Abstract
Kidney transplant program performance in the United States is commonly measured by posttransplant outcomes. Inclusion of pretransplant measures could provide a more comprehensive assessment of transplant program performance and necessary information for patient decision-making. In this study, we propose a new metric, the waitlisting rate, defined as the ratio of patients who are waitlisted in a center relative to the person-years referred for evaluation to a program. Furthermore, we standardize the waitlisting rate relative to the state average in Georgia, North Carolina, and South Carolina. The new metric was used as a proof-of-concept to assess transplant-program access compared to the existing transplant rate metric. The study cohorts were defined by linking 2017 United States Renal Data System (USRDS) data with transplant-program referral data from the Southeastern United States between January 1, 2012 and December 31, 2016. Waitlisting rate varied across the 9 Southeastern transplant programs, ranging from 10 to 22 events per 100 patient-years, whereas the program-specific waitlisting rate ratio ranged between 0.76 and 1.33. Program-specific waitlisting rate ratio was uncorrelated with the transplant rate ratio (r = −.15, 95% CI, −0.83 to 0.57). Findings warrant collection of national data on early transplant steps, such as referral, for a more comprehensive assessment of transplant program performance and pretransplant access.
Keywords: ethics and public policy, health services and outcomes research, kidney disease, kidney transplantation/nephrology, patient referral, quality of care/care delivery, registry/registry analysis, Scientific Registry for Transplant Recipients (SRTR)
1 |. INTRODUCTION
Transplant program quality metrics have focused historically on outcomes following transplantation, including the risk-adjusted 1-year graft and patient survival quality metrics used to compare their performance.1 However, due to a number of unintended consequences associated with a focus on these posttransplant outcomes, there has been a call by the transplant community to reform these metrics to improve transplant access.2 Although the Scientific Registry of Transplant Recipients (SRTR) program-specific reports have previously included metrics for transplant rate and waitlist mortality, these metrics were not featured previously on the SRTR patient-facing website until 2019.3,4 Prior data suggest that patients would preferentially use pretransplant measures over posttransplant outcomes when trying to identify a preferred transplant center,5 that patients favor multiple measures when assessing where they want to undergo transplantations,6 and that when told that transplant rate has the largest influence on survival, patients prefer centers with better transplant rates.7 These patient preferences are consistent with findings that a major determinant of patient outcomes for a waitlisted patient is whether they get a transplant.8
Currently, the transplant rate metric, calculated and reported by SRTR, is publicly available for patients to use to compare transplant centers, and was recently emphasized on the SRTR website as having the most impact on survival after listing.4,9 However, because the program-specific transplant rate metric only reflects patient access to transplant for waitlisted patients, it is dependent on center listing practices and may not adequately represent access for all patients referred to the center. This is particularly important in the current era for kidney transplant candidates, where allocation time starts on the end-stage renal disease (ESRD) certification date on the CMS 2728 form rather than at the time of addition to the waitlist, and when waitlisting rates have declined in recent years.10 For example, transplant centers could choose to not waitlist patients who are unlikely to get transplanted quickly, thereby decreasing the denominator and inflating the unadjusted transplant rate. In addition, given the current geographic variations in organ supply11,12 and ongoing adjustments to kidney allocation, understanding and reporting access at the level of the base patient populations in the centers is critical to ensuring equity and justice in organ allocation.
There are more than 600 000 patients with ESRD in the United States and less than one fifth of these individuals are on the active wait-list.10 The rate of attrition of patients at the various steps to transplant prior to waitlisting is currently unknown, with no national data available at the present time. Prior research has shown that, for ESRD patients, variation in multiple early transplant steps, including waitlisting, is often a culmination of multilevel factors such as a patient’s age, race, sex, comorbidities, geographical location, insurance status, and profit status of the dialysis provider, and that these barriers may differ depending on the transplant step.13,14 For example, barriers to referral from a dialysis facility to a transplant center may be different from barriers to waitlisting after referral.15,16 Lack of specific nationally standardized transplant eligibility criteria and regulations focused on posttransplant outcomes have resulted in increased transplant program patient selection and variability in waitlisting practices across center.17,18 All patients who want a kidney transplant, including those who get a living donor transplant, have to first be placed on the kidney transplant waitlist. Thus a waitlisting metric would be able to capture the need for both living and deceased donor organs for a particular center, and would be less dependent on organ availability for a particular region. However, there are no metrics available that examine waitlisting rates across transplant programs. Although the Centers for Medicare & Medicaid Services (CMS) introduced a new waitlisting quality measure for dialysis facilities, there is no similar metric for transplant programs.19 Here, we develop a new measure for a waitlisting rate—using a denominator of patients referred for transplant—as proof-of-concept for a potential new transplant center metric. We compare and contrast the performance of this new waitlisting metric with the existing SRTR transplant metric at all 9 transplant programs in Georgia, North Carolina, and South Carolina, the only states for which referral data are systematically collected at present.
2 |. METHODS
The study cohort for the existing kidney transplant rate metric was identified from the 2017 United States Renal Data System (USRDS) database and included incident ESRD patients in 1 of the 9 adult transplant programs in GA, NC, and SC between January 1, 2012 and December 31, 2015. This study compares 2 metrics: our newly proposed waitlisting metric and the existing kidney transplant rate metric currently used in SRTR program-specific reports. We used an incident, rather than prevalent, cohort of patients because referral data were not available prior to 2012.
For the proposed waitlisting rate metric, the cohort consisted of patients referred for evaluation from a dialysis facility to 1 of the 9 transplant programs in the GA, NC, and SC, between January 1, 2012 and December 31, 2015, followed through December 31, 2016 to ensure at least 1 year of follow-up for outcomes. Referral was defined as the date at which the transplant program received a transplant referral form from a dialysis facility or referring provider. The referral data were collected from transplant referral forms and electronic medical records in the transplant programs and linked to the USRDS data as described previously.16,20 Patients <18 or >80 years of age and those waitlisted, transplanted, or died before referral were excluded (Figure 1). The waitlisting rate metric was calculated as the number of patients who were waitlisted at the transplant program (numerator) divided by the total number of person-years after referral to that program (denominator).
FIGURE 1.
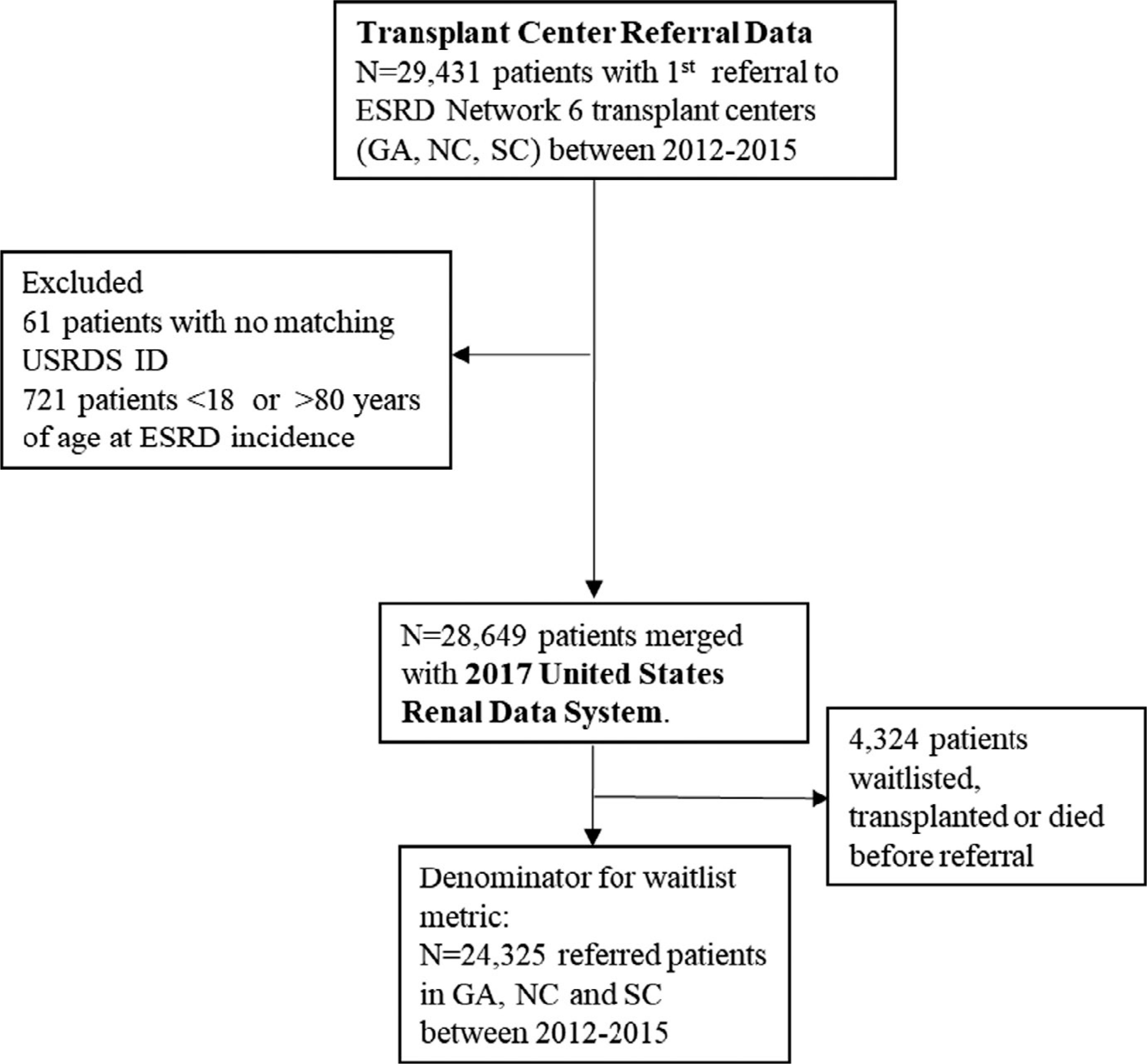
Waitlist rate denominator: all referred patients in GA, NC, and SC over study years 2012–2015
The transplant rate metric was calculated among waitlisted patients. Waitlisting was defined as the date a patient was placed on the national deceased donor waiting list at a transplant program. The cohort was followed from January 1, 2012 through December 31, 2016 to ensure a minimum of 1-year follow-up to observe the event of interest (transplantation). Patients <18 or >80 years of age and those who were transplanted or who died before waitlisting were excluded (Figure 2). As defined in the SRTR program-specific reports, the kidney transplant rate was calculated as the ratio of the number of patients who underwent deceased donor kidney transplantation (numerator) to the total number of person-years on the waiting list at that program (denominator).21
FIGURE 2.
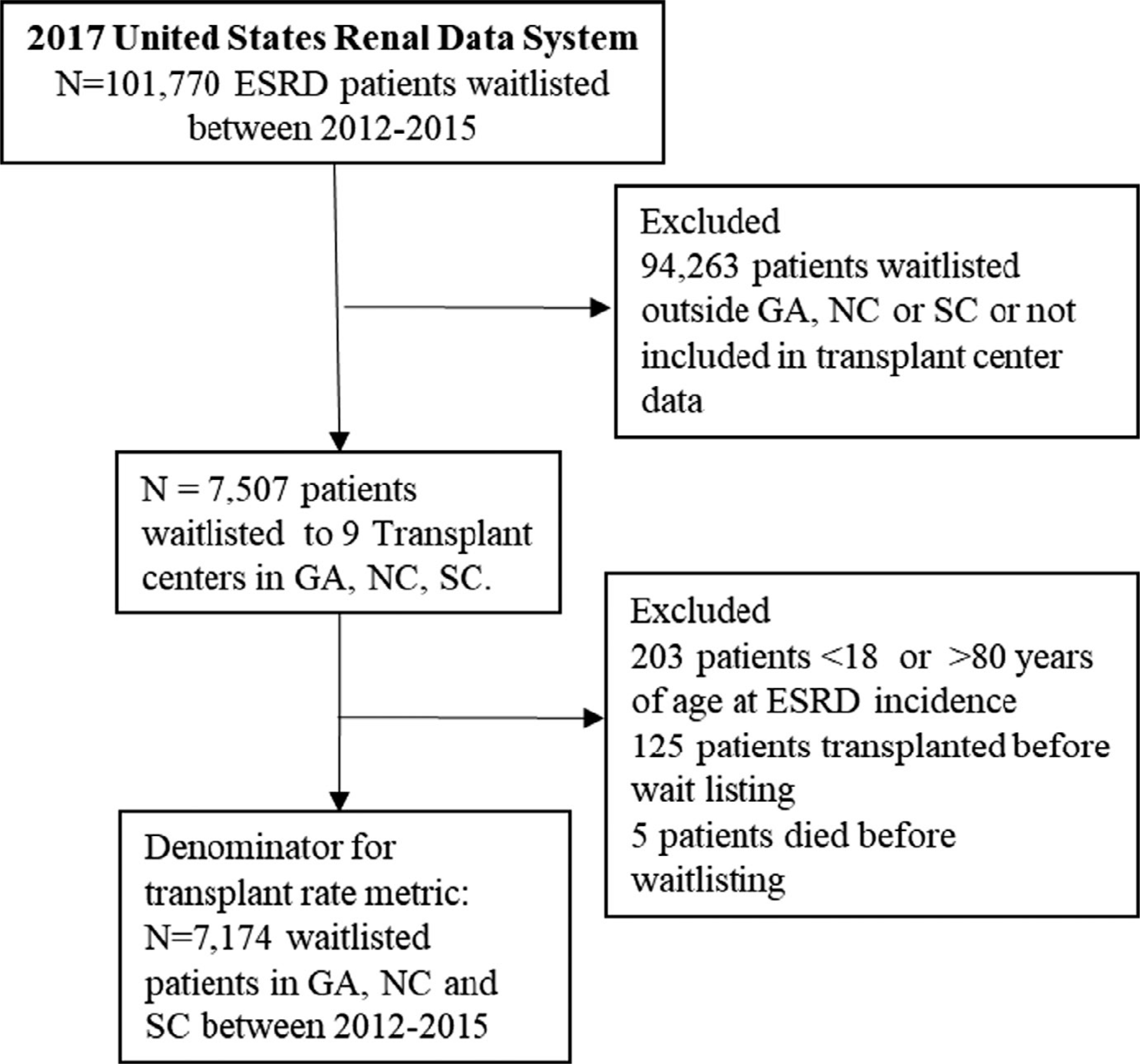
Transplant rate denominator: all waitlisted patients in GA, NC, and SC over study years 2012–2015
To adjust for patient-level variation among the 9 transplant programs, we computed an adjusted waitlisting rate ratio and adjusted transplant rate ratio metric relative to the performance in the southeastern region. First, for the waitlisting rate ratio, we fitted an exponential survival model for the cause-specific hazard of waitlisting to adjust for risk factors of age, race, sex, BMI, insurance status, and other comorbidities at ESRD start (eg, congestive heart failure, atherosclerotic heart disease, cardiovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, hypertension, diabetes, tobacco use, and cancer). In the next step, we used a Bayesian method to obtain the conditional posterior distribution of the hazard ratio (HR|O), where O is the observed number of waitlisting events among those referred.22 Assuming O|HR follows a Poisson distribution and that the HR has a prior distribution Gamma (2, 2), it can be shown that the posterior distribution of the HR is a gamma (shape = O+2, rate = E+2). The program-specific waitlisting rate ratio was estimated by the posterior mean, (O+2)/(E+2), and the 95% credible intervals were obtained by computing the 2.5 and 97.5 percentile of the corresponding gamma distribution. In addition, we adjusted for patient blood type and used a similar 2-stage method to obtain the program-specific transplant rate ratios.22
Summary statistics, bivariate correlations, and scatter plots were used to describe and compare the transplant programs based on the program-specific waitlist and transplant rate ratios, respectively. To account for the small sample size at the transplant program level (N = 9), 95% bootstrap confidence intervals were constructed for correlation coefficients. All analyses were performed in IBM SPSS (version 26) and SAS 9.4.
3 |. RESULTS
The denominator of the waitlisting rate metric consisted of 24 325 patients who were referred for transplantation to 1 of the 9 transplant programs in GA, NC, and SC between January 1, 2012 and December 31, 2015 (Figure 1). Table 1 shows the characteristics of transplanted patients relative to the waitlisted patient population. Of those referred for evaluation, 7538 (31%) were added to the waiting list by December 31, 2016. The waitlisted patients were 50.3 years (SD 13.2) at ESRD incidence; 60% were male, 58% were non-Hispanic Black, and 23%, 14%, and 36% had Medicare, Medicaid, and private insurance, respectively. Similar to the previous cohort, the waitlisted patients reported hypertension (88%), diabetes (43%), cerebrovascular disease (17%), and congestive heart failure (11%) at incidence (Table 1).
TABLE 1.
Selected characteristics of (a) waitlisted and (b) transplanted patients among all referred and waitlisted patients with ESRD, respectively, in transplant programs of GA, NC, and SC: 2012–2015
| Among patients referred to treatment programs in GA, NC, and SC between 2012 and 2015 N = 24 325 |
Among patients waitlisted in Tx-programs in GA, NC and SC between 2012 and 2015 N = 7174 |
|
|---|---|---|
| Characteristic N (%) | Waitlisted between 2012 and 2016 N = 7538 (31.0%) | Transplanted between 2012 and 2016 N = 1320 (18.4%) |
| Mean age, y (SD) | 50.3 (13.2) | 49.9 (13.5) |
| Age category, y | ||
| 18–29 | 616 (8.2) | 123 (9.3) |
| 30–39 | 1017 (13.5) | 195 (14.8) |
| 40–49 | 1755 (23.3) | 261 (19.8) |
| 50–59 | 2011 (26.7) | 374 (28.3) |
| 60–69 | 1768 (23.5) | 300 (22.7) |
| 70–80 | 371 (4.9) | 67 (5.1) |
| Male sex, N (%) | 4527 (60.1) | 759 (57.5) |
| Black race, N (%) | 4294 (58.3) | 697 (54.0) |
| Comorbidities, N (%) | ||
| Obesity (BMI ≥35 kg/m2) | 1440 (19.7) | 214 (16.7) |
| Congestive heart failure | 813 (10.8) | 128 (9.7) |
| Atherosclerotic heart disease | 321 (4.3) | 47 (3.6) |
| Other cardiac disease | 608 (8.1) | 93 (7.1) |
| Cerebrovascular disease (stroke) | 1267 (16.8) | 196 (14.9) |
| Peripheral vascular disease | 245 (3.3) | 34 (2.6) |
| Hypertension | 6646 (88.2) | 1153 (87.4) |
| Diabetes | 3208 (42.6) | 500 (37.9) |
| Chronic obstructive pulmonary disease | 99 (1.3) | 20 (1.5) |
| Cancer | 197 (2.6) | 30 (2.3) |
| Tobacco use | 293 (4.0) | 63 (4.9) |
| Primary health insurance provider, N (%) | ||
| Medicare | 1688 (22.9) | 279 (21.7) |
| Medicaid | 1037 (14.1) | 194 (15.1) |
| Employer group | 2637 (35.8) | 455 (35.3) |
| Other coverage | 1000 (13.6) | 170 (13.2) |
| No coverage | 1004 (13.6) | 190 (14.8) |
The denominator for the at-risk cohort for the transplant rate metric included 7174 patients waitlisted in 1 of the 9 transplant centers in GA, NC, and SC between January 1, 2012 and December 31 2015 (Figure 2). Of those, 1320 (18.4%) received a deceased donor transplant by December 31, 2016. The average age of the transplanted cohort at ESRD start was 49.9 (SD 13.5) years; 58% were male, 54% non-Hispanic Black, 15% had Medicaid, 22% Medicare, and 35% had private insurance. The patients reported comorbidities at baseline including hypertension (87%), diabetes (38%), cerebrovascular disease (15%), and congestive heart failure (10%) (Table 1).
The average waitlisting rate was 15.7 (median, 15.8; range, 9.5–21.6) events per 100 patient-years, and the program-specific waitlisting rate ratio metric had an SD of 0.24 (range, 0.76–1.33) (Table 2). In contrast, the average transplant rate (unadjusted) among waitlisted patients was 10.9 (median, 11.8; range, 3.9–19.6) events per 100 patient-years, and the risk-adjusted program-specific transplant rate ratio had a SD of 0.65 (range, 0.44–2.20) (Table 3).
TABLE 2.
Waitlisting rates and program-specific waitlisting rates ratios for programs in GA, NC, and SC, study years 2012–2015 (with follow-up through 2016)
| At-risk patients |
Numerator |
Denominator |
Waitlisting rate |
Waitlisting rate ratioa |
|
|---|---|---|---|---|---|
| Transplant program | Number referred 01/2012–12/2015 | Number waitlisted 01/2012–12/2016 | Referred patient-years | Per 100 patient-years | Observed/expected waitlisted (95% credible interval) |
| 1 | 3517 | 906 | 7897.31 | 11.47 | 0.76 (0.71, 0.81) |
| 2 | 1663 | 355 | 3728.92 | 9.52 | 0.77 (0.69, 0.85) |
| 3 | 2003 | 547 | 4093.22 | 13.36 | 0.77 (0.71, 0.84) |
| 4 | 1863 | 515 | 3996.99 | 12.88 | 0.79 (0.72, 0.86) |
| 5 | 3713 | 1237 | 7187.37 | 17.21 | 1.01 (0.95, 1.07) |
| 6 | 3087 | 935 | 5917.00 | 15.80 | 1.13 (1.06,1.21) |
| 7 | 5592 | 2055 | 10726.66 | 19.16 | 1.20 (1.15,1.25) |
| 8 | 1534 | 501 | 2482.32 | 20.18 | 1.29 (1.18,1.41) |
| 9 | 1353 | 487 | 2252.80 | 21.62 | 1.33 (1.22, 1.46) |
Expected waitlisted adjusted for patient age, sex, race, insurance status, and comorbidities at ESRD start.
TABLE 3.
Transplant rates and program-specific transplant rate ratios for programs in GA, NC, and SC, study years 2012–2015 (with follow-up through 2016)
| Transplant program | At-risk patients |
Numerator |
Denominator |
Transplant rate |
Transplant rate ratioa |
|---|---|---|---|---|---|
| Number waitlisted 01/2012–12/2015 | Number of deceased donor transplants 01/2012–12/2016 | Waitlisted patient-years | Per 100 patient-years | Observed/expected transplants (95% credible interval) | |
| 1 | 779 | 250 | 1312.98 | 19.04 | 2.20 (1.93, 2.49) |
| 2 | 334 | 97 | 698.86 | 13.88 | 1.63 (1.32, 1.97) |
| 3 | 537 | 142 | 1169.46 | 12.14 | 1.34 (1.13, 1.57) |
| 4 | 442 | 57 | 848.19 | 6.72 | 0.74 (0.56, 0.94) |
| 5 | 1262 | 151 | 2734.89 | 5.52 | 0.66 (0.56, 0.77) |
| 6 | 870 | 80 | 2082.66 | 3.84 | 0.44 (0.35, 0.54) |
| 7 | 1929 | 234 | 4153.30 | 5.63 | 0.63 (0.55, 0.72) |
| 8 | 522 | 196 | 1007.16 | 19.46 | 2.09 (1.81, 2.40) |
| 9 | 496 | 111 | 948.26 | 11.71 | 1.33 (1.09, 1.59) |
Expected transplanted adjusted for patient age, sex, race, blood type, insurance status, and comorbidities at ESRD start.
Overall, the transplant rate metrics varied substantially compared to the waitlist rate metrics among the 9 Southeastern transplant programs (5-fold vs 2-fold changes in mean). When comparing the performance of transplant programs with respect to the 2 risk-adjusted metrics, 3 programs (33%) indicated agreement, whereas 33% (3 programs) had higher waitlisting but lower transplant rate ratios and 33% had lower waitlisting but higher transplant rate ratios (Figure 3). In addition, neither the absolute transplant and waitlisting rates (r = −.14, 95% confidence interval [CI], −0.82 to 0.61) nor the risk-adjusted waitlisting and transplant rate ratio metrics were correlated (r = −.15, 95% CI, −0.83 to 0.57), at the transplant program level (Figure 4).
FIGURE 3.
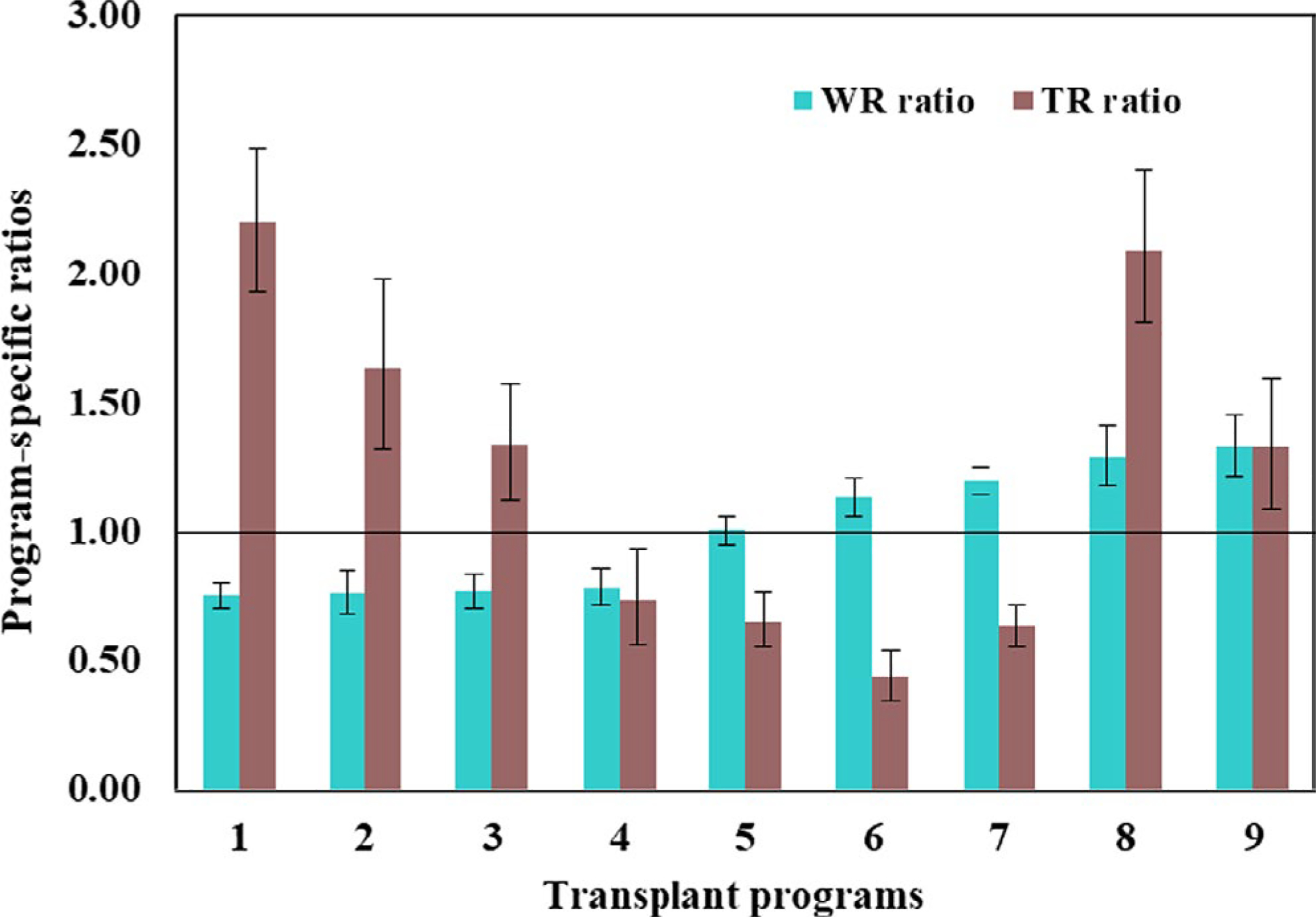
Program-specific ratios of observed to expected transplant and waitlisting rates in GA, NC, and SC, study years 2012–2015; transplant programs are arranged in ascending order of the waitlisting ratio metric. Expected rates adjusted for patient age, sex, race, insurance status, and comorbidities at ESRD start. The solid black line denotes perfect agreement between the observed and expected waitlist and transplant rates at the program, respectively [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
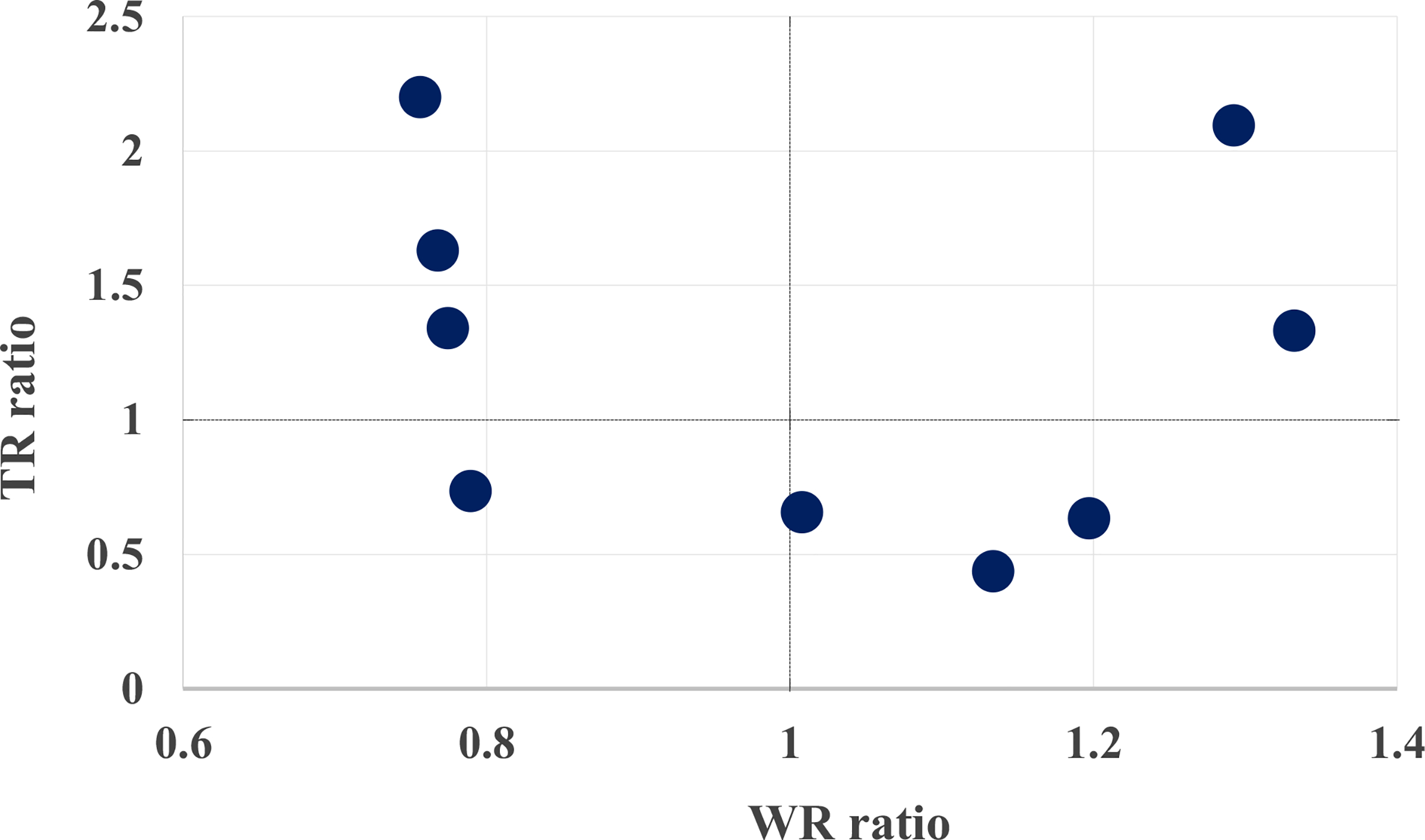
Association between transplant rate and waitlisting rate ratios at transplant programs in GA, NC, and SC, study years 2012–2016. Expected rates adjusted for patient age, sex, race, insurance status, and comorbidities at ESRD start. The dotted black lines denote agreement of observed and expected rates for waitlisting (vertical) and transplantation (horizontal), respectively [Color figure can be viewed at wileyonlinelibrary.com]
4 |. DISCUSSION
In this population-based study of ESRD patients in 3 states in the Southeast, we found a more than 2-fold change in waitlisting rates across 9 transplant programs. Furthermore, we report that centers that have high transplant rates do not necessarily have high waitlisting rates, and vice versa. In fact, programs 1, 2, and 3 with high transplant rate ratios performed poorly in terms of the waitlist rate ratio metric. This pattern suggests that the transplant rate metric currently utilized by SRTR may not be representative of patient access for all patients seeking to pick a transplant center based on current transplant rate metrics, at least in this particular region of the country. Furthermore, the lack of correlation suggests minimal overlap between the 2 metrics and that utilizing a waitlisting rate metric in addition to a transplant rate metric may be appropriate for evaluating access to transplantation for patients, patients’ family members, and their referring nephrologist or providers.
Declines in transplant rates and increases in waitlist removals over the last 15 years suggest that transplant centers could influence their transplant rate by changing waitlisting practices.17 This, however, would come at the cost of limiting access to care for patients who could benefit, especially those who are poor, older, or with certain comorbid conditions that would not otherwise be considered a contraindication for transplantation,23–25 as centers fear being flagged for poor performance by regulatory agencies and insurers. More recent analysis identifying dramatic secular trends in the survival of patients on the waitlist and the increasing rate at which patients are being removed from the waitlist point toward increasing selectivity on the part of transplant centers of patients that they are willing to waitlist. Of note, these trends appear to predate the elimination of the 1-year outcome provisions in the CMS conditions of participation for transplant centers. Although our descriptive analyses examined only 9 centers in 1 region, we did not see specific evidence that the transplant programs in this region were achieving improved transplant rates by being more selective and using stringent clinical criteria for waitlisting, given the lack of correlation between the waitlisting and transplant rate metrics. National data on waitlisting among referred patients are needed to determine the extent to which waitlisting and transplant rate metrics may be correlated.
Numerous calls to reform the transplant metrics system to include a more holistic evaluation of transplant center practices and to incentivize improving transplant rates have been made.2,26,27 The potential to reform kidney transplant metrics to improve kidney transplant access is even more complex, given regulatory oversight over both dialysis facilities and transplant programs and the conflicting nature of current quality measures.28 For example, the new Prospective Payment System Final Rule for ESRD introduced a new quality measure for dialysis facilities on the proportion of prevalent dialysis patients waitlisted that will go into effect in 2022. However, at the same time, waitlisting is declining nationwide after the implementation of the new kidney allocation system in 2014.29 Regulatory oversight policies and incentives aimed at improving access to kidney transplantation—such as new alternative payment models proposed in the Advancing American Kidney Health executive order—need to be coordinated at the federal level to ensure that incentives are well aligned across the continuum of care for patients with advanced chronic kidney disease and ESRD.
There are several limitations to our study that should be noted. First, this is a small sample size of only 9 transplant programs, which limits our ability to conduct rigorous statistical testing and generalize findings. However, these centers do have a large catchment area, and we intend the presentation of this new metric as a proof-of-concept to be further validated when there are more data available on referral rates to programs nationally. Second, to ensure anonymity of participating transplant centers, it is not possible to examine more transplant center–level factors or more specific regional factors. Third, due to the small sample size, we were unable to explore other important factors that may influence transplant rates such as organ supply, competitiveness within a donor service area region, and center- or provider-level factors that could influence the variability in transplant rates. Fourth, transplant programs in the Southeastern United States may not be representative of transplant centers across the nation. The Southeast has a higher prevalence of obesity, hypertension, and diabetes compared to the national average, and a higher proportion of non-Hispanic Black ESRD patients. The causes of variation in access to transplantation vary across geographic region. However, there is substantial evidence of geographic differences in waitlisting access when using all ESRD patients as a denominator,30 and variation in referral for transplant,13,31 which suggests it is likely that there may also be variation in waitlisting among a population referred for kidney transplantation.
The goal of the SRTR quality measures is to provide data on transplant center quality to patients and the general public. Waitlisting is a necessary step to receiving a transplant, but no information about transplant program waitlisting practices is currently available for patients. This study provides preliminary data in 1 region for a potential measure that could be used to help patients decide where to pursue transplantation, and could help transplant programs conduct quality improvement projects focused on increasing outreach and access among their referred patient populations. However, caution is warranted in using a measure such as this for regulatory purposes, as there are likely unintended consequences to transplant centers and/or patients in using this metric.
5 |. CONCLUSION
Findings from our study re-emphasize the need for collecting national level surveillance data at the early steps of transplantation (eg, referral to a transplant program) and warrants application across broader geographical regions for scientific rigor and generalizability. Transplant centers already collect referral data for all organs, and infrastructure to submit these data are already in place with UNOS. National policies should be amended to require the collection of these data from centers, and in the absence of this call, centers should consider voluntarily submitting data to the existing Transplant Access Registry maintained by the Southeastern Kidney Transplant Coalition.16 Patient-centered quality measures focusing on transplant access, in addition to outcomes, need to be considered to generate a comprehensive picture of quality of care in kidney transplantation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the National Institute on Minority Health and Health Disparities grant U01MD010611. The data reported here have been supplied in part by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. The authors acknowledge the assistance of the Southeastern Kidney Transplant Coalition and the Centers for Medicare & Medicaid Services (CMS) in providing data, which made this research possible. The Southeastern Kidney Transplant Coalition participated in data collection, and the authors would like to specifically acknowledge the Coalition members that heavily contributed to data collection: Randy Detwiler (University of North Carolina Center for Transplant), Derek DuBay (Medical University of South Carolina), Matthew Ellis (Duke University Transplant Center), Chris Fotiadis (Carolinas Health), Joseph Gulotta (IPRO ESRD Network of the South Atlantic), Erica Hartmann (Piedmont Transplant Institute), Heather Jones (Vidant Medical Center), Laura Mulloy (Augusta University Health Transplant Center), Amber Reeves-Daniel (Wake Forest Health), Brenda Thrasher (Carolinas Health), Al Wagner (University of North Carolina Center for Transplant), Shannon Wright (IPRO ESRD Network of the South Atlantic), and Carlos Zayas (Augusta University Health Transplant Center).
Funding information
National Institute on Minority Health and Health Disparities, Grant/Award Number: U01MD010611
Abbreviations:
- CMS
Centers for Medicare & Medicaid Services
- ESRD
end-stage renal disease
- HR
hazard ratio
- SRTR
Scientific Registry of Transplant Recipients
- USRDS
United States Renal Data System
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Brett KE, Ritchie LJ, Ertel E, Bennett A, Knoll GA. Quality metrics in solid organ transplantation: a systematic review. Transplantation. 2018;102(7):e308–e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandraker A, Andreoni KA, Gaston RS, et al. Time for reform in transplant program-specific reporting: AST/ASTS transplant metrics taskforce. Am J Transplant. 2019;19(7):1888–1895. [DOI] [PubMed] [Google Scholar]
- 3.Scientific Registry of Transplant Recipients. Program-specific reports. 2019. https://www.srtr.org/. Accessed May 20, 2019 [DOI] [PubMed]
- 4.Wey A, Gustafson SK, Salkowski N, et al. Association of pretransplant and posttransplant program ratings with candidate mortality after listing. Am J Transplant. 2019;19(2):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husain SA, Brennan C, Michelson A, et al. Patients prioritize waitlist over posttransplant outcomes when evaluating kidney transplant centers. Am J Transplant. 2018;18(11):2781–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaffhausen CR, Bruin MJ, Chu S, et al. The importance of transplant program measures: surveys of three national patient advocacy groups. Clin Transplant. 2018;32(12):e13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaffhausen CR, Bruin MJ, Chu S, et al. Comparing pretransplant and posttransplant outcomes when choosing a transplant center: focus groups and a randomized survey. Transplantation. 2020;104(1):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu R, Kim SJ, de Oliveira C, Coyte PC. An instrumental variable approach confirms that the duration of pretransplant dialysis has a negative impact on the survival of kidney transplant recipients and quantifies the risk. Kidney Int. 2019;96(2):450–459. [DOI] [PubMed] [Google Scholar]
- 9.Kasiske BL, Wey A, Salkowski N, et al. Seeking new answers to old questions about public reporting of transplant program performance in the United States. Am J Transplant. 2019;19(2):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.System USRD. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. https://www.usrds.org/annual-data-report/previous-adrs/ Accessed January 13, 2020. [Google Scholar]
- 11.Lynch RJ, Patzer RE. Geographic inequity in transplant access. Curr Opin Organ Transplant. 2019;24(3):337–342. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg D, Karp S, Shah MB, Dubay D, Lynch R. Importance of incorporating standardized, verifiable, objective metrics of organ procurement organization performance into discussions about organ allocation. Am J Transplant. 2019;19(11):2973–2978. [DOI] [PubMed] [Google Scholar]
- 13.Alexander GC, Sehgal AR; Transplant Task Force of The Renal Network, Inc. Variation in access to kidney transplantation across dialysis facilities: using process of care measures for quality improvement. Am J Kidney Dis. 2002;40(4):824–831. [DOI] [PubMed] [Google Scholar]
- 14.Patzer REPL, Krisher J, Pastan SO. Dialysis facility and network factors associated with low kidney transplantation rates among United States dialysis facilities. Am J Transplant. 2014;14(7):1562–1572. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patzer REPL, Paul S, Gander J, et al. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. J Am Med Assoc. 2015;314(6):582. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patzer RE, McPherson L, Wang Z, et al. Dialysis facility referral and start of evaluation for kidney transplantation among patients treated with dialysis in the Southeastern United States. Am J Transplant. 2020;20(8):2113–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schold JD, Buccini LD, Poggio ED, Flechner SM, Goldfarb DA. Association of candidate removals from the kidney transplant waiting list and center performance oversight. Am J Transplant. 2016;16(4):1276–1284. [DOI] [PubMed] [Google Scholar]
- 18.Schold JD, Arrigain S, Flechner SM, et al. Dramatic secular changes in prognosis for kidney transplant candidates in the United States. Am J Transplant. 2019;19(2):414–424. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Medicare & Medicaid Services. Medicare program; end-stage renal disease prospective payment system, payment for renal dialysis services furnished to individuals with acute kidney injury, end-stage renal disease quality incentive program. Fed Reg. 2018;83(220):56922–57073. [PubMed] [Google Scholar]
- 20.Patzer RE, Gander J, Sauls L, et al. The RaDIANT community study protocol: community-based participatory research for reducing disparities in access to kidney transplantation. BMC Nephrol. 2014;15(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SRTR. Technical methods for the program specific reports. 2019. https://www.srtr.org/about-the-data/technical-methods-for-theprogram-specific-reports#tableb4. Accessed July 18, 2019
- 22.Salkowski N, Snyder JJ, Zaun DA, Leighton T, Israni AK, Kasiske BL. Bayesian methods for assessing transplant program performance. Am J Transplant. 2014;14(6):1271–1276. [DOI] [PubMed] [Google Scholar]
- 23.Mohan S, Chiles MC, Patzer RE, et al. Factors leading to the discard of deceased donor kidneys in the United States. Kidney Int. 2018;94(1):187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schold JD, Arrington CJ, Levine G. Significant alterations in reported clinical practice associated with increased oversight of organ transplant center performance. Prog Transplant. 2010;20(3):279–287. [DOI] [PubMed] [Google Scholar]
- 25.Schold JD, Buccini LD, Srinivas TR, et al. The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant. 2013;13(1):67–75. [DOI] [PubMed] [Google Scholar]
- 26.Schold JD, Patzer RE, Pruett TL, Mohan S. Quality metrics in kidney transplantation: current landscape, trials and tribulations, lessons learned, and a call for reform. Am J Kidney Dis. 2019;74(3):382–389. [DOI] [PubMed] [Google Scholar]
- 27.Patzer RE. Quality metrics in transplantation - a new emphasis on transplant access. Am J Transplant. 2018;18(6):1301–1302. [DOI] [PubMed] [Google Scholar]
- 28.Schold JD, Buccini LD, Phelan MP, et al. Building an ideal quality metric for ESRD health care delivery. Clin J Am Soc Nephrol. 2017;12(8):1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Melanson TA, Plantinga LC, et al. Racial/ethnic disparities in waitlisting for deceased donor kidney transplantation 1 year after implementation of the new national kidney allocation system. Am J Transplant. 2018;18(8):1936–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathur AK, Ashby VB, Sands RL, Wolfe RA. Geographic variation in end-stage renal disease incidence and access to deceased donor kidney transplantation. Am J Transplant. 2010;10(4 Pt 2):1069–1080. [DOI] [PubMed] [Google Scholar]
- 31.Patzer RE, Plantinga LC, Paul S, et al. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. JAMA. 2015;314(6):582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


