Abstract
Background
Few studies have investigated the safety and efficacy of anti-PD-(L)1 antibodies in metastatic urothelial carcinoma (mUC) in daily clinical practice. Knowledge about the influence of baseline clinical and analytical factors on therapy outcomes is scarce.
Patients and methods
We conducted a multicenter retrospective study involving 119 previously treated or untreated mUC patients under anti-PD-(L)1 therapy in a real-world scenario. The objectives of this study were to confirm the safety and efficacy of anti-PD-(L)1 monotherapy and to identify pretreatment factors influencing therapy outcomes. In addition, an independent prognostic model for overall survival (OS) was developed and internally validated.
Results
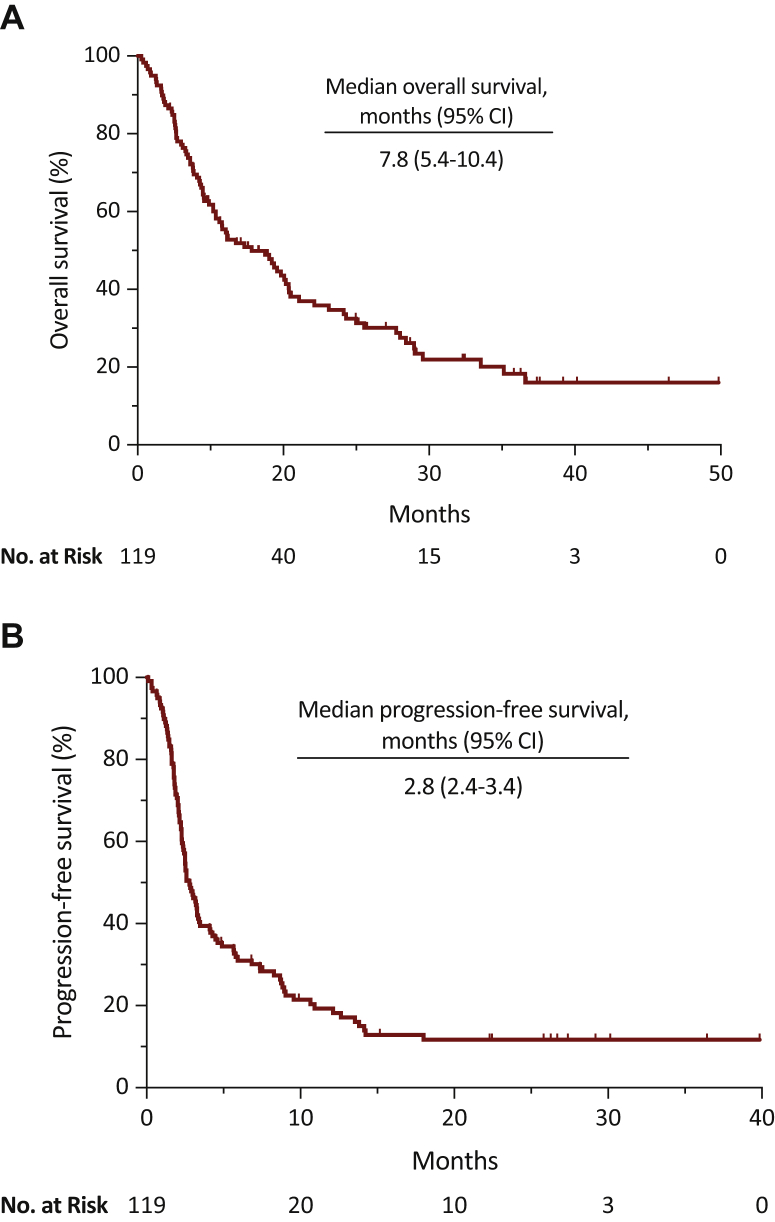
Median OS was 7.8 months [95% confidence interval (CI), 5.4-10.4], median progression-free survival (PFS) was 2.80 months (95% CI, 2.4-3.4), disease control rate (DCR) was 40% (95% CI, 31-49), and overall response rate (ORR) was 24% (95% CI, 15-31). Presence of peritoneal metastases was associated with poor OS [hazard ratio (HR) = 2.40, 95% CI, 1.08-5.33; P = 0.03]. Use of proton-pump inhibitors (PPI) was associated with poor OS (HR = 1.83, 95% CI, 1.11-3.02; P = 0.02) and PFS (HR = 1.94, 95% CI, 1.22-3.09; P = 0.005), and lower DCR (OR = 0.38, 95% CI, 0.17-0.89; P = 0.03) and ORR (OR = 0.18, 95% CI, 0.02-1.60; P = 0.002). The three risk category prognostic model developed included Eastern Cooperative Oncology Group performance status, PPI use, albumin level, presence of liver metastases, and presence of peritoneal metastases variables and was associated with higher risk of death (HR = 3.00, 95% CI, 1.97-4.56; P = 0.0001).
Conclusions
This study confirms anti-PD-(L)1 monotherapy as a safe and effective treatment option in daily clinical practice for mUC patients. It also describes the presence of peritoneal metastases as an independent prognostic factor for OS and underlines the association between PPI use and worse therapeutic outcomes. Finally, it proposes a new easy-to-use risk-assessment model for OS prediction.
Key words: immunotherapy, peritoneum, prognosis, proton-pump inhibitors, real world, urothelial carcinoma
Highlights
-
•
Monotherapy with anti-PD-(L)1 antibodies is a safe and effective treatment option in mUC.
-
•
Peritoneal metastases represent an independent prognostic factor in mUC.
-
•
The use of PPI correlates with poor therapeutic outcomes.
-
•
A new three risk category prognostic model enables OS prediction.
Introduction
Although urothelial carcinoma remains the 10th most common cancer worldwide, with an estimated 200 000 deaths in 2018,1 over the last few years, several advances have been made in the management of this aggressive disease. Immunotherapy, particularly the blockade of the programmed cell death-1 (PD-1)/programmed death-ligand 1 (PD-L1) axis, has been established as a standard treatment for locally advanced or metastatic urothelial carcinoma (mUC). To date, five anti-PD-1 or PD-L1 [PD-(L)1] antibodies have been approved by either the European Medicines Agency (EMA) and/or the US Food and Drug Administration (FDA) for those patients with disease progression during or following platinum-based chemotherapy: two anti-PD-1 drugs, pembrolizumab [which demonstrated and overall survival (OS) improvement in the KEYNOTE-045 phase III trial2] and nivolumab; and three anti-PD-L1 drugs, atezolizumab, avelumab, and durvalumab. Furthermore, pembrolizumab and atezolizumab have also been approved in the first-line setting for cisplatin-ineligible patients with PD-L1 positive tumors or platinum-ineligible patients. Recently, based on significant OS improvement demonstrated in the JAVELIN Bladder 100 phase III trial,3 avelumab has been approved by the FDA as first-line maintenance treatment of patients with mUC that has not progressed on first-line platinum-based chemotherapy.
While there are several well-established prognostic factors in patients with mUC treated with chemotherapy in the first-line (platinum-based therapy) and second-line (taxanes or vinflunine) setting,4, 5, 6, 7, 8, 9, 10 little is known about the influence of different baseline patient and disease characteristics in the outcome of monotherapy with immune checkpoint inhibitors (ICIs), regardless of the line of therapy. The relevance of the Eastern Cooperative Oncology Group performance status (ECOG-PS) in the efficacy of ICIs in mUC has been recently addressed in a real-world study conducted by Khaki et al.11 Moreover, the neutrophil-lymphocyte ratio (NLR), visceral metastases, and ECOG-PS have been proposed as independent predictors of OS in a cohort of 62 mUC patients treated with anti-PD-(L)1 antibodies. Similarly, Sonpavde et al.,12 after evaluating various clinical and analytical factors in the context of ICI monotherapy phase I/II trials on mUC, developed and validated a five-factor prognostic model integrating ECOG-PS, liver metastases, platelet count, NLR, and lactate dehydrogenase (LDH). Besides, atezolizumab has been recently evaluated in a real-world population of mUC patients in the SAUL trial, a single-arm, multicenter, international open-label phase IIIB study.13 In this study, pre-specified subgroup analyses in populations classically considered as difficult to treat, such as patients with ECOG-PS 2, presence of brain metastases, renal impairment, positive human immunodeficiency virus status, history of autoimmune disease, concomitant steroid use, and history of non-urothelial urinary tract carcinoma, proved the tolerance and effectivity of atezolizumab in these complex scenarios.14 On the one hand, other potentially relevant clinical factors, such as peritoneal metastases, associated with poor prognosis in other different tumor types,15,16 or the concomitant use of proton-pump inhibitors (PPIs), were recently associated with bad outcomes in two retrospective analyses of ICI-based clinical trials on advanced melanoma and non-small cell lung cancer patients (NSCLC),17,18 have not been systematically evaluated in mUC yet.
On the other hand, different molecular markers such as PD-L1,19 tumor mutation burden,20,21 copy-number and single-nucleotide variant counts,22 alterations in DNA damage response and repair genes,23 gene expression signatures,24, 25, 26, 27 and peripheral blood T-cell receptor clonality28 have been evaluated but, either there is still a lack of external validation of the results or these are inconsistent between different anti-PD-(L)1 antibodies depending, among other factors, on the methodology applied.29
Accordingly, we conducted this multicenter retrospective study in a cohort of mUC patients in order to confirm the safety and efficacy of monotherapy with anti-PD-(L)1 drugs and to better understand the influence of different pretreatment factors in therapy outcomes.
Patients and methods
Study design and patient population
We conducted a multicenter retrospective study of a cohort of 119 patients with mUC treated with anti-PD-(L)1 antibodies in either the first line or second line of therapy or beyond in the context of routine clinical practice and clinical trials between June 2016 and February 2020 in seven Galician medical centers (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100090). Patients received either atezolizumab 1200 mg every 3 weeks, pembrolizumab 200 mg every 3 weeks, nivolumab 3 mg/kg or 240 mg every 2 weeks, or durvalumab 10 mg/kg every 2 weeks intravenously until disease progression or unacceptable toxicity.
Complete blood cell counts and LDH and albumin levels were extracted from electronic medical records. Demographic, clinical, and pathological data, as well as the use of antibiotics, steroids, and PPI were also collected. Aiming to evaluate the influence of pretreatment factors in therapy efficacy, we considered laboratory parameters and any concomitant medication use within a window of 30 days before the start of first anti-PD-(L)1 antibody infusion.
The primary efficacy endpoint was OS. Secondary endpoints were progression-free survival (PFS), disease control rate (DCR), and overall response rate (ORR). Adverse events (AEs) were recorded by investigators from medical records and reported according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Tumor responses were assessed by the investigators according to Response Evaluation Criteria in Solid Tumors guidelines version 1.1 every 10 ± 2 weeks or before if medical reasons were indicated.
The study was approved by the Galician Research Ethics Committee (2019/386) and conducted in accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. All living patients provided written informed consent before enrollment. Informed consent was waived for dead patients before study initiation.
Statistical analysis
OS was calculated from the date of ICI initiation until death resulting from any cause or last known follow-up for living patients. PFS was calculated from the date of ICI initiation until disease progression or death resulting from any cause or last known follow-up for patients with no disease progression. DCR was defined as the proportion of patients who achieved a complete or partial response and stable disease, and ORR as the proportion of patients who achieved a complete or partial response. Patients who died before radiologic assessment were considered not evaluable for response. Pretreatment laboratory parameters were dichotomized into categoric variables using either the cut-off points of normal versus abnormal from each participating institution (LDH) or based on the criteria used in previous publications [for albumin, hemoglobin, absolute platelet count and derived neutrophil-lymphocyte ratio (dNLR), the cut-off was established at 3.5 g/dl, 10 g/dl, 400 000/μl, and 3, respectively]. In the context of a real-world experience, ECOG-PS was dichotomized into two categoric variables, 0-1 versus ≥2.1 Survival estimates were calculated by the Kaplan–Meier method, and groups were compared with the log-rank test. The Cox proportional hazards regression model was used to evaluate factors independently associated with OS and PFS. Variables included in the multivariate analysis (forced entry method) were selected according to their clinical relevance and statistical significance in univariate analysis (cut-off, P < 0.10). In the second step, the final prognostic model for OS was fit using a stepwise method to select variables retained among those significant (cut-off, P < 0.10) from the first multivariate Cox regression analysis step. The proportional hazard assumption was verified with the Schoenfeld residual method. Internal validation of the final prognostic model was verified with the bootstrap method (10 000 bootstrap samples). Comparisons of Cox proportional hazard regression models were made using the Akaike information criterion (AIC), with a smaller AIC value indicating the better model. Factors associated with DCR and ORR were tested with logistic regression in univariate analyses. Variables included in the final multivariate model were selected according to their clinical relevance and statistical significance in univariate analysis (cut-off, P < 0.10). Comparisons between patient and disease characteristics were carried out using Fisher's or chi-square tests (categorical variables) and t-test (continuous variables). All P values were two-sided, and those less than 0.05 were considered statistically significant. Statistical analyses were conducted using MedCalc version 19.4.1 (Broekstraat, Belgium), GraphPad Prism version 8.4.2 (GraphPad Software, San Diego, CA, USA), and R version 3.6.2 (Vienna, Austria).
Results
Patient population
Between 23 June 2016 and 21 February 2020, 119 patients were enrolled. Baseline patient and disease characteristics are summarized in Table 1. The median age was 69 years (range, 38-89 years). Nineteen percent (n = 23) of patients were female and 81% (n = 96) male. Eighty-seven percent (n = 103) of patients had usual urothelial carcinoma histology, 7% (n = 7) urothelial carcinoma variants, and 8% (n = 9) a mixed histology. Overall, 52% (n = 62) of patients were considered cisplatin ineligible, including 7% (n = 8) with ECOG-PS 2 and 37% (n = 44) with renal impairment. All the patients were TNM (tumor-node-metastasis) stage IV at ICI initiation; 2% (n = 2) had metastatic disease in the brain and 13% (n = 16) in the peritoneum. Sixteen percent (n = 19) of the patients had zero Bellmunt risk factors, while 58% (n = 69), 25% (n = 30), and 1% (n = 1) had one, two, and three risk factors, respectively. Eighteen percent (n = 22) of patients received ICI in the first-line metastatic setting, while the remaining 82% (n = 97) received at least one previous line of chemotherapy. Sixty-seven percent (n = 80) of patients received atezolizumab, 24% (n = 29) pembrolizumab, 6% (n = 7) nivolumab, and 3% (n = 3) durvalumab. Among the 119 patients enrolled, a total of 11 (9%) received antibiotics within the prior ICI initiation window; 7 (6%) received steroids, and 54 (45%) received PPI.
Table 1.
Baseline patient and disease characteristics
| Characteristics | Patients |
|---|---|
| Population size n | 119 |
| Age years | |
| Median (range) | 69 (38-89) |
| Sex n (%) | |
| Female | 23 (19) |
| Male | 96 (81) |
| ECOG-PS n (%) | |
| 0 | 22 (18) |
| 1 | 77 (65) |
| 2 | 19 (16) |
| 3 | 1 (1) |
| Smoking status n (%) | |
| Never | 31 (26) |
| Ever | 84 (71) |
| Current | 16 (13) |
| Former | 68 (57) |
| Missing | 4 (3) |
| Body mass index n (%) | |
| <25 | 41 (34) |
| ≥25 | 78 (66) |
| Primary tumor site n (%) | |
| Bladder | 104 (87) |
| Renal pelvis | 9 (8) |
| Ureter | 6 (5) |
| Histology n (%) | |
| Usual urothelial carcinoma | 103 (87) |
| Urothelial carcinoma variants | 7 (7) |
| Micropapillary | 1 (1) |
| Plasmacytoid | 4 (4) |
| Clear cell | 1 (1) |
| Nested | 1 (1) |
| Mixed | 9 (8) |
| Urothelial carcinoma plus squamous | 5 (4) |
| Urothelial carcinoma plus squamous and adenocarcinoma | 1 (1) |
| Urothelial carcinoma plus squamous and sarcomatoid | 1 (1) |
| Urothelial carcinoma plus squamous and neuroendocrine | 1 (1) |
| Urothelial carcinoma plus adenocarcinoma | 1 (1) |
| Histological grade at diagnosis n (%) | |
| Low | 10 (8) |
| High | 107 (90) |
| Missing | 2 (2) |
| TNM stage at diagnosis n (%) | |
| 0a | 2 (2) |
| I | 20 (17) |
| II | 16 (13) |
| III | 30 (25) |
| IV | 49 (41) |
| Missing | 2 (2) |
| TNM stage IV at ICI initiation n (%) | 119 (100) |
| Site of metastases n (%) | |
| Liver | 21 (18) |
| Lung | 46 (39) |
| Bone | 37 (31) |
| Lymph node | 83 (70) |
| Peritoneum | 16 (13) |
| Brain | 2 (2) |
| Othera | 13 (11) |
| Number of metastatic sites n (%) | |
| 1 | 51 (43) |
| 2 | 41 (34) |
| 3 | 21 (18) |
| 4 | 6 (5) |
| Intravesical BCG administered n (%) | 100 (84) |
| Previous primary tumor resection n (%) | 72 (61) |
| Cystectomy | 59 (50) |
| Nephroureterectomy | 13 (11) |
| Cisplatin ineligibility n (%) | 62 (52) |
| Renal impairment | 44 (37) |
| Ischemic cardiomyopathy | 10 (8) |
| Peripheral arteriopathy | 3 (3) |
| ECOG-PS 2 | 8 (7) |
| Frailty | 3 (3) |
| Previous therapy with platinum-based regimen n (%) | 105 (88) |
| Cisplatin plus gemcitabine | 44 (37) |
| Carboplatin plus gemcitabine | 52 (44) |
| MVAC | 3 (3) |
| Carboplatin | 3 (3) |
| Carboplatin plus paclitaxel | 3 (3) |
| Number of previous systemic regimens in the metastatic setting n (%) | |
| 0 | 22 (18) |
| 1 | 86 (72) |
| 2 | 7 (6) |
| 3 | 3 (3) |
| 4 | 1 (1) |
| Immune checkpoint antibody n (%) | |
| Atezolizumab | 80 (67) |
| Pembrolizumab | 29 (24) |
| Nivolumab | 7 (6) |
| Durvalumab | 3 (3) |
| Radiotherapy n (%) | |
| Yes | 11 (9) |
| No | 108 (91) |
| Albumin n (%) | |
| <3.5 g/dl | 13 (11) |
| ≥3.5 g/dl | 102 (86) |
| Missing | 4 (3) |
| LDH n (%) | |
| ≤ULN | 83 (70) |
| >ULN | 35 (29) |
| Missing | 1 (1) |
| Hemoglobin n (%) | |
| <10 g/dl | 16 (13) |
| ≥10 g/dl | 103 (87) |
| Platelet count n (%) | |
| <400 000/μl | 106 (89) |
| ≥400 000/μl | 13 (11) |
| dNLR n (%) | |
| ≥3 | 23 (19) |
| <3 | 96 (81) |
| Antibiotic use n (%) | |
| Yes | 11 (9) |
| No | 108 (91) |
| Steroid usebn (%) | |
| Yes | 7 (6) |
| No | 112 (94) |
| PPI use n (%) | |
| Yes | 54 (45) |
| No | 65 (55) |
| Number of Bellmunt risk factors n (%) | |
| 0 | 19 (16) |
| 1 | 69 (58) |
| 2 | 30 (25) |
| 3 | 1 (1) |
BCG, Bacillus Calmette-Guérin; dNLR, derived neutrophil-lymphocyte ratio; ECOG-PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; MVAC, methotrexate, vinblastine, adriamycin, and cisplatin; PPI, proton-pump inhibitors; TNM, tumor-node-metastasis; ULN, upper limit of normal.
Adrenal gland, pancreas, pericardium, soft tissue, ureter, and surgery site.
≥10 mg of prednisone or equivalent.
Treatment exposure and safety
At the time of data collection, after a median follow-up of 9.5 months (range, 0.3-39.8), the median of cycles administered was 4 (range, 1-48). At that time, 87% (n = 104) of patients had discontinued treatment, with disease progression (62%, n = 74) the most common cause for treatment discontinuation. Eleven percent (n = 13) of patients had discontinued treatment due to AEs, and 14% (n = 17) for other causes. Treatment was ongoing in 13% (n = 15) of patients at the time of data collection.
AEs of any cause and grade were reported in 70 (59%) of 119 patients. Most AEs were mild to moderate in nature, with asthenia (n = 33, 28%), diarrhea (n = 12, 10%), pruritus (n = 8, 7%), and dermatitis (n = 8, 7%) among the most common any-grade AEs. Twenty (17%) patients experienced a grade 3 or higher AE, with asthenia (n = 4, 3%), diarrhea (n = 3, 3%), and nephritis (n = 3, 3%) as the most common. There was one reported grade 5 event (1%, urinary tract infection) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100090).
Efficacy
OS
At the time of data collection, 71% (n = 85) of enrolled patients had died. Median OS was 7.8 months (95% CI, 5.4-10.4), and the 6-, 12-, 24-, and 36-month OS rates were 56% (95% CI, 47-65), 37% (95% CI, 29-48), 20% (95% CI, 13-31), and 16% (95% CI, 9-27), respectively (Table 2, Figure 1A). Of 29 baseline variables examined, five were independently associated with poor OS: ECOG-PS ≥2 (HR = 2.50, 95% CI, 1.24-5.03; P = 0.01), hemoglobin level <10 g/dl (HR = 1.99, 95% CI, 1.02 -3.89; P = 0.04), albumin level <3.5 g/dl (HR = 2.36, 95% CI, 1.14-4.90; P = 0.02), presence of peritoneal metastases (HR = 2.40, 95% CI, 1.08-5.33; P = 0.03; Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100090), and use of PPI (HR = 1.83, 95% CI, 1.11-3.02; P = 0.02; Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2021.100090) (Table 3 and Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100090).
Table 2.
Efficacy endpoints
| Endpoints | Results |
|---|---|
| Response n (%) | |
| Complete response | 6 (5) |
| Partial response | 22 (18) |
| Stable disease | 19 (16) |
| Progressive disease | 63 (53) |
| Not evaluable | 9 (8) |
| Overall response rate % (95% CI) | 24 (15-31) |
| Disease control rate % (95% CI) | 40 (31-49) |
| Median overall survival: months (95% CI) | 7.8 (5.4-10.4) |
| 6-month overall survival rate % (95% CI) | 56 (47-65) |
| 12-month overall survival rate % (95% CI) | 37 (29-48) |
| 24-month overall survival rate % (95% CI) | 20 (13-31) |
| 36-month overall survival rate % (95% CI) | 16 (9-27) |
| Median progression-free survival: months (95% CI) | 2.8 (2.4-3.4) |
CI, confidence interval.
Figure 1.
Efficacy in the overall population.
(A) Overall survival. (B) Progression-free survival.
CI, confidence interval.
Table 3.
Multivariate Cox regression analysis for overall survival
| Characteristics | HR (95% CI) | P value |
|---|---|---|
| ECOG-PS (≥2 versus 0-1) | 2.50 (1.24-5.03) | 0.01 |
| Metastatic sites (increment of one site) | 1.14 (0.77-1.69) | 0.51 |
| Lymph node metastases (yes versus no) | 0.72 (0.35-1.49) | 0.38 |
| Liver metastases (yes versus no) | 1.97 (0.89-4.36) | 0.09 |
| Bone metastases (yes versus no) | 1.40 (0.71-2.75) | 0.33 |
| Brain metastases (yes versus no) | 3.88 (0.53-28.51) | 0.18 |
| Peritoneal metastases (yes versus no) | 2.40 (1.08-5.33) | 0.03 |
| LDH (>ULN versus ≤ULN) | 0.93 (0.52-1.64) | 0.79 |
| Albumin (<3.5 g/dl versus ≥3.5 g/dl) | 2.36 (1.14-4.90) | 0.02 |
| Hemoglobin (<10 g/dl versus ≥10 g/dl) | 1.99 (1.02-3.89) | 0.04 |
| dNLR (≥3 versus <3) | 1.69 (0.91-3.13) | 0.09 |
| Antibiotic use (yes versus no) | 1.59 (0.73-3.45) | 0.24 |
| PPI use (yes versus no) | 1.83 (1.11-3.02) | 0.02 |
Bold numbers indicate statistically significant values.
CI, confidence interval; dNLR, derived neutrophil-lymphocyte ratio; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; LDH, lactate dehydrogenase; PPI, proton-pump inhibitors; ULN, upper limit of normal.
PFS
Median PFS based on 101 PFS events was 2.8 months (95% CI, 2.4-3.4) (Table 2, Figure 1B). Of 29 baseline variables examined, 3 were independently associated with poor PFS: increment of TNM stage (HR = 1.24, 95% CI, 1.01-1.52; P = 0.04), second line of therapy or beyond (HR = 2.09, 95% CI, 1.09-3.99; P = 0.03), and use of PPI (HR = 1.94, 95% CI, 1.22-3.09; P = 0.005; Supplementary Figure S2B and Supplementary Table S4A and B, available at https://doi.org/10.1016/j.esmoop.2021.100090).
DCR and ORR
DCR and ORR were 40% (95% CI, 31-49) and 24% (95% CI, 15-31), respectively, including 6 (5%) complete responses (Table 2). Of 29 baseline variables examined, 2 were independently associated with lower DCR [increment of number of metastatic sites (OR = 0.47, 95% CI, 0.24-0.92; P = 0.03) and use of PPI (OR = 0.38, 95% CI, 0.17-0.89; P = 0.03)], and 1 with lower ORR [use of PPI (OR = 0.17, 95% CI, 0.05-0.53; P = 0.002)] (Supplementary Table S5A-D, available at https://doi.org/10.1016/j.esmoop.2021.100090).
Prognostic model for OS
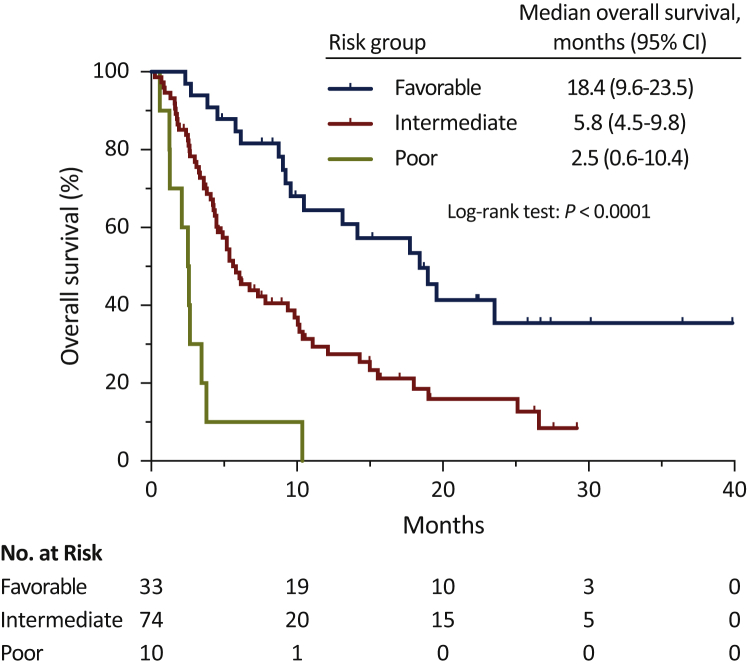
Five variables were retained in the final prognostic model for OS: ECOG-PS, PPI use, albumin level, presence of liver metastases, and presence of peritoneal metastases (Table 4 and Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2021.100090). The distribution of different patient and disease characteristics according to these five variables is shown in Supplementary Table S7A-E, available at https://doi.org/10.1016/j.esmoop.2021.100090. Considering the amount of these five prognostic factors, patients were segregated into three risk categories. Patients without any adverse prognostic factor were classified in the favorable-risk category [28%, n = 33; median OS = 18.4 months (95% CI, 9.6-23.5)]; patients with one or two adverse prognostic factors were classified in the intermediate-risk category [62%, n = 74; mOS = 5.8 months (95% CI, 4.5-9.8)]; and finally, patients with three to five adverse prognostic factors were classified in the poor-risk category [8%, n = 10; mOS = 2.5 months (95% CI, 0.6-10.4)] (log-rank test: P < 0.0001). Two cases (2%) could not be classified because the albumin level was not available. The Kaplan–Meier curves depicting these three risk categories are presented in Figure 2. Going up one category in our prognostic model triples the risk of death (HR = 3.00, 95% CI, 1.97-4.56; P = 0.0001).
Table 4.
Final multivariate Cox regression prognostic model for overall survival
| Characteristics | HR (95% CI) | P value |
|---|---|---|
| ECOG-PS (≥2 versus 0-1) | 3.20 (1.76-5.83) | 0.0001 |
| Liver metastases (yes versus no) | 2.81 (1.59-4.98) | 0.0004 |
| Peritoneal metastases (yes versus no) | 2.60 (1.40-4.83) | 0.002 |
| Albumin (<3.5 g/dl versus ≥3.5 g/dl) | 2.46 (1.28-4.74) | 0.01 |
| PPI use (yes versus no) | 1.64 (1.04-2.57) | 0.03 |
Bold numbers indicate statistically significant values.
CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; PPI, proton-pump inhibitors.
Figure 2.
Kaplan–Meier overall survival estimates according to risk-group.
CI, confidence interval.
To further appreciate the clinical significance of our risk-assessment model, we compared its goodness of fit with the three-factor prognostic model proposed by Bellmunt et al.5 Interestingly, our clinical model yielded smaller AIC (659.3 versus 679.1), confirming its better performance in this retrospective cohort.
Discussion
The treatment landscape of mUC has changed dramatically since the US FDA approved atezolizumab in May 2016. This approval was based on the results of the multicenter, single-arm, phase II trial, IMvigor210, where atezolizumab showed, in a cohort of 310 mUC patients with disease progression during or following platinum-based chemotherapy, similar ORR and longer duration of response (DoR) compared with historical chemotherapy controls. Three subsequent additional approvals of nivolumab, durvalumab, and avelumab were also based solely on the same surrogate endpoints in early-phase, single-arm clinical trials. In May 2017, pembrolizumab was approved by the US FDA in the same setting of mUC, being to date a unique anti-PD-(L)1 drug approved based on the positive results of an open-label randomized phase III trial in the post-platinum context. Unfortunately, another randomized phase III trial, the IMvigor211, failed to demonstrate a statistically significant OS advantage of atezolizumab compared with chemotherapy, although the DoR and safety profile were favorable to this drug. Furthermore, the safety and efficacy of atezolizumab have been recently confirmed in the SAUL study, a single-arm, multicenter, open-label phase IIIB trial conducted in a patient population more similar to the real-world setting. Apart from the SAUL trial, few studies have investigated the safety and efficacy of anti-PD-(L)1 antibodies in daily clinical practice.11,13,30, 31, 32, 33, 34 Taking this into account, we conducted a multicenter retrospective study in a cohort of 119 mUC patients treated with different anti-PD-(L)1 drugs. In our study, the safety and efficacy were consistent with previously reported experiences, and in line with the SAUL trial, patients with either brain metastases or ECOG-PS 2 also had worse efficacy outcomes.13,32
Identifying prognostic factors is of paramount importance as we move forward with the development of different immunotherapeutic agents. While several studies in mUC have evaluated prognostic factors in the platinum and post-platinum chemotherapy settings,4, 5, 6, 7, 8, 9, 10 not many studies have been conducted to evaluate specifically baseline pretreatment factors influencing anti-PD-(L)1 therapy outcomes.11,12,22 To address this gap in knowledge, we examined the influence of 29 pretreatment factors with perceived clinical importance on main ICI efficacy endpoints. Among the studied baseline prognostic factors, three of them require special attention, the presence of peritoneal and liver metastases, and the use of PPI.
Despite being associated with a poor prognosis in other tumor types such as gastric or colorectal cancers,15,16 the influence of the presence of peritoneal metastases has never been systematically evaluated in mUC. Herein, we described for the first time to the best of our knowledge the negative impact of peritoneal cancer spread on OS in a series of mUC patients treated with anti-PD-(L)1 antibodies. Although current evidence is scarce, one of the main biological aspects that can potentially explain the lack of efficacy of ICIs in this context is the extremely aberrant tumor vasculature observed in peritoneal carcinomatosis.35 An abnormal structure and function of tumor vessels drives an immunosuppressive tumor microenvironment characterized by hypoxia, acidosis, and high interstitial pressure. This situation generates a physicochemical barrier that makes difficult the tumor infiltration by immune cells and the delivery of many different types of therapeutic molecules.36, 37, 38 Interestingly, in our series, the percentage of cases with more metastatic sites involved was higher among those patients with peritoneal metastases, which probably underlines a more aggressive disease. On the other hand, although many different single-institution studies have correlated the plasmacytoid urothelial carcinoma variant with higher rates of peritoneal involvement,39 in our series, we did not find differences in the distribution of distinct histological subtypes based on the presence of peritoneal metastases.
Regarding the impact of liver metastases on systemic immunotherapy efficacy, recently, Yu et al.40 reported a detrimental effect in preclinical mouse models and patients. The authors found that patients with liver metastases present a reduced number of peripheral T cells and tumoral T-cell diversity and function, which means a limited benefit from immunotherapy independent of many other well-established predictive factors. Moreover, in preclinical models, activated CD8+ T cells underwent apoptosis following their interaction with FasL+CD11b+F4/80+ monocyte-derived macrophages presented in the liver.40 Similarly, in our series, the presence of liver metastases was independently associated with worse survival outcomes. Moreover, the percentage of patients with a dNLR ≥3 was higher among those with metastatic liver involvement. In accordance with the findings of Yu et al.,40 a higher dNLR could reflect a relatively small number of peripheral lymphocytes in these subgroups of patients with liver metastases.
Although previously investigated in a small retrospective study by Mukherjee et al.,41 the first solid correlation regarding the negative impact of PPI use on ICI efficacy was reported by Homicsko et al. in 2018.17 The authors retrospectively analyzed 140 melanoma patients from Checkmate 069 and found an independent significant detrimental effect of baseline PPI use on ipilimumab plus nivolumab efficacy, which was subsequently validated in an independent cohort of 68 advanced melanoma patients treated with anti-PD-1 monotherapy in the first-line setting. Recently, the same negative correlation has been described in a pooled post hoc analyses of the POPLAR and OAK studies, two randomized clinical trials which demonstrated the superior efficacy of atezolizumab over docetaxel in advanced NSCLC.18 OS and PFS were significantly shorter for PPI users in the atezolizumab group, although tests for interaction between PPI use and treatment (atezolizumab versus docetaxel) were not statistically significant. Correlation with ORR and DCR was not evaluated. Similarly, in our study, the use of PPI was associated not only with worse OS and PFS but also with lower DCR and ORR. Moreover, this correlation was confirmed after adjusting for various confounding factors in multivariate Cox and logistic regression analyses, respectively. Even lacking an external validation cohort, the clinical coherence and internal validation of these data reinforces the strength of our findings. Furthermore, our study confirms the results previously reported by Morales-Barrera et al.,42 who described a trend toward better outcomes in non-PPI users in a cohort of 95 mUC patients treated with anti-PD-(L)1 drugs alone or in combination with an anti-CTLA-4 antibody.
During the past few years, the gut microbiome has emerged as an important mediator associated with responsiveness to ICI therapy.43 Following the initial evidence in preclinical animal models for the key role in mediating anti-CTLA-4 and anti-D-L1 tumor responses, the importance of certain intestinal commensals has been subsequently substantiated in humans with different cancer types.44,45 A high diversity of the gut microbiome and abundance of certain commensal bacteria of the intestinal microbiome such as Faecalibacterium spp and Akkermansia muciniphila have been associated with improved ICI efficacy outcomes in various scenarios.46,47 This positive effect seems to be mediated by a systemic and tumoral modulation of the immune system driven by a favorable gut microbiome. On the other hand, there is available evidence suggesting the role of PPI in altering the functionality of the immune system through gut microbiome modulation.48, 49, 50 Together, these data may provide a rational explanation for the negative impact of PPI use on anti-PD-(L)1 efficacy. Considering the use of PPI a modifiable risk factor, these data should encourage physicians to carefully evaluate PPI use in mUC patients candidates for anti-PD-(L)1 monotherapy in advance. The prognostic impact of other co-medications such as antibiotics or steroids was not confirmed in our study, despite seeing a trend toward higher risk of death and disease progression among patients commencing the use of these drugs before anti-PD-(L)1 initiation.
To understand the clinical influence of the different independent prognostic factors altogether, we developed a simple model to segregate patients into three categories based on risk of death: favorable, intermediate, and poor prognostic groups. Among the factors traditionally included in the two best established prognostic models in mUC,4,5 only the presence of liver metastases and ECOG-PS were retained in our model. Again, ECOG-PS appears as the most consistent prognostic factor in oncology, regardless of the line and type of therapy.5 The other three baseline prognostic factors retained in our model were the use of PPI, albumin level, and presence of peritoneal metastases. Interestingly, we confirmed the best performance of our model compared with the three-factor prognostic model proposed by Bellmunt et al.5 Our work, along with a recent study conducted by Sonpavde et al.,12 represent the first steps in the development of clinical prognostic models in mUC in the immune checkpoint blockade era.
Together with the aspects already discussed, one of the potential limitations of our study is the use of only one retrospective cohort with limited sample size. Although the effect size of the described significant correlations was rather big, and a substantial number of important clinical and analytical factors were considered in multivariate analyses, validation in other independent retrospective datasets and prospective cohorts from randomized clinical trials will help to confirm their prognostic significance and to clarify their specific predictive nature in the ICI scenario.
This study, besides confirming the safety and efficacy of anti-PD-(L)1 monotherapy in a daily clinical practice scenario, positions the presence of peritoneal metastases as an independent prognostic factor for OS in mUC. Furthermore, this study confirms the correlation between the use of PPI before ICI therapy initiation with poor efficacy endpoints among these patients. Whether the association is prognostic and/or predictive should be investigated further in larger prospective cohorts from randomized clinical trials. Finally, we established an easy-to-use risk-assessment model composed of five readily available clinico-analytical factors which allow for predicting OS in mUC patients treated with anti-PD-(L)1 antibodies. If validated in further studies, our risk-assessment model may represent a useful tool not only for daily clinical practice but also for patient stratification in future ICI-based clinical trials.
Acknowledgements
The authors would like to thank the patients who participated in the study and their families.
Funding
JR-B is supported by a Río Hortega fellowship from the Institute of Health Carlos III [grant number CM19/00087].
Disclosure
JR-B: travel, accommodations, expenses: Bristol-Myers Squibb, Merck Sharp & Dohme, Ipsen, PharmaMar, and Roche; honoraria for educational activities: Roche; honoraria for consultancies: Boehringer Ingelheim; institutional research funding: Roche. AM-D: travel, accommodations, expenses: Novartis, Pierre Fabre, Bristol-Myers Squibb, Merck Sharp & Dohme, PharmaMar, Roche, Pfizer, Novartis, Bayer, Ipsen, Eisai, Janssen, Astellas, Sanofi, Lilly, Amgen, Kyowa-Kirin, Boehringer Ingelheim, and AstraZeneca; honoraria for educational activities: Novartis, Pierre Fabre, Bristol-Myers Squibb, Merck Sharp & Dohme, PharmaMar, Roche, Pfizer, Novartis, Bayer, Ipsen, Eisai, Janssen, Astellas, Sanofi, Lilly, Amgen, Kyowa-Kirin, Boehringer Ingelheim, and AstraZeneca; honoraria for consultancies: Pierre Fabre, PharmaMar, Pfizer, Merck Sharp & Dohme; institutional research funding: Merck Sharp & Dohme, and Bristol-Myers Squibb. OF-C: travel, accommodations, expenses: Bristol-Myers Squibb, Ipsen, and Astellas; honoraria for educational activities: Astellas, Roche, Pfizer, Bristol-Myers Squibb, Sanofi, and EUSA Pharma; honoraria educational activities: Pierre Fabre, Novartis, Bristol-Myers Squibb, Roche, Astellas, Bayer, and Janssen. LS: travel, accommodations, expenses: Roche, Pfizer, and Ipsen; honoraria for educational activities: Roche; honoraria for consultancies: Bristol-Myers Squibb and Boehringer Ingelheim. ML-Q: travel, accommodations, expenses: Roche, Merck, Lilly, Pfizer, Ipsen, Boehringer Ingelheim, and Takeda; honoraria for educational activities: Roche, Merck, AstraZeneca, Lilly, Janssen, Ipsen, and Boehringer Ingelheim; honoraria for consultancies: Roche, MSD Oncology, Bristol-Myers Squibb, GSK, Ipsen, Boehringer Ingelheim, Takeda, Sanofi, and Tesaro. RL-L: travel, accommodations, expenses: Lilly, Novartis, Pfizer, Merck, Roche, and Bristol-Myers Squibb; honoraria for educational activities: Lilly, Novartis, Pfizer, Merck, Roche, and Bristol-Myers Squibb; honoraria for consultancies: PharmaMar, Bayer, and Pierre Fabre. SV: travel, accommodations, expenses: Pfizer, Roche, and AstraZeneca; honoraria for consultancies/educational activities: Pfizer, Lilly, Astellas, Janssen, MSD Oncology, Bayer, Roche, Bristol-Myers Squibb, Boehringer Ingelheim, AstraZeneca, Ipsen, Novartis, EUSA Pharma, Eisai, and Sanofi. UA-H: travel, accommodations, expenses: Novartis, Pierre Fabre, Bristol-Myers Squibb, Roche, Pfizer, Bayer, Ipsen, Eisai, Janssen, Astellas, and Sanofi & Kyowa-Kirin; honoraria for educational activities: Sanofi and Ipsen; honoraria for consultancies: Novartis, Pfizer, Bayer, Roche, Ipsen, Eisai, and Sanofi; institutional research funding: Pierre Fabre. The other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Bellmunt J., de Wit R., Vaughn D.J. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powles T., Park S.H., Voog E. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 4.Bajorin D.F., Dodd P.M., Mazumdar M. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17(10):3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 5.Bellmunt J., Choueiri T.K., Fougeray R. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 6.Sonpavde G., Pond G.R., Fougeray R. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol. 2013;63(4):717–723. doi: 10.1016/j.eururo.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geller N.L., Sternberg C.N., Penenberg D., Scher H., Yagoda A. Prognostic factors for survival of patients with advanced urothelial tumors treated with methotrexate, vinblastine, doxorubicin, and cisplatin chemotherapy. Cancer. 1991;67(6):1525–1531. doi: 10.1002/1097-0142(19910315)67:6<1525::aid-cncr2820670611>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Lin C.-C., Hsu C.-H., Huang C.-Y. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology. 2007;69(3):479–484. doi: 10.1016/j.urology.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Roberts J.T., von der Maase H., Sengeløv L. Long-term survival results of a randomized trial comparing gemcitabine/cisplatin and methotrexate/vinblastine/doxorubicin/cisplatin in patients with locally advanced and metastatic bladder cancer. Ann Oncol. 2006;17:v118–v122. doi: 10.1093/annonc/mdj965. [DOI] [PubMed] [Google Scholar]
- 10.Bellmunt J., Albanell J., Paz-Ares L. Pretreatment prognostic factors for survival in patients with advanced urothelial tumors treated in a phase I/II trial with paclitaxel, cisplatin, and gemcitabine. Cancer. 2002;95(4):751–757. doi: 10.1002/cncr.10762. [DOI] [PubMed] [Google Scholar]
- 11.Khaki A.R., Li A., Diamantopoulos L.N. Impact of performance status on treatment outcomes: a real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors. Cancer. 2020;126(6):1208–1216. doi: 10.1002/cncr.32645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonpavde G., Manitz J., Gao C. Five-factor prognostic model for survival of post-platinum patients with metastatic urothelial carcinoma receiving PD-L1 inhibitors. J Urol. 2020;204(6):1173–1179. doi: 10.1097/JU.0000000000001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sternberg C.N., Loriot Y., James N. Primary results from SAUL, a multinational single-arm safety study of atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract. Eur Urol. 2019;76(1):73–81. doi: 10.1016/j.eururo.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Necchi A., Mariani L., Lo Vullo S. Impact of number of cycles of platinum-based first-line chemotherapy for advanced urothelial carcinoma. J Clin Oncol. 2018;36(suppl 6):426. doi: 10.1016/j.juro.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franko J., Shi Q., Meyers J.P. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–1719. doi: 10.1016/S1470-2045(16)30500-9. [DOI] [PubMed] [Google Scholar]
- 16.Thomassen I., van Gestel Y.R., van Ramshorst B. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134(3):622–628. doi: 10.1002/ijc.28373. [DOI] [PubMed] [Google Scholar]
- 17.Homicsko K., Richtig G., Tuchmann F. Proton pump inhibitors negatively impact survival of PD-1 inhibitor based therapies in metastatic melanoma patients. Ann Oncol. 2018;29:x40. [Google Scholar]
- 18.Chalabi M., Cardona A., Nagarkar D.R. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol. 2020;31(4):525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg J.E., Hoffman-Censits J., Powles T. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England) 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samstein R.M., Lee C.-H., Shoushtari A.N. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legrand F.A., Gandara D.R., Mariathasan S. Association of high tissue TMB and atezolizumab efficacy across multiple tumor types. J Clin Oncol. 2018;36(suppl 15):12000. [Google Scholar]
- 22.Nassar A.H., Mouw K.W., Jegede O. A model combining clinical and genomic factors to predict response to PD-1/PD-L1 blockade in advanced urothelial carcinoma. Br J Cancer. 2020;122(4):555–563. doi: 10.1038/s41416-019-0686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teo M.Y., Seier K., Ostrovnaya I. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. 2018;36(17):1685–1694. doi: 10.1200/JCO.2017.75.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariathasan S., Turley S.J., Nickles D. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powles T., Kockx M., Rodriguez-Vida A. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019;25(11):1706–1714. doi: 10.1038/s41591-019-0628-7. [DOI] [PubMed] [Google Scholar]
- 26.Kim J., Kwiatkowski D., McConkey D.J. The cancer genome atlas expression subtypes stratify response to checkpoint inhibition in advanced urothelial cancer and identify a subset of patients with high survival probability. Eur Urol. 2019;75(6):961–964. doi: 10.1016/j.eururo.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Ayers M., Lunceford J., Nebozhyn M. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder A., Nathanson T., Funt S.A. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: an exploratory multi-omic analysis. PLoS Med. 2017;14(5):e1002309. doi: 10.1371/journal.pmed.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penault-Llorca F., Tixier L., Adam J. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol. 2018;29(4):953–958. doi: 10.1093/annonc/mdy014. [DOI] [PubMed] [Google Scholar]
- 30.Albini B., Nadal D., Chen C. Assessment of engraftment and function of human tonsillar and blood mononuclear cells in immunodeficient mice. Adv Exp Med Biol. 1995;371A:85–89. doi: 10.1007/978-1-4615-1941-6_15. [DOI] [PubMed] [Google Scholar]
- 31.Barata P.C., Gopalakrishnan D., Koshkin V.S. Atezolizumab in metastatic urothelial carcinoma outside clinical trials: focus on efficacy, safety, and response to subsequent therapies. Target Oncol. 2018;13(3):353–361. doi: 10.1007/s11523-018-0561-6. [DOI] [PubMed] [Google Scholar]
- 32.Pal S.K., Hoffman-Censits J., Zheng H., Kaiser C., Tayama D., Bellmunt J. Atezolizumab in platinum-treated locally advanced or metastatic urothelial carcinoma: clinical experience from an expanded access study in the United States. Eur Urol. 2018;73(5):800–806. doi: 10.1016/j.eururo.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Mhatre S.K., Chuo C.-Y., Morgans A.K. Treatment (tx) characteristics of patients (pts) with locally advanced or metastatic urothelial cancer (mUC) receiving atezolizumab (atezo) monotherapy in United States clinical practice. J Clin Oncol. 2019;37(suppl 7):381. [Google Scholar]
- 34.Umang S., Benjamin H., Adam K. Comparative effectiveness of immune checkpoint inhibitors in patients with platinum-refractory advanced urothelial carcinoma. J Urol. 2021;205(3):709–717. doi: 10.1097/JU.0000000000001412. [DOI] [PubMed] [Google Scholar]
- 35.Solass W., Horvath P., Struller F. Functional vascular anatomy of the peritoneum in health and disease. Pleura Peritoneum. 2016;1(3):145–158. doi: 10.1515/pp-2016-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaaf M.B., Garg A.D., Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018;9(2):115. doi: 10.1038/s41419-017-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 38.Miller A.M., Lemke-Miltner C.D., Blackwell S. Intraperitoneal CMP-001: a novel immunotherapy for treating peritoneal carcinomatosis of gastrointestinal and pancreaticobiliary cancer. Ann Surg Oncol. 2021;28(2):1187–1197. doi: 10.1245/s10434-020-08591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rafael R.-G.R., Michael N., Neriman G., Sangoi A.R., Presti J.C., McKenney J.K. Plasmacytoid carcinoma of the bladder: a urothelial carcinoma variant with a predilection for intraperitoneal spread. J Urol. 2012;187(3):852–855. doi: 10.1016/j.juro.2011.10.145. [DOI] [PubMed] [Google Scholar]
- 40.Yu J., Green M.D., Li S. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27(1):152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee S., Ibrahimi S., Khalid B., Roman D., Zhao D., Aljumaily R. Do proton pump inhibitors modulate the efficacy of anti-PD-1/PD-L1 therapy? A retrospective study. J Oncol Pharm Pract. 2018;25(3):762–764. doi: 10.1177/1078155218771152. [DOI] [PubMed] [Google Scholar]
- 42.Morales-Barrera R., Vidal Casinello N., Bonfill T. Effect of concurrent proton pump inhibitors (PPI) use in patients (pts) treated with immune checkpoint inhibitors (ICI) for metastatic urothelial carcinoma (mUC) J Clin Oncol. 2020;38(suppl 6):500. [Google Scholar]
- 43.Ruiz-Bañobre J., Goel A. DNA mismatch repair deficiency and immune checkpoint inhibitors in gastrointestinal cancers. Gastroenterology. 2019;156(4):890–903. doi: 10.1053/j.gastro.2018.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sivan A., Corrales L., Hubert N. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vétizou M., Pitt J.M., Daillère R. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gopalakrishnan V., Spencer C.N., Nezi L. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Routy B., Le Chatelier E., Derosa L. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 48.Imhann F., Bonder M.J., Vich Vila A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackson M.A., Goodrich J.K., Maxan M.-E. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laheij R.J.F., Sturkenboom M.C.J.M., Hassing R.-J., Dieleman J., Stricker B.H.C., Jansen J.B.M.J. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. J Am Med Assoc. 2004;292(16):1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.