Abstract
Excess nutritional supply to the growing fetus, resulting from maternal diabetes and obesity, is associated with increased risks of fetal maldevelopment and adverse metabolic conditions in postnatal life. The placenta, interposed between mother and fetus, serves as the gateway between the two circulations and is usually considered to mediate maternal exposures to the fetus through a direct supply line. In this Perspective, however, we argue that the placenta is not an innocent bystander and mounts responses to fetal “signals of distress” to sustain its own adequate function and protect the fetus. We describe several types of protection that the placenta can offer the fetus against maternal metabolic perturbations and offer a theoretical model of how the placenta responds to the intrauterine environment in maternal diabetes and obesity to stabilize the fetal environment. Our approach supports growing calls for early screening and control of pregnancy metabolism to minimize harmful fetal outcomes.
Introduction
The placenta is a fetal organ interposed between the maternal and fetal circulation and thus is exposed to influences from both sides. The placenta develops alongside, and initially in advance of, the fetus throughout pregnancy. Various factors can disrupt this development, and in this context, much attention has focused on maternal undernutrition and preeclampsia (1). However, the metabolic, endocrine, and inflammatory effects of maternal diabetes or obesity are also associated with changes in placental structure and function (2). These changes are associated, potentially causally, with variability in fetal phenotype and may have long-term health effects (3). In particular, compared with neonates of lean mothers or mothers without diabetes, those of mothers with diabetes or obesity typically demonstrate excessive fat accretion (4,5).
However, at the individual level, neonates exposed to maternal metabolic dysfunction do not always show these changes. Why, therefore, are some mothers with diabetes or obesity prone to poorer neonatal outcomes? Beyond any heterogeneity in maternal metabolic phenotype, some reasons for variable fetal responses may relate to the placenta itself. However, this issue has received minimal attention.
The human fetus and neonate are unusual among mammals in demonstrating high fat accretion before birth (6), proposed to buffer the brain against potential malnutrition in early infancy (6). The human neonatal brain also consumes a much larger fraction of oxygen than any other species (6). These traits require adequate mechanisms of maternal oxygen supply across the placenta, not only under normal conditions, but also when fetal oxygen demand is high or maternal supply is impaired. The placenta develops and operates in a low oxygen environment. At the end of pregnancy, placental glucose metabolism is mostly glycolytic, i.e., nonoxidative (7). This may prevent the excessive generation of reactive oxygen species (ROS) by the mitochondria but also spares energy for the fetus through releasing a portion of placentally derived lactate, which may aid fetal brain metabolism.
Several aspects of placental function indicate some kind of adaptive response to variability in maternal phenotype, which may benefit fetal outcomes. Until recently, the primary focus was on maternal undernutrition. Under the lens of “genetic conflict theory,” the placenta was considered to act in the interests of the fetus, for example, releasing hormones into the maternal blood stream that elevate maternal blood pressure to force more nutrients across the placental interface (8,9). This would protect fetal fuel supply against low maternal circulating nutrient levels.
However, we suggest that such responses may also occur in the context of maternal diabetes or obesity. The placenta may respond to enhanced fetal oxygen demand by enlarging the surface area of exchange, thus facilitating maternal-to-fetal oxygen diffusion. Similarly, the placenta may remove cholesterol from its circulation, preventing formation of proatherosclerotic lesions that might impede fetoplacental blood flow. Manifold other placental changes have been described in diabetes and obesity at the end of gestation. However, little attempt has been made to interpret these changes within an adaptive framework.
Here, we describe in more detail how the placenta can protect the fetus against maternal metabolic perturbations and offer a conceptual model of how this provides a stable environment for the fetus. We use these concepts to support the growing call for early screening (and control) of pregnancy metabolism to minimize adverse fetal outcomes.
Placental Homeostatic Capacity
During intrauterine development, the homeostatic capacity of the fetus itself is immature and will only begin to fulfill this function after delivery (as shown by the heterogeneity of neonatal glucose metabolism [10]). Variations in fetal metabolism can serve as signals to the placenta, in particular to the endothelium, which lines the fetoplacental vasculature. These signals can induce placental responses, which can be interpreted as adapting placental structure and function to protect fetal development. Broadly, this allows the placenta to play a key role in fetal metabolic homeostasis.
The classical example is the placental response to fetal hypoxia. It has long been known that maternal overnutrition leads to fetal hyperinsulinism, which stimulates glucose utilization through aerobic metabolism. As a result, fetal oxygen demand rises. If this demand cannot be adequately satisfied because of maternal undersupply, fetal hypoxia may ensue. To protect the fetus, the placenta enlarges its surface area of exchange to facilitate oxygen transfer (11). One of the fetal signals that stimulates this vascular growth (angiogenesis) through various cooperating mechanisms is insulin (12,13). Thus, the same signal that causes the increased fetal demand for oxygen also stimulates the adaptive placental response.
At the same time, the fetus increases its number of red blood cells as acceptors for oxygen (14). In turn, this augmented erythropoiesis increases fetal iron demand, which is met by transplacental transfer of transferrin. This may be enhanced in diabetes, as placental transferrin receptor expression is increased in this condition (15). In sum, these adaptations link together metabolic responses in the fetus and placenta that allow higher oxygen uptake to be achieved.
A second example of placental adaptation relates to the protection of vessels from preatherosclerotic lesions in order to maintain blood flow and nutrient/oxygen supply. While the maternal-decidual blood vessels, the intervillous space (which harbors maternal blood), and the fetal arteries all show signs of preatherosclerotic lesions in situations of maternal overnutrition/hyperlipidemia (16–18), such lesions have never been reported in the vessels of the placenta itself. Thus, efficient mechanisms must exist to protect these vessels from any preatherosclerotic lesions/foam cells/plaques, which may compromise blood flow.
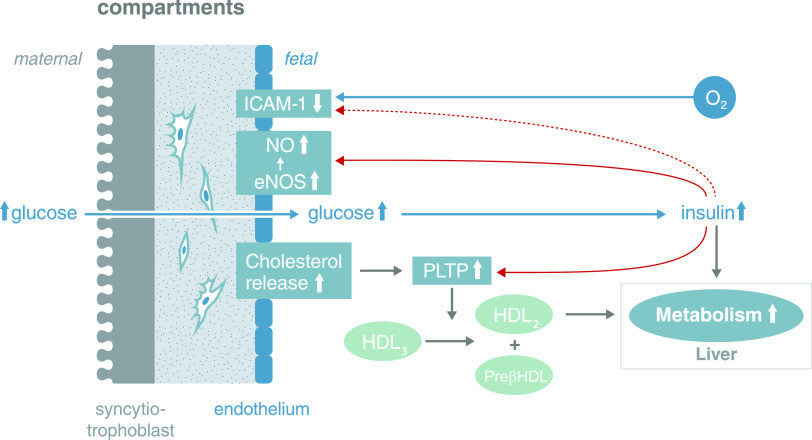
Recently, we found that oxidative stress in the fetal circulation and placental endothelial cells associated with gestational diabetes mellitus (GDM) lead to higher cholesterol synthesis in the endothelial cells (19). At the same time, the endothelial cells enhance their cholesterol efflux capacity by upregulating two efflux transporters in response to signals emerging from the adverse consequences of the diabetic environment (ROS-induced formation of oxysterols) as a stimulus for counterregulatory measures (Fig. 1). These serve to avoid intracellular toxic effects of cholesterol and, hence, maintain full function in the endothelial cells. Moreover, the formation of preatherosclerotic lesions is also avoided, as one enzyme on the surface of the endothelial cells, phospholipid transfer protein, is upregulated in GDM by insulin (20). Thus, this system efficiently removes free cholesterol from fetal endothelial cells and the fetoplacental circulation under conditions of GDM.
Figure 1.
Fetal signals related to excess nutritional supply facilitate placental adaptation to prevent atherosclerotic plaques being formed. Fetoplacental endothelial cells synthesize more cholesterol in GDM than in normal pregnancies (19). At the same time, two cholesterol efflux transporters (ABCA1, ABCG1) are upregulated through activation of the LXR transcription factor in response to higher concentrations of circulating and intraendothelial oxysterols formed by ROS-induced cholesterol oxidation. Cholesterol efflux from these endothelial cells is also enhanced by insulin-induced upregulation of phospholipid transfer protein (PLTP) on the surface of the endothelial cells in GDM (20,72). This enzyme transfers cholesterol from HDL3 to HDL2, while pre-β HDL remains. Pre-β HDL can pick up cholesterol from the endothelial cells, as it is a cholesterol acceptor (73). The majority of HDL2 will be taken up by the fetal liver and the cholesterol converted into bile acids. Thus, there is a very efficient system for removing free cholesterol from fetoplacental endothelial cells and the fetoplacental circulation to avoid fetal hypercholesterolemia under conditions of GDM. A second system to prevent formation of atherosclerotic plaques involves the downregulation of intercellular adhesion molecule 1 (ICAM-1) expressed on the surface of fetoplacental endothelial cells in GDM (74). ICAM-1 mediates the adhesion of leukocytes to endothelial cells, which then transmigrate into the subendothelial space. Outside pregnancy, this mechanism plays a pivotal role in the inflammatory component of atherosclerosis (75). Loss of endothelial cell surface and soluble ICAM-1 in GDM may be induced, among other factors, by fetal insulin (76). Fetal insulin also increases endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) synthesis (12) providing atheroprotection (77). These three systems, and probably more, act in concert to ultimately protect the fetoplacental circulation. Note: This is a schematic depicting the coordinated action of the players and not their precise location. PLTP is located on the endothelial surface.
These examples highlight the capacity of the placenta, at least at the end of pregnancy, to mount adaptive responses to the adverse fetal environment generated by maternal overnutrition, thereby protecting fetal development. Intriguingly, fetal insulin, which is elevated in GDM and metabolically abnormal obesity, seems to play a key role in inducing several of these adaptive changes at the end of pregnancy.
It is pertinent that early in pregnancy, the majority of insulin receptors are located on the syncytiotrophoblast, which represents the placental interface with the maternal circulation. Later in pregnancy, insulin receptor location undergoes a maternal-to-fetal shift, such that the receptor majority is then on the surface of the endothelial cells that interact with the fetal circulation and can receive fetal insulin signals (21). This shift makes the fetoplacental unit less susceptible to variations in maternal insulin levels. At the same time, it also establishes a mechanism for fetal protection, whereby “signals of distress” from the fetus induce an adaptive placental response. The timing of onset of this mechanism has not been established yet but will not be before pregnancy weeks 12–14, when insulin is secreted by the fetal pancreas into the circulation (22,23).
The Capacity-Load Model
Homeostasis is a key physiological principle referring to a range of metabolic regulatory processes that maintain a relatively stable internal state in the face of environmental fluctuations. The brain plays a key role but has also “outsourced” many activities to other organs and tissues while also protecting itself from metabolic perturbations through its resistant blood-brain barrier (24).
While many challenges to homeostasis emerge directly from the external environment (e.g., temperature, food supply, predators), other stresses relate more closely to “internal” components of metabolism. We have previously proposed a “capacity-load” model of metabolism, noting that many internal characteristics of the organism (e.g., lipogenic diet, abdominal adiposity, sedentary behavior, psychosocial stress, infection, and immune response), which we collectively term “metabolic load,” can pose major challenges to homeostasis. This load must be resolved by the functions of diverse organs, which we collectively term homeostatic “metabolic capacity.” Failure to resolve these challenges allows the early accumulation of risk factors for a range of noncommunicable diseases (24–26).
From a life-course perspective, the development of metabolic capacity occurs primarily in fetal life and early infancy under the protective metabolic milieu of maternal phenotype (24). During this period, development is dominated by hyperplastic growth, which encompasses organogenesis (27). Other critical developmental processes include the emergence of epigenetic effects in DNA expression and the development of hormonal set points (28,29). Interindividual variability in many of these traits tends to track on into later life and has life-long impact on the capacity for homeostasis (24).
In contrast to metabolic capacity, metabolic load largely develops from birth onward, though it may already be present in the later stages of fetal life, as demonstrated by the high fat content and hyperinsulinemia of neonates born to mothers with obesity or diabetes (30,31).
The capacity-load model assumes that cardiometabolic risk increases in association with both lower metabolic capacity and higher metabolic load (24–26). Numerous studies support the hypothesis; for example, birth weight (a proxy for metabolic capacity) shows an inverse association with the risk of type 2 diabetes or hypertension, whereas lipogenic diet, high BMI, and sedentary behavior in adulthood (markers of load) all increase the risk of these conditions (32). While sharing much in common with the “thrifty phenotype” hypothesis (33), a difference in the capacity-load model is that it assumes dose-response associations of fetal nutritional supply and early growth patterns with metabolic capacity. This helps explain why inverse associations of birth weight with the risk of noncommunicable diseases are observed across the majority of the range of birth weight rather than being evident only in those of low birth weight (32,34).
For most of the life course, both metabolic capacity and load can be considered properties of individual organisms. However, we suggest that this model should be adapted to address pregnancy, where metabolic load is broadly generated by the mother rather than the fetus and the fetus has very limited direct capacity for homeostasis.
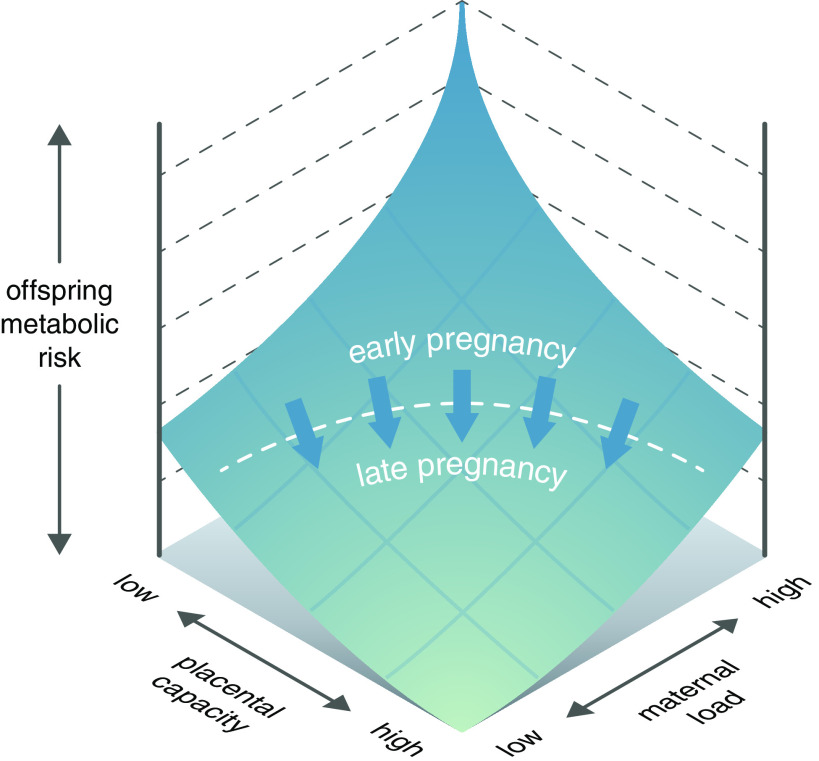
The examples of placental adaptability reviewed above may therefore be reconceptualized as components of placental metabolic capacity unique to the developmental stage of fetal life. When a mother develops a high metabolic load, the placenta is uniquely positioned to protect the fetus. We would then expect maternal metabolic aberrations, or their correlated responses in the fetus, to become vital signals for the placenta, eliciting protective responses. However, such placental protection is expected to have limits, above which it is overwhelmed by maternal and fetal metabolic perturbations with adverse consequences for the fetus (Fig. 2).
Figure 2.
Placental homeostatic capacity/efficiency model. During pregnancy, the placenta has a homeostatic capacity that will maintain fetoplacental homeostasis and determine the efficiency, with which the placenta protects the fetus from adverse consequences of a disturbed intrauterine environment. Up to a certain level of maternal metabolic load, the placenta can respond to signals such as insulin and orchestrate adaptive homeostatic responses that preserve an optimal metabolic milieu for fetal development. We hypothesize that this capacity is negligible during the early weeks in pregnancy and fully developed at the end. However, above the limiting threshold, such placenta capacity is exhausted and adverse fetal effects ensue. This threshold may differ according to both maternal and fetal traits (53).
Variation of Placental Tolerance
This model offers the advantage of incorporating several factors relating to both mother and offspring that may collectively modify the threshold of metabolic load that the placenta is capable of tolerating. These are briefly reviewed below.
Fetal Sex
In terms of the model illustrated in Fig. 2, substantial evidence indicates that females have a higher tolerance threshold than males, resulting in males being more susceptible to metabolic perturbations. These differences may ultimately be due to the two sexes being subject to contrasting selective pressures to maximize inclusive fitness, resulting in different growth strategies in fetal life (35). Male fetuses typically accrete more lean mass than females (36), who accrete slightly more fat and demonstrate a more central fat distribution (37).
Pregnancy outcomes have long been established to be worse in males than in females (38). Sex dichotomy in placentas may contribute to these different outcomes, as male and female placentas differ molecularly and functionally. The effect of fetal sex on gene expression differs even within the placenta in ways that are cell specific and related to different functional pathways. In male placentas, these encompass signaling pathways for graft-versus-host disease as well as immune function and inflammation, both of which parallel poorer pregnancy outcomes among male fetuses (39).
These molecular sex differences may underlie sex-dependent placental responses to environmental challenges. Supplementing mothers with n-3 fatty acids modifies the placental transcriptome in a sexually dimorphic manner, with female placentas being more responsive to treatment (40), potentially reflecting better plasticity to mount adaptive responses.
As detailed above, fetal insulin is a driver of placental adaptation (12,41,42). However, these findings were obtained using endothelial cells from female placentas, and males may respond differently. For example, placental vascularization in maternal diabetes shows a significant interaction with fetal sex (43). Similarly, the higher rate of stillbirths in males than females (44) can be interpreted as metabolic load exceeding placental homeostatic capacity and, thus, placental failure to adapt to fetal oxygen demand by hypervascularization.
Fetal responses also differ by sex. Female neonates born to GDM pregnancies are less insulin-sensitive than males (45). Since insulin is a key determinant of fetal phenotype in maternal overnutrition, this may represent a protective mechanism that is especially pronounced in females. In particular, the deposition of triglycerides in fetal/neonatal adipocytes may be reduced in females. Consistent with that hypothesis, a sex-dependent association between insulin and neonatal fat was found for some depots, indicating that fat deposition seems to be less affected by insulin in female neonates compared with males (46).
Such sex differences are also apparent during maternal undernutrition, as shown in the Dutch famine, where exposure early in gestation was associated with a greater reduction in placental area in boys than girls (47). In a situation of maternal overnutrition such as GDM, estrogen receptor α in the decidual vessels, in particular the extravillous trophoblast subpopulation, is downregulated in pregnancies with male but not female fetuses (48). The functional consequences are unclear.
Heterogeneity of the Placenta
The placenta is a heterogeneous organ composed of diverse different cell types. Their activity and spatial arrangement depend on the specific function of the anatomical key structures (villi) in which they are located. The surface repertoire of molecules such as receptors and transporters may therefore vary depending on the cells’ location within the placental tissue. Even among endothelial cells, phenotypic heterogeneity suggests differential functional responses along the vascular tree (49). Such heterogeneity may influence the placental capacity for protecting the fetus. For example, insulin receptors are heterogeneously distributed along the vascular tree (42); hence, insulin-mediated adaptive responses may vary by location.
Time Period in Gestation
The capacity of the placenta for adaptive responses will undoubtedly increase with gestational age. At the very beginning of pregnancy, i.e., the blastocyst stage, even minor environmental perturbations such as culture media used in in vitro fertilization influence placental growth (50), suggesting a high degree of sensitivity and vulnerability at this stage.
Although at week 12 of pregnancy the placenta weighs only 5% of its total final weight, it is in this period that it grows most rapidly (51). It is known that rapidly growing tissues/proliferating cells are most sensitive to environmental perturbations (52). Indeed, there is circumstantial evidence suggesting that placental growth is reduced in the first trimester of type 1 diabetes pregnancies (53).
The consequence of impaired placental growth as a response to the diabetic environment is a reduction in fetal growth rate. Given that placental size limits fetal growth early in pregnancy, shorter fetuses at this pregnancy stage may be the consequence of smaller placentas. However, later in pregnancy, the fetus can undergo catch-up growth, and at the end of pregnancy, its weight may exceed that of the neonate born to normal pregnancies (54). Parallel to its growth, the adaptive capacity of the placenta may increase, increasing its threshold for tolerating metabolic disturbances.
Genetic Factors
Aside from any maternal genes associated with maternal obesity and gestational diabetes mellitus (55), the placental response to metabolic load may also be determined by its genetic makeup. As a fetal tissue, the placenta is under the genetic influence of both parents, with the paternal genome promoting nutrient supply to the fetus while the maternal genome acts in the opposing direction (56,57). As discussed above, these asymmetric roles are assumed to have evolved in the evolutionary context of energy scarcity, though whether genomic imprinting evolved through overt conflict between the two parents regarding the magnitude of maternal nutritional investment (58,59) or whether it evolved more as a coadaptive process (60,61) remains unresolved. Importantly, genomic imprinting may have different effects depending on the trimester of pregnancy, for example, impacting placental structure at earlier gestational age and placental function at later gestational age (62,63).
While growth-promoting paternal genes may appear to play a disproportionate role in generating the need for placental protection, by increasing fetal exposure to maternal metabolic excess, maternal genes may still play an important role. Paternally expressed genes appear to promote growth of functional tissues in the fetus such as skeletal muscle, bone, and organ mass, whereas maternal-expressed genes favor fetal fat accretion, which is augmented in maternal obesity and diabetes (57). Moreover, it is not clear which of paternal and maternal genes might then drive the adaptive responses we have outlined above. On the one hand, paternal genes might themselves promote this protection, to “insure” against the costs of increasing nutrient demand. On the other hand, maternal genes might promote placental homeostasis to protect the fetus from such aggressive paternal tactics. It is also possible that the genes of both parents might contribute to the capacity for placental homeostasis.
Limits to Homeostatic Capacity
Any of these adaptive responses may, however, have a limited capacity. When the metabolic load exceeds this capacity, then the placental changes described above will not be enough to protect the fetus and fetal compromise may ensue. There are several examples for limited placental capacity and tolerance toward environmental changes/stresses:
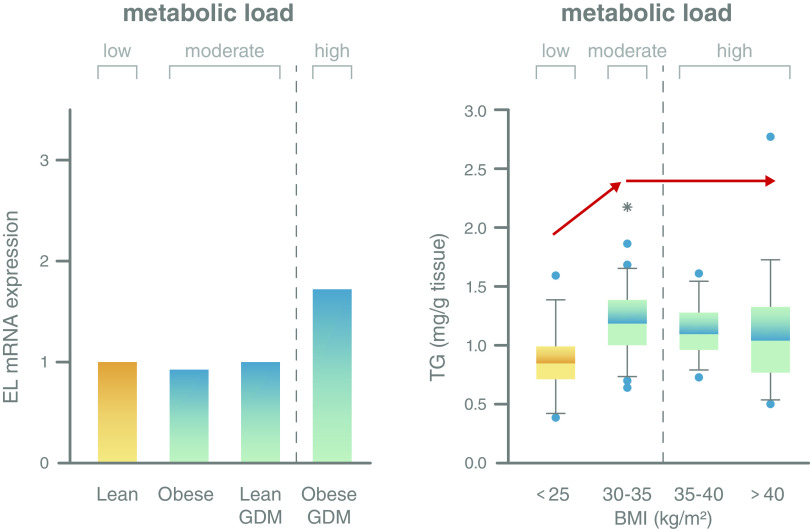
1. First, endothelial lipase (EL) is an enzyme on the surface of the syncytiotrophoblast and on placental endothelial cells. It mediates the uptake of fatty acids from high-density lipoproteins. Its expression is unchanged in GDM or obesity. Only when both conditions are combined (i.e., GDM and obesity) is EL upregulated, indicating that placental homeostatic capacity has been exceeded (Fig. 3, left panel). We have identified proinflammatory cytokines as key signals inducing this upregulation (64).
2. Second, the placenta can store fatty acids as triglycerides in lipid droplets, potentially serving as a protective mechanism to avoid lipotoxic effects in the placenta/syncytiotrophoblast (65). The storage capacity has a limit: with increasing BMI of the mother, i.e., with increasing metabolic load as reflected by increasing fatty acid and insulin concentrations, more triglycerides can be stored (BMI <25 vs. BMI 30–35). However, with further increasing BMI, the placenta does not store any more triglycerides (Fig. 3, right panel) (66), indicating that metabolic load has exceeded placental capacity.
3.Third, studies show that transmission of the stress generated by maternal obesity to the fetus may depend not only on the placenta but also on the mother’s own metabolic capacity. In a study of Swedish mothers, for example, the association of maternal obesity with childhood obesity in the offspring was exacerbated if the mother was also born with low birth weight (67). This suggests that the capacity to maintain homeostasis and regulate substrate supply to the fetus during pregnancy is reduced among mothers with lower metabolic capacity. Likewise, we propose that placental protection will be more quickly exhausted among mothers with obesity/diabetes who developed lower levels of metabolic capacity in early life.
Figure 3.
Placental homeostatic capacity has its limits resulting in potentially adverse changes when the metabolic load exceeds this limit. Left panel: Expression of endothelial lipase (EL), a gene that is involved in lipid metabolism and perhaps fatty acid supply to the fetus, is stable in maternal obesity and GDM and only responds when metabolic load is high, i.e., in GDM and obesity (64). Importantly, the association of maternal obesity with adverse outcomes such as high neonatal fat mass is independent of maternal (gestational) diabetes (5). Right panel: Placental accumulation of triglycerides (TG) as a means to protect against lipotoxicity (63) increases with increasing maternal metabolic load, i.e., between lean women and women with class I obesity (BMI 30–35 kg/m2). This capacity is exhausted with a further increase in load, i.e., in women with class II and III obesity (BMI 35–40 and >40 kg/m2), and no further additional triglycerides are stored (64).
Conclusion and Implications
During evolution, the human placenta developed mechanisms that support its role not only in sustaining fetal development but also in protecting the growing fetus from an affluent metabolic environment and potential adverse consequences. Although it is difficult to reconstruct maternal pregnancy metabolism in ancestral populations, apes such as orangutans are capable of storing fat to fund lactation (68), while high levels of female body fat in preagricultural human populations are indicated by figurines that clearly depict very high BMI (69). Therefore, it is plausible that natural selection could have favored the evolution of such protective mechanisms. Moreover, the prevalence of GDM worldwide is inversely associated with the historical duration over which high-glycemic diets have been consumed, indicating recent selection for blunted metabolic responses to such diets (70).
Key components of these protective mechanisms are fetal signals, which interact with placental endothelial cells to induce adaptive responses that maintain homeostasis at the fetoplacental interface. The homeostatic capacity of the placenta depends on various factors including gestational age and fetal sex and may regionally vary along the vascular tree. These protective effects, however, can be overwhelmed by an excessive metabolic load of maternal overnutrition associated with diabetes, obesity, or both. This model offers an opportunity to integrate future results on fetoplacental interactions into a framework aligned with concepts on human evolution.
Our approach highlights placental function as a new target to be considered in future intervention strategies, and the window of early pregnancy may be especially important, as the placenta develops ahead of the fetus. Although the experimental results used to develop this concept relate to the end of pregnancy, these mechanisms must be in place at earlier time periods. This perspective supports the growing call to control maternal metabolism as early as possible (71). Early disturbance of maternal metabolism especially in high-risk women such as those with obesity, history of GDM, and/or a family history of diabetes is associated with poorer pregnancy outcome of the newborn manifested, e.g., in excessive fat accretion in utero. We propose that this is in part the result of underdeveloped placental homeostatic capacity.
Article Information
Acknowledgments. The authors thank all collaborators for their contributions to the studies that support the concept presented here.
References
- 1.Myatt L. Placental adaptive responses and fetal programming. J Physiol 2006;572:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauster M, Desoye G, Tötsch M, Hiden U. The placenta and gestational diabetes mellitus. Curr Diab Rep 2012;12:16–23 [DOI] [PubMed] [Google Scholar]
- 3.Hirschmugl B, Crozier S, Matthews N, et al. Relation of placental alkaline phosphatase expression in human term placenta with maternal and offspring fat mass. Int J Obes 2018;42:1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Phenotype of infants of mothers with gestational diabetes. Diabetes Care 2007;30(Suppl. 2):S156–S160 [DOI] [PubMed] [Google Scholar]
- 5.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers 2019;5:47. [DOI] [PubMed] [Google Scholar]
- 6.Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol 1998;(Suppl. 27):177–209 [DOI] [PubMed] [Google Scholar]
- 7.Desoye G, Shafrir E. Placental metabolism and its regulation in health and diabetes. Mol Aspects Med 1994;15:505–682 [DOI] [PubMed] [Google Scholar]
- 8.Haig D. Genetic conflicts in human pregnancy. Q Rev Biol 1993;68:495–532 [DOI] [PubMed] [Google Scholar]
- 9.Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front Physiol 2018;9:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowett RM, Farrag HM. Selected principles of perinatal-neonatal glucose metabolism. Semin Neonatol 2004;9:37–47 [DOI] [PubMed] [Google Scholar]
- 11.Desoye G, Shafrir E. The human placenta in diabetic pregnancy. Diabetes Rev 1996;4:70–89 [Google Scholar]
- 12.Lassance L, Miedl H, Absenger M, et al. Hyperinsulinemia stimulates angiogenesis of human fetoplacental endothelial cells: a possible role of insulin in placental hypervascularization in diabetes mellitus. J Clin Endocrinol Metab 2013;98:E1438–E1447 [DOI] [PubMed] [Google Scholar]
- 13.Hiden U, Lassance L, Tabrizi NG, et al. Fetal insulin and IGF-II contribute to gestational diabetes mellitus (GDM)-associated up-regulation of membrane-type matrix metalloproteinase 1 (MT1-MMP) in the human feto-placental endothelium. J Clin Endocrinol Metab 2012;97:3613–3621 [DOI] [PubMed] [Google Scholar]
- 14.Widness JA, Susa JB, Garcia JF, et al. Increased erythropoiesis and elevated erythropoietin in infants born to diabetic mothers and in hyperinsulinemic rhesus fetuses. J Clin Invest 1981;67:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petry CD, Wobken JD, McKay H, et al. Placental transferrin receptor in diabetic pregnancies with increased fetal iron demand. Am J Physiol 1994;267:E507–E514 [DOI] [PubMed] [Google Scholar]
- 16.Kitzmiller JL, Watt N, Driscoll SG. Decidual arteriopathy in hypertension and diabetes in pregnancy: immunofluorescent studies. Am J Obstet Gynecol 1981;141:773–779 [DOI] [PubMed] [Google Scholar]
- 17.Nielsen FH, Jacobsen BB, Rolschau J. Pregnancy complicated by extreme hyperlipaemia and foam-cell accumulation in placenta. Acta Obstet Gynecol Scand 1973;52:83–89 [DOI] [PubMed] [Google Scholar]
- 18.Napoli C, D’Armiento FP, Mancini FP, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest 1997;100:2680–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Kopp S, Strutz J, et al. Gestational diabetes mellitus modulates cholesterol homeostasis in human fetoplacental endothelium. Biochim Biophys Acta Mol Cell Biol Lipids 2018;1863:968–979 [DOI] [PubMed] [Google Scholar]
- 20.Scholler M, Wadsack C, Lang I, et al. Phospholipid transfer protein in the placental endothelium is affected by gestational diabetes mellitus. J Clin Endocrinol Metab 2012;97:437–445 [DOI] [PubMed] [Google Scholar]
- 21.Hiden U, Maier A, Bilban M, et al. Insulin control of placental gene expression shifts from mother to foetus over the course of pregnancy. Diabetologia 2006;49:123–131 [DOI] [PubMed] [Google Scholar]
- 22.Kaplan SL, Grumbach MM, Shepard TH. The ontogenesis of human fetal hormones. I. Growth hormone and insulin. J Clin Invest 1972;51:3080–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desoye G, Nolan CJ. The fetal glucose steal: an underappreciated phenomenon in diabetic pregnancy. Diabetologia 2016;59:1089–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells JC. The Metabolic Ghetto: An Evolutionary Perspective on Nutrition, Power Relations and Chronic Disease. Cambridge, Cambridge University Press, 2016 [Google Scholar]
- 25.Wells JC. The thrifty phenotype: an adaptation in growth or metabolism? Am J Hum Biol 2011;23:65–75 [DOI] [PubMed] [Google Scholar]
- 26.Wells JCK. The capacity-load model of non-communicable disease risk: understanding the effects of child malnutrition, ethnicity and the social determinants of health. Eur J Clin Nutr 2018;72:688–697 [DOI] [PubMed] [Google Scholar]
- 27.Enesco M, LeBlond CP. Increase in cell number as factor in the growth of the organs and tissues of the young male rat. Development 1962;10:530–562 [Google Scholar]
- 28.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 2008;105:17046–17049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Gronert MS, Ozanne SE. Metabolic programming of insulin action and secretion. Diabetes Obes Metab 2012;14(Suppl. 3):29–39 [DOI] [PubMed] [Google Scholar]
- 30.Mitanchez D, Chavatte-Palmer P. Review shows that maternal obesity induces serious adverse neonatal effects and is associated with childhood obesity in their offspring. Acta Paediatr 2018;107:1156–1165 [DOI] [PubMed] [Google Scholar]
- 31.Schwartz R, Gruppuso PA, Petzold K, Brambilla D, Hiilesmaa V, Teramo KA. Hyperinsulinemia and macrosomia in the fetus of the diabetic mother. Diabetes Care 1994;17:640–648 [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Ley SH, Tobias DK, et al. Birth weight and later life adherence to unhealthy lifestyles in predicting type 2 diabetes: prospective cohort study. BMJ 2015;351:h3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992;35:595–601 [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Ley SH, VanderWeele TJ, et al. Joint association between birth weight at term and later life adherence to a healthy lifestyle with risk of hypertension: a prospective cohort study. BMC Med 2015;13:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lampl M, Jeanty P. Timing is everything: a reconsideration of fetal growth velocity patterns identifies the importance of individual and sex differences. Am J Hum Biol 2003;15:667–680 [DOI] [PubMed] [Google Scholar]
- 36.Catalano PM, Drago NM, Amini SB. Factors affecting fetal growth and body composition. Am J Obstet Gynecol 1995;172:1459–1463 [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez G, Samper MP, Ventura P, Moreno LA, Olivares JL, Pérez-González JM. Gender differences in newborn subcutaneous fat distribution. Eur J Pediatr 2004;163:457–461 [DOI] [PubMed] [Google Scholar]
- 38.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med 2007;4:19–30 [DOI] [PubMed] [Google Scholar]
- 39.Cvitic S, Longtine MS, Hackl H, et al. The human placental sexome differs between trophoblast epithelium and villous vessel endothelium. PLoS One 2013;8:e79233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sedlmeier EM, Brunner S, Much D, et al. Human placental transcriptome shows sexually dimorphic gene expression and responsiveness to maternal dietary n-3 long-chain polyunsaturated fatty acid intervention during pregnancy. BMC Genomics 2014;15:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson SM, Coan PM, Burton GJ, Lindsay RS. Placental structure in type 1 diabetes: relation to fetal insulin, leptin, and IGF-I. Diabetes 2009;58:2634–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiden U, Glitzner E, Hartmann M, Desoye G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J Anat 2009;215:60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayhew TM, Sørensen FB, Klebe JG, Jackson MR. The effects of mode of delivery and sex of newborn on placental morphology in control and diabetic pregnancies. J Anat 1993;183:545–552 [PMC free article] [PubMed] [Google Scholar]
- 44.Wells JC. Natural selection and sex differences in morbidity and mortality in early life. J Theor Biol 2000;202:65–76 [DOI] [PubMed] [Google Scholar]
- 45.Walsh JM, Segurado R, Mahony RM, Foley ME, McAuliffe FM. The effects of fetal gender on maternal and fetal insulin resistance. PLoS One 2015;10:e0137215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eder M, Csapo B, Wadsack C, et al. Sex differences in the association of cord blood insulin with subcutaneous adipose tissue in neonates. Int J Obes 2016;40:538–542 [DOI] [PubMed] [Google Scholar]
- 47.Roseboom TJ, Painter RC, de Rooij SR, et al. Effects of famine on placental size and efficiency. Placenta 2011;32:395–399 [DOI] [PubMed] [Google Scholar]
- 48.Knabl J, Hiden U, Hüttenbrenner R, et al. GDM alters expression of placental estrogen receptor α in a cell type and gender-specific manner. Reprod Sci 2015;22:1488–1495 [DOI] [PubMed] [Google Scholar]
- 49.Lang I, Pabst MA, Hiden U, et al. Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. Eur J Cell Biol 2003;82:163–173 [DOI] [PubMed] [Google Scholar]
- 50.Eskild A, Monkerud L, Tanbo T. Birthweight and placental weight; do changes in culture media used for IVF matter? Comparisons with spontaneous pregnancies in the corresponding time periods. Hum Reprod 2013;28:3207–3214 [DOI] [PubMed] [Google Scholar]
- 51.Molteni RA. Placental growth and fetal/placental weight (F/P) ratios throughout gestation--their relationship to patterns of fetal growth. Semin Perinatol 1984;8:94–100 [PubMed] [Google Scholar]
- 52.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child 1973;48:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desoye G. The human placenta in diabetes and obesity: friend or foe? The 2017 Norbert Freinkel Award lecture. Diabetes Care 2018;41:1362–1369 [DOI] [PubMed] [Google Scholar]
- 54.Hemachandra AH, Klebanoff MA. Use of serial ultrasound to identify periods of fetal growth restriction in relation to neonatal anthropometry. Am J Hum Biol 2006;18:791–797 [DOI] [PubMed] [Google Scholar]
- 55.Powe CE, Kwak SH. Genetic studies of gestational diabetes and glucose metabolism in pregnancy. Curr Diab Rep 2020;20:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angiolini E, Fowden A, Coan P, et al. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta 2006;27(Suppl. A):S98–S102 [DOI] [PubMed] [Google Scholar]
- 57.Crespi BJ. Why and how imprinted genes drive fetal programming. Front Endocrinol (Lausanne) 2020;10:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore T, Haig D. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet 1991;7:45–49 [DOI] [PubMed] [Google Scholar]
- 59.Haig D, Graham C. Genomic imprinting and the strange case of the insulin-like growth factor II receptor. Cell 1991;64:1045–1046 [DOI] [PubMed] [Google Scholar]
- 60.Curley JP, Barton S, Surani A, Keverne EB. Coadaptation in mother and infant regulated by a paternally expressed imprinted gene. Proc Biol Sci 2004;271:1303–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haig D. Coadaptation and conflict, misconception and muddle, in the evolution of genomic imprinting. Heredity 2014;113:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore GE, Ishida M, Demetriou C, et al. The role and interaction of imprinted genes in human fetal growth. Philos Trans R Soc Lond B Biol Sci 2015;370:20140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care 2007;30(Suppl. 2):S120–S126 [DOI] [PubMed] [Google Scholar]
- 64.Gauster M, Hiden U, van Poppel M, et al. Dysregulation of placental endothelial lipase in obese women with gestational diabetes mellitus. Diabetes 2011;60:2457–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 2003;100:3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirschmugl B, Desoye G, Catalano P, et al. Maternal obesity modulates intracellular lipid turnover in the human term placenta. Int J Obes 2017;41:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cnattingius S, Villamor E, Lagerros YT, Wikström AK, Granath F. High birth weight and obesity--a vicious circle across generations. Int J Obes 2012;36:1320–1324 [DOI] [PubMed] [Google Scholar]
- 68.Knott CD. Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. Int J Primatol 1998;19:1061–1079 [Google Scholar]
- 69.Conard NJ. A female figurine from the basal Aurignacian of Hohle Fels Cave in southwestern Germany. Nature 2009;459:248–252 [DOI] [PubMed] [Google Scholar]
- 70.Brown EA, Ruvolo M, Sabeti PC. Many ways to die, one way to arrive: how selection acts through pregnancy. Trends Genet 2013;29:585–592 [DOI] [PubMed] [Google Scholar]
- 71.McIntyre D, Desoye G, Dunne F, et al. FIGO analysis of research priorities in hyperglycemia in pregnancy. Diabetes Res Clin Pract 2018;145:5–14 [DOI] [PubMed] [Google Scholar]
- 72.Scholler M, Wadsack C, Metso J, et al. Phospholipid transfer protein is differentially expressed in human arterial and venous placental endothelial cells and enhances cholesterol efflux to fetal HDL. J Clin Endocrinol Metab 2012;97:2466–2474 [DOI] [PubMed] [Google Scholar]
- 73.Stefulj J, Panzenboeck U, Becker T, et al. Human endothelial cells of the placental barrier efficiently deliver cholesterol to the fetal circulation via ABCA1 and ABCG1. Circ Res 2009;104:600–608 [DOI] [PubMed] [Google Scholar]
- 74.Díaz-Pérez FI, Hiden U, Gauster M, et al. Post-transcriptional down regulation of ICAM-1 in feto-placental endothelium in GDM. Cell Adhes Migr 2016;10:18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies MJ, Gordon JL, Gearing AJ, et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol 1993;171:223–229 [DOI] [PubMed] [Google Scholar]
- 76.Aljada A, Saadeh R, Assian E, Ghanim H, Dandona P. Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. J Clin Endocrinol Metab 2000;85:2572–2575 [DOI] [PubMed] [Google Scholar]
- 77.Sharma A, Sellers S, Stefanovic N, et al. Direct endothelial nitric oxide synthase activation provides atheroprotection in diabetes-accelerated atherosclerosis. Diabetes 2015;64:3937–3950 [DOI] [PubMed] [Google Scholar]