Abstract
Background.
Tracheal cancer (TC) is a rare disease, and surgical treatment requires a high level of expertise. We sought to determine the treatment patterns and surgical outcomes of TC in the United States.
Methods.
The National Cancer Database was queried for all cases of primary invasive TC without distant metastatic disease between 2004 and 2015. Primary surgical treatment and outcomes were analyzed. Factors associated with utilization of surgery and overall survival were tested using regression analysis.
Results.
Of 1379 identified TC patients, 338 patients (25%) were treated surgically. Among resected patients, most had adenoid cystic (48%) or squamous cell (28%) carcinoma. Median length of hospital stay after resection was 7 days (interquartile range, 3–8), and 30-day mortality was 1.4%. Most nonsurgically managed patients underwent radiation (63%). Factors associated with surgical resection were younger age, higher education level, tumor size, and adenoid cystic histology. On multivariate analysis patients were also more likely to undergo surgery if they traveled a farther distance for treatment (>45 km; odds ratio, 1.53; 95% confidence interval, 1.09–2.13) or were treated at academic centers (odds ratio, 1.68; 95% confidence interval, 1.25–2.26). Five-year overall survival was 71% after resection, 39% after surgical debulking, and 31% without surgery (P < .001).
Conclusions.
National surgical outcomes for resection of TC demonstrate low perioperative mortality and excellent long-term prognosis. However, few nonmetastatic TC patients underwent surgery, indicating disparities in access to optimal surgical care and variability in practice patterns at a national level.
Primary tracheal cancer (TC) is a rare tumor, accounting for 0.2% of all respiratory malignancies.1 The incidence of TC in the United States has been estimated as 2.6 new cases per 1 million people per year.2 Squamous cell carcinoma (SCC) is the most common type and accounts for over 50% of primary tracheal malignancies.2–4 It typically presents in the sixth and seventh decades of life in men with a history of smoking and often involves the lower two-thirds of the trachea.5–7 Adenoid cystic carcinoma (ACC) is the second most common tracheal neoplasm and is characterized by a slowly progressive nature with a mean incidence in the fourth and fifth decades of life, with an equal distribution among men and women.1 Because of its rarity, there is no commonly accepted staging system for tracheal malignancies to date.
Surgical resection may be applicable to most patients presenting with localized disease and has been associated with the best long-term prognosis. For some patients (~20%) who present with metastatic disease, resection may be merely palliative to relieve airway obstruction in cases where a tracheostomy is not feasible.6,8
Although excellent perioperative and long-term outcomes for resection of TC have been reported, most data are derived from a few centers with case series spanning several decades.6,7 Treatment patterns and surgical outcomes of tracheal tumors on a national level in the United States are currently ill defined. Previous population-based studies have suggested an underutilization of tracheal resection in several other healthcare systems.3,9,10 Multiple possible barriers to surgical care have been hypothesized, including problems with access to surgical care.9 In this study we sought to describe contemporary patterns of care, explore factors associated with the utilization of surgery, and determine surgical outcomes after resection of nonmetastatic TC on the national level.
Patients and Methods
Data Acquisition and Patient Selection
We used the National Cancer Database (NCDB), a hospital-based nationwide cancer registry and joint program sponsored by the American College of Surgeon Commission on Cancer (ACS-CoC) and the American Cancer Society. The NCDB captures more than 70% of newly diagnosed cancer cases in the United States from over 1500 contributing hospitals. Data are entered by trained site-based cancer registrars using standardized coding definitions outlined by the ACS-CoC Facility Oncology Registry Data Standards manual. Cancer site–specific data were requested through a web-based application process and were provided in a deidentified NCDB file, exempt from institutional review board review or requirement of informed consent.
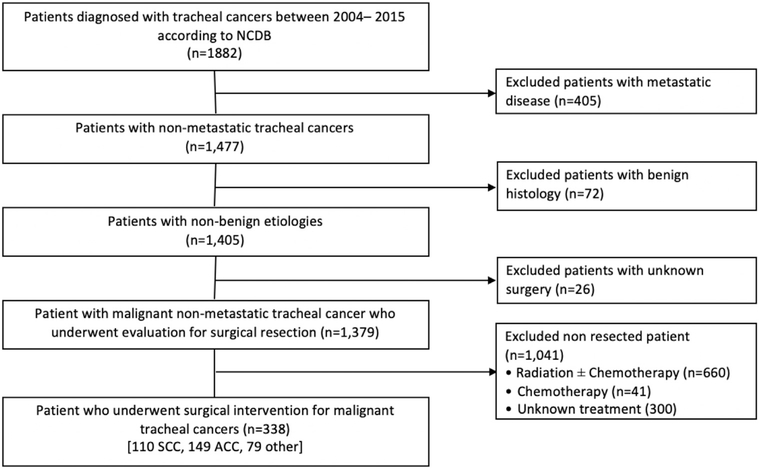
Data from adult patients with TC diagnosed between 2004 and 2015 were retrieved from the NCDB. We selected cases of primary TC based on the International Classification of Disease for Oncology, 3rd edition, topography code c33.9. Patients with noninvasive or in situ tumors, distant metastatic disease, and unknown surgical treatment status were excluded (Figure 1). Histology codes were used to distinguish ACC (8200, 8201), SCC (8052, 8070–74, 8083), or any other histology. Patients from regions of low education status were defined as residing in areas where more than 13% of the population has not achieved high school graduation. The cutoff for low versus high income was $48,000 per year. Demographic and clinical characteristics for the analysis included patient age, gender, race, education level, income, insurance type, facility type, distance traveled to the facility, and Charlson comorbidity score. Treatment facility type was defined between academic and nonacademic center based on the ACS-CoC accreditation program. Disease descriptive factors, such as tumor size, tumor extension, and lymph node status, were derived from the collaborative staging information.
Figure 1.
Consolidated Standards Of Reporting Trials (CONSORT) flow diagram describing the selection method of the patient cohort in this study. (ACC, adenoid cystic carcinoma; SCC, squamous cell carcinoma; NCDB, National Cancer Database.)
Utilization of Surgery
Primary surgical treatment of patients was determined, using site-specific procedure codes. Surgical resection was defined as all possibly curative procedures, including “simple/partial surgical removal,” “total surgical removal,” and “radical surgery.” Surgical debulking was analyzed separately. Patients who did not undergo resection and were treated with local tumor ablation/destruction procedure, polypectomy, or excisional biopsy were also recorded. Nonsurgical treatment with radiation and/or chemotherapy was specified. Patient and disease variables were analyzed to determine predictors of receiving surgical resection.
Surgical Outcomes
Outcomes of patients undergoing resection for TC were determined and reported by disease histology. Extent of resection and margin status were determined. Perioperative endpoints included length of hospital stay, 30-day readmission, 30- and 90-day mortality, and utilization of adjuvant therapy. Overall survival was calculated from the date of diagnosis to last follow-up or death.
Statistical Analysis
Descriptive statistics were summarized and compared between groups using χ2 tests for categorical variables and Student’s t tests or Kruskal-Wallis tests for continuous variables where relevant. The association of clinical factors with surgical resection was tested using univariate and multivariate binary logistic regression analyses. Univariate predictors with P < .1 were included in the multivariate model. Kaplan-Meier survival curves were created based on primary treatment, surgical treatment type (curative vs debulking), and histology. Survival estimates were compared using log-rank tests. Hypothesis testing was conducted at a 5% Type I error rate, and P < .05 was considered to be statistically significant. Cox proportional hazards regression models were fit to test factors associated with survival. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary NC).
Results
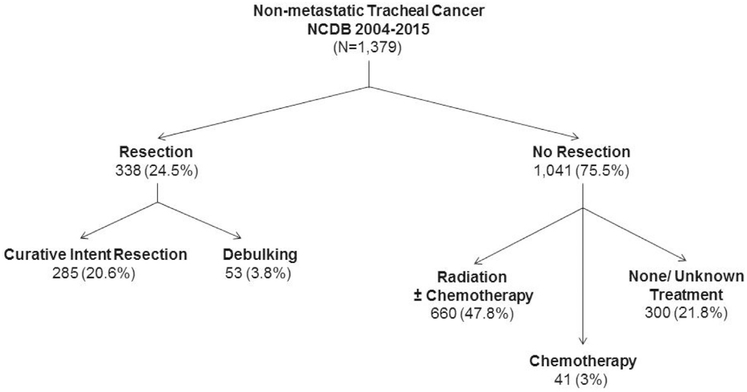
We identified 1379 patients with a diagnosis of nonmetastatic TC with a mean age of 62.8 years. The most frequent histology was SCC (55.9%) followed by ACC (22%). Various other histologies combined totaled 22.1%. Clinical characteristics are summarized in Supplemental Table 1. Surgery was performed for 338 patients (24.5%) (Figure 2). At the time of operation 84.3% of patients underwent curative resection and 15.7% had tumor debulking. Additionally 380 patients (27.6%) underwent local tumor destruction or excisional biopsy only. Among the 1041 patients who did not undergo resection, most were treated with concurrent chemoradiation (37.2%) or with radiation alone (26.2%) and a few with chemotherapy alone (3.9%) (Figure 2). Three hundred patients (21.8%) who did not undergo surgery received no other known treatment.
Figure 2.
Management of 1379 patients with nonmetastatic invasive primary tracheal cancer in the NCDB diagnosed between 2004 and 2015. (NCDB, National Cancer Database.)
Factors Predicting Utilization of Surgical Resection
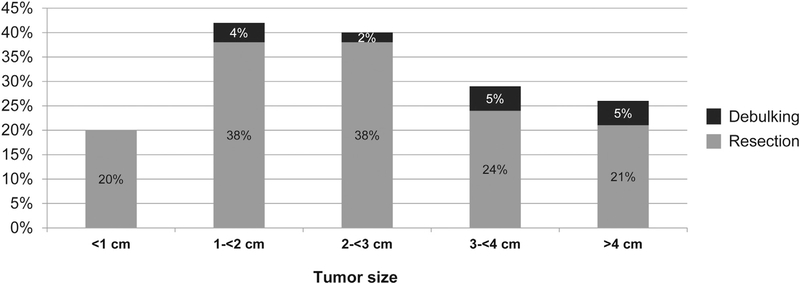
The strongest predictors of surgical resection were tumor size and histology. Patients with ACC had more than 4 times greater odds to undergo resection, as compared with SCC (Table 1). The utilization of surgery based on tumor size is depicted in Figure 3. The rate of resection was 20% for lesions <1 cm in size, increased to a high of 38% for tumors measuring between 2 and 3 cm, and decreased for larger tumors (Figure 3). Several patient and treatment site factors were associated with receiving surgical resection on univariate analysis (Table 1). On multivariate analysis controlling for disease factors, patients were more likely to undergo surgery if they resided in an area with a higher education level (odds ratio, 1.54; 95% confidence interval [CI], 1.15–2.07), traveled a farther distance to the treatment facility (>45 km; odds ratio, 1.53; 95% CI, 1.12–2.11), or were treated at academic centers (odds ratio, 1.69; 95% CI, 1.26–2.26) (Table 2).
Table 1.
Binary Logistic Regression Analysis of Factors Associated With Not Receiving Surgical Resection for Nonmetastatic Tracheal Cancer (N = 1379)
| Variable | Univariate Predictors |
P | Multivariate Predictors |
P |
|---|---|---|---|---|
| Crude Odds Ratio [95% Confidence Interval] | Adjusted Odds Ratio [95% Confidence Interval] | |||
| Age, y | 1.034 [1.025–1.044] | <.001 | 1.023 [1.011–1.035] | .001 |
| Gender, female vs male | 0.963 [0.751–1.235] | |||
| .76 | ||||
| Race, white vs nonwhite | ||||
| 1.211 [0.8831.662] | .23 | |||
| Education level | ||||
| Low | Reference | Reference | ||
| High | 0.664 [0.516–0.853] | .001 | 0.647 [0.484–0.864] | .003 |
| Unknown | 0.841 [0.27–2.62] | .77 | 0.682 [0.190–2.449] | .56 |
| Income | ||||
| High | Reference | |||
| Low | 1.164 [0.907–1.493] | .23 | ||
| Unknown | 1.217 [0.396–3.743] | .73 | ||
| Insurance | ||||
| Private | Reference | Reference | ||
| Nonprivate | 1.844 [1.426–2.384] | <.001 | 0.977 [0.701–1.361] | .89 |
| Uninsured/unknown | 1.138 [0.684–1.894] | .62 | 0.914 [0.506–1.653] | .77 |
| Facility type | ||||
| Academic center | Reference | Reference | ||
| Nonacademic center | 1.775 [1.385–2.274] | <.001 | 1.686 [1.261–2.255] | .001 |
| Distance to facility | ||||
| ≤45 km | Reference | Reference | ||
| >45 km | 0.543 [0.414–0.711] | <.001 | 0.650 [0.473–0.892] | .008 |
| Charlson comorbidity index | ||||
| 0 | Reference | Reference | ||
| 1 | 1.297 [0.973–1.730] | .076 | 1.010 [0.729–1.403] | .95 |
| >1 | 1.675 [1.060–2.648] | .027 | 1.050 [0.625–1.762] | .85 |
| Tumor size | ||||
| <2 cm | Reference | Reference | ||
| 2–5 cm | 1.105 [0.807–1.514] | .53 | 1.200 [0.846–1.703] | .31 |
| >5 cm | 2.751 [1.554–4.872] | .001 | 3.030 [1.623–5.657] | .001 |
| Unknown | 6.451 [4.315–9.644] | <.001 | 5.328 [3.463–8.197] | <.001 |
| Tumor histology | ||||
| Squamous cell carcinoma | Reference | Reference | ||
| Adenoid cystic carcinoma | 0.172 [0.127–0.233] | <.001 | 0.247 [0.177–0.345] | <.001 |
| Other | 0.476 [0.283–0.669] | <.001 | 0.529 [0.370–0.757] | .001 |
| Tumor extension | ||||
| Local/confined to trachea | Reference | Reference | ||
| Invades adjacent structures | 0.765 [0.592–0.987] | .039 | 0.779 [0.578–1.049] | .10 |
| Unknown | 5.143 [2.056–12.866] | .001 | 2.058 [0.786–5.391] | .14 |
Figure 3.
Rate of surgery based on the largest tumor size in centimeter increments (n = 865).
Table 2.
Clinicopathologic Characteristics and Surgical Outcomes of Tracheal Cancer Patients Undergoing Surgical Resection
| Characteristics | Squamous Cell Carcinoma (n = 81) | Adenoid Cystic Carcinoma (n = 137) | Other Malignancy (n = 67) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 65 (56–72) | 54 (44–63) | 59 (42–68) | <.001 |
| Gender | ||||
| Female | 20 (24.7) | 69 (50.4) | 27 (40.3) | .001 |
| Male | 61 (75.3) | 68 (49.6) | 40 (59.7) | |
| Race | ||||
| White | 68 (83.9) | 107 (78.1) | 57 (85.1) | .38 |
| Not white | 13 (16.1) | 30 (21.9) | 10 (14.9) | |
| High education level | ||||
| Yes | 48 (59.3) | 83 (60.6) | 41 (61.2) | .80 |
| No | 31 (38.3) | 52 (38.0) | 26 (38.8) | |
| Unknown | 2 (2.5) | 2 (1.5) | 0 (0.0) | |
| High income | ||||
| Yes | 43 (53.1) | 81 (59.1) | 40 (59.7) | .68 |
| No | 36 (44.4) | 54 (39.4) | 27 (40.3) | |
| Unknown | 2 (2.5) | 2 (1.5) | 0 (0.0) | |
| Charlson comorbidity score | ||||
| 0 | 51 (63.0) | 96 (70.1) | 54 (80.6) | .021 |
| 1 | 20 (24.7) | 36 (26.3) | 8 (11.9) | |
| >1 | 10 (12.3) | 5 (3.6) | 5 (7.5) | |
| Treating facility | ||||
| Academic/research center | ||||
| Yes | 49 (60.5) | 75 (54.7) | 38 (56.7) | .71 |
| No | 32 (39.5) | 62 (45.3) | 29 (43.3) | |
| Travel distance, miles | 18.7 (7.1–90.4) | 14.4 (9.0–56.4) | 45.0 (12.0–91.7) | .009 |
| Disease characteristics | ||||
| Tumor size | ||||
| <2 cm | 24 (29.6) | 31 (22.6) | 29 (43.3) | .024 |
| 2–5 cm | 51 (63.0) | 82 (59.9) | 31 (46.3) | |
| >5 cm | 1 (1.2) | 9 (6.6) | 4 (6.0) | |
| Unknown | 5 (6.2) | 15 (11.0) | 3 (4.5) | |
| Tumor extension | ||||
| Local/confined to trachea | 45 (55.6) | 74 (54.0) | 44 (65.7) | .30 |
| Invades adjacent structures | 35 (43.2) | 63 (46.0) | 22 (32.8) | |
| Unknown | 1 (1.2) | 0 (0.0) | 1 (1.5) | |
| Lymph node metastases | ||||
| No | 25 (30.9) | 52 (38.0) | 23 (34.3) | .26 |
| Yes | 12 (14.8) | 13 (9.5) | 3 (4.5) | |
| Unknown | 44 (54.3) | 72 (52.6) | 41 (61.2) | |
| Margins status | ||||
| Negative | 44 (54.3) | 49 (35.8) | 52 (77.6) | <.001 |
| Positive | 31 (38.3) | 80 (58.4) | 10 (14.9) | |
| Unknown | 6 (7.4) | 8 (5.8) | 5 (7.5) | |
| Treatment details | ||||
| Extent of resection | ||||
| Simple/total resection | 75 (92.6) | 122 (89.1) | 55 (82.1) | .13 |
| Radical resection (adjacent organs) | 6 (7.4) | 15 (10.9) | 12 (17.9) | |
| Lymphadenectomy | ||||
| Yes | 39 (48.2) | 68 (49.6) | 30 (44.8) | .97 |
| No | 40 (49.4) | 65 (47.5) | 35 (52.2) | |
| Unknown | 2 (2.5) | 4 (2.9) | 2 (3.0) | |
| Perioperative outcomes | ||||
| Length of stay, days | 6 (3–11) | 7 (4–10) | 7 (4–8) | .74 |
| Readmission in 30 days | ||||
| Yes | 5 (6.2) | 8 (5.8) | 4 (6.0) | .96 |
| No | 72 (88.9) | 121 (88.3) | 61 (91.0) | |
| Unknown | 4 (4.9) | 8 (5.8) | 2 (3.0) | |
| Mortality, 30 days | ||||
| Yes | 2 (2.5) | 2 (1.5) | 0 (0.0) | .20 |
| No | 72 (88.9) | 125 (91.2) | 56 (83.6) | |
| Unknown | 7 (8.6) | 10 (7.3) | 11 (16.4) | |
| Mortality, 90 days | ||||
| Yes | 5 (6.2) | 2 (1.5) | 0 (0.0) | .033 |
| No | 67 (82.7) | 124 (90.5) | 55 (82.1) | |
| Unknown | 9 (11.2) | 11 (8.0) | 12 (17.9) | |
| Adjuvant treatment | ||||
| Radiation | ||||
| Yes | 40 (49.4) | 102 (74.5) | 20 (29.9) | <.001 |
| No | 38 (46.9) | 32 (23.4) | 45 (67.2) | |
| Unknown | 3 (3.7) | 3 (2.2) | 2 (3.0) | |
| Chemotherapy | ||||
| Yes | 20 (24.7) | 13 (9.5) | 11 (16.4) | .041 |
| No | 57 (70.4) | 115 (83.9) | 54 (80.6) | |
| Unknown | 4 (4.9) | 9 (6.6) | 2 (3.0) | |
Values are median (interquartile range) or n (%).
Surgical Treatment Characteristics
Among 338 patients treated surgically, 32.5% of patients had SCC, 44.1% had ACC, and 23.4% had another histology (Table 2). SCC patients tended to be older, were more commonly men, and had higher comorbidity scores (Table 2). Most resections (57%) were performed in academic centers (Table 2). Most patients underwent a simple or total resection (75%), and 10% required a radical resection of adjacent organs. A complete (R0) resection was achieved in 55% of patients undergoing resection. Lymph node metastases were found in 37.1% of patients with known lymph node status (Table 2).
Surgical Outcomes
Median length of the index hospitalization after TC resection was 7 days (interquartile range, 3–8), and 17 patients (5.9%) required readmission. Thirty- and 90-day postoperative mortality was 1.4% and 2.5%, respectively. Utilization of adjuvant radiation was 74% after resection of ACC, 43% for SCC, and 65% for other resected histologies. Adjuvant chemotherapy was used in some patients and more frequently for SCC (24.7%) as compared with ACC or other tumors (16%, P = .041) (Table 2). Median follow-up among survivors was 72.6 months (95% CI, 63.1–82.4) for surgically treated patients and 65.8 months (95% CI, 59.8–70.8) for nonsurgical patients.
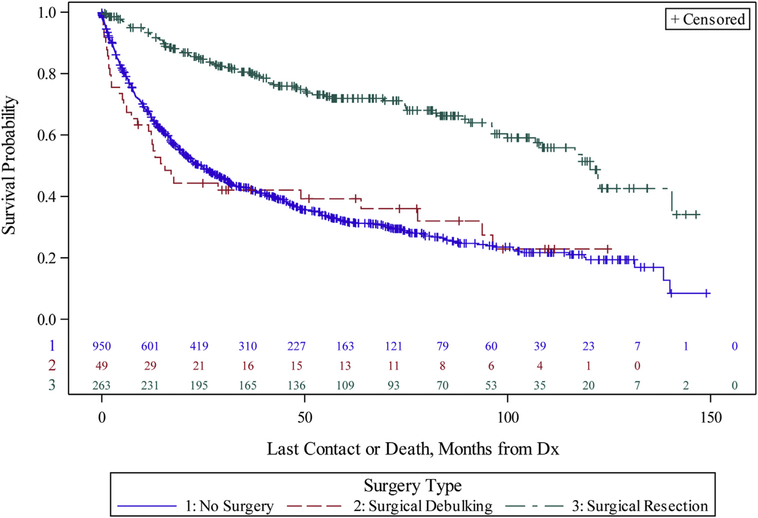
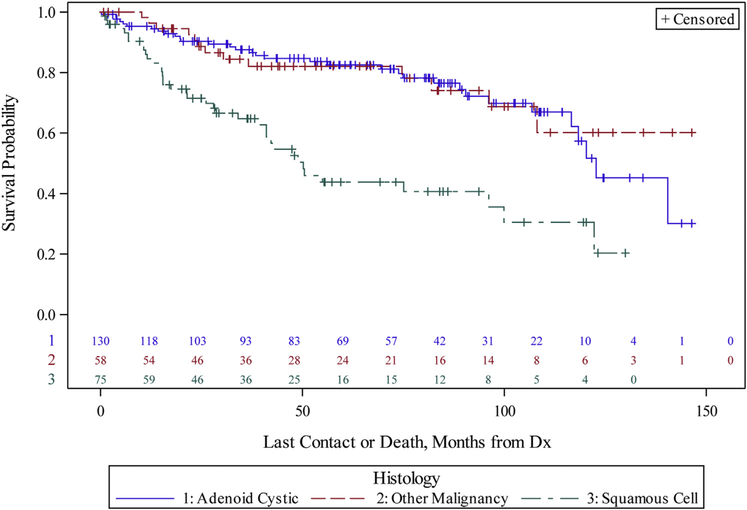
Survival after diagnosis was significantly longer for patients undergoing curative intent resection, with a median overall survival of 120.3 month, as compared with 15.6 months for patients who underwent a debulking procedure and 24.5 months for patients who did not undergo surgery (P < .001) (Figure 4). Survival varied based on histology (Figure 5). Estimated 3- and 5-year overall survival after resection was 80.2% and 74.1% for ACC, 52% and 30.3% for SCC, and 71.2% and 56.2% for other primary tracheal malignancies, respectively (P < .001). ACC histology and complete resection remained independent prognostic factors on multivariate survival analysis (Table 3).
Figure 4.
Kaplan-Meier curves of overall survival for nonmetastatic tracheal cancer patients, comparing patients who underwent curative resection (green), surgical debulking (red), or no surgery (blue) (P < .001). (Dx, diagnosis.)
Figure 5.
Kaplan-Meier curves of overall survival for curative intent resection patients (n = 285) by histology, comparing adenoid cystic carcinoma (blue), squamous cell carcinoma (green), and other histologies (red) (P < .001). (Dx, diagnosis.)
Table 3.
Multivariate Cox Regression Analysis of Overall Survival
| Variables | Adjusted Hazard Ratioa | 95% Confidence Interval | P |
|---|---|---|---|
| Age (per year) | 1.024 | 1.008–1.039 | .003 |
| Histology | |||
| Squamous cell | Reference | ||
| Adenoid cystic | 0.443 | 0.273–0.719 | .001 |
| Other | 0.626 | 0.356–1.104 | .68 |
| Surgery type | |||
| Simple resection | Reference | ||
| Total/radical surgery | 1.073 | 0.668–1.723 | .77 |
| Debulking | 2.566 | 1.402–4.696 | .002 |
| Margin status | |||
| Negative | Reference | ||
| Positive | 1.784 | 1.105–2.883 | .018 |
| Unknown | 1.790 | 0.902–3.552 | .095 |
Model adjusted for Charlson-comorbidity score, tumor size, lymph node status, and receipt of adjuvant therapy.
Comment
This comprehensive analysis of the NCDB provides insight on the national patterns of care and outcomes of nonmetastatic primary TC in the United States. Our results demonstrate that although resection for TC was associated with low perioperative mortality and excellent long-term prognosis, only a minority of all patients captured in the NCDB were treated with surgery. Patients were more likely to undergo surgery if they resided in areas with higher education levels, traveled farther distance to the treating center, and were treated at academic institutions, which points to disparities in treatment and barriers to access of optimal surgical care.
Our first objective was to examine the utilization of surgery. We found that only one-fourth of patients with nonmetastatic primary TC in NCDB underwent surgery. Previous population-based studies from European countries have shown even lower rates of surgery for primary TC in several other healthcare systems.3,5,10,11 A remote study from the Finnish Cancer Registry (1967–1985) showed a 6% rate of surgery for primary TC.11 Similarly analyses of national cancer registries from Denmark (1978–1995) and the Netherlands (1989–2002) reported surgery rates of 10% and 11.6%, respectively.3,5 A more contemporary report on management of primary TC from the National Health System in England included 686 patients with nonmetastatic TC, of which only 60 patients (8.7%) underwent curative resection between 1996 and 2011.10 Similar to our observation, studies uniformly showed that most patients received radiation therapy as the primary treatment with rates ranging from 24% to 63%. In contrast a much higher subset of patients treated surgically was reported in specialized centers, with resection rates of over 70% for TC.6,7 At Massachusetts General Hospital, Gaissert and colleagues6 reported a resection rate as high as 82% since 1992. Of note their study had a significantly higher proportion of ACC patients (50% vs 7% in national studies from Denmark and Netherlands), which was associated with a higher rate of resection in our analysis (Table 1).3,5,6
The concern remains that utilization of surgery for TC is limited by access to surgical care. A unique study by Honings and colleagues9 aimed to characterize the underutilization of surgery suggested by low resection rates in national registry studies. Their study closely examined 44 patients diagnosed with nonmetastatic and potentially resectable TC in the Netherlands between 2000 and 2005 and showed that 12 patients (27%) actually received an operation. Interestingly when the authors audited the cases retrospectively by review of imaging and medical records, they found that 28 patients (64%) appeared to have been surgical candidates for resection, based on their judgement of having resectable tumors and being in good general health. Furthermore the estimated resectability reached 72% for ACC and 50% for SCC.9 The difference between the number of surgical candidates and patients who underwent resection in this study was attributed to a lack of experience by treatment facilities. Given the rarity of tracheal neoplasms, early referral to tertiary centers with multidisciplinary experience may therefore improve selection of surgical candidates and maximize the chance for curative resection.
This current study analyzed factors associated with the utilization of surgery in the United States. Our results substantiate the concern that barriers to surgical care may contribute to low surgery rates. In addition to disease factors that determine resectability, such as tumor size and histology, we showed that longer travel distance to the treating center and treatment at an academic institution were associated with higher likelihood of surgical treatment (Table 1). Some authors have suggested a centralized referral system to specialized surgery units as a possible solution to increase resection rates, which may be possible in government-controlled healthcare systems but is not an option in countries with an uncontrolled referral system.5,10 Nevertheless a registry of surgeons skilled in airway surgery could provide a useful resource for referring providers and create an awareness of the available surgical expertise. Although the surgical volume with TC outside of few high-volume institutions is generally low, thoracic surgeons are now abundant across the country and maintain their skills of tracheal surgery primarily through resections for benign disease, such as for tracheal stenosis.
Our second objective was to determine surgical outcomes for TC at a national level. The overall 90-day mortality after surgery for TC was 2.5%, which compares favorably with the perioperative mortality reported from most single-institution series ranging from 3% to 14%, most of which include remote cases from previous decades.4,7,12–14 As demonstrated by Gaissert and colleagues,6 mortality from tracheal resections has decreased drastically over the past 3 decades. Although data on perioperative events are not captured in the NCDB, we report a median length of hospital stay of 7 days, which is consistent with the expectation for patients with an uncomplicated recovery from tracheal resection, because most surgeons will maintain patients in a flexed neck position for several days and perform a bronchoscopy typically 1 week after surgery before discharge.
With regards to oncologic outcomes we showed that margin rates after resection for TC varied based on histology, with the highest rate of positive surgical margins for ACC (58%), followed by SCC (38%), and lowest for other histologies (15%). It is difficult to ascertain how many patients underwent resection for palliation of an airway obstruction and who may be left with a positive airway margin after reaching the maximum length of resected trachea that allows for safe reconstruction. These factors likely account for the wide range of positive margin rates between studies. A French multicenter study reported lower positive margin rates of 38% for ACC, 26% for SCC, and 9% for other histologies.12 Higher positive margin rates of 93% for ACC and 40% for SCC were reported from Massachusetts General Hospital,6 which likely included many patients who were operated on for palliation. Most importantly survival in this study was excellent, with 5-year survival rates of 74%, 30%, and 56% for ACC, SCC, and other histologies, respectively. Our survival analysis further confirms that the completeness of resection is the strongest predictive factor of survival independent of histology (Table 3). Although surgical debulking may provide palliative benefits in selected cases, we found no survival benefit as compared with nonsurgically managed patients (Figure 4). Consistent with the varying rates of patients undergoing complete resections, comparative survival rates after resection also varied between previous studies, with 5-year survival rates of 52% to 100% for ACC and 39% to 53% for SCC.5–7,12
Adjuvant therapy was not associated with survival in resected patients in this study on multivariate analysis but may have contributed to additional local control in many cases where a negative margin was not achieved. Although the evidence for adjuvant therapy is inconclusive, most reports have demonstrated favorable outcomes.4,15,16
Our study has several limitations, which need to be borne in mind when interpreting the data. Although the NCDB provides an excellent resource to study rare disease types, such as TC, we are limited by a fixed subset of data. Specifically for TC we were unable to discern the exact surgical procedures, because the operations are reported as general procedure codes with no clear definitions of what specific procedure and approach were included in each procedure category. When examining the utilization of surgery it was not possible to determine the denominator of patients with resectable disease, because this consideration is complex and depends on multiple patient characteristics that are not available in the NCDB, such as the length of the trachea involved, body habitus, prior mediastinal surgery or radiation, or steroid use. In addition surgeon characteristics were not available, such as training and specialty, which may affect the assessment of resectability. Given the rarity of the disease and that most tracheal resections are performed for benign disease, we were unable to analyze the relationship between surgical volume in this dataset. Furthermore, given the lack of a defined TNM staging system, we had to rely on the collaborative staging information for TC, which is not as well defined. Previous studies have also pointed to a high rate of misclassification of TC in national cancer registries, in some instances >10%, which included misattribution of secondary tracheal malignancies due to cancer metastases and tumors originating from adjacent organs, such as the thyroid, or paratracheal lymph node metastases.3,9
In summary, this study demonstrates an overall low utilization of surgery for TC in the United States. Factors associated with surgical resection in this study were higher education level, longer travel distance to the treating facility, and treatment at academic centers, indicating problems with access to optimal surgical care. Given the low postoperative mortality and excellent long-term prognosis of patients who undergo curative resection for TC, our results should provide impetus to steer patients to centers with experience in airway surgery.
Supplementary Material
Acknowledgments
The NCDB is a joint project of the ACS-CoC and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the NCDB are the source of the deidentified data used herein. The interpretation and reporting of these data are the sole responsibility of the authors. The ACS-CoC and American Cancer Society have not verified the results and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the authors.
Footnotes
The Supplemental Table can be viewed in the online version of this article [https://doi.org/10.1016/j.athoracsur.2020.03.048] on http://www.annalsthoracicsurgery.org.
References
- 1.Gaissert HA, Mark EJ. Tracheobronchial gland tumors. Cancer Control. 2006;13:286–294. [DOI] [PubMed] [Google Scholar]
- 2.Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol. 2011;34:32–37. [DOI] [PubMed] [Google Scholar]
- 3.Licht PB, Friis S, Pettersson G. Tracheal cancer in Denmark: a nationwide study. Eur J Cardiothorac Surg. 2001;19:339–345. [DOI] [PubMed] [Google Scholar]
- 4.Webb BD, Walsh GL, Roberts DB, Sturgis EM. Primary tracheal malignant neoplasms: the University of Texas MD Anderson Cancer Center experience. J Am Coll Surg. 2006;202:237–246. [DOI] [PubMed] [Google Scholar]
- 5.Honings J, van Dijck JA, Verhagen AF, van der Heijden HF, Marres HA. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol. 2007;14:968–976. [DOI] [PubMed] [Google Scholar]
- 6.Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg. 2004;78:1889–1896 [discussion: 1896–1897]. [DOI] [PubMed] [Google Scholar]
- 7.Hazama K, Miyoshi S, Akashi A, et al. Clinicopathological investigation of 20 cases of primary tracheal cancer. Eur J Cardiothorac Surg. 2003;23:1–5. [DOI] [PubMed] [Google Scholar]
- 8.Honings J, Gaissert HA, van der Heijden HF, Verhagen AF, Kaanders JH, Marres HA. Clinical aspects and treatment of primary tracheal malignancies. Acta Otolaryngol. 2010;130: 763–772. [DOI] [PubMed] [Google Scholar]
- 9.Honings J, Gaissert HA, Verhagen AF, et al. Undertreatment of tracheal carcinoma: multidisciplinary audit of epidemiologic data. Ann Surg Oncol. 2009;16:246–253. [DOI] [PubMed] [Google Scholar]
- 10.Nouraei SM, Middleton SE, Nouraei SA, et al. Management and prognosis of primary tracheal cancer: a national analysis. Laryngoscope. 2014;124:145–150. [DOI] [PubMed] [Google Scholar]
- 11.Manninen MP, Pukander JS, Flander MK, Laippala PJ, Huhtala HS, Karma PH. Treatment of primary tracheal carcinoma in Finland in 1967–1985. Acta Oncol. 1993;32:277–282. [DOI] [PubMed] [Google Scholar]
- 12.Regnard JF, Fourquier P, Levasseur P. Results and prognostic factors in resections of primary tracheal tumors: a multicenter retrospective study. The French Society of Cardiovascular Surgery. J Thorac Cardiovasc Surg. 1996;111:808–813 [discussion: 813–814]. [DOI] [PubMed] [Google Scholar]
- 13.Cordos I, Bolca C, Paleru C, Posea R, Stoica R. Sixty tracheal resections—single center experience. Interact Cardiovasc Thorac Surg. 2009;8:62–65 [discussion: 65]. [DOI] [PubMed] [Google Scholar]
- 14.Shiraishi T, Yanagisawa J, Higuchi T, et al. Tracheal resection for malignant and benign diseases: surgical results and perioperative considerations. Surg Today. 2011;41:490–495. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Huang M, Xu Y, et al. Primary tracheal adenoid cystic carcinoma: adjuvant treatment outcome. Int J Clin Oncol. 2015;20:686–692. [DOI] [PubMed] [Google Scholar]
- 16.Xie L, Fan M, Sheets NC, Chen RC, Jiang GL, Marks LB. The use of radiation therapy appears to improve outcome in patients with malignant primary tracheal tumors: a SEER-based analysis. Int J Radiat Oncol Biol Phys. 2012;84: 464–470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.