Summary
Neuronal loss can considerably diminish neural circuit function, impairing normal behavior by disrupting information flow in the circuit. Here, we use genetically engineered electrical synapses to reroute the flow of information in a C. elegans damaged chemosensory circuit, in order to restore organism behavior. We impaired chemotaxis by removing one pair of interneurons from the circuit, then artificially coupled two other adjacent neuron pairs through ectopically expressing in them the gap junction protein connexin. This restored chemotaxis in the animals. We expected to observe linear and direct information flow between the connexin-coupled neurons in the recovered circuit, but also revealed a formation of new potent left-right lateral electrical connections within the connexin-expressing neuron pairs. Our analysis suggests that these additional electrical synapses help restore circuit function by amplifying weakened neuronal signals in the damaged circuit, in addition to emulating the wild-type circuit. A record of this paper’s Transparent Peer Review process is included in the Supplemental Information.
Graphical Abstract

eTOC blurb
Neuronal loss due to injury or disease could lead to considerable impairments. We asked whether such conditions could be alleviated by genetically inserting new synaptic connections into the damaged neural circuit, providing alternative pathways for information flow. We focused on the relatively simple and extensively studied olfactory circuit of the tiny nematode worm, C. elegans. Loss of a single pair of interneurons in this circuit diminished chemosensory performance. We designed a synaptic bypass, implemented by genetically inserting an electrical synapse into the circuit, which restored behavioral performance. We further found that the impact of the synthetic connection was due also to the amplification of weakened sensory signals in the damaged circuit, enabled by the formation of new lateral left-right electrical connections. Our findings demonstrate the power of engineered electrical synapses as a tool for analyzing neural circuit structure-function relations and as a potential strategy for the repair of damaged neural circuits.
Introduction
The pattern of synaptic connectivity between neurons delineates possible paths for information flow in neural circuits, shaping in this way the dynamics and function of the circuit. Loss of neurons due to injury or disease often causes a break in information flow, leading to behavioral deficiencies (Aerts et al., 2016; Caeyenberghs et al., 2017). Remarkably, in some cases, the brain is capable of spontaneous recovery from such damage through mechanisms of synaptic plasticity (Murphy and Corbett, 2009; Nudo, 2013), suggesting that alternative circuit configurations could replace at least in part the original damaged ones, and that information may be rerouted through alternative neural pathways, restoring signal propagation and circuit function. These observations raise questions as to how changes in synaptic connectivity impact information flow and whether such changes are sufficient for functional circuit recovery.
Here we address these questions by focusing on a well described neural circuit for chemosensation in the nematode worm C. elegans (Chalasani et al., 2007; Gray et al., 2005; Tsalik and Hobert, 2003). In this circuit, sensory information is transmitted from sensory neurons to a set of interneurons that modulate locomotion, enabling worms to locate chemical cues in the environment and migrate towards them through a process termed chemotaxis. Removal of neurons from the circuit has an adverse effect on basic chemotaxis abilities(Bargmann et al., 1993) as well as on more complex aspects of this behavior (Chalasani et al., 2010; Shinkai et al., 2011; Tomioka et al., 2006). We propose that a potential remedy for such circuit lesions could be the introduction of new synaptic connections into the circuit to bypass breaks in information flow and restore some of the circuit’s functionality.
To this end we applied an approach based on genetically inserting new electrical synapses between specific C. elegans neurons (Rabinowitch and Schafer, 2015; Rabinowitch et al., 2014. This is done by ectopically expressing the vertebrate gap junction protein, connexin (Beyer et al., 1990), in target neurons using cell-specific promoters. This procedure enables to create artificial electrical coupling between selected neurons. For example, we have previously shown that inserting a synthetic electrical synapse between the C. elegans ASH nociceptive neurons and the AVB forward premotor neurons is sufficient to cause worms to approach a noxious stimulus rather than escape it (Rabinowitch, 2019). Conversely, introducing a new electrical synapse between the AWC olfactory sensory neurons and either the AIY (Rabinowitch et al., 2014) or AIA (Rabinowitch, 2019) interneurons, can switch chemotaxis from attraction to avoidance. Here we examine the possibility of employing such synthetic electrical synapses as synaptic bypasses for neuronal information flow, as a means for circumventing neural circuit damage.
Results
Loss of AIA interneurons impairs chemotaxis and AIB functionality
In the relatively simple and well-characterized C. elegans chemosensory neural circuit (Chalasani et al., 2007; Gray et al., 2005; Ino and Yoshida, 2009; Luo et al., 2014; Pierce-Shimomura et al., 1999; Suzuki et al., 2008; Tsalik and Hobert, 2003), the AWC sensory neuron class sends parallel synaptic outputs to several interneuron classes, including AIA, AIB, and AIY (Fig. 1a). These primary interneurons act to modulate locomotion in an odor-dependent manner (Chalasani et al., 2007; Gray et al., 2005; Tsalik and Hobert, 2003). In coordination with additional interneurons and motor neurons, the circuit enables worms to detect attractive odors and migrate towards them. Loss of AIA, one of the key interneurons in the circuit (Larsch et al., 2015), has been shown to adversely affect several behavioral capacities, such as salt chemotaxis learning (Tomioka et al., 2006) and the integration of conflicting environmental cues (Shinkai et al., 2011). In addition to these, we found, following genetic ablation of AIA, a reduction in chemotaxis to AWC-sensed odors such as isoamyl alcohol (IAA) and benzaldehyde (Fig. 1b,c). According to the C. elegans synaptic wiring diagram (Cook et al., 2019; Varshney et al., 2011; White et al., 1986) a major fraction of AIA chemical synaptic outputs is directed to the AIB neuron class (Fig. 1a, inset). We thus wondered whether loss of AIA affects AIB function, and performed calcium imaging experiments to examine the responses of AWC, AIA, and AIB neurons to odor removal and presentation (Fig. 1d). As previously described (Chalasani et al., 2007), odor removal in wild type worms triggered the activation of AWC, leading to AIA silencing and AIB activation (Fig. 1d, top). Conversely, odor presentation resulted in a drop in AWC activity, which produced a transient rise in AIA activity and a decrease in AIB activity (Fig. 1d, bottom). These response patterns are attributed to inhibitory AWC→AIA and excitatory AWC→AIB synaptic transmission, respectively (Chalasani et al., 2007, 2010) (Fig. 1a). Since AIB receives input from both AWC and AIA, we examined the extent of AIA’s impact on AIB activity. We observed that AIA removal strongly diminishes AIB responses, suggesting that, in addition to direct AWC→AIB transmission, the indirect AWC→AIA→AIB pathway acts as a prominent driver of AIB activity (Fig. 1a). Removal of AIB in worms lacking AIA led to a further decrease in chemotaxis performance compared to AIA removal alone (Fig. 1e), indicating that AIB still maintains a residual functional role even in the absence of AIA, most likely enabled by the direct inputs received from AWC.
Figure 1. AIA plays an important role in chemosensory circuit function.

(a) Simplified chemosensory circuit diagram. Edge thickness is proportional to connection strength (number of electron microscope sections that they occupy). Inset, relative strengths of AIA chemical synaptic outputs. Source: www.wormwiring.org. (b) Impact of AIA removal (strain JN580) on chemotaxis to AWC-sensed odors, isoamyl alcohol (IAA; n=40,40) and benzaldehyde (n=28,28). (c) Similar to JN580, another AIA-ablated strain BJH8585 also shows decreased chemotaxis (n=28,28; see Methods). Inset shows AIA-specific expression produced by the split cGAL system (see Methods) and the intersection (∩) of two promoters (Pttx-3(intron7) and Pgcy-28d). (d) Calcium responses of AWC, AIA and AIB neurons to removal and presentation of IAA. AIB responses (n=59,56 and n=90,87) are shown also for worms lacking the AIA neurons (red). (e) Impact of AIA and AIB removal on chemotaxis (n=65,63,64, 63). Boxes represent mean ± 95% confidence intervals. * p<0.05, ** p<0.01, *** p<0.001.
Ectopic expression of connexin in AWC and AIB enhances chemotaxis in worms lacking AIA
We next asked whether reinforcing direct AWC→AIB transmission (Fig. 2a) could compensate for interrupted indirect AWC→AIA→AIB information flow in worms lacking AIA, thus enabling functional recovery of the circuit. To increase AWC and AIB coupling, we inserted a synthetic electrical synapse between the two neurons. We did this by ectopically expressing the vertebrate gap junction protein, connexin (Beyer et al., 1990), in each neuron (Fig. 2a). Connexin forms gap junctions that allow the passage of electrical signals between adjacent neurons (Fig. 2a, inset). Vertebrate connexin ectopically expressed in C. elegans neurons, is unlikely to interact with the endogenous invertebrate gap junction protein, innexin (Epstein and Gilula, 1977), and is thus expected to give rise to highly specific, new connections. We have previously established and validated this technique in different circuits and scenarios (Rabinowitch et al., 2013, 2014). Upon introducing connexin to AWC and AIB in AIA-ablated worms, we observed a substantive recovery of chemotaxis performance, exceeding even that of wild type worms (Fig. 2b). We also expressed connexin in AWC and AIB separately as controls, and were surprised to find that each of these was sufficient for restoring chemotaxis, equivalent in degree to that of the normal worms (Fig. 2b,c). It should be noted that the Podr-1 promoter that we used for AWC connexin expression, drives expression also in the pharyngeal I1 and chemosensory AWB neurons(Yu et al., 1997). However, unlike Podr-1 connexin expression (in AWC, AWB and I1), connexin expression in AWB alone using the Pstr-1 promoter (Troemel et al., 1997a) did not restore chemotaxis in worms lacking AIA (Fig. 2d), excluding a role for AWB neurons per se in behavioral recovery. In addition, the Pinx-1 promoter that we used for AIB connexin expression has been reported to drive expression in AIY and AIB neurons until the L3 larval stage and then solely in AIB (Altun et al., 2008), and has accordingly been used in several studies for AIB-specific expression (Attreed et al., 2016; Choi et al., 2020; Gordus et al., 2015. We indeed consistently observed specific AIB expression in over 40 worms that we tested (e.g., Fig. 2e).
Figure 2. Ectopic expression of connexin in AWC and AIB.

(a) Circuit diagram showing a desired new link between AWC and AIB formed by an engineered electrical synapse. Inset, a synthetic AWC-AIB electrical synapse, or gap junction, is constructed by expressing connexin in AWC and AIB. (b,c) Chemotaxis following AIA removal (strain JN580 in panel b, and strain BJH858 in panel c; see Methods) and ectopic expression of connexin in AWC alone, AIB alone or both (all n=21). (d) Chemotaxis following connexin expression in AWB compared to AWC in worms lacking AIA (n=15,16,14). (e) Examples of the Pinx-1 promoter, which we used for dirving connexin expression in AIB, exhibiting an AIB-specific expression pattern, as observed in adult worms from strain BJH1033 containing Pinx-1::GcAMP. Boxes represent mean ± 95% confidence intervals. * p<0.05, ** p<0.01, *** p<0.001.
Connexin forms synthetic electrical synapses between left-right neuron pairs within individual neuron classes
How could connexin expression in a single neuron class induce functional recovery in the damaged circuit? To address this question, we considered that many C. elegans neuron classes, including those in the olfactory circuit, are composed of bilateral left-right neuron pairs(Hobert et al., 2002) (Fig. 3a). Connexin expression in these neurons could thus lead to the formation of new electrical synapses between the left and right members of the class (Fig. 3a). An informative test of this possibility is to activate just one of the two individual neurons within a neuron pair and then examine whether connexin expression in both neurons equalizes their responses due to synthetic electrical coupling. To this end, we examined AIB connectivity. Although no synaptic connections exist between AIBL and AIBR (Fig. 3a), their two processes pass in close proximity to each other in their paths across the nerve ring (White et al., 1986). We sought to test whether connexin expression in the AIB pair results in the formation of synthetic electrical coupling between these neurons. Notably, an asymmetry can be ovserved in the connection strength between the salt sensing neuron, ASER, and the two AIB neurons, AIBL and AIBR (Fig. 3a). We asked whether this asymmetry in connectivity could lead to distinct AIBL and AIBR responses to ASER input. To examine this possibility, we activated ASER by exposing worms to a sudden decrease in salt concentration (Suzuki et al., 2008), and recorded AIBL and AIBR responses. Such ASER activation has been shown to elicit a rise in the average AIB calcium response (Kuramochi and Doi, 2019). Notabley, when we analyzed the left and right AIB neuron responses separately, we were able to observe an asymmetrical response to this stimulation: whereas AIBL strongly increased its activity, AIBR exhibited almost no response (Fig. 3b). We took advantage of this lateralization that we observed in the AIB salt response, and tested whether connexin expression in AIB would diminish this asymmetry. Indeed, this led to a much more similar response in AIBL and AIBR to the decrease in salt concentration. This result provides evidence that connexin expression in AIB, creates functional electrical coupling between AIBL and AIBR.
Figure 3. Synthetic electrical coupling between left-right neuron pairs.

(a) Circuit diagram showing individual neurons in the olfactory circuit from each bilateral symmetrical neuron class. In addition, an asymmetrical distribution of synaptic strengths between ASER and AIBR versus AIBL is shown. (b) Activation of ASER by reducing salt concentration, led to strong activation of AIBL and almost no response in AIBR. Ectopic connexin expression in AIB, equalized the response of the two neurons, as expected for an AIBL-AIBR electrical coupling (n=28,25,25,25). Boxes represent mean ± 95% confidence intervals. ** p<0.01.
Lateral electrical coupling can amplify weak signals
How could artificial left-right electrical coupling lead to restored chemotaxis in worms lacking AIA? Previous studies in the olfactory systems of drosophila (Huang et al., 2010; Yaksi and Wilson, 2010) and zebrafish (Zhu et al., 2013) have identified a role for electrical synapses in amplifying weak signals in these circuits through lateral excitation (Szczupak, 2016). We asked whether the synthetic left-right electrical coupling obtained through connexin expression within individual neuron classes could artificially amplify weak signals in the C. elegans olfactory circuit. To test this, we first studied the responses of AWC neurons to weak stimulation using low IAA concentrations. As expected, reduced odor intensity led to a decrease in the magnitude of AWC responses in normal worms (Fig. 4a, compare WT 1:10−5 and 1:10−7). This weakened response was considerably amplified in worms expressing connexin in AWC (Fig. 4a, top). Connexin expression in AWC produced also a corresponding behavioral effect, substantially increasing chemotaxis to dilute IAA, compared to normal worms (Fig. 4a, bottom). These findings suggest that electrical coupling between left and right AWC neurons can amplify weak signals.
Figure 4. Left-right electrical coupling can amplify weak signals.

(a) AWC responses to IAA removal considerably weaken when IAA concentration is decreased. However, ectopic expression of connexin in AWC increases the weakened AWC response (top; n=24,24) and enhanced chemotaxis to dilute IAA (bottom; n=26,23). (b) AIB weakened responses following AIA removal (n=59,56,61) and presentation (n=90,87,95) are partially restored by ectopic expression of connexin in AIB. Boxes represent mean ± 95% confidence intervals. * p<0.05, ** p<0.01, *** p<0.001.
We next considered the impact of synthetic AIBL-AIBR coupling on signal strength. As we have shown above, AIA removal strongly decreases AIB calcium responses (Fig. 4b). We asked whether expressing connexin in AIB mitigated the weakened response, amplifying AIB signaling. We found that AIB responses to both IAA removal and presentation were almost completely restored in worms expressing connexin in their AIB neurons (Fig. 4b), which correlated with the recovery of chemotaxis behavior (Fig. 2b). Taken together, our results suggest that inserting synthetic electrical synapses between left and right neuron class members could amplify weak signals in these neurons and lead to a circuit-level recovery from damage.
Reinforcing the direct AWC→AIB pathway contributes to functional recovery
Beyond the observed restorative effects of inserting synthetic electrical connections between left and right neuron pairs in the olfactory circuit, we sought to evaluate the impact of AWC-AIB electrical coupling on the direct AWC→AIB pathway (Fig. 2a). In particular, we wished to determine whether the high chemotaxis performance observed in worms expressing connexin in both AWC and AIB (Fig. 2b) was due, at least in part, to enhanced AWC-AIB coupling, or was solely the sum of the effects of independent connexin expression in AWC and AIB. To address this question, we engineered a synthetic electrical connection between just one of the two AWC neurons and AIB. We used the Pstr-2 promoter, specific to AWCON (as well as the ASI neurons (Troemel et al., 1999)), to selectively drive connexin expression in AWCON but not AWCOFF, in worms lacking AIA. These worms are not expected to display chemotaxis recovery because they do not form AWCL-AWCR connections . Indeed, no such recovery was observed (Fig. 5a). To the contrary, the defects in chemotaxis were even further exacerbated (Fig. 5a). This could be due to the formation of additional new electrical synapses with or within another neuron class. We thus proceeded to examine the effects of combined expression of connexin in AWCON and AIB, and found a significant increase in chemotaxis performance beyond the levels obtained by connexin expression in AIB alone (Fig. 5b). These results are consistent with AWCON-AIB synthetic coupling having a distinct role in functional recovery, and together with Fig. 2b,c provide further evidence for the role of AWC-specific connexin expression in behavioral recovery. It is worth noting that the observed effect may underestimate the full capacity of AWC-AIB synthetic coupling to bypass the break in information flow caused by the loss of AIA. First, because the AWCON-AIB connection includes only one of the two AWC neurons (AWCON). Thus, it is possible that linking both AWC neurons to AIB delivers enhanced stimulation to AIB with even greater impact. Second, as described (Fig. 5a), the AWCON connexin worms show diminished chemotaxis, which could undermine a larger positive contribution of AWCON-AIB.
Figure 5. Direct synthetic coupling of AWC and AIB contributes to functional recovery in worms lacking AIA.

(a) Ectopic connexin expression using an AWCOFF promoter does not restore chemotaxis behavior (all n=18). (b) Ectopic expression of connexin in AWCON and AIB results in an increased recovery compared to connexin expression in AIB only (all n=64). Boxes represent mean ± 95% confidence intervals. * p<0.05, ** p<0.01, *** p<0.001.
Discussion
It is convenient to describe information flow in the nervous system as originating from the sensory neurons, passing through interneurons, and terminating in motor neurons. The C. elegans olfactory circuit is frequently depicted in this manner (Fig. 6, gray): chemosensory information is transmitted from the AWC sensory neurons to several interneurons, including AIA and AIB, and then from there downstream to premotor and motor neurons. However, our findings support a more distributed view of information flow in neural circuits, whereby an indirect pathway, such as AWC→AIA→AIB, appears to be more prominent than the direct AWC→AIB stream, and lateral electrical coupling within neuron classes such as AIBL-AIBR can regulate signal strength in the circuit (Fig. 6, black). Indeed, it is tempting to speculate that one of the reasons why AIA plays a powerful role in the olfactory circuit is because of the electrical synapses that naturally couple AIAL and AIAR (Fig. 3a), and perhaps provide this neuron class with a level of gain control. Interestingly, AIB activation has been shown to be probabilistic due to stochastic feedback received from the RIM neurons, accounting for substantial variability in worm chemotaxis (Gordus et al., 2015). It is possible that synthetic AIBL-AIBR electrical coupling may increase AIB sensitivity by reducing this inherent source of noise, and thus counteracting the decrease in chemotaxis performance in worms lacking AIA. In addition, the two AWC neurons exhibit functional asymmetry, including differential expression of olfactory receptors (Hsieh et al., 2014). Synthetically coupling the AWCL and AWCR should disrupt this asymmetry, possible affecting the responses to odors normally sensed by just one of the two neurons. It will be revealing to examine these effects in comparison, for example, to mutant worms, in which both AWC neurons assume the same functional role (Wes and Bargmann, 2001). Unraveling such circuit features should play an important part in the design of synthetic synapses.
Figure 6. Distributed information flow in the C. elegans olfactory circuit.
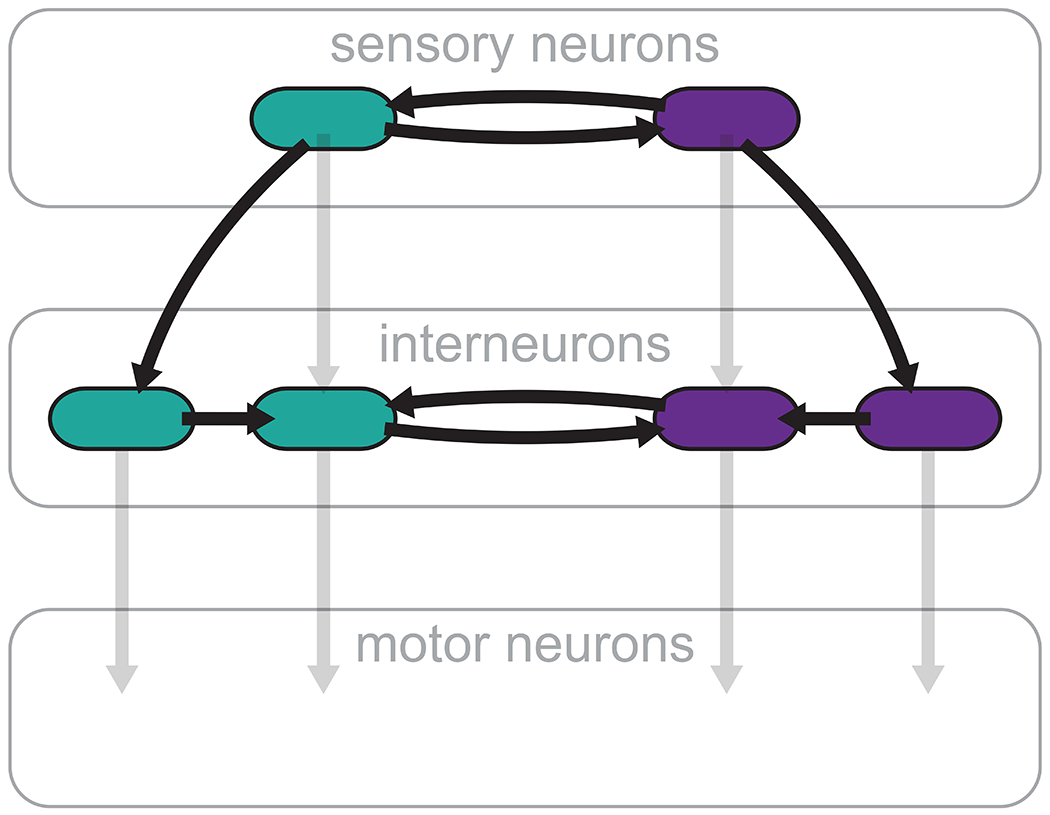
Neural information flow is often described as progressing from sensory neurons through interneurons to motor neurons (light gray). Our study highlights the importance of a more distributed flow of information between interneurons and within neuron pairs (dark arrows).
The general architecture of the C. elegans olfactory circuit has previously been compared to that of retinal circuits of photoreceptor cells and their postsynaptic partners, the ON and OFF bipolar cells (Chalasani et al., 2007). Our analysis reveals additional analogies with the retinal circuit. Similarly to the indirect links between AWC and AIB in the C. elegans olfactory circuit, photoreceptor cells form indirect links with ON and OFF bipolar cells (Popova, 2015). Furthermore, many neuron classes in the retina are interconnected by electrical synapses (Bloomfield and Völgyi, 2009). Such electrical coupling could play a role in signal amplification as in the olfactory circuit. This conserved feature of electrical synapses, shared with other organisms (Huang et al., 2010; Yaksi and Wilson, 2010; Zhu et al., 2013), may offer a potential strategy for using synthetic electrical coupling as a means for signal amplification in damaged circuits.
The notion of creating artificial links to overcome neural circuit damage is proving to be especially effective in brain-machine-brain interfaces (BMBIs) (Lebedev and Nicolelis, 2017). These electronic devices composed of electrodes and computers, monitor and decode neural signals from one brain region, and then encode and deliver corresponding stimulation to a second region (Widge and Moritz, 2014). Such systems appear to be capable of bridging disconnected neural pathways, and thus restoring function after neural damage (Fetz, 2015; Guggenmos et al., 2013). Our current work follows a similar logic, but offers a different, complementary implementation of this basic approach. It focuses on synthetic neuronal coupling at the microscopic level of small local circuits rather than general macroscopic brain regions linked by BMBIs. Thus, the design and implementation of genetically encoded synapses, could complement BMBIs in promoting novel strategies for coping with neuronal damage and for uncovering circuit principles. It is important to note, however, that the behavioral recovery that we have demonstrated following synthetic electrical coupling applies to basic chemotaxis behavior. In effect, the olfactory circuit, and in particular AIA, underlie also substantially more elaborate functionality, such as olfactory adaptation (Chalasani et al., 2010), associative learning (Tomioka et al., 2006) and sensory integration (Shinkai et al., 2011). Further analysis will be necessary to determine the extent to which deficiencies in these behaviors due to AIA removal may be circumvented by synthetic electrical synapses, or whether additional synthetic biological strategies may have to be applied to more comprehensivley restore circuit function.
STAR★Methods
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ithai Rabinowitch (ithai.rabinowitch@mail.huji.ac.il).
Materials Availability
To request reagents (plasmids and C. elegans strains generated in this study) please contact the Lead Contact.
Data and Code availability
Behavioral and calcium imaging source data have been deposited at Mendeley Data and are publicly available at http://dx.doi.org/10.17632/6mgwk57bbt.1.
All code used to track the imaged neurons and then analyze the resulting traces is publicly available at Github via https://github.com/ithairab/NeuronTracker and https://github.com/ithairab/SpikeFinder, respectively.
Scripts were not used to generate the figures reported in this paper.
Any additional information required to reproduce this work is available from the Lead Contact.
Experimental Model and Subject Details
C. elegans Growth and Maintenance
C. elegans strains were maintained under standard conditions at room temperature (20-21°C) on nematode growth medium (NGM) 2% agar plates seeded with E. coli strain, OP50. The N2 strain (Bristol, England) was used as the wild type reference strain. All other strains are listed in Table 1.
Table 1.
C. elegans strains appearing in this study and the experiments in which they were used.
| Strain | Purpose | Figure |
|---|---|---|
| JN580★ peIs580[Pins-1(short)::casp1, Pins-1(short)::venus, Punc-122::gfp] | AIA genetic ablation | 1b,e; 2b,d; 5a |
| BJH1045 pekEx186[Pttx-3(intron7)::NLS::GAL-4_DBD::gp41-1-N-intein::tbb-2utr, Pgcy-28d::NLS::gp41-1-C-intein::cGAL(AD)::tbb-2utr, 15xUAS-CX36::mScarlet-let-858 3′UTR, Prpl-28S::neoR::rpl-28UTR, Pttx-3(intron7)::GFP, Punc-122::gfp] | AIA specific expression through promoter intersection | 1c |
| BJH858 pekls103[Pttx-3(intron7)::NLS::GAL-4_DBD::gp41-1-N-intein::tbb-2utr, Pgcy-28d::NLS::gp41-1-C-intein::cGAL(AD)::tbb-2utr, 15xUAS::Δpes-10::ICE::tbb-2utr, Prpl-28S::neoR::rpl-28UTR, Punc-122::gfp, Pttx-3 (intron7)::wmCherry::tbb-2utr] I (4X outcross) | AIA genetic ablation through promoter intersection | 1c; 2c |
| AQ2632 ljEx351[Podr-1::YC3.60*, Pelt-2::mCherry] | AWC calcium imaging | 1d; 4a |
| CX11073★ kyEx2916[Pgcy-28.d::GCamp2.2b, Punc-122::gfp] | AIA calcium imaging | 1d |
| BJH1033 pekEx176[Pinx-1::GCaMP6s, Punc122::mCherry, Pgcy-5::ChrimsonCel::SL2::mCherry] | AIB calcium imaging and visualization | 1d; 2e; 3b; 4b |
| BJH2190 peIs580[Pins-1(short)::casp1, Pins-1(short)::venus, Punc-122::gfp]; pekEx176[Pinx-1::GCaMP6s, Punc122::mCherry, Pgcy-5::ChrimsonCel::SL2::mCherry] | AIB calcium imaging, AIA ablated | 1d; 4b |
| JN578 peIs578[Pnpr-9::casp1, Pnpr-9::venus, Punc-122::mCherry] | AIB genetic ablation | 1e |
| BJH2161 peIs580[Pins-1(short)::casp1, Pins-1(short)::venus, Punc-122::gfp]; peIs578[Pnpr-9::casp1, Pnpr-9::venus, Punc-122::mCherry] | AIA and AIB genetic ablation | 1e |
| BJH2176 peIs580[Pins-1(short)::casp1, Pins-1(short)::venus, Punc-122::gfp]; pekSi46[cb-unc-119(+), Podr-1::Cx36::mCherry, Pvha-6::mRuby] ttTi5605 II; pekSi54[cb-unc-119(+), Pinx-1::Cx36::mCherry, Pvha-6::GFP] cxTi10816 IV | AIA ablated and connexin in AWC and AIB | 2b |
| BJH2173 peIs580[Pins-1(short)::casp1, Pins-1(short)::venus, Punc-122::gfp]; pekSi46[cb-unc-119(+), Podr-1::Cx36::mCherry, Pvha-6::mRuby] ttTi5605 II | AIA ablated and connexin in AWC | 2b,d; 5a |
| BJH2168 peIs580[Pins-1(short)::casp1, Pins-1(short)::venus, Punc-122::gfp]; pekSi54[cb-unc-119(+), Pinx-1::Cx36::mCherry, Pvha-6::GFP] cxTi10816 IV | AIA ablated and connexin in AIB | 2b; 5b |
| BJH2555 pekls103[Pttx-3(intron7)::NLS::GAL-4_DBD::gp41-1-N-intein::tbb-2utr, Pgcy-28d::NLS::gp41-1-C-intein::cGAL(AD)::tbb-2utr, 15xUAS::Δpes-10::ICE::tbb-2utr, Prpl-28S::neoR::rpl-28UTR, Punc-122::gfp, Pttx-3 (intron7)::wmCherry::tbb-2utr] I (4X outcross); pekSi46[cb-unc-119(+), Podr-1::Cx36::mCherry, Pvha-6::mRuby] ttTi5605 II | AIA genetic ablation through promoter intersection and AWC connexin | 2c |
| BJH2556 pekls103[Pttx-3(intron7)::NLS::GAL-4_DBD::gp41-1-N-intein::tbb-2utr, Pgcy-28d::NLS::gp41-1-C-intein::cGAL(AD)::tbb-2utr, 15xUAS::Δpes-10::ICE::tbb-2utr, Prpl-28S::neoR::rpl-28UTR, Punc-122::gfp, Pttx-3 (intron7)::wmCherry::tbb-2utr] I (4X outcross); pekSi54[cb-unc-119(+), Pinx-1::Cx36::mCherry, Pvha-6::GFP] cxTi10816 IV | AIA genetic ablation through promoter intersection and AIB connexin | 2c |
| BJH2506 peIs580[Pins-1(short)::casp1, Pins-1(short)::venus, Punc-122::gfp]; pekSi50[cb-unc-119(+), Pstr-1::Cx36::mCherry, Punc-54::mCherry] ttTi4348 I | AIA ablated and connexin in AWB | 2d |
| BJH2192 pekSi54[cb-unc-119(+), Pinx-1::Cx36::mCherry, Pvha-6::GFP] cxTi10816 IV; pekEx176[Pinx-1::GCaMP6s, Punc122::mCherry, Pgcy-5::ChrimsonCel::SL2::mCherry] | AIB calcium imaging and connexin in AIB | 3b |
| BJH1055 pekEx196[Podr-1::GCaMP6s-SL2-mCherry + Punc122::mCherry] | AWC calcium imaging | 4a |
| BJH2463 pekEx196[Podr-1::GCaMP6s-SL2-mCherry + Punc122::mCherry]; pekSi46[cb-unc-119(+), Podr-1::Cx36::mCherry, Pvha-6::mRuby] ttTi5605 II | AWC calcium imaging and connexin in AWC | 4a |
| BJH918 pekSi46[cb-unc-119(+), Podr-1::Cx36::mCherry, Pvha-6::mRuby] ttTi5605 II | Connexin in AWC | 4a |
| BJH2204 peIs580[Pins-1(short)::casp1, Pins-1(short)::venus, Punc-122::gfp]; pekEx176[Pinx-1::GCaMP6s, Punc122::mCherry, Pgcy-5::ChrimsonCel::SL2::mCherry]; pekSi54[cb-unc-119(+), Pinx-1::Cx36::mCherry, Pvha-6::GFP] cxTi10816 IV | AIB calcium imaging, AIA ablated and connexin in AIB | 4b |
| BJH2170★★ peIs580[Pins-1(short)::casp1, Pins-1(short)::venus, Punc-122::gfp]; pekSi47[cb-unc-119(+), Pstr-2::Cx36::mCherry, Pvha-6::mCherry] cxTi10816 IV | AIA ablated and connexin in AWCON | 5a,b |
| BJH2554★★★ peIs580[Pins-1(short)::casp1, Pins-1(short)::venus, Punc-122::gfp]; pekSi47[cb-unc-119(+), Pstr-2::Cx36::mCherry, Pvha-6::mCherry] cxTi10816 IV / -; pekSi54[cb-unc-119(+), Pinx-1::Cx36::mCherry, Pvha-6::GFP] cxTi10816 IV / - | AIA ablated and connexin in AIB and AWCON | 5b |
Strain JN580 kind gift of Yuichi lino. Strain CX11073 kind gift of Cori Bargmann.
Strain BJH2170 is heterozygotic for pekSi47.
Strain BJH2554 is heterozygotic for pekSi47 and pekSi54.
Method Details
Genetic Engineering of Electrical Synapse
New electrical synaptic connections were inserted into the chemotaxis circuit as previously described (Rabinowitch et al., 2014) by ectopically expressing the gene for connexin 36 (Cx36) in specific neurons. However, rather than generating multi-copy extrachromosomal transgenes, we used the MosSCI system for single copy targeted chromosomal integration (Frøkjær-Jensen et al., 2008), obtaining in this way more uniform expression and avoiding over-expression. A codon-optimized synthesized cDNA sequence of Mus musculus gap junction protein, delta 2 (Gjd2), known also as connexin 36 (Cx36), was fused to relevant upstream promoters (Podr-1 for AWC (Yu et al., 1997), Pinx-1 for AIB (Altun et al., 2008), Pstr-2 for AWCON (Troemel et al., 1999), Pstr-1 for AWB (Troemel et al., 1997b)) and a downstream gene encoding mCherry, and inserted into a MosSCI vector using Gateway (Thermo Fisher Scientific). The injection mix included pCFJ601 Peft-3::mos1 transposase::tbb-2utr at 50 ng/μl for extracting the Mos1 transposon, a DNA repair template including the connexin construct at 30 ng/μl, and pMA122 Phsp-16.41::peel-1::tbb-2 UTR at 10 ng/μl for eliminating extrachromosomal transformants. To combine different transgenic strains we performed standard crossings. For unknown reasons, the cross between peIs580 (genetically ablated AIA) and pekSi47 (connexin expressed in AWCON) yielded homozygotes only for peIs580, and so the resultant crossed strain (BJH2170) that we used was heterozygotic for pekSi47, and strain BJH2554 containing peIs580, pekSi47 and pekSi54 (connexin expressin AIB) was heterozygotic for both pekSi47 and pekSi54, since these two transgenes occupy the same genetic locus (cxTi10816 IV).
Calcium Imaging Strain Generation
Strains expressing genetically encoded calcium indicators were generated by standard microinjection of DNA constructs at 50-70 ng/μl together with specific co-injection markers at 10-30 ng/μl.
Genetic Ablation of AIA
For most experiments we used the strain JN580, kindly provided to us by Dr. Yuichi lino. As previously reported (Satoh et al., 2014), this strain carries an integrated cell death caspase gene driven by an AlA-specific promotor, Pins-1(short). In addition, we also generated AIA-specific strains BJH1045 and BJH858 using the Split cGAL method (Wang et al., 2018). To this end, we combined the the 7th intron of the ttx-3 gene, which drives expression in AIA and NSM (Zhang et al., 2014) with the Pgcy-28d promoter, which drives expression in AIA but also to a variable degree in AVF, ASI, and IL2, I1, or M3 pharyngeal neurons (Cho et al., 2016). The transgenes were first transformed extrachromosomally and then integrated into the genome using UV irradiation. Strain BJH1045 was used to visualize AIA (Fig. 1c), and strain BJH858 as an additional AIA-ablated strain (Fig. 1c, Fig. 2c).
Chemotaxis Assay
To evaluate chemotaxis, we performed standard behavioral assays (Bargmann et al., 1993). Approximately 200 adult worms were washed with M9 buffer onto 9 cm NGM plates. A 1 μl drop of 1M sodium azide was placed at two spots on either side of the plate. A 10 μl drop of Isoamyl alcohol (IAA) or benzaldehyde (Bz) diluted in ethanol to a concentration of 1:1,000 was applied at one spot and a 10 μl drop of ethanol was placed at the other spot as a control. We found that this relatively large volume of drops helped reduce variance. The plate was divided into three regions. The number of worms immobilized within the two regions containing the spots was counted approximately 24 hr after the beginning of the assay (it took the AIA- worms a prolonged period of time to uncluster and disperse from the center of the plate) and used to calculate the chemotaxis index (the difference in worm number between stimulus region and control region, divided by the sum of worms found in these two regions). Each experiment was repeated in at least 3 different days. The total number of repetitions for each condition is indicated in the figure legends.
Calcium Imaging
Calcium imaging was performed using a microfluidic PDMS chip designed to deliver chemical stimuli under a fluorescent microscope (Chronis et al., 2007). We placed the chip on top of a 63x oil objective of a Leica DMI3000B inverted microscope and a QImaging OptiMOS camera. 10 sec after the beginning of each recording, we switched on the stimulus channel presenting the worm with either odor (isoamyl alcohol, AIA) or salt, diluted in S-basal medium lacking cholesterol. Imaging continued for an additional 30 sec. We then kept the stimulus channel open for 5 min and resumed imaging. After an additional 10 sec, we switched the stimulus off and continued the recording for 30 sec. Images were captured at a rate of 5 frames per second with a 100ms exposure time. For each recording, a region of interest was defined as a square-shaped area surrounding the desired cell body. Background-subtracted fluorescence intensity values were collected from every sample’s ROI and stored into MATLAB formatted files. Changes in fluorescence intensity (ΔF/F%) were calculated by dividing each value by the average intensity of the first 3 seconds of imaging. For statistical analysis we calculated the ratio of the average fluorescence intensity over the ten seconds after and before stimulus presentation/removal.
Neuronal Visualization
To observe the expression pattern of Pinx-1::GCaMP6 (Fig. 2e) young adult worms from strain BJH1033 were mounted on 2% agar pads supplemented with 40 mM sodium for immobilization. Worms were observed under an inverted Zeiss Axio Observer fluorescent microscope using a 40X air objective.
Quantification and Statistical Analysis
Statistical analysis was performed using Microsoft Excel. Statistical details of the results can be found in the figure legends. In general, one-way ANOVA was performed to compare between multiple group means. T-tests were carried out for comparison between two specific groups. Bonferroni corrections were applied for multiple comparisons. Each experiment was repeated in at least 3 different days. The total number of repetitions, n, for each condition is indicated in the figure legends. All data figures show individual data points (repetitions), as well as the mean and ±95% confidence intervals.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical Commercial Assays | ||
| Phusion polymerase | NEB | Cat#M0530S |
| Miniprep DNA extractiton kit | Quiagen | Cat#27104 |
| Deposited Data | ||
| Behavioral and imaging raw data | Ithai Rabinowitch | http://dx.doi.org/10.17632/6mgwk57bbt.1 |
| Experimental Models: Organisms/Strains | ||
| C. elegans strain JN580 peIs580 | Yuichi Iino | N/A |
| C. elegans strain CX11073 kyEx2916 | Cori Bargmann | N/A |
| C. elegans strain BJH1033 pekEx176 | Jihong Bai | This study |
| C. elegans strain BJH2190 peIs580; pekEx176 | Jihong Bai | This study |
| C. elegans strain JN578 peIs578 | Yuichi Iino | N/A |
| C. elegans strain BJH2161 peIs580; peIs578 | Jihong Bai | This study |
| C. elegans strain BJH2176 peIs580; pekSi46; pekSi54 | Jihong Bai | This study |
| C. elegans strain BJH2173 peIs580; pekSi46 | Jihong Bai | This study |
| C. elegans strain BJH2168 peIs580; pekSi54 | Jihong Bai | This study |
| C. elegans strain BJH2555 pekIs103; pekSi46 | Jihong Bai | This study |
| C. elegans strain BJH2556 pekIs103; pekSi54 | Jihong Bai | This study |
| C. elegans strain BJH2506 peIs580; pekSi50 | Jihong Bai | This study |
| C. elegans strain BJH2192 pekSi54; pekEx176 | Jihong Bai | This study |
| C. elegans strain BJH1055 pekEx196 | Jihong Bai | This study |
| C. elegans strain BJH2463 pekEx196; pekSi46 | Jihong Bai | This study |
| C. elegans strain BJH918 pekSi46 | Jihong Bai | This study |
| C. elegans strain BJH2204 peIs580; pekEx176; pekSi54 | Jihong Bai | This study |
| C. elegans strain BJH2170 peIs580; pekSi47/- | Jihong Bai | This study |
| C. elegans strain BJH2554 peIs580; pekSi47 / -; pekSi54 / - | Jihong Bai | This study |
| C. elegans strain BJH1045 pekEx186 | Jihong Bai | This study |
| C. elegans strain BJH858 pekIs103 | Jihong Bai | This study |
| C. elegans strain AQ2632 ljEx351 | William Schafer | N/A |
| Recombinant DNA | ||
| BJP-I185 ttTi5605 II Podr-1::Cx36::mCherry, Pvha-6::mRuby | Jihong Bai | This study |
| BJP-I103 Phsp-16.41::peel-1::tbb-2 UTR | Addgene | pMA122 |
| BJP-I230 ttTi10816 IV Pinx-1::CX36::mCherry, Pvha-6::GFP | Jihong Bai | This study |
| BJP-I197 cxTi10816 IV Pstr-1::Cx36::mCherry, Pvha-6::mRuby | Jihong Bai | This study |
| BJP-I122 Peft-3::mos1 transposase | Addgene | pCFJ601 |
| BJP-C213 Pttx-3 (intron7)::NLS::GAL-4_DBD::gp41-1-N-intein::tbb-2utr | Jihong Bai | This study |
| BJP-C231 Pgcy-28(d)::NLS::gp41-1-C-intein::cGAL(AD)::tbb-2utr | Jihong Bai | This study |
| BJP-C295 15xUAS-CX36::mScarlet-let-858 3’UTR | Jihong Bai | This study |
| BJP-C252 15xUAS::Δpes-10::ICE::tbb-2utr | Han Wang | pHW499 |
| BJP-BU6 Pinx-1::GCaMP6 | Jihong Bai | This study |
| BJP-BU3 Podr-1::GCaMP6s-SL2-mCherry | Jihong Bai | This study |
| Software and Algorithms | ||
| Microsoft Excel | Microsoft | N/A |
| Adobe Illustrator | Adobe | N/A |
| NeuronTracker (Matlab code) | Rabinowitch et al., Current Biology (2013) | N/A |
| SpikeFinder (Matlab code) | Rabinowitch et al., Current Biology (2013) | N/A |
Highlights.
Neuron loss in a C. elegans chemosensory circuit disrupts chemosensation
A genetically inserted electrical synapse circumvents the damage
Alternative pathways for information flow are established
Weakened signaling is enhanced due to new lateral left-right electrical coupling
Acknowledgments
This research was supported by Hartwell Innovation Fund, R21DC016158, and R01GM127857 to JB and Israel Science Foundation grant No. 1465/20 to IR. The authors thank the Caenorhabditis elegans Genetic Consortium (funded by NIH Office of Research Infrastructure Programs P40 OD010440) and Dr. Shohei Mitani, Dr. Yuichi Iino and Dr. Cornelia Bargmann for worm strains and Dr. Han Wang for plasmid DNA. We thank the following people for technical assistance: Lin Zhang, Norman Nguyen, Carli Wightman and Abby Tan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Aerts H, Fias W, Caeyenberghs K, and Marinazzo D (2016). Brain networks under attack: Robustness properties and the impact of lesions. Brain 3063–3083. [DOI] [PubMed] [Google Scholar]
- Altun ZF, Chen B, Wang ZW, and Hall DH (2008). High resolution map of Caenorhabditis elegans gap junction proteins. Dev. Dyn 238, 1936–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attreed M, Saied-Santiago K, and Bülow HE (2016). Conservation of anatomically restricted glycosaminoglycan structures in divergent nematode species. Glycobiology 26, 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, and Horvitz HR (1993). Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515–527. [DOI] [PubMed] [Google Scholar]
- Beyer EC, Paul DL, and Goodenough DA (1990). Connexin family of gap junction proteins. J. Membr. Biol 116, 187–194. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, and Völgyi B (2009). The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat. Rev. Neurosci 10, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K, Verhelst H, Clemente A, and Wilson PH (2017). Mapping the functional connectome in traumatic brain injury: What can graph metrics tell us? Neuroimage 160, 113–123. [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, and Bargmann CI (2007). Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70. [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, and Bargmann CI (2010). Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat. Neurosci 13, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CE, Brueggemann C, L’Etoile ND, and Bargmann CI (2016). Parallel encoding of sensory history and behavioral preference during Caenorhabditis elegans olfactory learning. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MK, Liu H, Wu T, Yang W, and Zhang Y (2020). NMDAR-mediated modulation of gap junction circuit regulates olfactory learning in C. elegans. Nat. Commun 11, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis N, Zimmer M, and Bargmann CI (2007). Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat. Methods 4, 727–731. [DOI] [PubMed] [Google Scholar]
- Cook SJ, Jarrell TA, Brittin CA, Wang Y, Bloniarz AE, Yakovlev MA, Nguyen KCQ, Tang LT-H, Bayer EA, Duerr JS, et al. (2019). Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 571, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein ML, and Gilula NB (1977). A study of communication specificity between cells in culture. J. Cell Biol 75, 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE (2015). Restoring motor function with bidirectional neural interfaces. In Progress in Brain Research, (Elsevier; ), pp. 241–252. [DOI] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Wayne Davis M, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, and Jorgensen EM (2008). Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet 40, 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordus A, Pokala N, Levy S, Flavell SW, and Bargmann CI (2015). Feedback from network states generates variability in a probabilistic olfactory circuit. Cell 161, 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, and Bargmann CI (2005). A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci 102, 3184–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenmos DJ, Azin M, Barbay S, Mahnken JD, Dunham C, Mohseni P, and Nudo RJ (2013). Restoration of function after brain damage using a neural prosthesis. Proc. Natl. Acad. Sci 110, 21177–21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O, Johnston RJ, and Chang S (2002). Left-right asymmetry in the nervous system: The Caenorhabditis elegans model. Nat. Rev. Neurosci 3, 629–640. [DOI] [PubMed] [Google Scholar]
- Hsieh YW, Alqadah A, and Chuang CF (2014). Asymmetric neural development in the Caenorhabditis elegans olfactory system. Genesis 52, 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang W, Qiao W, Hu A, and Wang Z (2010). Functional connectivity and selective odor responses of excitatory local interneurons in drosophila antennal lobe. Neuron 67, 1021–1033. [DOI] [PubMed] [Google Scholar]
- Ino Y, and Yoshida K (2009). Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J. Neurosci 29, 5370–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi M, and Doi M (2019). An excitatory/inhibitory switch from asymmetric sensory neurons defines postsynaptic tuning for a rapid response to NaCl in Caenorhabditis elegans. Front. Mol. Neurosci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsch J, Flavell SW, Liu Q, Gordus A, Albrecht DR, and Bargmann CI (2015). A Circuit for Gradient Climbing in C. elegans Chemotaxis. Cell Rep. 12, 1748–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev MA, and Nicolelis MAL (2017). Brain-Machine Interfaces: From Basic Science to Neuroprostheses and Neurorehabilitation. Physiol. Rev 97, 767–837. [DOI] [PubMed] [Google Scholar]
- Luo L, Wen Q, Ren J, Hendricks M, Gershow M, Qin Y, Greenwood J, Soucy ER, Klein M, Smith-Parker HK, et al. (2014). Dynamic encoding of perception, memory, and movement in a C. elegans chemotaxis circuit. Neuron 82, 1115–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, and Corbett D (2009). Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci 10, 861–872. [DOI] [PubMed] [Google Scholar]
- Nudo RJ (2013). Recovery after brain injury: Mechanisms and principles. Front. Hum. Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Morse TM, and Lockery SR (1999). The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J. Neurosci 19, 9557–9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova E (2015). ON-OFF Interactions in the Retina: Role of Glycine and GABA. Curr. Neuropharmacol 12, 509–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitch I (2019). Synthetic biology in the brain: A vision of organic robots. In Proceedings of the 2019 Conference on Artificial Life: How Can Artificial Life Help Solve Societal Challenges, ALIFE 2019, (Cambridge, MA: MIT Press; ), pp. 654–655. [Google Scholar]
- Rabinowitch I, and Schafer WR (2015). Engineering new synaptic connections in the C. elegans connectome. Worm 4, e992668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitch I, Chatzigeorgiou M, and Schafer WR (2013). A gap junction circuit enhances processing of coincident mechanosensory inputs. Curr. Biol 23, 963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitch I, Chatzigeorgiou M, Zhao B, Treinin M, and Schafer WR (2014). Rewiring neural circuits by the insertion of ectopic electrical synapses in transgenic C. elegans. Nat. Commun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh Y, Sato H, Kunitomo H, Fei X, Hashimoto K, and Iino Y (2014). Regulation of experience-dependent bidirectional chemotaxis by a neural circuit switch in Caenorhabditis elegans. J. Neurosci 34, 15631–15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Yamamoto Y, Fujiwara M, Tabata T, Murayama T, Hirotsu T, Ikeda DD, Tsunozaki M, Iino Y, Bargmann CI, et al. (2011). Behavioral choice between conflicting alternatives is regulated by a receptor guanylyl cyclase, GCY-28, and a receptor tyrosine kinase, SCD-2, in AIA interneurons of Caenorhabditis elegans. J. Neurosci 31, 3007–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Thiele TR, Faumont S, Ezcurra M, Lockery SR, and Schafer WR (2008). Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature 454, 114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczupak L (2016). Functional contributions of electrical synapses in sensory and motor networks. Curr. Opin. Neurobiol 41, 99–105. [DOI] [PubMed] [Google Scholar]
- Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, and Iino Y (2006). The Insulin/PI 3-Kinase Pathway Regulates Salt Chemotaxis Learning in Caenorhabditis elegans. Neuron 51, 613–625. [DOI] [PubMed] [Google Scholar]
- Troemel ER, Kimmel BE, and Bargmann CI (1997a). Reprogramming chemotaxis responses: Sensory neurons define olfactory preferences in C. elegans. Cell 91, 161–169. [DOI] [PubMed] [Google Scholar]
- Troemel ER, Kimmel BE, and Bargmann CI (1997b). Reprogramming chemotaxis responses: Sensory neurons define olfactory preferences in C. elegans. Cell 91, 161–169. [DOI] [PubMed] [Google Scholar]
- Troemel ER, Sagasti A, and Bargmann CI (1999). Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell 99, 387–398. [DOI] [PubMed] [Google Scholar]
- Tsalik EL, and Hobert O (2003). Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J. Neurobiol 56, 178–197. [DOI] [PubMed] [Google Scholar]
- Varshney LR, Chen BL, Paniagua E, Hall DH, and Chklovskii DB (2011). Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput. Biol 7, e1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu J, Yuet KP, Hill AJ, and Sternberg PW (2018). Split cGAL, an intersectional strategy using a split intein for refined spatiotemporal transgene control in Caenorhabditis elegans. Proc. Natl. Acad. Sci 115, 201720063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, and Bargmann CI (2001). C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature 410, 698–701. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, and Brenner S (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B. Biol. Sci 314, 1–340. [DOI] [PubMed] [Google Scholar]
- Widge AS, and Moritz CT (2014). Pre-frontal control of closed-loop limbic neurostimulation by rodents using a brain-computer interface. J. Neural Eng 11, 024001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksi E, and Wilson RI (2010). Electrical Coupling between Olfactory Glomeruli. Neuron 67, 1034–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Avery L, Baude E, and Garbers DL (1997). Guanylyl cyclase expression in specific sensory neurons: A new family of chemosensory receptors. Proc. Natl. Acad. Sci 94, 3384–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Bhattacharya A, Nelson JC, Abe N, Gordon P, Lloret-Fernandez C, Maicas M, Flames N, Mann RS, Colón-Ramos DA, et al. (2014). The LIM and POU homeobox genes ttx-3 and unc-86 act as terminal selectors in distinct cholinergic and serotonergic neuron types. Dev. 141, 422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Frank T, and Friedrich RW (2013). Equalization of odor representations by a network of electrically coupled inhibitory interneurons. Nat. Neurosci 16, 1678–1686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Behavioral and calcium imaging source data have been deposited at Mendeley Data and are publicly available at http://dx.doi.org/10.17632/6mgwk57bbt.1.
All code used to track the imaged neurons and then analyze the resulting traces is publicly available at Github via https://github.com/ithairab/NeuronTracker and https://github.com/ithairab/SpikeFinder, respectively.
Scripts were not used to generate the figures reported in this paper.
Any additional information required to reproduce this work is available from the Lead Contact.


