Abstract
Abstract
Bronchopulmonary dysplasia (BPD) is a major complication in prematurely born infants. Pulmonary hypertension (PH) associated with BPD (BPD-PH) is characterized by alveolar diffusion impairment, abnormal vascular remodeling, and rarefication of pulmonary vessels (vascular growth arrest), which lead to increased pulmonary vascular resistance and right heart failure. About 25% of infants with moderate to severe BPD develop BPD-PH that is associated with high morbidity and mortality. The recent evolution of broader PH-targeted pharmacotherapy in adults has opened up new treatment options for infants with BPD-PH. Sildenafil became the mainstay of contemporary BPD-PH therapy. Additional medications, such as endothelin receptor antagonists and prostacyclin analogs/mimetics, are increasingly being investigated in infants with PH. However, pediatric data from prospective or randomized controlled trials are still sparse. We discuss comprehensive diagnostic and therapeutic strategies for BPD-PH and briefly review the relevant differential diagnoses of parenchymal and interstitial developmental lung diseases. In addition, we provide a practical framework for the management of children with BPD-PH, incorporating the modified definition and classification of pediatric PH from the 2018 World Symposium on Pulmonary Hypertension, and the 2019 EPPVDN consensus recommendations on established and newly developed therapeutic strategies. Finally, current gaps of knowledge and future research directions are discussed.
Impact
PH in BPD substantially increases mortality. Treatment of BPD-PH should be conducted by an interdisciplinary team and follow our new treatment algorithm while still kept tailored to the individual patient.
We discuss recent developments in BPD-PH, make recommendations on diagnosis, monitoring and treatment of PH in BPD, and address current gaps of knowledge and potential research directions.
We provide a practical framework, including a new treatment algorithm, for the management of children with BPD-PH, incorporating the modified definition and classification of pediatric PH (2018 WSPH) and the 2019 EPPVDN consensus recommendations on established and newly developed therapeutic strategies for BPD-PH.
Introduction
Bronchopulmonary dysplasia (BPD) represents a major cardiopulmonary complication in survivors of premature birth. The incidence of BPD is on the rise despite/because of the advances in neonatal intensive care.1 Besides impaired proximal airway and bronchoalveolar development, BPD is often associated with pulmonary vascular disease (PVD) and secondary pulmonary hypertension (PH). PH associated with BPD (BPD-PH) is characterized by abnormal vascular remodeling and rarefication of the pulmonary vasculature (vascular growth arrest),2 leading to increased pulmonary vascular resistance (PVR) and right heart failure. About 25% of infants with moderate to severe BPD develop PH3,4 that affects heart and lungs, greatly increasing mortality (47% of BPD infants die 2 years after diagnosis of PH).5–7
Compared to BPD without PH, BPD-PH is associated with suboptimal somatic growth and neurodevelopmental outcome,8,9 and higher rates of tracheostomy, increased use of supplemental oxygen, feeding problems, and frequent hospital admissions.10 Young adults born preterm (very low birth weight ≤1500 g; average gestational age 28 weeks) are at increased risk for PVD, PH, and right ventricular (RV) dysfunction.11
The increased survival of even the most immature infants makes infants with BPD-PH an ever-growing population among pediatric PH patients, requiring highly specialized care. Nevertheless, there is still a paucity of published data supporting the current management of children with BPD-PH.
In this article, we briefly review evolving concepts on the pathobiology of BPD-PH. We further discuss comprehensive diagnostic strategies, including the differential diagnosis of rather rare parenchymal and interstitial developmental lung diseases associated with PH. We provide a practical framework for the clinical management of children with BPD-PH, including established and newly developed therapeutic strategies, based on the 2018 World Symposium on Pulmonary Hypertension, and the 2019 updated consensus recommendations of the European Pediatric Pulmonary Vascular Disease Network (EPPVDN).12,13 Finally, we discuss current gaps of knowledge and potential future research directions.
Definition and classifications of pediatric PH
The WSPH 2018 modified the definition and classification of PH presented in the “2015 ESC/ERS Guidelines.”13,14 Specifically, the lower limit of normal mean pulmonary arterial pressure (mPAP) was decreased from 24 to 20 mmHg.15 In adults, even mildly elevated mPAP values (20–24 mmHg, prognostic threshold 17 mmHg) were found to be independent predictors of poor survival.16 For consistency, this new definition of PH was also used in the pediatric WSPH 2018 document13 and the current 2019 EPPVDN guidelines,12 although the cut-off mPAP >20 mmHg remains arbitrary for children (Table 1). The Pulmonary Vascular Research Institute’s (PVRI) Panama Classification divided pulmonary hypertensive vascular disease (PHVD = PVD + PH) into 10 main categories, including BPD-PH, and more than 100 subcategories (2011).17 It should be noted that due to the physiologic postnatal elevation and subsequent decline of PVR after birth, the new mPAP cut-off value of 20 mmHg only applies to infants beyond 3 months of age. However, the aforementioned definition of PH does not specify whether chronological or corrected age should be used in preterm infants. Furthermore, prognostic studies on the relevance of borderline-elevated mPAP in older infants are currently lacking. Due to this ambiguity, BPD patients at risk for or with confirmed PH must be evaluated and cared for by a multidisciplinary team of PH specialists, including neonatologists, pediatric cardiologists, and pulmonologists, with experience in the care of infants with PH.12,18,19
Table 1.
Developmental lung diseases associated with pulmonary hypertension.
| Developmental defect | Vascular pathology | Diagnosis and treatment |
|---|---|---|
| Alveolar capillary dysplasia (ACD) with or without misalignments of veins (MPV) | Reduction of alveolar capillaries, thickening of alveolar septal tissue, and failure of the capillaries to make contact with the alveolar epithelium. Familial cases occur. Genomic and genetic deletions of the FOX transcription factor gene cluster at 16p24.1 and inactivating point mutations of FOXF1 were identified | Usually diagnosed post mortem by histological examination. Positive family history? Genetic testing. Symptomatic treatment to decrease PVR (O2, iNO, ECMO), bilateral lung transplant |
| Bronchopulmonary dysplasia (BPD) | Pre- and postnatal impact of exogenous risk factors on a structural and functional immature lung lead to postnatal impairment of angiogenesis and alveolarization associated with abnormal vascular function (increased tone, altered reactivity, impaired metabolism) and structure (smooth muscle cell proliferation, altered extra cellular matrix structure) | Defined by criteria based on supplemental oxygen at 36 weeks postmenstrual age. Typical chest X-ray findings. Supportive treatment including respiratory support, O2, corticosteroids, in case of PH sildenafil, diuretics (hydrochlothiazide, spironolactone), and ERA might be considered |
| Congenital diaphragmatic hernia (CDH) | Developmental defect leading to severe vascular remodeling and rarefication of the vascular bed. The defect is associated with variable degrees of lung hypoplasia | Clinical and radiological diagnosis. Surgical repair. Supportive treatment involves ventilation with permissive hypercapnia, HFOV, surfactant, pre- or postnatal glucocorticoids without clear benefit. Early repair on ECMO might be beneficial |
| Lung hypoplasia (primary and secondary) | Genetic abnormalities, severe reduction in amniotic fluid leading to reduced prenatal alveolar and vascular development. Secondary to congenital pulmonary malformations (CPAM, etc.) | Diagnosis by HR-CT depending on underlying cause. Supportive treatment (consider mechanical ventilation including HFOV, iNO, and surfactant). PH-specific medications often with only minimal effect |
| Pulmonary interstitial glycogenosis (PIG) | Rare non-lethal pediatric form of interstitial lung disease, possible male predominance. Infants present with respiratory distress | Diffuse interstitial infiltrates on chest radiography. Lung biopsy with histological cytoplasmic accumulation of glycogen in interstitial cells and associated lung growth abnormalities. Corticosteroids have been used with various efficacies. Spontaneous regression in some cases |
| Pulmonary alveolar proteinosis (PAP) | Rare lung disease in which abnormal accumulation of surfactant occurs within the alveoli, interfering with gas exchange and affecting lung growth. Possible cause anti-GM-CSF autoantibodies. GM-CSF receptor mutations in hereditary PAP | PAS-positive dense bodies in the distal airways on lung biopsy. Supportive treatment including repeated bronchioalveolar lavage and lung transplantation. GM-CSF might be considered in autoimmune PAP. Transplantation of macrophage progenitors in hereditary PAP (experimental) |
| Pulmonary lymphangiectasia | Rare developmental pulmonary disorder characterized by pulmonary subpleural, interlobar, perivascular and peribronchial lymphatic dilatation | Pleural (chylous) effusions with or without generalized edema. Radiologic progression of generalized hazy infiltrates in the neonatal period to a more perihilar interstitial pattern with hyperinflation (X-ray, CT, MRI). Lymphangiectasia on lung biopsy (periartierial, subpleural, interlobar). Supportive and symptomatic treatment (respiratory support, drainage). MCT nutrition. Octreotide and antiplasmin (experimental) |
| Surfactant protein (SP) abnormalities (SP-B and SP-C deficiency, ATP-binding cassette A3 mutation, thyroid transcription factor 1/Nkx2.1 homeobox mutation) | Genetic inheritance of surfactant deficiency leading to impaired lung development | Diagnosis by genetic testing. Might present as interstitial lung disease on chest imaging. Surfactant replacement has only temporary effect, lung transplantation. Supportive treatment. Corticosteroids might show some effect. Hydroxychloroquine (experimental, SP-C deficiency) |
Current concepts of the pathobiology of BPD-PH
PH associated with BPD mainly develops in survivors of extreme preterm birth as a result of incomplete lung development (abnormal alveolarization), postnatal hyperoxia-/hypoxia-triggered vascular remodeling, and the rarefication of pulmonary blood vessels (vascular growth arrest).2 Dysbalanced TGFβ/BMP20 and VEGF signaling in BPD are pathobiological hallmarks of BPD-PH.2 Risk factors for BPD include placental anomalies, extreme prematurity, very low birth weight, intra-uterine growth restriction, perinatal infections, oxygen toxicity, and mechanical ventilation.2,21 While the precise mechanisms of PH associated with BPD are still unknown, it is likely they involve insults such as inflammation and endothelial dysfunction, perpetuated through alveolar hypoxia.2,21 In older infants, a cycle of progressive respiratory insufficiency, hypoxia, and compromised pulmonary perfusion aggravates PH, contributing to the increased mortality observed in BPD-PH infants.
Diagnosis of BPD and BPD-PH
Clinical suspicion of BPD-PH should be raised in every infant with a history of prematurity who requires supplemental oxygen and/or invasive or non-invasive respiratory support. Traditionally, BPD is defined clinically as supplemental oxygen use either at a corrected age of 36 gestational weeks (p.m.) or after 28 days of postnatal life in infants born <32 weeks of gestational age.22 However, this definition has recently been challenged and a new definition of BPD was proposed that includes infants on any form of respiratory support (such as high-flow nasal cannula) at 36 corrected weeks (p.m.), even in the absence of supplemental oxygen use.23 The significance of this new, alternative BPD definition for the frequency and morbidity of BPD-PH still needs to be investigated, and has not been used yet for this review article. In any case, PH/PHVD might occur before the formal diagnosis of BPD can be made. Indeed, echocardiographic screening may help identify infants at risk for BPD-PH as early as 7 days of life.4
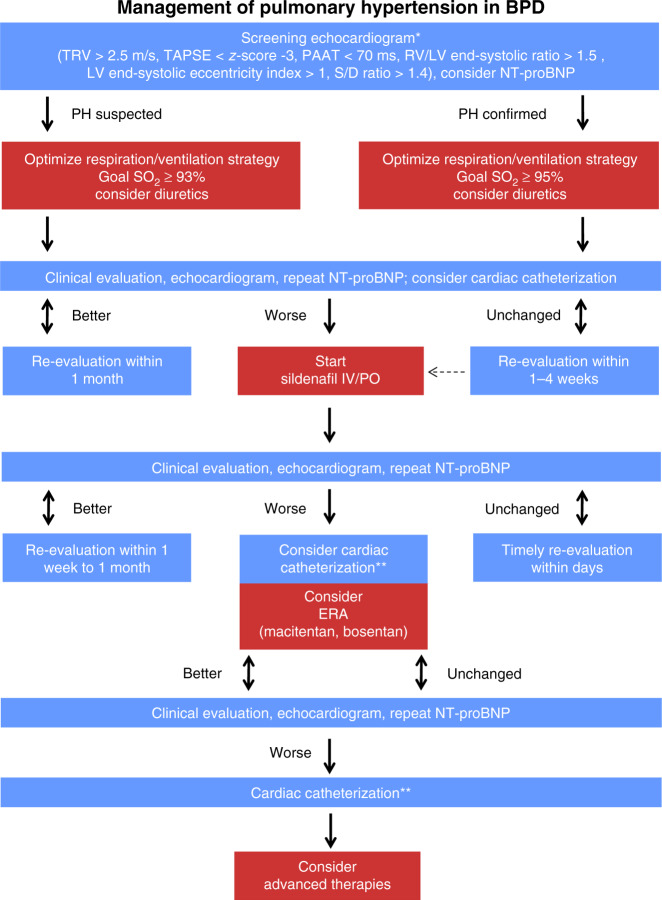
While BPD-specific changes are detected on conventional chest X-ray, the initial assessment of suspected PH is based on transthoracic echocardiography (TTE) (Fig. 1). Here, one should not rely on a single TTE parameter, but follow a multiparametric approach. Even with a history of immaturity, certain (rare) interstitial or parenchymal developmental lung diseases that can mimic BPD/BPD-PH must be included in the differential diagnosis (Table 1). Cardiac anomalies should be ruled out as these might affect therapy: for example, the presence of hemodynamically significant cardiovascular post-tricuspid shunts, such as large ventricular septal defects, often preclude the use of pulmonary vasodilators due to an aggravation of the shunt volume resulting from decreased PVR.12
Fig. 1. Management of pulmonary hypertension in bronchopulmonary dysplasia.
BPD—bronchopulmonary dysplasia; ERA—endothelin receptor antagonist; iNO—inhaled nitric oxide; LV—left ventricle; RV—right ventricle; PAAT—pulmonary artery acceleration time; PH—pulmonary hypertension; S/D ratio—systolic to diastolic duration ratio; TAPSE—tricuspid annular posterior systolic excursion; TRV—tricuspid regurgitation velocity. *Screening for pulmonary hypertension by transthoracic echocardiography is indicated (1) if severe respiratory compromise is present in any preterm infant <28 weeks of gestation, (2) in any infant with established BPD at corrected age of 36 weeks gestation (p.m.) and before discharge, (3) in any infant with prolonged oxygen requirement, poor growth, and unsatisfactory clinical improvement. **Cardiac catheterization should be considered before (i) introduction of a second PH-specific medication and is advised if PH worsens under dual therapy or if unsatisfactory treatment response is seen in any infant with PH at a corrected postnatal age of >3 months.
Echocardiography for diagnosis and monitoring of PH
TTE is an important diagnostic and monitoring modality.12,24–27 Screening for PH by TTE is indicated (1) if severe respiratory compromise is present in any preterm infant <28 weeks of gestation, (2) in any infant with established BPD at corrected age of 36 weeks gestation (p.m.) and before discharge, (3) in any infant with prolonged oxygen requirement, poor growth, unsatisfactory clinical improvement,12 and has also been suggested at 7 days of life to identify infants at risk for BPD-PH.4 Comprehensive TTE allows for complete initial assessment of cardiovascular anatomy and may also confirm RV pressure elevation through the measurement of the maximal velocity of the tricuspid regurgitation (TR) jet by continuous wave Doppler; TTE can detect impaired RV systolic function through qualitative assessment in several views, and quantitatively by means of a reduced age-related tricuspid annular plane systolic excursion as surrogate of longitudinal systolic RV function.12 Of note, TTE variables have different age-related reference ranges and variable impact on the accuracy of the diagnosis “PH” in children vs. adults, especially in young infants.24 The presence of a hemodynamically significant post-tricuspid shunt makes TTE estimation of PAP elevation less accurate.28
The relevant non-invasive imaging risk variables currently evaluated for infants and young children with PH,12 including those with BPD-PH, are listed in Table 2.
Table 2.
Echocardiographic determinants of PH risk in infants.
| Lower risk | Determinants of risk | Higher risk |
|---|---|---|
|
Minimal RA/RV enlargement No RV systolic dysfunction RV/LV ratio <1 (PSAX) TAPSE normal (z >−2) S/D ratio <1.0 (TR jet) PAAT >100 ms (>1 year old) |
Echocardiography |
Severe RA/RV enlargement RV systolic dysfunction RV/LV ratio >1.5 (PSAX) TAPSE ↓↓ (z <−3) S/D ratio >1.4 (TR jet) PAAT <70 ms (>1 year old) Pericardial effusion |
| TTE measurement | To estimate | Comment |
|---|---|---|
| TRV (m/s) | RVSP | Depends on the angle of continuous wave Doppler interrogation71 (full Doppler envelopes) |
| S/D ratio | PAP | Requires presence of well-defined TR71 (full Doppler envelopes) |
| PRV (m/s), diastolic max. | Mean PAP | Requires presence of well-defined PR71 (full Doppler envelopes) |
| PRV (m/s), diastolic min. | Diastolic PAP | Requires presence of well-defined PR71 (full Doppler envelopes) |
| PAAT (ms) | PAP, PVR | Measured in PSAX. PAAT was suggested to be an adequate follow-up parameter for assessing BPD-PH72,73 despite the notion that the normal reference range for PAAT in first year of life is particularly broad (54–116 ms).74 Heart rate dependent |
| RVOT VTI (cm) | PAP, PVR | Depends on the angle of PW Doppler interrogation. Increased TRV/RVOT VTI ratio in PH75 |
| RA area (cm2), end systole | RA dilation | Requires standard, on-axis four-chamber imaging25 |
| RA/LA ratio, end systole | RA dilation | Requires standard, on-axis four-chamber imaging |
| RV/LV ratio, end systole | RV dilation | Requires standard, on-axis PSAX imaging76 |
| RVES RI | RV remodeling | Useful especially in the absence of reliable TR and PR jets77 |
| LV eccentricity index, end systole | RV end-systolic pressure vs. LV end-systolic pressure; LV filling and compression | Requires standard, on-axis PSAX imaging78 |
| TAPSE | Systolic longitudinal RV function | Not a surrogate of segmental or radial changes in RV function71 |
LV left ventricle, RA right atrium, RV right ventricle, PAAT pulmonary artery acceleration time, PH pulmonary hypertension, PR pulmonary regurgitation, PRV pulmonary artery regurgitation velocity in diastole, PSAX parasternal short axis view, PVR pulmonary vascular resistance, RVES RI right ventricular end-systolic remodeling index, RVSP right ventricular systolic pressure, S/D ratio systolic to diastolic duration ratio, TAPSE tricuspid annular posterior systolic excursion, TTE transthoracic echocardiography, TR tricuspid regurgitation, TRV tricuspid regurgitation velocity, Z z-score.
Magnetic resonance imaging and chest computed tomography
Magnetic resonance imaging (MRI) and computed tomography (CT) have become essential non-invasive imaging modalities in suspected or confirmed PH.12 MRI offers the ability to assess pulmonary and systemic blood flow, pulmonary regional perfusion, PA and aortic diameters, (bi-) ventricular cardiac function, and myocardial tissue characteristics.29 More recently, lung MRI (e.g., TWIST, time-resolved angiography with stochastic trajectories) was introduced to assess lung perfusion in PHVD.30,31 A recent study on MRI in BPD-PH revealed that the pulmonary artery to aorta ratio (PA/AO) increased with BPD severity. An increased PA/AO and MR-derived LV end-systolic eccentricity index (MR-LVEI) were associated with increased length of stay and duration of respiratory support in BPD infants.31 Both increased PA/AO ratio and MR-LVEI were associated with PH therapy during hospitalization and at discharge. The primary role of chest CT is to detect lung parenchymal disorders, thromboembolic disease, and vascular abnormalities, such as pulmonary vein stenosis, which may be causally linked to evident PH. The key role of chest imaging, including newer MRI techniques in differentiating interstitial and parenchymal developmental lung disease has been discussed elsewhere.32 The significant risk of sedation/general anesthesia in PH patients need to be balanced against the potential gain of information of MRI or CT studies, and their impact on the future therapy of the individual PH patient.12
Diagnostic cardiac catheterization in suspected or confirmed BPD-PH
Cardiac catheterization is rarely necessary to make the diagnosis of BPD-PH early on. Although cardiac catheterization with acute pulmonary vasoreactivity testing (AVT) generally is recommended in pediatric PH prior to the initiation of pulmonary vasodilator therapy,11–13 exceptions may be made when the risk of the cardiac catheterization outweighs the potential benefits; for instance, severe hemodynamic instability (premature infants) or evidence of systemic vasculopathies. However, cardiac catheterization for the diagnosis and assessment of BPD-PH should be considered before introduction of a second PH-targeted medication; cardiac catheterization is advised if PH worsens under dual PH-targeted therapy or if an unsatisfactory response to PH-targeted pharmacotherapy is seen in any infant with PH at a corrected postnatal age of >3 months (Fig. 1).13 Additional indications include concern for vascular abnormalities (e.g., pulmonary vein stenosis),33 other features of an atypical response to therapy, and/or failure to thrive.34
A systematic cardiac catheterization protocol for pediatric PH is available.12 However, the complexity of pediatric PH/PVD often requires individualized adaptations of these recommendations. A recent study found that AVT may aid in the assessment of disease severity and management of BPD-PH. In this cohort of 26 infants with BPD, 35% had positive AVT, which was associated with better long-term outcomes. AVT also distinguished higher from lower risk PH in infants with BPD better than baseline pulmonary hemodynamics.35 Others found that the severity of lung disease as assessed by impaired oxygenation at cardiac catheterization did not correlate with mortality while the presence of pulmonary vein stenosis was associated with death.33
Biomarkers
Blood biomarker concentrations contribute to tailoring longitudinal medical care of PH patients.12 Brain natriuretic peptide (BNP) and its N-terminal cleavage product (NT-proBNP) are secreted by cardiomyocytes in response to ventricular wall stress due to pressure overload and/or volume expansion. Both biomarkers exhibit similar relative stress-induced release and age dependency, but NT-proBNP has the longer half-life (118 vs. 18 min). In a meta-analysis of 25 small-scale pediatric studies, serum NT-proBNP was identified as significant prognostic factor in pediatric PH and suggested to aid in the longitudinal assessment of patients36 (reference values of NTproBNP-BNP37). Recent studies have also shown the applicability of serum NT-proBNP as a suitable biomarker in infants with BPD-PH.38–40 Based on our experience, especially in infants, absolute values of NT-proBNP have a high inter-individual variance, are strongly dependent on postnatal and gestational age, and thus have limited value in establishing the diagnosis or severity of PH, especially in young infants. However, longitudinal assessment of NT-proBNP in combination with echocardiography is very useful to assess disease progression and/or response to treatment.41–44
Meanwhile, additional biomarkers are under consideration and may contribute to future PH management.12,40
Treatment
Supportive measures
Supplemental oxygen should be supplied when target oxygen saturations are >93% for infants with suspected and >95% for infants with proven PH (Fig. 1).12 Maximizing non-invasive respiratory support as much as possible in order to avoid mechanical ventilation and therefore aggravation of lung injury is advisable. However, atelectasis causes an increased ventilation/perfusion mismatch and local hypoxic vasoconstriction, and thus needs to be avoided. In addition, treatment with diuretics, that is, hydrochlorothiazide and spironolactone may be considered in infants with severe BPD.12 However, diuretic therapy in preterm infants remains controversial, given its negative impact on growth and the risk for metabolic bone disease. Thus, indications and duration of diuretic treatment should be discussed in a multidisciplinary team, involving cardiologists, neonatologists, pulmonologists, and nutritionists. Infants with severe BPD have higher caloric requirements, which should be accounted for in their nutritional plans (up to 160 kcal/kg). Infants with BPD-PH are prone to a sudden elevation of PVR when undergoing stressful procedures (“PH crisis”) and often require sufficient sedation or even analgesia/relaxation before such procedures are performed in an intensive care setting. Administration of inhaled nitric oxide and phosphodiesterase-3 inhibitors, such as milrinone, which are frequently used for acute treatment of PH in infants (e.g., in PPHN or acute deterioration of BPD-PH), are beyond the scope of this review and discussed elsewhere.12
Pharmacotherapy
General considerations
The overall goal of therapy for PH patients, adult or pediatric, is (1) to induce pulmonary arterial vasodilation, (2) to pressure unload and support the RV, (3) to avoid coronary ischemia and heart failure, (4) improve clinical outcomes and quality of life, and (5) to improve signs and symptoms, and thus the aggravation of PH.12,14,45–47 Of note, especially oral pulmonary vasodilator therapy may amplify ventilation and perfusion mismatch in any type of PH, especially in the setting of lung disease.
Regardless of a patient’s age or type of PH (group 1–5 PH),15 PH-targeted therapy currently focuses on three main molecular pathways: the nitric oxide pathway, the endothelin pathway, and the prostacyclin pathway (Table 3). While only two drugs have so far been approved by the regulatory European Medicines Agency (EMA) for pediatric patients with PAH, that is, sildenafil (body weight ≥8 kg and >1 year old) and bosentan (age >1 year), only bosentan has been approved by the Food and Drug Administration (FDA) for chronic use in PH children > 3 years of age. However, both sildenafil and bosentan are frequently used for acute and long-term treatment of infants with BPD-PH (Fig. 1).41,42 In the absence of randomized clinical trial data, use of PH-targeted medications in infants is based on expert opinion and experience, underlining the necessity of comprehensive evaluation in PH expert centers according to current international recommendations.12,48 The primary treatment goal for patients with PH associated with developmental lung diseases (group 3 PH)15 is to treat the underlying lung disease.12,48 However, BPD-PH patients are likely to benefit from vasodilator treatment.12,48,49 A recent, retrospective analysis showed clinical and echocardiographic improvements with a survival rate of 95% during a median follow-up time of 2 years in infants treated with PH-targeted therapy for BPD complicated by PH.42
Table 3.
Pharmacotherapy of BPD-PH.
| Drug | Mechanism | Comment |
|---|---|---|
| Sildenafil | PDE5 inhibitor | Most widely used in BPD-PH. Aggravates gastroesophageal reflux. EMA approved for children >1 year. The EPPVDN consensus (2019) recommends sildenafil at a maximum dose of 1 mg/kg/dose every 6 h in the first year of life, followed by the EMA drug recommendations for sildenafil: 10 mg every 8 h for patients ≥10 kg bodyweight and >12 months old, and 20 mg every 8 h for patients ≥20 kg bodyweight) |
| Tadalafil | PDE5 inhibitor | Alternative to sildenafil, less frequent dosing. No data on BPD-PH |
| Riociguat | Stimulator of soluble guanylatcyclase (sGC) | “Dual” mode of action (sGC activator and stimulator). No data on BPD-PH |
| Bosentan | ERA |
ET1A- and ET1B-receptor antagonist. Most widely used in BPD-PH in combination with PDE-5 inhibitor. May increase liver transaminases. Lowers circulating sildenafil levels. EMA approved for children with PAH >1 year based on BREATHE-3 and FUTURE-1 trials FDA-approved for children with PAH >3 years Maximum target bosentan dose is 2 mg/kg body weight twice daily (4 mg/kg/day) |
| Macitentan | ERA | ET1A- und ET1B-receptor antagonist. No liver toxicity. Does not lower sildenafil levels. Only limited experience in BPD-PH |
| Ambrisentan | ERA | Selective ET1A-receptor inhibition. May increase liver transaminases. Does not lower sildenafil levels. No experience in BPD-PH |
| Iloprost | PCA | Some experience in infants with PPHN and CHD-PAH either in combination with sildenafil or as monotherapy. May cause airway hyperreactivity |
| Epoprostenol | PCA | No experience in BPD-PH |
| Trepostinil | PCA | No experience in BPD-PH |
| Selexipag | Oral IP-receptor agonist | Only few reports on pediatric use in PAH, and CHD-PAH. Offers potential of oral “triple”-combination therapy. See EPPVDN pediatric selexipag study (2020)43 |
BPD-PH bronchopulmonary dysplasia-associated pulmonary hypertension, CHD congenital heart disease, EMA European Medicines Agency, ERA endothelin receptor antagonist, ET endothelin, IP prostacyclin receptor, PDE5 phosphodiesterase 5, PAH pulmonary arterial hypertension, PCA prostacyclin analog, PPHN persistent pulmonary hypertension of the newborn, FDA Food and Drug Administration.
Pharmacotherapy to modify the nitric oxide pathway
Phosphodiesterase-5 (PDE5) inhibitors are the most commonly employed drugs acting on the nitric oxide (NO) pathway, namely sildenafil and tadalafil. PDE5 inhibitors inhibit primarily PDE5 (NO/cGMP pathway), thus inducing vasodilation through vascular smooth muscle cell relaxation, and also exhibit anti-proliferative effects.50 STARTS-1 and its extension phase, STARTS-2,51 were the first pediatric randomized, placebo-controlled clinical trials in PAH. The STARTS study demonstrated improvement in secondary outcomes among those PH patients on medium- or high-dose sildenafil, including functional class and PVR. Controversially, the STARTS trial detected an increase in mortality among those subjects receiving high-dose sildenafil vs. placebo group, which in 2013 led to a warning of the EMA not to use higher doses, and rejection of approval by the FDA. Nevertheless, sildenafil is—by most centers and experts—regarded as a safe and efficacious first-of-choice drug for PH/PVD in children, and its widespread use and recommendation in formal guidelines continues.12,42 Sildenafil at standard dose should be considered in children with BPD-PH as improvements of clinical status and PAP have been demonstrated for oral sildenafil therapy.41 Tadalafil is reported to be widely used in North America due to its reduced dosing frequency, compared to sildenafil. Common to all PDE5 inhibitors, gastroesophageal reflux is an often reported unwanted effect that is also highly relevant to neonates and infants.46 Riociguat is a novel oral agent with dual mode of action that acts in synergy with endogenous NO and directly stimulates soluble guanylyl cyclase, with only very limited pediatric experience.47
Pharmacotherapy to modify the endothelin pathway
Bosentan, an oral non-selective endothelin A- and B-receptor antagonist (ERA), counteracts the vasoconstrictive and mitogenic effects of endothelin-1 in PAH patients. Elevated liver aminotransferases may occur with bosentan use as a serious adverse event, but seem to be less frequent in children under 12 years than in adults and children ≥12 years of age (2.7% vs. 7.8%).52–55 Nevertheless, monthly liver enzyme and function testing should be performed in children receiving bosentan.12,14 ERA treatment may also be considered in BPD infants.12 Macitentan provides an alternative to bosentan that shows a more favorable safety profile (less liver toxicity, no teratogenicity). Macitentan has been studied in a small prospective pediatric study and was shown to be safe and efficacious as add-on therapy.44 Off-label use of macitentan also has been reported in BPD-PH patients.56 Of note, bosentan lowers circulating sildenafil levels, while the other two ERA, ambrisentan and macitentan, do not.12
Pharmacotherapy to modify the prostacyclin pathway: prostacyclin and prostacyclin analogs (PGI2 and PGI2 analogs; PCA)
Prostacyclin (PGI2) and its analogs activate the PGI2receptor (IP receptor) and lead to vasodilation, inhibit vascular smooth muscle cell proliferation, and have anti-inflammatory effects. PCAs were developed which facilitate intravenous, but also subcutaneous, inhaled, and more recently oral delivery routes.12 As with other pulmonary vasodilators, PGI2 or PCAs may have off-target effects prompting side effects, including headache, jaw pain, nausea, vomiting, abdominal pain, diarrhea, and flushing. Inhaled PGI2 or PCAs may cause airway hyperreactivity,57 in addition to headaches and flushing. However, the use of especially inhaled PCAs has been reported in infants, for example, inhaled iloprost might be used as monotherapy or as adjuvant.58 Selexipag is a new oral selective prostacyclin receptor (IP receptor) agonist, but not yet systematically studied in “BPD-PH”. The first prospective, pediatric multicenter study on PAH-combination therapy indicates that selexipag can be used safely even in small children (4–10 kg bodyweight), and that a positive drug response can be expected in half of the children treated with selexipag.43
Interventional and surgical procedures
In children with BPD, large left-to-right atrial or ventricular shunts are not well tolerated, and may be closed early if PVD is mild (i.e., no or only mild PVR elevation at cardiac catheterization).59 However, aorto-pulmonary connections (e.g., a patent ductus arteriosus, PDA) may facilitate decompressing a pressure-loaded RV in severe PH/PVD.12 Procedures for palliation of children with severe PH and RV failure include the creation of a right-to-left shunt with the aim to decompress the right heart and to increase cardiac output. These interventions decompress the right atrium only (balloon atrial septostomy, BAS) or the RV (reverse Potts shunt). While the interatrial shunt resulting from BAS only secures some cardiac output in critical supra-systemic PAP elevations, the “endogenous” reverse Potts shunt (e.g., through stenting of a small PDA) clearly decomprresses the RV and lowers RV pressure.
The resulting hemodynamic situation after both interventional procedures is similar to the physiology of Eisenmenger patients. In imminent RV failure, an existing PDA can be balloon-dilated and stented (endogenous Potts shunt), representing an elegant procedure to decompress a failing RV and may be considered in patients with supra-systemic PH refractory to any medical treatment, including combination PH-targeted therapy.12,60 Similarily, in acute deterioration, PGE1 may be used to keep the PDA open at the expense of hypoxemia in the lower half of the body.
Prevention of BPD-PH
Two recent studies on clinical risk factors for BPD-PH identified these to be similar to the risk factors known to be associated with BPD: extreme prematurity, mechanical ventilation, tracheostomy, tracheitis, intraventricular hemorrhage (grade ≥3) and systemic steroid use, hyperoxia, and inflammation/sepsis.61,62 Thus, preventive measures to decrease BPD frequency and severity are of pivotal importance in order to lower the BPD-PH burden among preterm infants. These preventive measures include, but are not limited to: less use of invasive mechanical ventilation and predominant use of non-invasive respiratory support strategies, minimally invasive surfactant application, avoidance of hyper- and hypoxia, caffeine, corticosteroids, and reduction of infections.63–66 Further research is required to determine the role of caffeine and corticosteroids on the developing pulmonary vasculature. The same is true for a possible contribution of a hemodynamically significant PDA (here: sole left-to-right shut) to PH and PVD in BPD infants. In addition, early biomarkers, detection, and treatment of PH + PVD might hold the potential to decrease BPD-PH disease severity (Table 4).
Table 4.
Future research directions in BPD-PH.
| New discoveries in the pathobiology |
| Mechanistic studies on the pathobiology of BPD-PH and the role of common neonatal conditions such as respiratory support, surfactant, nutritional care, ductus arteriosus and inflammation on the developing pulmonary vasculature |
| Investigating mechanisms of right heart adaptation and remodeling in preterm infants with and without BPD |
| Identification of risk factors |
| Identification of pre- and postnatal risk factors relevant for the development of PHVD/PH in BPD infants |
| Identification of preventive measures against PHVD/PH during the fetal and early neonatal periods |
| Role of genetic risk factors in the pathogenesis of BPD-PH |
| Role of cardiac catheterization and advanced imaging (CT/CMR) in severe BPD-PH |
| Early detection |
| Clinical and echocardiographic parameters for early detection of PHVD/PH in preterm infants at risk for BPD |
| Innovative and efficient therapies |
| Research on underlying beneficial mechanisms, mode of delivery, and efficacy of stem-cell-based therapies, including cell-free preparation (e.g., conditioned media, exosome-based therapies) |
| Trials on the safety and efficacy of pulmonary vasodilatory therapy in preterm infants with BPD-PH |
| Potential role of vasodilatory combination therapy for BPD-PH |
| Potential role of new PAH-drugs, such as macitentan, selexipag, riociguat in BPD-PH |
| Clinical follow-up |
| Further research on biomarkers in BPD-PH (early detection and follow-up) |
| Definition of comprehensive multidisciplinary follow-up protocols, including neurodevelopmental aspects |
BPD-PH bronchopulmonary dysplasia-associated pulmonary hypertension, CT computerized tomography, CMR cardiac magnetic resonance, PHVD pulmonary hypertensive vascular disease.
Conclusions and future research directions
BPD-PH represents a significant burden to pediatric PH programs, but has been relatively understudied to date. The current wide ambiguity regarding optimal diagnosis, treatment, and monitoring of disease progression creates several high yield opportunities for study and practice improvement (Table 4). These include, but are not limited to: (1) comprehensive observational research cohort studies of the epidemiology of PH among high risk individuals with lung disease; (2) formal therapeutic trials incorporating PH-specific therapies in concert with traditional disease-specific approaches (e.g., oxygen and nutritional support); (3) novel therapies, including those directed at the underlying lung disease and those directed toward the pulmonary vasculature must be developed and studied, including modern oral combination pharmacotherapy; and, (4) studies designed to prevent and detect PH earlier among those with significant lung disease. Finally, cell-based therapies such as the use of mesenchymal stem cells (MSCs), MSC-conditioned media (cell media) or MSC extracellular vesicles/exosomes have shown promising preclinical results in reversing BPD-PH and several clinical trials are underway.67–70 A phase 1 study on the use of mesenchymal stem cell-derived extracellular vesicles in preterm neonates at high risk for BPD is underway (NCT03857841).
Acknowledgements
This work was supported by the European Pediatric Pulmonary Vascular Disease Network (www.pvdnetwork.org). Dr. Hansmann receives financial support from the German Research Foundation (DFG; HA4348/2-2, HA4348/6-2 KFO311), and the Federal Ministry of Education and Research (BMBF ViP+ program 03VP08053; BMBF 01KC2001B). SK was supported in part by NIH grants R01 HL055454 and R01HL146128. Open access funding provided by Projekt DEAL.
Author contributions
Concept, design, and drafting of the manuscript: G.H., H.S., and M.K. Critical editing and revising the manuscript for important intellectual content: all authors (G.H., H.S., C.C.R., S.K., E.D.A., M.K.)
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stoll BJ, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lignelli E, Palumbo F, Myti D, Morty RE. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;317:L832–L887. doi: 10.1152/ajplung.00369.2019. [DOI] [PubMed] [Google Scholar]
- 3.Weismann CG, et al. Pulmonary hypertension in preterm infants: results of a prospective screening program. J. Perinatol. 2017;37:572–577. doi: 10.1038/jp.2016.255. [DOI] [PubMed] [Google Scholar]
- 4.Mourani PM, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2015;191:87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khemani E, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 6.Altit, G., Bhombal. S., Feinstein, J., Hopper, R. K. & Tacy, T. A. Diminished right ventricular function at diagnosis of pulmonary hypertension is associated with mortality in bronchopulmonary dysplasia. Pulm. Circ. 9, 2045894019878598 (2019). [DOI] [PMC free article] [PubMed]
- 7.Ong MS, et al. Racial and ethnic differences in pediatric pulmonary hypertension: an analysis of the Pediatric Pulmonary Hypertension Network Registry. J. Pediatr. 2019;211:63–71.e6. doi: 10.1016/j.jpeds.2019.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakanishi H, Uchiyama A, Kusuda S. Impact of pulmonary hypertension on neurodevelopmental outcome in preterm infants with bronchopulmonary dysplasia: a cohort study. J. Perinatol. 2016;36:890–896. doi: 10.1038/jp.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi EK, Shin SH, Kim EK, Kim HS. Developmental outcomes of preterm infants with bronchopulmonary dysplasia-associated pulmonary hypertension at 18-24 months of corrected age. BMC Pediatr. 2019;19:26. doi: 10.1186/s12887-019-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagatta JM, et al. The impact of pulmonary hypertension in preterm infants with severe bronchopulmonary dysplasia through 1 year. J. Pediatr. 2018;203:218–224.e3. doi: 10.1016/j.jpeds.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goss KN, et al. Early pulmonary vascular disease in young adults born preterm. Am. J. Respir. Crit. Care Med. 2018;198:1549–1558. doi: 10.1164/rccm.201710-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansmann G, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J. Heart Lung Transplant. 2019;38:879–901. doi: 10.1016/j.healun.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig EB, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur. Respir. J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galie N, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douschan P, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am. J. Respir. Crit. Care Med. 2018;197:509–516. doi: 10.1164/rccm.201706-1215OC. [DOI] [PubMed] [Google Scholar]
- 17.Cerro MJ, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: report from the PVRI Pediatric Taskforce, Panama 2011. Pulm. Circ. 2011;1:286–298. doi: 10.4103/2045-8932.83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallmon H, et al. Recommendations from the Association for European Paediatric and Congenital Cardiology for training in pulmonary hypertension. Cardiol. Young. 2019;29:1323–1327. doi: 10.1017/S104795111900235X. [DOI] [PubMed] [Google Scholar]
- 19.Duijts L, et al. European Respiratory Society guideline on long term management of children with bronchopulmonary dysplasia. Eur. Respir. J. 2020;55:1900788. doi: 10.1183/13993003.00788-2019. [DOI] [PubMed] [Google Scholar]
- 20.Calvier L, et al. PPARγ links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell. Metab. 2017;25:1118–1134.e7. doi: 10.1016/j.cmet.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Thébaud B, et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 2019;5:78. doi: 10.1038/s41572-019-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 23.Jensen EA, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am. J. Respir. Crit. Care Med. 2019;200:751–759. doi: 10.1164/rccm.201812-2348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ploegstra MJ, et al. Echocardiography in pediatric pulmonary arterial hypertension: early study on assessing disease severity and predicting outcome. Circ. Cardiovasc. Imaging. 2015;8:e000878. doi: 10.1161/CIRCIMAGING.113.000878. [DOI] [PubMed] [Google Scholar]
- 25.Koestenberger M, et al. Echocardiographic reference values for right atrial size in children with and without atrial septal defects or pulmonary hypertension. Pediatr. Cardiol. 2016;37:686–695. doi: 10.1007/s00246-015-1332-0. [DOI] [PubMed] [Google Scholar]
- 26.Burkett DA, et al. Impact of pulmonary hemodynamics and ventricular interdependence on left ventricular diastolic function in children with pulmonary hypertension. Circ. Cardiovasc. Imaging. 2016;9:e004612. doi: 10.1161/CIRCIMAGING.116.004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansmann G. Left ventricular diastolic dysfunction in pediatric pulmonary hypertension. Circ. Cardiovasc. Imaging. 2016;9:e005527. doi: 10.1161/CIRCIMAGING.116.005527. [DOI] [PubMed] [Google Scholar]
- 28.Nawaytou H, et al. Clinical utility of echocardiography in former preterm infants with bronchopulmonary dysplasia. J. Am. Soc. Echocardiogr. 2020;33:378–388.e1. doi: 10.1016/j.echo.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Rajaram S, et al. 3D contrast-enhanced lung perfusion MRI is an effective screening tool for chronic thromboembolic pulmonary hypertension: results from the ASPIRE Registry. Thorax. 2013;68:677–678. doi: 10.1136/thoraxjnl-2012-203020. [DOI] [PubMed] [Google Scholar]
- 30.Schoenfeld C, et al. MR imaging-derived regional pulmonary parenchymal perfusion and cardiac function for monitoring patients with chronic thromboembolic pulmonary hypertension before and after pulmonary endarterectomy. Radiology. 2016;279:925–934. doi: 10.1148/radiol.2015150765. [DOI] [PubMed] [Google Scholar]
- 31.Critser PJ, et al. Cardiac magnetic resonance imaging evaluation of neonatal bronchopulmonary dysplasia-associated pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2020;201:73–82. doi: 10.1164/rccm.201904-0826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato S, et al. Prognostic value of cardiovascular magnetic resonance derived right ventricular function in patients with interstitial lung disease. J. Cardiovasc. Magn. Reson. 2015;17:10. doi: 10.1186/s12968-015-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steurer MA, et al. Mortality in infants with bronchopulmonary dysplasia: data from cardiac catheterization. Pediatr. Pulmonol. 2019;54:804–813. doi: 10.1002/ppul.24297. [DOI] [PubMed] [Google Scholar]
- 34.Ploegstra MJ, et al. Growth in children with pulmonary arterial hypertension: a longitudinal retrospective multiregistry study. Lancet Respir. Med. 2016;4:281–290. doi: 10.1016/S2213-2600(16)00069-2. [DOI] [PubMed] [Google Scholar]
- 35.Frank BS, et al. Acute vasoreactivity testing during cardiac catheterization of neonates with bronchopulmonary dysplasia-associated pulmonary hypertension. J. Pediatr. 2019;208:127–133. doi: 10.1016/j.jpeds.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Ploegstra MJ, Zijlstra WM, Douwes JM, Hillege HL, Berger RM. Prognostic factors in pediatric pulmonary arterial hypertension: a systematic review and meta-analysis. Int. J. Cardiol. 2015;184:198–207. doi: 10.1016/j.ijcard.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 37.Nir A, et al. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr. Cardiol. 2009;30:3–8. doi: 10.1007/s00246-008-9258-4. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez-Blanco S, et al. N-terminal-probrain natriuretic peptide as a biomarker of moderate to severe bronchopulmonary dysplasia in preterm infants: a prospective observational study. Pediatr. Pulmonol. 2018;53:1073–1081. doi: 10.1002/ppul.24053. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, et al. N-terminal Pro-B-type natriuretic peptide as a biomarker of bronchopulmonary dysplasia or death in preterm infants: a retrospective cohort analysis. Front. Pediatr. 2019;7:166. doi: 10.3389/fped.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery AM, et al. Biochemical screening for pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Neonatology. 2016;109:190–194. doi: 10.1159/000442043. [DOI] [PubMed] [Google Scholar]
- 41.Cohen JL, et al. Sildenafil use in children with pulmonary hypertension. J. Pediatr. 2019;205:29–34.e1. doi: 10.1016/j.jpeds.2018.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadmon G, et al. Pulmonary hypertension specific treatment in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 2017;52:77–83. doi: 10.1002/ppul.23508. [DOI] [PubMed] [Google Scholar]
- 43.Hansmann, G., Meinel, K., Bukova, M., Chouvarine, P., Wåhlander, H. & Koestenberger, M.; European Pediatric Pulmonary Vascular Disease Network (EPPVDN). Selexipag for the Treatment of Children With Pulmonary Arterial Hypertension: First Multicenter Experience in Drug Safety and Efficacy. J. Heart Lung Transplant. (2020). 10.1016/j.healun.2020.03.029. [Online ahead of print]. [DOI] [PubMed]
- 44.Schweintzger S, et al. Safety and efficacy of the endothelin receptor antagonist macitentan in pediatric pulmonary hypertension. Cardiovasc. Diagn. Ther. 2020 doi: 10.21037/cdt.2020.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J. Pediatr. 2009;154:379–384. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakshminrusimha S, Mathew B, Leach CL. Pharmacologic strategies in neonatal pulmonary hypertension other than nitric oxide. Semin. Perinatol. 2016;40:160–173. doi: 10.1053/j.semperi.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghofrani HA, et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 48.Abman SH, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–2099. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 49.Krishnan U, et al. Evaluation and management of pulmonary hypertension in children with bronchopulmonary dysplasia. J. Pediatr. 2017;188:24–34.e1. doi: 10.1016/j.jpeds.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 50.Tzoumas, N., Farrah, T. E., Dhaun, N. & Webb, D. J. Established and emerging therapeutic uses of phosphodiesterase type 5 inhibitors in cardiovascular disease. Br. J. Pharmacol. 10.1111/bph.14920 (2019). [DOI] [PMC free article] [PubMed]
- 51.Barst RJ, et al. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation. 2014;129:1914–1923. doi: 10.1161/CIRCULATIONAHA.113.005698. [DOI] [PubMed] [Google Scholar]
- 52.Rosenzweig EB, et al. Effects of long-term bosentan in children with pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2005;46:697–704. doi: 10.1016/j.jacc.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 53.Barst RJ, et al. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin. Pharmacol. Ther. 2003;73:372–382. doi: 10.1016/s0009-9236(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 54.Beghetti M, et al. Pharmacokinetic and clinical profile of a novel formulation of bosentan in children with pulmonary arterial hypertension: the FUTURE-1 study. Br. J. Clin. Pharmacol. 2009;68:948–955. doi: 10.1111/j.1365-2125.2009.03532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berger RM, et al. FUTURE-2: results from an open-label, long-term safety and tolerability extension study using the pediatric FormUlation of bosenTan in pUlmonary arterial hypeRtEnsion. Int. J. Cardiol. 2016;202:52–58. doi: 10.1016/j.ijcard.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 56.Koestenberger, M. et al. Ventricular–ventricular interaction variables correlate with surrogate variables of clinical outcome in children with pulmonary hypertension. Pulm. Circ. 9, 2045894019854074 (2019). [DOI] [PMC free article] [PubMed]
- 57.Ivy DD, et al. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2008;51:161–169. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piastra M, et al. Nebulized iloprost and noninvasive respiratory support for impending hypoxaemic respiratory failure in formerly preterm infants: a case series. Pediatr. Pulmonol. 2012;47:757–762. doi: 10.1002/ppul.21619. [DOI] [PubMed] [Google Scholar]
- 59.Thomas VC, et al. Transcatheter closure of secundum atrial septal defect in infants less than 12 months of age improves symptoms of chronic lung disease. Congenit. Heart Dis. 2012;7:204–211. doi: 10.1111/j.1747-0803.2010.00442.x. [DOI] [PubMed] [Google Scholar]
- 60.Baruteau AE, et al. Palliative Potts shunt for the treatment of children with drug-refractory pulmonary arterial hypertension: updated data from the first 24 patients. Eur. J. Cardiothorac. Surg. 2015;47:e105–e110. doi: 10.1093/ejcts/ezu445. [DOI] [PubMed] [Google Scholar]
- 61.Nagiub M, Kanaan U, Simon D, Guglani L. Risk factors for development of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review and meta-analysis. Paediatr. Respir. Rev. 2017;23:27–32. doi: 10.1016/j.prrv.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Sheth S, Goto L, Bhandari V, Abraham B, Mowes A. Factors associated with development of early and late pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. J. Perinatol. 2020;40:138–148. doi: 10.1038/s41372-019-0549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sweet DG, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology. 2019;115:432–450. doi: 10.1159/000499361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vento M, Bohlin K, Herting E, Roehr CC, Dargaville PA. Surfactant administration via thin catheter: a practical guide. Neonatology. 2019;116:211–226. doi: 10.1159/000502610. [DOI] [PubMed] [Google Scholar]
- 65.Belkhatir K, et al. Variations in preterm stabilisation practices and caffeine therapy between two European tertiary level neonatal units. Acta Paediatr. 2020;109:488–493. doi: 10.1111/apa.15011. [DOI] [PubMed] [Google Scholar]
- 66.Zivanovic S, Roehr CC. One step further toward defining the optimal respiratory care package for neonates: interventions to successfully extubate preterm infants. JAMA Pediatr. 2017;171:120–121. doi: 10.1001/jamapediatrics.2016.3271. [DOI] [PubMed] [Google Scholar]
- 67.Hansmann G, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm. Circ. 2012;2:170–181. doi: 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang YS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J. Pediatr. 2014;164:966–972 e966. doi: 10.1016/j.jpeds.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Willis GR, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am. J. Respir. Crit. Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rüdiger, M., Kirpalani, H., Steinhorn, R., Davis, J. M. & Thebaud, B. How to introduce MSC-based therapy for the developing lung safely into clinical care? Pediatr. Res. 10.1038/s41390-020-0758-0 (2020). [DOI] [PubMed]
- 71.Nagiub M, Lee S, Guglani L. Echocardiographic assessment of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review of literature and a proposed algorithm for assessment. Echocardiography. 2015;32:819–833. doi: 10.1111/echo.12738. [DOI] [PubMed] [Google Scholar]
- 72.Levy PT, Patel MD, Choudhry S, Hamvas A, Singh GK. Evidence of echocardiographic markers of pulmonary vascular disease in asymptomatic infants born preterm at one year of age. J. Pediatr. 2018;197:48–56.e2. doi: 10.1016/j.jpeds.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel MD, et al. Echocardiographic assessment of right ventricular afterload in preterm infants: maturational patterns of pulmonary artery acceleration time over the first year of age and implications for pulmonary hypertension. J. Am. Soc. Echocardiogr. 2019;32:884–894.e4. doi: 10.1016/j.echo.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koestenberger M, et al. Normal reference values and z scores of the pulmonary artery acceleration time in children and its importance for the assessment of pulmonary hypertension. Circ. Cardiovasc. Imaging. 2017;10:e005336. doi: 10.1161/CIRCIMAGING.116.005336. [DOI] [PubMed] [Google Scholar]
- 75.Koestenberger M, et al. Right ventricular outflow tract velocity time integral (RVOT VTI) and tricuspid regurgitation velocity/RVOT VTI ratio in pediatric pulmonary hypertension. Int. J. Cardiol. 2016;212:274–276. doi: 10.1016/j.ijcard.2016.03.111. [DOI] [PubMed] [Google Scholar]
- 76.Jone PN, Hinzman J, Wagner BD, Ivy DD, Younoszai A. Right ventricular to left ventricular diameter ratio at end-systole in evaluating outcomes in children with pulmonary hypertension. J. Am. Soc. Echocardiogr. 2014;27:172–178. doi: 10.1016/j.echo.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koestenberger, M. et al. Right ventricular end-systolic remodeling index in the assessment of pediatric pulmonary arterial hypertension. The European Pediatric Pulmonary Vascular Disease Network (EPPVDN). Pediatr. Res. 10.1038/s41390-020-0748-2 (2020). [DOI] [PubMed]
- 78.McCrary AW, et al. Differences in eccentricity index and systolic-diastolic ratio in extremely low-birth-weight infants with bronchopulmonary dysplasia at risk of pulmonary hypertension. Am. J. Perinatol. 2016;33:57–62. doi: 10.1055/s-0035-1556757. [DOI] [PMC free article] [PubMed] [Google Scholar]