Abstract
Eleven piperazine-containing 1,3-diphenylprop-2-en-1-one derivatives (PC1-PC11) were evaluated for their inhibitory activities against monoamine oxidases (MAOs), cholinesterases (ChEs), and β-site amyloid precursor protein cleaving enzyme 1 (BACE-1) with a view toward developing new treatments for neurological disorders. Compounds PC10 and PC11 remarkably inhibited MAO-B with IC50 values of 0.65 and 0.71 μM, respectively. Ten of the eleven compounds weakly inhibited AChE and BChE with > 50% of residual activities at 10 μM, although PC4 inhibited AChE by 56.6% (IC50 = 8.77 μM). Compound PC3 effectively inhibited BACE-1 (IC50 = 6.72 μM), and PC10 and PC11 moderately inhibited BACE-1 (IC50 =14.9 and 15.3 μM, respectively). Reversibility and kinetic studies showed that PC10 and PC11 were reversible and competitive inhibitors of MAO-B with Ki values of 0.63 ± 0.13 and 0.53 ± 0.068 μM, respectively. ADME predictions for lead compounds revealed that PC10 and PC11 have central nervous system (CNS) drug-likeness. Molecular docking simulations showed that fluorine atom and trifluoromethyl group on PC10 and PC11, respectively, interacted with the substrate cavity of the MAO-B active site. Our results suggested that PC10 and PC11 can be considered potential candidates for the treatment of neurological disorders such as Alzheimer’s disease and Parkinson’s disease.
Keywords: Piperazine, Chalcone, Monoamine oxidase, Acetylcholinesterase
Introduction
Of the heterogeneous and complex neurodegenerative disorders (NDDs) that largely affect the elderly, Alzheimer’s disease (AD) and Parkinson’s disease (PD) are the most prevalent and their pathogenesis have been attributed to a variety of genomic, epigenomic, and environmental factors (Van Bulck et al. 2019). Mounting evidence indicates that drugs targeting a single pathway cannot adequately address the multifactorial pathophysiologies of NDDs (Geldenhuys et al. 2011). Oxidative stress, mitochondrial dysfunction, and imbalances in the levels of enzymes that control the metabolism of biogenic amines may promote NDD progression (Barnham et al. 2004; Lin and Beal 2006). On the other hand, several molecular scaffolds have been designed to simultaneously target entities such as choline esterase (ChE), monoamine oxidases (MAOs), and β-site amyloid precursor protein cleaving enzyme 1 (β-secretase, BACE-1), to retard NDD progression (Zhang et al. 2019).
Piperazine is a heteromonocyclic, six-membered ring containing two secondary nitrogen atoms, and diazacycloalkane with a non-planar, flexible nature that can interact hydrophobically and by hydrogen bonding with target enzymes. Furthermore, piperazine is a privileged structure with acknowledged “drug-likeness” and balanced pharmacodynamic and pharmacokinetic properties (Rathi et al. 2016). Currently, more than 40 piperazine-containing drugs have been FDA-approved as antianginals, antidepressants, antiserotonergics, urologicals, anthelmintics, antineoplastic agents, nootropics, and tranquillizers (Brito et al. 2019). Furthermore, the substitution of one or both nitrogen atoms in the piperazine ring system with various structural motifs can result in significant MAO-A, MAO-B, and acetylcholinesterase (AChE) inhibitions (Pettersson et al. 2012; Kaya et al. 2017; Kumar et al. 2018; Özdemir et al. 2020; Sağlık et al. 2020; Jevtić et al. 2020; Modh et al. 2013; Sahin et al. 2018).
On the other hand, 1,3-diphenylprop-2-en-1-one is a chalcone that contains two phenyl rings separated by rotatable three-carbon units. This linker is an α, β-unsaturated ketone with trans-orientated olefinic linkage (Zhuang et al. 2017). More than 90% of the chalcones synthesized exhibit selective MAO-B inhibition (Chimenti et al. 2009; Guglielmi et al. 2020), and analogs of chalcones with furan, thiophene, indole, imidazole, or morpholine heterocyclic entities on the A ring of α, β-unsaturated ketones have been reported to act as competitive, selective, and reversible MAO-B inhibitors (Robinson et al. 2013; Mathew et al. 2016; Sasidharan et al. 2016, 2018; Mathew et al. 2019a). However, the effect of introducing the piperazine pharmacophore into the chalcone framework has not been explored in the context of MAO-B inhibition. Previously, new chalcones containing the piperazine or 2,5-dichlorothiophene moiety were synthesized and evaluated for antimicrobial activities (Tomar et al. 2007). Herein, we describe the synthesis of a series of piperazine-derived chalcones and inspected for their abilities to inhibit MAOs, ChEs, and BACE-1 in an effort to identify novel treatments for NDDs.
Materials and methods
Synthesis
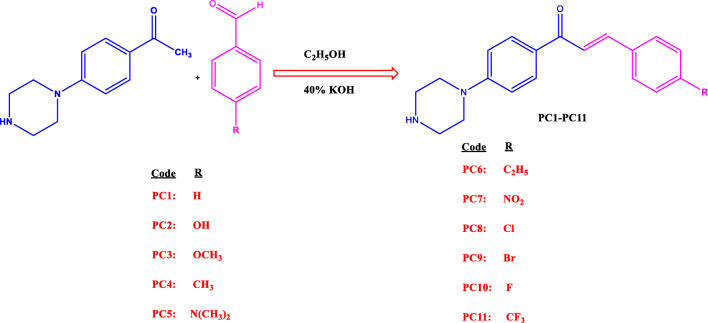
Derivatives were synthesized using base-catalyzed Claisen-Schmidt condensation reaction between various aromatic aldehydes and 4′-piperazinoacetophenone (Tomar et al. 2007). Briefly, the synthesis was initiated by adding 0.01 M 4′-piperazinoacetophenone to an ethanol (20 ml)/40% of KOH (8 ml) mix and then adding 0.01 M of an aromatic benzaldehyde and stirring for 10–12 h (Scheme 1). Resulting solutions were poured into ice-cold water, and the precipitates were washed with water. The formation of the products was checked by thin layer chromatography with a solvent system of ethyl acetate: hexane (1:9). Recrystallization was done with ethanol.
Scheme 1.
The synthetic route used
Enzyme assays
Kynuramine (0.06 mM) and benzylamine (0.3 mM) were used as substrates to assay the activities of recombinant human MAO-A and MAO-B, respectively [Mathew et al. 2018]. AChE and butyrylcholinesterase (BChE) activities were assayed using Electrophorus electricus Type VI-S and Equine serum Type, respectively, in the presence of 0.5 mM acetylthiocholine iodide (ATCI) or 0.05 mM butyrylthiocholine iodide (BTCI), respectively, by adding 0.5 mM 5, 5′-dithiobis (2-nitrobenzoic acid) (DTNB) [Ellman et al. 1961; Baek et al. 2018a; Lee et al. 2019]. Enzymes and inhibitors were preincubated for 15 min before measuring inhibitory activities. BACE-1 activity was determined using a β-secretase (BACE-1) activity detection kit, which included the 7-methoxycoumarin-4-acetyl-[Asn670,Leu671]-amyloid β/A4 protein fragment 667-676-(2,4-dinitrophenyl)Lys-Arg-Arg amide trifluoroacetate as a substrate. The reaction was performed for 2 h at 37°C and the signals were measured using a fluorescence spectrometer (FS-2, Scinco, Seoul, Korea) with an excitation wavelength of 320 nm and an emission wavelength of 405 nm. Chemicals, enzymes, and BACE-1 activity detection kits were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Enzyme inhibitory and kinetic studies
The inhibitory activities of the 11 compounds were initially measured at a concentration of 10 μM against MAO-A, MAO-B, AChE, BChE, or BACE-1. IC50 values for MAO-A and MAO-B by the compounds were determined first, and then, those for AChE, BChE, and BACE-1 by compounds with residual activities of < 50% were investigated. Kinetic studies were carried out on PC10 and PC11, which most potently inhibited MAO-B, at five substrate concentrations and three inhibitor concentrations, as previously described [Çeçen et al. 2020].
Inhibitor reversibility analysis
The reversibility of MAO-B inhibitions by PC10 and PC11 was assessed by dialysis after preincubating them at 0.15 μM with MAO-B for 30 min, as described previously [Baek et al. 2018b]. For comparison purposes, MAO-B was preincubated with lazabemide (a reference reversible MAO-B inhibitor) or pargyline (a reference irreversible MAO-B inhibitor) at 0.20 and 0.30 μM, respectively. Reversibility patterns were assessed by comparing the activities of dialyzed (AD) and undialyzed (AU) samples.
ADME prediction
ADME parameters including pharmacokinetic data and physical-chemical properties such as lipophilicity and water solubility of PC10 and PC11 were predicted in silico using free software available at http://www.swissadme.ch/ (Daina et al. 2017).
Molecular docking
The 3D-coordinates of MAO-A, MAO-B, and AChE crystals were collected from the Protein Data Bank by selecting 2Z5X, 2V5Z, and 4EY7 entries, respectively (Son 2008; Binda et al. 2007; Cheung et al. 2012). Protein Preparation Wizard tools were employed to optimize and minimize crystal structures (Schrödinger 2020c; Madhavi Sastry et al. 2013). To explore the chirality, ionization states, ring conformations, and tautomers of each input structure, PC4, PC10, and PC11 were treated by using the Ligprep tool (Schrödinger 2020b). Docking simulations were carried out using GLIDE (Friesner et al. 2004; Schrödinger 2020a), and the centers of mass of cognate ligands were used to generate the enclosing boxes. The standard precision docking protocol was used with default Force Field OPLS_2005 and detailed analysis of ligand binding affinities was performed by calculating binding free energies (ΔG) between protein and ligands using the Molecular Mechanics/Generalized Born Surface Area (MM-GBSA) method (Banks et al. 2005; Genheden and Ryde 2015). The ΔG values were calculated using: ΔGbind = ΔEMM + ΔGsolv + ΔGSA, where ΔEMM = minimized energy of the ligand-protein complex, ΔGsolv = solvation energy, and ΔGSA = surface area energy.
Results
Inhibitory activities against MAOs, ChEs, and BACE-1
All eleven compounds exhibited high inhibitory activities against MAO-B with residual activities of <50% at the concentration of 10 μM (Table 1). PC10 exhibited the greatest inhibitory activity against MAO-B (IC50 value = 0.65 μM), followed by PC11, which had an IC50 value of 0.71 μM. PC1 inhibited MAO-B least (IC50 = 7.62 μM), and the other 8 compounds had IC50 values ranging from 1.09 to 3.65 μM. A comparison of the IC50 value of PC1 with those of PC10 and PC11 indicated that the presence of a –F or –CF3 group instead of –H increased MAO-B inhibitory activity. Other substituents such as –OCH3 of PC3, –Cl of PC8, and –OH of PC2 moderately enhanced MAO-B inhibition. However, all compounds much less effectively inhibited MAO-A at 10 μM and achieved residual activities of > 65% (Table 1). PC3 had the lowest IC50 value of 27.9 μM. Regarding selectivity index (SI), PC10 and PC11 had the highest values of 48.3 and 49.2, respectively, for MAO-B over MAO-A.
Table 1.
Inhibitions of recombinant human MAO-A, MAO-B, AChE, BChE, and BACE-1 by piperazine-substituted chalconesa
| Compounds | Residual activity at 10 μM (%) | IC50 (μM) | SIb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MAO-A | MAO-B | AChE | BChE | BACE-1 | MAO-A | MAO-B | AChE | BChE | BACE-1 | ||
| PC1 | 96.6 ± 4.77 | 40.6 ± 3.93 | 72.9 ± 3.84 | 96.0 ± 4.86 | 90.6 ± 0.11 | > 40 | 7.62 ± 0.70 | - | - | - | > 5.25 |
| PC2 | 76.4 ± 1.59 | 13.3 ± 3.14 | 56.8 ± 0.96 | 97.1 ± 1.87 | 51.2 ± 0.060 | 30.1 ± 0.14 | 1.56 ± 0.14 | - | - | 9.86 ± 0.10 | 19.3 |
| PC3 | 65.7 ± 1.80 | 15.0 ± 0.79 | 58.8 ± 3.20 | 94.2 ± 1.50 | 28.9 ± 0.27 | 27.9 ± 0.55 | 1.09 ± 0.21 | - | - | 6.72 ± 0.061 | 25.6 |
| PC4 | 82.9 ± 7.20 | 29.5 ± 1.93 | 43.4 ± 2.61 | 99.8 ± 0.31 | 55.5 ± 0.34 | 29.4 ± 1.22 | 2.72 ± 0.32 | 8.77 ± 0.20 | - | 15.5 ± 0.088 | 10.8 |
| PC5 | 80.1 ± 1.96 | 23.2 ± 0.64 | 64.2 ± 2.98 | 93.7 ± 8.26 | 49.3 ± 0.83 | 34.1 ± 2.51 | 2.31 ± 0.46 | - | - | 11.6 ± 0.0011 | 14.8 |
| PC6 | 93.1 ± 0.65 | 27.3 ± 3.86 | 63.2 ± 2.23 | 82.9 ± 5.82 | 61.0 ± 0.71 | 35.6 ± 1.24 | 2.59 ± 0.18 | - | - | 16.4 ± 0.011 | 13.8 |
| PC7 | 98.1 ± 5.24 | 35.1 ± 1.75 | 62.8 ± 1.07 | 98.7 ± 4.40 | 90.0 ± 0.71 | 35.3 ± 2.51 | 3.65 ± 0.47 | - | - | - | 9.67 |
| PC8 | 99.2 ± 2.62 | 11.6 ± 1.17 | 61.4 ± 2.14 | 94.7 ± 2.51 | 40.3 ± 0.38 | > 40 | 1.37 ± 0.027 | - | - | 9.76 ± 0.041 | > 29.2 |
| PC9 | 98.6 ± 7.86 | 26.9 ± 0.58 | 71.2 ± 1.07 | 89.1 ± 0.31 | 105.0 ± 0.93 | > 40 | 3.07 ± 0.81 | - | - | - | > 13.0 |
| PC10 | 94.5 ± 7.79 | 9.91 ± 1.27 | 72.1 ± 0.10 | 69.6 ± 0.31 | 51.1 ± 0.74 | 31.4 ± 3.50 | 0.65 ± 0.023 | 28.0 ± 2.43 | 36.4 ± 2.36 | 14.9 ± 0.36- | 48.3 |
| PC11 | 80.9 ± 4.19 | 10.4 ± 0.64 | 68.4 ± 2.41 | 70.9 ± 0.31 | 52.2 ± 0.31 | 34.9 ± 4.10 | 0.71 ± 0.0035 | 26.3 ± 1.29 | 36.2 ± 3.65 | 15.3 ± 0.89 | 49.2 |
| Toloxatone | 1.08 ± 0.025 | - | - | - | - | ||||||
| Lazabemide | - | 0.11 ± 0.016 | - | - | - | ||||||
| Clorgyline | 0.0070 ± 0.00070 | - | - | - | - | ||||||
| Pargyline | - | 0.14 ± 0.0059 | - | - | - | ||||||
| Tacrine | - | - | 0.27 ± 0.019 | 0.060 ± 0.0022 | - | ||||||
| Donepezil | - | - | 0.0095 ± 0.0019 | 0.18 ± 0.0038 | - | ||||||
| Quercetin | - | - | - | - | 13.4 ± 0.035 | ||||||
aResults are expressed as the means ± standard errors of experiments performed in duplicate or triplicate
bSI values were calculated by dividing IC50 values for MAO-A by MAO-B values
Values for reference compounds were determined after preincubating them for 30 min with enzymes
All compounds weakly to moderately inhibited AChE and BChE by < 50% at 10 μM, except PC4, which inhibited AChE by 56.6% (IC50 = 8.77 μM) (Table 1). PC10 and PC11 had IC50 values of 28.0 and 26.3 μM, respectively, for AChE, and showed moderate inhibitory activities against BChE with IC50 values of 36.4 and 36.2 μM, respectively. Interestingly, some of the compounds effectively inhibited BACE-1 (Table 1). PC3 effectively inhibited BACE-1, and PC10 and PC11 showed moderate BACE-1 inhibitory activities with IC50 values of 14.9 and 15.3 μM, respectively; these two compounds contained fluoro and trifluoromethyl pharmacophores, respectively, on a piperazine-substituted chalcone framework.
MAO-B inhibition and its kinetics
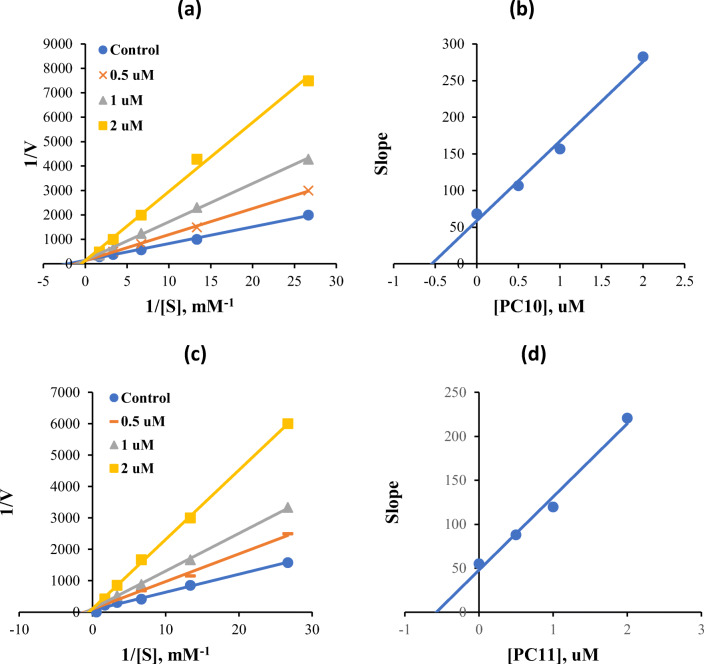
MAO-B inhibitions by PC10 and PC11 were subjected to kinetic analysis. Lineweaver-Burk and secondary plots showed that PC10 and PC11 competitively inhibited MAO-B (Fig. 1a and c), with Ki values of 0.63 ± 0.13 and 0.53 ± 0.068 μM, respectively (Fig. 1b and d). These experimental observations showed that PC10 and PC11 are competitive and selective inhibitors of MAO-B.
Fig. 1.
Lineweaver-Burk plots for MAO-B inhibition by PC10 (a) or PC11 (c), and respective secondary plots (b and d) of slopes vs. inhibitor concentrations
Reversibility studies of MAO-B inhibition
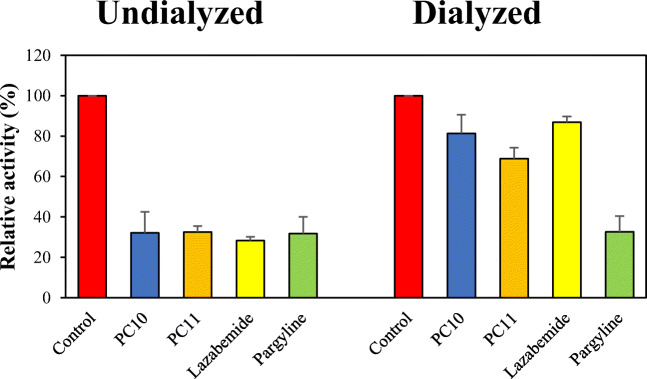
MAO-B inhibitions by PC10 and PC11 were also subjected to reversibility studies. The results obtained showed that inhibition of MAO-B by PC10 was recovered after dialysis from 32.1 (AU value) to 81.2% (AD value), and that inhibition by PC11 recovered from 32.6 (AU) to 68.9% (AD). These values were close to those of the reversible reference lazabemide (from 28.3 to 86.9%). On the other hand, inhibition of MAO-B by the irreversible reference pargyline was not recovered by dialysis (from 31.8 to 32.7%) (Fig. 2). The above data revealed that MAO-B inhibitions by PC10 and PC11 were recovered to reversible reference values and showed that PC10 and PC11 reversibly inhibit MAO-B.
Fig. 2.
Recoveries of MAO-B inhibitions by PC10 and PC11 using dialysis experiments
ADME prediction
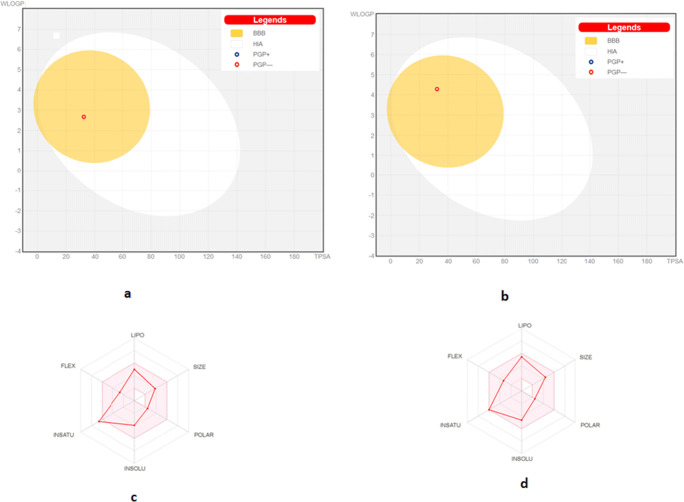
In silico ADME prediction revealed that PC10 and PC11 fully complied with the limits of Lipinski’s rule and supported their oral use and drug-likenesses (Table 2). In addition, the boiled-egg and bioavailability radar plots generated by the SwissADME tool also provided two clear ADME characteristics (Fig. 3). Boiled-egg pictures represent passive absorption in the gastrointestinal (GI) tract (shown as the white area) and the ability to cross the blood-brain barrier (BBB; yellow area). These plots showed that PC10 and PC11 were located in the yellow area, suggesting ability to cross the BBB, and indicated that both would be passively absorbed in the GI system (Fig. 3a and b). Bioavailability radar also placed the drug-likeness representation of PC11 within the pink area, which is an indicator of optimal physicochemical properties, such as size, polarity, solubility, lipophilicity, saturation, and suitability for oral administration. In this respect, PC10 showed a slight deviation in physicochemical property of saturation (Fig. 3c and d).
Table 2.
ADME predictions for PC10 and PC11
| Properties | PC10 | PC11 |
|---|---|---|
| No. H-bond acceptor | 3 | 5 |
| No. H-bond donor | 1 | 1 |
| LogPO/W(iLOGP) | 2.97 | 3.30 |
| No. rotatable bonds | 4 | 5 |
| TPSA | 32.34 | 32.34 |
| LogKP (skin permeation) | −7.39 | −6.96 |
| Lipinski’s rule violation | No | No |
| Bioavailability score | 0.55 | 0.55 |
| GI absorption | Yes | Yes |
| BBB permeation | High | Yes |
| PAINS alerts | Zero | Zero |
| P-pg substrate | No | No |
Fig. 3.
Representations of the boiled-egg graphs (a and b) and bioavailability radar plots (c and d) for PC10 and PC11 produced using the SwissADME web-tool
Molecular docking studies
Molecular docking simulations and binding free energies calculations were carried out to investigate molecular interactions between PC4, PC10, or PC11 and the binding sites of MAOs and AChE. PC10 and PC11 had docking scores of −5.73 and −4.81 kcal/mol, respectively, toward MAO-A, and of −7.29 and −7.11 kcal/mol, respectively, toward MAO-B (Table 3). In addition, the MM-GBSA values of PC10 and PC11 were −28.77 and −52.60 kcal/mol, respectively, toward MAO-A, and −64.19 and 66.06 kcal/mol, respectively, toward MAO-B (Table 3). Regarding AChE inhibition, although docking simulation returned comparable scores for the three compounds, PC4 had the lowest binding free energy (i.e., −55.81, −39.82, and −52.18 kcal/mol for PC4, PC10, and PC11, respectively) (Table 3).
Table 3.
Docking scores and MM-GBSA values for interaction between PC4, PC10, or PC11 and MAO-A, MAO-B, or AChE
| Compounds | Docking Score (kcal/mol) | MM-GBSA (kcal/mol) | ||||
|---|---|---|---|---|---|---|
| MAO-A | MAO-B | AChE | MAO-A | MAO-B | AChE | |
| PC4 | −5.85 | −7.29 | −6.10 | −12.43 | −66.34 | −55.81 |
| PC10 | −5.73 | −7.29 | −6.25 | −28.77 | −64.19 | −39.82 |
| PC11 | −4.81 | −7.11 | −5.97 | −52.60 | −66.06 | −52.18 |
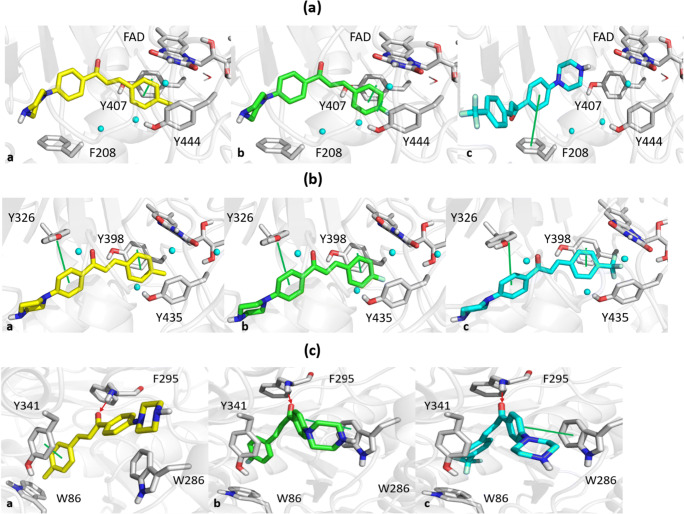
The para-methyl and para-fluorine rings of PC4 and PC10 faced FAD and interacted through π–π contacts with Y407 of MAO-A (Fig. 4a). Conversely, although slightly larger, the −CF3 substituent of PC11 did not allow its styrene moiety to face FAD, which resulted in the phenyl ring of chalcone establishing π–π interactions with F208 of MAO-A. All three compounds assumed similar binding poses within the MAO-B binding pocket (Fig. 4b), which was ascribed to the existence of two π–π interactions between chalcone aromatic rings and Y398 or Y326 of MAO-B selective residues. The styrene portion of PC4 established a π–π interaction with Y341 of AChE and the carbonyl group of its chalcone moiety formed a hydrogen bond with F295 of AChE (Fig. 4c). On the other hand, the chalcone moieties of PC10 and PC11 formed π–π interactions with W286 and hydrogen bonds with the main chain of F295.
Fig. 4.
Molecular dockings with the binding pockets of MAO-A (a), MAO-B (b), or AChE (c) by PC4 (a, yellow sticks), PC10 (b, green sticks), or PC11 (c, cyan sticks), respectively. Green lines and red arrows indicate π–π contacts and hydrogen bonds, respectively. Water molecules are depicted as cyan spheres. Docking score values for PC4, PC10, or PC11 with MAO-A were −5.845, −5.730, and −4.811 kcal/mol, respectively; with MAO-B were −7.293, −7.285, and −7.113 kcal/mol, respectively; and with AChE were −6.099, −5.584, and −6.681 kcal/mol, respectively
Discussion
Chalcones are considered versatile scaffolds for many of the CNS-related agents, such as anti-depressants, anxiolytics, β-amyloid plaque imaging agents, adenosine receptor antagonists, and MAO-B and AChE inhibitors (Mathew et al. 2019b). The three rotatable bonds available in the Michael acceptor between the two phenyl systems of chalcones provide different mode interactions in the inhibitor binding cavities of the enzymes, which are highly dependent on the natures and bulkinesses of groups bearing on the A and B chalcone rings (Matos et al. 2015).
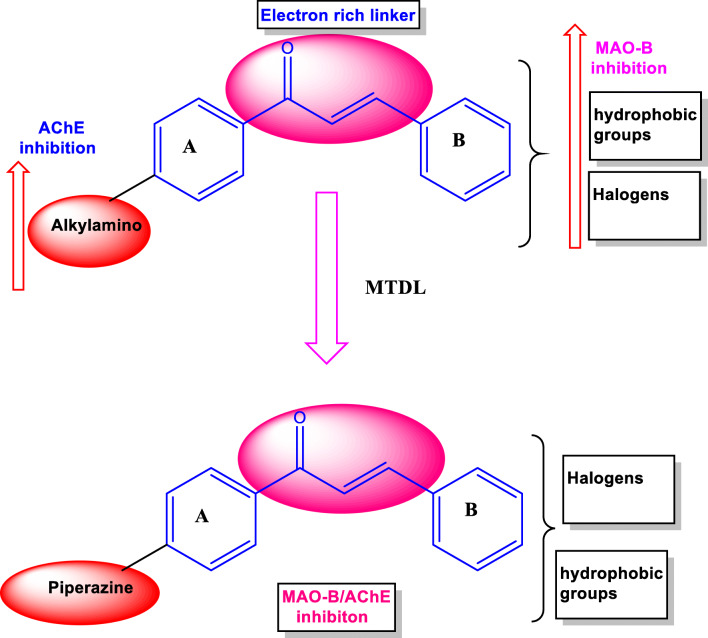
Recent studies have reported that the presence of electron-donating groups, such as methyl, methoxy, ethyl, dimethylamino, and ethyl acetohydroxamate, at the para position of the phenyl B ring of chalcones confers greater MAO-B inhibition than MAO-A inhibition. Lipophilic halogen atoms (fluorine, chlorine, and bromine) at the same position also resulted in outstanding MAO-B inhibition (Morales-Camilo et al. 2015; Reeta et al. 2019; Shalaby et al. 2019). In addition, the presence of an aliphatic or methyl-containing amino group or a nitrogen-derived pharmacophore in chalcones is required for AChE inhibition (Liu et al. 2016; Xiao et al. 2017; Bai et al. 2019). In this respect, the present design strategy explores the effects of the presence of both pharmacophores in the chalcone framework (Fig. 5) by introducing a piperazine nucleus at the para position of the phenyl A ring and various electron-donating or electron-withdrawing substituents on the B ring of the chalcone scaffold. Recently, Sasidharan et al., reported that morpholine-bearing chalcones exhibited dual-acting inhibitory activities, i.e., selective MAO-B inhibition with moderate AChE inhibition (Sasidharan et al. 2021).
Fig. 5.
Design strategy used to produce piperazine-based multi-target directed ligands (MTDLs)
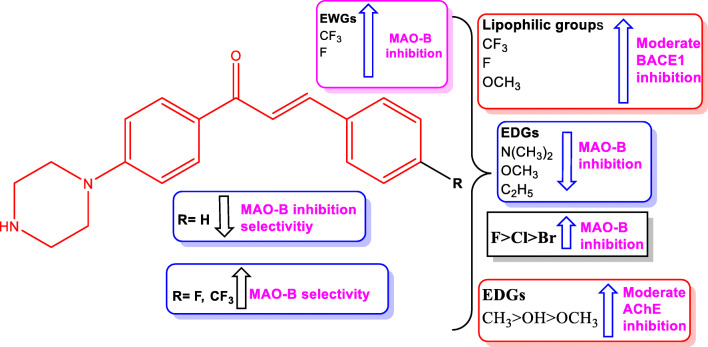
In the present study, the unsubstituted piperazine-based chalcone (PC1) exhibited moderate MAO-B inhibition with an IC50 value of 7.62 μM but high residual activities for MAO-A, AChE, BChE, and BACE-1 (96.6%, 72.9%, 96.0%, and 90.6%, respectively) at 10 μM. The introductions of small groups on the B ring of the phenyl system slightly impacted activity ratio toward multi-targets, which emphasizes the importance of substituents on the B ring. Some interesting structure-activity relationships (SARs) were derived, as depicted in Fig. 6.
Fig. 6.
SAR analyses of the 11 piperazine-based chalcones produced
The SAR studies revealed that all piperazine-substituted chalcones inhibited MAO-B substantially or moderately better than MAO-A. The presence of electron-withdrawing groups like trifluoromethyl or fluorine provided good MAO-B inhibition and the presence of a methyl group (electron-donating) on the para position of the phenyl B ring provided optimal activity as exemplified by PC4, which had IC50 values for MAO-B and AChE of 2.72 and 8.77 μM, respectively, and inhibited BACE-1 by 44.5% at 10 μM. Whereas the introduction of electron-donating groups like methyl (PC4), hydroxyl (PC2), or methoxy (PC3) groups resulted in moderate AChE inhibition (residual activities of 43.4%, 56.8%, and 58.8%, respectively, at 10 μM). Moreover, these electron-donating groups showed moderately selective inhibition of MAO-B. Regarding the effects of halogen substitution at phenyl ring on MAO-B inhibition, fluorine (PC10) had a greater effect than chlorine (PC8) or bromine (PC9). The high electronegativity of fluorine substituted phenyl in various molecular frameworks has been recently reported to increase hydrophobic interactions with Tyr398 and Tyr435 markedly in the active site of MAO-B (Mathew et al. 2020).
MAO-B, AChE, and BACE-1 are important enzyme targets and can produce neurotoxic free radical by-products, degrade acetylcholine, and generate amyloid β (Aβ), and thus, the development of highly selective ligands that target these enzymes is of considerable interest to those involved in the development of drugs for AD (Moussa-Pacha et al. 2019; Benny and Thomas 2019). Recently, many researchers have used ligand or structure-based drug strategies to design dual-acting MAO-B/AChE inhibitors (Mathew 2020). However, this is the first study to focus on the inhibitory profiles of piperazine containing chalcone-based compounds on MAO-A, MAO-B, AChE, BChE, and BACE-1. Our findings show that the lead molecules PC4, PC10, and PC11 selectively inhibit MAO-B at the submicromolar level and moderately inhibit AChE and BACE-1.
Reversible MAO-B inhibitors have shorter action durations than irreversible inhibitors because they dissociate from targets (Tipton 2018). Many new reversible MAO-B inhibitors have been developed using various structural scaffolds, such as chromones, coumarins, chalcones, phenyloxazolidinones, and pyrazolines (Carradori and Silvestri 2015; Mathew et al. 2017; Mathew 2020). Our reversibility studies on PC10 or PC11 inhibition of MAO-B indicate that the reversible natures of their interactions cause minimal target disruption and provide improved ADME profiles.
Computational results of interactions between PC4, PC10, or PC11 and MAO-A, MAO-B, or AChE targets provided satisfactory explanations of experimental data and agreed well with experimental IC50 values. Furthermore, they showed that π–π interaction with Y326 of MAO-B (a selective residue), which is changed to I335 in MAO-A (Mangiatordi et al. 2017), is a prerequisite for interaction in its binding pocket, as previously reported (Oh et al. 2020).
Conclusion
We report the results of an investigation of the neuro-related, multi-enzyme targeting profiles of piperazine-bearing α,β-unsaturated ketones. The abilities of MAOs and ChEs inhibitions by the compounds were found to depend on the natures of substituents at the para position of the B phenyl ring of the chalcone framework. All eleven compounds inhibited MAO-B more than MAO-A, and compounds PC4, PC10, and PC11 potentially inhibited MAO-B with IC50 values of 2.72, 0.65, and 0.71 μM, respectively, and moderately inhibited AChE with IC50 values of 8.77, 28.0, and 26.3 μM, respectively. Furthermore, these experimental results were also supported by molecular docking studies. In addition, PC4, PC10, and PC11 moderately inhibited BACE-1 with IC50 values of 15.5, 14.9, and 15.3 μM, respectively, which further supports their potential use for the development of novel drugs against various neurological disorders.
Acknowledgements
This work was supported by Taif University Researchers Supporting Program (project number: TURSP-2020/269), Taif University, Saudi Arabia.
Author contribution
B. Mathew synthesized the molecules, planned the study, and edited the manuscript. J.M. Oh, H. Kim performed the biological evaluation. R.S. Baty, G.E. Batiha, D.G.T. Parambi, N. Gambacorta, O. Nicolotti conducted the computational studies. Data curation and writing the original draft preparation done by B. Mathew and H. Kim.
Funding
The authors extend their appreciation to the researchers supporting project number (TURSP-2020/269) Taif University, Taif, Saudi Arabia. This research was also supported by Amrita Vishwa Vidyapeetham University. Seed Grant Number: K‐PHAR‐20‐628 (B. Mathew).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bijo Mathew and Jong Min Oh contributed equally to this work.
Contributor Information
Bijo Mathew, Email: bijomathew@aims.amrita.edu, Email: bijovilaventgu@gmail.com.
Hoon Kim, Email: hoon@sunchon.ac.kr, Email: hoon@scnu.ac.kr.
References
- Baek SC, Park MH, Ryu HW, Lee JP, Kang MG, Park D, Park CM, Oh SR, Kim H. Rhamnocitrin isolated from Prunus padus var. seoulensis: a potent and selective reversible inhibitor of human monoamine oxidase A. Bioorg Chem. 2018;28:317–325. doi: 10.1016/j.bioorg.2018.10.051. [DOI] [PubMed] [Google Scholar]
- Baek SC, Lee HW, Ryu HW, Kang MG, Park D, Kim SH, Cho ML, Oh SR, Kim H. Selective inhibition of monoamine oxidase A by hispidol. Bioorg Med Chem Lett. 2018;15:584–588. doi: 10.1016/j.bmcl.2018.01.049. [DOI] [PubMed] [Google Scholar]
- Bai P, Wang K, Zhang P, Shi J, Cheng X, Zhang Q, Zheng C, Cheng Y, Yang J, Lu X, Sang Z. Development of chalcone-O-alkylamine derivatives as multifunctional agents against Alzheimer’s disease. Eur J Med Chem. 2019;183:111737. doi: 10.1016/j.ejmech.2019.111737. [DOI] [PubMed] [Google Scholar]
- Banks JL, Beard HS, Cao Y, Cho AE, Damm W, Farid R, Felts AK, Halgren TA, Mainz DT, Maple JR, Murphy R, Philipp DM, Repasky MP, Zhang LY, Berne BJ, Friesner RA, Gallicchio E, Levy RM. Integrated Modeling Program, Applied Chemical Theory (IMPACT) J Comput Chem. 2005;26:1752–1780. doi: 10.1002/jcc.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Benny A, Thomas J. Essential oil as treatment for Alzheimer’s disease: current and future perspectives. Planta Med. 2019;85:239–248. doi: 10.1055/a-0758-0188. [DOI] [PubMed] [Google Scholar]
- Binda C, Wang J, Pisani L, Caccia C, Carotti A, Salvati P, Edmondson DE, Mattevi A. Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: safinamide and coumarin analogs. J Med Chem. 2007;50:5848–5852. doi: 10.1021/jm070677y. [DOI] [PubMed] [Google Scholar]
- Brito AF, Moreira LKS, Menegatti R, Costa EA. Piperazine derivatives with central pharmacological activity used as therapeutic tools. Fundam Clin Pharmacol. 2019;33:13–24. doi: 10.1111/fcp.12408. [DOI] [PubMed] [Google Scholar]
- Carradori S, Silvestri R. New frontiers in selective human MAO-B inhibitors. J Med Chem. 2015;58:6717–6732. doi: 10.1021/jm501690r. [DOI] [PubMed] [Google Scholar]
- Çeçen M, Oh JM, Özdemir Z, Büyüktuncel SE, Uysal M, Abdelgawad MA, Musa A, Gambacorta N, Nicolotti O, Mathew B, Kim H. Design, synthesis, and biological evaluation of pyridazinones containing the (2-Fluorophenyl) Piperazine Moiety as Selective MAO-B Inhibitors. Molecules. 2020;25:5371. doi: 10.3390/molecules25225371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung J, Rudolph MJ, Burshteyn F, Cassidy MS, Gary EN, Love J, Franklin MC, Height JJ. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem. 2012;55:10282–10286. doi: 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- Chimenti F, Fioravanti R, Bolasco A, Chimenti P, Secci D, Rossi F, Yáñez M, Orallo F, Ortuso F, Alcaro S. Chalcones: a valid scaffold for monoamine oxidases inhibitors. J Med Chem. 2009;52:2818–2824. doi: 10.1021/jm801590u. [DOI] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Geldenhuys WJ, Youdim MB, Carroll RT, Van der Schyf CJ. The emergence of designed multiple ligands for neurodegenerative disorders. Prog Neurobiol. 2011;94:347–359. doi: 10.1016/j.pneurobio.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Genheden S, Ryde U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discovery. 2015;10:449–461. doi: 10.1517/17460441.2015.1032936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmi P, Mathew B, Secci D, Carradori S. Chalcones: unearthing their therapeutic possibility as monoamine oxidase B inhibitors. Eur J Med Chem. 2020;205:112650. doi: 10.1016/j.ejmech.2020.112650. [DOI] [PubMed] [Google Scholar]
- Jevtić II, Lai TH, Penjišević JZ, Dukić-Stefanović S, Andrić DB, Brust P, Kostić-Rajačić SV, Teodoro R. Newly synthesized fluorinated cinnamylpiperazines possessing low in vitro MAO-B binding. Molecules. 2020;25:4941. doi: 10.3390/molecules25214941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya B, Yurttaş L, Sağlik BN, Levent S, Özkay Y, Kaplancikli ZA. Novel 1-(2-pyrimidin-2-yl)piperazine derivatives as selective monoamine oxidase (MAO)-A inhibitors. J Enzyme Inhib Med Chem. 2017;32:193–202. doi: 10.1080/14756366.2016.1247054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, Sheetal MAK, Kumar V. Synthesis, biological evaluation and molecular modeling studies of phenyl-/benzhydrylpiperazine derivatives as potential MAO inhibitors. Bioorg Chem. 2018;77:252–262. doi: 10.1016/j.bioorg.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Lee JP, Kang MG, Lee JY, Oh JM, Baek SC, Leem HH, Park D, Cho ML, Kim H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorg Chem. 2019;89:103043. doi: 10.1016/j.bioorg.2019.103043. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu H, Fan H, Gao X, Huang X, Liu X, Liu L, Zhou C, Tang J, Wang Q, Liu W. Design, synthesis and preliminary structure-activity relationship investigation of nitrogen-containing chalcone derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors: a further study based on Flavokawain B Mannich base derivatives. J Enzyme Inhib Med Chem. 2016;31:580–589. doi: 10.3109/14756366.2015.1050009. [DOI] [PubMed] [Google Scholar]
- Madhavi Sastry G, Adzhigirey M, Day T, Annabhimoju R, Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Mangiatordi GF, Alberga D, Pisani L, Gadaleta D, Trisciuzzi D, Farina R, Carotti A, Lattanzi G, Catto M, Nicolotti O. A rational approach to elucidate human monoamine oxidase molecular selectivity. Eur J Pharm Sci. 2017;101:90–99. doi: 10.1016/j.ejps.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Mathew B. Privileged pharmacophore of FDA approved drugs in combination with chalcone framework: a new hope for Alzheimer’s treatment. Comb Chem High Throughput Screen. 2020;23:842–846. doi: 10.2174/1386207323999200728122627. [DOI] [PubMed] [Google Scholar]
- Mathew B, Haridas A, Uçar G, Baysal I, Adeniyi AA, Soliman ME, Joy M, Mathew GE, Lakshmanan B, Jayaprakash V. Exploration of chlorinated thienyl chalcones: a new class of monoamine oxidase-B inhibitors. Int J Biol Macromol. 2016;91:680–695. doi: 10.1016/j.ijbiomac.2016.05.110. [DOI] [PubMed] [Google Scholar]
- Mathew B, Mathew GE, Petzer JP, Petzer A. Structural exploration of synthetic chromones as selective MAO-B inhibitors: a mini review. Comb Chem High Throughput Screen. 2017;20:522–532. doi: 10.2174/1386207320666170227155517. [DOI] [PubMed] [Google Scholar]
- Mathew B, Baek SC, Grace Thomas Parambi D, Lee JP, Joy M, Annie Rilda PR, Randev RV, Nithyamol P, Vijayan V, Inasu ST, Mathew GE, Lohidakshan KK, Kumar Krishnan G, Kim H. Selected aryl thiosemicarbazones as a new class of multi-targeted monoamine oxidase inhibitors. MedChemComm. 2018;9:1871–1881. doi: 10.1039/c8md00399h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew B, Baek SC, Thomas Parambi DG, Lee JP, Mathew GE, Jayanthi S, Vinod D, Rapheal C, Devikrishna V, Kondarath SS, Uddin MS, Kim H. Potent and highly selective dual-targeting monoamine oxidase-B inhibitors: fluorinated chalcones of morpholine versus imidazole. Arch Pharm (Weinheim) 2019;352:e1800309. doi: 10.1002/ardp.201800309. [DOI] [PubMed] [Google Scholar]
- Mathew B, Parambi DGT, Sivasankarapillai VS, Uddin MS, Suresh J, Mathew GE, Joy M, Marathakam A, Gupta SV. Perspective design of chalcones for the management of CNS disorders: a mini-review. CNS Neurol Disord Drug Targets. 2019;18:432–445. doi: 10.2174/1871527318666190610111246. [DOI] [PubMed] [Google Scholar]
- Mathew B, Carradori S, Guglielmi P, Uddin MS, Kim H. New aspects of monoamine oxidase B inhibitors: the key role of halogens to open the golden door. Curr Med Chem. 2020;28:266–283. doi: 10.2174/0929867327666200121165931. [DOI] [PubMed] [Google Scholar]
- Matos MJ, Vazquez-Rodriguez S, Uriarte E, Santana L. Potential pharmacological uses of chalcones: a patent review (from June 2011 - 2014) Expert Opin Ther Pat. 2015;25:351–366. doi: 10.1517/13543776.2014.995627. [DOI] [PubMed] [Google Scholar]
- Modh RP, Kumar SP, Jasrai YT, Chikhalia KH. Design, synthesis, biological evaluation, and molecular modeling of coumarin-piperazine derivatives as acetylcholinesterase inhibitors. Arch Pharm (Weinheim) 2013;346:793–804. doi: 10.1002/ardp.201300242. [DOI] [PubMed] [Google Scholar]
- Morales-Camilo N, Salas CO, Sanhueza C, Espinosa-Bustos C, Sepúlveda-Boza S, Reyes-Parada M, Gonzalez-Nilo F, Caroli-Rezende M, Fierro A. Synthesis, biological evaluation, and molecular simulation of chalcones and aurones as selective MAO-B inhibitors. Chem Biol Drug Des. 2015;85:685–695. doi: 10.1111/cbdd.12458. [DOI] [PubMed] [Google Scholar]
- Moussa-Pacha NM, Abdin SM, Omar HA, Alniss H, Al-Tel TH. BACE1 inhibitors: current status and future directions in treating Alzheimer’s disease. Med Res Rev. 2019;40:339–384. doi: 10.1002/med.21622. [DOI] [PubMed] [Google Scholar]
- Oh JM, Rangarajan TM, Chaudhary R, Singh RP, Singh M, Singh RP, Tondo AR, Gambacorta N, Nicolotti O, Mathew B, Kim H. Novel class of chalcone oxime ethers as potent monoamine oxidase-B and acetylcholinesterase inhibitors. Molecules. 2020;25:2356. doi: 10.3390/molecules25102356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir Z, Alagöz MA, Uslu H, Karakurt A, Erikci A, Ucar G, Uysal M. Synthesis, molecular modelling and biological activity of some pyridazinone derivatives as selective human monoamine oxidase-B inhibitors. Pharmacol Rep. 2020;72:692–704. doi: 10.1007/s43440-020-00070-w. [DOI] [PubMed] [Google Scholar]
- Pettersson F, Svensson P, Waters S, Waters N, Sonesson C. Synthesis and evaluation of a set of para-substituted 4-phenylpiperidines and 4-phenylpiperazines as monoamine oxidase (MAO) inhibitors. J Med Chem. 2012;55:3242–3249. doi: 10.1021/jm201692d. [DOI] [PubMed] [Google Scholar]
- Rathi AK, Syed R, Shin HS, Patel RV. Piperazine derivatives for therapeutic use: a patent review (2010-present) Expert Opin Ther Pat. 2016;26:777–797. doi: 10.1080/13543776.2016.1189902. [DOI] [PubMed] [Google Scholar]
- Reeta, Baek SC, Lee JP, Rangarajan TM, Ayushee, Singh RP, Singh M, Mangiatordi GF, Nicolotti O, Kim H, Mathew B. Ethyl acetohydroxamate incorporated chalcones: unveiling a novel class of chalcones for multitarget monoamine oxidase-B inhibitors against Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2019;18:643–654. doi: 10.2174/1871527318666190906101326. [DOI] [PubMed] [Google Scholar]
- Robinson SJ, Petzer JP, Petzer A, Bergh JJ, Lourens AC. Selected furanochalcones as inhibitors of monoamine oxidase. Bioorg Med Chem Lett. 2013;23:4985–4989. doi: 10.1016/j.bmcl.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Sağlık BN, Cebeci O, Acar Çevik U, Osmaniye D, Levent S, Kaya Çavuşoğlu B, Ilgın S, Özkay Y, Kaplancıklı ZA. Design, synthesis, in vitro and in silico studies of new thiazolylhydrazine-piperazine derivatives as selective MAO-A inhibitors. Molecules. 2020;25:E4342. doi: 10.3390/molecules25184342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin Z, Ertas M, Bender C, Bülbül EF, Berk B, Biltekin SN, Yurttaş L, Demirayak Ş. Thiazole-substituted benzoylpiperazine derivatives as acetylcholinesterase inhibitors. Drug Dev Res. 2018;79:406–425. doi: 10.1002/ddr.21481. [DOI] [PubMed] [Google Scholar]
- Sasidharan R, Manju SL, Uçar G, Baysal I, Mathew B. Identification of indole-based chalcones: discovery of a potent, selective, and reversible class of MAO-B inhibitors. Arch Pharm (Weinheim) 2016;349:627–637. doi: 10.1002/ardp.201600088. [DOI] [PubMed] [Google Scholar]
- Sasidharan R, Baek SC, Sreedharannair Leelabaiamma M, Kim H, Mathew B. Imidazole bearing chalcones as a new class of monoamine oxidase inhibitors. Biomed Pharmacother. 2018;106:8–13. doi: 10.1016/j.biopha.2018.06.064. [DOI] [PubMed] [Google Scholar]
- Sasidharan R, Eom BH, Heo JH, Park JE, Abdelgawad MA, Musa A, Gambacorta N, Nicolotti O, Manju SL, Mathew B, Kim H. Morpholine based chalcones as dual acting monoamine oxidase-B and acetylcholinesterase inhibitors. Synthesis and biochemical investigations. J Enzyme Inhib Med Chem. 2021;36:188–197. doi: 10.1080/14756366.2020.1842390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger Release 2020-4 (2020a) Glide, Schrödinger. LLC
- Schrödinger Release 2020-4 . LigPrep, Schrödinger. New York: LLC; 2020. [Google Scholar]
- Schrödinger Release 2020-4: (2020c) Protein Preparation Wizard; Epik, Schrödinger, LLC, New York, NY, 2016; Impact, Schrödinger, LLC, New York, NY, 2016; Prime, Schrödinger, LLC, New York, NY.
- Shalaby R, Petzer JP, Petzer A, Ashraf UM, Atari E, Alasmari F, Kumarasamy S, Sari Y, Khalil A. SAR and molecular mechanism studies of monoamine oxidase inhibition by selected chalcone analogs. J Enzyme Inhib Med Chem. 2019;34:863–876. doi: 10.1080/14756366.2019.1593158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son SY. Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T (2008). Structure of human monoamine oxidase A at 2.2-A resolution: the control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci U S A. 2008;105:5739–5744. doi: 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton KF. 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna) 2018;125:1519–1551. doi: 10.1007/s00702-018-1881-5. [DOI] [PubMed] [Google Scholar]
- Tomar V, Bhattacharjee G, Kamaluddin KA. Synthesis and antimicrobial evaluation of new chalcones containing piperazine or 2,5-dichlorothiophene moiety. Bioorg Med Chem Lett. 2007;17:5321–5324. doi: 10.1016/j.bmcl.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Van Bulck M, Sierra-Magro A, Alarcon-Gil J, Perez-Castillo A, Morales-Garcia JA. Novel approaches for the treatment of Alzheimer’s and Parkinson’s disease. Int J Mol Sci. 2019;20:719. doi: 10.3390/ijms20030719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Li Y, Qiang X, Xu R, Zheng Y, Cao Z, Luo L, Yang X, Sang Z, Su F, Deng Y. Design, synthesis and biological evaluation of 4'-aminochalcone-rivastigmine hybrids as multifunctional agents for the treatment of Alzheimer's disease. Bioorg Med Chem. 2017;25:1030–1041. doi: 10.1016/j.bmc.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Zhang P, Xu S, Zhu Z, Xu J. Multi-target design strategies for the improved treatment of Alzheimer’s disease. Eur J Med Chem. 2019;176:228–247. doi: 10.1016/j.ejmech.2019.05.020. [DOI] [PubMed] [Google Scholar]
- Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z. Chalcone: a privileged structure in medicinal chemistry. Chem Rev. 2017;117:7762–7810. doi: 10.1021/acs.chemrev.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.