Abstract
Purpose:
Lung protective ventilation (LPV), defined as a tidal volume (Vt) ≤8cc/kg of predicted body weight, reduces ventilator-induced lung injury but is applied inconsistently.
Materials and Methods:
We conducted (1) a prospective, quasi-experimental, cohort study of adults mechanically ventilated admitted to intensive care units (ICU) in the year before, year after, and second year after implementation of an electronic medical record based LPV order, and (2) a cross-sectional qualitative study of ICU providers regarding their perceptions of the order. We applied the Reach, Efficacy, Adoption, Implementation, and Maintenance (RE-AIM) framework to evaluate the implementation.
Results:
There were 1405, 1424, and 1342 in the control, adoption, and maintenance cohorts, representing 95% of mechanically ventilated adult ICU patients. The overall prevalence of LPV increased from 65% to 73% (p<0.001, adjusted-OR for LPV adherence: 1.9, 95% CI 1.5-2.3), but LPV adherence in women was approximately 30% worse than in men (women: 44% to 56% [p<0.001],men: 79% to 86% [p<0.001]). ICU providers noted difficulty obtaining an accurate height measurement and mistrust of the Vt calculation as barriers to implementation. LPV adherence increased further in the second year post implementation.
Conclusion:
We designed and implemented an LPV order that sustainably improved LPV adherence across diverse ICUs.
Keywords: Lung Protective Ventilation (LPV), Low tidal volume ventilation (LTVV), Mechanical Ventilation, Clinical decision support tool (CDS), Implementation science
INTRODUCTION
Lung protective ventilation (LPV) with low tidal volumes (Vt) of 4-8 cc/kg based on predicted body weight (PBW) improves mortality in patients with acute respiratory distress syndrome (ARDS) (1, 2). In patients without ARDS, when compared to higher tidal volumes of 10-12 cc/kg of PBW, lower tidal volumes of 4-8cc/kg of PBW are associated with decreased atelectasis, fewer pulmonary infections, decreased risk of developing ARDS, decreased length of stay (1, 3-11). While a recent trial showed no difference between mortality or ventilator days in patients without ARDS using low tidal volume ventilation versus intermediate tidal volume ventilation, median tidal volumes in the intermediate tidal volume ventilation arm were less than 8cc/kg PBW (12). In daily clinical practice, recognition of ARDS and adherence to LPV remains low (13-16). Barriers to adherence include under-recognition of LPV guidelines, perceived contraindications to LPV, and clinician overestimation of LPV use (17, 18).
Clinical decision support (CDS) tools provide clinicians with patient-specific recommendations that help clinical decision making (19). CDS tools within the electronic medical record (EMR) have been shown to improve adherence to clinical practice guidelines for deep vein thrombosis prophylaxis and other preventative care services (19-21). In prior studies, EMR-based LPV CDS tools were shown to improve LPV adherence (5, 15, 22-24). However, these prior studies have been limited to single ICUs, did not seek to identify barriers to its implementation nor factors that contributed to its success, and did not assess long-term sustainability of LPV adherence using an EMR-based LPV CDS tool. To address these knowledge gaps, the under-recognition of ARDS by clinicians, and decreased use of LPV when there is proven benefit, we implemented an LPV order with an embedded CDS tool at a quaternary-care medical center with five types of ICUs. We measured adherence to LPV one year before implementation, and for two years after implementation. We also conducted qualitative interviews with providers from the Emergency Department (ED), and from medical, surgical, cardiothoracic, cardiac, and neurological ICUs to identify factors that contributed to the LPV order success, and barriers that limited LPV adherence.
METHODS
Study Overview
We conducted a two-part, quality improvement, mixed-methods, implementation science study. First, we conducted a prospective, quasi-experimental, cohort study at NewYork- Presbyterian/Columbia University Irving Medical Center (NYP-CUIMC). On January 1, 2017, we implemented an LPV order that automatically calculated Vt based on PBW using patient height as entered in the EMR. We sought to determine whether this intervention would initially improve and then maintain better adherence to LPV among adults admitted to one of five intensive care units (ICUs). Second, we conducted a cross-sectional study, where we asked focus group participants comprised of ED and ICU providers for their opinions about the utility of and potential patient benefits from the LPV order. We applied the implementation science analytic framework of Reach, Efficacy, Adoption, Implementation, and Maintenance (RE-AIM) to evaluate the LPV order and its effect on LPV adherence (25-29).
Pre- versus Post-Lung Protective Ventilation Order Cohort Study
Patients
We included all adults age ≥18 years who newly required invasive mechanical ventilation (IMV) and were admitted to a medical, surgical, cardiothoracic, cardiac, or neurological ICU during the pre-implementation period (January – June 2016, control cohort), post-implementation period (January – June 2017, adoption cohort), and maintenance period (January – June 2018, maintenance cohort). We excluded patients who were not mechanically ventilated via Volume Assist Control mode since their intended tidal volumes were not prescribed, or who required chronic mechanical ventilation, which we defined as having a tracheostomy at the time of admission to the ICU. The institutional review board of Columbia University Irving Medical Center approved the study (Protocol Number AAAR4739).
Lung Protective Ventilation (LPV) Order
Prior to implementing the LPV order, the original EMR-based mechanical ventilation order required the user to enter the Vt in cubic centimeters (cc) without any reference to the patient’s height. We developed software within the Allscripts EMR (Allscripts, Chicago, Il) that contains a CDS tool imbedded in the mechanical ventilation order (Figure E1). In order for the mechanical ventilation order to generate an LPV tidal volume, the patient’s height had to be already entered into the EMR. First, the CDS tool states the definition of ARDS using Berlin Criteria in a dialog box (30), and asks the prescriber to indicate whether or not the patient has ARDS. Second, an initial Vt of 6cc/kg or 8cc/kg of PBW is suggested based on the presence or absence of ARDS, respectively. Third, the provider selects from a dropdown menu a Vt in a range of 4-8 cc/kg of PBW. The order has a function where it automatically calculates and displays the Vt based on the patient’s entered height. There are two “override options” for ordering Vt in the dropdown menu: the “other cc/kg” allows the provider to enter a numeric value in cc/kg of PBW, which then calculates a Vt accordingly; and the “direct entry” option that allows the prescriber to manually enter the Vt in cc.
Cross-Sectional Qualitative Study of Providers
We conducted focus groups with resident physicians, nurse practitioners (NP), and physician assistants (PA) from the ED and each ICU during the maintenance study period in March 2018. We did not include attending physicians, since they do not regularly enter orders for ICU patients at our medical center. Participation was voluntary and uncompensated, and we obtained written informed consent. We asked providers open-ended questions about (1) their perceptions of the clinical benefit of LPV, (2) applicability to their ICU patients, (3) factors and clinical situations that limit the effectiveness of the LPV order, and (4) ways to further improve and maintain the delivery of LPV at our medical center. We reviewed transcripts using the constant comparison method, which involved coding transcripts in three phases in order to identify common themes, and to assess for theme saturation across the focus groups (31).
RE-AIM Model
We used the RE-AIM framework to organize our study of the LPV order implementation. The framework focuses on the reach of the intervention to the target population; whether the intervention produces expected results when it is delivered (efficacy); adoption of the intervention across a broad and representative proportion of settings; whether the intervention is delivered as intended (implementation); and, whether changes are maintained over a period time and whether there is an infrastructure to ensure the sustainability of the intervention (maintenance) (32).
RE-AIM Measurements
We defined reach as the study population, which included all adult patients receiving invasive mechanical ventilation (IMV) who were admitted to ICU’s at NYP-CUIMC during each of the study periods (Table 1). Our primary measure of efficacy was the proportion of patients receiving volume control IMV with a delivered Vt that was adherent to LPV (defined as ≤8 cc/kg of PBW) after an initial mechanical ventilation order. We measured delivered Vt as the Vt that was set on the ventilator and recorded in the EMR by the respiratory therapist. The delivered Vt was the first Vt recorded in the EMR by the respiratory therapist after the order was placed. Our secondary measure of efficacy was the proportion of patients receiving volume control IMV whose initial ordered Vt was adherent to LPV. We defined ordered Vt as the Vt that was calculated or entered using the LPV order. We measured both delivered and ordered Vt because we did not want to assume that what was ordered in the EMR was actually what was delivered to the patient. Of note, several patients admitted to the ICUs were first ordered and started on mechanical ventilation in the ED, post-anesthesia care unit, or hospital floor, and we used the initial ordered and delivered Vt recorded while patients were in these locations. We measured adoption as the proportion of adoption cohort patients receiving volume control IMV who had an IMV order that was placed using the implemented LPV order. We measured implementation as the proportion of volume control IMV orders with a pre-calculated Vt of 4-8 cc/kg of PBW, rather than having a Vt entered by the provider using the “direct entry” option. We measured maintenance as the adherence to LPV in the second year after the LPV order was implemented. We used focus group feedback to assess the utility of the LPV order in the ED and each of the different specialty ICUs (reach), to identify factors that contributed to the successful delivery of LPV (efficacy), to identify specific benefits and barriers to the use of the LPV order (adoption, implementation), and to identify ways to maintain improved LPV delivery using the LPV order (maintenance).
Table 1:
| RE-AIM Dimension |
Description | Measurements |
|---|---|---|
| Reach | Is the intervention reaching the target population? | Newly intubated patients admitted to the ICUc |
| Effectiveness | Does the intervention accomplish its goals? | |
| Adoption | Are those targeted to deliver the intervention participating? | Proportion of patients for which EMR-based LPV order was used |
| Implementation | Is the intervention consistently implemented? | Proportion of orders where the pre-calculated Vt was used rather than the provider entering Vt (in ccf) directly |
| Maintenance | Did the intervention become part of the routine organizational practices and maintain effectiveness? | Measured as adherence to LPV order use by providers and adherence to LPV in patients one year after implementation of the EMR-based LPV order |
Definition of abbreviation:
Electronic medical record
Lung productive ventilation
Intensive Care Unit
Tidal Volume
Predicted body weight
cubic centimeters
Variables and Statistical Analysis
We ascertained patient demographics, clinical data, ventilator orders, and ventilator Vt from the EMR. ICU Admission diagnosis were defined by groups of diagnosis codes in the International Classification of Diseases 10th edition, according to the Clinical Classifications Software by the Health Cost and Utilization Project (33). We calculated the Sequential Organ Failure Assessment (SOFA) score for each patient based on the first 24 hours after initiation of IMV (34). We made comparisons between the control, adoption, and maintenance cohorts using chi square, Student’s t-, Wilcoxon rank sum, analysis of variance, and Kruskal-Wallis tests, as appropriate. We used logistic regression models with adoption versus control cohort membership as the dependent variable, and LPV adherence as the independent variable of interest to estimate the odds of being adherent to LPV after LPV order implementation while controlling for age, sex, height, SOFA score, and type of ICU.
We conducted a pre-specified stratified analysis by sex, because we assumed the risk of being nonadherent to LPV would vary by height, and because women are, on average, shorter than men. In unadjusted sex-stratified analyses, we report the percentage of Vt orders and percentage of Vt delivered that are LPV-adherent for men and for women in each of the three cohorts. In adjusted sex-stratified analyses, we estimate the odds of receiving an LPV-adherent order and having LPV delivered for men and for women, while controlling for age, height, SOFA score, and ICU. We tested for the presence of interaction between sex and adoption vs. control cohort, while controlling for age, height, SOFA score, and ICU. To examine whether the LPV orderset was more effective for ARDS patients than non-ARDS patients, we conducted a post-hoc analysis stratifying patients by PaO2:FiO2 (P:F) ratio <300 (a liberal estimate of the patients who potentially had ARDS) versus ≥300 (unlikely to have ARDS) during the first 24 hours of IMV. We compared the OR of ordered and delivered Vts adherent to LPV in adoption versus control cohort patients with P:F<300 and P:F≥300. We tested for the presence of interaction between P:F ratio category and adoption vs. control cohort, while controlling for age, height, sex, SOFA score, and ICU. For all analyses, a p <0.05 was considered significant. We used SAS version 9.4 software for analyses (SAS Institute, Cary, NC).
RESULTS
Reach
There were 1405, 1424, and 1342 patients in the control, adoption, and maintenance cohorts, respectively (Figure 1). For the entire study population, the mean (SD) age was 63 (16) years, and 40% were women. There were no significant differences in age, sex, height, BMI, or SOFA scores between the cohorts (Table 2). More patients in the adoption and maintenance cohorts than in the control cohort had a P:F ratio <300 (p = 0.002).
Fig. 1.
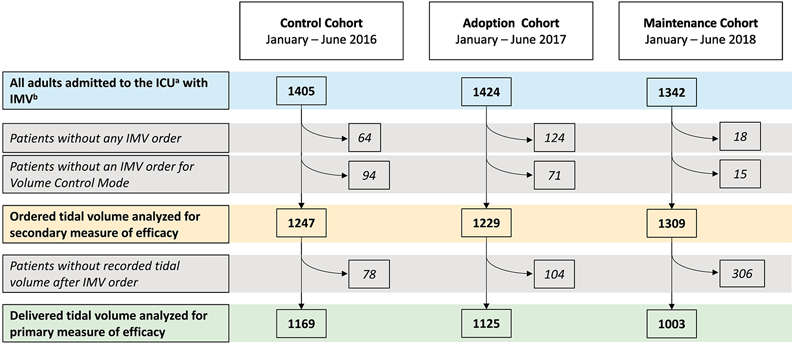
Study flowsheet.
Table 2:
Patient Characteristics
| Control Cohort | Adoption Cohort | Maintenance Cohort | P value | |
|---|---|---|---|---|
| Number of Participants | 1405 | 1424 | 1342 | |
| Age, years, mean ±SDa | 62.3±15.2 | 62.5±15.6 | 63.2±16.2 | 0.77 |
| Sex, n (%) | 0.98 | |||
| Male | 842 (60) | 853 (59.9) | 819 (61) | |
| Female | 563 (40) | 571 (40.1) | 523 (39) | |
| Height, cmb, median (IQRc) | 169 (160-177) | 168 (162-177) | 19 (162-177) | 0.96 |
| Weight (kgd), median (IQR) | 78.9 (66.3-90.7) | 78.4 (61.2-92) | 79.4 (67-94) | 0.41 |
| BMIe, n (%) | 0.16 | |||
| Underweight, <18 | 45 (3.2) | 59 (4.1) | 22 (1.6) | |
| Normal, 18-24 | 330 (23.5) | 342 (24.0) | 345 (25.7) | |
| Overweight, 25-30 | 593 (42.2) | 549 (38.6) | 553 (41.3) | |
| Obese, >30 | 437 (31.1) | 474 (33.3) | 422 (31.4) | |
| Vtf, cc/kg of PBW, median (IQR) | 7.5 (6.7-8.4) | 7.2 (6.4-8.1) | 7.02 (6.03-7.98) | <0.0001 |
| Location of first ventilator order, n (%) | <0.0001 | |||
| Cardiac | 79 (5.6) | 49 (3.5) | 119 (8.9) | |
| Cardiothoracic | 644 (45.8) | 400 (28.1) | 591 (44.0) | |
| Medical | 185 (13.2) | 177 (12.4) | 285 (21.2) | |
| Surgical | 144 (10.3) | 76 (5.3) | 145 (10.8) | |
| Neurological | 100 (7.1) | 68 (4.8) | 125 (9.3) | |
| PACUg | 42 (3.0) | 25 (1.7) | 53 (4.0) | |
| EDh/Floor | 211 (15.0) | 629 (44.2) | 24 (1.8) | |
| SOFAi Score, median (IQR) | 10 (7,12) | 10 (7,12) | 10 (7,12) | 0.53 |
| SOFA, Respiratory Component, n (%) | 0.002 | |||
| 0: P:Fj>= 400 | 226 (18.8) | 158 | 161 (12.0) | |
| 1: P:F <400 | 227 (18.9) | 188 (16.7) | 227 (16.9) | |
| 2: P:F <300 | 308 (25.7) | 297 (26.3) | 378 (28.2) | |
| 3: P:F <200 | 287 (23.9) | 330 (29.3) | 421 (31.4) | |
| 4: P:F <100 | 153 (12.7) | 156 (13.8) | 155 (11.5) | |
| Primary ICUi admission diagnosis category, n (%) | ||||
| Cardiac | 592 (46.5) | 651 (46.4) | 619 (46.5) | |
| Pulmonary | 91 (7.2) | 111 (7.9) | 119 (9.0) | |
| Sepsis or infection | 139 (10.9) | 168 (12.0) | 184 (13.7) | |
| Gastrointestinal | 82 (6.5) | 78 (5.6) | 54 (4.1) | |
| Neurological | 79 (6.2) | 85 (6.1) | 96 (7.2) | |
| Oncology | 76 (6.0) | 72 (5.1) | 70 (5.3) | |
| Endocrine | 17 (1.3) | 17 (1.2) | 17 (1.3) | |
| Hematologic | 5 (0.4) | 4 (0.2) | 3 (0.2) | |
| Musculoskeletal or Rheumotologic | 25 (2.0) | 27 (1.9) | 17 (1.3) | |
| Renal | 8 (0.6) | 7 (0.5) | 6 (0.5) | |
| Injury, poisoning or other external cause | 103 (8.1) | 127 (9.0) | 98 (7.4) | |
| Pregnancy | 3 (0.2) | 6 (0.4) | 4 (0.3) | |
| Other | 52 (4.1) | 52 (3.7) | 42 (3.2) | |
Definition of abbreviation:
Standard deviation
Centimeters
Interquartile range
kilograms
Body mass index
Tidal volume
Post anesthesia care unit
Emergency department
Sequential organ failure assessment
PaO2:FiO2
Intensive Care Unit
Efficacy
Comparing the control cohort to the adoption cohort, the percentage of ordered Vts adherent to LPV increased from 70% to 82% (p <0.001), and the percentage of delivered Vts adherent to LPV increased from 65% to 73% (p <0.001), respectively (Figure 2). After controlling for age, sex, height, SOFA score, and ICU, the adoption cohort had about twice the odds of having an order adherent to LPV (OR 2.4, 95% CI 2.0-3.0) and having LPV delivered (OR 1.9, 95% CI 1.5-2.3) compared to the control cohort (Figure 2, Table E1). Scatter plots of delivered Vts by height show that control cohort Vts are more likely to be in increments of 50 cc (e.g. 400, 450, 500, 550); suggesting that they were estimated without performing a height measurement (Figure 3A,C). Adoption cohort Vts appeared less likely to be in increments of 50cc and corresponded more to the patient’s calculated PBW, especially for ordered Vts (Figure 3B, D). Indeed, 91% versus 34% of ordered Vts in the control versus adoption cohorts were in increments of 50 cc (p <0.001), and 85% versus 81% of delivered Vts in the control versus adoption were in increments of 50cc (p=0.009).
Figure 2: Adherence to lung protective ventilation in delivered and ordered tidal volume.
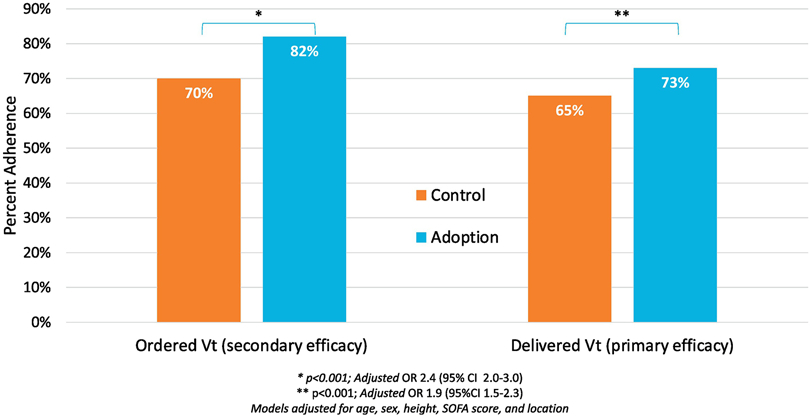
Difference in adherence to lung protective ventilation (LPV) in control and adoption cohorts. Adherence shown in both the ordered tidal volume (Vt) and delivered Vt. Both ordered and delivered models were adjusted from age, sex, height, SOFA score and ICU location and show a significant improvement in adherence between control and adoption cohorts.
Figure 3: Tidal volumes plotted by height in control and adoption cohorts.
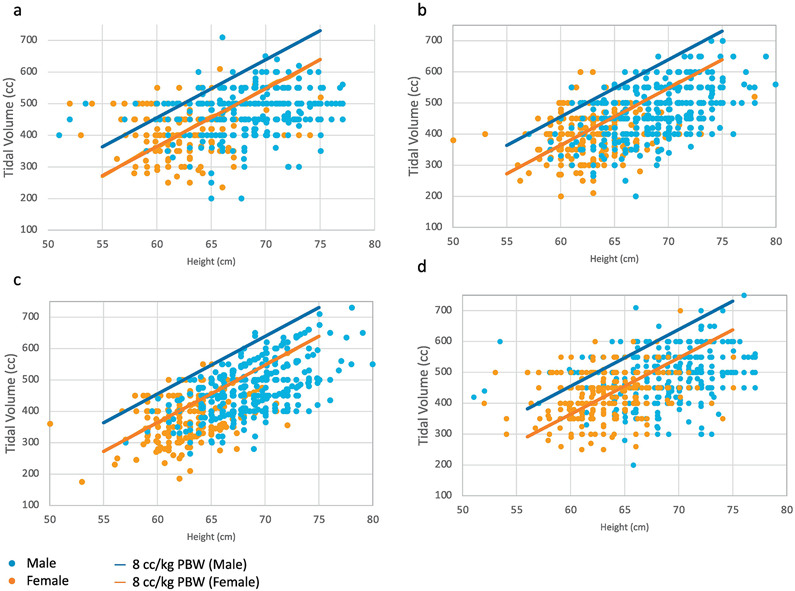
Each individual dot represents the initial tidal volume (Vt) in cubic centimeters (cc) for a unique patient plotted against the patient’s height in centimeters. Women are represented in orange, and men are represented in blue. The orange and blue lines indicate 8cc/kg of predicted body weight (PBW) for women and men respectively. Adherence to lung protective ventilation (LPV) is below these lines. Panel 3a depicts ordered Vt in the control cohort, 3b depicts delivered Vt in the control cohort. Panel 3c depicts ordered Vt in the adoption cohort and 3d depicts delivered Vt in the adoption cohort.
Despite the overall improvement in ordered and delivered LPV across the control and adoption cohorts, an underlying sex disparity in LPV orders and delivery persisted. In the control cohort, women were significantly less likely than men to have ordered Vts adherent to LPV (44% vs 87%, p <0.001), and to have LPV delivered (43% vs. 79% p <0.001) (Figure 4). While the percentage of ordered and delivered Vt adherent to LPV improved in women in the adoption cohort, these were still significantly lower than for men (ordered LPV adherence: 75% vs 87%, delivered: 55% vs. 86%, p-value for both <0.001). Still, ordered LPV adherence improved more significantly in women than in men after the intervention. After the intervention and controlling for age, height, SOFA score, and ICU, women had a fivefold greater odds of having ordered Vts adherent to LPV in the adoption cohort compared to the control cohort (OR: 5.3, 95% CI 3.8-7.3), whereas men showed no difference in the adoption vs. control cohort (OR: 0.98, 95% CI 0.71-1.35) (p-for-interaction <0.001). Ultimately, the relatively greater improvement in ordered Vts adherent to LPV for women in the adoption cohort did not fix the sex-disparity of a lower rate of delivered LPV for women. After controlling for age, height, SOFA score, and ICU, women were no more likely than men to have LPV delivered post-intervention (OR for women: 1.9, 95% CI 1.4-2.6 vs. OR for men: 1.8, 95% CI 1.3-2.5, p-for-interaction = 0.72). In multivariable analyses that include controlling for height, this sex disparity in LPV adherence seen in the adoption cohort became non-significant (OR for ordered Vt adherent to LPV in women vs. men was 0.8 [95% CI 0.6-1.3] and for delivered Vt adherent to LPV was 0.9 [95% CI 0.6-1.3]).
Figure 4: Adherence to lung protective ventilation (LPV) in men and women.
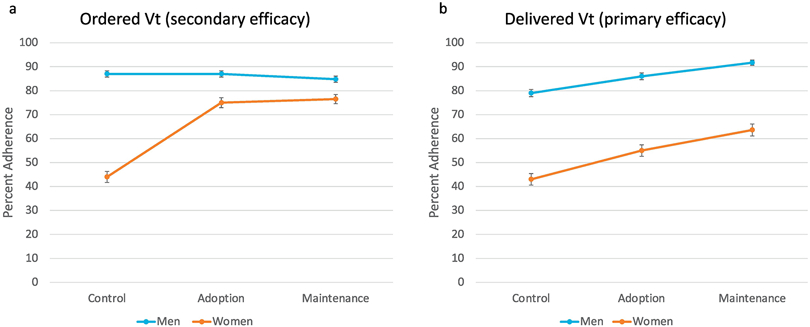
Percentage of men and women adherence to LPV in control, adoption and maintenance cohorts with standard error for each cohort. 4a: Adherence to LPV in ordered Vt. Adherence to LPV in men in each cohort: 86%, 87% and 85% for control, adoption and maintenance respectively; adherence to LPV in women in each cohort: 42%, 73% and 77% in control, adoption and maintenance respectively. 4b: Adherence in men and women to LPV in delivered tidal volume (Vt). Adherence to LPV in men in each cohort: 79%, 86% and 92% for control, adoption and maintenance respectively; adherence to LPV in women in each cohort: 44%, 56% and 64% in control, adoption and maintenance respectively
Post-hoc stratified analyses suggested that the LPV orderset was as effective at improving LPV adherence among those with P:F <300 compared to those with P:F ≥300. Specifically, the adjusted odds of ordered Vts adherent to LPV in the adoption versus control cohorts for patients with P:F <300 and ≥300 were 2.4 (95% CI 1.8-3.1) and 2.7 (95% CI 1.8-4.1), (p-for-interaction=0.75). The adjusted odds of delivered LPV adherence in the adoption versus control cohorts for patients with P:F <300 and ≥300 were 1.9 (95% CI 1.5-2.5) and 1.9 (95% CI 1.2-2.9), (p-for-interaction=0.91).
Adoption
In the adoption cohort, the implemented LPV order was used 83% of the time. The original EMR-based IMV order was used 8% of the time prior to it being removed from the EMR two weeks into the post-implementation period. Nine percent of IMV patients had no IMV order placed, which was most often observed in surgical ICU patients.
Implementation
After implementation of the LPV order, providers used the order as intended by selecting the pre-calculated Vt from the drop-down menu 79% of the time and used the “direct entry” order 21% of the time. Comparing pre-calculated Vt orders to “direct entry” Vt orders, the pre-calculated Vt orders were more often adherent to LPV in both unadjusted and adjusted analyses (83% vs. 73% (p = 0.002), adjusted-OR 2.74, 95% CI 1.8-4.2).
Maintenance
Comparing the adoption cohort to the maintenance cohort, the proportion of ordered Vts adherent to LPV remained the same at 81%, and the proportion of delivered Vt adherent to LPV increased from 73% to 81% (p<0.0001). The improvement in delivered LPV during the maintenance phase occurred in both women and men, but LPV adherence in women still remained significantly worse than in men (women: 56% to 64% [p <0.001], men: 86% to 92% [p <0.001]).
Focus Group Findings
We conducted five focus groups with a total of 19 participants from the ED, medical, surgical, cardiac, and cardiothoracic ICUs. Participants included 13 internal medicine, emergency medicine, and anesthesia residents, two cardiac ICU nurse practitioners, one cardiac ICU physician assistant, and three medical ICU nurse practitioners. Regarding barriers to efficacy, providers raised concerns that respiratory therapists who set the Vt on the ventilator do not have time to check the ventilator order in the EMR for a newly mechanically ventilated patient who is clinically unstable. Regarding barriers to adoption, providers reported that while the LPV order was easy to use, some were reluctant to use it because they believed LPV would not confer a clinical benefit to their non-ARDS patients. Regarding barriers to implementation, providers reported (1) a preference to calculate LPV independently; (2) a preference to have an ordered Vt match the delivered Vt when mechanical ventilation was urgently initiated prior to an order being placed; (3) difficulties in obtaining accurate height measurements; and 4) mistrust of the pre-calculated Vt in the order tool (Table E2).
DISCUSSION
This study of over 4,000 patients in ICUs of multiple specialties is the first to apply the RE-AIM framework to evaluate the implementation of an LPV order. After implementation, mechanically ventilated adult patients had twice the adjusted odds of having ordered and delivered Vts adherent to LPV, and LPV adherence increased from 65% to 73% to 81% across the cohorts. Our intervention increased ordered Vts adherent to LPV significantly more in women than in men. However, women still remained about 30% less likely than men to have LPV delivered. Our findings show that an LPV order can significantly and sustainably improve LPV adherence. However, to maximize LPV adherence, clinical education interventions are needed to ensure that accurate height measurements are obtained, to counter misconceptions about LPV, and to increase awareness that women are at higher risk for receiving high tidal volumes during mechanical ventilation.
The RE-AIM framework was developed to provide a stepwise approach to evaluating health behavior interventions, with the goal of elucidating program elements that can improve the sustainable adoption and implementation of effective, generalizable, evidence-based interventions (25, 35). Our RE-AIM analysis revealed several barriers to the adoption of our LPV order and to the efficacy of LPV delivery at our medical center. In order to maximize adoption of the LPV order, we developed the order following the format shown to be preferred by critical care providers (36). We created a default setting for Vt based on the entered height, but also allowed providers to choose an alternative Vt. We discovered that this override option led to lower LPV adherence, as directly entered Vts were less likely to be LPV-adherent than Vts that were selected based on patients’ heights. Providers in our focus group sessions identified barriers to LPV adherence that have been reported previously, including patient comfort, perceived lack of benefit, and under recognition of clinical guidelines (4, 17, 18, 37). In addition, providers identified novel barriers to LPV implementation, including mistrust of the entered height or calculated Vt, and desire for the ordered Vt to match the set Vt that the patient was already receiving. Ultimately, the RE-AIM analytic approach enabled us to systematically identify barriers to LPV adoption and implementation that were related to the EMR-based order itself, provider misconceptions about LPV and a lack of proper communication with nurses regarding height measurement and with respiratory therapists regarding Vts that were set on the ventilator.
Prior interventions to improve LPV adherence, including CDS tools, provider education and provider feedback have not shown consistent or long-lasting improvement in LPV adherence (5, 15, 22-24). Furthermore, these studies have not addressed how to best modify existing LPV initiatives to remove barriers and improve LPV delivery. Our RE-AIM analysis suggests that in order to maximize and sustain LPV delivery, implementation of an LPV order with an imbedded CDS tool needs to be combined with educational interventions for the multi-disciplinary team of care providers. Since delivery of a Vt beyond the point of EMR order entry involves nursing and respiratory therapy, a successful educational intervention will include physicians, physician assistants, nurse practitioners, nurses, and respiratory therapists (38). Additionally, our analysis suggests ways to improve the LPV order itself. We should remove the option for directly entering a Vt and require Vt to be entered in cc/kg PBW so that we shift our thinking towards setting the Vt to the height-based PBW. Educational interventions should not only seek to correct misconceptions about LPV benefits but identify ways to ensure that an accurate supine height measurement is made, and that the Vt set on the ventilator matches an LPV-adherent Vt order.
Any educational intervention aimed at improving LPV delivery should also teach about patients who are less likely to receive LPV. Prior work shows that short people are less likely to receive LPV (16). We found in the control cohort that women were 36% less likely to receive LPV than men. While women had a five-fold greater odds than men of having ordered Vts adherent to LPV after implementation of the LPV order, this did not fix the underlying sex-disparity. Women were still about 30% less likely to receive LPV than men in the adoption cohort. Since about one fifth of Vts ordered were directly entered by providers, and since there appeared to be a default to Vts of 400, 450 and 500 cc based on our dot-plot analyses, we suspect that the manual entry of Vt orders may be a major reason as to why there was less LPV adherence in women, who are on average shorter than men. Similar to prior work (16, 39), we found that the sex-disparity in LPV adherence became statistically non-significant after adjusting for height, which underscores the importance of obtaining an accurate height measurement, particularly in women.
Our study has limitations. The observed associations could be effected by residual confounding. However, we controlled for age, sex, height, severity of illness, and ICU location. This study is limited in its generalizability because it was performed at a single center. However, it has a large sample size from multiple types of ICUs over three years. We relied on the patient height recorded in the EMR, which may have been measured by nurses, reported by family members, or estimated by providers. Future studies aimed at improving LPV delivery should investigate how to accurately and reliably measure height in supine critically ill patients. The presence of ARDS was not captured in this dataset nor in the order, but rather was decided upon by the ordering provider, which may have led to some misclassification of ARDS (14) and limited our ability to discover any differential effect of the intervention on patients with and without ARDS. However, the Berlin Criteria were listed in the LPV order to minimize such misclassification (30). The LPV order did not require plateau pressures to be entered. Therefore, some patients may have had Vts of 4-8 cc/kg PBW ordered and delivered, but still did not meet LPV based on a plateau pressure threshold <30 cm H2O (40). To minimize the possibility of plateau pressures >30 cm H2O (40) in ARDS patients, the LPV order recommended an initial Vt of 6cc/kg PBW when the provider indicated that the patient had ARDS. Minimizing driving pressure is another important aspect of LPV that was not measured in the EMR data that was used to conduct this study. Future iterations of the CDS should consider algorithms that help providers minimize driving pressure. Lastly, our focus groups did not include attending physicians. However, in ICUs at our medical center, IMV orders are placed by nurse practitioners, physician assistants, residents, or fellows, and not by attendings.
CONCLUSIONS
We found that implementation of an LPV order can significantly improve adherence to LPV in a sustainable way in patients across a variety of critical care settings. Our RE-AIM analysis suggests that in order to maximize LPV adherence with implementation of an LPV order, educational interventions should be simultaneously implemented that teach the importance of obtaining accurate height measurement, address LPV misconceptions and sex-disparities, and encourage communication with nurses and respiratory therapists.
Supplementary Material
Figure E1: Example of electronic medical record-based lung protective ventilation order.
Table E1: Odds ratios for adherence to lung protective ventilation for demographic and clinical covariables in the control and adoption cohorts combined.
Table E2: Focus group results
Highlights.
Women are 30% less likely than men to receive lung protective ventilation
REAIM framework allowed a better understandings of barriers to implementation
An electronic clinical decision support tool improves lung protective ventilation
Acknowledgments
Funding: This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040. MRB is supported by K23 AG045560 and UL TR001873 . The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest: There are no conflicts of interest or competing interest regarding this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Acute Respiratory Distress Syndrome N, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 2.Needham DM, Yang T, Dinglas VD, Mendez-Tellez PA, Shanholtz C, Sevransky JE, Brower RG, Pronovost PJ, Colantuoni E. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med 2015; 191: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant JY, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin JE, Pereira B, Jaber S, Group IS. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013; 369: 428–437. [DOI] [PubMed] [Google Scholar]
- 4.Neto AS, Simonis FD, Barbas CS, Biehl M, Determann RM, Elmer J, Friedman G, Gajic O, Goldstein JN, Linko R, Pinheiro de Oliveira R, Sundar S, Talmor D, Wolthuis EK, Gama de Abreu M, Pelosi P, Schultz MJ, Investigators PRVN. Lung-Protective Ventilation With Low Tidal Volumes and the Occurrence of Pulmonary Complications in Patients Without Acute Respiratory Distress Syndrome: A Systematic Review and Individual Patient Data Analysis. Crit Care Med 2015; 43: 2155–2163. [DOI] [PubMed] [Google Scholar]
- 5.Bagga S, Paluzzi DE, Chen CY, Riggio JM, Nagaraja M, Marik PE, Baram M. Better ventilator settings using a computerized clinical tool. Respir Care 2014; 59: 1172–1177. [DOI] [PubMed] [Google Scholar]
- 6.Fuller BM, Mohr NM, Drewry AM, Carpenter CR. Lower tidal volume at initiation of mechanical ventilation may reduce progression to acute respiratory distress syndrome: a systematic review. Crit Care 2013; 17: R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinert CR, Gross CR, Marinelli WA. Impact of randomized trial results on acute lung injury ventilator therapy in teaching hospitals. Am J Respir Crit Care Med 2003; 167: 1304–1309. [DOI] [PubMed] [Google Scholar]
- 8.investigators LV. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS - an observational study in 29 countries. Eur J Anaesthesiol 2017; 34: 492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, Damasceno MC, Schultz MJ. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012; 308: 1651–1659. [DOI] [PubMed] [Google Scholar]
- 10.Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F, Hofstra JJ, de Graaff MJ, Korevaar JC, Schultz MJ. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care 2010; 14: R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu WJ, Wang F, Liu JC. Effect of lung-protective ventilation with lower tidal volumes on clinical outcomes among patients undergoing surgery: a meta-analysis of randomized controlled trials. CMAJ 2015; 187: E101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Writing Group for the PI, Simonis FD, Serpa Neto A, Binnekade JM, Braber A, Bruin KCM, Determann RM, Goekoop GJ, Heidt J, Horn J, Innemee G, de Jonge E, Juffermans NP, Spronk PE, Steuten LM, Tuinman PR,de Wilde RBP, Vriends M, Gama de Abreu M, Pelosi P, Schultz MJ. Effect of a Low vs Intermediate Tidal Volume Strategy on Ventilator-Free Days in Intensive Care Unit Patients Without ARDS: A Randomized Clinical Trial. JAMA 2018; 320: 1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunkhorst FM, Engel C, Ragaller M, Welte T, Rossaint R, Gerlach H, Mayer K, John S, Stuber F, Weiler N, Oppert M, Moerer O, Bogatsch H, Reinhart K, Loeffler M, Hartog C, German Sepsis Competence N. Practice and perception--a nationwide survey of therapy habits in sepsis. Crit Care Med 2008; 36: 2719–2725. [DOI] [PubMed] [Google Scholar]
- 14.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, Investigators LS, Group ET. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016; 315: 788–800. [DOI] [PubMed] [Google Scholar]
- 15.Kalhan R, Mikkelsen M, Dedhiya P, Christie J, Gaughan C, Lanken PN, Finkel B, Gallop R, Fuchs BD. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med 2006; 34: 300–306. [DOI] [PubMed] [Google Scholar]
- 16.Han S, Martin GS, Maloney JP, Shanholtz C, Barnes KC, Murray S, Sevransky JE. Short women with severe sepsis-related acute lung injury receive lung protective ventilation less frequently: an observational cohort study. Crit Care 2011; 15: R262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss CH, Baker DW, Tulas K, Weiner S, Bechel M, Rademaker A, Fought A, Wunderink RG, Persell SD. A Critical Care Clinician Survey Comparing Attitudes and Perceived Barriers to Low Tidal Volume Ventilation with Actual Practice. Ann Am Thorac Soc 2017; 14: 1682–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umoh NJ, Fan E, Mendez-Tellez PA, Sevransky JE, Dennison CR, Shanholtz C, Pronovost PJ, Needham DM. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med 2008; 36: 1463–1468. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005; 330: 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, Sam J, Haynes RB. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005; 293: 1223–1238. [DOI] [PubMed] [Google Scholar]
- 21.Eslami S, de Keizer NF, Abu-Hanna A, de Jonge E, Schultz MJ. Effect of a clinical decision support system on adherence to a lower tidal volume mechanical ventilation strategy. J Crit Care 2009; 24: 523–529. [DOI] [PubMed] [Google Scholar]
- 22.Eslami S, Abu-Hanna A, Schultz MJ, de Jonge E, de Keizer NF. Evaluation of consulting and critiquing decision support systems: effect on adherence to a lower tidal volume mechanical ventilation strategy. J Crit Care 2012; 27: 425 e421–428. [DOI] [PubMed] [Google Scholar]
- 23.Bourdeaux CP, Thomas MJ, Gould TH, Malhotra G, Jarvstad A, Jones T, Gilchrist ID. Increasing compliance with low tidal volume ventilation in the ICU with two nudge-based interventions: evaluation through intervention time-series analyses. BMJ Open 2016; 6: e010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castellanos I, Martin M, Kraus S, Burkle T, Prokosch HU, Schuttler J, Toddenroth D. Effects of staff training and electronic event monitoring on long-term adherence to lung-protective ventilation recommendations. J Crit Care 2018; 43: 13–20. [DOI] [PubMed] [Google Scholar]
- 25.Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health 2013; 103: e38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palinkas LA, Aarons GA, Horwitz S, Chamberlain P, Hurlburt M, Landsverk J. Mixed method designs in implementation research. Adm Policy Ment Health 2011; 38: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss CH, Krishnan JA, Au DH, Bender BG, Carson SS, Cattamanchi A, Cloutier MM, Cooke CR, Erickson K, George M, Gerald JK, Gerald LB, Goss CH, Gould MK, Hyzy R, Kahn JM, Mittman BS, Moseson EM, Mularski RA, Parthasarathy S, Patel SR, Rand CS, Redeker NS, Reiss TF, Riekert KA, Rubenfeld GD, Tate JA, Wilson KC, Thomson CC, Science ATSAHCoI. An Official American Thoracic Society Research Statement: Implementation Science in Pulmonary, Critical Care, and Sleep Medicine. Am J Respir Crit Care Med 2016; 194: 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forman J, Heisler M, Damschroder LJ, Kaselitz E, Kerr EA. Development and application of the RE-AIM QuEST mixed methods framework for program evaluation. Prev Med Rep 2017; 6: 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glasgow RE, Askew S, Purcell P, Levine E, Warner ET, Stange KC, Colditz GA, Bennett GG. Use of RE-AIM to Address Health Inequities: Application in a low-income community health center based weight loss and hypertension self-management program. Transl Behav Med 2013; 3: 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 31.Onwuegbuzie AJ DW, Leech NL, Zoran AG. A qualitative framework for collecting and analyzing data in focus group research. Int J Qual Methods 2009; 8: 1–21. [Google Scholar]
- 32.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999; 89: 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowen ME, Dusseau DJ, Toth BG, Guisinger C, Zodet MW, Shyr Y. Casemix adjustment of managed care claims data using the clinical classification for health policy research method. Med Care 1998; 36: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 34.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 35.King DK, Glasgow RE, Leeman-Castillo B. Reaiming RE-AIM: using the model to plan, implement, and evaluate the effects of environmental change approaches to enhancing population health. Am J Public Health 2010; 100: 2076–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta M, Veith J, Szymanski S, Madden V, Hart JL, Kerlin MP. Clinicians' Perceptions of Behavioral Economic Strategies to Increase the Use of Lung-Protective Ventilation. Ann Am Thorac Soc 2019; 16: 1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med 2004; 32: 1289–1293. [DOI] [PubMed] [Google Scholar]
- 38.Radosevich MA, Wanta BT, Meyer TJ, Weber VW, Brown DR, Smischney NJ, Diedrich DA. Implementation of a Goal-Directed Mechanical Ventilation Order Set Driven by Respiratory Therapists Improves Compliance With Best Practices for Mechanical Ventilation. J Intensive Care Med 2019; 34: 550–556. [DOI] [PubMed] [Google Scholar]
- 39.Walkey AJ, Wiener RS. Risk factors for underuse of lung-protective ventilation in acute lung injury. J Crit Care 2012; 27: 323.e321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Artis KA, Dweik RA, Patel B, Weiss CH, Wilson KC, Gagliardi AR, Huckson S, Nothacker M, Adhikari NKJ, Kajdacsy-Balla Amaral AC, Barbash IJ, Carlos WG, Costa DK, Metersky ML, Mularski RA, Sjoding MW, Thomson CC, Hyzy RC. Performance Measure Development, Use, and Measurement of Effectiveness Using the Guideline on Mechanical Ventilation in Acute Respiratory Distress Syndrome. An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc 2019; 16: 1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1: Example of electronic medical record-based lung protective ventilation order.
Table E1: Odds ratios for adherence to lung protective ventilation for demographic and clinical covariables in the control and adoption cohorts combined.
Table E2: Focus group results


