Abstract
A novel actinobacterium, designated ASO4wetT, was isolated from the unidentified sponge (SO4) in the deep sea collected of the North Atlantic Ocean. Study of 16S rRNA gene sequences indicated that strain ASO4wetT is a member of the genus Streptomyces and showed the closest similarities to Streptomyces karpasiensis K413T (98.87 %), Streptomyces glycovorans YIM M 10366T (98.38 %), and Streptomyces abyssalis YIM M 10400T (97.53 %). Strain ASO4wetT contained MK-9(H8) as the predominant menaquinone and the major fatty acids are iso-C16:0, anteiso-C15:0, and iso-C15:0. Polyphasic taxonomy was carried out between strain ASO4wetT and its phylogenetically closely related Streptomyces strains, which further elucidated their relatedness and revealed that strain ASO4wetT could be distinguished from currently known Streptomyces species. Strain ASO4wetT clearly represents a novel species in genus Streptomyces. We propose the name Streptomyces bathyalis sp. nov., with the type strain ASO4wetT (= DSM 106605T = NCCB 100657T). Analysis of the whole-genome sequence of S. bathyalis revealed that genome size is 7,377,472 bp with 6332 coding sequences.
Keywords: Deep‐sea sponge, The North Atlantic Ocean, Polyphasic taxonomy, Streptomyces
Introduction
In the effort of finding new bioactive compounds from novel Streptomyces, some studies recently are focused on the neglected and unexplored regions in order to enlarge the successful isolation of new species (Goodfellow et al. 2017). The deep sea is one of the underexplored areas on Earth. One of the reasons is probably because of its extreme environments such as high pressure, low temperature, less oxygen concentration, and lack of light intensity. More than 30,000 marine natural products have been isolated and about 2 % of those are from deep-sea organisms, including Actinobacteria from the genus Streptomyces, Marinactinospora, and Verrucosispora (Tortorella et al. 2018).
Previously, some strains and species such as Streptomyces sp. NTK 937 (Hohmann et al. 2009), Streptomyces olivaceus FXJ8.012 (Liu et al. 2013), Streptomyces sp. SCSIO 04496 (Luo et al. 2015), Streptomyces indicus (Luo et al. 2011), and Streptomyces nanhaiensis (Tian et al. 2012) were reported to be isolated from the deep-sea sources. Streptomyces nanhaiensis was found in the northern South China Sea at 1632 m below sea level (Tian et al. 2012), while Streptomyces indicus was isolated from the Indian Ocean depth of 2434 m (Luo et al. 2011).
Streptomyces is a genus of aerobic Gram-positive bacteria and one of its characters is the morphology that contains substrate and aerial mycelia (Williams et al. 1983). A minor amount of species of this genus was reported having no aerial mycelia such as Streptomyces somaliensis (Brumpt 1906) Waksman and Henrici 1948 (Approved Lists 1980) (Skerman et al. 1980) and Streptomyces sudanensis (Quintana et al. 2008). Streptomyces is one of the genera from Actinobacteria and many of them are isolated from soil (Ritacco et al. 2003; Risdian et al. 2018); however, in some previous studies, they are also reported to be found in the rhizosphere of the plant (Xiao et al. 2009), mangrove sediment (Handayani et al. 2018), and marine sediment (Xu et al. 2012). Streptomyces is one of the important producers of antibiotics, considering that more than half of the antibiotics used nowadays are produced by this group of bacteria (Lucas et al. 2013). However, they are mainly terrestrial strains (Kemung et al. 2018).
In the course of our investigation of Actinobacteria from the deep sea in the extended Continental shelf of Portugal, near Madeira Islands, strain ASO4wetT was isolated from an unidentified sponge (SO4) collected by ROV (remotely operated vehicle) from the North Atlantic Ocean (36°15.19038 N, 14°32.99767 W) at 1092 m water depth.
Materials and methods
Actinobacteria isolation and morphological study
The isolation of actinobacteria was performed using 5336-ASW medium (soluble starch 10.0 g, casein 1.0 g, K2HPO4 0.5 g, MgSO4.7H2O 5.0 g, artificial seawater (ASW) 1000 ml, agar 20.0 g, pH 7.3) and incubated at 30 °C. Artificial seawater (ASW) contained 3.9 % (w/v) of sea salt from ATI Coral Ocean. Morphological observations of spores and mycelia on International Streptomyces Project 2 or ISP2 agar (yeast extract-malt extract), ISP3 agar (oatmeal), ISP4 agar (inorganic salt- starch) agar, ISP5 agar (glycerol-asparagine), ISP6 agar (peptone-yeast extract-iron), and ISP7 agar (tyrosine) (Shirling and Gottlieb 1966) at 30 °C for 14 days. The colours of mycelium (aerial and substrate) and diffusible pigments were evaluated by comparison with the RAL-code (https://www.ral-farben.de) (Charousová et al. 2015). Spore chain morphology and spore-surface ornamentation of strain ASO4wetT were observed after growing on ISP 3 agar medium (Shirling and Gottlieb 1966) for 4 weeks at 30 °C by Zeiss Merlin field emission scanning electron microscope (SEM) (Landwehr et al. 2018).
Physiological and biochemical studies
Growth of strain ASO4wetT at different temperatures (15, 20, 25, 30, 37 and 44 °C) on GYM medium (glucose-yeast extract-malt extract) and pH range (pH 2, 3, 4, 5, 6, 7, 8, 9 and 10) on ISP2 medium were evaluated after incubation for 14 days. Utilisation of carbohydrate was examined on ISP9 medium supplemented with 1 % carbon sources (Shirling and Gottlieb 1966), the sodium chloride tolerance was investigated as described by Kutzner (1981), and the enzymatic activity profile analysis was conducted by using API ZYM strips (Humble et al. 1977). Antibiotic susceptibility was investigated by the disc-diffusion plate method (Bauer et al. 1966) using antibiotic discs on ISP2 agar medium incubated for 7 days at 30 °C. Eight antibiotic discs were used: ampicillin (10 µg/disc), erythromycin (15 µg/disc), gentamycin 30 (µg/disc), tetracycline (30 µg/disc), vancomycin (30 µg/disc), cefotaxime (30 µg/disc), rifampicin (5 µg/disc), and penicillin G (6 µg/disc).
Phylogenetic analysis
Extraction of DNA, PCR amplification, and purification of the 16S rRNA gene sequence was performed as described by Landwehr et al. (2016). PCR amplified templates were sequenced using a 96-capillary-system from Applied Biosystems (ABI), a 3730xl DNA Analyzer. Sequence data were compiled with the BioEdit program (Hall 1999) (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). The almost-complete 16S rRNA gene sequence (1,417 nucleotides) of strain ASO4wetT was obtained and submitted for BLAST analysis (Altschul et al. 1990) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The 16S rRNA gene sequence was deposited in GenBank as MT036271. The similarity and homology of the 16S rRNA gene sequence were examined for sequence homology with the database of 16S rRNA gene sequences from the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/). The 16S rRNA gene sequences of strain ASO4wetT and some related type strains were aligned using the CLUSTAL W algorithm (Thompson et al. 1994) from the MEGA X software package (Kumar et al. 2018). Phylogenetic analyses were performed using the maximum-likelihood (Felsenstein 1981) algorithm from MEGA X (Kumar et al. 2018). The topologies of the inferred trees were examined by bootstrap analyses (Felsenstein 1985) based on 1000 replicates. The resulting phylogenetic trees were rooted using the 16S rRNA gene sequence of Actinospica robiniae GE134769T (AJ865863).
Chemotaxonomy
Biomass for the chemical analyses was collected by cultivation in glucose-yeast-malt extract (GYM) medium in flasks on a rotary shaker (160 revolutions per minute) at 30 °C for 3–7 days. The freeze-dried cells from biomass were used for chemical analysis. The whole-cell diaminopimelic acid isomers and sugars were evaluated based on the method of Staneck and Roberts (1974). Menaquinones were extracted as described by Minnikin et al. (1984) and were analysed by high-performance liquid chromatography (Wink et al. 2017) equipped with diode-array detection and mass spectrometry (HPLC-DAD-MS). High-resolution electron spray ionisation mass spectrometry (HR-ESI-MS) data were recorded on a MaXis ESI-TOF-MS spectrometer (Bruker) equipped with an Agilent 1260 series RP-HPLC system using XBridge C18 column 2.1 × 100 mm, 1.7µm. Solvent A was isopropanol and solvent B was acetonitrile. The gradient system was 100 % B for 5 min, 35 % B in 5 to 15 min, and 50 % B in 16–20 min with the flow rate was 0.6 mL/min. The temperature of the column was 40 °C and the UV-detection was at 270 nm. The molecular formula of menaquinones was calculated using the Smart Formula algorithm, including the isotopic pattern (Bruker). The polar lipids were extracted according to Minnikin et al. (1977) and identified by two-dimensional thin-layer chromatography as described previously by Collins and Shah (1984).
Fatty acids were extracted, methylated and analysed using the Sherlock Microbial Identification (MIDI) system and the ACTIN version 6 database (Sasser 1990). For matrix-assisted linear desorption/ionisation-time-of-flight mass spectrometry (MALDI-TOF MS) analysis, the isolate ASO4wetT was incubated at 30 °C for 6–8 days. The samples were prepared using ethanol/formic acid extraction, as described by Schumann and Maier (2014).
DNA-DNA hybridisation and ribotyping analysis
DNA–DNA hybridisation was performed based on the method of Ziemke et al. (1998), except that for nick translation, 2 µg DNA was labelled during 3 h of incubation at 15 °C. This method was carried out for the DNA of strain ASO4wetT and the strain Streptomyces karpasiensis DSM 42068T, Streptomyces glycovorans DSM 42021T, and Streptomyces abyssalis DSM 42024T. Standardised and automated ribotyping analysis was conducted using the RiboPrinter system (Hygiena) involving PvuII as a restriction enzyme (Bruce 1996; Schumann and Pukall 2013).
DNA extraction and complete genome sequencing
The complete genome sequence of strain ASO4wetT was obtained via a combination of long-read PacBio and short-read Illumina-Sequencing. Therefore, DNA was isolated using Qiagen Genomic-tip 100/G (Qiagen, Hilden Germany) according to the instructions of the manufacturer. SMRTbell™ template library was prepared according to the instructions from PacificBiosciences, Menlo Park, CA, USA, following the Procedure & Checklist – Greater Than 10 kb Template Preparation. Briefly, for preparation of 15 kb libraries 8µg genomic DNA was sheared using g-tubes™ from Covaris, Woburn, MA, USA according to the manufacturer´s instructions. DNA was end-repaired and ligated overnight to hairpin adapters applying components from the DNA/Polymerase Binding Kit P6 from Pacific Biosciences, Menlo Park, CA, USA. Reactions were carried out according to the manufacturer´s instructions. For the bacterial DNAs, BluePippin™ Size-Selection to greater than 4 kb was performed according to the manufacturer´s instructions (Sage Science, Beverly, MA, USA). Conditions for annealing of sequencing primers and binding of polymerase to purified SMRTbell™ template were assessed with the Calculator in RS Remote, PacificBiosciences, Menlo Park, CA, USA. 1 SMRT cell was sequenced on the PacBio RSII (PacificBiosciences, Menlo Park, CA, USA) taking one 240-minutes movie.
Bacterial DNAs libraries for sequencing on Illumina platform were prepared to apply Nextera XT DNA Library Preparation Kit (Illumina, San Diego, USA) with modifications according to Baym et al. (2015). Samples were sequenced on NextSeq™ 500.
Genome assembly and annotation
Genome assembly performed applying the RS_HGAP_Assembly.3 protocol included in SMRT Portal version 2.3.0 using default parameters. The assembly revealed a single linear chromosome with a coverage value of 117x. Error-correction was performed by a mapping of Illumina short reads onto finished genome using Burrows-Wheeler Alignment bwa 0.6.2 (Li and Durbin 2009) in paired-end (sample) mode using default settings with subsequent variant and consensus calling using VarScan 2.3.6 (Koboldt et al. 2012) Automated genome annotation was carried out using the NCBI Prokaryotic Genome Annotation Pipeline PGAP (Tatusova et al. 2016). The assembly was also uploaded to RAST (Rapid Annotation using Subsystem Technology) server (https://rast.nmpdr.org/) (Aziz et al. 2008) and antiSMASH server (https://antismash.secondarymetabolites.org/) (Medema et al. 2011; Blin et al. 2019) for metabolic reconstruction analysis and prediction of secondary metabolite gene clusters, respectively. The complete genome sequence of strain ASO4wetT was deposited at NCBI GenBank under accession number CP048882 in the NCBI Genome database (https://www.ncbi.nlm.nih.gov/genome).
Results and discussion
Strain ASO4wetT was found to grow well on ISP2, ISP3, ISP4, and ISP5, while sparse in ISP6 and ISP7 (Table 1). The aerial mycelium can be seen in ISP3 and ISP7. The diffusible pigment was not detected on all tested medium. The strain formed aerial mycelium, albeit no spore was detected on ISP3 agar (Fig. 1).
Table 1.
Characteristics of strain ASO4wetT on various ISP agar media after incubation for 14 days at 30 °C
| Agar medium | Growth | Substrate mycelium colour | Aerial mycelium colour | Soluble pigment |
|---|---|---|---|---|
| Yeast extract-malt extract (ISP2) | Good | Light ivory | None | None |
| Oatmeal (ISP3) | Good | Light ivory | Grey white | None |
| Inorganic salt- starch (ISP4) | Good | Ivory | None | None |
| Glycerol-asparagine (ISP5) | Good | Light ivory | None | None |
| Peptone-yeast extract-iron (ISP6) | Sparse | Sandy yellow | None | None |
| Tyrosine (ISP7) | Sparse | Nutbrown | Light grey | None |
Fig. 1.

Scanning electron micrographs of aerial mycelium with no spore detected of strain ASO4wetT after incubation on ISP 3 agar for 4 weeks at 30 °C
Strain ASO4wetT grew on medium ISP2 at 15-37oC (optimum at 25-30oC) and at pH 6–9 (optimum at pH 7). The strain grew on CYE medium (10 g casein peptone l− 1, 5 g yeast extract l− 1, 20 g agar l− 1, pH 7) supplemented with up to 10 % NaCl. Antibiotic susceptibility test indicated that the strain was sensitive to ampicillin, erythromycin, gentamycin, penicillin G, tetracycline, vancomycin, and rifampicin. However, it was resistant to cefotaxime (30 µg/disc).
According to the result from the NCBI server, isolate ASO4wetT related to the genus Streptomyces. The strain closely related to Streptomyces karpasiensis K413T (98.87 %), Streptomyces glycovorans YIM M 10366T (98.38 %), and Streptomyces abyssalis YIM M 10400T (97.53 %). Strain ASO4wetT formed a stable clade with Streptomyces karpasiensis K413T that was supported by 82 % bootstrap value in the maximum-likelihood tree based on the 16S rRNA gene sequence (Fig. 2).
Fig. 2.
Maximum-likelihood tree based on 16S rRNA gene sequences (1408 positions in the final dataset) showing relationships between strain ASO4wetT and the related type strains of Streptomyces species. The phylogenetic trees were rooted using the 16S rRNA gene sequence of Actinospica robiniae GE134769T (AJ865863). The evolutionary distances were computed using the Tamura-Nei method (Tamura and Nei 1993). Numbers at the nodes are percentage bootstrap values with 1,000 replicates, only values above 70 % are shown. Bar 0.10 substitutions per nucleotide position
Cell-wall hydrolysates of strains ASO4wetT contained LL-diaminopimelic acid, which is suggested that it belongs to cell-wall type I (Lechevalier and Lechevalier 1970). Whole-cell hydrolysates of strains ASO4wetT contained glucose and xylose. The major fatty acids of strain ASO4wetT were iso-C16:0 (35. 1 %), anteiso-C15:0 (22 %), iso-C15:0 (13.8 %), anteiso-C17:0 (8.8 %), and iso-C14:0 (6.3 %). The menaquinone composition was identified as MK-9(H8) and MK-9(H6) in a ratio of 12:1. The polar lipids were identified as diphosphatidylglycerol, phosphatidylglycerol, phosphatidylethanolamine, phosphatidyl-N-methyl-ethanolamine, phosphatidylinositol mannoside, and four unidentified polar lipids (Fig. 3). These chemotaxonomic properties of strain ASO4wetT had similar profiles to some species from the genus Streptomyces that have been reported previously (Kämpfer et al. 2008; Busarakam et al. 2014; Ayed et al. 2018).
Fig. 3.

Polar lipid observed in strain ASO4wetT. DPG: diphosphatidylglycerol; PME: phosphatidyl-N-methyl-ethanolamine; PE: phosphatidylethanolamine; PL: unknown phospholipid; GL1-3: unknown glycolipid; PGL: unknown phosphoglycolipid; PIM: phosphatidylinositol mannoside
The MALDI-TOF analysis result also suggested that strain ASO4wetT formed a clade with Streptomyces karpasiensis DSM 42068T (Fig. 4). The comparison of the fingerprints using the BioNumerics software (version 7.6.1; Applied Maths, Belgium) exhibited the differences between strain ASO4wetT, Streptomyces karpasiensis DSM 42068T, Streptomyces glycovorans DSM 42021T, and Streptomyces abyssalis DSM 42024T (Fig. 5). All strains displayed different band patterns.
Fig. 4.
MALDI-TOF dendrogram of the strain ASO4wetT and its most closely related strains. Streptomyces griseus DSM 40236T was used as an outgroup
Fig. 5.
Dendrogram of RiboPrinter® patterns (restriction enzyme PvuII, BioNumerics Software) of the strain ASO4wetT and its closely related Streptomyces strains
To determine whether strain ASO4wetT represent a novel species, DNA-DNA hybridisation (DDH) was conducted to further delineate the relatedness between strain ASO4wetT and its closely related type strains, i.e., Streptomyces karpasiensis DSM 42068T, Streptomyces glycovorans DSM 42021T, and Streptomyces abyssalis DSM 42024T. The levels of DNA-DNA relatedness between strain ASO4wetT and Streptomyces karpasiensis DSM 42068T, Streptomyces glycovorans DSM 42021T, and Streptomyces abyssalis DSM 42024T were 40.4/54.7 %, 40.5/44.4 %, and 35.8/28.2 % respectively. These values are below the threshold value of 70 %, as suggested by Wayne et al. (1987) for determining novel species for bacterial strains.
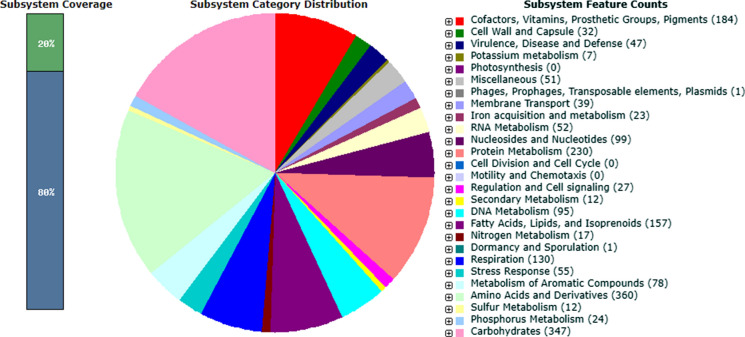
Genome sequencing of strain ASO4wetT resulted in a single linear chromosome typical for members of the genus Streptomyces consisting of 7,377,472 bp. The G + C content was 70.24 mol%. 6,332 coding sequences, 59 tRNA genes, and six rRNA operons were found after NCBI PGAP annotation. Analysis by using RAST server revealed that only 20 % of the annotated genes were assigned to subsystems (Fig. 6). Among the subsystem categories present in the genome, amino acids and derivatives metabolism had the highest feature counts (360), followed by carbohydrates metabolism which had 347 feature counts. However, only one feature count detected for dormancy and sporulation, which is different from the some other Streptomyces strains that have at least 10 feature counts (Busarakam et al. 2014; Ser et al. 2018; Quinn et al. 2020). The antiSMASH server predicted 23 secondary metabolite biosynthesis gene clusters, with six clusters showed more than 60 % similarities to known biosynthetic gene clusters: hopene biosynthetic gene cluster (61 %), planosporicin biosynthetic gene cluster (100 %), geosmin biosynthetic gene cluster (100 %), isorenieratene biosynthetic gene cluster (62 %), ectoine biosynthetic gene cluster (100 %), and desferrioxamine E biosynthetic gene cluster (100 %).
Fig. 6.
Subsystem category distribution of strain ASO4wetT based on RAST annotation server (https://rast.nmpdr.org/)
Besides the result of genotypic studies such as 16S rRNA gene analysis and DNA-DNA hybridisation, strain ASO4wetT can also be discriminated from its closely related type strains by some phenotypic properties (Table 2). Lipase (C14) activity could not be observed for strain ASO4wetT, whereas Streptomyces glycovorans DSM 42021T was positive. There was the β-galactosidase activity for strain ASO4wetT, while in the all compared type strains, it was not detected. Strain ASO4wetT had no β-glucosidase activity, while all of the tested type strains possessed it. Strain ASO4wetT exhibited good growth on the ISP 9 medium supplemented with arabinose, while Streptomyces karpasiensis DSM 42068T showed no growth. Phosphatidylinositol mannoside was detected in strain ASO4wetT but not in Streptomyces karpasiensis DSM 42068T based on data previously reported by Veyisoglu et al. (2014).
Table 2.
Phenotypic properties that distinguish strain ASO4wetT from the most closely related Streptomyces species
| Characteristics | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Esterase (C4) | + | (+) | (+) | + |
| Lipase (C14) | − | − | + | − |
| Trypsin | + | + | + | − |
| Chymotripsin | + | + | + | − |
| α-galactosidase | − | − | − | − |
| β-galactosidase | + | − | − | − |
| β-glucoronidase | - | − | (+) | (+) |
| α-glucosidase | + | + | + | + |
| β-glucosidase | − | (+) | + | + |
| Glucose | + | + | + | + |
| Arabinose | + | − | ++ | + |
| Sucrose | + | + | ++ | ++ |
| Xylose | ++ | ++ | ++ | ++ |
| Inositol | ++ | + | ++ | ++ |
| Mannose | (+) | + | − | + |
| Fructose | (+) | + | − | + |
| Rhamnose | + | + | − | + |
| Raffinose | ++ | + | ++ | ++ |
| Cellulose | − | + | − | − |
| Polar lipids | DPG, PG, PE, PME, PIM, PGL, 3 GLs | DPG, PG, PE, PME, PI, 3 PLs, 2 PGLs, 2 GLs* | DPG, PG, PME, PE, PIM, PI, 6PLs** | DPG, PG, PME, PIM, PI, 5PLs** |
| Predominant Menaquinone | MK-9(H8) | MK-9(H8)* | MK-9(H8)** |
MK-9(H4), MK-9(H6), MK-9(H8)** |
Strains: 1, ASO4wetT; 2, Streptomyces karpasiensis DSM 42068T (= K413T); 3, Streptomyces glycovorans DSM 42021T(= YIM M 10366T); 4, Streptomyces abyssalis DSM 42024T (= YIM M 10400T)
++ better growth; + positive result or good growth; - negative result or no growth; (+) weakly positive result or weak growth. DPG, diphosphatidylglycerol; PG, phosphatidylglycerol; PE, phosphatidylethanolamine; PME, phosphatidyl-N-methyl-ethanolamine; PI, phosphatidylinositol; PIM, phosphatidylinositol mannoside; PL, unknown phospholipid; PGL, unknown phosphoglycolipid; GL, unknown glycolipid. *Data from Veyisoglu et al. (2014); ** data from Xu et al. (2012)
In conclusion, strain ASO4wetT represents a novel species in the genus Streptomyces, for which the name Streptomyces bathyalis sp. nov. is proposed.
Description of Streptomyces bathyalis sp.nov.
Streptomyces bathyalis (ba.thy.al’is. L. neutrum substantive from the Greek bathys (deep) the part of the pelagic zone between 1,000 and 4,000 m).
Aerobic, Gram-positive actinomycete that forms branched substrate mycelium. Aerial hyphae can be seen only in ISP3 and ISP7. Spores are not detected in any medium tested even after 4 weeks of incubation at 30 °C. It grows well on ISP2, ISP3, ISP4, and ISP5 after 2 weeks incubation at 30 °C. Optimum growth occurs at 25–30 °C and at pH 7. The NaCl tolerance is 0–10 % (w/v) NaCl. Positive for esterase (C4), trypsin, chymotrypsin, β-galactosidase, and α-glucosidase. Negative for lipase (C14), α-galactosidase, β-glucuronidase, and β-glucosidase. Glucose, arabinose, sucrose, xylose, inositol, mannose, fructose, rhamnose, raffinose are used as sole carbon sources, but not cellulose. Major fatty acids are iso-C16:0 (35.0 %), anteiso-C15:0 (22.0 %), and iso-C15:0 (13.8 %). The major menaquinone is MK-9(H8). The diagnostic amino acid in the peptidoglycan is LL-diaminopimelic acid. Glucose and xylose are present in whole-cell hydrolysates. The type strain is ASO4wetT (= DSM 106605T = NCCB 100657 T), isolated from a sponge collected from the North Atlantic Ocean at 1092 m depth. The genomic DNA G + C content of the type strain is 70.24 mol%. The genome size is 7,377,472 bp with 6,332 coding sequences, 59 tRNA genes, and six rRNA operons. The complete genome and the 16S rRNA sequence of strain ASO4wetT were deposited at NCBI GenBank with accession number CP048882 and MT036271, respectively.
Acknowledgements
The authors thank Romy Schade for excellent technical assistance, Stephanie Schulz and Gabriele Pötter for chemotaxonomic analysis, and Aileen Gollasch for recording the HRESIMS data.
Authors’ contributions
CR and WL carried out the experiments, analysed the data, and drafted manuscript. CR and WL contributed equally to this work. MR performed scanning electron microscopy. PS conducted ribotyping and MALDI-TOF. RLH carried out fatty acid analysis. CS and BB carried out complete genome analysis. PK performed DDH analysis. PJS collected the sponge and supervised the project. JW supervised the project. MR, PS, RLH, CS, BB, PK, PJS, and JW corrected and reviewed the draft.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by German Federal Ministry of Education and Research (BMBF) under the German-Indonesian anti-infective cooperation (GINAICO) project, a fellowship awarded by the German Academic Exchange Service (German: Deutscher Akademischer Austauschdienst or DAAD), and The President’s Initiative and Networking Funds of the Helmholtz Association of German Research Centres (German: Helmholtz Gemeinschaft Deutscher Forschungszentren or HGF) under Contract Number VH-GS-202.
Data availability
The GenBank accession number for the 16S rRNA gene sequence of strain ASO4wetT is MT036271. The GenBank accession number for complete genome of strain ASO4wetT is CP048882.
Compilance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
This article does not contain any studies with human participants and/or animals performed by any of the authors. The formal consent is not required in this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chandra Risdian and Wiebke Landwehr contributed equally to this work.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ayed A, Slama N, Mankai H, Bachkouel S, ElKahoui S, Tabbene O, Limam F. Streptomyces tunisialbus sp. nov., a novel Streptomyces species with antimicrobial activity. Antonie Van Leeuwenhoek. 2018;111:1571–1581. doi: 10.1007/s10482-018-1046-4. [DOI] [PubMed] [Google Scholar]
- Aziz RK, et al. The RAST server: rapid annotations using subsystems technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A, Kirby W, Sherris J, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- Baym M, Kryazhimskiy S, Lieberman TD, Chung H, Desai MM, Kishony R. Inexpensive multiplexed library preparation for megabase-sized genomes. PLoS One. 2015;10:e0128036. doi: 10.1371/journal.pone.0128036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, Medema MH, Weber T. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids ResOxford University Press. 2019;47:W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J. Automated system rapidly identifies and characterizes microorganisms in food. Food Tech. 1996;50:77–81. [Google Scholar]
- Busarakam K, Bull AT, Girard G, Labeda DP, van Wezel GP, Goodfellow M. Streptomyces leeuwenhoekii sp. nov., the producer of chaxalactins and chaxamycins, forms a distinct branch in Streptomyces gene trees. Antonie Van Leeuwenhoek. 2014;105:849–861. doi: 10.1007/s10482-014-0139-y. [DOI] [PubMed] [Google Scholar]
- Charousová I, Javoreková S, Wink J. Isolation and characterization of Streptomyces rishiriensis (VY31) with antibiotic activity against various pathogenic microorganisms. J Microbiol Biotechnol Food Sci. 2015;04:23–27. doi: 10.15414/jmbfs.2015.4.special1.23-27. [DOI] [Google Scholar]
- Collins MD, Shah HN. Fatty acid, menaquinone and polar lipid composition of Rothia dentocariosa. Arch Microbiol. 1984;137:247–249. doi: 10.1007/BF00414552. [DOI] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- Goodfellow M, Busarakam K, Idris H, Labeda DP, Nouioui I, Brown R, Kim B-Y, del Carmen Montero-Calasanz M, Andrews BA, Bull AT (2017) Streptomyces asenjonii sp. nov., isolated from hyper-arid Atacama Desert soils and emended description of Streptomyces viridosporus Pridham et al. 1958. Antonie Van Leeuwenhoek 110: 1133–1148. 10.1007/s10482-017-0886-7 [DOI] [PMC free article] [PubMed]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Handayani I, Ratnakomala S, Lisdiyanti P, Alanjary M, Wohlleben W, Mast Y. Complete genome sequence of Streptomyces sp. strain BSE7F, a Bali mangrove sediment actinobacterium with antimicrobial activities. Genome Announc. 2018;6:1–3. doi: 10.1128/genomeA.00618-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann C, Schneider K, Bruntner C, Irran E, Nicholson G, Bull AT, Jones AL, Brown R, Stach JEM, Goodfellow M, Beil W, Krämer M, Imhoff JF, Süssmuth RD, Fiedler H. Caboxamycin, a new antibiotic of the benzoxazole family produced by the deep-sea strain Streptomyces sp. NTK 937. J Antibiot (Tokyo) 2009;62:99–104. doi: 10.1038/ja.2008.24. [DOI] [PubMed] [Google Scholar]
- Humble MW, King A, Phillips I. API ZYM: a simple rapid system for the detection of bacterial enzymes. J Clin Pathol. 1977;30:275–277. doi: 10.1136/jcp.30.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämpfer P, Huber B, Buczolits S, Thummes K, Grün-Wollny I, Busse HJ. Streptomyces specialis sp. nov. Int J Syst Evol Microbiol. 2008;58:2602–2606. doi: 10.1099/ijs.0.2008/001008-0. [DOI] [PubMed] [Google Scholar]
- Kemung HM, Tan LTH, Khan TM, Chan KG, Pusparajah P, Goh BH, Lee LH. Streptomyces as a prominent resource of future anti-MRSA drugs. Front Microbiol. 2018;9:1–26. doi: 10.3389/fmicb.2018.02221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzner H. The family Streptomycetaceae. In: Starr M, Stolp H, Trüper H, Balons A, Schlegel H, editors. The Prokaryotes – A handbook on habitats, isolation and identification of bacteria. Berlin: Springer Verlag; 1981. pp. 2028–2090. [Google Scholar]
- Landwehr W, Kämpfer P, Glaeser SP, Rückert C, Kalinowski J, Blom J, Goesmann A, Mack M, Schumann P, Atasayar E, Hahnke RL, Rohde M, Martin K, Stadler M, Wink J. Taxonomic analyses of members of the Streptomyces cinnabarinus cluster, description of Streptomyces cinnabarigriseus sp. nov. and Streptomyces davaonensis sp. nov. Int J Syst Evol Microbiol. 2018;68:382–393. doi: 10.1099/ijsem.0.002519. [DOI] [PubMed] [Google Scholar]
- Landwehr W, Karwehl S, Schupp PJ, Schumann P, Wink J. Biological active rakicidins A, B and E produced by the marine Micromonospora sp. isolate Guam1582. Adv Biotech Micro. 2016;1:555558. doi: 10.19080/AIBM.2016.01.555558. [DOI] [Google Scholar]
- Lechevalier MP, Lechevalier H. Chemical composition as a criterion in the classification of aerobic actinomycetes. Int J Syst Bacteriol. 1970;20:435–443. doi: 10.1099/00207713-20-4-435. [DOI] [Google Scholar]
- Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform 25: 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed]
- Liu N, Shang F, Xi L, Huang Y. Tetroazolemycins A and B, two new oxazole-thiazole siderophores from deep-sea Streptomyces olivaceus FXJ8.012. Mar Drugs. 2013;11:1524–1533. doi: 10.3390/md11051524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas X, Senger C, Erxleben A, Grüning BA, Döring K, Mosch J, Flemming S, Günther S. StreptomeDB: a resource for natural compounds isolated from Streptomyces species. Nucleic Acids Res. 2013;41:D1130–D1136. doi: 10.1093/nar/gks1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Tang G, Ju J, Lu L, Huang H. A new diketopiperazine derivative from a deep sea-derived Streptomyces sp. SCSIO 04496. Nat Prod Res. 2015;30:138–143. doi: 10.1080/14786419.2015.1045509. [DOI] [PubMed] [Google Scholar]
- Luo Y, Xiao J, Wang Y, Xu J, Xie S. Streptomyces indicus sp. nov., an actinomycete isolated from deep-sea sediment. Int J Syst Evol Microbiol. 2011;61:2712–2716. doi: 10.1099/ijs.0.029389-0. [DOI] [PubMed] [Google Scholar]
- Medema MH, Blin K, Cimermancic P, De Jager V, Zakrzewski P, Fischbach MA, Weber T, Takano E, Breitling R. AntiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011;39:339–346. doi: 10.1093/nar/gkr466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnikin DE, O’Donnell AG, Goodfellow M, Alderson G, Athalye M, Schaal A, Parlett JH. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J Microbiol Methods. 1984;2:233–241. doi: 10.1016/0167-7012(84)90018-6. [DOI] [Google Scholar]
- Minnikin DE, Patel PV, Alshamaony L, Goodfellow M. Polar lipid composition in the classification of Nocardia and related bacteria. Int J Syst Bacteriol. 1977;27:104–117. doi: 10.1099/00207713-27-2-104. [DOI] [Google Scholar]
- Quinn GA, Abdelhameed AM, Alharbi NK, Cobice D, Adu SA, Swain MT, Castro HC, Facey PD, Bakshi HA, Tambuwala MM, Banat IM. The isolation of a novel Streptomyces sp. CJ13 from a traditional Irish folk medicine alkaline grassland soil that inhibits multiresistant pathogens and yeasts. Appl Sci. 2020;11:173. doi: 10.3390/app11010173. [DOI] [Google Scholar]
- Quintana ET, Wierzbicka K, Mackiewicz P, Osman A, Fahal AH, Hamid ME, Zakrzewska-Czerwinska J, Maldonado LA, Goodfellow M. Streptomyces sudanensis sp. nov ., a new pathogen isolated from patients with actinomycetoma. Antonie Van Leeuwenhoek. 2008;93:305–313. doi: 10.1007/s10482-007-9205-z. [DOI] [PubMed] [Google Scholar]
- Risdian C, Primahana G, Mozef T, Dewi RT, Ratnakomala S, Lisdiyanti P, Wink J (2018) Screening of antimicrobial producing actinobacteria from Enggano Island, Indonesia. AIP Conf Proc 2024: 020039. 10.1063/1.5064325
- Ritacco FV, Haltli B, Janso JE, Greenstein M, Bernan VS. Dereplication of Streptomyces soil isolates and detection of specific biosynthetic genes using an automated ribotyping instrument. J Ind Microbiol Biotechnol. 2003;30:472–479. doi: 10.1007/s10295-003-0038-0. [DOI] [PubMed] [Google Scholar]
- Sasser M. Identification of bacteria by gas chromatography of cellular fatty acids. Newark: MIDI Inc.; 1990. [Google Scholar]
- Schumann P, Maier T (2014) MALDI-TOF Mass Spectrometry Applied to Classification and Identification of Bacteria. in Goodfellow, M., Sutcliffe, I., and Chun, J. (eds) Methods in Microbiology. Academic Press: 275–306. 10.1016/bs.mim.2014.06.002
- Schumann P, Pukall R. The discriminatory power of ribotyping as automatable technique for differentiation of bacteria. Syst Appl MicrobiolElsevier GmbH: 2013;36:369–375. doi: 10.1016/j.syapm.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Ser H, Ab Mutalib N-S, Yin W, Goh B-H, Lee L-H, Chan K. Genome sequence of Streptomyces antioxidans MUSC 164T isolated from mangrove forest. Prog Microbes Mol Biol. 2018;1:4–6. doi: 10.36877/pmmb.a0000001. [DOI] [Google Scholar]
- Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Evol Microbiol. 1966;16:313–340. [Google Scholar]
- Skerman VBD, McGowan V, Sneath PHA. Approved Lists of Bacterial Names. Int J Syst Evol Microbiol. 1980;30:225–420. doi: 10.1099/00207713-30-1-225. [DOI] [Google Scholar]
- Staneck JL, Roberts GD. Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl Microbiol. 1974;28:226–231. doi: 10.1128/AM.28.2.226-231.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Long L, Wang F, Xu Y, Li J, Zhang J, Zhang C, Zhang S, Li W. Streptomyces nanhaiensis sp. nov ., a marine streptomycete isolated from a deep-sea sediment. Int J Syst Evol Microbiol. 2012;62:864–868. doi: 10.1099/ijs.0.031591-0. [DOI] [PubMed] [Google Scholar]
- Tortorella E, Tedesco P, Esposito FP, January GG, Fani R, Jaspars M, De Pascale D. Antibiotics from deep-sea microorganisms: current discoveries and perspectives. Mar Drugs. 2018;16:1–16. doi: 10.3390/md16100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyisoglu A, Tatar D, Cetin D, Guven K, Sahin N. Streptomyces karpasiensis sp. nov., isolated from soil. Int J Syst Evol Microbiol. 2014;64:827–832. doi: 10.1099/ijs.0.056275-0. [DOI] [PubMed] [Google Scholar]
- Wayne LG, Brenner DJ, Colwell RR, Grimont PAD, Kander O, Krichevsky MI, Moore LH, Moore WEC, Murray RGE, Stackebrandt E, Starr MP, Truper HG. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol. 1987;37:463–464. doi: 10.1099/00207713-37-4-463. [DOI] [Google Scholar]
- Williams ST, Goodfellow M, Alderson G, Wellington EMH, Sneath PHA, Sackin MJ. Numerical classification of Streptomyces and related genera. J Gen Microbiol. 1983;129:1743–1813. doi: 10.1099/00221287-129-6-1743. [DOI] [PubMed] [Google Scholar]
- Wink J, Schumann P, Atasayar E, Klenk HP, Zaburannyi N, Westermann M, Martin K, Glaeser SP, Kämpfer P (2017) ‘Streptomyces caelicus’, an antibiotic-producing species of the genus Streptomyces, and Streptomyces canchipurensis Li et al. 2015 are later heterotypic synonyms of Streptomyces muensis Ningthoujam et al. 2014. Int J Syst Evol Microbiol 67: 548–556. 10.1099/ijsem.0.001612 [DOI] [PubMed]
- Xiao J, Wang Y, Luo Y, Xie SJ, Ruan JS, Xu J. Streptomyces avicenniae sp. nov., a novel actinomycete isolated from the rhizosphere of the mangrove plant Avicennia mariana. Int J Syst Evol Microbiol. 2009;59:2624–2628. doi: 10.1099/ijs.0.009357-0. [DOI] [PubMed] [Google Scholar]
- Xu Y, He J, Tian XP, Li J, Yang LL, Xie Q, Tang SK, Chen YG, Zhang S, Li WJ (2012) Streptomyces glycovorans sp. nov., Streptomyces xishensis sp. nov. and Streptomyces abyssalis sp. nov., isolated from marine sediments. Int J Syst Evol Microbiol 62: 2371–2377. 10.1099/ijs.0.035386-0 [DOI] [PubMed]
- Ziemke F, Hofle MG, Lalucat J, Rossello-Mora R. Reclassification of Shewanella putrefaciens Owen’s genomic group II as Shewanella baltica sp. nov. Int J Syst Bacteriol. 1998;48:179–186. doi: 10.1099/00207713-48-1-179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The GenBank accession number for the 16S rRNA gene sequence of strain ASO4wetT is MT036271. The GenBank accession number for complete genome of strain ASO4wetT is CP048882.