms and further enables the estimation of an ideal PSF image from an acquired one. In 3D TSE imaging, each image volume formed by the ith echo can be written as , where Nv and Ne corresponds to the total number of voxels and echoes within each TSE shot. With a spatial temporal subspace model, xi can be decomposed as
| (1) |
where indicates the spatial components, ϕl,i represents the ith element within the temporal subspace basis , and L describes the model order. Given a specific VFA refocusing pulse train and other sequence parameters (e.g. repetition time, echo spacing), the subspace basis ϕl, l ∈ {1,2, ∙∙∙, L} can be established by applying singular value decomposition (SVD) on a simulated dictionary consisting of various echo signal evolution curves derived from different T1 and T2 values. Furthermore, the model order L can be determined by counting the number of singular values above a proper threshold.
With Eq. (1) and a specific k-space sampling ordering, the TSE image acquired across the echo train can be synthesized accordingly by
| (2) |
where is a diagonal matrix with diagonal entries of 0 or 1 indicating the k-space sampling mask for the ith echo, and F, correspond to the Fourier and inverse Fourier transform operator respectively. Eq. (2) demonstrates that the temporal subspace basis ϕl will induce an acquired PSF that applies signal modulation in k-space onto the corresponding spatial component αl. Furthermore, the blurred image xacq can be synthesized by a mixture of different PSF blurred spatial components. In contrast, a T1w TSE image with an ideal PSF can be realized by acquiring all k-space samples at the first echo time, which can be derived similarly as
| (3) |
Note that ϕl,1 is used to modulate all k-space samples for spatial component αl. By comparing Eq. (2) and Eq. (3), it becomes obvious that a linear transform from xacq to xideal cannot simultaneously convert all of the acquired PSFs to the corresponding ideal PSFs. Therefore, a nonlinear transform is required to achieve such PSF enhancement.
2.3. Neural Network based PSF Enhancement
The neural network is a powerful non-parametric model to construct a nonlinear mapping from a given input to an expected output via supervised learning. Figure 2 illustrates the deep CNN model for PSF enhancement and the overall flowchart for its training/inference process. Since the relaxation induced PSF blurring for 3D Cartesian TSE occurs only within the phase- and slice-encoding plane, a 2D residual learning based CNN architecture was developed to restore the penalized high frequency components during SNR priority VFA data acquisition. Except for the global skip connection between input and output [21], residual blocks with local skip connection [19] and pre-activation [27] were also exploited to reduce the difficulties for network training. With the pair of xacq and xideal sampled from training dataset and , the CNN parameters θ can be optimized by minimizing the following L1 and adversarial loss functions
| (4) |
where Gθ(xacq) approximates the corresponding ideal PSF modulated image given input xacq, ∥∙∥1 implies the L1-norm metric that quantifies the pixel-wise image similarities, Dφ(∙) denotes a discriminator with parameters φ that measures the image structural similarities from a distribution perspective, λ indicates the weight to tradeoff the L1 and adversarial loss, and Ex{∙} represents the expectation operator over variable x. The parameters φ in classifier Dφ(∙) can be progressively updated by maximizing the following loss function to discriminate the PSF enhanced image from the ideal image
| (5) |
This discriminator network Dφ exploited similar residual blocks between stride convolutions for dimension reduction (Fig. 2 (B)), but using a leaky rectified linear unit (ReLU) [28]. During training, Eq. (5) and Eq. (4) were solved in an interleaved manner for this saddle-point optimization [29] of φ and θ. Also, random Rician noise with variable standard deviations was added to xacq, to reduce the adverse noise amplification effects during the Gθ restoration process. The paired training samples were synthesized by simulation. Given the sharp image sample xideal, its corresponding blurred sample xacq can be simulated by exploiting the relations in Eq. (2) and Eq. (3). Tikhonov regularization was used to solve the underdetermined problem in Eq. (3) for spatial components αl, l ∈ {1,2, ···, L} estimation:
| (6) |
where γl is the regularization weight for spatial component αl and can be calibrated from a short and a long echo train pre-scans. With the estimated αl, l ∈ {1,2, ···, L}, xacq can be further synthesized by using Eq. (2). More detailed regularization weight calibration and image patch synthesis process are discussed in the supplementary material.
Figure 2:
The architecture of convolutional neural network (CNN) for point spread function (PSF) enhancement and the flowchart of its training/inference process. (A) Given the reference image, the PSF blurred image can be generated by a developed simulation process (purple arrow pathway), which further synthesizes the PSF enhanced image by the trained CNN Gθ (dark blue arrow pathway). During training, the PSF blurred image was corrupted by different levels of Rician noise, and both L1 and adversarial losses were used as the similarity metric (orange arrow pathway). (B) The architecture of CNN Dϕ used for the discriminator in adversarial loss. (C) The basic network building blocks used in (A) and (B).
2.4. Learning with Synthesized Database
A whole brain isotropic 0.25mm T1w MPRAGE image dataset [30] was downloaded and used as the sharp image reference, and this high-resolution image was resampled to lower resolutions (e.g. 0.3mm, 0.6mm and 0.9mm) for training patch preparation. Wavelet based image denoising [31] was first applied to reduce the noise level of the original dataset, and the denoised image was normalized within the range between 0 and 1. To create the echo signal evolution dictionary, five different echo spacings from 2ms to 8ms were sampled for each designed VFA pulse train with an ETL 40. For each echo spacing, twenty samples of T1 values ranging from 200ms to 3000ms and T2 values between 20ms and 200ms were selected independently to simulate the echo signal curves as dictionary atoms. This EPG simulation results in a dictionary with 2000 atoms that can extract the temporal subspace bases by SVD to cover a broad variation of TSE signal evolutions. With the extracted temporal subspace bases and the centric k-space ordering, blurred images can be synthesized by solving Eq. (6) and Eq. (2) sequentially. Considering the difference between acquisition and reconstruction resolutions, 2-fold decimation and interpolation were also applied before and after PSF blurring to cover such situation. Furthermore, the paired training images were cropped into 41×41 sized patches to exclude background and ensure sufficient structural complexity, where 41 fulfilled the receptive field of the CNN with 20 convolutional layers and 3×3 kernel size for each layer. This simulation process generated 64*750 cropped image patch pairs with a batch size of 64, while 80% and 20% were used as training and validation datasets respectively. 20 and 16 convolutional layers with 64 feature channels in each layer were used for network Gθ and Dφ, respectively. In this work, different training datasets were simulated for different VFA refocusing pulse trains to learn the corresponding CNN for each TSE sequence. λ in Eq. (4) was empirically set to 3 × 102 for balancing the L1 and adversarial loss. The Adam stochastic optimization [32] was used to solve Eq. (4) and Eq. (5) for CNN training, with a learning rate of 2 × 10−4 in 60 epochs. The training and inference process were implemented using Tensorflow on a Linux workstation with a 6-core Intel Xeon E5–1650 3.6GHz CPU, 96GB memory and a single Nvidia Titan Xp 12GB GPU.
2.5. MR Scans
All scans were performed with institutional review board approval on a 3T Ingenia CX MR scanner (Philips, Best, Netherlands) using a 32-channel head coil. 7 healthy volunteers were scanned for IVWI optimization, including 3 subjects for SNR improved acquisition and PSF enhancement assessment in low-resolution scans, and 4 subjects for PSF enhancement comparison in high-resolution scans. In addition, 10 patients with carotid plaques were recruited to evaluate the performance of optimized 3D T1w TSE for whole brain isotropic 0.5mm IVWI. The scan parameters are summarized in Table 1. The ETL 40 was used to provide reasonable scan efficiency and signal decay during TSE acquisition window (∼200ms) for optimized high-resolution (isotropic 0.5mm) 3D T1w IVWI. To optimize imaging SNR and PSF enhancement, the lowpass filtering shaped PSF profiles with different cutoff values between 0.4 and 0.6 were evaluated, while fixing the signal attenuation ratio between the acquired first and last echo to be 10-fold. The maximum refocusing flip angle during EPG calculation was constrained to be less than 150 degrees to reduce specific absorption rate issues. The low- and high-resolution 3D T1w TSE scans with different VFA PSF profile designs were compared with resolution-matched shorter ETL of TSE scans as the reference. In patients, 3D time-of-flight (TOF) bright blood MR angiography was acquired additionally as a reference for intracranial vessel lumen shape.
Table 1:
Summary of MR Imaging Parameters
| 3D T1w TSE | 3D TOF | ||||
|---|---|---|---|---|---|
| Low-resolution | High-resolution | ||||
| Field of viewa | 230x230x210mm3 | 230x210x180mm3 | 105x180x190mm3 | ||
| Voxela (Acq./Rec.) | 1.5x1.5x1.5mm3/0.72x0.72x0.75mm3 | 0.5x0.5x0.5mm3/0.24x0.24x0.25mm3 | 1.0x0.5x0.5mm3/0.5x0.25x0.25mm3 | ||
| Flip angle | 90° | 18° | |||
| Echo train lengthb | 5 | 40 | 30 | 40 | N/A |
| VFA designc | SNR prior | PSF prior | SNR prior | N/A | |
| Echo spacing/timed | 3ms | 5ms | 3.45ms | ||
| Repetition time | 800ms | 20ms | |||
| Driven-equilibrium | Anti-drive | N/A | |||
| Fat suppression | Spectral adiabatic inversion recovery | No | |||
| Orientation | Sagittal | Axial | |||
| Acceleration | CS-SENSE x5 | CS-SENSE x6 | SENSE(RL) x2.2 Halfscan(RL) 0.75 |
||
| Scan time | 7:39 | 0:58 | 8:50 | 6:38 | 6:37 |
The imaging field of view and voxel size is represented in a format of FHxAPxRL.
The echo train length for turbo spin echo (TSE) scan only considers the number of acquired echoes, and the skipped start-up echoes at the beginning are not counted.
The SNR and PSF prior used in this VFA design category correspond to SNR and PSF priority VFA design approach, respectively. The PSF priority approach is the default VFA design on our scanner that tries to achieve an ideal/flat PSF (the first 11 echoes) given the minimum flip angle (50 degree) and start to decay once the calculated flip angle over a prescribed threshold (120 degree).
Echo spacing and echo time correspond to TSE and time-of-flight (TOF) scan respectively. TE was not a fixed value for TSE scans since different number of skipped echoes were investigated.
2.6. Assessment of SNR Improved Acquisition and PSF Enhancement
For a long ETL 3D TSE scan, the effectiveness of SNR priority VFA design to improve SNR and the CNN based PSF enhancement to restore similar image sharpness to a short ETL scan was investigated. In this experiment, TSE scans with 40 and 5 acquired echoes were compared. Due to the low scan efficiency of the reference 5 ETL TSE scan, this comparison was performed with low-resolution 3D T1w TSE scans. Three ETL 40 scans using different cutoff frequencies from 0.4 to 0.6 were acquired to compare the relative signal enhancement within the volume of brain and evaluate the CNN based image sharpness restoration performance.
2.7. Optimization of High-Resolution 3D T1w TSE for Whole Brain IVWI
Short excitation and refocusing RF pulses were applied to minimize the echo spacing for the high-resolution 3D T1w TSE scan with SNR priority VFA design. The first two echoes of TSE were skipped to tradeoff between the SNR, T1 contrast and flow suppression for IVWI.
Since SNR drops remarkably in high-resolution scans, the image quality restored from CNN enhanced 3D T1w TSE scan with SNR priority VFA design was evaluated. Two ETL 40 scans with cutoff frequencies of 0.4 and 0.6 were acquired and compared with a reference ETL 30 scan using a PSF priority VFA design. The reference scan was expected to provide a better SNR and PSF performance, although requiring a longer scan time. Both acquired and CNN enhanced images from the ETL 40 TSE scans were compared with the reference for intracranial vessel wall delineation. In addition, the CNN based PSF enhancement was compared to the traditional linear PSF enhancement method [33] without and with additional wavelet based image denoising [31]. In the linear PSF enhancement, the acquired PSF was generated for vessel wall (T1/T2=844ms/39ms) [34] and used for deblurring with an empirically tuned L2-norm regularization to reduce the noise amplification.
Curved planar reformatted (CPR) images were used to visualize the intracranial vessels from proximal to distal segments. In this study, All CPR images for vessel wall visualization across multiple vascular beds were generated by Horos (a free and open source software, horosproject.org).
2.8. Evaluation of Optimized High-Resolution 3D T1w TSE IVWI on Patients
The optimized isotropic 0.5mm 3D T1w TSE IVWI with CNN based PSF enhancement was investigated on patients for evaluation of diseased vessel wall visibility. For performance comparison, the linear PSF enhancement with wavelet denoising approach was also performed as the reference. The CPR images were compared across different imaging and processing methods for the delineation of intracranial lumen, vessel wall and plaques. Quantitative measurements, including mean local gradient as well as contrast-to-noise ratio (CNR) between wall and lumen/background, were compared at selected vessel segments to evaluate the performance of PSF enhancement for vessel wall image quality improvement.
2.9. Image Quality Analysis
In sequence optimization experiments, the relative signal enhancement was compared by calculating the mean and standard deviation of signal ratio between the test image and its reference (e.g. image acquired with a short ETL) within the entire brain volume. To evaluate the SNR improvement for different cutoffs of SNR priority VFA design, the vessel wall SNR was measured on both acquired images with cutoff = 0.4 and cutoff = 0.6 for the high resolution ETL = 40 TSE volunteer scans. For the analysis of patient scans, 8 cross-sectional vessel images were reformatted from different major intracranial vessel segments including internal carotid artery (ICA), middle cerebral artery (M1, M2), anterior cerebral artery (A1, A1), basilar artery (BA), and posterior cerebral artery (P1, P2). The region of interest (ROI) for vessel wall, lumen, background surrounding tissue, and local noise (e.g. homogeneous region in CSF or white matter) were manually chosen with the ImageJ software [35]. The mean value of image gradients within the local vessel region was calculated to compare the overall image sharpness for vessel wall delineation. Also, the CNR between wall and lumen/background was measured to evaluate the inner/outer wall contrast (the higher the better). Since the acquired and enhanced images are naturally co-registered, the ROIs can be shared by both images and provide a fair quantitative image sharpness and contrast comparison. The Mann-Whitney U-test was used to test the statistical significance (p-value < 0.05) of those paired measurements on the acquired and/or enhanced image.
3. Results
3.1. Assessment of SNR Improved Acquisition and PSF Enhancement
Figure 3 (B)–(D) illustrated that a smaller cutoff value designed VFA TSE sequences could provide higher signal level, and the signal ratio measurements in Fig. 3 (I) also show that the ETL 40 TSE scan with a cutoff value of 0.4 in SNR priority VFA design can achieve similar signal level to an ETL 5 TSE scan. Longer ETL scans with smaller cutoff values also lead to more severe blurring issues (Fig. 3 (F)–(H)) compared to a short ETL scan (Fig. 3 (E)). After CNN based PSF enhancement, the visibility of detailed image structures can be largely restored particularly in the vessel wall of basilar artery and other structural tissues around cerebellum as shown in Fig. 3 (J)–(L). Similar effects on signal enhancement and PSF enhancement were also reproduced on the other 2 test cases.
Figure 3:
Comparison of 3D turbo spin echo (TSE) imaging with different variable flip angle (VFA) pulse trains. (A) Reference 3D TSE image acquired with an echo train length (ETL) of 5. (B)-(D) Example images acquired by 3D TSE sequences with ETL 40 but using different signal-to-noise ratio priority VFA pulse trains designed by cutoff frequencies of 0.4, 0.5 and 0.6. (E)-(H) The corresponding zoomed-in images from the local regions (blue box in (A)) in (A)-(D). (J)-(L) The same zoomed-in region comparison after convolutional neural network based PSF enhancement. Images in (A)-(D) are shown with the acquired signal scaling, and panel (I) summarizes the signal ratio within the region of interest (ROI) of whole head volume using ETL 5 image as the reference. Images in (F)-(H) and (J)-(L) are scaled to match the signal intensity in (E). Red arrows show that the vessel boundaries and structures can be better delineated in (J)-(L) compared to (F)-(H) with respect to the reference (E).
3.2. Optimization of High-Resolution 3D T1w TSE for Whole Brain IVWI
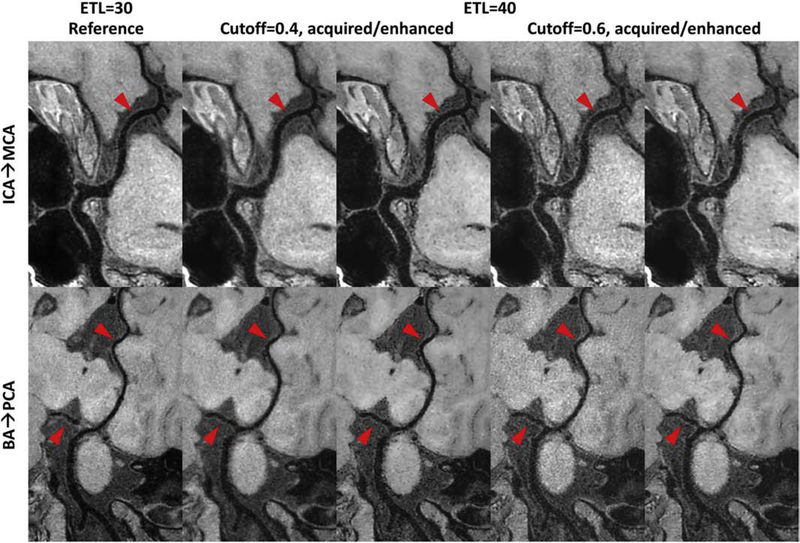
For high-resolution 3D T1w TSE scans, severe SNR drop was observed on the images acquired by a cutoff of 0.6 in SNR priority VFA design in comparison to the case of cutoff = 0.4. The measured vessel wall SNR on cutoff = 0.4 images (mean±std: 9.14±2.34) also demonstrate the significant improvement (p < 0.05) compared to the vessel wall SNR measure on cutoff = 0.6 images (mean±std: 6.38±1.59). Compared to the ETL 30 reference with conventional PSF priority VFA design, images acquired by a cutoff of 0.4 in SNR priority VFA design show more improved SNR and ∼25% reduced scan time for whole brain IVWI (>2 mins reduction in table 1). Although the blurring effect and noise level are different for cutoff = 0.4/0.6, the CNN enhanced images can show consistently superior image sharpness restoration and noise suppression for improved intracranial vessel wall depiction, compared to the ETL 30 reference and their originally acquired images (red arrows in Fig. 4). The similar CNN enhanced vessel wall results for cutoff = 0.4 and cutoff = 0.6 also support the effectiveness of the proposed CNN based PSF correction approach.
Figure 4:
Comparison of curved planar reformatted (CPR) images for different 3D turbo spin echo (TSE) intracranial vessel wall imaging sequences. Column 1 shows the reference image acquired with an echo train length (ETL) 30, while other columns are images acquired with ETL 40 but using different cutoff values to design the TSE refocusing pulse train (cutoff = 0.4 for column 2 and 3, cutoff = 0.6 for column 4 and 5). Column 2/4 shows the acquired CPR image, and column 3/5 shows the convolutional neural network enhanced CPR image. The top CPR images cover from the left C6 segment of internal carotid artery (ICA) to the M1 segment of middle cerebral artery (MCA), while the bottom CPR images cover from the end of right vertebral artery to the right posterior cerebral artery (PCA). Note the reduced noise level in brain tissue regions and the improved vessel wall delineation in column 3/5 shown by the red arrows compared to column 2/4.
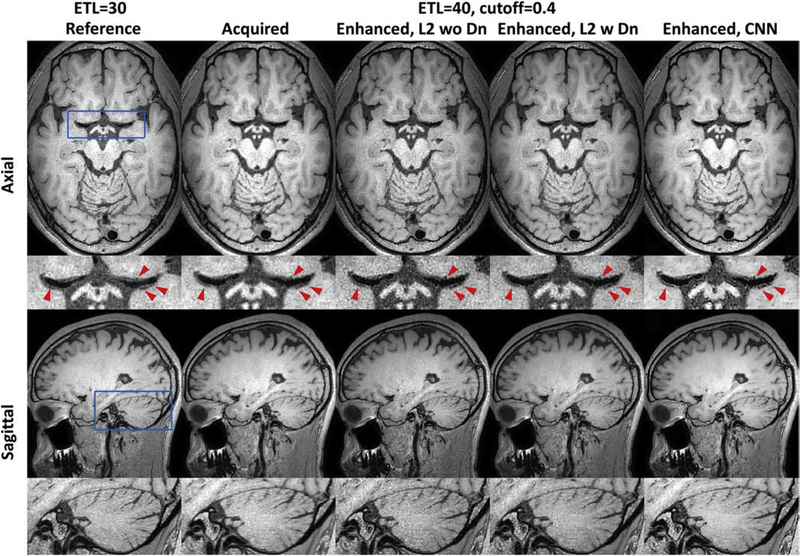
Figure 5 illustrates that CNN based PSF enhancement can provide much better detailed structure delineation (e.g. vessel wall of MCA, junction between MCA and lenticulostriate artery, cerebellum) in comparison to the traditional linear PSF enhancement approach without/with denoising. By comparing all test cases, the CNN enhanced image for cutoff = 0.4 outperforms the other acquired or enhanced images in terms of the scan efficiency or overall image quality, which was regarded as the optimized CNN enhanced 3D T1w TSE sequence for further IVWI evaluation on patients.
Figure 5:
Comparison of different point spread function (PSF) enhancement methods. Column 1 shows the reference image acquired with an echo train length (ETL) 30, and column 2 is the image acquired with an ETL 40 and a cutoff value of 0.4 for refocusing pulse train design. Columns from 3 to 5 correspond to the linear based PSF enhancement without or with wavelet denoising, and the convolutional neural network (CNN) based PSF enhancement. Row 1 show the axial images (phase- and slice-encoding plane), while row 3 show the sagittal images. Row 2/4 shows the zoom-in local images (blue box in Row 1/3) from the Row 1/3. CNN based PSF enhancement shows improved vessel wall delineation (red arrows in row 2) and cerebellum (row 4) over the acquired and linear based PSF enhancement results.
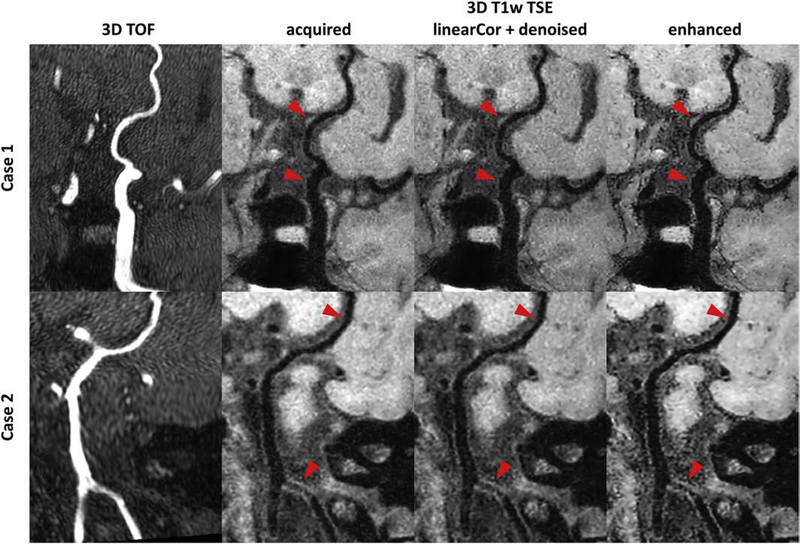
3.3. Evaluation of Optimized High-Resolution 3D T1w TSE IVWI on Patients
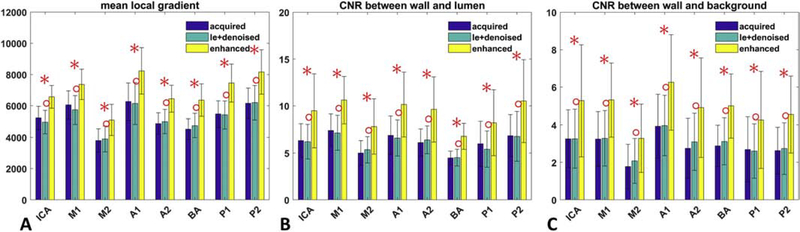
10 patients were scanned consecutively with acceptable image quality for diagnosis and image evaluation. Figure 6 shows two example cases with identified intracranial plaques and/or vessel wall thickening. An intracranial plaque was found in one case on the optimized T1w TSE image at the vessel wall of left posterior communicating artery, while the 3D TOF image did not show apparent luminal stenosis at the same location. Another case with vessel wall thickening at the right vertebral artery was found on the optimized T1w TSE image, while the 3D TOF image showed slight narrowing within the lesion segment. The boundaries of vessel wall and plaque were more sharply defined on the CNN enhanced CPR image compared to both acquired and the linear PSF enhanced CPR images. In Fig. 7, all quantitative measurements, including mean local gradient, CNR between wall and lumen and CNR between wall and background, at all segments on the 10 CNN enhanced images showed significant improvement over the acquired and linear PSF enhanced images, indicating sharper and better contrast for vessel wall depiction on the CNN enhanced images.
Figure 6:
Example curved planar reformatted (CPR) images from intracranial vessel scans on patients. The top case shows CPR images covering from the left internal carotid artery to posterior communicating artery, and the bottom case shows CPR images covering from vertebral arteries to right posterior cerebral artery. Different columns from left to right correspond to 3D time-of-flight (TOF) CPR images, the acquired CPR images with the optimized 3D T1 weighted (T1w) turbo spin echo (TSE) sequence, the linear enhanced CPR images with wavelet denoising, and the convolutional neural network (CNN) enhanced CPR images. The local vessel wall regions (red arrows) can be better delineated on the CNN enhanced images.
Figure 7:
Quantitative image quality comparison of the optimized 3D T1 weighted turbo spin echo imaging on patients. The ‘acquired’, ‘le+denoised’ and ‘enhanced’ corresponds to the acquired image, linear enhanced image with wavelet denoising, and convolutional neural network (CNN) enhanced image, respectively. 8 different segments covering the major anterior and posterior intracranial vessels are analyzed. (A) The comparison of mean image gradient within a local vessel region indicating the image sharpness for vessel wall depiction. (B) The comparison of contrast-to-noise ratio (CNR) measurements between vessel wall and lumen. (C) The comparison of CNR measurements between vessel wall and background surrounding tissue. The red star/circle marker indicates the statistical significance (p < 0.05) in Mann-Whitney U-test between CNN enhanced method and acquired/linear enhanced and denoised method.
4. Discussion
3D TSE scan with VFA refocusing pulse train allows certain compromise between SNR and PSF blurring. A deep CNN that enables a retrospective nonlinear approximation of the ideal PSF image from a PSF blurred image has been demonstrated to further optimize the TSE acquisition and reconstruction strategy in a complementary manner. The multilayer structure of an end-to-end deep CNN can extract various levels of image features to discriminate different blurring patterns from noise and synthesize the residuals for enhancement, which could be a powerful solution to reduce the tissue dependent PSF blurring in TSE imaging compared to the conventional linear PSF correction methods. With this CNN enhancement approach, it is possible to design a SNR priority VFA pulse train for longer ETL and/or SNR improved TSE acquisition. The combination of optimized TSE acquisition and deep CNN based PSF enhancement was evaluated for whole brain isotropic 0.5mm T1w 3D TSE IVWI at 3T. The preliminary results have demonstrated that the developed approach can accelerate TSE scans with a longer ETL and achieve similar delineation of intracranial vessel wall to a shorter ETL TSE scan.
3D isotropic imaging is essential to generate multi-planar reformatted or CPR images for visualization of tortuous intracranial vessels without severe morphological distortion. However, there will be additional PSF blurring problems within the phase- and slice-encoding plane for 3D TSE scans, which may limit the accuracy of following image analysis for intracranial vessel tracing [36] and wall segmentation [37]. The proposed CNN was trained as an end-to-end classifier to differentiate the blurred edges from the noise and then approximate its original sharpness. With the spatial temporal subspace representation for TSE imaging, a simulation process was developed to synthesize the co-registered pair image database for CNN training, which can span certain variations of echo spacing, T1 and T2 values. This simulation process can be applied flexibly on any type of high quality sharp images to generate its simulated TSE blurred image, which can easily increase the size of training database even with natural images [38] to train more complex network models. It implies that the accuracy of the proposed simulation process to approximate the PSF blurring in VFA TSE acquisition is much more critical than the image content (e.g. image contrast, anatomy). To ensure a more accurate PSF blurring simulation for the specific TSE sequence, the regularization weights for different spatial components in Eq. (6) were calibrated from a pair of short and long ETL TSE scans. During inference phase, the entire processing time takes ∼60 secs with our hardware settings for the acquired high-resolution whole brain 3D IVWI datasets, which shows promising potential for clinical translation.
To achieve high-resolution whole brain 3D IVWI, the isotropic voxel acquisition may raise a critical challenge to SNR and scan time. With retrospective PSF enhancement, the imaging SNR can be improved prospectively by a SNR priority VFA design method that can significantly enhance the echo signals during central k-space acquisition. Without the constraint to achieve the ideal PSF profile, the scan time can be also reduced by using a longer ETL. Our in vivo experiments demonstrated that the PSF enhanced image acquired with a SNR improved and longer ETL scan is able to approximate similar SNR level and image sharpness compared to a shorter ETL scanned image. The Butterworth lowpass filtering shaped PSF profile was empirically selected during the design of SNR priority VFA pulse train. This can be further optimized in future work. In addition, the noise level was retrospectively reduced by the trained CNN since the input was noise corrupted image patches during CNN training. Furthermore, various noise levels (i.e. standard deviations) between 0 and 10% of maximum intensity was randomly chosen to extend the capability of the trained CNN for spatially varying denoising, so that it can better perform on parallel imaging [39] and compressed sensing [40] accelerated images with non-uniform noise amplification. Compared to traditional linear PSF enhancement approaches, the CNN has demonstrated more powerful sharpness improvement and noise reduction for superior detailed structure delineation. One extension for future investigation is to exploit the impact of g-factor maps as another CNN input channel for improved spatially variant noise suppression [41]. To replace the Rician noise distribution by a more realistic noise pattern in the acquired images might be another direction to further improve the noise suppression performance. Moreover, no additional flow suppression prepulse was exploited in this work to keep the theoretical modeling and numerical simulation simple. Thus, the presented approach can be further combined with DANTE prepulse [42] which might result in better image quality by minimizing the echo time of the TSE scan and improving the blood and CSF suppression for IVWI [43]. The developed methodology can be extended to other TSE image contrast weightings with different k-space acquisition orderings, and large coverage screening applications for joint intra- and extra-cranial [44,45] as well as peripheral [46] vessel wall imaging.
There are some limitations in this study. Firstly, the spatial temporal subspace model is established based on Bloch simulation for static tissues only. Therefore, the simulation generated blurred image may have some inconsistencies with the acquired TSE blurring features, leading to potential PSF correction errors of the learned CNN. Secondly, the CNN must be re-trained for different TSE sequence parameters, particularly when changing ETL, repetition time, and driven equilibrium condition. In this case, the pre-scan calibration has to be performed as well for training database preparation. This might not be a significant concern for clinical routine TSE imaging protocols using fixed parameters. However, a more versatile CNN based PSF enhancement model that can adapt for different TSE scans can be explored in future. Thirdly, the number of recruited subjects in this study is small. Follow-up studies with more subjects and different applications are required to comprehensively evaluate the clinical value of this approach. Lastly, it is difficult to validate the in vivo PSF enhancement results with a single echo spin echo sequence due to intolerably long scan times. The recent shuffling TSE technique [26] may provide a useful alternative for further validation and comparison instead of the short ETL TSE imaging as reference used in this work.
5. Conclusion
We demonstrated a neural network enhanced TSE imaging approach that can further improve scan efficiency and image quality by a retrospective CNN based PSF enhancement and a prospective SNR improved acquisition scheme. This approach has the potential to be applied for all different TSE scans with a sequence adaptive CNN training. With further evaluation on a larger population size and different clinical applications, the TSE scan as a widely used clinical sequence might better serve for improved diagnosis confidence and patient comfort. Its initial application on whole brain isotropic 0.5mm 3D T1w TSE IVWI shows promising results at 3T for intracranial vessel wall and plaque delineation.
Supplementary Material
SNR and sharpness have to be compromised for long echo train length 3D TSE scans
Reduced SNR and sharpness may obscure intracranial vessel wall (IVW) depiction
Network enhanced TSE can improve IVW image quality than conventional TSE
Acknowledgements
This study was supported in part by the National Institutes of Health [grant number R01NS092207]. We would also like to acknowledge the support of Nvidia Corporation for donating the Titan Xp GPU.
Footnotes
Declaration of Competing Interest
Zechen Zhou and Peter Börnert are employees of Philips Research. Other authors have no relevant conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- [2].Lindenholz A, van der Kolk AG, Zwanenburg JJM, Hendrikse J. The Use and Pitfalls of Intracranial Vessel Wall Imaging: How We Do It. Radiology. 2018;286:12–28. doi: 10.1148/radiol.2017162096. [DOI] [PubMed] [Google Scholar]
- [3].Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017;38:218–229. doi: 10.3174/ajnr.A4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging. 2011;34:22–30. doi: 10.1002/jmri.22592. [DOI] [PubMed] [Google Scholar]
- [5].Qiao Y, Steinman DA, Qin Q, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. 2014;271:534–542. doi: 10.1148/radiol.13122812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang H, Zhang X, Qin Q, Liu L, Wasserman BA, Qiao Y. Improved cerebrospinal fluid suppression for intracranial vessel wall MRI. J Magn Reson Imaging. 2016;44:665–672. doi: 10.1002/jmri.25211. [DOI] [PubMed] [Google Scholar]
- [7].Fan Z, Yang Q, Deng Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid attenuated T1-weighted 3D turbo spin echo. Magn Reson Med 2017;77:1142–1150. doi: 10.1002/mrm.26201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang Q, Deng Z, Bi X, et al. Whole-brain vessel wall MRI: A parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo. J Magn Reson Imaging. 2017;46:751–757. doi: 10.1002/jmri.25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li D, Dai W, Cai Y, et al. Atherosclerosis in stroke-related vascular beds and stroke risk: A 3-D MR vessel wall imaging study. Ann Clin Transl Neurol 2018;5:1599–1610. doi: 10.1002/acn3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xie G, Chen H, He X, et al. Black-blood thrombus imaging (BTI): a contrast-free cardiovascular magnetic resonance approach for the diagnosis of non-acute deep vein thrombosis. J Cardiovasc Magn Reson 2017;19:4. doi: 10.1186/s12968-016-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yang Q, Duan J, Fan Z, et al. Early Detection and Quantification of Cerebral Venous Thrombosis by Magnetic Resonance Black-Blood Thrombus Imaging. Stroke. 2016;47:404–409. doi: 10.1161/STROKEAHA.115.011369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Quan K, Song J, Yang Z, et al. Validation of Wall Enhancement as a New Imaging Biomarker of Unruptured Cerebral Aneurysm. Stroke. 2019;50(6):1570–1573. doi: 10.1161/STROKEAHA.118.024195. [DOI] [PubMed] [Google Scholar]
- [13].Hennig J, Weigel M, Scheffler K. Calculation of flip angles for echo trains with predefined amplitudes with the extended phase graph (EPG)-algorithm: principles and applications to hyperecho and TRAPS sequences. Magn Reson Med 2004;51:68–80. doi: 10.1002/mrm.10658. [DOI] [PubMed] [Google Scholar]
- [14].Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med 2006;55:1030–1037. doi: 10.1002/mrm.20863. [DOI] [PubMed] [Google Scholar]
- [15].Mugler JP 3rd. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging. 2014;39:745–767. doi: 10.1002/jmri.24542. [DOI] [PubMed] [Google Scholar]
- [16].Krizhevsky A, Sutskever I, Hinton GE. Imagenet classification with deep convolutional neural networks. In Proceedings of the 25th International Conference on Neural Information Processing Systems (NIPS) 2012, pp. 1097–1105. [Google Scholar]
- [17].Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. In Proceedings of the International Conference on Learning Representations (ICLR) 2015. [Google Scholar]
- [18].Szegedy C, Liu W, Jia Y, et al. Going deeper with convolutions. In Proceedings of the IEEE conference on Computer Vision and Pattern Recognition (CVPR) 2015, pp. 1–9. [Google Scholar]
- [19].He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. IEEE Conference on Computer Vision and Pattern Recognition (CVPR) 2016, pp. 770–778. [Google Scholar]
- [20].Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS. Image reconstruction by domain-transform manifold learning. Nature. 2018;555(7697):487–492. doi: 10.1038/nature25988. [DOI] [PubMed] [Google Scholar]
- [21].Kim J, Lee JK, Lee KM. Accurate image super-resolution using very deep convolutional networks. IEEE Conference on Computer Vision and Pattern Recognition (CVPR) 2016, pp. 1646–1654. [Google Scholar]
- [22].Lim B, Son S, Kim H, Nah S, Lee KM. Enhanced deep residual networks for single image super-resolution. In Proceedings of the IEEE conference on Computer Vision and Pattern Recognition (CVPR) workshops 2017, pp. 1132–1140. [Google Scholar]
- [23].Chaudhari AS, Fang Z, Kogan F, et al. Super-resolution musculoskeletal MRI using deep learning. Magn Reson Med 2018;80:2139–2154. doi: 10.1002/mrm.27178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liang ZP. Spatiotemporal imaging with partially separable functions. In Proceedings of IEEE Int Symp Biomed Imaging. 2007:988–991. doi: 10.1109/NFSI-ICFBI.2007.4387720. [DOI] [Google Scholar]
- [25].Zhao B, Haldar JP, Christodoulou AG, Liang ZP. Image reconstruction from highly undersampled (k, t)-space data with joint partial separability and sparsity constraints. IEEE Trans Med Imaging. 2012;31(9):1809–1820. doi: 10.1109/TMI.2012.2203921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tamir JI, Uecker M, Chen W, et al. T2 shuffling: Sharp, multicontrast, volumetric fast spin-echo imaging. Magn Reson Med 2017;77(1):180–195. doi: 10.1002/mrm.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].He K, Zhang X, Ren S, Sun J. Identity mappings in deep residual networks. European Conference on Computer Vision (ECCV) 2016, pp. 630–645. [Google Scholar]
- [28].Ledig C, Theis L, Huszár F, et al. Photo-realistic single image super-resolution using a generative adversarial network. In Proceedings of the IEEE conference on Computer Vision and Pattern Recognition (CVPR) workshops 2017, pp. 4681–4690. [Google Scholar]
- [29].Goodfellow I, Pouget-Abadie J, Mirza M, et al. Generative adversarial nets. Proceedings of the international conference on Neural Information Processing Systems (NIPS) 2014, pp. 2672–2680. [Google Scholar]
- [30].Lüsebrink F, Sciarra A, Mattern H, Yakupov R, Speck O. T1-weighted in vivo human whole brain MRI dataset with an ultrahigh isotropic resolution of 250 μm. Sci Data. 2017;4:170032. doi: 10.1038/sdata.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Crouse M, Robert DN, Richard GB. Wavelet-based statistical signal processing using hidden Markov models. IEEE Trans Signal Processing. 1998;46(4):886–902. doi: 10.1109/78.668544 [DOI] [Google Scholar]
- [32].Kingma DP, Ba J. Adam: A method for stochastic optimization. In Proceedings of the 3rd International Conference on Learning Representations (ICLR) 2015. [Google Scholar]
- [33].Zhao L, Chang CD, Alsop DC. Controlling T2 blurring in 3D RARE arterial spin labeling acquisition through optimal combination of variable flip angles and k-space filtering. Magn Reson Med 2018;80(4):1391–1401. doi: 10.1002/mrm.27118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Coolen BF, Poot DH, Liem MI, et al. Three-dimensional quantitative T1 and T2 mapping of the carotid artery: Sequence design and in vivo feasibility. Magn Reson Med 2016;75(3):1008–17. doi: 10.1002/mrm.25634. [DOI] [PubMed] [Google Scholar]
- [35].Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen L, Mossa-Basha M, Balu N, et al. Development of a quantitative intracranial vascular features extraction tool on 3D MRA using semiautomated open-curve active contour vessel tracing. Magn Reson Med 2018;79(6):3229–3238. doi: 10.1002/mrm.26961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shi F, Yang Q, Guo X, et al. Intracranial Vessel Wall Segmentation Using Convolutional Neural Networks. IEEE Trans Biomed Eng 2019;66(10):2840–2847. doi: 10.1109/TBME.2019.2896972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhou Z, Chen S, Wu J, Zhao X, Börnert P, Yuan C. Deep convolutional neural network enhanced 3D high resolution turbo spin echo intracranial vessel wall imaging. In Proceedings of the 26th Annual Meeting of ISMRM 2018. p0824. [Google Scholar]
- [39].Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42(5):952–62. doi: . [DOI] [PubMed] [Google Scholar]
- [40].Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182–95. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- [41].Zhang K, Zuo W, Zhang L. FFDNet: Toward a fast and flexible solution for CNN-based image denoising. IEEE Trans on Image Process. 2018;27(9):4608–4622. doi: 10.1109/TIP.2018.2839891. [DOI] [PubMed] [Google Scholar]
- [42].Li L, Miller KL, Jezzard P. DANTE-prepared pulse trains: a novel approach to motionsensitized and motion-suppressed quantitative magnetic resonance imaging. Magn Reson Med 2012;68(5):1423–38. doi: 10.1002/mrm.24142. [DOI] [PubMed] [Google Scholar]
- [43].Wang J, Helle M, Zhou Z, Börnert P, Hatsukami TS, Yuan C. Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI. Magn Reson Med 2016;75(2):831–838. doi: 10.1002/mrm.25667. [DOI] [PubMed] [Google Scholar]
- [44].Zhou Z, Li R, Zhao X, et al. Evaluation of 3D multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2015;17:41. doi: 10.1186/s12968-015-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xie Y, Yang Q, Xie G, Pang J, Fan Z, Li D. Improved black-blood imaging using DANTESPACE for simultaneous carotid and intracranial vessel wall evaluation. Magn Reson Med 2016;75(6):2286–2294. doi: 10.1002/mrm.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mihai G, Chung YC, Merchant A, Simonetti OP, Rajagopalan S. T1-weighted-SPACE dark blood whole body magnetic resonance angiography (DB-WBMRA): initial experience. J Magn Reson Imaging. 2010;31(2):502–9. doi: 10.1002/jmri.22049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.