Abstract
Despite a collaborative effort towards developing suitable oral drug products for pediatrics over the past decade, appropriate pediatric dosage forms have remained lacking due to special considerations in dose flexibility, swallowability, palatability, and safety of excipients for pediatrics. The present research aims to develop a nanoparticle-based orodispersible pediatric drug delivery platform to improve oral bioavailability and taste of poorly water-soluble and unpalatable therapeutics. Two Biopharmaceutics Classification System II/IV compounds lopinavir (LPV) and ritonavir (RTV) with unpleasant taste were chosen as the model compounds. LPV and RTV Eudragit® E PO nanoparticles (NP) were prepared using a nanoprecipitation method and their key quality attributes and taste-masking effect were evaluated. Moreover, in vitro dissolution testing was conducted at simulated gastrointestinal pH conditions. The in vivo bioavailability of the developed NP formulations was assessed using a rat model. Following the formulation optimization, over 98% encapsulation efficiency was obtained for both LPV and RTV NP and both drugs remained amorphous in its respective NP. LPV/RTV NP combination (4/1, w/w) showed comparable in vitro dissolution to that of the commercial LPV/RTV tablet (Kaletra®). In addition, the taste-masking effect of the developed NP formulations was confirmed by an E-tongue study. The lyophilized LPV and RTV NP were completely dispersible in water within 7 sec and remained stable at 4 ± 2 °C over three months. Lastly, the pharmacokinetic study demonstrated that the LPV/RTV NP combination (4/1, w/w) had improved oral bioavailability compared to Kaletra® and their corresponding raw drug powders. The results demonstrated a novel nanoparticle-based orodispersible platform that is capable of improving oral bioavailability and taste of poorly water-soluble and unpalatable therapeutics for pediatric use.
Keywords: Bioavailability, Eudragit® E PO, Nanoparticles, Oral pediatric formulation, Orodispersible, Taste masking
1. Introduction
Despite advances in pharmaceutical formulation and manufacturing technology in the past few decades, the availability of age-appropriate oral drug products for pediatric patients has remained a challenge (Ivanovska et al., 2014; Lopez et al., 2015). This may be partially due to a diverse patient population (ranging from birth to adolescence age) with specific needs for various sub-populations (Thabet et al., 2018). For example, children under 12 years of age often have difficulty in swallowing capsules, whereas those under four years generally cannot swallow tablets. In addition, the toxicity of excipients and taste preferences may differ in children compared to adults. Acceptable palatability of oral pediatric medicinal products is critical to ensure acceptability and patient adherence. Due to the lack of appropriate pediatric products, extemporaneous compounding (e.g., breaking tablets and opening capsules) is being commonly used to convert medications approved for use in adults into pediatric formulations, which may lead to inaccurate doses, unproved clinical efficacy, as well as challenges in masking the unpleasant taste of many drugs. Consequently, the welfare of children has been greatly affected. Over the past decade, a collaborative effort towards developing child-friendly, easy-to-swallow, and palatable pediatric drug products has been made by various public authorities including the United States (U.S.) Food and Drug Administration (FDA), the National Institutes of Health (NIH), the European Medicines Agency (EMA, 2005, 2013), and the World Health Organization (WHO, 2011), to improve adherence and therapeutic effectiveness of children medicines and hence children’s health. Owing to dose flexibility and ease of administration, flavored liquid dosage forms (e.g. drops, solutions, and suspensions), are recommended for infants and younger children. However, the development of these liquid dosage forms is limited by drug solubility, stability and taste, the use of preservatives, as well as high transportation costs and special storage requirements (e.g. under refrigeration). Accordingly, there is an urgent need to develop solid dosage forms with dose flexibility and ease of swallowing for children at different developmental stages, and for other populations with swallowing difficulties. For example, small-sized tablets/multiparticulates (Harris et al., 2020; Lopez et al., 2016) and orally disintegrating films (Preis, 2015) have been recently studied for pediatric use.
Orodispersible dosage forms (e.g., orodispersible tablets, orodispersible films and oral lyophilisates) (Cilurzo et al., 2018; Douroumis, 2011; Slavkova and Breitkreutz, 2015) can be rapidly dissolvable or dispersible in the oral cavity for small children to swallow. They can also be prepared as an oral liquid for precise dosage and dose titration for children at different developmental stages. Finally, orodispersible dosage forms can be stored in a solid form and hence the stability is improved without the use of preservatives. Excipients (e.g., pH sensitive polymers and cyclodextrins) (Buckley et al., 2018; Felton, 2018; Walsh et al., 2014) and technologies (e.g., microencapsulation) (Sosnik and Augustine, 2016; Sosnik and Muhlebach, 2018) for taste masking and drug dissolution improvement are critical in the development of orodispersible formulations, in particular for Biopharmaceutical Classification System (BCS) Class II and IV compounds with poor taste.
Nanotechnology-based drug delivery systems (e.g. crystalline/amorphous nanosuspensions and polymeric or lipid nanoparticles) (Jog and Burgess, 2017; Rawal et al., 2019; Sosnik and Carcaboso, 2014) have attracted extensive attention since they can enhance dissolution rate and/or solubility and hence oral bioavailability of poorly soluble drugs. To address highly unmet medical needs in suitable pediatric formulations, the objective of the present work was to develop a nanoparticle-based orodispersible and palatable drug delivery platform for pediatrics with improved palatability and dose flexibility. A generally recognized as safe (GRAS) pH-sensitive methacrylate copolymer (Eudragit®E PO) with a taste-masking effect (Draskovic et al., 2017; Felton, 2018) was used to fabricate nanoparticles. BCS class II/IV compounds with unpleasant tastes i.e., lopinavir (LPV) and ritonavir (RTV) (Sosnik and Augustine, 2016) that are on the NIH’s Best Pharmaceuticals for Children Act (BPCA) Priority List of Pediatric Therapeutic Needs (in need of pediatric antiretroviral formulations) (NIH, 2020) were chosen as the model drugs. LPV- and RTV-loaded nanoparticles (NP) were prepared via nanoprecipitation (Martínez Rivas et al., 2017). Quality-by-design (QbD) principles were implemented for formulation and process optimizations. The critical quality attributes (CQA) of the drug-loaded NP such as particle size, drug loading and taste-masking effect were assessed. Furthermore, in vitro dissolution and in vivo bioavailability of the developed LPV and RTV NP were investigated.
2. Materials and methods
2.1. Materials
Lopinavir (LPV, ≥98.0%) and Ritonavir (RTV, ≥97.0%) were purchased from LGM Pharma (Erlanger, KY). Eudragit® E PO was kindly provided by Evonik Corporation (Piscataway, NJ). Kolliphor® P188, trifluoroacetic acid (TFA), sodium hydroxide (NaOH), hydrochloric acid (HCl), potassium phosphate monobasic (KH2PO4) were procured from Sigma Aldrich (St. Louis, MO). Trehalose was kindly gifted by Pfanstiehl, Inc. (Waukegan, IL). Kaletra® tablets (LPV/RTV: 200 mg/50 mg) were kindly provided by Professor Fatemeh Akhlaghi (University of Rhode Island). Unless otherwise specified, all organic solvents were of high-performance liquid chromatography (HPLC) grade and purchased from Fisher Scientific (Waltham, MA).
2.2. Animals
Male Sprague Dawley rats (200–250 g) were obtained from the Experimental Animal Centre at the Fujian Medical University, China. All care and handling of animals were performed in accordance with the Institutional Authority of the Fujian Medical University. All animal experiments were evaluated and approved by the Animal and Ethics Review Committee of Faculty of the Fujian Medical University, China.
2.3. Preparation of drug-loaded nanoparticles
Drug-loaded NP was prepared using a nanoprecipitation method. Risk assessment and formulation optimization were conducted based on QbD principles. Following the formulation optimization, drug (either LPV or RTV) and Eudragit® E PO (1/1, w/w) were accurately weighed and completely dissolved in methanol. The polymer/drug solution was then injected into an aqueous Kolliphor® P 188 solution (5%, w/v) dropwise (approximately 1 mL/min) using a syringe with a G25 needle under magnetic stirring. The resultant suspension was stirred for 1 hr in an ice bath and the organic solvent was then removed under vacuum using an RV 10 rotary evaporator system (IKA Works, Inc., Wilmington, NC, USA) at room temperature. The obtained NP were purified via ultracentrifugation (Beckman Coulter, Inc., Indianapolis, IN, USA) at 20,000 rpm for 30 min at 4 °C. The pellets were resuspended in a trehalose solution (NP/trehalose: 1/5, w/w) and lyophilized (FreeZone Triad Benchtop, Labconco Corporation, Kansas City, MO). The dry NP were stored at 4 °C and protected from moisture until further use. Blank NP (without drug) were prepared using the same procedures described above as a control.
2.4. High performance liquid chromatography (HPLC) analysis
LPV and RTV were quantified using Hitachi LaChrom Elite® HPLC with a DAD detector set at 210 nm. The mobile phase consists of trifluoroacetic acid (0.1%, w/v) and acetonitrile (40/60, v/v). A Phenomenex XB-C18 column (250 × 4.6 mm, 5 μm, 100 Å) was used with a flow rate of 1 mL/min and the injection volume was 20 μL. The chromatographs were analyzed using the EZChrom Elite (Version 3.3.1SP1). Good linearity was obtained in the concentration range of 0.02 to 10 μg/mL for both LPV and RTV (R2 = 0.9999). The method demonstrated adequate inter- and intra-day precision (RSD (%) < 3%) for both drugs.
2.5. Physicochemical characterization of NP
2.5.1. Particle size, particle size distribution and zeta potential
Particle size, particle size distribution (polydispersity index, PDI), and zeta potential of the prepared NP (before and after lyophilization) were measured using a Malvern Zetasizer Nano ZS90 particle sizing system (Malvern Instruments, Ltd., Malvern, UK). 10 mg of lyophilized NP was dispersed in 5 mL of water at room temperature and redispersion time was recorded (n = 3) prior to analysis. With respect to the liquid NP samples, the samples were diluted in distilled water (50-fold) prior to analysis.
2.5.2. Drug loading/encapsulation efficiency
Approximately 4 mg of NP were weighed into a 2 mL glass vial and 1 mL of methanol was added to the vial. The samples were vortexed for 2 min and sonicated until the particles were fully dissolved. The solution was filtered using polyvinylidene fluoride (PVDF, 0.45 μm) syringe filter and diluted with the mobile phase. The LPV or RTV concentration was then determined via HPLC. Drug loading was calculated based on Equation (1):
| (1) |
The NP dispersion was centrifuged at 20,000 rpm for 30 min at 4 °C and the supernatant was withdrawn and diluted using methanol. The free drug in the supernatant was analyzed using HPLC. Encapsulation efficiency (EE, %) was calculated using the following Equation (2):
| (2) |
2.5.3. X-ray crystallography
X-ray crystallography of the drug-loaded NP was determined using a Rigaku Multiplex X-ray diffractometer (XRD) (The Woodlands, TX) with a Cu Kα radiation source of 40 kV and 44 mA. Samples were placed on a horizontal quartz glass holder prior to analysis. Each diffraction pattern was collected with a step width of 0.2 in the 2θ range of 5–60°.
2.5.4. Differential scanning calorimetry (DSC)
DSC analysis was performed using a TA Q10 calorimeter (TA Instruments, New Castle, DE, USA) equipped with a refrigerated cooling accessory. The instrument was calibrated for enthalpy and heat capacity using indium and sapphire, respectively. Around 5 mg of sample was hermetically sealed in an aluminum pan and equilibrated at 0 °C and then heated up to 200 °C at a heating rate of 10 °C/min. Nitrogen gas was used for purging at a flow rate of 50 mL/min. Data was analyzed using TA universal analysis software.
2.5.5. Transmission electron microscope (TEM)
The NP was visualized using TEM (JEM 2100, JEOL Ltd, Japan) bright filed. Samples were deposited on copper grids, dried in air for 3 hr at room temperature, stained with UranyLess negative stain for 30 sec, and dried in air for 1 hr before TEM imaging.
2.6. In vitro dissolution testing
In vitro dissolution profiles of LPV and RTV NP were determined using a two-stage dissolution method in a water bath shaker with a rotation speed of 100 rpm at 37 °C (n = 3). Kaletra® tablets were tested as a control. The mixture of LPV/RTV NP (4/1, w/w) was transferred into 75 mL of 0.1 N HCl (pH 1.2) in reagent bottles (150 mL) and the dissolution testing was conducted at 37 °C for 2 hr followed by neutralization of the medium using 18.5 mL of 0.5 M NaOH and 3 mL of 2 M KH2PO4. The dissolution testing was continued for another 6 hr. At pre-determined time intervals, 1 mL of the release medium was withdrawn and replenished with fresh media. The samples of the NP groups were filtrated through a syringe filter (0.05 μm, Whatman Nuclepore™ hydrophilic membrane) to remove NP (if any). For the control group (Kaletra® tablets), the release samples were filtered using a PVDF syringe filter (0.45 μm). The release samples were analyzed via the developed HPLC method.
2.7. Taste masking assessment
2.7.1. Taste evaluation using e-tongue system
Taste-masking effect of LPV- and RTV-NP was evaluated using an ISENSO®SmarTongue e-tongue system (Isenso Group Corporation, New York, USA). The e-tongue system consists of six metallic disc electrodes (i.e., platinum, gold, palladium, titanium, tungsten, nickel, and silver) as working electrodes, a Ag/Cl electrode as the reference electrode and a platinum counter electrode as the auxiliary electrode for standard three-electrode systems. NP samples and Kaletra® were suspended in water and filtered through PVDF membrane (0.45 μm) prior to analysis. Drug solutions in 5% (w/v) Tween 80 and blank NP were studied as controls. Before analysis, the sensors were rinsed for 10 sec using deionized water to minimize and correct the drift of sensors. The detection voltage was between −1 to 1 V, with an interpulse interval of 100 mV and a sensitivity of 10−4 mol. The acquisition time was 120 sec at room temperature. The signal was collected and analyzed using the principal component analysis (PCA) (n = 6 for each sample). By using the “Masking Efficiency” in the software, we further calculated the distance that is the Euclidean distance (Choi du et al., 2014) between the center of the cluster of testing samples (e.g., LPV or RTV NP and solution, and Kaletra®) and the center of the cluster of the blank NP (the placebo).
2.7.2. Drug leakage study at the simulated saliva pH
A 30-minute drug leakage study of lyophilized LPV- and RTV-NP in 60 mM phosphate buffered saline (PBS, pH 6.8) at 37 °C was carried out to demonstrate whether NP can protect LPV and RTV from dissolution under the pH environment of saliva, thus masking the taste of LPV and RTV. Lyophilized drug-loaded NP (approximately 50 mg) was placed in 5 mL of PBS under stirring. At pre-determined intervals (e.g., 5 mins, 10 mins, 15 mins, 20 mins and 30 mins), 200 μL of sample was withdrawn and filtrated through a syringe filter (0.05 μm, Whatman Nuclepore™ hydrophilic membrane) to remove NP (if any). The samples were then analyzed via the developed HPLC method.
2.8. In vivo bioavailability study
In vivo bioavailability of the prepared NP was evaluated using a rat model. Rats were randomly divided into six groups (n = 6): LPV raw drug; RTV raw drug; Kaletra®; LPV NP; RTV NP; and LPV/RTV NP combination (4/1: w/w) (doses: 10 and 2.5 mg/kg for LPV and RTV, respectively). The prepared NP (i.e., LPV NP, RTV NP, and LPV/RTV NP combination) and Kaletra® were dispersed in distilled water for oral administration. The LPV or RTV raw drug was dispersed in sodium carboxymethylcellulose (0.5%, w/v) to facilitate administration. Blood samples were collected at pre-determined time points (e.g., 0.083, 0.17, 0.25, 0.5, 1, 2, 4, 8, 12, and 24 h following oral administration) into heparinized tubes. The blood was centrifuged at 8,000 rpm for 5 min to obtain plasma. The plasma samples were stored at −80 °C until assay.
RTV and LPV were extracted from the plasma samples using a protein-precipitation method in methanol. Diazepam was used as an internal standard (IS). Briefly, the IS solution (40 ng/mL,150 μL) was added into 50 μL of plasma samples. The samples were vortex-mixed for 2 min followed by centrifugation (4 °C) at 15,000 rpm for 15 min. The supernatant was then centrifuged at 15,000 r/min for 5 min and analyzed using LC-MS/MS consists of an Agilent 1290 Infinity II LC system and an Agilent 6410 Triple quadrupole Mass Spectrometer with an electrospray ionization (ESI) ion source. Chromatographic separation was performed on an Ultimate XDB C18 (2.1 × 50 mm, 3.5 μm, Phenomenex) through gradient elution at 30 °C. Mobile phases A and B were 5 mM ammonium formate in water and methanol, respectively. The gradient started at 80% mobile phase B which was increased to 95% over 0.7 min and held constant for an additional 3.3 min. At 4.1 min, the gradient was set back to 80% mobile phase B and the column was equilibrated with 80% mobile phase B for 2.9 min. The flow rate was 0.20 mL/min and the injection volume was 1 μL. The following MS detection parameters were used: 3,500 V capillary voltage; 30 psi nebulizer gas pressure; 10 L/min drying gas (N2) flow; and 320 °C gas temperature. The collision energy and fragmentor voltage for LPV, RTV, and IS were 43, 30, 36 eV and 130, 175, 120 V, respectively. Detection of ions was conducted in a positive-ion mode with the following transitions in a multi-reaction monitoring (MRM) mode. The monitoring ions (precursor ion → product ion) had an m/z of 629.3 → 155.0 for LPV, an m/z of 721.8 → 268.0 for RTV, and an m/z of 285.0 → 193.1 for IS. The data acquisition was ascertained by MassHunter software Version B.03.01 (Agilent, USA). Calibration curves were established on the day when the analysis was conducted. These curves ranged from 20 to 2,500 ng/mL for LPV and 6.25 to 300 ng/mL for RTV with correlation coefficients over 0.99. The relative bioavailability (Frel) was calculated using the following Eq. (3):
| (3) |
Where Frel and D are the relative bioavailability and dose, respectively. AUC0-t is the area under the plasma concentration–time curve from time zero to t of the drug (either LPV or RTV). Reference and test represent reference and test formulations, respectively.
2.9. Storage stability study
According to the Guidance for Industry Q1A (R2) Stability Testing of New Drug Substances and Products, the obtained drug-loaded NP were stored in a sealed container at 4 ± 2 °C/60%±5% relative humidity (RH). The samples were evaluated in terms of particle size, PDI, zeta potential, drug loading and solid state characteristics over a three-month storage period.
2.10. Statistical analysis
All data were expressed as a mean ± standard deviation (S.D.). Statistical analysis of the data was performed using Student’s t-test (unpaired two-sample t-tests) with p < 0.05 as the minimal level of significance.
3. Results and discussion
3.1. Development of drug-loaded NP
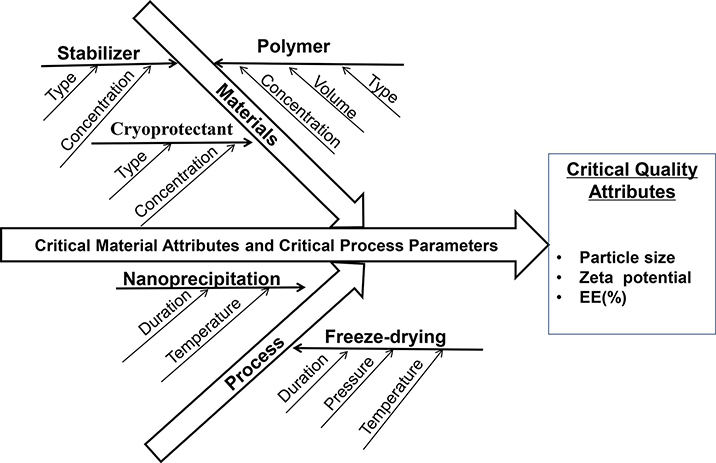
The goals for developing the present nanoparticle-based orodispersible drug delivery platform are to mask taste of unpalatable drugs, improve dissolution and hence oral bioavailability of poorly water-soluble compounds, as well as provide dose flexibility and orodispersity (e.g., disintegrating rapidly in the mouth to enable oral administration without water or chewing). In order to attain these desired features, a systematic approach to analyze and optimize formulation and processing parameters is essential. QbD (ICH, 2009) is a scientific and risk-based systematic method that can effectively shorten research time, reduce risks and improve product quality. As such, QbD principles were implemented in the present research to identify critical formulation and processing parameters that affect dissolution, palatability, and stability of the nano-sized orodispersible systems.
As shown in Fig. 1, material attributes (e.g. type of polymer, stabilizer, cryoprotectant) and processing parameters (e.g. duration and temperature of the nanoprecipitation process) may affect nanoparticle CQA (e.g. particle size, zeta potential and surface properties), thus influencing oral absorption of drug in the gastrointestinal (GI) tract. The nanoparticles with a particle size of 50 to 300 nm, positively charged and hydrophobic surface were found to have favorable absorption in the GI tract (Banerjee et al., 2016; Ghadi and Dand, 2017; Rawal et al., 2019; Yellepeddi et al., 2019). Based on the desired CQA, Eudragit® E PO, a cationic polymer and generally considered nontoxic and nonirritant, was chosen to develop the nanoparticle-based orodispersible formulations. LPV and RTV NP was designed to be formulated separately to provide the flexibility of either single or combination therapy of LPV/RTV (4/1, w/w) that is commercially available as a fixed-dose combination product Kaletra®.
Fig. 1.
Ishikawa fishbone diagram highlighting parameters that affect the critical quality attributes of nanoparticles. EE: encapsulation efficiency.
In order to improve the stability of NP without the addition of preservatives that may potentially cause safety concerns in pediatrics, dry NP were obtained via lyophilization. The effect of different cryoprotectants (e.g. sucrose, trehalose, mannitol, and lactose) on the particle size, PDI and zeta potential of NP was studied. Following the formulation and process optimizations, LPV or RTV NP were lyophilized using trehalose as the cryoprotectant (NP/trehalose: 1/5, w/w).
3.2. Physicochemical characteristics of drug-loaded NP
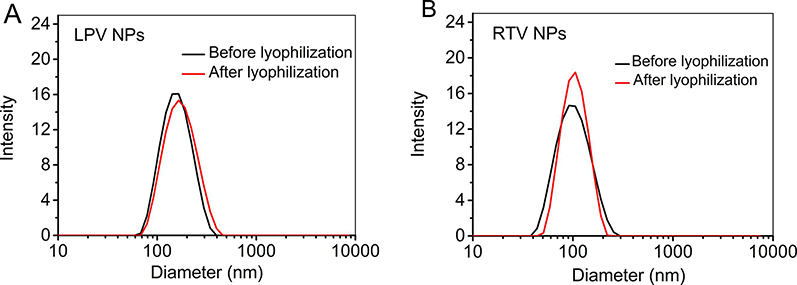
Lyophilized LPV and RTV NP were rapidly dispersible in water in 6.66 ± 1.5 sec (n = 3), indicating that solid NP-based preparations can disintegrate rapidly in the oral cavity. After reconstitution, the particle size of LPV and RTV NP was 158.2 ± 1.1 nm (PDI: 0.120) and 125.2 ± 3.7 nm (PDI: 0.241), respectively, which was similar to that of the corresponding liquid NP (Fig. 2 and Table 1). Both LPV and RTV NP (before and after lyophilization) were positively charged with a zeta potential of 36.9 mV and 47.6 mV, respectively (Table 1). This confirmed that the lyophilization process did not lead to NP aggregation in the presence of trehalose (Abdelwahed et al., 2006). Due to the strong hydrophobicity of LPV and RTV, both drug-loaded NP exhibited excellent encapsulation efficiency (over 98%).
Fig. 2.
The particle size and particle size distribution of lopinavir (LPV) (A) and ritonavir (RTV) (B) nanoparticles (NP) before and after lyophilization (n = 3).
Table 1.
Physicochemical properties of lopinavir (LPV) and ritonavir (RTV) nanoparticles (NP) (n = 3).
| LPV NP |
RTV NP |
|||
|---|---|---|---|---|
| Before lyophilization | After lyophilization | Before lyophilization | After lyophilization | |
| Drug Loading (%, w/w) | 8.21 ± 0.08 | 8.13 ± 0.03 | 8.15 ± 0.04 | 8.09 ± 0.08 |
| Encapsulation Efficiency (%) | 99.2 ± 0.14 | 98.9 ± 0.24 | 99.1 ± 0.19 | 98.9 ± 0.17 |
| Zeta Potential (mV) | 32.2 ± 0.5 | 36.9 ± 0.6 | 45.0 ± 0.7 | 47.6 ± 2.6 |
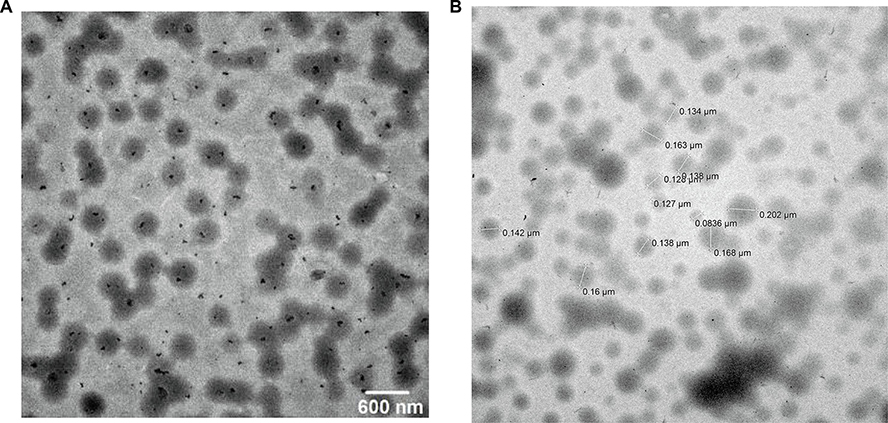
The TEM images show that the prepared NP were monodispersed spherical particles (Fig. 3A and B). The particle size of LPV and RTV NP was in good agreement with the dynamic light scattering results as shown in Fig. 2.
Fig. 3.
Transmission electron microscope (TEM) images of lopinavir (LPV) (A) and ritonavir (RTV) (B) nanoparticles (NP).
3.3. Solid-state characterization of drug-loaded NP
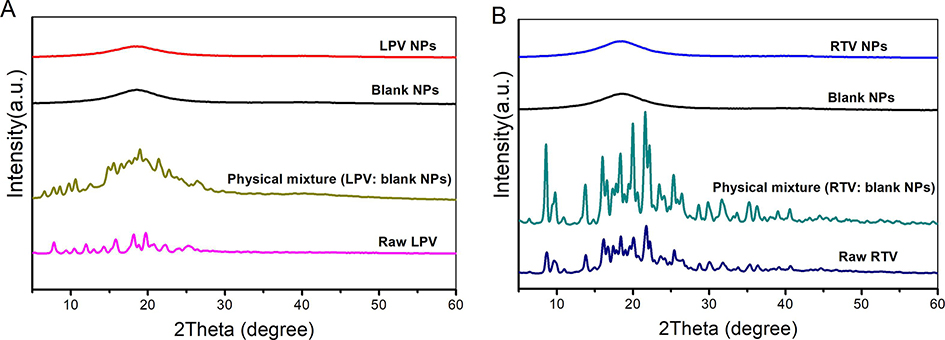
Solid-state of LPV and RTV in their respective NP was characterized via XRD and DSC. The XRD patterns of the raw drugs, physical mixtures of blank NP and drug, drug-loaded NP, as well as blank NP are shown in Fig. 4. Both raw LPV and RTV exhibit a multitude of strong peaks between 5 and 30°, indicating that raw LPV and RTV are crystalline. All the strong peaks of raw drugs were present in the physical mixtures. On the contrary, the blank NP and drug-loaded NP exhibit broad peaks around 18°, indicating that both drugs were amorphous in the NP.
Fig. 4.
X-ray diffraction patterns of raw drug, physical mixture of blank nanoparticles (NP) and raw drug, blank NP and drug-loaded NP. (A) Lopinavir (LPV) and (B) ritonavir (RTV).
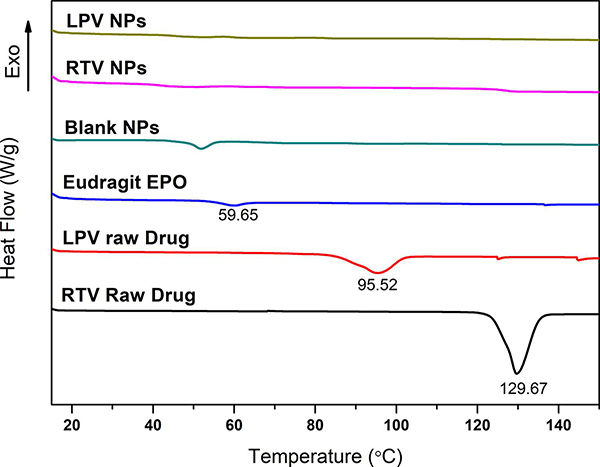
Thermal analysis results of the NP and raw drugs are shown in Fig. 5. Endothermic peaks at 95.52 °C and 129.67 °C were observed for raw LPV and RTV, respectively, indicating the crystalline nature of the raw LPV and RTV. The melting temperatures of LPV and RTV observed were consistent with that reported previously (Pham et al., 2016). The Eudragit® E PO exhibited a melting temperature (Tg) near 59.65 °C. The endothermic peaks at 95.52 °C and 129.67 °C disappeared for the LPV and RTV NP samples, indicating the amorphous nature of LPV and RTV in the NP, which was consistent with the XRD results (Fig. 4).
Fig. 5.
Differential scanning calorimetry (DSC) thermograms of raw lopinavir (LPV) and ritonavir (RTV), Eudragit® E PO, blank nanoparticles (NP), and LPV- and RTV-loaded NP.
3.4. In vitro dissolution profiles
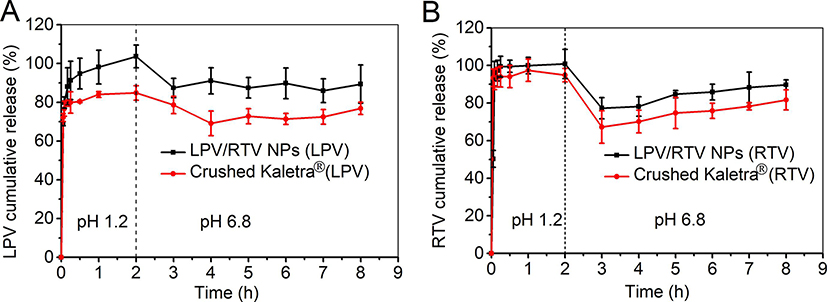
A two-stage dissolution experiment was conducted to mimic gastric and intestinal environments. The sink conditions were maintained throughout the in vitro dissolution testing. In order to monitor drug release from NP, a suitable sample-and-separate procedure (e.g. filtration) is essential to separate free drug (or released drug) from NP (Nothnagel and Wacker, 2018; Shen and Burgess, 2013). In the case of a filtration method, it is important to select an appropriate filter membrane that is compatible with the drug. Based on the membrane compatibility studies, polycarbonate membrane (0.05 μm, for drug-loaded NP) and PVDF (0.45 μm, for Kaletra® tablet) were chosen for the in vitro dissolution studies. As shown in Fig. 6, close to 100% drug dissolution was achieved for both RTV and LPV NP within 1 hr. RTV release from Kaletra® was similar to that of RTV NP under an acidic environment, which can be explained by the high RTV solubility (174.30 ± 2.91 μg/mL) under an acidic pH environment (Fig. 6A). Moreover, LPV dissolution was improved by formulating NP compared to Kaletra® (Fig. 6B). The cumulative release percentage of both LPV and RTV decreased following pH adjustment from pH 1.2 to pH 6.8. This can be explained by a drastic decrease in drug solubility at neutral pH compared to that at pH 1.2. The solubility of LPV was decreased from 0.87 ± 0.16 μg/mL at pH 1.2 to 0.52 ± 0.14 μg/mL at pH 6.8, and that of RTV was decreased from 174.30 ± 2.91 μg/mL at pH 1.2 to 0.75 ± 0.01 μg/mL at pH 6.8. These results were consistent with the previous report (Trasi et al., 2019). Overall, the LPV and RTV NP combination showed improved LPV dissolution and comparable RTV dissolution compared to Kaletra® under both pH conditions.
Fig. 6.
In vitro dissolution profiles of lopinavir (LPV) (A) and ritonavir (RTV) (B) from LPV/RTV nanoparticle (NP) combination (4/1, w/w) and Kaletra® tested in 0.1 N HCl (pH 1.2) for 2 hr and in PBS (pH 6.8) for another 6 hr in a water bath shaker with a rotation speed of 100 rpm at 37 °C (n = 3).
3.5. Storage stability results
A three-months stability study was carried out to determine whether the lyophilized NP were stable. As presented in Table 2, no significant changes in the CQA of NP were observed under the storage conditions (4 ± 2 °C/60%±5%RH) studied over three months. Moreover, the variation (<5%) of particle size, PDI, drug loading and zeta potential was within the acceptable range. Therefore, the optimized NP formulations had adequate physicochemical stability. A longer-term stability will be conducted in the future.
Table 2.
Physicochemical properties of dry lopinavir (LPV) and ritonavir (RTV) nanoparticle (NP) following a three-month storage stability study (n = 3).
| Formulation | Months | Size (nm) | PDI | Zeta potential (mv) | Drug loading (%, w/w) |
|---|---|---|---|---|---|
| LPV NP | 0 | 159.4 ± 1.1 | 0.120 ± 0.013 | 36.9 ± 0.6 | 8.17 ± 0.07 |
| 1 | 163.4 ± 4.2 | 0.136 ± 0.018 | 39.6 ± 1.33 | 7.99 ± 0.22 | |
| 2 | 165.3 ± 2.1 | 0.133 ± 0.015 | 38.5 ± 1.81 | 8.12 ± 0.13 | |
| 3 | 168.6 ± 3.6 | 0.137 ± 0.018 | 39.1 ± 1.25 | 8.03 ± 0.15 | |
| RTV NP | 0 | 125.2 ± 3.7 | 0.241 ± 0.023 | 51.2 ± 1.50 | 8.13 ± 0.18 |
| 1 | 131.9 ± 3.8 | 0.259 ± 0.015 | 50.6 ± 0.09 | 7.99 ± 0.13 | |
| 2 | 129.2 ± 1.6 | 0.245 ± 0.035 | 50.9 ± 1.21 | 8.03 ± 0.22 | |
| 3 | 135.2 ± 2.5 | 0.261 ± 0.013 | 49.2 ± 3.51 | 8.01 ± 0.11 | |
3.6. Taste masking assessment
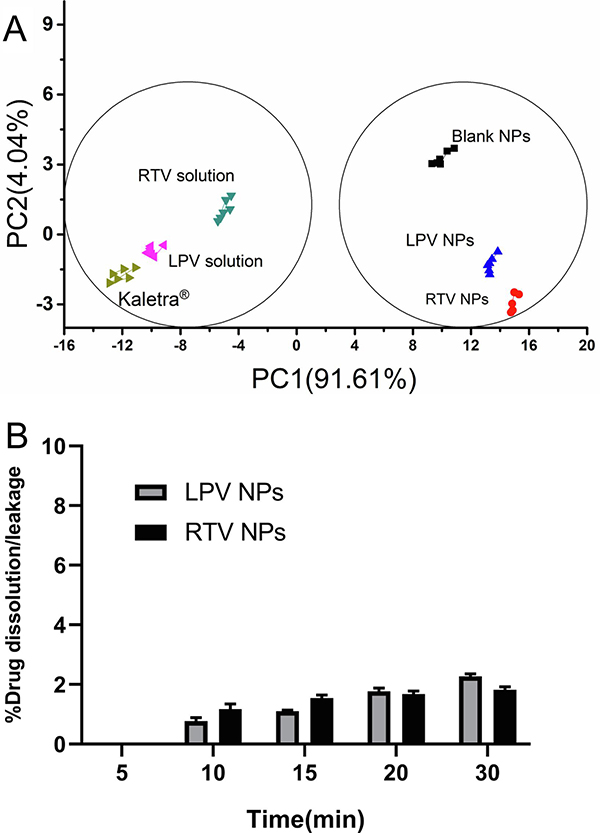
Palatability is one of the key factors to ensure acceptability and patient adherence in pediatric populations. Therefore, it is very important to consider taste-masking when developing oral pediatric medications. The e-tongue technology has been extensively used to assess taste masking effect (Choi du et al., 2014; Rachid et al., 2010; Zheng and Keeney, 2006). The assessment was based on the comparison of the distances between testing samples and placebo(s). Eudragit® E PO is GRAS and has been successfully used as a tablet-coating agent to mask taste. Therefore, blank NP was chosen as the placebo. NP was dispersed in water before the assessment. PCA, an effective statistical analysis method, was used to differentiate samples on a two-dimensional graph representing the first two principal components. The axis that contains the most variance is referred to as the first principal component (PC1); the second is the second principal component (PC2). According to the PCA chart (Fig. 7A), the relative contribution for PC1 was 91.61%, meaning that the distance along the x-axis (PC1) is the most important in distinguishing the samples. Accordingly, we assumed that PC1 represents the intensity of bitterness. It can be seen in Fig. 7A that Kaletra® and the pure drug solutions were in the same cluster, indicating that they have a similar taste. On the other hand, the cluster of LPV- and RTV-NP were close to the placebo (the blank NP), suggesting that drug-loaded NP and blank NP had a similar taste. The distance between the blank NP and tested samples were calculated and shown in Table 3. The distance value of between the blank NP and drug-loaded NP was approximately a half of that between the blank NP and the corresponding pure drug solution, as well as much less than that between the blank NP and Kaletra®, indicating that drug-loaded NP exhibited a good taste masking effect. Furthermore, <2.2% of drug (either LPV or RTV) was released/leaked from drug-loaded NP during a 30-min dissolution/leakage study at the simulated saliva pH environment, demonstrating that Eudragit® E PO NP can effectively protect both drugs from dissolution in the mouth and hence good taste masking effect (Fig. 7B). The good taste masking effect can be attributed to high EE (over 98%). Since both drugs are encapsulated in NP and have minimal or no direct contact with taste buds, the bitter taste of the drugs may be masked (Sosnik and Augustine, 2016).
Fig. 7.
Taste evaluation of lopinavir (LPV) and ritonavir (RTV) nanoparticles (NP). (A) Principal component analysis (PCA) chart of taste evaluation using an e-tongue for blank NP, lopinavir (LPV) and ritonavir (RTV) NP or solutions, and Kaletra®. (B) In vitro leakage study of lyophilized LPV- and RTV-NP in 60 mM PBS (pH 6.8) at 37 °C (n = 3).
Table 3.
Distance between blank nanoparticles (NP) and testing samples (lopinavir (LPV) and ritonavir (RTV) NP and solutions and Kaletra® tablet) based on the e-tongue measurement analyzed by “Masking Efficiency” (n = 6).
| Sample | Reference | Distances (mean ± SD) |
|---|---|---|
| RTV NP | Blank NP | 9.37 ± 1.05 |
| RTV solution | Blank NP | 18.31 ± 5.19 |
| LPV NP | Blank NP | 8.48 ± 3.34 |
| LPV solution | Blank NP | 22.61 ± 4.10 |
| Kaletra® | Blank NP | 28.52 ± 5.69 |
3.7. In vivo bioavailability
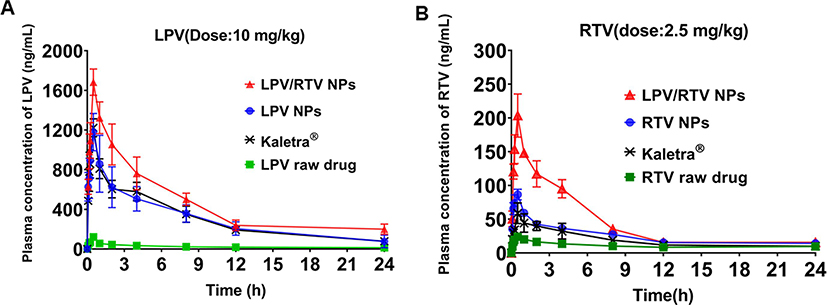
To demonstrate whether the developed NP formulations can improve oral bioavailability of LPV and RTV, a pharmacokinetic study was conducted using a rat model. The mean plasma concentration–time profiles of the drug (LPV and RTV) following oral administration are shown in Fig. 8 and the main pharmacokinetic parameters are listed in Tables 4 and 5. The AUC0-t and Cmax of either LPV or RTV in different formulations studied followed a similar rank order: the LPV/RTV NP combination > LPV or RTV NP > Kaletra® > LPV or RTV raw drug. A significant increase in LPV oral bioavailability was obtained for the LPV/RTV NP combination (21.4 folds) and LPV NP (12.4 folds) compared to the raw LPV (P < 0.0001). Moreover, the LPV/RTV NP combination and RTV NP resulted in a significant increase (P < 0.05) in RTV AUC0−t compared to the raw RTV (a 4.04- and 2.27-fold, respectively). Notably, the LPV/RTV NP combination displayed 158% and 240% relative bioavailability for LPV and RTV compared to Kaletra® with statistical significance (P < 0.05). Similarly, LPV and RTV NP demonstrated comparable bioavailability (100% and 135% relative bioavailability for LPV and RTV) to Kaletra® (P > 0.05). Formulating LPV- and RTV-NP improved dissolution of both drugs at pH 1.0. As a result, solubilized drugs were available for absorption in the intestine region, resulting in enhanced bioavailability for LPV and RTV. In addition, RTV is a protease inhibitor, albeit a much less potent one than LPV, and is capable of inhibiting CYP3A4 enzymes (Molla et al., 2002), resulted in significantly improved plasma concentration and bioavailability of LPV. Accordingly, the LPV/RTV NP combination demonstrated better absorption compared to LPV and RTV NP. Overall, the improved oral bioavailability of the NP-based platform was ascribed to the merits of the developed NP with a proper particle size (100–200 nm), positively charged surface (35–40 mv) and superior EE (over 98%).
Fig. 8.
Mean concentration–time curves of different lopinavir (LPV) (A) and ritonavir (RTV) (B) testing groups following oral administrations in rats (n = 6).
Table 4.
Pharmacokinetic parameters of lopinavir (LPV) in rats (n = 6).
| Formulations | AUC0-t (mg × h/L) | T1/2 (h) | Cmax (mg/L) | Frel (%) a | Frel (%) b |
|---|---|---|---|---|---|
| LPV/RTV NP | 12.64 ± 1.47 | 8.26 ± 3.28 | 1.68 ± 0.13 | 1897.18 ± 449.33 | 158.12 ± 44.66 |
| LPV NP | 7.29 ± 1.30Δ | 6.05 ± 2.02 | 1.22 ± 0.09Δ | 1272.67 ± 324.94 | 102.66 ± 29.65 |
| Kaletra® | 7.11 ± 1.02 | 6.91 ± 1.91 | 1.18 ± 0.19 | / | / |
| LPV raw drug | 0.59 ± 0.08 | 13.79 ± 6.81 | 0.12 ± 0.02 | / | / |
LPV: lopinavir; NP: nanoparticle
No significant difference in both AUC0-t and Cmax between the LPV NP and the commercial product (Kaletra®) (P > 0.05) while significant difference (P < 0.0001) was observed when comparing all other testing groups.
calculated based on AUC0-t by comparing to the LPV raw drug group
based on AUC0-t by comparing to Kaletra®
Table 5.
Pharmacokinetic parameters of ritonavir (RTV) in rats (n = 6).
| Formulations | AUC0-t * (mg × h/L) | T1/2 (h) | Cmax (ng/mL) | Frel (%) a | Frel (%) b |
|---|---|---|---|---|---|
| LPV/RTV NP | 1.05 ± 0.10 | 13.12 ± 0.52 | 203.54 ± 31.95 | 415.57 ± 95.67 | 239.90 ± 42.08 |
| RTV NP | 0.59 ± 0.06 | 12.38 ± 4.37 | 87.01 ± 7.90Δ | 230.90 ± 44.25 | 134.71 ± 28.75 |
| Kaletra® | 0.45 ± 0.10 | 9.02 ± 1.22 | 58.73 ± 15.32 | / | / |
| RTV raw drug | 0.26 ± 0.04 | 13.85 ± 4.89 | 24.10 ± 3.85 | / | / |
RTV: ritonavir; NP: nanoparticle
No significant difference in Cmax between the RTV NP and the commercial product (Kaletra®) (P > 0.05) while significant difference (P < 0.05) was observed when comparing all other testing groups.
Significant difference in AUC0-t was observed when comparing all testing groups (P < 0.05).
calculated based on AUC0-t by comparing to the RTV raw drug
calculated based on AUC0-t by comparing to Kaletra®
4. Conclusions
An orodispersible delivery platform composed of palatable Eudragit® E PO NP was successfully developed for potential pediatric use. The developed nanoparticle-based delivery platform offers a unique combination feature of taste masking and solubility/dissolution and absorption enhancement. The developed orodispersible platform does not require water to aid disintegration/dissolution, thus ease of swallowing. It can help improve the adherence and therapeutic efficacy of children’s medicines. The developed orodispersible and taste-masked nanoparticles can be further customized into mini-tablets or films for age-appropriate pediatric use. Taken together, the developed NP orodispersible palatable pediatric formulations is a promising pediatric drug delivery platform for poorly water-soluble and unpalatable drugs. The food/beverage effect on the taste masking effect and drug absorption of the developed NP-based pediatric drug delivery platform will be studied in the future.
Acknowledgments
The authors would like to acknowledge Dr. Irene Andrea for assisting with the TEM imaging study. This work was supported in part by the IPEC-Americas Foundation Emerging Researcher Award and the Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103430.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdelwahed W, Degobert G, Stainmesse S, Fessi H, 2006. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv. Drug Deliv. Rev 58, 1688–1713. 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S, 2016. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 238, 176–185. 10.1016/j.jconrel.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley LA, Salunke S, Thompson K, Baer G, Fegley D, Turner MA, 2018. Challenges and strategies to facilitate formulation development of pediatric drug products: Safety qualification of excipients. Int. J. Pharm 536, 563–569. 10.1016/j.ijpharm.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi du H, Kim NA, Nam TS, Lee S, Jeong SH, 2014. Evaluation of taste-masking effects of pharmaceutical sweeteners with an electronic tongue system. Drug Dev. Ind. Pharm 40, 308–317. 10.3109/03639045.2012.758636. [DOI] [PubMed] [Google Scholar]
- Cilurzo F, Musazzi UM, Franzé S, Selmin F, Minghetti P, 2018. Orodispersible dosage forms: biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 23, 251–259. 10.1016/j.drudis.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Douroumis D, 2011. Orally disintegrating dosage forms and taste-masking technologies. Expert Opin. Drug Deliv 8, 665–675. 10.1517/17425247.2011.566553. [DOI] [PubMed] [Google Scholar]
- Draskovic M, Medarevic D, Aleksic I, Parojcic J, 2017. In vitro and in vivo investigation of taste-masking effectiveness of Eudragit E PO as drug particle coating agent in orally disintegrating tablets. Drug Dev. Ind. Pharm 43, 723–731. 10.1080/03639045.2016.1220572. [DOI] [PubMed] [Google Scholar]
- EMA, 2005. Reflection paper : formulations of choice for the paediatric population (EMEA/CHMP/PEG/194810/2005). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatricuse_en.pdf (2005).
- EMA, 2013. Guideline on pharmaceutical development of medicines for paediatric use (EMA/CHMP/QWP/805880/2012 Rev.2). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicinespaediatric-use_en.pdf (15 February 2014).
- Felton LA, 2018. Use of polymers for taste-masking pediatric drug products. Drug Dev. Ind. Pharm 44, 1049–1055. 10.1080/03639045.2018.1430822. [DOI] [PubMed] [Google Scholar]
- Ghadi R, Dand N, 2017. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release 248, 71–95. 10.1016/j.jconrel.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Harris D, Hermans E, Klein S, Wagner-Hattler L, Walsh J, 2020. Age-appropriate solid oral formulations for pediatric applications with a focus on multiparticulates and minitablets: Summary of September 2019 EuPFI workshop. Eur. J. Pharm. Biopharm 153, 222–225. 10.1016/j.ejpb.2020.06.012. [DOI] [PubMed] [Google Scholar]
- ICH, 2009. Q8(R2):Pharmaceutical Development. https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (22 March 2009).
- Ivanovska V, Rademaker CM, van Dijk L, Mantel-Teeuwisse AK, 2014. Pediatric drug formulations: a review of challenges and progress. Pediatrics 134, 361–372. 10.1542/peds.2013-3225. [DOI] [PubMed] [Google Scholar]
- Jog R, Burgess DJ, 2017. Pharmaceutical Amorphous Nanoparticles. J. Pharm. Sci 106, 39–65. 10.1016/j.xphs.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Lopez FL, Bowles A, Gul MO, Clapham D, Ernest TB, Tuleu C, 2016. Effect of formulation variables on oral grittiness and preferences of multiparticulate formulations in adult volunteers. Eur. J. Pharm. Sci 92, 156–162. 10.1016/j.ejps.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Lopez FL, Ernest TB, Tuleu C, Gul MO, 2015. Formulation approaches to pediatric oral drug delivery: benefits and limitations of current platforms. Expert Opin. Drug Deliv 12, 1727–1740. 10.1517/17425247.2015.1060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez Rivas CJ, Tarhini M, Badri W, Miladi K, Greige-Gerges H, Nazari QA, Galindo Rodríguez SA, Román RÁ, Fessi H, Elaissari A, 2017. From encapsulation to drug delivery. Int. J. Pharm 532, 66–81. 10.1016/j.ijpharm.2017.08.064. [DOI] [PubMed] [Google Scholar]
- Molla A, Mo H, Vasavanonda S, Han L, Lin CT, Hsu A, Kempf DJ, 2002. In vitro antiviral interaction of lopinavir with other protease inhibitors. Antimicrob. Agents Chemother 46, 2249–2253. 10.1128/aac.46.7.2249-2253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH, 2020. Best Pharmaceuticals for Children Act (BPCA) Priority List of Needs in Pediatric Therapeutics for 2020 to 2021. https://www.nichd.nih.gov/sites/default/files/inline-files/2020PriorityListFeb20.pdf (04 April 2020).
- Nothnagel L, Wacker MG, 2018. How to measure release from nanosized carriers? Eur. J. Pharm. Sci 120, 199–211. 10.1016/j.ejps.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Pham K, Li D, Guo S, Penzak S, Dong X, 2016. Development and in vivo evaluation of child-friendly lopinavir/ritonavir pediatric granules utilizing novel in situ self-assembly nanoparticles. J. Control. Release 226, 88–97. 10.1016/j.jconrel.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Preis M, 2015. Orally disintegrating films and mini-tablets-innovative dosage forms of choice for pediatric use. AAPS PharmSciTech. 16, 234–241. 10.1208/s12249-015-0313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachid O, Simons FER, Rawas-Qalaji M, Simons KJ, 2010. An electronic tongue: evaluation of the masking efficacy of sweetening and/or flavoring agents on the bitter taste of epinephrine. AAPS PharmSciTech. 11, 550–557. 10.1208/s12249-010-9402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal M, Singh A, Amiji MM, 2019. Quality-by-Design Concepts to Improve Nanotechnology-Based Drug Development. Pharm. Res 36, 153. 10.1007/s11095-019-2692-6. [DOI] [PubMed] [Google Scholar]
- Shen J, Burgess DJ, 2013. In Vitro Dissolution Testing Strategies for Nanoparticulate Drug Delivery Systems: Recent Developments and Challenges. Drug Deliv. Transl. Res 3, 409–415. 10.1007/s13346-013-0129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavkova M, Breitkreutz J, 2015. Orodispersible drug formulations for children and elderly. Eur. J. Pharm. Sci 75, 2–9. 10.1016/j.ejps.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Sosnik A, Augustine R, 2016. Challenges in oral drug delivery of antiretrovirals and the innovative strategies to overcome them. Adv. Drug Deliv. Rev 103, 105–120. 10.1016/j.addr.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Sosnik A, Carcaboso AM, 2014. Nanomedicines in the future of pediatric therapy. Adv. Drug Deliv. Rev 73, 140–161. 10.1016/j.addr.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Sosnik A, Muhlebach S, 2018. Editorial: Drug Nanoparticles and Nano-Cocrystals: From Production and Characterization to Clinical Translation. Adv. Drug Deliv. Rev 131, 1–2. 10.1016/j.addr.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Thabet Y, Klingmann V, Breitkreutz J, 2018. Drug Formulations: Standards and Novel Strategies for Drug Administration in Pediatrics. J. Clin. Pharmacol 58, S26–S35. 10.1002/jcph.1138. [DOI] [PubMed] [Google Scholar]
- Trasi NS, Bhujbal S, Zhou QT, Taylor LS, 2019. Amorphous solid dispersion formation via solvent granulation - A case study with ritonavir and lopinavir. Int. J. Pharm.: X 1, 100035. 10.1016/j.ijpx.2019.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J, Cram A, Woertz K, Breitkreutz J, Winzenburg G, Turner R, Tuleu C, European Formulation I, 2014. Playing hide and seek with poorly tasting paediatric medicines: do not forget the excipients. Adv. Drug Deliv. Rev 73, 14–33. 10.1016/j.addr.2014.02.012. [DOI] [PubMed] [Google Scholar]
- WHO, 2011. Development of paediatric medicines: points to consider in pharmaceutical development. https://www.who.int/childmedicines/partners/SabineKopp_Partners.pdf?ua=1 (9 July 2018). [DOI] [PubMed]
- Yellepeddi VK, Joseph A, Nance E, 2019. Pharmacokinetics of nanotechnology-based formulations in pediatric populations. Adv. Drug Deliv. Rev 151–152, 44–55. 10.1016/j.addr.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JY, Keeney MP, 2006. Taste masking analysis in pharmaceutical formulation development using an electronic tongue. Int. J. Pharm 310, 118–124. 10.1016/j.ijpharm.2005.11.046. [DOI] [PubMed] [Google Scholar]