Recently, Feldmann et al. in Lancet suggested investigating TNF alpha blocker therapies for patients with COVID-19 at high risk of developing a life-threatening form [1]. The cytokine release syndrome and immuno-thrombosis induced by Sars-CoV2 mostly explain the severity of coronavirus disease 2019 (COVID-19) [2,3]. In a recent issue of Nature Medicine, Del Valle et al. reported the plasma levels of IL-6, IL-8, tumour necrosis factor (TNF)-α and IL-1β in patients hospitalised for COVID-19 [7]. They highlighted in a comprehensive multivariate analysis that high plasma levels of IL-6 and TNF-α remained significantly associated with a poor prognosis of patients with COVID-19 [7]. They proposed to measure IL-6 and TNF-α to stratify patients in further prospective clinical trials treating COVID-19 patients [7]. Targeting interleukin-6 (IL6) – one of the more proinflammatory cytokine – with the anti-IL6 receptor (anti-IL6R) have shown encouraging, although controversial, preliminary efficacy in the treatment of severe COVID-19 patients, while definitive results of clinical trials are still pending (COVACTA trial [NCT04320615]; CORIMUNO-TOCI trial [NCT04331808]; RECOVERY trial [NCT04381936] [[4], [5], [6]].
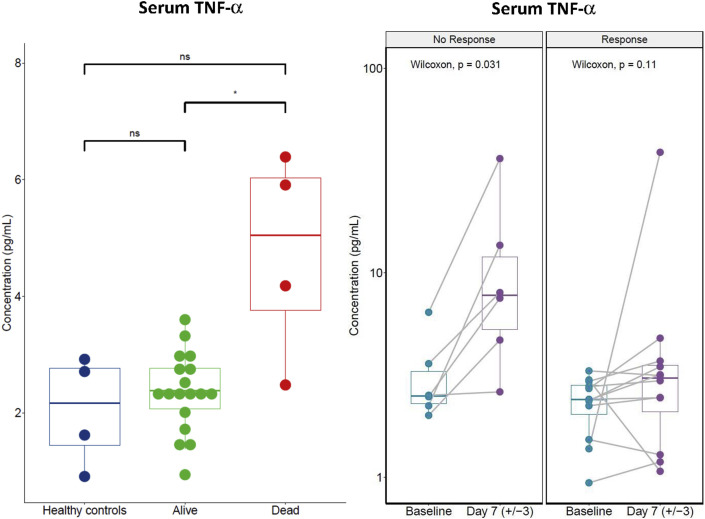
In order to better understand the effects of the anti-IL6R tocilizumab (Roche, Switzerland) on Covid19, we measured the serum cytokines levels before and during the tocilizumab therapy of 25 consecutive patients hospitalised for severe respiratory symptoms of COVID-19 at Gustave Roussy and Foch hospitals in the Paris area in France. Patients received tocilizumab (one infusion 8 mg/kg, intravenously, repeated once 12 h later at the same dose in case of absence of clinical response) in an off-label use between 20th March and 20th April 2020 (i.e. at the time of the first epidemic peak in France). All patients were treated because of worsening of their respiratory symptoms and their increase in oxygen therapy requirements ≥4 L/mn. Cytokine titration was performed on serum samples collected for routine biological assessments. Patients and healthy donors were informed of those analyses, and this project was run in compliance with the French law on human research (Loi Jardé, hors RIPH, conformité MR004). The measurements of serum IL-6 and TNF-α were done at two time points: before tocilizumab infusion ‘baseline’ and 7 days after tocilizumab. Serum IL-6 and TNF-α titrations were performed with the Meso Scale Discovery® VPlex proinflammatory assay (Meso Scale Diagnostics, USA). The main outcomes were mortality 30 days after treatment and favourable clinical response to tocilizumab. Clinical response was defined by improvement of respiratory symptoms related to COVID-19 and decrease in oxygen requirements and a WHO clinical progression scale [8] ≤ 5, following tocilizumab therapy. Patients were mainly men (22/25), median age was 56 [32–77] years old, median body mass index was 27.6 [22.7–39.0] and 13 (25%) patients had at least one comorbidity (cancer [n = 6, 24%], high blood pressure [n = 5, 20%], obesity [n = 4, 16%] or diabetes [n = 4, 16%]). Before tocilizumab, the median [range] of oxygen therapy was 9 [4–15] L/min. The median time between first COVID19 symptoms and tocilizumab infusion was 10 days (3–19). Fourteen patients received one dose of tocilizumab and the 11 other patients were treated with two doses. During follow-up, 16 (64%) patients achieved favourable clinical response to tocilizumab. Four (16%) patients died of COVID-19 in the first 30 days. Before tocilizumab, median C-reactive protein was 186 [26–396] mg/l, procalcitonin 0.15 [0.05–21.27] ng/mL, ferritin 1313 [232–4148] μg/L, d-dimer 858 [232–20,412] μg/L and fibrinogen 6.8 [2.2–8.9] g/L. The baseline serum IL-6 level did not significantly differ between patients who eventually died and those who survived (21.4 [5.1–38.2] pg/mL and 23.0 [5.4–56.7] pg/mL respectively, p = 0.652, Wilcoxon test) as between those with or without response to tocilizumab (19.4 [9.6–45.1] pg/mL and 27.5 [5.1–56.7] pg/mL respectively, p = 0.471, Wilcoxon test). However, the baseline serum TNF-α level was significantly higher in patients who eventually died of COVID-19 within 30 days post-tocilizumab treatment (4.7 [2.5–6.4] pg/mL] and 2.4 [0.9–3.6] pg/mL respectively, p = 0.015, Wilcoxon test). Additionally, 7 days after treatment, serum TNF-α increased from 3.5 [1.7–6.4] pg/mL to 12.1 [2.6–36.1] pg/mL in patients who did not favourably respond to tocilizumab, whereas patients favourably responding to tocilizumab were keeping their serum TNF-α at the same levels than healthy controls (Fig. 1 ).
Fig. 1.
TNF-α levels were measured in serum collected from Healthy controls and COVID-19 patients before ‘baseline’ and 7 days after tocilizumab. Data shown are those of all the patients treated with tocilizumab and who were able to provide a pre-treatment (baseline) and post-treatment serum (up-to-date serum +7). Of the 25 patients treated with tocilizumab, pre-treatment and post-treatment serum analyses were available for 18 patients represented according to their outcome; mortality before day 30 (left panel) and response to tocilizumab (right panel). Baseline TNF-α serum levels were significantly higher at baseline in patients who eventually died of Covid19 (left panel). Post-tocilizumab TNF-α serum levels were significantly increasing in patients who did not favourably respond to tocilizumab (right panel).
Huang and et al. reported that COVID-19 patients in the intensive care unit had higher plasma concentrations of TNF-α than non-ICU patients [2]. Del Valle and et al. reported serum IL-6 and TNF-alpha as significant predictors of survival [4]. Furthermore, a pivotal role of calprotectin, an inflammation protein upstream of tumour necrosis factor-alpha (TNF-a), has been recently demonstrated in severe forms of COVID-19 [9]. Our results confirm TNF-α as a biomarker of severity in a cohort of patients treated with tocilizumab and support that serum levels of TNF-α should help to stratify patients according to their severity in further prospective clinical trials. These data reinforce Feldmann and et al. view [1] supporting further investigations of TNF-α blocking therapies as potential drugs for patients with a severe and life-threatening form of COVID-19 and provide a putative rationale for tocilizumab resistance.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Over the last 5 years, AM and JMM have participated as principal investigators and sub-investigators of oncology clinical trials sponsored by manufacturers of anti-IL6 and anti-IL6R therapies Roche/Genentech, Sanofi/Regeneron, J&J/Janssen. AM has received honoraria from Roche / Genentech, J&J and Sanofi for consulting in the field of cancer immunotherapy. AM has received grants for oncology research from Sanofi. Gustave Roussy has received funding, grants and drug supply from Roche / Genentech, Sanofi / Regeneron, Janssen for running clinical trials or research projects in oncology. FXD, FA, JR and MR have no conflict of interest to disclose.
Acknowledgements
We would like to thank the following collaborators for their effort during this Covid19 crisis and their contribution to the generation, collection and analysis of our dataset: Dr Laurence Albiges, Dr Fanny Pommeret, Dr Emeline Colomba, Dr Lisa de Rosa, Pr Laurence Zitvogel, Pr Guido Kroemer, Dr Marc Vasse, Dr Christophe Willekens, Dr Antoine Hollebecque, Delphine Bredel, Dr Thomas Hueso, Severine Mouraud, Chifaou Mohamed-Djalim, Dr Giulia Baciarello, Prof Benjamin Besse, Prof Fabrice Barlesi, Prof Fabrice André, Prof Jean-Charles Soria. This work could have been done thanks to the funding support of Fondation Philanthropia, Gustave Roussy Cancer Center and Fondation Gustave Roussy.
References
- 1.Feldmann M., Maini R.N., Woody J.N., et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guaraldi G., Meschiari M., Cozzi-Lepri A., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciascia S., Aprà F., Baffa A., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 7.Del Valle D.M., Kim-Schulze S., Huang H.-H., et al. An inflammatory cytokine signature predicts COVID- 19 severity and survival. Nat Med. 2020 doi: 10.1038/s41591-020-1051-9. published online Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall J.C., Murthy S., Diaz J., et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silvin A., Chapuis N., Dunsmore G., et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.08.002. published online Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]