Abstract
Timeliness of vaccinations is rarely part of monitoring in a routine immunization program. We reviewed infant immunization and conducted caregiver interviews in three regions in the Philippines from January to October 2016. We randomly selected thirty public health centers, one for each region. We defined timeliness of the receipt of antigen as within 4 weeks after the recommended age at vaccination. We assessed a total of 986 infants for timeliness of vaccination. The median age of receipt of vaccine was at 2.7 weeks (BCG), 10.1 weeks (Penta 1), and 21.7 weeks (Penta 3) compared to the recommended 0, 6, and 14 weeks of age, respectively. We found timely receipt only in 74.4% for BCG, 70.3% for Penta 1, and 39.1% for Penta 3 recipients. Thus, alongside declining immunization coverage, the infants in the Philippines had substantial delays in vaccine receipt.
Electronic supplementary material
The online version of this article (10.1057/s41271-020-00255-w) contains supplementary material, which is available to authorized users.
Keywords: Immunization, Routine immunization, Immunization program, Immunization schedule, Philippines
Introduction
Immunization is one of the most cost-effective public health interventions. According to the World Health Organization (WHO), immunization prevents between 2 and 3 million deaths every year from diphtheria, tetanus, pertussis (whooping cough), and measles [1, 2]. The WHO established the Expanded Program on Immunization (EPI) in 1974 to reduce morbidity and mortality by improving vaccine availability globally. The program initially targeted six diseases: diphtheria, pertussis, tetanus, measles, poliomyelitis, and tuberculosis [2]. Subsequently, EPI introduced newer vaccines into the program to lessen the burden of other vaccine-preventable diseases caused by hepatitis B virus, Haemophilus influenza type b (Hib), and Streptococcus pneumoniae. The EPI includes policies on evidence-based recommended vaccine schedules [2]. These schedules contain the age at which the child is most vulnerable to the disease and at which the vaccine can be given safely to achieve adequate protection.
Monitoring of immunization program performance has typically been assessed through measurements of vaccine coverage (the proportion of individuals who have received a vaccine by a benchmark age) regardless of the timing of administration [3, 4]. These do not take into account the timeliness, which detects adherence to vaccination schedules. Delays in immunization have potentially serious consequences. First, children with delayed vaccination will be unprotected from the vaccine-preventable diseases at a time when they are most at risk [5]. Second, substantial delays may contribute to diminished herd immunity or the indirect protection received by the unimmunized population when a large proportion is immunized [5]. Third, previous studies have demonstrated the association of delayed vaccination with increased risk of pertussis, measles, and Haemophilus influenzae type b infections and outbreaks [5–10]. Fourth, delays increase the risk of failing to achieve full immunization of the child [5, 11, 12]. Thus, adherence to immunization schedules or timeliness of vaccination is also an important indicator of a successful national immunization program aside from vaccine coverage.
In the Philippines, only one published study examined the timeliness of immunization [13]. The Philippines EPI had also been monitoring program implementation using vaccine coverage rates. In this study, we explored the timeliness of infant immunization and assess factors that may be associated with delayed vaccination using immunization records review and caregiver interviews.
Methods
Study setting and population
The Philippines is an archipelago with three major island groups (Luzon, Visayas, and Mindanao). The healthcare system is divided into 17 autonomous regional health offices charged with implementation of the immunization program. The country follows the recommended schedule by the Expanded Programme of Immunization by the World Health Organization (WHO).
We analyzed the immunization records and caregiver interviews from a study conducted on the introduction of inactivated poliovirus vaccine (IPV) in the Philippines [14]. The study collected immunization data on the following vaccines: Bacillus Calmette-Guérin vaccine (BCG) birth dose, Hepatitis B vaccine (HepB) birth dose, pentavalent vaccine series (includes vaccines for diphtheria, whole-cell pertussis, HepB, and Hib, referred to as Penta in this study), pneumococcal conjugate vaccine (13-valent) (PCV13) series, oral polio vaccine (OPV) series, and inactivated poliovirus vaccine (IPV). Because the immunization records did not contain any sociodemographic variables about the infants, we supplemented data collection with a structured interview and investigated factors affecting vaccine timeliness.
We conducted the study from January to October 2016 in three regions, representing the three major island groups in the country: Luzon (Region 3), the Visayas (Region 6), and Mindanao (Region 10) (Fig. 1). The Philippine Department of Health selected these three regions for pilot implementation of IPV introduction. Region 3 (Central Luzon Region) has a population of 11,124,400, with an infant population (< 1-year-old) of 300,300. It has 119 public health centers (PHCs) that serve the 7 provinces and 2 cities. Region 6 (Western Visayas Region) has a total population of 8,317,800, with an infant population of 224,600. It has 147 PHCs that serve the 6 provinces and 2 cities. Region 10 (Northern Mindanao Region) has a total population of 4,799,700, with an infant population of 129,600. It has 122 PHCs that serve the five provinces and 2 cities.
Fig. 1.
Location of the three study sites (Region 3 Central Luzon, Region 6 Western Visayas, and Region 10 Northern Mindanao)
Field data collection
We calculated the sample size of PHCs for each region to test whether there was a 5% or greater decrease in the proportion of eligible infants receiving all the recommended injectable vaccines with the PHCs as the primary unit of analysis. We determined 29 PHCs are needed to attain a power of 81%. We selected 30 PHCs by simple random sampling from a list in each region.
We deployed field staff already trained for interviews, data collection, and data entry in accordance with the guidelines set by the study team. We carried out additional training for interview conduct in vernacular language to maximize comprehension by interview respondents. We scheduled our staff to visit a PHC on an immunization day for data collection. An immunization day is a specific day within the week when infants received their immunizations in a PHC.
For each PHC, we prospectively reviewed the records of at least 10 infants at the site on an immunization day. If fewer than 10 infants came that day, a staff member made a return visit to comply with the minimum number. We interviewed 5 caregivers selected through convenience sampling on each immunization day in a PHC. We determined that caregivers would be eligible for interviews if ≥ 18 years and if she or he brought an infant for that child’s 14-week visit. If the study team did not achieve 5 interviews, a member of the staff had to make a return visit until having completed the minimum number. We obtained Informed consent from the caregiver. We conducted standardized interviews in an isolated place to ensure the confidentiality of the responses by the caregivers. Field staff asked the questions in vernacular language—a native dialect or form of speech of specific people or a specific region—and responses were recorded and classified for data analysis.
We collected the data using password-protected Android tablets with a preinstalled application developed for the study. The study team tabulated the answers in real time and sent them to a secured server at the study institution. We conducted data quality checks in real time to ensure accurate data entry in the application. We provided immediate feedback for any data inconsistencies and resolved these issues within the day of the visit.
The previous study focused on the introduction of IPV given at the 14th week visit and hence had data limited up to that visit only. In addition, infants may have received different vaccine dose numbers in those given in a series at that visit, depending on caregiver or healthcare provider preference or availability of vaccines during the previous visits. For example, an infant may have received Penta 1, PCV13 2, OPV 3, and IPV at this visit if s/he had not yet received these vaccines. One year after the survey, in September 2017, the study staff revisited the PHCs to gather vaccination information on the enrolled infants from the immunization records retrospectively. The PHC revisit determined whether these infants received all the recommended vaccines. We merged these data with the original dataset from the previous study to determine the timeliness of vaccination.
Definition of timely receipt and timely completion of vaccination
In this study, we used the recommended age of vaccine receipt as defined by the Philippine EPI. For infants < 6 months old, the EPI recommends these doses at birth (BCG and HepB) and visits at 6 weeks of age (Penta/PCV13/OPV dose 1), at 10 weeks (Penta/PCV13/OPV dose 2), and at 14 weeks (Penta/PCV13/OPV dose 3 and IPV). Based on this, we defined timely receipt for each vaccine, as the receipt of the vaccine within 4 weeks after the recommended age, and we defined ‘delays’ as receipt of the recommended dose for > 4 weeks after the recommended age. Thus, we set age cut-off for delays at 4 weeks for BCG and HepB (recommended at birth), 10 weeks for Penta/PCV13/OPV dose 1 (recommended at 6 weeks), 14 weeks for Penta/PCV13/OPV dose 2 (recommended at 10 weeks), and 18 weeks for Penta/PCV13/OPV dose 3 and IPV (recommended at 14 weeks).
We defined ‘complete vaccination’ as an infant receiving all the vaccines included in the study before 6 months of age. This is an arbitrary cut-off in most immunization programs. Since maternal antibodies in an infant disappear at 6 months, most programs aim to complete the primary infant series before this age.
Data analysis
We summarized the baseline characteristics of the study population by frequency and proportion for the categorical variables. We computed for the age of receipt of vaccine for each child (expressed in weeks) by using their respective birthdate and corresponding dates of vaccination. We compared the mean age of receipt to the recommended schedule in the EPI using one-sample t test. Also, we used time-to-event analysis to determine the proportion of children having received the vaccination at each time point, as previously described in a study [15, 16]. To assess timely completion, we determined the number of infants who received all the vaccines (BCG, HepB, Penta 1, and Penta 3) on or before 6 months of age. We used logistic regression to compute the odds ratio on the timely completion and sociodemographic determinant. The sociodemographic variables included were caregiver sex (male or female), relationship to the infant, age group, number of children, and level of education. We also used the frequency of immunization days of PHCs as an additional variable in the logistic regression. As a sensitivity analysis, we performed multilevel logistic regression to determine if clustering of data had occurred because of the enrollment by regions. For statistical analysis, we used STATA 15.1 (STATA Corp, Texas, US) with two-tailed tests, and considered a P value < 0.05 significant.
Ethical consideration
Ethical clearance was obtained from the University of the Philippines Manila Research Ethics Board (UPM-REB-2015-349-01) and the WHO Regional Office of the Western Pacific Ethical Review Committee (2015.25.PHL.5.EPI) prior to the study conduct.
Results
Study population and baseline demographic data
We assessed a total of 986 infant records to determine the timeliness of vaccination, of which 465 (47.2%) had been paired with interview data (sociodemographic factors). There was an equal distribution of infants across the different regions: 328 infants (33.3%) were from Region 3, 316 infants (32.0%) from Region 6, and 342 infants (34.7%) from Region 10. Most of the infant caregivers were females (97.8%) belonging to the 25–34 age group (46.5%) and the majority were parents of the infants (88.2). Most had at least attended secondary school (92.5%). More than half of the PHCs give routine immunizations to infants on a weekly basis (Table 1).
Table 1.
Demographic data of infants and infant caregivers, and frequency of immunization in the RHUs included in the study
| Characteristics | n (%) |
|---|---|
| Number of infants included | |
| Overall | 986 |
| Region 3 | 328 (33.3) |
| Region 6 | 316 (32.0) |
| Region 10 | 342 (34.7) |
| Infant caregiver data | |
| Caregivers interviewed | 465 (47.2) |
| Sex | |
| Male | 10 (2.2) |
| Female | 455 (97.8) |
| Age | |
| 18–24 years | 159 (34.2) |
| 25–34 years | 216 (46.5) |
| 35 years and above | 90 (19.4) |
| Relationship to infant | |
| Parents | 410 (88.2) |
| Othersa | 55 (11.8) |
| Number of childrenb | |
| 0–2 children | 291 (62.6) |
| 3–4 children | 122 (26.8) |
| 5 or more children | 52 (11.4) |
| Level of education | |
| Attended primary/never attended school | 35 (7.5) |
| Attended secondary school | 246 (52.9) |
| Attended post-secondary school | 184 (39.6) |
| Frequency of immunization in the PHCc | |
| Weekly | 301 (64.7) |
| 2–3 times per month | 30 (6.5) |
| Once a month | 134 (28.8) |
PHC public health center
aThese include the grandparent, aunt, uncle, and other caregivers
bOnly female caregivers were asked regarding number of children in the household
cReflects the frequency in infants whose caregivers were interviewed
Proportion of infants vaccinated and mean age of receipt of vaccines
The vaccine coverage rates and mean age of vaccine receipt overall and per region are shown in Table 2. At the end of follow-up, all infants received Penta 1, all OPV dose series, and IPV. The proportion of vaccinated infants exceeded 95% for the rest of the vaccines except HepB, at 88%. This was true for all regions excluding Region VI, where HepB coverage exceeded 95%. The mean ages of receipt of vaccines overall were beyond the accepted range of timely receipt in the study except for BCG, HepB, and OPV 1; a similar trend was observed in all 3 regions except for Penta 1 in Regions 3 and 6. All mean values were significantly different compared to the recommended age of vaccine receipt in the Philippine EPI (Table 2).
Table 2.
Vaccination schedule and mean age of receipt among infants
| Vaccine | Recommended age of vaccine receipt in the Philippine EPI | Overalla N = 986 |
Region IIIa N = 328 |
Region VIa N = 316 |
Region Xa N = 342 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Proportion vaccinated at follow-up, % | Mean age at receipt, weeks (SD) | Proportion vaccinated at follow-up, % | Mean age at receipt, weeks (SD) | Proportion vaccinated at follow-up, % | Mean age at receipt, weeks (SD) | Proportion vaccinated at follow-up, % | Mean age at receipt, weeks (SD) | ||
| BCG birth dose | At birth | 99.8 | 2.7 (4.2) | 100.0 | 2.6 (4.1) | 99.7 | 1.8 (3.1) | 99.7 | 3.7 (5.0) |
| HepB birth dose | At birth | 88.1 | 0.8 (3.0) | 89.0 | 1.2 (2.7) | 96.5 | 0.4 (3.2) | 79.5 | 1.0 (3.2) |
| Penta 1 | 6 weeks | 100.0 | 10.1 (5.0) | 100.0 | 9.6 (4.4) | 100.0 | 9.9 (5.3) | 100.0 | 10.6 (5.3) |
| Penta 2 | 10 weeks | 99.9 | 15.8 (6.3) | 100.0 | 14.8 (4.7) | 100.0 | 15.7 (6.4) | 99.7 | 16.9 (7.2) |
| Penta 3 | 14 weeks | 99.0 | 21.7 (7.7) | 99.1 | 20.1 (5.6) | 100.0 | 22.5 (8.1) | 98.0 | 22.4 (8.6) |
| PCV13 1 | 6 weeks | 99.3 | 10.5 (5.3) | 98.5 | 10.0 (4.8) | 100.0 | 10.7 (5.6) | 99.4 | 10.7 (5.4) |
| PCV13 2 | 10 weeks | 99.0 | 16.4 (6.6) | 98.2 | 15.1 (4.9) | 100.0 | 17.3 (7.6) | 98.3 | 16.9 (6.9) |
| PCV13 3 | 14 weeks | 97.8 | 22.5 (7.6) | 97.9 | 20.2 (5.3) | 99.4 | 24.2 (9.0) | 96.2 | 23.1 (7.5) |
| OPV 1 | 6 weeks | 100.0 | 9.1 (3.6) | 100.0 | 9.0 (3.5) | 100.0 | 8.9 (3.7) | 100.0 | 9.4 (3.7) |
| OPV 2 | 10 weeks | 100.0 | 14.7 (4.8) | 100.0 | 14.2 (3.9) | 100.0 | 14.6 (4.5) | 100.0 | 15.4 (5.6) |
| OPV 3 | 14 weeks | 100.0 | 20.7 (6.1) | 100.0 | 19.4 (4.4) | 100.0 | 21.2 (6.6) | 100.0 | 21.4 (6.7) |
| IPV | 14 weeks | 100.0 | 21.0 (6.4) | 100.0 | 19.6 (4.5) | 100.0 | 21.6 (7.2) | 100.0 | 21.7 (7.0) |
BCG Bacillus Calmette-Guérin, HepB Hepatitis B vaccine, IPV inactivated poliovirus vaccine, OPV oral poliovirus vaccine, PCV13 pneumococcal conjugate vaccine (13-valent), SD standard deviation
aOne sample t test using the recommended age of receipt as fixed value showed a P value of < 0.05 for all vaccines
The proportion of infants who received the vaccines at the recommended age tended to decrease with vaccine doses in a series given at a later age, as shown in the Kaplan–Meier plots (Fig. 2). At birth, only 28.1% and 62.5% of infants received BCG and HepB birth doses, with a median age of receipt of 2.7 and 0 weeks, respectively. In the primary infant series, only 3.7% of the children received Penta 1 at 6 weeks, with a median age of the receipt of 8.9 weeks of age and a delay of 2.9 weeks from the recommended age. Similarly, the proportion of children who received a timely dose of Penta 3 was smaller at 0.5%. The median age of the population that received Penta 3 was 19.7 weeks, with a delay of 5.7 weeks from the recommendation (Fig. 2a). We observed a similar trend for the other vaccine series: only 3.3% and 3.9% of infants received PCV13 1 and OPV 1 at 6 weeks, with a median delay of 3.0 and 2.4 weeks, respectively; and only 0.4% and 0.5% received PCV13 3 and IPV at 14 weeks, with a median delayed receipt of 7.7 and 5.6 weeks, respectively (Fig. 2b, c).
Fig. 2.
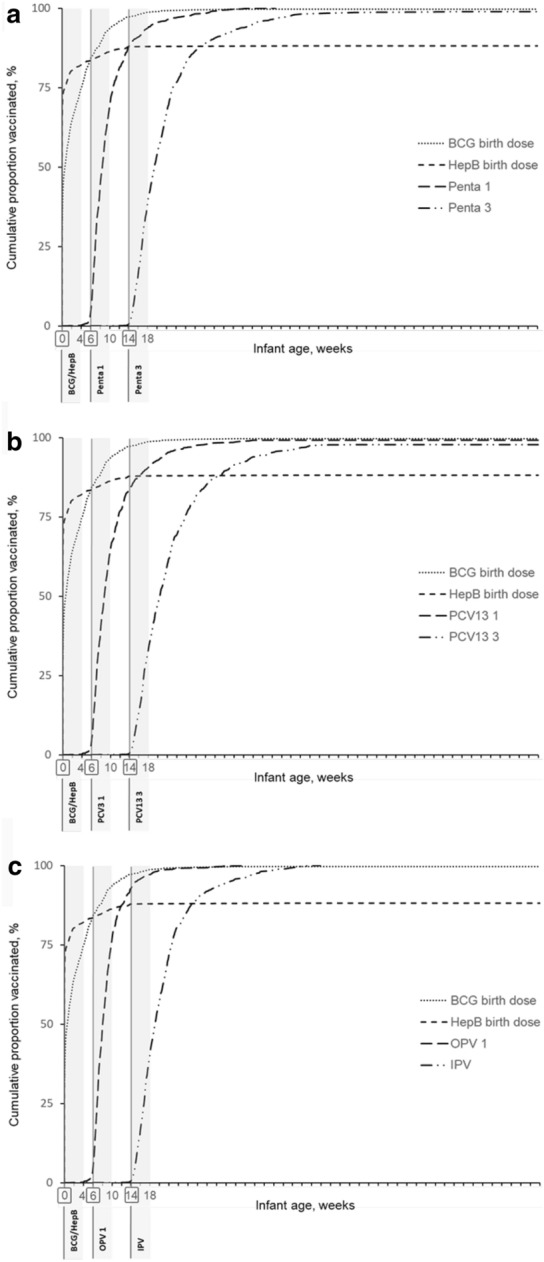
Cumulative proportions of infants receiving vaccines by age in weeks. Data grouped as cumulative proportion by a BCG, Hepatitis B, Penta 1, and Penta 3, b BCG, Hepatitis B, PCV13 1, and PCV13 3, and c BCG, Hepatitis B, OPV 1, and IPV. Gray vertical line and boxed infant age (weeks) correspond to the recommended age of vaccine receipt. Gray area corresponds to the timely receipt as defined in this study (+ 4 weeks from recommended age). BCG, Bacillus Calmette-Guérin; HepB, Hepatitis B vaccine; IPV, inactivated poliovirus vaccine; OPV, oral poliovirus vaccine; PCV13, pneumococcal conjugate vaccine (13-valent); Penta, Pentavalent vaccine
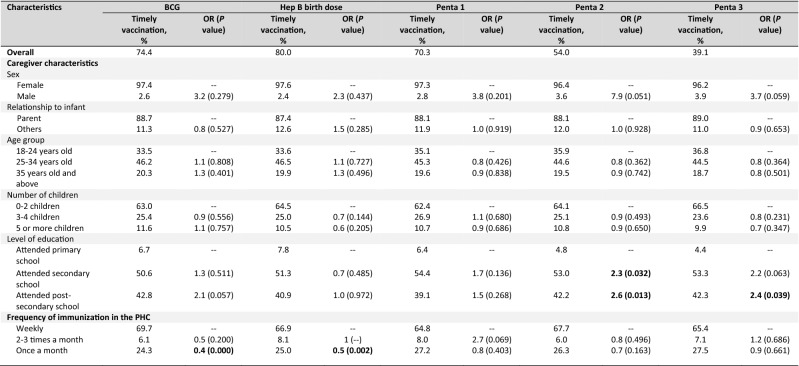
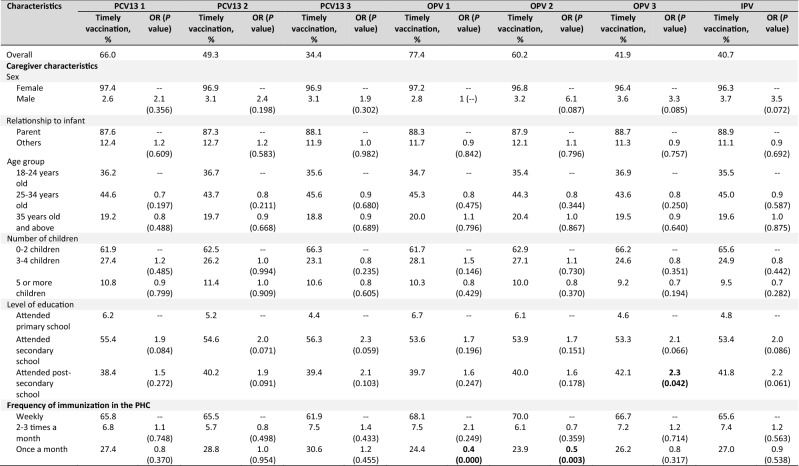
Timely receipt of vaccines and factors for delay in receipt of vaccines
Overall, the proportion of infants who received timely vaccination was low and below 90% for all vaccines analyzed (Tables 3). Similarly, this decreases with vaccine doses given in a series at a later age. Among the factors analyzed, a higher level of education and more frequent immunization schedule in the PHCs were associated with timely receipt of some of the vaccines. Infants of caregivers who attended secondary school were twice more likely to have infants in their care receive Penta 2 in a timely manner, while those whose caregivers attended post-secondary school were at least twice more likely to receive Penta 2, Penta 3, and OPV 3 compared to those with lower levels of education. We observed a similar trend with Penta 3 and PCV13 series among caregivers who attended secondary school, and with BCG, PCV13 2, OPV 3, and IPV among those who attended post-secondary school. Infants at PHCs that offered only a monthly immunization schedule were 40% and 50% less likely to receive BCG and HepB birth doses, respectively, compared to those with more frequent immunization schedules. On the contrary, we observed a trend towards lower chances of immunization with Penta 2, Penta 3, OPV 2, OPV 3, and IPV in infants brought to the PHCs by a female caregiver.
Table 3.
Factors for timely receipta of vaccines among infants whose caregivers were interviewed (N = 465)
Bold represents the P value of < 0.05 is considered statistically significant
BCG Bacillus Calmette-Guérin, HepB Hepatitis B vaccine, IPV inactivated poliovirus vaccine, OPV oral polio vaccine, OR odds ratio, Penta pentavalent vaccine, PHC public health center, IPV inactivated poliovirus vaccine, PCV13 pneumococcal conjugate vaccine (13-valent)
aTimely receipt of vaccines is defined as receipt of vaccines within 4 weeks from recommended age based on the Philippine EPI
Because we conducted the sampling by region, we performed a multilevel logistic regression model (Supplement Table 1) as part of our sensitivity analyses. In this analysis using regions as clusters, we found no statistically relevant factors for the delay in vaccine receipt.
Timely completion of immunization and factors for delayed completion
Of the 465 infants whose caregivers we interviewed, only 60.7% completed all the vaccines on time. We observed no statistically significant difference among caregivers’ age, sex, and educational status for the delayed completion of primary infant series. However, there was a trend towards delayed completion of immunization for infants in the care of females with 5 or more children in the household (Table 4).
Table 4.
Factors for timely completiona of vaccines among infants whose caregivers were interviewed (N = 465)
| Caregiver characteristics | Timely completion, n (%) | Delayed completion, n (%) | OR (P value) |
|---|---|---|---|
| Overall | 282 (60.7) | 183 (39.4) | |
| Sex | |||
| Female | 274 (97.2) | 181 (98.9) | – |
| Male | 8 (2.8) | 2 (1.1) | 2.6 (0.2) |
| Relationship to infant | |||
| Parent | 245 (86.9) | 165 (90.2) | – |
| Others | 37 (13.1) | 18 (9.8) | 1.4 (0.3) |
| Age group | |||
| 18–24 years | 93 (33.0) | 66 (36.1) | – |
| 25–34 years | 127 (45.0) | 89 (48.6) | 1.0 (1.0) |
| 35 years and above | 62 (22.0) | 28 (15.3) | 1.6 (0.1) |
| No. of children of female caregivers | |||
| 0–2 children | 185 (65.6) | 106 (57.9) | – |
| 3–4 children | 71 (25.2) | 51 (27.9) | 0.8 (0.3) |
| 5 or more children | 26 (9.2) | 26 (14.2) | 0.6 (0.07) |
| Level of education | |||
| Attended primary school/never attended school | 119 (42.2) | 65 (35.5) | – |
| Attended secondary school | 145 (51.4) | 101 (55.2) | 1.4 (0.4) |
| Attended post-secondary school | 18 (6.4) | 17 (9.3) | 1.7 (0.1) |
BCG Bacillus Calmette-Guérin, HepB Hepatitis B vaccine, IPV inactivated poliovirus vaccine, OPV oral poliovirus vaccine, OR odds ratio, Penta pentavalent vaccine, PCV13 pneumococcal conjugate vaccine (13-valent)
aTimely completion of vaccines is defined as 3 doses of Penta, PCV13, and OPV, and one dose each of BCG birth dose, HepB birth dose, and IPV received within 6 months of age
Discussion
In most developing countries with problems on immunization coverage, information about timeliness of immunization is not routinely collected. Instead, most countries monitor receipt of EPI vaccines at 12 or 24 months. In the Philippines, the EPI monitors ‘fully immunized children’ (FIC), defined as infants who received BCG, 3 doses of DPT, 3 doses of OPV, 3 doses of HepB, and one dose of anti-measles vaccine before reaching 1 year of age [17]. However, this information does not provide insights into the extent to which vaccinations are administered on time. Our study revealed a substantial delay in the vaccine receipt for infants. The receipt of age-appropriate vaccination was delayed with only 60.7% having completed vaccination at 6 months of age, and the mean age of vaccine receipt more than 4 weeks later than the recommended age (Penta 1 recommended at 6 weeks but received at 10.1 weeks, Penta 2 recommended at 10 weeks but received at 15.8 weeks, and Penta 3 recommended at 14 weeks but received at 21.7 weeks).
We conducted the study after a 6- to 9-month pentavalent stockouts that occurred in 2015, and this resulted in untimely vaccination. Hence, the information on the extent of the delays may not be representative of the current state of immunization. The World Health Organization and UNICEF estimated that repeated stockouts of vaccines contributed to a 15% point reduction in the Philippine vaccine coverage for 2015, but the coverage marginally improved in the subsequent years [18]. According to the 2017 National Demographic and Health Survey in the Philippines, only 70% of children aged 12–23 months received all the recommended vaccinations [19]. With low immunization coverage and delayed vaccination, a rise in the number of vaccine-preventable diseases appeared in the following years. Following the repeated Pentavalent vaccine stockouts in the Philippines, the WHO Representative Office of the Philippines reported a rise in the number of diphtheria cases in the country in 2015 [20]. The declining immunization coverage also resulted in a measles outbreak in January 2019 [21] and the re-emergence of polio in September 2019 [22].
We identified further limitations in our study. First, our analysis is limited to primary infant immunization series (BCG, Hep B birth dose, DTwP-Hib-HepB, PCV, and OPV-IPV), which limits our analysis up to children 6 months of age. Equally important are the vaccination delays and coverage among older infants receiving measles and measles-mumps-rubella (MMR) vaccine. Studies have shown increased dropouts with increasing infant age [23, 24]. Second, we did not obtain other important maternal and social factors, namely, family income, distance from the PHCs, and other indicators for health-seeking behaviors. Third, our study has a small sample size, as we only included infants nested in a survey. A larger study to determine when children are being vaccinated in the Philippines will be useful for disease control and prevention efforts as well as for policymakers, as this will provide information in ascertaining the appropriate vaccination schedule.
Our findings confirm delays in immunization in the Philippines that are similar to other developing countries [24–28]. The combination of low immunization coverage and the delayed vaccine receipt among fully immunized children has negative repercussions on the control of vaccine-preventable diseases. These may result in a reduction in the benefits of the immunization program if there are delays in protecting high-risk children. With the delays, the age of the susceptible population becomes younger, and further disease transmission is shifted to a much younger population.
Our results explored the timeliness of vaccination in a country with a hugely growing and young population. In 2015, the Philippines added an additional injectable vaccine, IPV, to the schedule. Concomitant multiple injectable vaccination may have been a factor in changing and/or delaying scheduled immunizations, and healthcare providers’ perceptions and attitudes may have been a major contributing factor [14]. The work schedule of each PHCs is a major driving factor. In some PHCs, immunization visits are scheduled once per week up to once per month. The latter precludes an increased number of opportunities for the infant to be vaccinated at PCHs with less frequent immunization sessions. This then translates to low immunization coverage and further delays in vaccination.
Another finding of our study is the delay in the receipt of BCG and HepB birth doses. For BCG, one of the most commonly cited reasons for disparity in the delay is the health worker’s striving to open a new vial only when a minimum number of vaccines/infants are reached to be cost-efficient. A BCG multidose vial is only available for 6 h after reconstitution, and the vial should be discarded if not used [1]. Thus, these providers advised caregivers to go to the nearest immunization facility for the infant to receive the vaccine in order to minimize the vaccine wasting. This practice is not allowed in the current immunization guidelines. The guideline requires health workers to open vials and vaccinate infants regardless of whether the minimum number of vaccines/infants is reached. This practice is not a problem with HepB vaccine as the multidose vial can be used within 28 days after opening, and the utility of each vial can be maximized easily. Disparities in Hepatitis B delays are brought about by difficulties in reviewing records. The vaccine is usually given in birthing facilities while the immunization registries and immunization cards are being distributed in immunization facilities [29]. PHCs serve as both birthing facilities and immunization facilities, but this practice may differ across all regions.
Conclusion
We have updated the data on the timeliness of immunization for infants in the Philippines and explored delays in vaccinations that could be common for low-middle income countries with a huge vaccine target population. With the looming threat of vaccine hesitancy that may cause more delays in vaccination or even vaccine refusals, the gains achieved against vaccine-preventable diseases are at risk [30]. Timeliness of receipt is crucial to confer early protection to an infant, to sustain herd immunity in the population, and to minimize the lost opportunity for vaccine completion. With the resurgence of vaccine-preventable diseases worldwide, we highlight the need for continued monitoring of vaccination, including delays in the timing of vaccines. Timeliness of vaccine receipt is not only a surrogate measure for vaccine hesitancy in the population but has an impact on the control of vaccine-preventable disease in the population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge the help of Vincent Sumergido (Region 6 Western Visayas), Shiela Laquindanum (Region 3 Central Luzon), and Karen Hojas (Region 10 Northern Mindanao) for their support in the continuing data collection activities. We would like to thank Luzviminda Garcia for her coordinating work in the Department of Health Central Office and Brendalyn Red for her administrative help in the National Institutes of Health-UP Manila. We would also like to thank Abigail Marasigan for rendering the figures used in this paper.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention. The authors have no conflicts of interest.
Biographies
Peter Francis Raguindin
is a Vaccinologist and Epidemiologist who works on vaccine-preventable diseases and immunization system in the Philippines. He is a Senior Researcher at the Institute of Child Health and Human Development, National Institutes of Health – University of the Philippines, Manila, Philippines. He is also a Ph.D. student at the Institute of Social and Preventive Medicine - University of Bern, Bern, Switzerland.
Merrylle Morales-Dizon
is a Pediatrician and a Senior Researcher at the Institute of Child Health and Human Development, National Institutes of Health – University of the Philippines, Manila, Philippines.
Josephine G. Aldaba
is a Pediatrician and a Research Associate Professor at the Institute of Child Health and Human Development, National Institutes of Health – University of the Philippines, Manila, Philippines.
Lailani P. Mangulabnan
is a Medical Officer at the Center for Health Development Region 3, Department of Health, San Fernando City, Pampanga, Philippines.
Renilyn P. Reyes
is a Pediatrician and a Medical Officer at the Center for Health Development Region 6, Department of Health, Iloilo City, Iloilo, Philippines.
Batmunkh Nyambat
is a Medical Officer for Vaccine Preventable Disease and Immunization at the World Health Organization Regional Office for the Western Pacific, Manila, Philippines.
Maria Joyce Ducusin
is a Public Health Practitioner, who was the past Director of the Disease Prevention and Control Bureau, Department of Health, Manila, Philippines.
Anna Lena Lopez
is a Pediatric Infectious Disease specialist and was the past Director of the Institute of Child Health and Human Development, National Institutes of Health, University of the Philippines, Manila, Philippines.
Author contributions
PFR: Conceptualization, Methodology, Analysis, Writing, Editing. MPM: Methodology, Data curation, Analysis, Writing, Editing. JA: Methodology, Analysis, Writing, Editing. LM: Resources, Supervision, Analysis, Editing. RR: Resources, Supervision, Analysis, Editing. NB: Methodology, Analysis, Writing, Reviewing Editing. ALL: Conceptualization, Methodology, Analysis, Writing, Editing, Funding acquisition.
Funding
Open access funding provided by University of Bern. Open access funding provided by University of Bern. Open access funding provided by University of Bern. The study used funds from a project supported by the U.S. Centers for Disease Control and Prevention. The funding agency has no had no role in the design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and was ultimately responsible for the decision to submit this work for publication.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
The study obtained clearance from the University of the Philippines Manila Research Ethics Board (UPM-REB-2015-349-01) and the WHO Regional Office for the Western Pacific Ethical Review Committee (2015.25.PHL.5.EPI).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peter Francis Raguindin, Email: pnraguindin@up.edu.ph.
Merrylle Morales-Dizon, Email: mpmorales@up.edu.ph.
Josephine Aldaba, Email: jgaldaba@up.edu.ph.
Lailani P. Mangulabnan, Email: lpmangulabnan@gmail.com
Renelyn P. Reyes, Email: reyesrenilyn@yahoo.com
Nyambat Batmunkh, Email: batmunkhn@who.int.
Maria Joyce Ducusin, Email: jducusin@gmail.com.
Anna Lena Lopez, Email: allopez2@up.edu.ph.
References
- 1.World Health Organization . Immunization in practice: a practical guide for health staff. Geneva: WHO Press; 2015. [Google Scholar]
- 2.Feikin DR, Flannery B, Hamel MJ, Stack M, Hansen PM. Vaccines for Children in Low- and Middle-Income Countries. In: Black RE, Laxminarayan R, Temmerman M, Walker N, editors. Reproductive, maternal, newborn, and child health: disease control priorities. 3. The International Bank for Reconstruction and Development/The World Bank: Washington (DC); 2016. [PubMed] [Google Scholar]
- 3.Luman ET, Barker LE, Shaw KM, McCauley MM, Beuhler JW, Pickering LK. Timeliness of childhood caccinations in the United States: days undervaccinated and number of vaccines delayed. J Am Med Assoc. 2005;293(10):1024–1211. doi: 10.1001/jama.293.10.1204. [DOI] [PubMed] [Google Scholar]
- 4.Masters NB, Wagner AL, Boulton ML. Vaccination timeliness and delay in low- and middle-income countries: a systematic review of the literature, 2007-2017. Hum Vacc Immunother. 2019;15(12):2790–2805. doi: 10.1080/21645515.2019.1616503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerra FA. Delays in immunization have potentially serious health consequences. Paediatr Drugs. 2007;9(3):143–148. doi: 10.2165/00148581-200709030-00002. [DOI] [PubMed] [Google Scholar]
- 6.Choudhary TS, Reddy NS, Apte A, Sinha B, Roy S, Nair NP, et al. Delayed vaccination and its predictors among children under 2 years in India: insights from the national family health survey-4. Vaccine. 2019;37(17):2331–2339. doi: 10.1016/j.vaccine.2019.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant CC, Roberts M, Scragg R, Stewart J, Lennon D, Kivell D, et al. Delayed immunisation and risk of pertussis in infants: unmatched case-control study. BMJ. 2003;326(7394):852–853. doi: 10.1136/bmj.326.7394.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolos V, Menzies R, McIntyre P. Higher pertussis hospitalization rates in indigenous Australian infants, and delayed vaccination. Vaccine. 2007;25(4):588–590. doi: 10.1016/j.vaccine.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Siedler A, Hermann M, Schmitt HJ, Von Kries R. Consequences of delayed measles vaccination in Germany. Pediatr Infect Dis J. 2002;21(9):826–830. doi: 10.1097/00006454-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 10.von Kries R, Böhm O, Windfuhr A. Haemophilus influenzae b-vaccination: the urgency for timely vaccination. Eur J Pediatr. 1997;156(4):282–287. doi: 10.1007/s004310050601. [DOI] [PubMed] [Google Scholar]
- 11.Kiely M, Boulianne N, Talbot D, Ouakki M, Guay M, Landry M, et al. Impact of vaccine delays at the 2, 4, 6 and 12 month visits on incomplete vaccination status by 24 months of age in Quebec, Canada. BMC Public Health. 2018;18(1):1364. doi: 10.1186/s12889-018-6235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieu T, Black S, Sorel M, Ray P, Shinefield H. Would better adherence to guidelines improve childhood immunization rates? Pediatrics. 1997;98:1062–1068. [PubMed] [Google Scholar]
- 13.Wallace AS, Sobel H, Ryman TK, Mantaring JB, 3rd, Silvestre M, Thorley M, et al. Timing of hepatitis B vaccination and impact of non-simultaneous vaccination with DTP vaccine following introduction of a hepatitis B birth dose in the Philippines. J Pub Health Policy. 2012;33(3):368–381. doi: 10.1057/jphp.2012.18. [DOI] [PubMed] [Google Scholar]
- 14.Lopez AL, Harris JB, Raguindin PF, Aldaba J, Morales M, Sylim P, et al. Introduction of inactivated poliovirus vaccine in the Philippines: effect on health care provider and infant caregiver attitudes and practices. Vaccine. 2018;36(48):7399–7407. doi: 10.1016/j.vaccine.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayan GH, Shaw KM, Baughman AL, Orellana LC, Forlenza R, Ellis A, et al. Assessment of delay in age-appropriate vaccination using survival analysis. Am J Epidemiol. 2006;163(6):561–570. doi: 10.1093/aje/kwj074. [DOI] [PubMed] [Google Scholar]
- 16.Laubereau B, Hermann M, Schmitt HJ, Weil J, von Kries R. Detection of delayed vaccinations: a new approach to visualize vaccine uptake. Epidemiol Infect. 2002;128(2):185–192. doi: 10.1017/S0950268801006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epidemiology Bureau DOH. Electronic field health service information system 2011: manual of operations. Manila: DOH; 2011. https://uhmis.doh.gov.ph/index.php/downloads/155-manual-of-operation/167-electronic-field-health-service-information-system-2011.
- 18.WHO, UNICEF. WHO-UNICEF Joint report form for immunization Geneva: WHO and UNICEF; 2017. https://data.unicef.org/wp-content/uploads/country_profiles/Philippines/Immunization_phl.pdf.
- 19.Philippine Statistics Authority, ICF. Philippines National Demographic and Health Survey 2017: key indicators. Quezon City, Philippines: PSA and ICF; 2018.
- 20.WHO Representative Office Philippines. Lessons learned from recent diphtheria cases: Working towards sustainable prevention and treatment of vaccine-preventable diseases Manila: WHO; 2017. http://www.wpro.who.int/philippines/mediacentre/features/lessons_learned_diphtheria_cases/en/. Accessed 31 Mar 2019.
- 21.International Federation of Red Cross. Philippines: Re-emergence of vaccine preventable diseases—Measles outbreak. Geneva, Switzerland: International Federation of Red Cross and Red Crescent Societies; 2019. Contract No.: MDRPH032.
- 22.International Federation of Red Cross. Philippines: Re-emergence of vaccine preventable diseases (polio). Geneva, Switzerland: International Federation of Red Cross and Red Crescent Societies; 2019. Contract No.: MDRPH032.
- 23.Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Commun Health. 2012;66(7):e14. doi: 10.1136/jech.2010.124651. [DOI] [PubMed] [Google Scholar]
- 24.Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373(9674):1543–1549. doi: 10.1016/S0140-6736(09)60317-2. [DOI] [PubMed] [Google Scholar]
- 25.Mbengue MAS, Mboup A, Ly ID, Faye A, Camara FBN, Thiam M, et al. Vaccination coverage and immunization timeliness among children aged 12-23 months in Senegal: a Kaplan-Meier and Cox regression analysis approach. Pan Afr Med J. 2017;27(Suppl 3):8. doi: 10.11604/pamj.supp.2017.27.3.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akmatov MK, Kretzschmar M, Kramer A, Mikolajczyk RT. Timeliness of vaccination and its effects on fraction of vaccinated population. Vaccine. 2008;26(31):3805–3811. doi: 10.1016/j.vaccine.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Zaidi SM, Khowaja S, Kumar Dharma V, Khan AJ, Chandir S. Coverage, timeliness, and determinants of immunization completion in Pakistan: evidence from the Demographic and Health Survey (2006-07) Hum Vaccin Immunother. 2014;10(6):1712–1720. doi: 10.4161/hv.28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson DG, Ochieng B, Kagucia EW, Obor D, Odhiambo F, O’Brien KL, et al. Individual level determinants for not receiving immunization, receiving immunization with delay, and being severely underimmunized among rural western Kenyan children. Vaccine. 2015;33(48):6778–6785. doi: 10.1016/j.vaccine.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Sobel HL, Mantaring JB, 3rd, Cuevas F, Ducusin JV, Thorley M, Hennessey KA, et al. Implementing a national policy for hepatitis B birth dose vaccination in Philippines: lessons for improved delivery. Vaccine. 2011;29(5):941–945. doi: 10.1016/j.vaccine.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald NE, Hesitancy SWGoV. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.