Abstract
Severe acute respiratory syndrome–associated coronavirus 2 has caused a global public health crisis with high rates of infection and mortality. Treatment and prevention approaches include vaccine development, the design of small-molecule antiviral drugs, and macromolecular neutralizing antibodies. Polymers have been designed for effective virus inhibition and as antiviral drug delivery carriers. This review summarizes recent progress and provides a perspective on polymer-based approaches for the treatment and prevention of coronavirus infection. These polymer-based partners include polyanion/polycations, dendritic polymers, macromolecular prodrugs, and polymeric drug delivery systems that have the potential to significantly improve the efficacy of antiviral therapeutics.
Keywords: Antiviral, Polyanion, Polycation, Drug delivery, Biomaterials
Graphical abstract
1. Introduction
Severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2) belongs to the β family of coronaviruses and infects humans by the fusion of viral and cell membranes, facilitated by binding between the SARS-CoV-2-related spike (S) protein and angiotensin-converting enzyme 2 (ACE2) [[1], [2], [3]]. The β coronavirus family is also responsible for the common cold, severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome–associated coronavirus (MERS-CoV) [4,5]. Strategies to reduce coronavirus infection include wearing masks [[6], [7], [8], [9], [10]], small-molecule drugs [11,12], vaccines, neutralizing antibodies [[13], [14], [15]], RNA interference therapy [16,17], and other mitigation factors [18]. These treatment methods span in vitro prevention to in vivo treatment, including blocking the spread of the virus, inhibiting the translation of viral RNA in host cells, activating the immune system and inhibiting the expression of the S protein gene. Some of these interventions, however, would benefit from a polymer ‘modulator’ to improve efficacy.
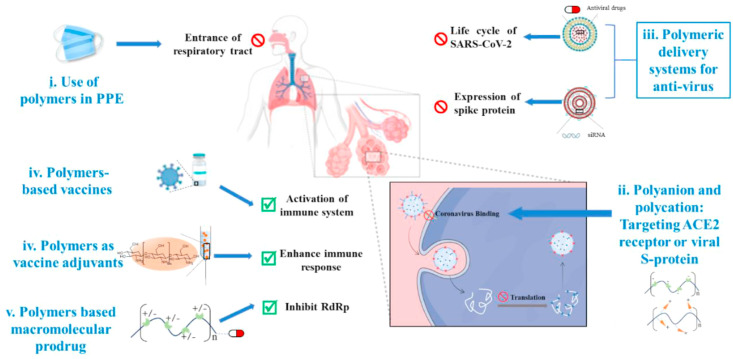
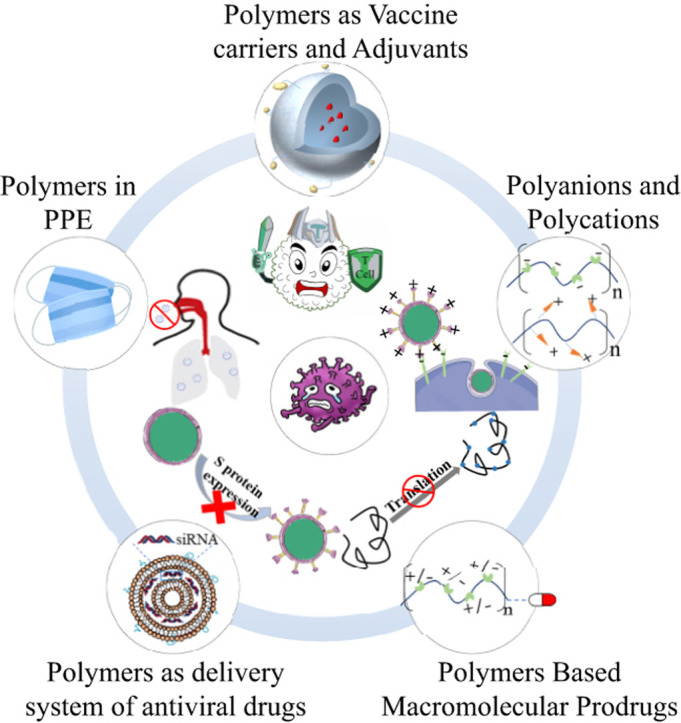
There has been some work in literature that describes disinfection as well as antimicrobial materials [[19], [20], [21], [22], [23], [24]]. Recently, polymer materials have demonstrated antiviral capabilities. As shown in Fig. 1 , polymers can prevent or inhibit the spread of a virus by (1) providing a semipermeable barrier (e.g. mask or face-shield), (2) interfering with binding to the glycoprotein surface of host cells, (3) augmenting small molecular antiviral drug therapies, (4) enhancing the response of the immune system as a vaccine adjuvant, or (5) as a vehicle for other therapeutic molecules to improve the water solubility or stability of antiviral therapeutics. Based on the structural characteristics and uses of the previously mentioned polymers, this review will summarize recent polymeric antiviral approaches based on polyanion/polycation, dendritic polymer, macromolecular prodrugs, and polymer-based delivery system, as well as provide a short perspective of polymeric approach toward the treatment of new type of coronavirus.
Fig. 1.
Polymeric approaches for the prevention or treatment of coronavirus. (i) Integrating functional polymers into personal protective equipment (PPE) can prevent the entrance of virus into the respiratory system. (ii) Cellular binding of viral particles at the alveoli can be inhibited using polyanion and polycation against viral S protein or angiotensin-converting enzyme 2 (ACE2) receptors. (iii) Polymers could also be used to deliver antivirus drugs. (iv) Polymers could also be useful when being covalently combined with small-molecule drugs to form macromolecular prodrugs. (v) Polymer-based vaccines or vaccine adjuvants can be used to prevent virus infection or even to boost the immune response during infection [25]. SARS-CoV-2, Severe acute respiratory syndrome–associated coronavirus 2.
2. Polymer-based in Vitro prevention strategy
Coronavirus disease 2019 is spread by droplets or contact, and a proven strategy for preventing this spread is daily use of personal protective equipment [6,26]. Masks, gloves, and protective clothing are made from various polymers, such as polystyrene, polypropylene, polyethylene, polyvinyl chloride, polyethylene terephthalate etc. [17]. During outbreaks of severe acute respiratory syndrome (SARS), masks and isolation gowns are effective in protecting health care workers from the risk of infection [27]. As a typical example, common medical masks are usually composed of three layers of non-woven fabrics, which are divided into an outer layer (for insulating the sprayed liquid), an inner layer (for moisture absorption), and the middle filter layer (the core part, as a barrier to block viral particles). The core filter layer of the mask is generally made from electret-treated polypropylene melt-blown non-woven fabric, which can reach 95% filterability. Melt-blown, non-woven fabrics have a much higher filtration efficiency than fabrics of cotton, polyester, nylon, or silk. The polypropylene is triboelectrically charged to enhance filtration efficiency. Polypropylene is more hydrophobic and so repels moisture in the air, providing a protective environment [28]. In another case, the effect of polytetrafluoroethylene/polyurethane membranes in chemical protective clothing was evaluated. Excellent isolation performance against poliovirus was demonstrated, and a possible protective mechanism against the SARS virus was inferred [29]. This ultra-high filtration efficiency has proved to be an effective block to the intrusion of coronavirus.
3. Polymeric structures with direct coronavirus binding
Polymers can directly interfere with the interactions of a virus with the host cell. The high molecular weight and multivalent binding of specifically designed polymers can sterically shield the viral surface or competitively inhibit virus—host cell interactions [30]. The next section summarizes recent advances in this field by category of polymer.
3.1. Synthetic polymers
3.1.1. Polyelectrolytes
The surface of a virus is rich in amino acid residues. For example, the envelope protein gp120 of HIV is rich in positively charged arginine and lysine. Polyanions therefore can electrostatically bind to the gp120 protein and prevent HIV from interacting with the host cell's surface. Similarly, SARS-CoV or SARS-CoV-2 has a spike (S) glycoprotein, which binds to the ACE2 protein as an important step in host epithelial cell invasion. SARS-CoV-2 has the D480→S456 mutation relative to SARS-CoV, which removes some negatively charged amino acids in the former, leading to an even stronger electrostatic interaction between ACE2 on epithelial cell membranes and SARS-CoV-2 (as shown in Fig. 2 A) [31]. Disrupting this strong electrostatic interaction will inhibit the virus from entering the host cell, and polyanions and polycations can potentially disrupt the binding between the virus and host cell.
Fig. 2.
(A) Electrostatic potential maps (in kT/e) SARS-CoV-2 and ACE2 shown in a cartoon view [31]. (B) The structure of PVBzA, PPAA, PVPA, and PAEP and antiviral activities of 14 polyanions [34]. (C) Synthesis and characterization of PEI-mann [42]. (a) Synthetic scheme and chemical structure of mannose-functionalized carbonate-modified PEI polymers. (b) Antiviral activity (EC50), cytotoxicity (CC50), selectivity index (SI, CC50/EC50), and pH neutralization capacity of unmodified and mannose-functionalized PEI polymers. Prevention of DENV-2 infection in human primary peripheral blood mononuclear cells (PBMCs) (c) and macrophages (d) by PEI-man65. ACE2, angiotensin-converting enzyme 2; PEI, poly(ethylene imine); PPAA, poly(propylacrylic acid); PVPA, poly(vinylphosphonic acid); PVBzA, poly(vinylbenzoic acid); SARS-CoV-2, severe acute respiratory syndrome–associated coronavirus 2.
Polyanions are the largest class of biomaterials that have been investigated for the blocking of viral adsorption and infection. As early as the 1960s, poly(methacrylic acid) (PMAA) was demonstrated to inhibit the infectivity of enveloped vesicular stomatitis, Simbis, and vaccinia viruses [32,33]. More and more polyanions were subsequently shown to exhibit antiviral activity. Poly(propylacrylic acid), poly(vinylbenzoic acid) (PVBzA), poly(vinylphosphonic acid) (PVPA), and poly(2-acrylamidoethyl)phosphate have exhibited an inhibitory effect on the SARS virus. PVPA has the strongest inhibitory effect on SARS. However, PVBzA, a carboxylate, exhibits broad-spectrum antiviral activity in antienveloped viruses, with a lesser inhibitory effect on enveloped viruses (as shown in Fig. 2B) [34]. Polyanions target the receptor-binding domain of the coronavirus S protein that binds to the ACE2 receptor of potential host cells in bats or humans.
Polycations may also act as antiviral agents through electrostatic interaction with negatively charged cell membranes or lipid-encapsulated virus envelopes, thereby preventing viruses from adsorbing to the cell surface or directly inactivating virus particles. Poly(amidoamine)s (PAMAMs), phosphonium-type cationic polyacrylamide, poly(ethylene imine) (PEI), and their derivatives have significant inhibitory effects on influenza virus and herpes simplex virus (HSV) [[35], [36], [37], [38], [39], [40], [41]]. Polycations interact with negatively charged cell membranes or with lipid-encapsulated virus envelopes. For example, the envelope of HSV contains glycoproteins with anionic amino acid side chains, and cationic PEI serves as an effective HSV inhibitor by interacting with HSV glycoproteins [40]. Moreover, PEI with glycosyl modification bound to TIM-1/TIM-3 receptors, thereby inhibiting viral infection. More interestingly, this glycosyl PEI derivative could also (1) neutralize the pH of the cell nucleus and prevent virus replication, (2) reduced the toxicity of PEI, and (3) improved selectivity and helped overcome drug resistance. Glycosyl-modified PEI imparted extraordinary resistance to infection by RNA, DNA, and enveloped or non-enveloped viruses (as shown in Fig. 2C) [42]. ACE2 receptors have a negative electrostatic potential and could also be a potential target of polycations to prevent virus binding. The previously mentioned PEI with glycosyl modification has unique potential in anticoronavirus.
Although PVBzA and PEI both exhibit a broad-spectrum antiviral effect, which helps reduces drug resistance, their limitation is potential toxicity that has not been assessed, and large-scale clinical still needs to be conducted.
3.1.2. Dendritic polymers
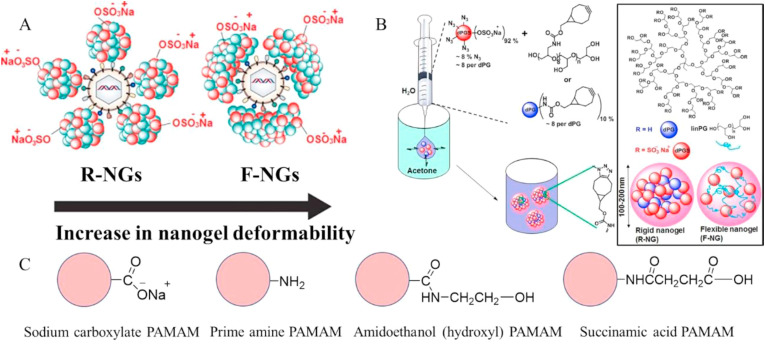
Dendritic or highly branched polymers possess greater solubilities, larger surface areas, and tunable shapes, including the possibility of hydrophobic cavities, relative than their linear counterparts. These structural features can impart enhanced antimicrobial or antiviral activity, resulting from ultrastrong interaction with viruses [43]. Kandeel et al. have reported three anionic dendritic polymers, including hydroxyl, carboxyl, and succinic acid–terminated PAMAM dendrimers, and cationic dendritic polymers containing primary amine end groups. These dendrimers inhibited MERS-CoV, with the G(1.5)-16COONa (carboxyl derivative) and G(5)-128SA (succinic acid derivative) exhibiting the best performances (as shown in Fig. 3 C) [44]. Nanogels based on dendritic polyglycerol sulfate, an analog of heparan sulfate (HS), have proved to be non-toxic with broad-spectrum antiviral activity, inhibiting viruses from binding to the cell surface. HS is a co-receptor for SARS-CoV-2 mediating entry to host cells [45]. Non-toxic and broad-spectrum dendritic nanogels could therefore be potential components of a coronavirus therapy (as shown in Fig. 3A and B) [46].
Fig. 3.
(A) Sulfide nanogels (simulating HS) to shield virus particles (rigid nanogel [R-NG] and flexible nanogel [F-NG]) [46]. (B) Schematic representation of flexible and rigid dPGS-based nanogels. Using linPG and dPG as cross-linkers, respectively, they were prepared by strain-promoted azide–alkyne ring addition reaction via reverse nanoprecipitation technique. The scheme shows the structure of dPG and the models of rigid and flexible nanogels [46]. (C) The terminal groups of the PAMAM dendrimers used were sodium carboxylate, primary amine, hydroxyl, and succinic acid. PAMAM, polyamidoamine [44].
3.2. Natural polymers
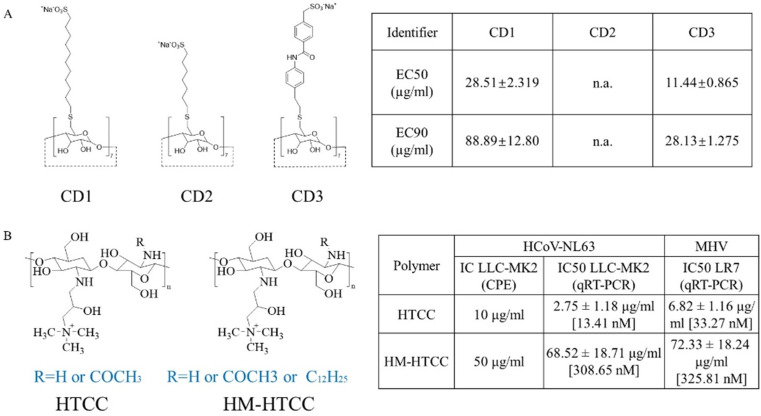
Natural polymers have also been extensively studied as potential antiviral agents [47,48]. Polysaccharides such as carrageenan from seaweed have good antiviral activity after sulfation or sulfonation. Cyclodextrins (CDs) and derivatives such as methyl-β-cyclodextrin (M-β-CD) are reported to inhibit the attachment of coronavirus to host cells because of their ability to remove cholesterol from cell membranes [[49], [50], [51]]. In another example, CD modified with mercaptoundecane sulfonic acid exhibited broad-spectrum antiviral effects and possessed high biocompatibility (as shown in Fig. 4 A) [52]. Chitosan is a natural polycationic polysaccharide that exhibits an inhibitory effect on coronavirus infection. Milewska et al. have reported that chitosan derivative N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride and a hydrophobically modified derivative could effectively disturb the replication of human coronavirus strain (HCoV-NL63) or murine hepatitis virus (as shown in Fig. 4B) [53,54].
Fig. 4.
(A) Structures of modified CDs and relative effective concentrations of inhibition of HSV-2 growth [52]. (B) Structures of HTCC and HM-HTCC and inhibition of HCoV-NL63 and MHV replication in vitro [54]. CD, cyclodextrin; HSV, herpes simplex virus; HTCC, N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride; HCoV-NL63, human coronavirus strain; MHV, murine hepatitis virus.
Naturally occurring polymers are generally less toxic with greater biocompatibility than synthetic polymers. Moreover, polysaccharides can activate T lymphocytes, B lymphocytes, macrophages, and other immune cells to elicit a beneficial immune response.
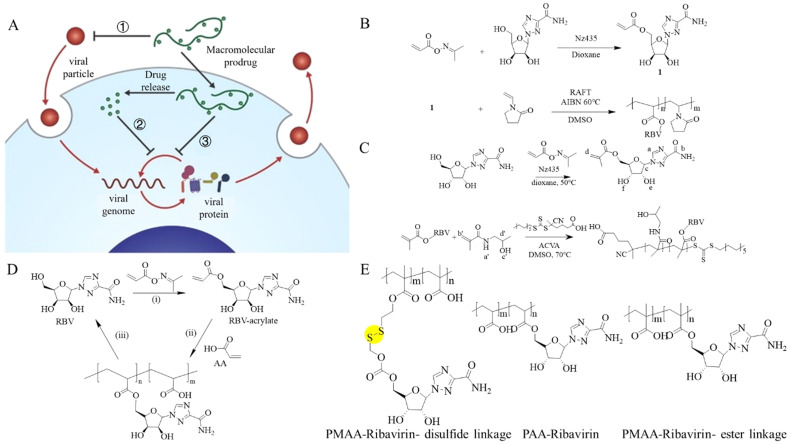
4. Polymer-based macromolecular prodrugs
Polymer-based excipients can improve the performance of -molecule antivirus drugs by modifying release kinetics and/or exerting complementary antiviral activity, achieving a synergistic antivirus effect and significantly improving the therapeutic index of the small-molecule drugs [55]. For example, ribavirin, a guanosine nucleoside with virus messenger RNA synthesis interfering ability, has been coupled with polyanions (polyacrylic acid, PMAA, poly(N-vinylpyrrolidone), and poly(N-(2-hydroxypropyl) methacrylamide) to give ribavirin-based macromolecular prodrugs. This prodrug design could maintain the efficacy of ribavirin while reducing its toxicity to expand its therapeutic range (as shown in Fig. 5 B–D) [[56], [57], [58]]. Ribavirin can accumulate in red blood cells, causing hemolytic anemia. It is therefore only clinically available to hospitalized patients with severe respiratory syncytial virus (RSV) or anti–hepatitis C virus (HCV) infections. Ribavirin polyanionic macromolecular prodrug (PAMP) has been used as a broad-spectrum antiviral drug against HIV, HCV, RSV, influenza, measles, mumps, dengue fever, and Ebola virus. PAMP's antiviral effect is associated with its ability to inhibit virion binding to the host cell receptor because of both the polyanion component and the inhibitory effect of the nucleoside analog (as shown in Fig. 5E) [59]. A prodrug made of PMAA and ribavirin linked with a disulfide bond achieved rapid intracellular release compared with other macromolecular prodrugs [60].
Fig. 5.
(A) Mechanism of macromolecular prodrugs inhibiting viruses [59]. (B) Ribavirin acrylate monomer is synthesized via a chemotaxis pathway (top). RAFT controlled the copolymerization of RBV acrylate with N-vinylpyrrolidone (NVP) to provide a macromolecular precursor for RBV (bottom). Phthalimidomethyl-O-ethyl xanthate was used as an RAFT agent [56]. (C) Synthesis of ribavirin (RBV( methacrylate and macromolecular prodrugs for RBV based on HPMA [57]. (D) Proposed synthesis of macromolecular prodrugs of RBV. The polymerizable acrylate of RBV was synthesized by a chemoenzymatic method using Nz435/CAL-B in dioxane (i) for RAFT polymerization and AA as a co-monomer to obtain a macromolecular precursor (ii). The synthesized polymer released the original RBV on hydrolysis (iii) [58]. (E) Structures of polyanionic macromolecular prodrugs of ribavirin based on these polymers, whereby RBV is conjugated to the polymer via an ester linkage or a disulfide linkage to achieve ultrafast intracellular drug release [59].
5. Polymeric delivery systems for antivirus applications
5.1. Polymers as vaccine carriers and adjuvants
Polymers can serve as delivery systems and adjuvants for improving the safety and effectiveness of vaccines. A suitable excipient can increase the accumulation of vaccine at a disease site and elevate an immune response. Polymeric structures can improve the safety and effectiveness of a vaccine as well as protecting the integrity of encapsulated antigen to inhibit degradation. Polymeric encapsulation can facilitate mucosal administration, rather than injection, resulting in higher patient compliance. Polymers can also be designed with a low immunogenic risk, good biocompatibility, a large specific surface area, biodegradability, and a reduction in the therapeutic dose required [[61], [62], [63], [64], [65], [66]]. Several natural and synthetic polymers have been used for preparing nanoparticle vaccine delivery vehicles (Table 1 ).
Table 1.
Summary of polymers for vaccine carriers and adjuvants.
| Polymers | Compounds | Virus | Animal model | Effect | Advantage | References |
|---|---|---|---|---|---|---|
| PLGA | CpG ODN 2007 | Infectious bronchitis virus (IBV) | Chickens | Improved innate and long-term immunostimulatory effects in vivo and in vitro | Degradability, renewable, non-toxic, and completely biodegradable and recognized by the Food and Drug Administration (FDA) | [67] |
| PLGA | STING agonists | Middle East respiratory syndrome coronavirus (MERS-CoV) | C57BL/6 mice | Coordinated delivery of antigen and adjuvant in vitro and in vivo; significantly enhances antigen-specific humoral and cellular responses | [68] | |
| DSPE-PEG-maleimide | MERS-CoV RBD | |||||
| PLGA | PEDV killed vaccine antigens (KAg) | Porcine epidemic diarrhea virus (PEDV) | Pregnant sows and suckling piglets | Induced systemic and mucosal immunity; efficiently protected suckling piglets against challenge with PEDV | [69] | |
| PLGA | Inactivated PRRSV vaccine (NP-KAg) | Porcine reproductive and respiratory syndrome virus (PRRSV) | Piglets | Reduce greatly the required vaccine dose; the entrapped antigen was released at a much slower rate and triggers a robust effect and memory immune response | [70] | |
| PLGA | DNA vaccines | Newcastle disease virus (NDV) | Chickens | Induces stronger immune responses, and achieve sustained release | [71] | |
| PLGA | Killed PRRSV vaccine (Nano-KAg) | PRRSV | Pigs | The potential to generate anti-PRRSV immune response and in better clearance of viremia | [72] | |
| PEG-PLGA | Diphyllin | Feline coronaviruses (FCoVs) | Mice | Higher safety and increased inhibitory activity against FIPV | [73] | |
| O-2′-HACC | Live Newcastle disease vaccine | NDV | Chickens | Long release, low toxicity, high safety | High antimicrobial activity, low toxicity, and a high safety level | [74] |
| N-2-HACC-CMC | NDV/La Sota + IBV/H120 | NDV and IBV | Chickens | Induces greater IgG and IgA antibody potency; significantly promotes lymphocyte proliferation and induces higher levels of cytokines | N-2-HACC was more cost-effective than O-2′-HACC, and N-2-HACC has superior water solubility and more suitable size than chitosan and O-2′-HACC | [75] |
| NDV F gene plasmid DNA with C3d6 molecular adjuvant | NDV | Chickens | Increased production of anti-NDV IgG and IgA antibodies; significantly stimulated lymphocyte proliferation, triggering higher levels of IL-2, IL-4, and IFN-γ | [76] | ||
| HACC and SCS | As vaccine adjuvants to prepare NDV-loaded nanoparticles | NDV | Chickens | Qualified levels of humoral immunity (HI > 5) and higher levels of cellular immunity compared with the commercial oil emulsion vaccine; these nanoparticles provide 100% protection against virulent NDV | SCS nanoparticles were not active as CS and HACC nanoparticles for the adjuvant effect of NDV | [77] |
| Chitosan | Inactivated NDV vaccine | NDV | Chickens | Adjuvant effects of Chitosan, CS particles efficiently changed mucosal and humoral immunity and protective activity | Chitosan has superior biocompatibility and biodegradability and can bind to negatively charged proteins or DNA plasmids through the electrostatic interaction, forming polymer composites to protect proteins and DNA from degradation | [78] |
| Live NDV vaccine | NDV | Chickens | Induced greater protection of immunized specific pathogen | [79] | ||
| NDV F gene deoxyribonucleic acid (DNA) vaccine | NDV | Pathogen-free chickens | Induced significantly higher mucosal and humoral immune responses; protect the plasmid DNA from degradation and help the expression of the plasmid DNA encapsulated | [80] | ||
| NDV vaccines | NDV | Chickens | Produce higher mucosal immunity titers by taking vaccine orally; meanwhile, it can induce humoral and cell-mediated immune response and mucosal immunity strongly | [81] | ||
| Chitosan (CS)-coated poly(lactic-co-glycolic) acid (PLGA) | DNA (the F gene) of NDV | NDV | Chickens | The immunogenicity and protective immunity can be improved | CS reduces burst release of encapsulated proteins or DNA; increases the stability of biomolecules; enhances zeta potential reversal and promotes cell adhesion and retention of the delivery system at the target site; provides the possibility of conjugating targeting ligands to free amino acids on the surface | [82] |
| Polyethylenimine (PEI) | SARS DNA vaccine | Severe acute respiratory syndrome (SARS-CoV) | BALB/c mice | PEI/pci-S nanoparticles induce antigen-specific humoral and cellular immune responses | High transfection efficiency and buffering capacity; PEI is a very effective gene delivery vehicle for lung transfection producing high antibody titers against the encoded protein | [83] |
| pci-S/PEI | SARS DNA vaccine | SARS-CoV | BALB/c mice | Induce antigen-specific humoral and cellular immune responses | [84] |
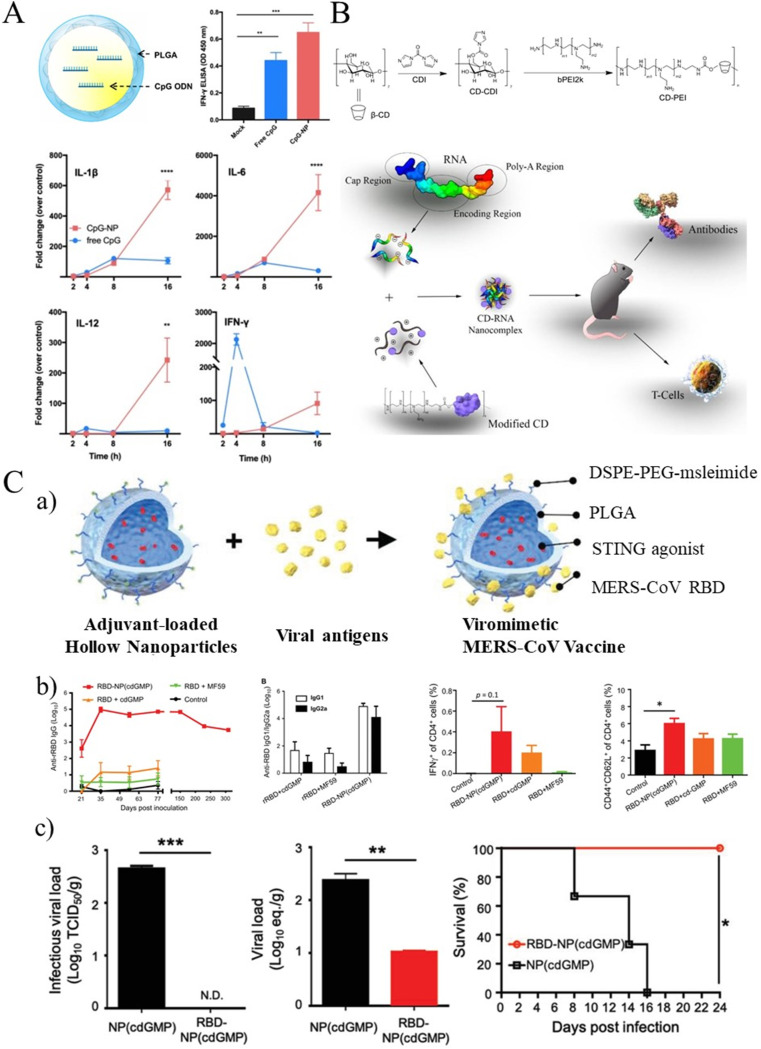
CDs have also been used as delivery systems and adjuvants in virus-induced disease treatments. For example, a common influenza vaccine containing 30% 2-hydroxylpropyl-beta-CD (HP-β-CD) as adjuvant generated increased antibody production against virus infection in mice [85]. Survival rates in infected mice reached 100%. In the presence of thiomersal and alum, CD was found to minimize the degradation of IPV (inactivated polio vaccine or virus) and reduce the loss of D antigen titer of mixed IPV (as shown in Fig. 6 B) [86,87].
Fig. 6.
(A) Schematic illustration of a PLGA hollow nanoparticle encapsulating CpG (CpG-NP). And CpG (CpG-NP) showed results of more effective and long-lasting immune activation in chBMDCs [67]. (B) The synthetic route to CD-PEI conjugates and a CD-based mRNA vaccine platform [86,87]. (C, a) Preparation of viromimetic nanoparticle vaccine. Hollow PLGA nanoparticles with encapsulated adjuvant and surface maleimide linkers were prepared using a double emulsion technique. Recombinant viral antigens were then coupled to the surface of nanoparticles via thiol-maleimide bonds. Synthetic viral-like nanoparticles facilitate coordinated delivery of antigens and adjuvants in vitro and in vivo. (b) Viromimetic nanoparticle induces robust and long-lasting humoral and CD4+ T cell responses. (c) Viral-like nanoparticles vaccine grants protection against MERS-CoV infection in DPP4-transplanted mice [68].
5.2. Delivery systems for antiviral drugs
Many polymers have been used for antiviral drug delivery, including natural polysaccharides, poly(ethylene glycol) (PEG) and PEI derivatives, and emerging dendrites. Polymeric delivery vehicles reduce the side effects of encapsulated drugs, increase their water solubility, and improve efficiency. Natural polysaccharides (e.g. CD or chitosan) are popular for the delivery of antiviral drugs, such as nitazoxanide [76], ribavirin [88], camostat mesylate [89,90], lopinavir, and ritonavir [91], which all exhibit inhibitory effects on coronavirus. Polymeric structures are also helpful for the delivery of therapeutic small interfering RNA (siRNA), improving stability, pharmacokinetics, and cellular uptake [92]. siRNA can be physically encapsulated in nanoparticles made of PEG [93,94] or poly(lactic acid-history polymer co-glycolic acetic acid) (PLGA) [95]. This stability is enhanced by the electrostatic interaction between the negatively charged phosphates of the siRNA and the positively charged groups on polymer chain such linear poly(ethylene imine) (LPEI) and branched poly(ethylene imine) (BPEI) [96,97]. With more folding options, BPEI exhibited stronger siRNA loading ability than LPEI, indicating the importance of branched polymeric structures. Inspired by this discovery, researchers have synthesized highly branched architectures of natural polymer such as CD and chitosan. CD-based, self-assembled polymer nanoparticles improve siRNA delivery [98,99]. Likewise, higher molecular weight chitosan provided better complexation ability and increase stability of siRNA polyelectrolyte complexes (polyplex) [100,101]. Dendritic macromolecules have also been used as non-viral vectors for siRNA delivery-based virus treatments [102,103]. siRNA could effectively and specifically inhibit gene expression of S protein in SARS-CoV-infected cells, and so, a RNAi strategy might have potential for SARS-CoV inhibition [[104], [105], [106]]. Conti et al. have reported that poly(amide)-based dendrimer nanocarriers as an aerosol based siRNA delivery system could effectively transfect lung epithelial cells for coronavirus treatment [107,108]. Ciliated cells of the human lung are the main site of SARS-CoV-2 infection. siRNA delivery systems based on dendritic macromolecules might therefore might have potential as a treatment for SARS-CoV-2–induced pneumonia (Fig. 7 ).
Fig. 7.
(A) Schematic diagram of the targeted nanoparticles. Polyethylene glycol (PEG) molecules were endowed with adamantane (AD) to form inclusion complexes with surface CDs, which decorated the nanoparticle surface with PEG for steric stabilization and PEG-TF for targeting [99]. (B) 7C1 Synthesis scheme.7C1 nanoparticles were mixed with C14PEG2000 and siRNA in a high-throughput microfluidic chamber [97]. (C, a) Synthesis program for siRNA-PLGA conjugates via cleavable disulfide linkers. (b) Schematic illustration of the preparation of surface crosslinked siRNA-PLGA–conjugated microbubbles with cationic LPEI and their efficient intracellular uptake by polyelectrolyte charge interaction [95]. (D) Preparation of mannitol microparticles loaded with dendriplexes [107].
6. Conclusions and future perspectives
SARS-CoV-2 has caused a global public health crisis with high rates of infection and mortality. In conventional treatment programs, there are disadvantages such as time consuming and high cost. There is also an added worry that the next pandemic may be even more dangerous. Thoughts around a superinfectious Disease X needing ultra-low virus dose to infect and working on the combination of the aerosol transmission as well as the asymptomatic infection. This will be a disastrous recipe that will make a pandemic of a Disease X very difficult to control. As an emerging field of antiviral properties, polymers have inestimable prospects. Polymers will play an important role in the fight against coronavirus infections, from providing better semipermeable barriers to air-borne particles to important partners in chemotherapeutic treatments. Polymer vaccine adjuvants provide improved humoral immunity and administration routes. Polymeric nanocarriers of small-molecule antiviral drugs assist local or sustained delivery and assist to overcome poor aqueous solubility and drug resistance. Last, but not least, polymeric structures with targeted gene delivery ability have the potential to silence or disturb the activity of coronaviruses. Polyanions/polycations, dendritic polymers, macromolecular prodrugs, and polymeric drug delivery systems have a bright future in this respect. The current evidence shows that its advantages are irreplaceable in conventional treatment programs. There will be new polymer discoveries, some enabled by new technologies or new scientific capabilities and knowledge, which did not exist before. It is hoped that the development of polymers will advance rapidly in the future and move toward clinical treatment as soon as possible. We hope that this summary of recent advances in polymer bioscience will stimulate more discoveries to meet an increasing challenge for a growing human population. We can learn about the technological solutions, research into new technologies, and dig into the our archives to find and develop solutions. Some could be new research from which we derive early research data, some of these will be built on research done over the past several decades.
Authors’ contributions
C.W, Y.W., and X.L. proposed the ideas; X.J., Z.L, and D.J.Y. searched the references and wrote the article; all the authors have critically revised the scientific content of this article and approved the final version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
X.J. and Z.L. contributed equally to this work. This project is supported by the Natural Science Foundation of China (81773688, U1903119, 81971724, and 81773661) and the National Major Scientific and Technological Special Project for ‘Significant New Drugs Development’ (No. 2020ZX09201005), and Agency for Science, Technology and Research (A∗STAR).
References
- 1.Wang Q.H., Zhang Y.F., Wu L.L., Niu S., Song C.L., Zhang Z.Y., Lu G.W., Qiao C.P., Hu Y., Yuen K.Y., Wang Q.S., Zhou H., Yan J.H., Qi J.X. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4) doi: 10.1016/j.cell.2020.03.045. 894-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Totura A.L., Bavari S. Broad-spectrum coronavirus antiviral drug discovery. Expet Opin. Drug Discov. 2019;14(4):397–412. doi: 10.1080/17460441.2019.1581171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song W.F., Gui M., Wang X.Q., Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14(8) doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L., Yan Y., Wang L.N. Coronavirus disease 2019: coronaviruses and blood safety. Transfus. Med. Rev. 2020;34(2):75–80. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou R., Dana T., Jungbauer R., Weeks C., McDonagh M.S. Masks for prevention of respiratory virus infections, including SARS-CoV-2, in health care and community settings: a living rapid review. Ann. Intern. Med. 2020;173(7):542–555. doi: 10.7326/M20-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seto W.H., Tsang D., Yung R.W., Ching T.Y., Ng T.K., Ho M., Ho L.M., Peiris J.S., Advisors of Expert S.g. o.H.A. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361(9368):1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan L., Wang C., Jin H., Li J., Yang L., Zheng Y., Wen Y., Tan B.H., Loh X.J., Chen X. Lab-on-mask for remote respiratory monitoring. ACS Mater. Lett. 2020;2(9):1178–1181. doi: 10.1021/acsmaterialslett.0c00299. [DOI] [PubMed] [Google Scholar]
- 9.Su X., Sutarlie L., Loh X.J. Sensors and analytical technologies for air quality: particulate matters and bioaerosols. Chem.-Asian J. 2020;15(24):4241–4255. doi: 10.1002/asia.202001051. [DOI] [PubMed] [Google Scholar]
- 10.Chua M.H., Cheng W., Goh S.S., Kong J., Li B., Lim J.Y., Mao L., Wang S., Xue K., Yang L. Face masks in the new COVID-19 normal: materials, testing, and perspectives. Research. 2020:2020. doi: 10.34133/2020/7286735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinner C.D., Gottlieb R.L., Criner G.J., Arribas Lopez J.R., Cattelan A.M., Soriano Viladomiu A., Ogbuagu O., Malhotra P., Mullane K.M., Castagna A., Chai L.Y.A., Roestenberg M., Tsang O.T.Y., Bernasconi E., Le Turnier P., Chang S.C., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wang H., Gaggar A., Brainard D.M., McPhail M.J., Bhagani S., Ahn M.Y., Sanyal A.J., Huhn G., Marty F.M., Investigators G.-U.-. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate covid-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drozdzal S., Rosik J., Lechowicz K., Machaj F., Kotfis K., Ghavami S., Los M.J. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist. Updates. 2020;53:100719. doi: 10.1016/j.drup.2020.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H., He X., Martinez D.R., Rutten L., Bos R., van Manen D., Vellinga J., Custers J., Langedijk J.P., Kwaks T., Bakkers M.J.G., Zuijdgeest D., Rosendahl Huber S.K., Atyeo C., Fischinger S., Burke J.S., Feldman J., Hauser B.M., Caradonna T.M., Bondzie E.A., Dagotto G., Gebre M.S., Hoffman E., Jacob-Dolan C., Kirilova M., Li Z., Lin Z., Mahrokhian S.H., Maxfield L.F., Nampanya F., Nityanandam R., Nkolola J.P., Patel S., Ventura J.D., Verrington K., Wan H., Pessaint L., Van Ry A., Blade K., Strasbaugh A., Cabus M., Brown R., Cook A., Zouantchangadou S., Teow E., Andersen H., Lewis M.G., Cai Y., Chen B., Schmidt A.G., Reeves R.K., Baric R.S., Lauffenburger D.A., Alter G., Stoffels P., Mammen M., Van Hoof J., Schuitemaker H., Barouch D.H. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Tureci O., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Sahin U., Gruber W.C. Safety and immunogenicity of two rna-based covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41(5):355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He M.L., Zheng B., Peng Y., Peiris J.S., Poon L.L., Yuen K.Y., Lin M.C., Kung H.F., Guan Y. Inhibition of SARS-associated coronavirus infection and replication by RNA interference. JAMA. 2003;290(20):2665–2666. doi: 10.1001/jama.290.20.2665. [DOI] [PubMed] [Google Scholar]
- 17.Jung S., Lee S., Dou X., Kwon E.E. Valorization of disposable COVID-19 mask through the thermo-chemical process. Chem. Eng. J. 2021;405:126658. doi: 10.1016/j.cej.2020.126658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suwardi A., Ooi C.C., Daniel D., Tan C.K.I., Li H., Liang O.Y.Z., Tang Y.K., Chee J.Y., Sadovoy A., Jiang S.-Y., Ramachandran S., Ye E., Kang C.W., Cheong W.C.D., Lim K.H., Loh X.J. The efficacy of plant-based ionizers in removing aerosol for COVID-19 mitigation. Research. 2021;2021 doi: 10.34133/2021/2173642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayandi V., Wen Choong A.C., Dhand C., Lim F.P., Aung T.T., Sriram H., Dwivedi N., Periayah M.H., Sridhar S., Fazil M.H.U.T. Multifunctional antimicrobial nanofiber dressings containing ε-polylysine for the eradication of bacterial bioburden and promotion of wound healing in critically colonized wounds. ACS Appl. Mater. Interfaces. 2020;12(14):15989–16005. doi: 10.1021/acsami.9b21683. [DOI] [PubMed] [Google Scholar]
- 20.Dhand C., Ong C.Y., Dwivedi N., Varadarajan J., Halleluyah Periayah M., Jianyang Lim E., Mayandi V., Goh E.T.L., Najjar R.P., Chan L.W. Mussel-inspired durable antimicrobial contact lenses: the role of covalent and noncovalent attachment of antimicrobials. ACS Biomater. Sci. Eng. 2020;6(5):3162–3173. doi: 10.1021/acsbiomaterials.0c00229. [DOI] [PubMed] [Google Scholar]
- 21.Mayandi V., Sridhar S., Fazil M.H.U.T., Goh E.T.L., Htoon H.M., Orive G., Choong Y.K., Saravanan R., Beuerman R.W., Barkham T.M.S. Protective action of linear polyethylenimine against Staphylococcus aureus colonization and exaggerated inflammation in vitro and in vivo. ACS Infect. Dis. 2019;5(8):1411–1422. doi: 10.1021/acsinfecdis.9b00102. [DOI] [PubMed] [Google Scholar]
- 22.Leung C.M., Dhand C., Mayandi V., Ramalingam R., Lim F.P., Barathi V.A., Dwivedi N., Orive G., Beuerman R.W., Ramakrishna S. Wound healing properties of magnesium mineralized antimicrobial nanofibre dressings containing chondroitin sulphate–a comparison between blend and core–shell nanofibres. Biomater. Sci. 2020;8(12):3454–3471. doi: 10.1039/d0bm00530d. [DOI] [PubMed] [Google Scholar]
- 23.Liow S.S., Chee P.L., Owh C., Zhang K., Zhou Y., Gao F., Lakshminarayanan R., Loh X.J. Cationic poly ([R]-3-hydroxybutyrate) copolymers as antimicrobial agents. Macromol. Biosci. 2019;19(4):1800466. doi: 10.1002/mabi.201800466. [DOI] [PubMed] [Google Scholar]
- 24.Lin Q., Lim J.Y., Xue K., Yew P.Y.M., Owh C., Chee P.L., Loh X.J. Sanitizing agents for virus inactivation and disinfection. View. 2020;1(2):e16. doi: 10.1002/viw2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M., Scott J.A., Vitale F., Unal M.A., Mattevi C., Bedognetti D., Merkoci A., Tasciotti E., Yilmazer A., Gogotsi Y., Stellacci F., Delogu L.G. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14(6):6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 26.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schunemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M., Cheng S.-Z., Xu K.-W., Yang Y., Zhu Q.-T., Zhang H., Yang D.-Y., Cheng S.-Y., Xiao H., Wang J.-W. Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: cross sectional study. BMJ. 2020;369 doi: 10.1136/bmj.m2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao M., Liao L., Xiao W., Yu X., Wang H., Wang Q., Lin Y.L., Kilinc-Balci F.S., Price A., Chu L., Chu M.C., Chu S., Cui Y. Household materials selection for homemade cloth face coverings and their filtration efficiency enhancement with triboelectric charging. Nano Lett. 2020;20(7):5544–5552. doi: 10.1021/acs.nanolett.0c02211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao X., Zhang J., Guo Y. Study of new protective clothing against SARS using semi-permeable PTFE/PU membrane. Eur. Polym. J. 2004;40(4):673–678. [Google Scholar]
- 30.Li J., Yu F., Chen Y., Oupicky D. Polymeric drugs: advances in the development of pharmacologically active polymers. J. Contr. Release. 2015;219:369–382. doi: 10.1016/j.jconrel.2015.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amin M., Sorour M.K., Kasry A. Comparing the binding interactions in the receptor binding domains of SARS-CoV-2 and SARS-CoV. J. Phys. Chem. Lett. 2020;11(12):4897–4900. doi: 10.1021/acs.jpclett.0c01064. [DOI] [PubMed] [Google Scholar]
- 32.Jensen L.S., Maurice D.V. Influence of sulfur amino acids on copper toxicity in chicks. J. Nutr. 1979;109(1):91–97. doi: 10.1093/jn/109.1.91. [DOI] [PubMed] [Google Scholar]
- 33.De Somer P., De Clercq E., Billiau A., Schonne E., Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J. Virol. 1968;2(9):886–893. doi: 10.1128/jvi.2.9.886-893.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schandock F., Riber C.F., Rocker A., Muller J.A., Harms M., Gajda P., Zuwala K., Andersen A.H.F., Lovschall K.B., Tolstrup M., Kreppel F., Munch J., Zelikin A.N. Macromolecular antiviral agents against Zika, Ebola, SARS, and other pathogenic viruses. Adv. Healthc. Mater. 2017;6(23) doi: 10.1002/adhm.201700748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue Y., Pan Y.F., Xiao H.N., Zhao Y. Novel quaternary phosphonium-type cationic polyacrylamide and elucidation of dual-functional antibacterial/antiviral activity. RSC Adv. 2014;4(87):46887–46895. [Google Scholar]
- 36.Wegmann F., Gartlan K.H., Harandi A.M., Brinckmann S.A., Coccia M., Hillson W.R., Kok W.L., Cole S., Ho L.P., Lambe T., Puthia M., Svanborg C., Scherer E.M., Krashias G., Williams A., Blattman J.N., Greenberg P.D., Flavell R.A., Moghaddam A.E., Sheppard N.C., Sattentau Q.J. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat. Biotechnol. 2012;30(9) doi: 10.1038/nbt.2344. 883-U116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larson A.M., Oh H.S., Knipe D.M., Klibanov A.M. Decreasing herpes simplex viral infectivity in solution by surface-immobilized and suspended N,N-dodecyl,methyl-polyethylenimine. Pharm. Res.-Dordr. 2013;30(1):25–31. doi: 10.1007/s11095-012-0825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashi K., Onoue H., Sasaki K., Lee J.B., Kumar P.K.R., Gopinath S.C.B., Maitani Y., Kai T., Hayashi T. Topical application of polyethylenimine as a candidate for novel prophylactic therapeutics against genital herpes caused by herpes simplex virus. Arch. Virol. 2014;159(3):425–435. doi: 10.1007/s00705-013-1829-x. [DOI] [PubMed] [Google Scholar]
- 39.Haldar J., An D.Q., de Cienfuegos L.A., Chen J.Z., Klibanov A.M. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc. Natl. Acad. Sci. U.S.A. 2006;103(47):17667–17671. doi: 10.1073/pnas.0608803103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donalisio M., Ranucci E., Cagno V., Civra A., Manfredi A., Cavalli R., Ferruti P., Lembo D. Agmatine-containing poly(amidoamine)s as a novel class of antiviral macromolecules: structural properties and in vitro evaluation of infectivity inhibition. Antimicrob. Agents Chemother. 2014;58(10):6315–6319. doi: 10.1128/AAC.03420-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donalisio M., Quaranta P., Chiuppesi F., Pistello M., Cagno V., Cavalli R., Volante M., Bugatti A., Rusnati M., Ranucci E., Ferruti P., Lembo D. The AGMA1 poly(amidoamine) inhibits the infectivity of herpes simplex virus in cell lines, in human cervicovaginal histocultures, and in vaginally infected mice. Biomaterials. 2016;85:40–53. doi: 10.1016/j.biomaterials.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 42.Ichiyama K., Yang C., Chandrasekaran L., Liu S.Q., Rong L.J., Zhao Y., Gao S.J., Lee A., Ohba K., Suzuki Y., Yoshinaka Y., Shimotohno K., Miyakawa K., Ryo A., Hedrick J., Yamamoto N., Yang Y.Y. Cooperative orthogonal macromolecular assemblies with broad spectrum antiviral activity, high selectivity, and resistance mitigation. Macromolecules. 2016;49(7):2618–2629. [Google Scholar]
- 43.Itani R., Tobaiqy M., Al Faraj A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 2020;10(13):5932–5942. doi: 10.7150/thno.46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kandeel M., Al-Taher A., Park B.K., Kwon H.J., Al-Nazawi M. A pilot study of the antiviral activity of anionic and cationic polyamidoamine dendrimers against the Middle East respiratory syndrome coronavirus. J. Med. Virol. 2020;92:1665–1670. doi: 10.1002/jmv.25928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., Thacker B.E., Glass C.A., Yang Z., Torres J.L., Golden G.J., Bartels P.L., Porell R.N., Garretson A.F., Laubach L., Feldman J., Yin X., Pu Y., Hauser B.M., Caradonna T.M., Kellman B.P., Martino C., Gordts P., Chanda S.K., Schmidt A.G., Godula K., Leibel S.L., Jose J., Corbett K.D., Ward A.B., Carlin A.F., Esko J.D. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043–1057 e15. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dey P., Bergmann T., Cuellar-Camacho J.L., Ehrmann S., Chowdhury M.S., Zhang M., Dahmani I., Haag R., Azab W. Multivalent flexible nanogels exhibit broad-spectrum antiviral activity by blocking virus entry. ACS Nano. 2018;12(7):6429–6442. doi: 10.1021/acsnano.8b01616. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez M.E., Alarcon B., Carrasco L. Polysaccharides as antiviral agents - antiviral activity of carrageenan. Antimicrob. Agents Chemother. 1987;31(9):1388–1393. doi: 10.1128/aac.31.9.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derby N., Lal M., Aravantinou M., Kizima L., Barnable P., Rodriguez A., Lai M., Wesenberg A., Ugaonkar S., Levendosky K., Mizenina O., Kleinbeck K., Lifson J.D., Peet M.M., Lloyd Z., Benson M., Heneine W., O'Keefe B.R., Robbiani M., Martinelli E., Grasperge B., Blanchard J., Gettie A., Teleshova N., Fernandez-Romero J.A., Zydowsky T.M. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat. Commun. 2018;9(1):3881. doi: 10.1038/s41467-018-06349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin J.J., Holguera J., Sanchez-Felipe L., Villar E., Munoz-Barroso I. Cholesterol dependence of newcastle disease virus entry. Biochim. Biophys. Acta. 2012;1818(3):753–761. doi: 10.1016/j.bbamem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi K.S., Aizaki H., Lai M.M. Murine coronavirus requires lipid rafts for virus entry and cell-cell fusion but not for virus release. J. Virol. 2005;79(15):9862–9871. doi: 10.1128/JVI.79.15.9862-9871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo H., Huang M., Yuan Q., Wei Y., Gao Y., Mao L., Gu L., Tan Y.W., Zhong Y., Liu D., Sun S. The important role of lipid raft-mediated attachment in the infection of cultured cells by coronavirus infectious bronchitis virus Beaudette strain. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones S.T., Cagno V., Janecek M., Ortiz D., Gasilova N., Piret J., Gasbarri M., Constant D.A., Han Y., Vukovic L., Kral P., Kaiser L., Huang S., Constant S., Kirkegaard K., Boivin G., Stellacci F., Tapparel C. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020;6(5) doi: 10.1126/sciadv.aax9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milewska A., Kaminski K., Ciejka J., Kosowicz K., Zeglen S., Wojarski J., Nowakowska M., Szczubialka K., Pyrc K. HTCC: broad range inhibitor of coronavirus entry. PloS One. 2016;11(6) doi: 10.1371/journal.pone.0156552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milewska A., Ciejka J., Kaminski K., Karewicz A., Bielska D., Zeglen S., Karolak W., Nowakowska M., Potempa J., Bosch B.J., Pyrc K., Szczubialka K. Novel polymeric inhibitors of HCoV-NL63. Antivir. Res. 2013;97(2):112–121. doi: 10.1016/j.antiviral.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wohl B.M., Smith A.A., Jensen B.E., Zelikin A.N. Macromolecular (pro)drugs with concurrent direct activity against the hepatitis C virus and inflammation. J. Contr. Release. 2014;196:197–207. doi: 10.1016/j.jconrel.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 56.Wohl B.M., Smith A.A., Kryger M.B., Zelikin A.N. Narrow therapeutic window of ribavirin as an inhibitor of nitric oxide synthesis is broadened by macromolecular prodrugs. Biomacromolecules. 2013;14(11):3916–3926. doi: 10.1021/bm401048s. [DOI] [PubMed] [Google Scholar]
- 57.Smith A.A.A., Zuwala K., Kryger M.B.L., Wohl B.M., Guerrero-Sanchez C., Tolstrup M., Postma A., Zelikin A.N. Macromolecular prodrugs of ribavirin: towards a treatment for co-infection with HIV and HCV. Chem. Sci. 2015;6(1):264–269. doi: 10.1039/c4sc02754j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kryger M.B., Wohl B.M., Smith A.A., Zelikin A.N. Macromolecular prodrugs of ribavirin combat side effects and toxicity with no loss of activity of the drug. Chem. Commun. 2013;49(26):2643–2645. doi: 10.1039/c3cc00315a. [DOI] [PubMed] [Google Scholar]
- 59.Hinton T.M., Zuwala K., Deffrasnes C., Todd S., Shi S., Marsh G.A., Dearnley M., Wohl B.M., Tolstrup M., Zelikin A.N. Polyanionic macromolecular prodrugs of ribavirin: antiviral agents with a broad spectrum of activity. Adv. Healthc. Mater. 2016;5(5):534–540. doi: 10.1002/adhm.201500841. [DOI] [PubMed] [Google Scholar]
- 60.Zuwala K., Riber C.F., Lovschall K.B., Andersen A.H.F., Sorensen L., Gajda P., Tolstrup M., Zelikin A.N. Macromolecular prodrugs of ribavirin: polymer backbone defines blood safety, drug release, and efficacy of anti-inflammatory effects. J. Contr. Release. 2018;275:53–66. doi: 10.1016/j.jconrel.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han J., Zhao D., Li D., Wang X., Jin Z., Zhao K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers. 2018;10(1) doi: 10.3390/polym10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xue K., Liu Z., Jiang L., Kai D., Li Z., Su X., Loh X.J. A new highly transparent injectable PHA-based thermogelling vitreous substitute. Biomater. Sci. 2020;8(3):926–936. doi: 10.1039/c9bm01603a. [DOI] [PubMed] [Google Scholar]
- 63.Luo Z., Wu Y.L., Li Z., Loh X.J. Recent progress in polyhydroxyalkanoates-based copolymers for biomedical applications. Biotechnol. J. 2019;14(12):1900283. doi: 10.1002/biot.201900283. [DOI] [PubMed] [Google Scholar]
- 64.Lim J.Y., Lin Q., Liu C.K., Guo L., Xue K., Loh X.J. Zinc diethyldithiocarbamate as a catalyst for synthesising biomedically-relevant thermogelling polyurethanes. Mater. Adv. 2020;1(9):3221–3232. [Google Scholar]
- 65.Lim J., Lin Q., Xue K., Loh X. Recent advances in supramolecular hydrogels for biomedical applications. Mater. Today Adv. 2019;3:100021. [Google Scholar]
- 66.Lin Q., Lim J.Y., Xue K., Chee C.P., Loh X.J. Supramolecular thermogels from branched PCL-containing polyurethanes. RSC Adv. 2020;10(64):39109–39120. doi: 10.1039/d0ra07426h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin S.Y., Yao B.Y., Hu C.J., Chen H.W. Induction of robust immune responses by CpG-ODN-loaded hollow polymeric nanoparticles for antiviral and vaccine applications in chickens. Int. J. Nanomed. 2020;15:3303–3318. doi: 10.2147/IJN.S241492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin L.C., Huang C.Y., Yao B.Y., Lin J.C., Agrawal A., Algaissi A., Peng B.H., Liu Y.H., Huang P.H., Juang R.H., Chang Y.C., Tseng C.T., Chen H.W., Hu C.J. Viromimetic STING agonist-loaded hollow polymeric nanoparticles for safe and effective vaccination against Middle East respiratory syndrome coronavirus. Adv. Funct. Mater. 2019;29(28):1807616. doi: 10.1002/adfm.201807616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li B., Du L., Yu Z., Sun B., Xu X., Fan B., Guo R., Yuan W., He K. Poly (d,l-lactide-co-glycolide) nanoparticle-entrapped vaccine induces a protective immune response against porcine epidemic diarrhea virus infection in piglets. Vaccine. 2017;35(50):7010–7017. doi: 10.1016/j.vaccine.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 70.Binjawadagi B., Dwivedi V., Manickam C., Ouyang K., Wu Y., Lee L.J., Torrelles J.B., Renukaradhya G.J. Adjuvanted poly(lactic-co-glycolic) acid nanoparticle-entrapped inactivated porcine reproductive and respiratory syndrome virus vaccine elicits cross-protective immune response in pigs. Int. J. Nanomed. 2014;9:679–694. doi: 10.2147/IJN.S56127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao K., Li W., Huang T., Luo X., Chen G., Zhang Y., Guo C., Dai C., Jin Z., Zhao Y., Cui H., Wang Y. Preparation and efficacy of Newcastle disease virus DNA vaccine encapsulated in PLGA nanoparticles. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dwivedi V., Manickam C., Binjawadagi B., Renukaradhya G.J. PLGA nanoparticle entrapped killed porcine reproductive and respiratory syndrome virus vaccine helps in viral clearance in pigs. Vet. Microbiol. 2013;166(1–2):47–58. doi: 10.1016/j.vetmic.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu C.J., Chang W.S., Fang Z.S., Chen Y.T., Wang W.L., Tsai H.H., Chueh L.L., Takano T., Hohdatsu T., Chen H.W. Nanoparticulate vacuolar ATPase blocker exhibits potent host-targeted antiviral activity against feline coronavirus. Sci. Rep. 2017;7(1):13043. doi: 10.1038/s41598-017-13316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dai C., Kang H., Yang W., Sun J., Liu C., Cheng G., Rong G., Wang X., Wang X., Jin Z., Zhao K. O-2'-hydroxypropyltrimethyl ammonium chloride chitosan nanoparticles for the delivery of live Newcastle disease vaccine. Carbohydr. Polym. 2015;130:280–289. doi: 10.1016/j.carbpol.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 75.Zhao K., Li S., Li W., Yu L., Duan X., Han J., Wang X., Jin Z. Quaternized chitosan nanoparticles loaded with the combined attenuated live vaccine against Newcastle disease and infectious bronchitis elicit immune response in chicken after intranasal administration. Drug Deliv. 2017;24(1):1574–1586. doi: 10.1080/10717544.2017.1388450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao K., Han J., Zhang Y., Wei L., Yu S., Wang X., Jin Z., Wang Y. Enhancing mucosal immune response of newcastle disease virus DNA vaccine using N-2-hydroxypropyl trimethylammonium chloride chitosan and N,O-carboxymethyl chitosan nanoparticles as delivery carrier. Mol. Pharm. 2018;15(1):226–237. doi: 10.1021/acs.molpharmaceut.7b00826. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y., Xing R., Liu S., Qin Y., Li K., Yu H., Li P. Chitosan, hydroxypropyltrimethyl ammonium chloride chitosan and sulfated chitosan nanoparticles as adjuvants for inactivated Newcastle disease vaccine. Carbohydr. Polym. 2020;229:115423. doi: 10.1016/j.carbpol.2019.115423. [DOI] [PubMed] [Google Scholar]
- 78.Volkova M.A., Irza A.V., Chvala I.A., Frolov S.F., Drygin V.V., Kapczynski D.R. Adjuvant effects of chitosan and calcium phosphate particles in an inactivated Newcastle disease vaccine. Avian Dis. 2014;58(1):46–52. doi: 10.1637/10510-020413-Reg.1. [DOI] [PubMed] [Google Scholar]
- 79.Zhao K., Chen G., Shi X.M., Gao T.T., Li W., Zhao Y., Zhang F.Q., Wu J., Cui X., Wang Y.F. Preparation and efficacy of a live newcastle disease virus vaccine encapsulated in chitosan nanoparticles. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao K., Zhang Y., Zhang X., Li W., Shi C., Guo C., Dai C., Chen Q., Jin Z., Zhao Y., Cui H., Wang Y. Preparation and efficacy of Newcastle disease virus DNA vaccine encapsulated in chitosan nanoparticles. Int. J. Nanomed. 2014;9:389–402. doi: 10.2147/IJN.S54226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhai R.L., Xu H.Y., Wang Y.L., Qin Z.M., Jiang S.J. [Study on immune efficacy of Newcastle disease chitosan microsphere vaccine] Wei Sheng Wu Xue Bao. 2007;47(4):692–696. [PubMed] [Google Scholar]
- 82.Zhao K., Zhang Y., Zhang X., Shi C., Wang X., Wang X., Jin Z., Cui S. Chitosan-coated poly(lactic-co-glycolic) acid nanoparticles as an efficient delivery system for Newcastle disease virus DNA vaccine. Int. J. Nanomed. 2014;9:4609–4619. doi: 10.2147/IJN.S70633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shim B.S., Park S.M., Quan J.S., Jere D., Chu H., Song M.K., Kim D.W., Jang Y.S., Yang M.S., Han S.H., Park Y.H., Cho C.S., Yun C.H. Intranasal immunization with plasmid DNA encoding spike protein of SARS-coronavirus/polyethylenimine nanoparticles elicits antigen-specific humoral and cellular immune responses. BMC Immunol. 2010;11:65. doi: 10.1186/1471-2172-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yun C.-H., Shim B.-S., Park S.-M., Han H.H. DNA vaccine encoding spike protein of SARS-coronavirus loaded polyethylenimine elicits antigen-specific immune responses in mice immunized through intranasal route (42.8) J. Immunol. 2009;182(1 Supplement) 42.48–42.8. [Google Scholar]
- 85.Kim S.K., Yun C.H., Han S.H. Induction of dendritic cell maturation and activation by a potential adjuvant, 2-Hydroxypropyl-beta-cyclodextrin. Front. Immunol. 2016;7:435. doi: 10.3389/fimmu.2016.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan L., Zheng T., Li M., Zhong X., Tang Y., Qin M., Sun X. Optimization of an mRNA vaccine assisted with cyclodextrin-polyethyleneimine conjugates. Drug Deliv. Transl. Res. 2020;10(3):678–689. doi: 10.1007/s13346-020-00725-4. [DOI] [PubMed] [Google Scholar]
- 87.Garrido P.F., Calvelo M., Blanco-Gonzalez A., Veleiro U., Suarez F., Conde D., Cabezon A., Pineiro A., Garcia-Fandino R. The lord of the NanoRings: cyclodextrins and the battle against SARS-CoV-2. Int. J. Pharm. 2020;588:119689. doi: 10.1016/j.ijpharm.2020.119689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grancher N., Venard V., Kedzierewicz F., Ammerlaan W., Finance C., Muller C.P., Le Faou A. Improved antiviral activity in vitro of ribavirin against measles virus after complexation with cyclodextrins. Antivir. Res. 2004;62(3):135–137. doi: 10.1016/j.antiviral.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Kwon S., Lee W., Shin H.J., Yoon S.I., Kim Y.T., Kim Y.J., Lee K., Lee S. Characterization of cyclodextrin complexes of camostat mesylate by ESI mass spectrometry and NMR spectroscopy. J. Mol. Struct. 2009;938(1–3):192–197. [Google Scholar]
- 90.Chen J., Liu C., Shan W., Xiao Z., Guo H., Huang Y. Enhanced stability of oral insulin in targeted peptide ligand trimethyl chitosan nanoparticles against trypsin. J. Microencapsul. 2015;32(7):632–641. doi: 10.3109/02652048.2015.1065920. [DOI] [PubMed] [Google Scholar]
- 91.Adeoye O., Conceicao J., Serra P.A., Bento da Silva A., Duarte N., Guedes R.C., Corvo M.C., Aguiar-Ricardo A., Jicsinszky L., Casimiro T., Cabral-Marques H. Cyclodextrin solubilization and complexation of antiretroviral drug lopinavir: in silico prediction; effects of derivatization, molar ratio and preparation method. Carbohydr. Polym. 2020;227:115287. doi: 10.1016/j.carbpol.2019.115287. [DOI] [PubMed] [Google Scholar]
- 92.Dong Y., Siegwart D.J., Anderson D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019;144:133–147. doi: 10.1016/j.addr.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oishi M., Nagasaki Y., Itaka K., Nishiyama N., Kataoka K. Lactosylated poly(ethylene glycol)-siRNA conjugate through acid-labile beta-thiopropionate linkage to construct pH-sensitive polyion complex micelles achieving enhanced gene silencing in hepatoma cells. J. Am. Chem. Soc. 2005;127(6):1624–1625. doi: 10.1021/ja044941d. [DOI] [PubMed] [Google Scholar]
- 94.Kim S.H., Jeong J.H., Lee S.H., Kim S.W., Park T.G. Local and systemic delivery of VEGF siRNA using polyelectrolyte complex micelles for effective treatment of cancer. J. Contr. Release. 2008;129(2):107–116. doi: 10.1016/j.jconrel.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Lee S.H., Mok H., Lee Y., Park T.G. Self-assembled siRNA-PLGA conjugate micelles for gene silencing. J. Contr. Release. 2011;152(1):152–158. doi: 10.1016/j.jconrel.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 96.Schroeder A., Dahlman J.E., Sahay G., Love K.T., Jiang S., Eltoukhy A.A., Levins C.G., Wang Y., Anderson D.G. Alkane-modified short polyethyleneimine for siRNA delivery. J. Contr. Release. 2012;160(2):172–176. doi: 10.1016/j.jconrel.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dahlman J.E., Barnes C., Khan O., Thiriot A., Jhunjunwala S., Shaw T.E., Xing Y., Sager H.B., Sahay G., Speciner L., Bader A., Bogorad R.L., Yin H., Racie T., Dong Y., Jiang S., Seedorf D., Dave A., Sandu K.S., Webber M.J., Novobrantseva T., Ruda V.M., Lytton-Jean A.K.R., Levins C.G., Kalish B., Mudge D.K., Perez M., Abezgauz L., Dutta P., Smith L., Charisse K., Kieran M.W., Fitzgerald K., Nahrendorf M., Danino D., Tuder R.M., von Andrian U.H., Akinc A., Schroeder A., Panigrahy D., Kotelianski V., Langer R., Anderson D.G. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 2014;9(8):648–655. doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 99.Davis M.E., Zuckerman J.E., Choi C.H., Seligson D., Tolcher A., Alabi C.A., Yen Y., Heidel J.D., Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Howard K.A., Rahbek U.L., Liu X., Damgaard C.K., Glud S.Z., Andersen M.O., Hovgaard M.B., Schmitz A., Nyengaard J.R., Besenbacher F., Kjems J. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol. Ther. 2006;14(4):476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 101.Lavertu M., Methot S., Tran-Khanh N., Buschmann M.D. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials. 2006;27(27):4815–4824. doi: 10.1016/j.biomaterials.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 102.Du X.J., Wang Z.Y., Wang Y.C. Redox-sensitive dendrimersomes assembled from amphiphilic Janus dendrimers for siRNA delivery. Biomater. Sci. 2018;6(8):2122–2129. doi: 10.1039/c8bm00491a. [DOI] [PubMed] [Google Scholar]
- 103.Cai X., Zhu H., Zhang Y., Gu Z. Highly efficient and safe delivery of VEGF siRNA by bioreducible fluorinated peptide dendrimers for cancer therapy. ACS Appl. Mater. Interfaces. 2017;9(11):9402–9415. doi: 10.1021/acsami.6b16689. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Y., Li T., Fu L., Yu C., Li Y., Xu X., Wang Y., Ning H., Zhang S., Chen W., Babiuk L.A., Chang Z. Silencing SARS-CoV Spike protein expression in cultured cells by RNA interference. FEBS Lett. 2004;560(1–3):141–146. doi: 10.1016/S0014-5793(04)00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu C.J., Huang H.W., Liu C.Y., Hong C.F., Chan Y.L. Inhibition of SARS-CoV replication by siRNA. Antivir. Res. 2005;65(1):45–48. doi: 10.1016/j.antiviral.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li B.J., Tang Q., Cheng D., Qin C., Xie F.Y., Wei Q., Xu J., Liu Y., Zheng B.J., Woodle M.C., Zhong N., Lu P.Y. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat. Med. 2005;11(9):944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Conti D.S., Brewer D., Grashik J., Avasarala S., da Rocha S.R. Poly(amidoamine) dendrimer nanocarriers and their aerosol formulations for siRNA delivery to the lung epithelium. Mol. Pharm. 2014;11(6):1808–1822. doi: 10.1021/mp4006358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghosh S., Firdous S.M., Nath A. siRNA could be a potential therapy for COVID-19. EXCLI J. 2020;19:528–531. doi: 10.17179/excli2020-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]