Abstract
Background
Colon cancer is a top lethal cancer in man and women worldwide and drug resistance is the major cause of cancer-related death. Combinational therapy and drug delivery with nanoparticles have been shown to effectively overcome drug resistance in many cancers. We previously reported that nanoemulsion (NE) loaded paclitaxel (PTX) and BEZ235 could synergistically inhibit colon cancer cell growth.
Purpose
To investigate whether NE loaded PTX and BEZ235 can overcome drug resistance and synergistically inhibit drug-resistant colon cancer cell growth in vitro and in vivo.
Methods
The in vitro treatment effect on cell viability was assayed using CCK8 kit, cell morphological change was detected by β-tubulin immunofluorescence staining, drug resistance-related proteins were analyzed by Western blotting, and in vivo tumor growth test was performed in nude mice xeno-transplanted with 2 drug-resistant colon cancer cell lines HCT116-LOHP and HT29-DDP.
Results
Both cell lines were sensitive to PTX but relatively insensitive to BEZ235. PTX combined with BEZ235 synergistically inhibited the proliferation of both cell lines. Nanoemulsion loaded PTX (NE-PTX) reduced the IC50 of PTX to approximately 2/5 of free PTX, indicating a high inhibitory efficacy of NE-PTX. When NE-PTX combined with a low concentration of BEZ235 (50 nM), the IC50 was decreased to approximately 2/3 of free PTX. Moreover, NE-PTX+BEZ235 treatment increased apoptosis, decreased Pgp and ABCC1 expression, and reduced tumor weights compared to the single drug treatment and the control group. These results suggest that nanoemulsion loaded PTX+BEZ235 can overcome drug resistance and improve the inhibitory effect on cancer cell proliferation and tumor growth.
Conclusion
Our study thus provides a possible new approach to treat colon cancer patients with drug resistance.
Keywords: colon cancer, nanoemulsion, paclitaxel, BEZ235, drug-resistance
Introduction
Colon cancer is one of the most common malignant tumors in both men and women. Most cases of colon cancer are diagnosed at advanced stages.1 Chemotherapy is the common treatment approach and the first-line drugs include 5-FU, platinum-based drugs, and EGFR receptor blockers. However, multi-drug resistance caused by 5-FU and platinum drugs is a great challenge in the successful treatment of colon cancer. Several mechanisms of multidrug resistance in colon cancer have been reported to date. These include enhanced efflux caused by the high expression of ATP-binding transporters (such as P-glycoprotein, Pgp), decreased intracellular drug concentration, decreased sensitivity of tumor cells to chemotherapeutic drugs, changes in activities and content of drug-resistance-related enzymes, and anti-apoptosis.2–7 About 46% of colorectal cancers have K-ras gene mutations,8,9 making targeted therapies by EGFR receptor blockers, such as cetuximab, inefficient.1 The incidence of multidrug resistance in patients with colorectal cancer metastases is more than 90%.7,8 Existing chemotherapy drugs for colorectal cancer have varying degrees of toxicity and side effects. Therefore, overcoming chemotherapy resistance, finding new, effective, and low toxicity drugs or approaches have a great clinical significance.
Paclitaxel (PTX) can effectively promote cancer cell apoptosis and is effective to tumor cells with K-ras gene mutation.10–15 PI3K/Akt/mTOR signaling pathway is thought to be closely related to multidrug resistance in human colon cancer. It was reported that blockage of PI3K/AKT signaling pathway could enhance drug sensitivity of HCT-116/L-OHP resistant cells and reverse Pgp-mediated multidrug resistance in human colon cancer.16,17 BEZ235, a PI3K/Akt/mTOR dual-pathway inhibitor, has been reported to enhance the sensitivity of colon cancer cell line HCT-116 to 5-FU and inhibit the growth of colon cancer stem cells.18–20 Combinational therapy with multiple drugs is a common strategy to overcome drug resistance. Herein, we speculated that the combination of BEZ235 and PTX may synergistically act on multiple targets of colon cancer cells to reduce drug resistance and promote cell apoptosis. Previously, we demonstrated that PTX and PI3K/mTOR inhibitor BEZ235 had a good synergistic inhibitory effect on the growth of colon cancer cells; and a nanoemulsion loaded with PTX and BEZ235 combination showed even more markable improvement in inhibitory activity.21 However, PTX are highly hydrophobic molecules, which hamper the maximally effective dosage delivery to patients, limiting treatment efficacy in the clinic. Their application for cancer treatment is limited by their low solubility and bioavailability. We further encapsulated PTX into nanoemulsion and then mixed with BEZ235 and have demonstrated that PTX nanoemulsion-BEZ235 combination therapy further significantly inhibited the growth of HCT-116 and HT-29 colon cancer cells due to the increase of solubility and bioavailability of PTX.21 Nanoemulsion nanoparticles consist of oils, surfactants/co-surfactants and water with the size in the range of 10–200 nm. They are able to encapsulate various hydrophobic drugs together if these drugs are soluble in the oil phase to enhance drug solubility in water. Importantly, the hydrophobic drugs encapsulated in nanoemulsion can be taken up by the cancer cells efficiently and have a long circulation time in the body. Studies have reported that paclitaxel nanoemulsion not only reduces the toxicity of the drug, but also prolongs the action time of the drug in the rat.22,23
Herein, we hypothesize that the combination of PTX and BEZ235 may synergistically act on multiple targets of colon cancer cells to reduce drug resistance and promote cell apoptosis. However, there have been no reports on the effect of nanoemulsion loaded PTX (NE-PTX) and BEZ235 combination on drug-resistant colon cancer cells. Therefore, we aimed to investigate whether the combination of NE-PTX and BEZ235 could show a synergistic inhibitory effect on drug-resistant colon cancer cell growth (both in vitro and in vivo), with reduced drug toxicity and side effects. Morphological changes and their underlying mechanisms were also analyzed. The results confirm that combination of NE-PTX and BEZ235 can significantly enhance the inhibition of the growth of drug-resistant colon cancer cells, providing great potential in colon cancer treatment for the future clinical application.
Materials and Methods
Materials
The oxaliplatin-resistant (HCT116-LOHP) and cisplatin-resistant (HT29-DDP) human colon cancer cell lines were purchased from Shanghai Aurora Technology Co., Ltd. (Shanghai, China). The cisplatin and oxaliplatin used to maintain the resistance of the two colon cancers were purchased from Sigma, USA. Both BEZ235 and PTX were purchased from Selleck Chemicals, USA. Oxaliplatin and cisplatin are dissolved in saline for storage, PTX and BEZ235 are dissolved in DMSO for storage. The sources of other reagents related to cell culture are as follows: RPMI 1640 medium (Invitrogen, USA), 0.25% EDTA trypsin (Beijing Soleibao, China), fetal bovine serum (Biolnd, Israel), penicillin streptomycin Mixture (Beijing Soleibao Company, China), DMSO (Beijing Soleibao Company, China), PBS (Shanghai Shenggong Company, China); CCK-8 cell proliferation-toxicity detection kit (Tongren Institute of Chemistry, Japan) is used to detect cell proliferation; the cytoskeleton-related reagents are anti-beta tubulin antibody, FITC-labeled anti-rabbit IgG (Abcam, US), propidium iodide (Sigma, US), cell cycle detection kit purchased From Lianke Biotechnology, China; Western Blotting assay antibodies and their concentrations are Pgp (1:1000, CST, US), ABCC1 (1:1000, CST, USA), β-actin (1:1000, Abcam, USA), Bcl-2 (1:1000, CST, USA).
Methods
Preparation of Paclitaxel Nanoemulsion21
Paclitaxel was dissolved in DMSO. It was then mixed with Capryol 90, Tween 20, and propylene glycol (Tween 20 to propylene glycol volume ratio was 2:1) on a vortex mixer to form an emulsified mixture. Water was added to this mixture and the mixture was stirred at the highest speed for 5 minutes to obtain the nanoemulsion. The mass ratio of Capryol 90, Tween 20 and propylene glycol to water was 20:35:45. Dynamic light scattering (Nano ZS, Zetasizer, Nano series, Malvern Instruments) was used to measure the average particle size and distribution of paclitaxel nanoemulsions. The stability was investigated by measuring its size distribution characteristics in serum-containing medium, PBS and PH5.8 buffer for 6 consecutive days. The prepared paclitaxel nanoemulsions (2 mM) were stored at 4°C for further assays.
CCK8 (Cell Counting Kit-8) Cell Viability Assay
Both cell lines were culture in RPMI-1640 medium containing 10% fetal bovine serum and 5% penicillin/streptomycin. Medium was supplemented with cisplatin (1.5 μg/mL) for culture of HT29DDP cell line and with oxaliplatin (10 μg/mL) for culture of HCT116-LOHP cell line to maintain the corresponding resistance phenotype of the two drug-resistant cells. Cells were incubated in a constant temperature humidified incubator at 37°C and 5%CO2. For subculture, cells were digested by 0.25% trypsin. HCT116-LOHP or HT29DDP cells were seeded 96-well plates with cell density of 4⨯103 cells per well. After incubating overnight, cells were attached and grown in the wells. Then, RPMI 1640 medium was removed and replaced by fresh RPMI-1640 medium containing BEZ235, PTX, NE-PTX or NE-PTX+BEZ235 (50 nM) with different concentration, respectively. Among them, the details of mixing 50nM BEZ235 with different concentrations of PTX or NE-PTX medium are as follows, when BEZ235 was added to the cell culture medium, we mixed the 1mg/mL BEZ235 concentrate with the cell culture medium to obtain a cell culture medium containing 50nM BEZ235, and then added PTX or PTX to the cell culture medium containing 50nMBEZ235. NE-PTX, obtain cell culture medium containing 100nM PTX or NE-PTX and 50nMBEZ235, and then add the cell culture medium containing BEZ235 to dilute in order to prepare corresponding medium containing 50nmBEZ235 and different concentrations of PTX and NE-PTX, And then classified according to the experimental group, change the medium, and then observe and determine. After 48 hours of treatment, 10μL of CCK-8 reagent was added to each well in the dark, then the 96-well plate was wrapped with foil and put it in the incubator. After 3 hours, the absorbance OD values were measured at A450nm with xMark microplate reader (BIO-RAD, USA), and the cell survival rate was calculated according to the following formula:
Cell survival rate% = (absorbance OD value of experimental group-absorbance of blank group) ÷ (absorbance OD value of control group-absorbance of blank group) × 100%
A combination index was calculated to determine the synergetic effect of combination treatment based on the following formula:
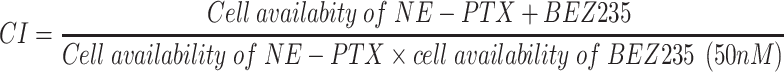 |
CI < 1, = 1 and > 1 indicated synergic, additive and antagonistic effect, respectively.
β-Tubulin Staining Experiment
The colon cancer HT29DDP and HCT116-LOHP cells were cultured in 6-well plates (2⨯105 cells per well). After 24 hours, cells were treated with drug directly added to the medium. After 2 hours treatment, the medium was removed. The cells were fixed with 4% paraformaldehyde, permeated with 0.5% Triton X-100 for 20 minutes, and blocked with 3% goat serum at room temperature for 30 minutes. Cells were then incubated with diluted rabbit anti-human β-tubulin antibodies (Abcam, USA) overnight at 4°C. Subsequently, cells were washed with PBST three times, and incubated with FITC (green fluorescence) labeled anti-rabbit fluorescent antibody (Abcam, USA) at 37°C for 1 hour. Then, cells were washed with PBST three times and incubated with PI (propidium iodide purchased from Sigma) to stain the nuclei (red fluorescence). Excess PI was removed by washing with PBS. Cells were then mounted onto glass slides with an anti-fluorescent quenching agent, and then observed and photographed under a fluorescence microscope.
Apoptosis Analysis
Apoptosis was analyzed by cell cycle kit (Biotech Corp). HT29DDP and HCT116-LOHP cells were cultured in 6-well plates. On the second day, cells were treated with PTX, BEZ235, or a combination of the two drugs. After 24 hours, 2⨯105 - 1⨯106 cells were collected by centrifugation and washed with PBS. Subsequently, 1 mL of DNA staining solution and 10mL of permeabilization solution were added to cells and mixed by vortexing for 5–10 seconds. Then, the cells were incubated at room temperature for 30 minutes and analyzed by flow cytometry. The apoptotic cells were gated as sub-G1/G0 population from single cells.
Western Blotting for Determination of Multidrug Resistance and Anti-Apoptosis Protein Expression
After treating HT29DDP and HCT116-LOHP cells with different drugs for 24 hours, cells were washed twice with PBS and the total protein was extracted. The protein concentration was determined by using Nano Drop spectrophotometer. The proteins were separated by gel electrophoresis (BIO-RAD, USA) and transferred to PVDF membranes. The membranes were blocked at room temperature (5% BSA) for 2 hours and then incubated with rabbit anti-human Pgp and ABCC1 and mouse anti-human Bcl-2 and β-actin antibodies overnight at 4 °C. Then, the membranes were washed at room temperature, treated with horseradish peroxidase conjugated anti-rabbit and anti-mouse IgG antibodies for 2 hours, and exposed to ECL for observation and imaging.
Tumor Growth and Treatment in Nude Mice
Our animal experiments were approved by the Animal Ethic Committee of Affiliated Hospital, Shihezi University School of Medicine. According to China’s “Guidelines for the Ethical Review of Laboratory Animal Welfare” (GB/T 35892–2018), as well as the “3Rs” animal welfare and ethical principles, which refers to replacing and reducing animals in experiments, and refining procedures to make them less harmful, eighty female BALB/C-nu nude mice were evenly divided into two halves (one was injected with HT29DDP and the other injected HCT116-LOHP cells; 2⨯106 cells per injection in 50 uL). When the tumor size reached 50–100 mm3, animals in each of these halves were randomly divided into five groups (PBS control group, PTX group-10mg/kg, BEZ235 group-20mg/kg, NE-PTX group-6.6mg/kg, NE-PTX+BEZ235 group-6.6 mg/kg, NE-PTX+BEZ235-20 mg/kg; 8 mice per group). EDTA was used to prepare the drug solutions. The drugs were injected every two days. The tumor was injected with a single injection of 50 ul. The longest diameter (a) and the shortest diameter (b) of the tumor were measured by vernier caliper after every 2 days, whereas the weight of nude mice was measured after every 3 days. After three weeks, the mice were euthanized, and the volume of tumors was measured (V (mm3) = ab2/2).
Statistical Analyses
Data collected from experimental and control groups were expressed as mean ± SD. One-way analysis of variance and the unpaired Student’s t-test (GraphPad Prism 6 program) were used to analyze the differences between groups and identify the significant differences (two-tail, P< 0.05) between experimental and control groups.
Results
Characterization of NE-PTX
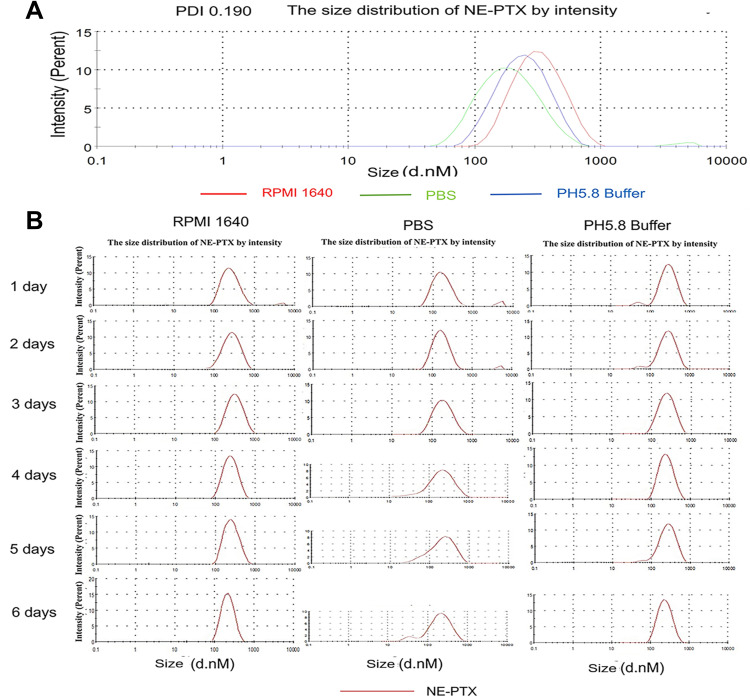
The homogenous NE-PTX was prepared by low-energy spontaneous emulsification. At an oil/emulsifier/water ratio of 20:35:45, a clear and uniform suspension was obtained, the final concentrations of NE-PTX were 2.0 mM. After loading the drugs, the size and polydispersity index of the drug-NE remained unchanged.21 The dynamic light scattering results in Figure 1A show that the NE-PTX had a narrow size distribution and polydispersity index of 0.19, It has similar particle size and PDI in medium, PBS and pH5.8 buffer. In the subsequent 6 days of contact measurement (Figure 1B), we found that the distribution and particle size of the NE-PTX remained in the same zone without significant changes, especially in the medium, indicating that the NE-PTX has a good stability.
Figure 1.
Particle size of NE-PTX from DLS analysis. (A) The NE-PTX had a narrow size distribution and polydispersity index of 0.19, It has similar particle size and PDI in RPMI 1640 medium, PBS and pH5.8 buffer. (B) In the subsequent 6 days of contact measurement, the distribution and particle size of the NE-PTX remained in the same zone without significant changes, especially in the medium, indicating that the NE-PTX has good stability.
Abbreviations: DLS, dynamic light scattering; NE, nanoemulsion; PTX, paclitaxel.
Inhibitory Effects of PTX, BEZ235, and the Combination of the Two Drugs on Drug-Resistant Colon Cancer Cells
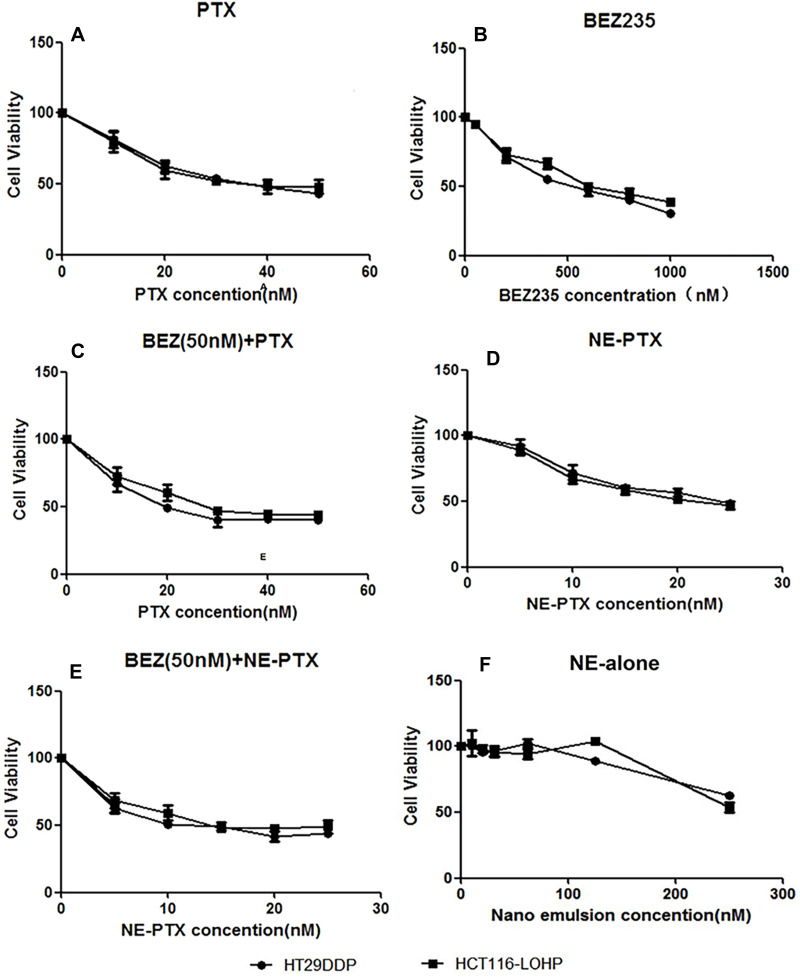
To investigate whether the combination of PTX and BEZ235 has synergistic effects in drug-resistant colon cancer cells, we first investigated the inhibitory effects of individual drugs. Both drug-resistant cell lines HT29DDP and HCT116-LOHP were sensitive to PTX treatment (IC50 = 34.78 nM and 37.56 nM (Table 1, Figure 2A), respectively). However, they were not very sensitive to BEZ235 (IC50 = 483.8 nM and 651.9 nM, respectively; Figure 2B). Because BEZ235 is not sensitive to colon cancer drug-resistant cells, it is worth exploring whether low concentrations of BEZ235 can enhance the sensitivity of colon cancer drug-resistant cells to PTX. Therefore, both cells were treated with PTX combined with BEZ235 at 50 nM (9–13 folds lower). As compared with PTX alone, the combination treatment decreased the IC50 of PTX to one third in both cell lines (21.48 nM and 26.42 nM in HT29DDP and HCT116-LOHP cell lines, respectively; Figure 2C), suggesting that BEZ235 can increase the sensitivity of drug-resistant cells to PTX. The combination index of 50nM BEZ235+10nM PTX are 0.86 and 0.92 in HT29DDP and HCT116-LOHP cell lines, respectively (Table 2). These data indicate that the combination approach with PTX and BEZ235 has synergistic treatment effect on drug-resistant colon cancers.
Table 1.
IC50s of Free or NE-Loaded BEZ235 and PTX in Drug-Resistant Colon Cancer Cell Treatment
| Treatment | HT-29 DDP (nM) | HCT116-LOHP (nM) |
|---|---|---|
| PTX | 34.70 | 37.50 |
| BEZ235 | 483.80 | 651.90 |
| BEZ235-50nM + PTX | 21.48 | 26.42 |
| NE-PTX | 21.57 | 22.77 |
| BEZ235-50nM + NE-PTX | 11.84 | 14.78 |
Abbreviations: IC50, 50% inhibitory concentrations; NE, nanoemulsion; PTX, paclitaxel.
Figure 2.
Dose-dependent inhibitory effects of PTX, BEZ235, NE-PTX, and their combinations on HT29DDP and HCT116-LOHP growth measured by CCK8 assay. (A) CCK8 assay results in HT29DDP and HCT116-LOHP cells treated with PTX at various concentrations. (B) CCK8 assay results in both cell lines treated with BEZ235 at different concentrations. (C) Different concentrations of PTX combined with 50nM-BEZ355 improved sensitivity of both cell lines to the treatment. (D) NE-PTX showed the improved inhibitory effects in both cell lines. (E) NE-PTX combined with BEZ235 (NE-PTX + BEZ235) synergistically inhibit the growth of both cell lines. (F) The control of NE; no obvious toxic effects were observed in the two cell lines at the dose lower than 150 nM.
Table 2.
Analysis of Combination Index (CI) of BEZ235 and PTX/NE-PTX Treatment Using the Mean Values of Cell Viability (%)
| Cell Lines | Concentrations | Cell Viability | CI* | |||
|---|---|---|---|---|---|---|
| BEZ235 (nM) | PTX (nM) | BEZ235 | PTX | BEZ235/PTX | ||
| HT29 DDP | 50 | 10 | 0.97 | 0.80 | 0.61 | 0.85 |
| HCT116LOPH | 50 | 10 | 0.96 | 0.79 | 0.70 | 0.93 |
| BEZ235 (nM) | NE-PTX (nM) | BEZ235 | NE-PTX | BEZ235/NE-PTX | ||
| HT29 DDP | 50 | 10 | 0.97 | 0.70 | 0.54 | 0.79 |
| HCT116LOPH | 50 | 10 | 0.96 | 0.72 | 0.61 | 0.88 |
Note: *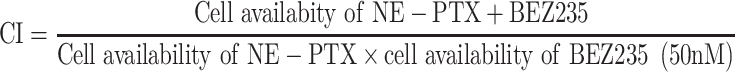 CI < 1, = 1 and > 1 indicated synergic, additive, and antagonistic effect, respectively.
CI < 1, = 1 and > 1 indicated synergic, additive, and antagonistic effect, respectively.
Abbreviations: PTX, paclitaxel; NE, nanoemulsion.
Nanoemulsion Delivery More Effectively Inhibits the Growth of Drug-Resistant Colon Cancer Cells
Nanoemulsion can reduce toxicity and side effects by increasing the solubility and efficacy of chemotherapeutic drugs.21 Therefore, we prepared nanoemulsion to deliver PTX to further improve their efficiency. Compared with PTX alone (free drug), NE-PTX showed an improved inhibitory effect in both HT29DDP and HCT116-LOHP cells (IC50 = 21.57 nM and 22.77 nM, respectively, which were about 2/5 (40%) times lower than that of PTX alone; Figure 2D). Combination of NE-PTX with 50 nM BEZ235 further decreased the IC50 of PTX to 11.84 nM and 14.78 nM in HT29DDP and HCT116-LOHP cells (Figure 2E), respectively. These IC50 were about 1/3 times lower than that of PTX alone, 1/2 times lower than that of PTX combined with BEZ235 at 50 nM, showing that NE-PTX combined with low concentration BEZ235 also showed a more obvious sensitization effect. The combination index of 50nM BEZ235+10nM NE-PTX are 0.79 and 0.88 in HT29DDP and HCT116-LOHP cell lines, respectively (Table 2), indicating that nanoemulsion loaded with PTX combined with low concentrations of BEZ235 can synergistically inhibit the growth of the drug-resistant colon cancer cells. This significant reduction in the dose of chemotherapeutic drugs will dramatically reduce their toxicity and side effects, As NE alone did not show much cytotoxicity, even at 120 nM (Figure 2F).
PTX and BEZ235 Induce Morphological Changes in Drug-Resistant Colon Cancer Cells
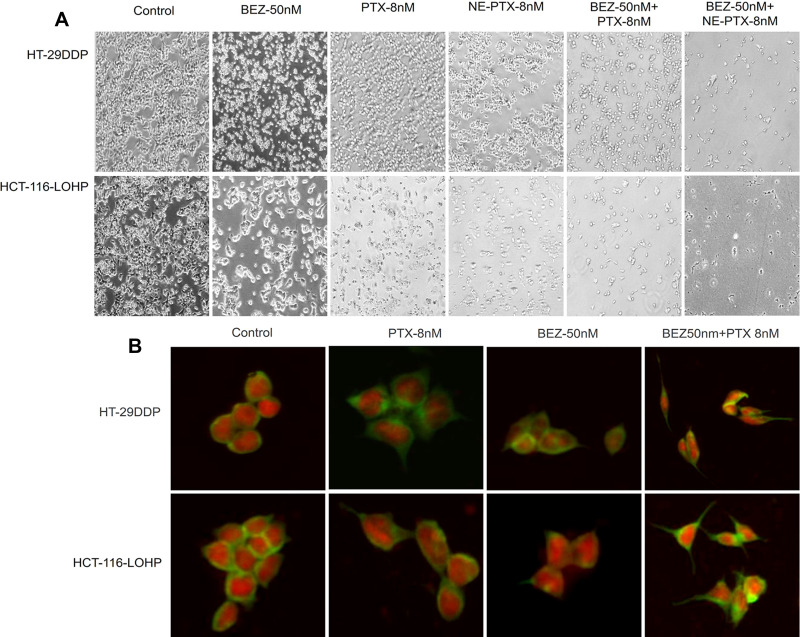
In addition to the significant cell death after 24 hours treatment (Figure 3A), cell morphological changes were observed in both HT29DDP and HCT116-LOHP cells. Using β-tubulin staining (green fluorescence), we could see that the round-shaped cells in controls became more fibroblast-like cells in the treatment samples. The red fluorescence shows the nucleus stained by PI, the morphological changes were more obvious in PTX+BEZ235 groups (Figure 3B). The morphological changes were the results from BEZ235 treatment that is consistent with previous reports.21
Figure 3.
Cell number and morphological changes after BEZ235 and PTX treatment. (A) Bright-field images of cell death induced by PTX and BEZ235 alone or combination treatment. Significant reduction in the cell number was observed in the treated groups, compared to the control. The images were taken using an Olympus IX51 fluorescent microscope at 100⨯ magnification. (B) The morphological changes of cells treated with PTX or BEZ235 or both for 2 hours. Using β-tubulin staining (green fluorescence) and nucleus stained by PI (red fluorescence), the morphological change from round-shaped cells in controls to fibroblast-shaped cells in the treatment groups was observed. The images were taken using an Olympus IX51 fluorescent microscope at 400⨯ magnification.
Combination Treatment Significantly Decreased the Expressions of Pgp and ABCC1 Proteins
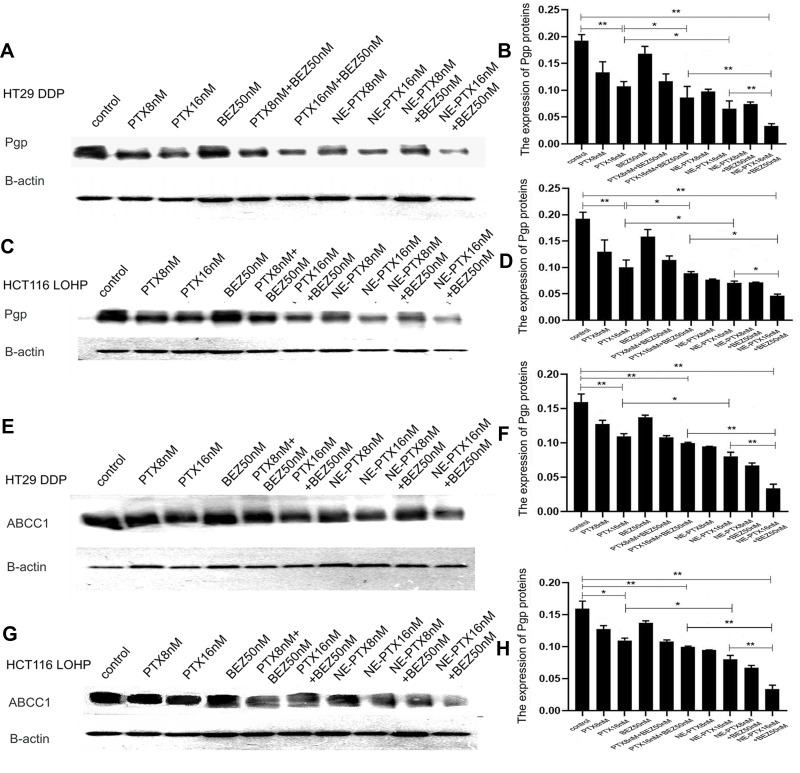
Colon cancer cells are prone to multidrug resistance. To study the mechanism of action of PTX and BEZ235 on HT29DDP and HCT116-LOHP colon cancer cells, we compared the expression of Pgp and multidrug resistance-associated protein (ABCC1) between different treatment groups by Western blotting analysis. Compared to the untreated control group, the expression of Pgp (Figure 4A–D) and ABCC1 (Figure 4E–H) was markedly decreased after the single drug and NE-PTX treatment. With the drug dose increased, the protein expression decreased more significantly. Although there was no significant difference in the expression of Pgp and ABCC1 protein in the BEZ235 50nM group, the combination therapy decreased even more (P < 0.05) (Figure 4A, B, E, F and Figure 4C, D, G, H for HT29DDP and HCT116-LOHP cells, respectively). The effect of PTX combined with BEZ235 and NE-PTX combined with BEZ235 in reducing the expression of drug-resistant protein was more obvious than that of single drug application. Among them, the NE-PTX combined with BEZ235 group showed the most obvious effect on reducing the expression of resistance protein.
Figure 4.
The expression of Pgp and ABCC1 in HT29DDP and HCT116-LOHP cells. The Western Blotting (WB) assay results show that compared to the untreated control, the expression of Pgp (A–D) and ABCC1 (E–H) was significantly decreased after single PTX and NE-PTX treatment; the combination therapy decreased even more significantly (P < 0.05) (A, B, E, F and C, D, G, H) for both HT29DDP and HCT116-LOHP cells. The graphs of b, d, f. and h are the densitometry results of corresponding WB assays.
Notes: *P<0.05; **P<0.01.
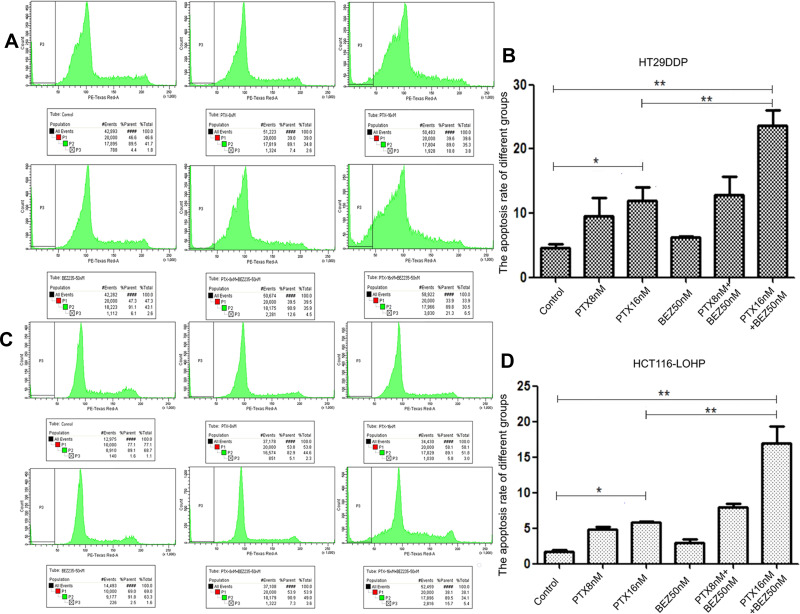
Combination Treatment Promoted Apoptosis in Drug-Resistant Colon Cancer Cells
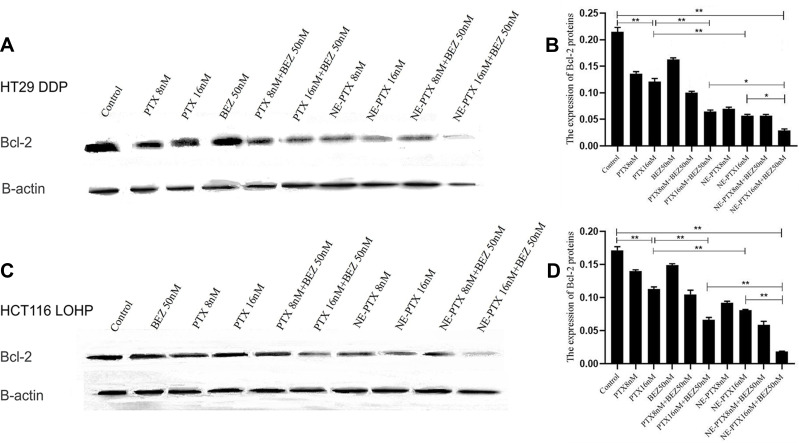
We further analyzed the effect of combinational therapy on apoptosis of colon cancer cells. The results (Figure 5A–D) for HT29DDP and HCT116-LOHP cells, respectively) showed that the apoptotic rates of different treatment groups were higher than those of the untreated control group; the difference between PTX-16nM group and the control group was statistically significant (P <0.05). The difference between combination therapy group (PTX-8nM + BEZ 50nM group and PTX-16nM + BEZ 50nM group) and the control group was also statistically significant (P < 0.01). These data indicate that PTX combined with BEZ235 can more effectively promote apoptosis in drug-resistant colon cancer as compared with PTX alone. We then further analyzed the levels of Bcl-2 protein (an anti-apoptosis protein associated with mitochondria apoptosis pathways) and found that the treatment with a single drug led to a reduction in this protein, but the more significant reduction was seen in the combination treatment (Figure 6). Compared to the untreated control, the expression of Bcl-2 was significantly decreased after single paclitaxel and NE-PTX treatment; the combination therapy decreased it even more significantly (P < 0.05) for HT29DDP and HCT116-LOHP cells, respectively). Among them, the NE-PTX combined with BEZ235 group showed the most obvious effect of reducing the expression of resistance protein, which is consistent with the above cell death results.
Figure 5.
Apoptosis analysis by sub-G1/G0 population with PI staining. After different treatments with PTX and BEZ235 in HT29DDP (A and B) and HCT116-LOHP (C and D) cancer cells, different degrees of apoptosis were observed. Graph a and c show the apoptotic sub-G1/G0 (P3) populations. Graphs c and d show the summary of 3 individual assays.
Notes: *P < 0.05, **P < 0.01.
Figure 6.
Apoptosis analysis by the expression of Bcl-2 (B-cell lymphoma 2) protein in HT29DDP and HCT116 LOHP cell lines. After different treatments with PTX and BEZ235, the expression of Bcl-2 was significantly decreased after single paclitaxel (PTX) and NE-PTX treatment; while the combination therapy decreased it even more significantly (for HT29DDP (A and B) and HCT116-LOHP cells (C and D). Among them, the NE-PTX combined with BEZ235 group showed the most obvious effect of reducing the expression of this protein.
Notes: *P<0.05; **P<0.01.
Combination Therapy Significantly Inhibited the Tumor Growth in Xenotransplant Tumor Model
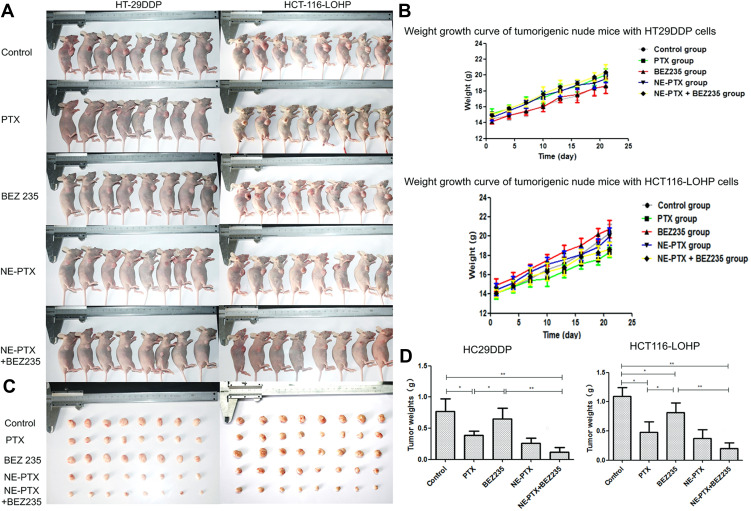
To further verify the in vitro results, we employed the xenotransplant tumor model in mice. HT29DDP and HCT116-LOHP cells were subcutaneously injected into nude mice. The mice in each group were photographed after 3 weeks of tumor formation (Figure 7A). Mice with both HT29DDP and HCT116-LOHP tumors gained weight, which was similar between all animals, allowing for modeling (Figure 7B). After the tumors reached a predefined volume, drugs were injected into the tumors. After 3 weeks, the tumors were dissected and weighed (Figure 7C). Compared to the untreated group, the tumor weight of different treatment groups decreased, with more significant decrease observed in the combination treatment group (Figure 7D) (P<0.005). This result confirms that the combination therapy can significantly inhibit the tumor growth in vivo, which was consistent with the results obtained in vitro.
Figure 7.
Inhibition of tumor growth in nude mice after drug treatments. (A) tumors on nude mice: (1) the control group, injected with PBS; (2) PTX, injected with 10mg/kg PTX; (3) BEZ235, injected with 20mg/kg BEZ235; (4) NE-PTX, injected with 6.6mg/kg; (5) NE-PTX + BEZ235, injected with 20mg/kg. The mice were imaged at day 21 post cancer cell injection. (B) The body weight of nude mice in different groups from day 0 to day 25. (C) Images of dissected tumors from each group of (A). (D) Comparison of tumor weights among different groups.
Notes: *P < 0.05; **P < 0.01.
Discussion
Our previous studies have shown that PTX and BEZ235 have a synergistic inhibitory effect on the growth of K-ras mutant or non-mutant colorectal cancer cells. Nanoemulsion loaded with PTX and BEZ235 combination was shown to further enhance the sensitivity and synergism.21 However, these studies used ordinary colon cancer cells. Nanoemulsion (NE) is a stable, transparent, low-viscosity, homogeneous and thermodynamically stable dispersion system formed by oil phase, water phase, surfactant and co-surfactant in appropriate proportions. Nanoemulsions can increase the solubility of poorly soluble drugs, improve the stability and bioavailability of drugs.21–23,25 In the present study, we aimed to explore whether NE-PTX combined with BEZ235 could exert the same synergistic inhibitory effect on the growth of colon cancer drug-resistant cell lines. The NE-PTX prepared by us shows good stability in culture medium, PBS and pH5.8 buffer. Cell viability results show that the combination treatment can improve inhibitory effects of PTX by reducing its IC50 and inhibit the tumor growth in animal model. This suggests that the approach has the potential to reduce the dosage of PTX and reduce its toxicity and side effects, which proves its potential clinical application value in the treatment of colon cancer.
The cellular mechanism of PTX acting on cancer cells (including these with K-ras gene mutations) includes interfering with the dynamic balance between microtubules and tubulin dimers, arresting the cell cycle into G2/M phase, and promoting apoptosis.24,26,27 BEZ235 is a dual inhibitor of PI3K/Akt/mTOR signaling pathway that down-regulates the expression of ATP-binding transporters (such as Pgp), reverses Pgp-mediated multidrug resistance in colorectal cancer, and promotes apoptosis.20,28 Therefore, we hypothesized that PTX combined with BEZ235 could simultaneously act on the growth, proliferation, apoptosis, and drug resistance in colon cancer cells to achieve synergistic antitumor and anti-drug resistance effects. The results of the present study confirmed our hypothesis. To further improve the sensitivity of tumor cells to PTX, we used nanoemulsion delivery as we reported before (Hong Zou et al 2016), and we prove that nanoemulsion delivery is more effective at reducing IC50 and inhibiting cancer cell growth in vitro and in vivo. Moreover, it is clear seen that PTX+BEZ235 exhibits a synergistic inhibition of cancer cells that is advantageous to individual drug. For future application, nanoemulsion loaded both drugs together may benefit this treatment approach.
The mechanism of colon cancer resistance involves resistance of tumor cells against apoptosis. It is known that nearly half of the colon cancer patients have K-ras gene mutations. Therefore, the EGFR receptor blockers, such as cetuximab, become inefficient, which is also a major issue in clinical practice. PTX can effectively promote apoptosis, and tumor cells with K-ras mutation are also sensitive to PTX.10,11,29 The main targets and function of PTX include: (1) PTX is localized in the tubulin/microtubule system. It promotes microtubule polymerization, inhibits microtubule degradation, and arrests cell division in G2/M phase. (2) PTX causes abnormalities in autophagic transport and localization. Moreover, it inhibits degradation of autophagy, thereby inducing apoptosis and degradation of cells.10 BEZ235 can also inhibit apoptosis in colon cancer cells. Therefore, we used flow cytometry to analyze the apoptosis of HT29DDP and HCT116-LOHP cells in different treatment groups of PTX and BEZ235. The results showed that the apoptosis rates of HT29DDP and HCT116-LOHP cells in treatments groups were higher than those in the negative control group. This indicated that PTX and BEZ235 could promote apoptosis in both colon cancer cell lines, albeit at different extents. The rate of apoptosis in PTX-16nM group was significantly different from the control group (P < 0.05), indicating that high PTX concentration induced relatively high apoptosis in both drug-resistant colon cancer cells. This suggested that PTX induced apoptosis in a concentration-dependent manner, which was consistent with the results of CCK8 experiment. For example, PTX-8nM+BEZ50nM group and PTX-16nM+BEZ50nM group had significantly higher degree of apoptosis than that in the control group (P < 0.01). This further confirmed that the combination of PTX and BEZ235 could promote apoptosis of resistant colon cancer cell lines more obviously. Moreover, the combination treatment showed stronger inhibitory effect on tumor growth and could significantly promote apoptosis, and inhibit tumor growth.
In tumors with MDR, ABC membrane transporter family helps tumor cells to pump out intracellular chemotherapy drugs to the outside of cells, which is an important mechanism of drug resistance in tumor cells.30 ABCB1/Pgp are the most important members of MDR involved in cell membrane transport function, and are the main target of tumor MDR inhibitors.31 ABCC1/MRP1 and ABCB1/Pgp are energy-dependent drug efflux pumps with ATP binding sites and drug binding sites. ATP can be hydrolyzed by binding to ABCC1 and Pgp to provide energy. This energy is used to pump out drugs entered the cells, lead to reducing the intracellular drug concentration or formation of septal distribution. Therefore, the intracellular drug concentration fails to reach the effective levels that will result in drug resistance and to the failure of chemotherapy. Thus, it is necessary to find new treatment approaches for drug-resistant colon cancers. In this study, the protein expressions of Pgp and ABCC 1 in both cell lines were detected by Western blotting assay. The results showed that the expression of both proteins was down-regulated compared to the untreated control after treatment with combination of PTX and BEZ235, indicating that the combination treatment can reduce multidrug resistance of both resistant colon cancer cell lines by suppressing the protein levels of the transporter. For instance, the expressions of Pgp and ABCC1 in PTX-16nM, BEZ50nM, and combination groups were all lower than those in PTX-8nM group, indicating their superior in inhibitory effect to PTX-8nM group (Figure 3). In addition, the expressions of Pgp and ABCC1 in PTX-8nM+BEZ50nM and PTX-16nM+BEZ50nM groups were lower than those in BEZ50nM group, providing evidence supporting that the combination treatments have a stronger inhibitory effect than BEZ235 alone treatment. This is further supported by the expressions of Pgp and ABCC1 in PTX-16nM+BEZ50nM group were significantly lower than those in PTX-16nM group (P < 0.01). Thus, the combination of PTX and BEZ235 could inhibit or reduce drug resistance of colon cancer by inhibiting the expression of ABCB1/Pgp. To validate the in vitro results, we tested the synergistic effect of PTX and BEZ235 in nude mice. The results showed that the tumor weights of the nude mice treated with PTX, BEZ235, NE-PTX, and NE-PTX+BEZ235 were lower than that of the untreated (control group) mice; the tumor weight of the nude mice treated with NE-PTX and BEZ235 was the lowest. These results corroborated with those of in vitro experiments. Compared with the PTX treatment group, the tumor weights of NE-PTX and NE-PTX+BEZ235 treatment groups decreased more significantly. Together, our data indicate that PTX loaded in nanoemulsions and combines with BEZ235 can effectively overcome drug resistance, increase drug sensitivity of drug resistant colon cancer cells, which promise a potential approach to treat drug-resistant colon cancer.
Conclusions
PTX combined with BEZ235 could effectively and synergistically inhibit the proliferation of drug-resistant colon cancer HT29DDP and HCT116-LOHP cells in vitro and in vivo. This mechanism is mainly related to the increased apoptosis and the inhibition of multidrug resistance. Together with previous research,21 it suggests that PTX combined with low concentration of BEZ235 is a promising approach to treat not only ordinary colon cancer cells but also platinum-resistant colon cancer cells. The use of nanoemulsion delivery system has further increased the sensitivity of tumor cells to PTX, and NE loaded PTX has the characteristics of good stability and increased solubility. Therefore, this approach should be explored further for clinical application, in the treatment of colon cancer.
Funding Statement
This research was funded by the National Natural Science Foundation of China [grant numbers 81660411, 81460383], the International Cooperation Project of Xinjiang Production and Construction Corps of China [grant number 2019BC001], the Key Areas Innovation Team Project of Xinjiang Production and Construction Corps of China [grant number 2018CB002].
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Yali Hu, Kunpeng Zhang, and Xingyao Zhu are co-first authors for this study. The authors declare no conflicts of interest.
References
- 1.Engstrom P. Update: NCCN Colon Cancer Clinical Practice Guidelines. JNCCN. 2005:3;S25. [PubMed] [Google Scholar]
- 2.Kozovska Z, Gabrisova V, Kucerova L. Colon cancer: cancer stem cells markers, drug resistance and treatment. Biomed Pharmacother. 2014;68(8):911–916. doi: 10.1016/j.biopha.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 3.Ding Z, Yang L, Xie X, et al. Expression and significance of hypoxia-inducible factor-1 alpha and MDR1/P-glycoprotein in human colon carcinoma tissue and cells. J Cancer Res Clin Oncol. 2010;136(11):1697–1707. doi: 10.1007/s00432-010-0828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia Z, Zhu Z, Zhang L. Specific reversal of MDR1/P-gp-dependent multidrug resistance by RNA interference in colon cancer cells. Oncol Rep. 2008:20(6):1433. [PubMed] [Google Scholar]
- 5.Neerati P, Sudhakar YA, Kanwar JR. Curcumin regulates colon cancer by inhibiting p-glycoprotein in in-situ cancerous colon perfusion rat model. J Cancer Sci Ther. 2013;5(9):313–319. [PMC free article] [PubMed] [Google Scholar]
- 6.Sodani K, Patel A, Kathawala RJ, Chen ZS. Multidrug resistance associated proteins in multidrug resistance, Chinese. J Cancer. 2012:31(2);58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian D, Li ZP, Huang CM, Zhen L, Tang QL. Effect and mechanism of DNMT inhibitor on the reversal of multidrug resistance in human colon cancer cell line sw620/L-OHP. J Sichuan Univ. 2010;41(6):975–979. [PubMed] [Google Scholar]
- 8.Starmans MHW, Pintilie M, Chan-Seng-Yue M, Moon NC, Boutros PC. Integrating RAS Status into Prognostic Signatures for Adenocarcinomas of the Lung. Clin Cancer Res Official J Am Assoc Cancer Res. 2015;21(6):1477–1486. doi: 10.1158/1078-0432.CCR-14-1749 [DOI] [PubMed] [Google Scholar]
- 9.Bozkurt O, Inanc M, Turkmen E, Karaca H, Ozkan M. Clinicopathological characteristics and prognosis of patients according to recurrence time after curative resection for colorectal cancer. Asian Pac J Cancer Prev. 2014;15(21):9277–9281. doi: 10.7314/APJCP.2014.15.21.9277 [DOI] [PubMed] [Google Scholar]
- 10.Veldhoen RA, Banman SL, Hemmerling DR, et al. The chemotherapeutic agent paclitaxel inhibits autophagy through two distinct mechanisms that regulate apoptosis. Oncogene. 2013;32(6):736–746. doi: 10.1038/onc.2012.92 [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Kaiser CE, Frett B, Li H-Y. Targeting Mutant KRAS for anticancer therapeutics: a review of novel small molecule modulators. J Med Chem. 2013;56(13):5219–5230. doi: 10.1021/jm3017706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adeberg S, Baris D, Habermehl D, et al. Evaluation of chemoradiotherapy with carbon ions and the influence of p53 mutational status in the colorectal carcinoma cell line HCT 116. Tumori J. 2014;100(6):675. [DOI] [PubMed] [Google Scholar]
- 13.Anagnostakos K, Kelm J, Schmitt E. Paclitaxel and gemcitabine combinational drug-loaded mucoadhesive delivery system in the treatment of colon cancers. Drug Res. 2015;65(4):199–204. doi: 10.1055/s-0034-1375665 [DOI] [PubMed] [Google Scholar]
- 14.Gon A, Alves D, Braguer G, Carles N, André C, Briand C. Briand, Caspase-8 activation independent of CD95/CD95-L interaction during paclitaxel-induced apoptosis in human colon cancer cells (HT29-D4). Biochem Pharmacol. 2000;60(11):1579–1584. doi: 10.1016/s0006-2952(00)00481-0 [DOI] [PubMed] [Google Scholar]
- 15.Xu R, Sato N, Yanai K, Akiyoshi T, Katano M. Enhancement of paclitaxel-induced apoptosis by inhibition of mitogen-activated protein kinase pathway in colon cancer cells. Anticancer Res. 2009;29(1):261–270. [PubMed] [Google Scholar]
- 16.Sui H, Xiao-Ling FU, Pan SF, et al. PI3K/Akt/NF-κB regulate ABCB1/P-glycoprotein–mediated multidrug resistance in colon carcinoma cells. China Oncol. 2014:24(2):106. [Google Scholar]
- 17.Abdul-Ghani R, Serra V, Györffy B. The PI3K inhibitor LY294002 blocks drug export from resistant colon carcinoma cells overexpressing MRP1. Oncogene. 2006;25(12):1743–1752. doi: 10.1038/sj.onc.1209201 [DOI] [PubMed] [Google Scholar]
- 18.Gu W, Jiezhong C, Renfu S, Li L, Zhiping X. Effective inhibition of colon cancer cell growth with MgAl-layered double hydroxide (LDH) loaded 5-FU and PI3K/mTOR dual inhibitor BEZ-235 through apoptotic pathways. Int J Nanomedicine. 2014;9:3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Shao R, Li F, et al. PI3K/Akt/mTOR pathway dual inhibitor BEZ235 suppresses the stemness of colon cancer stem cells. Clin Exp Pharmacol Physiol. 2015;42(12):1317–1326. doi: 10.1111/1440-1681.12493 [DOI] [PubMed] [Google Scholar]
- 20.Roper J, Richardson MP, Wei VW, et al. The Dual PI3K/mTOR Inhibitor NVP-BEZ235 induces tumor regression in a genetically engineered mouse model of PIK3CA Wild-Type Colorectal Cancer. PLoS One. 2011;6(9):e25132. doi: 10.1371/journal.pone.0025132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu W, Hong Z, Li L, Ines GC, Ping XZ, Michael M. Synergistic inhibition of colon cancer cell growth with nanoemulsion-loaded paclitaxel and PI3K/mTOR dual inhibitor BEZ235 through apoptosis. Int J Nanomedicine. 1947:11:1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivas G, Mansoor A. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6(3). 928. [DOI] [PubMed] [Google Scholar]
- 23.Choudhury H, Gorain B, Karmakar S, et al. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int J Pharm. 2014;460(1–2):131–143. doi: 10.1016/j.ijpharm.2013.10.055 [DOI] [PubMed] [Google Scholar]
- 24.Schwarz R. The dual PI3K/mTOR inhibitor NVP-BEZ235 enhances nab-paclitaxel antitumor response in experimental gastric cancer, International. J Oncol. 2013:43(5):1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu W, Zou H, Li L, Carcedo IG, Xu ZP, Monteiro M. Synergistic inhibition of colon cancer cell growth with nanoemulsion-loaded paclitaxel and PI3K/mTOR dual inhibitor BEZ235 through apoptosis. Int J Nanomedicine. 2016;1947. doi: 10.2147/IJN.S100744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokunaga Y, Hosogi H, Hoppou T, Nakagami M, Tokuka A, Ohsumi K. Effects of MDR1/P-glycoprotein expression on prognosis in advanced colorectal cancer after surgery. Oncol Rep. 2001. doi: 10.3892/or.8.4.815 [DOI] [PubMed] [Google Scholar]
- 27.Kudoh A, Oishi T, Itamochi H, et al. Dual Inhibition of Phosphatidylinositol 3′-Kinase and Mammalian Target of Rapamycin Using NVP-BEZ235 as a novel therapeutic approach for mucinous adenocarcinoma of the ovary, international. J Gynecol Cancer. 2014;24(3):444–453. doi: 10.1097/IGC.0000000000000091 [DOI] [PubMed] [Google Scholar]
- 28.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7(7):1851–1863. doi: 10.1158/1535-7163.MCT-08-0017 [DOI] [PubMed] [Google Scholar]
- 29.Ciombor KK, Wu C, Goldberg RM. Recent Therapeutic Advances in the Treatment of Colorectal Cancer. Annu Rev Med. 2015;66(1):83–95. doi: 10.1146/annurev-med-051513-102539 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Teng JS. Increased multi-drug resistance and reduced apoptosis in osteosarcoma side population cells are crucial factors for tumor recurrence. Exp Ther Med. 2016;12(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwabedissen HEMZ, Kroemer HK. In vitro and in vivo evidence for the importance of breast cancer resistance protein transporters (BCRP/MXR/ABCP/ABCG2). Handb Exp Pharmacol. 2011;201(201):325–371. [DOI] [PubMed] [Google Scholar]