Abstract
Aspirin-exacerbated respiratory disease (AERD) is characterized by the clinical triad of chronic rhinosinusitis with nasal polyps, asthma, and an intolerance to medications that inhibit the cycloxgenase-1 enzyme. Patients with AERD on average have more severe respiratory disease compared with patients with chronic rhinosinusitis with nasal polyps and/or asthma alone. Although patients with AERD traditionally develop significant upper and lower respiratory tract symptoms on ingestion of cycloxgenase-1 inhibitors, most of these same patients report clinical benefit when desensitized to aspirin and maintained on daily aspirin therapy. This Work Group Report provides a comprehensive review of aspirin challenges, aspirin desensitizations, and maintenance aspirin therapy in patients with AERD. Identification of appropriate candidates, indications and contraindications, medical and surgical optimization strategies, protocols, medical management during the desensitization, and recommendations for maintenance aspirin therapy following desensitization are reviewed. Also included is a summary of studies evaluating the clinical efficacy of aspirin therapy after desensitization as well as a discussion on the possible cellular and molecular mechanisms explaining how this therapy provides unique benefit to patients with AERD.
Keywords: Aspirin-exacerbated respiratory disease, AERD, NSAID-exacerbated respiratory disease, Samter triad, aspirin desensitization
Aspirin-exacerbated respiratory disease (AERD), also referred to as Widal syndrome, Samter triad, aspirin-sensitive asthma, aspirin-induced asthma, and nonsteroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease, is an acquired inflammatory syndrome that is characterized by the clinical triad of asthma, eosinophilic chronic rhinosinusitis with nasal polyps (CRSwNP), and the development of respiratory reactions following exposure to all cyclooxygenase (COX)-1 inhibitors including aspirin and other NSAIDs (Table I). AERD is characterized in part by a dysregulation in arachidonic acid metabolism leading to elevated levels of cysteinyl leukotrienes (CysLTs), reduced levels of prostaglandin E2 (PGE2), and elevated levels of prostaglandin D2 (PGD2).1–4 AERD is estimated to affect 7% to 15% of patients with asthma and 10% to 16% of patients with CRSwNP.5,6 However, the condition is probably underdiagnosed and is not readily recognized by most physicians.7
TABLE I.
List of medications that inhibit COX-1 and/or COX-2
| Highly selective COX-1 inhibitors | ||
| Acetylsalicylic acid | Flurbiprofen | Metamizole |
| Antipyrine/benzocaine | Ibuprofen | Mefanamic acid |
| Benoxaprofen | Indomethacin | Naproxen |
| Diclofenac | Ketoprofen | Oxaprozin |
| Etodolac | Ketorolac | Piroxicam |
| Fenoprofen | Meclofenamate | Tolmetin |
| Weakly selective COX-1 inhibitors | ||
| Acetaminophen | Diflunisal | |
| Choline magnesium trisalicylate | Salsalate | |
| Highly selective COX-2 inhibitors | ||
| Celecoxib | Lumiracoxib | |
| Etoricoxib | Parecoxib | |
| Preferentially selective COX-2 inhibitors (COX-1 inhibition at high dose) | ||
| Meloxicam | Nimesulide | |
| Nabumetone | ||
The delay in diagnosing AERD is not harmless. On average, patients with AERD tend to have more severe upper and lower respiratory tract disease when compared with aspirin-tolerant patients with CRSwNP with or without asthma.6 Standard medical treatment for AERD focuses on managing the upper and lower respiratory tract symptoms and can include the use of corticosteroids, leukotriene-modifying drugs (LTMDs), and biologics that target type 2 inflammatory cytokines.8,9 Functional endoscopic sinus surgery (FESS) is also used to debulk nasal polyps and improve topical penetration of saline or corticosteroids. Despite these medical and surgical interventions, patients with AERD tend to undergo repeated sinonasal surgeries and are more likely to be dependent on chronic or frequent oral corticosteroids to manage their disease.6,10,11 Overall, this contributes to increased health care costs as well as enhanced physical and emotional burden of patients with AERD and their families.
Aspirin therapy after desensitization (ATAD) is a unique therapeutic option that can be offered to patients with AERD. This treatment can result in improved quality of life, delayed nasal polyp regrowth, reduced need for additional sinus surgeries, and improved control of upper and lower respiratory tract symptoms.12–16 In this report, the clinical evidence supporting the use of aspirin desensitization followed by maintenance oral aspirin therapy in patients with AERD will be reviewed. Expanding on the European Academy of Allergy and Clinical Immunology article on the diagnosis and management of NSAID-exacerbated respiratory disease,17 this report will provide an in-depth discussion on medical and surgical indications, desensitization protocols, and oral aspirin maintenance regimens available and commonly used in the United States (Fig 1).
FIG 1.
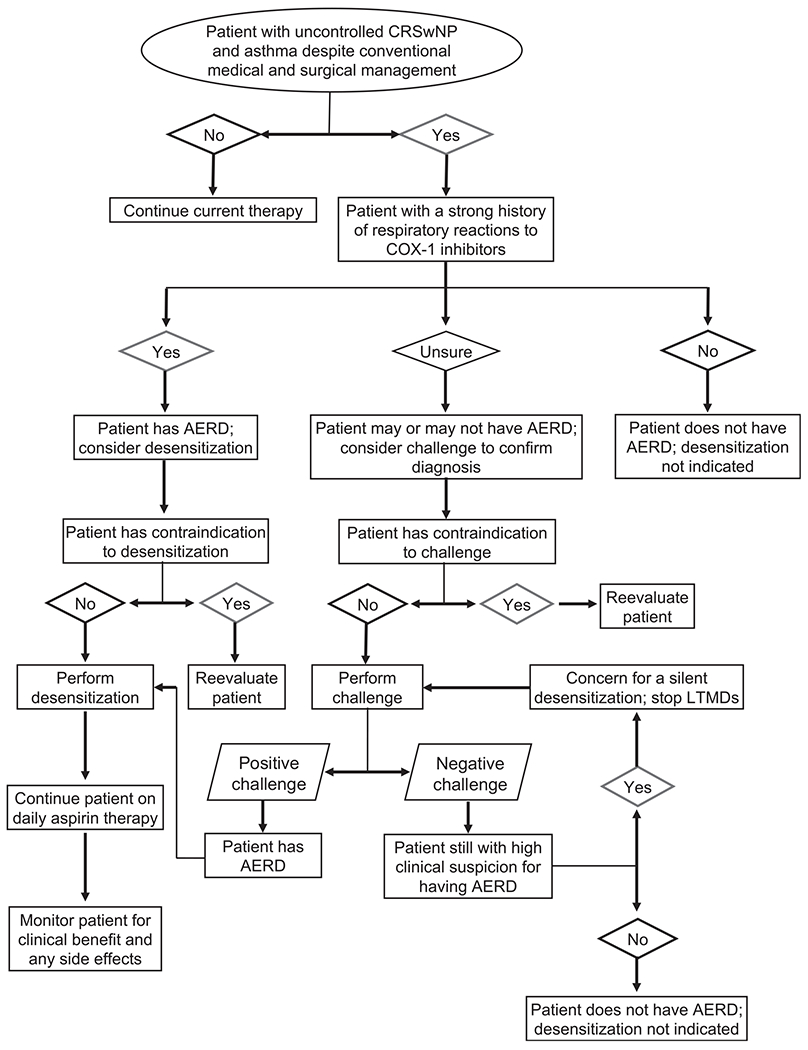
Algorithm for evaluating patients for an aspirin desensitization followed by maintenance aspirin therapy.
EVIDENCE SUPPORTING THE BENEFITS OF ASPIRIN DESENSITIZATION FOLLOWED BY DAILY MAINTENANCE THERAPY IN AERD
Case reports and retrospective analyses
In 1922, the first aspirin desensitization was described in a 37-year-old woman with asthma and nasal polyps who noted worsening asthma symptoms following the ingestion of aspirin.18 In this report, the “desensitization to aspirin was attained very easily … by administering infinitesimal doses [of aspirin] then progressively increasing them in a continuous fashion,” which then allowed the patient to tolerate subsequent doses of aspirin without complications.18–20 In the 1970s, this phenomenon was again described by Zeiss and Lockey21 in an asthmatic patient with nasal polyps who reacted during the first challenge to aspirin but not on the following day when given another dose of aspirin as part of a double-blind double-dummy protocol.
These early studies indicated that patients could tolerate aspirin following desensitization but did not comment further on the course of their asthma or sinonasal disease. In 1980, Stevenson et al22 published a report in which 2 patients with AERD remained desensitized on a daily aspirin regimen for several months and reported improvement in their clinical symptoms over the same time period.22 One patient was able to significantly reduce her dose of daily oral corticosteroids while the other patient reported improved nasal congestion, reduced asthma flares, and discontinuation of oral corticosteroids altogether.22 This work suggested that desensitizing patients with AERD and continuing them on daily aspirin had therapeutic benefits. One limitation of these early seminal studies was the small sample size including as few as 1 to 2 patients each.
Subsequent observational studies have examined larger cohorts of patients with AERD who were desensitized and maintained on daily aspirin for weeks, months, or years 12–14,23–33 (Table II). Patients reported subjective improvement in upper and lower respiratory tract symptoms and objective reductions in daily oral steroid requirements as early as 4 weeks following desensitization while on maintenance therapy.25 Even 10 years following desensitization, 85% of patients with AERD still taking daily aspirin found the therapy to be very or extremely helpful in controlling their sinonasal and asthma symptoms and in improving their quality of life.14 In this study, fewer patients with AERD on daily aspirin (32%) required an additional sinus surgery compared with those patients with AERD who discontinued the therapy (79%), but aspirin therapy did not significantly reduce the total number of sinus surgeries needed nor did it delay the time of the next sinus surgery between the 2 groups.14 In a separate study, 95% of patients with AERD had sustained endoscopic and symptomatic improvement when sinus surgery was combined with aspirin desensitization/maintenance therapy and the latter treatment provided additional significant benefit to what was already observed from surgery alone.27 Furthermore, daily aspirin therapy was found to be effective at slowing the rate of polyp regrowth and reducing the need for repeat sinus surgery.31,34,35
TABLE II.
Summary of case series evaluating the clinical efficacy of ATAD in patients with AERD
| Study | Level of evidence* | Study description | Study duration | No. enrolled / completed the study | Sinus surgery required before desensitization? | Daily maintenance oral aspirin dose | Overview of results |
|---|---|---|---|---|---|---|---|
| Lumry et al,23 1983 | 4 | Prospective | 2-12 mo | 17 / 17 | No | 325-2600 mg | 47% reported persistent sinonasal symptom improvement and tolerated daily aspirin |
| Sweet et al,30 1990 | 4 | Retrospective | 32-102 mo | 65 on aspirin therapy and 42 avoiding aspirin | No | 325-2600 mg | 54% (35 of 60 subjects) tolerated aspirin Subjects on ATAD had significantly fewer ED visits, reduced number of steroid bursts, improved nasal symptoms, and fewer sinus surgeries compared with subjects avoiding aspirin |
| Stevenson et al,24 1996 | 4 | Longitudinal | 1-6 y | 78 / 65 | No | 325-1950 mg | 83% (65 of 78 subjects) tolerated aspirin Subjects on ATAD had fewer sinus surgeries, reduced oral/intranasal steroid use, and improved sense of smell compared with before their ATAD |
| Berges-Gimeno et al,25 2003 | 4 | Longitudinal | 4 wk | 38 / 38 | No | 1300 mg | Subjects on ATAD reported improved nasal and asthma symptoms and reduced oral steroid use when compared with before their ATAD |
| Berges-Gimeno et al,12 2003 | 4 | Longitudinal | 1-5 y | 172 / 126 | No | 1300 mg | 67% (115 of 172 subjects) tolerated aspirin and reported improved sinonasal and asthma symptoms, reduced steroid use, reduced number of sinus surgeries, and decreased ED visits for asthma compared with before their ATAD |
| Lee et al,26 2007 | 4 | Longitudinal | 1 y | 137 / 105 | No | 650-1300 mg | Both doses of aspirin were associated with improvement in nasal and asthma symptoms, reduced hospitalizations for asthma, and reduced need for sinus surgery. However, some patients on the lower dose required an increase to 1300 mg daily to see improvement |
| Rozsasi et al,31 2008 | 4 | Prospective | 12 mo | 14 / 14 | No | 100 mg | 100% of subjects had recurrent nasal polyps and had no improvement in sense of smell or lung function |
| 300 mg | 0% of subjects had recurrent nasal polyps with significant improvement in sense of smell and lung function | ||||||
| Havel et al,13 2013 | 4 | Retrospective | 18-84 mo | 51 on aspirin therapy and 33 avoiding aspirin | Yes (4-6 wk prior) | 500 mg | Subjects on ATAD had less polyp regrowth and reported sinonasal and/or asthma symptom improvement compared with subjects avoiding aspirin |
| Comert et al,32 2013 | 4 | Prospective | 3 y | 40 / 18 | No | 300 mg | Subjects on ATAD had reduced systemic steroid bursts, fewer sinus surgeries, and noted improvement in some sinonasal symptoms compared with before their ATAD |
| Cho et al,27 2014 | 4 | Retrospective | 6 mo | 28 / 21 | Yes (4-6 wk prior) | 650-975 mg | 95% (20 of 21 subjects) tolerated aspirin Subjects reported a further improvement in sinonasal symptoms on ATAD than what they noted after sinus surgery |
| Adappa et al,28 2018 | 4 | Retrospective | 30 mo | 32 / 32 | Yes (3-6 wk prior) | 325-1300 mg | 84% (27 of 32 subjects) tolerated aspirin Subjects reported a further improvement in sinonasal symptoms on ATAD than what they noted after sinus surgery |
| Jerschow et al,33 2017 | 4 | Prospective | 4 wk | 39 / 39 | No | 1300 mg | 31% (12 of 39 subjects) had improved symptoms and lung function on ATAD 49% (19 of 39 subjects) could not complete the desensitization or had worsening symptoms and lung function on ATAD |
| Walters et al,14 2018 | 4 | Retrospective | 10 y | 92 / 57 | No | 325-650 mg | 85% of subjects on ATAD reported that this treatment was very or extremely helpful in controlling their airway disease and improving their quality of life |
| Shah et al,29 2019 | 4 | Prospective | 6 mo | 40 / 40 | Yes | 1300 mg for the first 4 wk, then 650 mg | 19 subjects who benefited from and 21 subjects who failed ATAD with a distant history of ESS were identified 24 of these 40 patients required subsequent ESS and underwent another ATAD 3-4 wk after repeat ESS. 100% (24 patients) tolerated this ATAD |
ED, Emergency department; ESS, endoscopic sinus surgery.
Level of evidence: 1 (systematic review of randomized trials or n-of-1 trials); 2 (randomized trial or observational study with dramatic effect); 3 (nonrandomized controlled cohort/follow-up study); 4 (case series, case-control studies, or historically controlled studies).
As with all therapies, aspirin desensitization followed by maintenance aspirin will not uniformly benefit all patients with AERD. In a 5-year follow-up of desensitized patients with AERD at a large tertiary care center, 67% reported a subjective improvement with aspirin therapy, with 22% of the patients reporting either no improvement in their symptoms or discontinuing aspirin because of adverse side effects.12 If patients who experienced adverse reactions were excluded from analysis, 78% of patients with AERD reported improvement in clinical symptoms.12 In a 10-year survey from the same institution, 38% of patients with AERD reported discontinuing aspirin treatment predominantly because of adverse reactions (26%), a lack of clinical benefit (26%), or a need to undergo a surgical procedure (23%). Of the remaining 62% of patients who continued daily aspirin therapy, the vast majority reported significant clinical benefit.14 Some patients have worsening of respiratory symptoms (measured by respiratory questionnaires) and decline in FEV1 while on high-dose aspirin treatment.33
Double-blind placebo-controlled studies
Double-blind placebo-controlled studies remain the criterion standard for evaluating the safety and efficacy of a proposed therapy. However, such studies are inherently difficult to perform in patients with AERD. First, the desensitization itself cannot be easily blinded because patients with AERD develop symptoms on ingestion of aspirin but not placebo. The second option is to perform a true desensitization followed by a period of placebo treatment. The subjects then lose their desensitized status and would need to undergo another desensitization at a future date. This is a considerable impediment to enrollment.
Despite these limitations, there have been several published double-blind placebo-controlled trials involving aspirin desensitization and maintenance therapy in patients with AERD32,36–40 (Table III). In these, most patients reported a clinical improvement in upper and lower respiratory tract symptoms as well as overall disease control while on maintenance aspirin therapy compared with placebo. The first double-blind placebo-controlled cross-over study of aspirin in AERD was performed in the early 1980s.36 Patients were challenged to aspirin, then desensitized, and maintained on aspirin (or placebo) for 3 months. Following this, they underwent a 1-month wash-out period, were then redesensitized, and assigned to the other treatment category for another 3 months. Of the patients who completed the study, 67% noted improvement in nasal symptoms and 48% noted improved asthma symptoms while on aspirin.36 Finally, a more recent double-blind placebo-controlled study compared 20 desensitized patients with AERD who were assigned to daily aspirin maintenance therapy or placebo for 6 months. Patients with AERD on aspirin reported subjective improvements in sinonasal and asthma symptoms (including smell) and a reduction in inhaled corticosteroid dose when compared with patients with AERD on placebo.37 Taken together, these studies highlight the utility of aspirin desensitization followed by daily maintenance therapy as a unique treatment option for patients with AERD.41
TABLE III.
Summary of double-blind placebo-controlled studies evaluating the clinical efficacy of ATAD in patients with AERD
| Study | Level of evidence* | Study description | Study duration | No. enrolled / completed the study | Sinus surgery required before desensitization? | Daily maintenance oral aspirin dose | Overview of results |
|---|---|---|---|---|---|---|---|
| Stevenson et al,36 1984 | 3 | Double-blind placebo-controlled cross-over | 7 mo | 38 / 25 | No | 325 mg | 57% (4 of 7 subjects) reported sinonasal and/or asthma symptom improvement and tolerated daily aspirin |
| 1300 mg | 60% (3 of 5 subjects) reported sinonasal and/or asthma symptom improvement and tolerated daily aspirin | ||||||
| 2600 mg | 69% (9 of 13 subjects) reported sinonasal and/or asthma symptom improvement and tolerated daily aspirin | ||||||
| Fruth et al,39 2013 | 2 | Double-blind placebo-controlled | 36 mo | 70 / 31 | Yes (6 wk prior) | 100 mg | Subjects on ATAD reported improved quality of life compared with controls, but there was no significant difference in rate of nasal polyp regrowth or size |
| Swierczynska-Krepa et al,37 2014 | 2 | Double-blind placebo-controlled | 6 mo | 20 / 15 | No | 624 mg | Subjects on ATAD reported improved asthma control, improved sinonasal symptoms, and reduced doses of inhaled steroids compared with placebo |
| Esmaeilzadeh et al,38 2015 | 2 | Double-blind placebo-controlled | 6 mo | 34 / 32 | No | 650-1300 mg | Subjects on ATAD had improvement in lung function, improved sinonasal symptoms, and reported less medication use compared with placebo |
| Mortazavi et al,40 2017 | 2 | Double-blind placebo-controlled | 6 mo | 41 / 38 | No | 1300 mg for the first 4 wk, then 650 mg daily for 5 mo | Subjects on ATAD reduced symptoms, improved FEV1, and improved quality of life compared with placebo |
| Chu et al,41 2019 | 1 | Meta-analysis25,29,31,33,34,36,40 | NA | NA | NA | NA | In patients with AERD, aspirin desensitization reduced symptoms of rhinosinusitis and improved quality of life. Adverse event rates were higher than in the placebo arm |
NA, Not applicable.
Level of evidence: 1 (systematic review of randomized trials or n-of-1 trials); 2 (randomized trial or observational study with dramatic effect); 3 (nonrandomized controlled cohort/follow-up study); 4 (case series, case-control studies, or historically controlled studies).
Proposed mechanisms for clinical benefit
The cellular and molecular mechanisms driving the improvement following aspirin desensitization/maintenance therapy remain largely speculative (Fig 2). One of the central features of AERD is the high constitutive production of CysLTs and their further surge following ingestion of aspirin and other nonselective inhibitors of the COX-1 enzyme. This observation might suggest that therapeutic efficacy would be associated with modulation of CysLT concentrations. However, studies have consistently shown that aspirin desensitization is not associated with decreases in CysLT concentrations.4,42,43 Cahill et al44 paradoxically found increased levels of urinary LTE4 in patients with AERD taking high-dose aspirin therapy for 8 weeks.44 In contrast, desensitization is associated with decreased expression of the CysLT1 receptor,45 and an approximately 20-fold reduction in sensitivity to inhaled LTE4 is observed following desensitization,46 presumably reflecting a decreased expression of the putative LTE4 receptor.47 Given the evidence for a critical role for LTE4 in the pathogenesis of AERD,48,49 it is reasonable to speculate that decreased expression of the LTE4 receptor could be a central mechanism of desensitization, although this concept has not been explored. Similarly, the mechanisms by which aspirin therapy modulates CysLT receptor expression are not immediately apparent.
FIG 2.
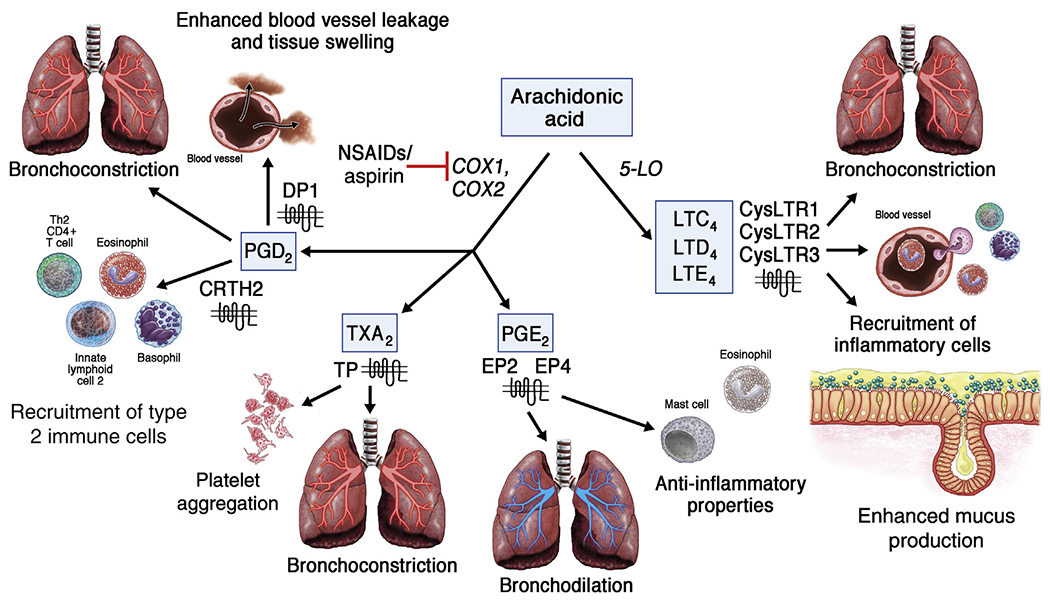
Arachidonic acid metabolism is dysregulated in AERD. Compared with aspirin-tolerant patients, patients with AERD at baseline have elevated levels of CysLTs (LTC4, LTD4, LTE4) and PGD2 that mediate bronchospasm, vascular leak, inflammatory cell recruitment, and enhanced mucus production. In contrast, levels of PGE2, which has anti-inflammatory properties and can induce bronchodilation, are reduced in patients with AERD. Although the precise mechanism of benefit from aspirin therapy is unclear, the effects of PGD2 and CysLTs are dominant components. During an aspirin desensitization, urinary levels of PGD2 further increase but later decrease while on high-dose aspirin therapy. In contrast, urinary levels of CysLTs have not been found to decrease while on high-dose aspirin therapy, but expression of the CysLT receptor 1 (CysLTR1) has been shown to be reduced. CRTH2, Chemoattractant receptor-homologous molecule expressed on TH2 cells; CysLTR2, CysLT receptor 2; CysLTR3, CystLT receptor 3; DP1, prostaglandin D2 receptor 1; EP2, prostaglandin E2 receptor 2; EP4, prostaglandin E2 receptor 4; TP, thromboxane receptor; TXA2, thromboxane A2.
There are also studies demonstrating off-target (COX-independent) mechanisms of aspirin, including those demonstrating its ability to inhibit signaling pathways (eg, Stat6) and expression of cytokines (eg, IL-4 and IL-13) that have been linked to upregulation of CysLT receptors.50–54 Although plausible, many of these studies used pharmacologically excessive concentrations of aspirin, leading to uncertainty of the biological significance. Aspirin maintenance therapy has also been associated with increased numbers of circulating eosinophils and basophils, suggesting that these inflammatory cells may no longer be recruited into the tissue to exert their inflammatory effects. 34,44,55 However, patients receiving aspirin therapy who developed worsening respiratory symptoms and FEV1 decline had a significantly greater increase in peripheral eosinophil numbers.33
An alternative explanation for why aspirin desensitization/maintenance therapy is beneficial in AERD is that aspirin would act through its primary biological pathway (ie, inhibiting COX and downstream prostanoids) to block the recruitment and activation of inflammatory cells. PGD2 has been extensively studied in AERD. It is the primary prostanoid secreted by mast cells, but in AERD, it may also be produced by eosinophils.56 PGD2 acts through 3 distinct receptors, all of which are overexpressed in AERD57 to induce vasodilatation, recruit type 2 immune cells, and elicit bronchospasm. PGD2 concentrations are elevated in AERD, further increase on desensitization, and then markedly diminish during maintenance aspirin therapy.4,58,59 The increase in PGD2 during the desensitization may account for the clinical symptoms observed, whereas the reduced levels of PGD2 on maintenance therapy may account for reduced inflammatory cell infiltrate, type 2 cytokine concentrations, and reduced expression of CysLT receptors.
In addition to elevated levels of CysLTs and PGD2, AERD is characterized by increased levels of thromboxane A2,4 reduced levels and function of PGE2,60 downregulation of the PGE2 receptor, EP2,61,62 and increased numbers of platelet-adherent leukocytes.63 Although outside the scope of this report, each of these observations may serve as potential targets by which aspirin desensitization/maintenance therapy could mediate its effects. Future studies are thus warranted to further investigate the dysregulated arachidonic acid metabolism observed in AERD and how aspirin desensitization/maintenance therapy may provide clinical benefit.
INDICATIONS FOR ASPIRIN DESENSITIZATION AND THERAPY
Indications for initiation of a desensitization followed by maintenance aspirin therapy include rapidly recurring nasal polyps following sinus surgery, uncontrolled rhinosinusitis despite use of standard medical therapies, and the need for frequent bursts of systemic corticosteroids to control respiratory or sinus symptoms. Importantly, this therapy should only be recommended for patients with AERD because no clinical benefit has been described for aspirin-tolerant patients with CRSwNP and/or asthma.37 As such, it is essential to confirm the diagnosis of AERD before desensitization. Although the criterion standard for diagnosing AERD is a formal aspirin challenge, the diagnosis is more commonly made clinically. For patients with known physician-diagnosed asthma, documented nasal polyps, and a clearly-reported history of a respiratory reaction after ingestion of an NSAID, the diagnosis of AERD does not need to be confirmed by a formal aspirin challenge.
However, the presence or absence of NSAID reactions cannot always be established on the basis of patient-report alone. Up to 15% of patients with AERD are not able to identify whether or not they are intolerant to NSAIDs.64 Many of these patients actually report that they have taken NSAIDs without noting any hypersensitivity reactions—these patients do not become aware of their hypersensitivity until a physician-administered provocation challenge induces a reaction.64,65 As such, there are subsets of patients for whom a provocative aspirin challenge is required to determine proper diagnosis, and these patients usually fall into 1 of 4 categories:
Patients who have not used NSAIDs recently. Patients who have not used any NSAIDs recently, or have not used NSAIDs since the development of their nasal polyps or respiratory disease, may not know whether they are hypersensitive.
Patients who are on a leukotriene-modifying drug. The use of the leukotriene receptor antagonist montelukast, or the 5-lipooxygenase inhibitor zileuton, can in some instances pharmacologically blunt or sufficiently prevent the clinical manifestations of NSAID-induced reactions so that the reactions are not noticed by the patient.66,67
Patients who are less perceptive to their reactions. There are patients with AERD who present with both asthma and severe nasal polyps, but report that they can use aspirin or NSAIDs without adverse effects. For such patients who chronically live with complete nasal obstruction, intermittent episodes of worsened nasal congestion (as seen in the setting of NSAID ingestion) may go unnoticed. Patients with AERD also tend to underestimate the severity of their disease, and their perception of disease burden is in stark contrast to that of their providers.68 Therefore, a physician-observed provocative challenge may be required.
Patients already on daily low-dose aspirin. Patients with AERD who were already taking 81 mg aspirin for cardiovascular or cerebrovascular protection at the time of initial clinical evaluation may not report symptoms of NSAID-induced hypersensitivity. These patients tend to have very mild baseline asthma symptoms, and tolerate their current low-dose aspirin, but after stopping low-dose aspirin for at least 10 days, do develop aspirin-induced respiratory symptoms during a provocative oral aspirin challenge.69 The initial tolerance of low-dose aspirin may be because the aspirin-induced symptoms on initiation of low-dose aspirin were mild enough that they went unnoticed by the patient, or because they had started low-dose aspirin before the development of their respiratory disease.
CONTRAINDICATIONS FOR DESENSITIZATION AND INITIATION OF HIGH-DOSE ASPIRIN THERAPY
Most contraindications to ATAD are relative or temporary but should be considered when evaluating patients with AERD for therapy (Table IV).
TABLE IV.
Indications and contraindications for an aspirin desensitization and aspirin therapy in patients with AERD
| Indications |
| Persistent sinonasal and asthma symptoms in a patient with AERD despite conventional medical and surgical therapy |
| Contraindications |
| Poorly controlled asthma |
| Significant nasal polyp burden at time of desensitization |
| Pregnancy |
| History of eosinophilic esophagitis |
| History of gastric and/or peptic ulcer disease |
| History of a bleeding disorder or coagulopathy |
| History of medication nonadherence |
| Relative contraindication |
| History of anaphylaxis to aspirin or other NSAID* |
Patients with a history of anaphylaxis to aspirin or other NSAID may undergo an aspirin desensitization and aspirin therapy, but this decision merits careful discussion with the patient and physician.
Planned sinus surgery
Given the potential for increased intraoperative bleeding and associated decrease in intraoperative visualization accompanying NSAID use, it is advisable to delay aspirin desensitization until the completion of planned sinus surgery. In addition, the reactions to aspirin are also generally less severe when the desensitization is performed following sinus surgery.70,71 Although controlled studies are lacking, general expert consensus suggests that long-term clinical outcomes are improved when the initiation of aspirin therapy occurs after sinus surgery. Therefore, whenever possible and appropriate, it is often recommended that a sinus surgery to debulk the inflammatory nasal polyp tissue precede aspirin desensitization.
Pregnancy
Studies suggest that low-dose aspirin is safe to use in second and third trimesters.72 Low-dose aspirin may be prescribed for prevention of miscarriage in the first trimester and for prevention of preeclampsia.72,73 Generally, the use of aspirin doses above 81 mg daily in pregnant patients should be avoided because it may contribute to maternal and fetal bleeding and premature closure of the ductus arteriosus.74,75 Therefore, it is advisable to defer aspirin desensitization and/or discontinue aspirin treatment in patients who are pregnant.
Gastric ulcers or history of gastrointestinal bleeding
Patients with a history of gastric or peptic ulcers or with active ulcers should not initiate aspirin therapy before consulting a gastroenterologist. If their gastroenterologist confirms that aspirin treatment would be safe, these patients can be desensitized and aspirin treatment started. Current guidelines suggest that in patients with history of bleeding ulcers associated with low-dose aspirin, initiation of low-dose aspirin treatment for secondary prevention of cardiovascular disease is advisable but daily proton-pump inhibitors (PPIs) are recommended.76 Because there is limited evidence for aspirin therapy in patients with AERD with a history of gastric ulcers, the decision should be made on a case-by-case basis in consultation with the treating gastroenterologist.
Bleeding disorders and coagulopathies
Patients who have a history of bleeding disorders or coagulopathies (including deep venous thrombosis and pulmonary embolism) and/or who are currently receiving anticoagulation therapy should first discuss aspirin desensitization and maintenance therapy with their physician who manages their anticoagulation therapy.
Uncontrolled asthma
Patients with poorly controlled and symptomatic asthma should not undergo aspirin desensitization until asthma control is optimized.
Eosinophilic esophagitis
Patients with a history of eosinophilic esophagitis might be at risk for worsening gastrointestinal symptoms on initiation of aspirin therapy.77 Although evidence is lacking, it is possible that some of these patients may tolerate aspirin therapy if concomitantly treated with agents targeting IL-5 or IL-4/13.
SPECIAL CONSIDERATIONS IN SPECIFIC PATIENT POPULATIONS
Pediatric patients
AERD occurs infrequently in patients younger than 20 years. There is a concern for Reye syndrome in children with viral infections and salicylate use.78 Reye syndrome has several peaks of incidence around the ages of 1, 5, and 13 years.78 Thus, it is advisable not to treat patients younger than 14 years with aspirin. Aspirin treatment in children older than 14 years should be decided on a case-by-case basis with the intention of preventing polyp recurrence.
Elderly patients
Most of the aspirin desensitization and treatment outcome studies included patients younger than 70 years. 13,33,37,79–82 Although at least 1 study included patients with AERD up to 81 years of age,83 the presence of comorbid conditions in the elderly that may be contraindications to aspirin therapy warrant further study. The use of biological therapies may be considered as alternative treatment options for disease control in older patients, although data for biologics in advanced age are also lacking.
Black and Latino patients
In a recent study of 39 patients, black and Latino patients were more likely than white patients to fail to tolerate the initial aspirin desensitization due to persistent bronchospasm or persistent gastrointestinal symptoms (nausea and vomiting).33 They were also more likely to have worsening upper and lower respiratory tract symptoms that continued for several weeks after initiation of high-dose aspirin. ATAD should still be offered to these patients, but additional counseling may be appropriate for these specific patient populations.
COST CONSIDERATIONS
An economic analysis of outpatient ATAD found that it was a cost-effective therapy for patients with moderate to severe AERD, even after accounting for the initial cost of the aspirin desensitization procedure.84 Compared with the estimated annual costs of biological agents conservatively ranging from $31,000 to $39,000, aspirin is an inexpensive treatment that is beneficial for many patients with AERD.85
MANAGEMENT BEFORE ASPIRIN DESENSITIZATION
Medical management
Aspirin desensitization is a procedure that will often induce symptoms that mimic allergic reactions affecting the upper and lower airways. Therefore, before aspirin desensitization, it is recommended that all coexisting cardiopulmonary conditions be optimized. This is especially true regarding asthma given lung function can sharply decline and lower respiratory tract symptoms can worsen during the procedure. Although there are no randomized trials to support requisite spirometric values, most authors recommend a prebronchodilator FEV1 percent predicted value of at least 70% to safely perform desensitizations. In addition, sinonasal and asthma symptoms should be optimally controlled for the week before desensitization. There are several medical options that can be considered to ensure a patient’s upper and lower respiratory tract symptoms are appropriately managed before desensitization as follows.
Leukotriene-modifying drugs
It is recommended that all patients take standard doses of either a leukotriene receptor antagonist (LTRA) or 5-lipoxygenase (5-LO) inhibitor for approximately 3 days before and then during the aspirin desensitization. These medications typically do not completely eliminate the aspirin-induced reaction, but rather decrease lower airway symptoms (eg, wheezing, shortness of breath, and decrease in FEV1) while preserving upper airway symptoms (eg, rhinorrhea and conjunctivitis).66,86,87 In rare cases, the use of an LTMD will completely block both upper and lower airway symptoms during a desensitization as discussed in the Approach to silent desensitizations section below.
Few trials have attempted to determine differences of an LTRA as compared with a 5-LO inhibitor in the attenuation of lower airway symptoms. In an observational study, an LTRA was found to be superior to a 5-LO inhibitor (zileuton),88 and another small study of 6 subjects found that zileuton had minimal effects in 4 of 6 subjects.89 There is also limited data on the effects of combining a 5-LO inhibitor with an LTRA before desensitization. LTMDs serve as an important component of the medical management of patients with AERD, particularly before aspirin desensitization.
Corticosteroids
Inhaled corticosteroids (ICSs) and ICSs combined with long-acting β2-agonists for asthma should be continued during aspirin desensitization. These medications have a less pronounced effect as an LTMD because they can shift the aspirin-induced reaction from the lower to the upper airway.86 If a patient is already on ICSs with stable disease, they should be continued at the same dose before and throughout the protocol. If a patient has well-controlled asthma and does not routinely take ICSs, the added benefit of starting an ICS before the desensitization is unclear. Likewise, the benefit of starting systemic corticosteroids in a patient who is not chronically dependent and whose asthma is controlled is also unknown. However, select patients with poorly controlled asthma may need a short course of oral corticosteroids to improve asthma control before desensitization. There is little data on the use of intranasal corticosteroids during oral aspirin desensitization, though many practitioners continue their use.
Antihistamines
The use of histamine 1 receptor antagonists before aspirin desensitization is controversial. Some authors have advocated that they be discontinued before challenge and desensitization90 because they may blunt a reaction to aspirin and potentially mask the sentinel reaction during the procedure.91 In contrast, others have performed aspirin desensitization while continuing antihistamines, especially in shorter desensitization protocols.92
Biologics
Biologic therapy for asthma (eg, mAbs against IgE, IL-4 receptor alpha [IL-4Rα]), IL-5, and IL-5 receptor alpha [IL-5Rα]) may also decrease both upper and lower airway symptoms during the desensitization. One randomized controlled trial of 11 subjects found that after use of omalizumab for 16 weeks, 70% of subjects randomized to the intervention arm had neither upper nor lower respiratory tract symptoms during aspirin desensitization, as compared with 0% of subjects randomized to the placebo arm.93 The mechanism may be related to decreased mast cell activation and decreased PGD2 and CysLT levels.94 Standard practice is to continue biologics during the procedure if needed for asthma control, with the understanding that aspirin-induced reactions may be blunted or not occur.93,95 Consideration can also be given to biologic therapy as pretreatment for patients who have previously failed aspirin desensitization due to frequent or severe reactions, though this requires further research.
Surgical management
Endoscopic sinus surgery is often completed before desensitization. Although this historically was done because of the universal prevalence of nasal polyps and the relative contraindication of surgery while taking high-dose aspirin, there is emerging evidence for the improved safety and tolerance of aspirin desensitization when preceded by FESS. In a prospective observational trial, Jerschow et al70 compared aspirin-induced reaction severity among subjects undergoing repeated aspirin desensitizations before and 3 and 4 weeks after FESS. Among this cohort of 28 subjects, all demonstrated decreased severity of clinical reactions following FESS. Strikingly, despite having positive reactions preoperatively, 43% of these patients developed no symptoms during the postoperative aspirin desensitization. These findings were validated in a separate retrospective study by Huang et al71 who reported a decreased risk of significant airway reactions following sinus surgery (odds ratio, 9; P = .033).
Patients with AERD are more likely to receive complete FESS.96 This is likely related to the extent of inflammatory polyp burden and the relatively high rate of surgical failure among patients with AERD.97,98 Given the emerging evidence of decreased reaction severity associated with postoperative aspirin desensitization, complete FESS with debulking of inflammatory polyp burden should be considered before desensitization in any surgically naive patient or those with significant polyp burden.70,71
SELECTING A LOCATION FOR AN ASPIRIN DESENSITIZATION
Aspirin desensitization protocols can be safely performed in an outpatient setting for most patients with AERD. The outpatient setting is generally recommended as appropriate for patients who have well-controlled asthma, a baseline FEV1 greater than or equal to 70% of predicted (and >1.5 L absolute), are not currently using an oral beta-receptor blocker, and do not have any underlying comorbidities that would make management of severe reaction symptoms more difficult.83,92,99 Patients with very stable asthma despite an FEV1 less than 70% could be considered for a desensitization on a case-by-case basis. In addition, in select cases, increased caution and access to inpatient-level care may be preferred, including for patients with poorly controlled asthma or hemodynamic instability. The inpatient setting may also be appropriate for patients who are not able to stop oral beta-blockers, because this may heighten the risk of severe anaphylaxis during reactions due to the poor response to rescue medications such as epinephrine100 though the clinical significance of this can be variable.101
Although earlier guidelines had recommended that a peripheral intavenous (IV) line be placed before provocative aspirin challenge procedures, 102,103 currently available safety data are strong enough that at most major academic centers in the United States that routinely perform aspirin provocation tests, they are done without a peripheral line in place. However, some practitioners continue to place an IV line before the procedure especially in the event that severe gastrointestinal upset and vomiting occur, so that IV rescue medications can be used.
ASPIRIN DESENSITIZATION PROTOCOLS
There are several published aspirin challenge and desensitization protocols for patients with AERD, with variations in dose escalation time (60 minutes, 90 minutes, 3 hours), starting dose (20.25 mg, 40.5 mg), and route of provocative drug administration (oral aspirin, intranasal ketorolac, intranasal lysine-aspirin).79,99,103–108 Although inhalational and IVaspirin desensitizations are performed at a few European and Asian sites, these modalities generally require the administration of nasal lysine-aspirin, which is not approved for use in the United States.102,109–111 The most common US protocols involve orally administered aspirin or a combination of both intranasally administered ketorolac and orally administered aspirin and will be the focus of this report (Table V).
TABLE V.
Select aspirin desensitization protocols
| 2-d protocols |
1-d protocols |
|||||
|---|---|---|---|---|---|---|
| Day | Time | Oral aspirin29 | Intranasal ketorolac and oral aspirin96 | Oral aspirin95 | Oral aspirin84 | Oral aspirin90 |
| Day 1 | 8:00 am | 20-40 mg | 1.26 mg ketorolac (1 spray) | 20.25 mg | 41 mg | 40 mg |
| 8:30 am | 2.52 mg ketorolac (2 sprays) | |||||
| 9:00 am | 5.04 mg ketorolac (4 sprays) | 80 mg | ||||
| 9:30 am | 7.56 mg ketorolac (6 sprays) | 40.5 mg | 81 mg | 80 mg | ||
| 10:00 am | 160 mg | |||||
| 10:30 am | 60 mg aspirin | |||||
| 11:00 am | 40-60 mg | 81 mg | 161 mg | 325 mg | ||
| 11:30 am | ||||||
| 12:00 pm | 60 mg aspirin | Desensitization complete* | ||||
| 12:30 pm | 162.5 mg | 325 mg | ||||
| 1:00 pm | ||||||
| 1:30 pm | Instructions and discharge | |||||
| 2:00 pm | 60-100 mg | 325 mg | Desensitization complete* | |||
| 2:30 pm | ||||||
| 3:00 pm | Desensitization complete* | |||||
| 3:30 pm | ||||||
| 4:00 pm | ||||||
| 4:30 pm | ||||||
| 5:00 pm | Instructions and discharge | |||||
| 5:00 pm | Instructions and discharge | |||||
| Day 2 | 8:00 am | 100 mg | 150 mg | |||
| 11:00 am | 160 mg | 325 mg | ||||
| 2:00 pm | 325 mg | Desensitization complete* | ||||
| 5:00 pm | Desensitization complete* | |||||
Actual time needed for the protocol to be completed may vary on the basis of severity of reaction and the time needed for recovery.
Two-day oral aspirin desensitization protocol
One of the earliest publications to describe an oral aspirin desensitization suggested a 2-day procedure that required a dosing interval of 3 hours.36 Commercially available 81-mg aspirin tablets can be cut with a pill cutter and used as the first dose, 40.5 mg. Doses are given at 3-hour intervals up to 325 mg (day 1: 40.5 mg, 81 mg, 162 mg, then 3 hours of observation before discharge; day 2: 325 mg). Placebo can be used on the first day of challenge, especially for those patients who are particularly anxious and there is concern for a false-positive reaction to the procedure.
Two-day intranasal ketorolac + oral aspirin desensitization protocol
In this protocol,105 parenteral ketorolac (Toradol) is diluted in saline and administered intranasally. Ketorolac solution is made by mixing ketorolac 60 mg/2 mL with 2.75 mL of normal saline. The solution is placed in a meter-dose nasal spray bottle such that each spray delivers 0.1 mL and 1.26 mg of ketorolac. The intervals between the ketorolac doses are 30 minutes. One spray in 1 nostril (1.26 mg), 1 spray in both nostrils (2.52 mg), 2 sprays in both nostrils (5.04 mg), and 3 sprays in both nostrils (7.56 mg) are given. After 60 minutes, 60-mg aspirin tablets are given twice with a 90-minute observation period between doses. After the second 60-mg dose, the patient is observed for 3 hours before discharge. The next day, 150 mg and then 325 mg of aspirin are administered with either a 90-minute or 3-hour interval, with another 3-hour observation period following the last oral aspirin dose before discharge.
The pros of the intranasal ketorolac protocol are that it has an excellent safety profile including increasing the percentage of patients who develop only naso-ocular reactions during the desensitization.105 Cons for this protocol include more limited access to parenteral ketorolac and metered-dose nasal spray bottles when compared with availability of oral aspirin.
One-day aspirin desensitization protocols
Two recent publications from academic medical centers in the United States have highlighted the safety and efficacy of completing an aspirin desensitization with oral aspirin in a single day.92,99 For both protocols, the procedure begins with 40 to 40.5 mg of oral aspirin, and proceeds with increasing doses of 81 mg, 162 mg, and then 325 mg of oral aspirin every 60 to 90 minutes. The main difference between these 2 protocols is the form of aspirin used. In the 60-minute protocol, dissolved tablets of Alka-Seltzer in solution are used,99 whereas in the 90-minute protocol, aspirin tablets are cut using a pill cutter to achieve the desired lower doses of aspirin.92 The predissolved nature of the aspirin in the Alka-Seltzer solution may allow for faster absorption112 and may decrease the time from provocative dose to reaction, therefore shortening the overall length of the procedure.
Regardless of the protocol, there is now general agreement that the desensitization can end with a maximum final dose of 325 mg, because patients are expected to display symptoms of reaction by this point and can tolerate higher doses without additional symptoms.81 In addition, patients who react at 162 mg or less during the 2-day desensitization protocol rarely react again at 325 mg, and consideration can be given to administer this dose at home.113 In terms of the starting dose for 1-day oral aspirin protocols, studies report that patients rarely react to very low oral aspirin doses, suggesting that it is appropriate and safe in most circumstances to begin these challenges at a dose of 40.5 mg.114 Most patients react after receiving the highest dose (7.56 mg) of intranasal ketorolac (and sometimes even at lower doses), and so it is not recommended to start with higher doses when following the intranasal ketorolac protocol. Before escalating to the next dose of medication within any desensitization protocol, the patient should be clinically evaluated by physical examination and vital signs as well as lung function should be measured by spirometry or peak expiratory flow readings. Monitoring peak nasal inspiratory flow can also be an informative assessment of the upper airways.
EXPECTED REACTIONS DURING A DESENSITIZATION
By definition, patients with AERD should become symptomatic during an aspirin desensitization. AERD is confirmed if they develop clinical symptoms and/or have a decrease in FEV1 by more than 15% and/or a reduction in peak nasal inspiratory flow by more than 20%. Reactions typically are observed between 60 and 80 mg of aspirin but this differs on an individual basis.
Typically, patients will develop a combination of the following upper respiratory tract symptoms: nasal congestion, rhinorrhea, postnasal drip, sneezing, nasal, ocular, or aural pruritus, conjunctival injection, and lacrimation. Reactions are typically self-limited within 3 to 4 hours of symptom onset. Lower respiratory tract symptoms include cough, shortness of breath, and wheezing. As noted previously, clinically significant declines in FEV1 of 20% or more were observed in 37% of patients with AERD despite being on concurrent montelukast therapy.115
Patients may also develop cutaneous symptoms including flushing, urticaria, angioedema, and macular pruritic eruptions on the extremities.4 Gastrointestinal manifestations including dyspepsia, nausea, vomiting, diarrhea, and crampy abdominal pain are also associated with evidence of systemic mast cell activation55,116 and occur in 10% to 30% of cases depending on the desensitization protocol used.55,105 As with the respiratory symptoms, the severity of these symptoms varies widely from mild and transient to severe and persistent such that aspirin must be discontinued. Hypotension and laryngeal angioedema are rare, but physicians performing aspirin desensitizations need to be prepared to treat such events.
Factors that may predict the severity of aspirin-induced reactions
The severity of symptoms during an aspirin desensitization protocol vary widely from patient to patient. Unfortunately for treating clinicians, there are very few known factors that predict whether a patient will develop a clinically significant reaction or that predict the severity of reaction. Although the tests are not traditionally part of routine clinical evaluation, the degree of elevation of baseline LTE4 and PGD2 in the urine is known to be associated with the severity of respiratory reactions and the extent to which FEV1 falls during aspirin-induced reactions.4,49,117 From a clinical standpoint, duration of challenge,118 the current state of the sinuses (preoperative vs postoperative),70,71 and pretreatment with leukotriene modifiers,66,86 antihistamines,91 misoprostol,119 and mast cell stabilizers such as cromolyn sodium120 and presumably ketotifen can all impact the severity of symptoms observed during a desensitization, and rarely can completely block both upper and lower airway symptoms from developing. If an aspirin challenge performed on 1 of these medications is negative, and the diagnosis of AERD is still unclear, there may be benefit in repeating the procedure off these medications, assuming asthma and rhinitis control remain stable, as discussed below.
There are various protocols used for challenges as well as combinations of challenges with desensitization, all of which can have an effect on the level of reactivity to aspirin.79,92,103,105 Younger age and shorter duration of AERD have been associated with more severe reactions to aspirin challenge.81,117 An FEV1 less than 80% predicted, no LTMD use at the time of the challenge, and a history of asthma exacerbations that require emergency room visits have all been associated with an increased likelihood of positive reactions.80,81 Importantly, however, a history of severe reactions to NSAIDs was not predictive of the reaction severity during office challenges.79,83,121
Treatment options for aspirin-induced reactions
The selection of treatment options during aspirin-induced reactions depends on the symptoms reported by patient and clinical observation (Table VI). Nasal congestion can be treated with intranasal antihistamines, intranasal corticosteroids, nasal decongestants, and/or oral antihistamines. Ocular reactions may be treated with topical antihistamines. Laryngeal reactions can be treated with nebulized racemic epinephrine or intramuscular epinephrine. Respiratory reactions with shortness of breath and wheezing can be treated with nebulized or meter-dose inhaled short-acting beta agonists, short-acting anticholinergics, short-acting beta-agonist/anticholinergic combination therapy, or intramuscular epinephrine. Gastrointestinal symptoms can be treated with antiemetics, PPIs, and histamine 2 receptor antagonists, though zileuton, if available, has been found to be particularly efficacious in preventing or treating the aspirin-induced gastrointestinal symptoms.66 Urticaria and angioedema can be treated with histamine 1 receptor antagonists. Systemic reactions should be treated with intramuscular epinephrine, and very occasionally intravenous fluids are needed.
TABLE VI.
Recommended pharmacologic treatment options for managing reactions induced during an aspirin desensitization in patients with AERD
| Type of reaction | Pharmacologic options |
|---|---|
| Ocular | HlR antagonists (ocular, oral) |
| Nasal | Decongestants (intranasal) H1R antagonists (intranasal, oral) Corticosteroids (intranasal) |
| Laryngeal | Racemic epinephrine (inhaled) Epinephrine (intramuscular) |
| Respiratory | β2-Agonists (inhaled) Anticholinergics (inhaled) Corticosteroids (oral) |
| Gastrointestinal | Antiemetics (oral) H2R antagonists (oral) PPIs (oral) Epinephrine (intramuscular) |
| Skin | H1R antagonists (oral) |
| Systemic | Epinephrine (intramuscular) |
H1R, Histamine 1 receptor.
PROTOCOL MODIFICATIONS FOR ASPIRIN-INDUCED REACTIONS
Regardless of the protocol, after a clinical reaction has developed, the patient should be treated and observed for a 3-hour period to ensure complete resolution of symptoms. Following symptom resolution, the provocative dose of aspirin (or ketorolac) should be repeated before continuing with the next-higher dose during a desensitization. Most patients have achieved a desensitized state once they have tolerated both the repeated administration of the provocative dose and an additional higher dose without developing further symptoms.92
ASPIRIN CHALLENGE VERSUS ASPIRIN DESENSITIZATION
Aspirin challenges may be indicated to confirm whether a patient truly has AERD. Aspirin desensitizations are performed such that a patient may be started on daily maintenance aspirin therapy. The protocol used for either an aspirin challenge or a desensitization is the same. In addition, if the patient has AERD, it is expected that they will develop a clinical reaction during the procedure. Following the development of a reaction in both an aspirin challenge and desensitization, the patient should be treated and monitored for at least 3 hours until their symptoms have resolved. The important difference between a challenge and desensitization is what occurs after the patient’s clinical reaction has resolved. In a challenge, the protocol is discontinued and the patient is discharged. In a desensitization, the provocative aspirin dose is repeated and the protocol is continued through the 325-mg dose of aspirin as described above.
ORAL ASPIRIN THERAPY FOLLOWING ASPIRIN DESENSITIZATION
Maintenance of tolerance to aspirin
Following aspirin desensitization, patients are cross-desensitized to nonselective COX inhibitors as long as they continue on aspirin 325 mg daily or higher doses.122 Patients can also change from one COX inhibitor to another without losing the desensitized state.26 If patients discontinue aspirin treatment, their NSAID tolerance may continue for several days. However, in one study, repeating intranasal aspirin-lysine challenges 24 hours after the initial challenge resulted in positive reactions in 98% of the patients.82 Therefore, if a desensitized patient misses more than 2 days of aspirin it is strongly recommended they be evaluated for repeat desensitization before resuming therapy.122
Dose of aspirin therapy
After patients have been desensitized to aspirin, they should continue taking aspirin daily. A uniform consensus is lacking on the exact daily dose of aspirin that should be offered to the patients. However, several groups agree that the aspirin dose should be at least 300 mg and possibly 650 mg or 1300 mg of aspirin daily. 12,25,26,31,32,37 It is recommended to start with aspirin 650 mg twice daily for 1 to 6 months. Patients who are doing well can then reduce the dose to 325 mg twice or once daily to see whether they maintain benefit.12,26 If symptoms worsen on 325 mg once or twice a day, it is then recommended to increase the dose back to 650 mg twice daily.26
For doses less than 325 mg twice daily, one study showed that dose reduction less than 325 mg twice daily led to recurrence of nasal congestion.36 Another study looking at 100 mg versus 300 mg daily showed no improvement in symptoms on the lower dose (100 mg daily) and recurrence of nasal polyposis. Subjects on 300 mg daily had decrease in nasal polyposis, and of 35 patients who continued taking 300 mg aspirin daily, none required a repeat sinus surgery during a median follow-up for 27 months.31 Therefore, doses of 300 mg of daily aspirin or greater may be helpful for sinonasal symptoms, but doses lower than 300 mg may not provide clinical benefit.31,32
Duration of aspirin treatment
It is recommended to attempt an initial trial period of 6 months on aspirin treatment to determine whether the patient notes clinical improvement. For patients who respond to aspirin, we recommend continuing aspirin indefinitely.14,123 For patients who show no signs of clinical benefit, high-dose aspirin may be discontinued after 6 months. However, the patient may elect to maintain aspirin tolerance by taking 325 mg of aspirin daily, be it for cardio-protection or freedom to use other NSAIDs as needed. Aspirin doses lower than 325 mg daily may not provide crosstolerance to other NSAIDs.
Aspirin should be discontinued at any time if the patient is having significant side effects despite medical management. For those patients who discontinue aspirin therapy, they should immediately resume avoiding all COX-1 inhibitors. In these patients, alternative medical management options should be reviewed. In aggressive recurrent nasal polyposis, referral for an evaluation by a rhinologist should be considered. Antecedent endoscopic sinus surgery performed 3 to 4 weeks before desensitization improves aspirin treatment response in patients with AERD and may convert patients who failed initial aspirin treatment to a more responsive phenotype.29
Patients on aspirin therapy who require surgery are presented with 2 options. First, the aspirin can be completely discontinued 2 weeks before the procedure. In this situation, aspirin cannot be restarted after the procedure unless done with a desensitization. A second option is to lower the dose of aspirin to 325 mg once daily. Aspirin can then be held the day before the procedure and restarted in the evening after the procedure. This allows aspirin to be restarted within the 48-hour refractory period. The decision on which protocol to use is based on surgical considerations and is usually made by the surgeon. Many minor procedures such as a colonoscopy can be done on daily aspirin. A small case series suggests that stopping aspirin and bridging with ibuprofen might be another option to maintain desensitization but will limit the antiplatelet effect of aspirin.124
Currently, there are no data on alternative dose escalation protocols following aspirin withdrawal nor on safety regarding bleeding complications with this approach. Alternative approaches to this question merit further study.
Risks and side effects
There are a number of risks and side effects associated with maintenance aspirin therapy that can generally be mitigated with appropriate patient selection. Reports suggest that discontinuation of aspirin is frequently due to the gastrointestinal side effects, nasal or ear bleeding, and skin rash induced by aspirin.12,37 There are patients who discontinue aspirin treatment because of the lack of effectiveness or worsening of the upper or lower respiratory tract symptoms.33,125
Gastrointestinal toxicity is the most common reason for discontinuation of aspirin, most likely due to gastritis. 12,37,125 Clinicians should ensure careful review of patient risk factors for gastroduodenal toxicity from aspirin/NSAIDs before initiating high-dose aspirin.126,127 For patients at a higher risk of gastrointestinal toxicity or those who develop gastrointestinal side effects after starting high-dose aspirin, adding a PPI or sucralfate can be beneficial. 128–130 In patients with persistent gastrointestinal symptoms following aspirin desensitization on high-dose PPI, clinicians should consider whether or not they may benefit from an esophagogastroduodenoscopy.
Risk of bleeding has not specifically been studied in AERD.131 In the general population, bleeding secondary to aspirin most commonly occurs in the gastrointestinal tract. Aspirin is associated with approximately a 50% increased risk of gastrointestinal bleeding in patients on aspirin for primary prevention of cardiovascular disease.132,133 Intracerebral hemorrhage is very rare in the general population.134 In patients on aspirin for primary prevention of cardiovascular or cerebrovascular disease, the risk of hemorrhagic stroke is very low.135,136 However, studies examining the bleeding risk while taking high doses of aspirin are warranted.
Peripheral eosinophilia frequently occurs in patients with AERD on high-dose aspirin.4,33 Generally, patients are asymptomatic and can be monitored safely. Coronary artery vasospasm, a rare but clinically important side effect, has been described in patients with AERD before and following aspirin desensitization. Vasospasm is responsive to oral corticosteroids and is presumed to be eosinophil related.137 Esophageal eosinophilia has also been described after aspirin desensitization.77,138
Approach to silent desensitizations
A clinical dilemma can exist if a patient with an excellent history and sinonasal polyposis does not “react” during the desensitization despite the fact that clinical criteria for AERD are met. One strategy that is frequently used in a patient who undergoes a nonreactive challenge would be to assume that they do have AERD and experienced a “silent desensitization”139 related to the use of LTMDs during the desensitization or recent FESS. Subsequently, a trial of aspirin therapy would be initiated and if the patient responded well, aspirin would be continued. However, because multiple interventions are likely taking place at the same time, it may be very difficult to tell whether the patient truly has AERD by this method, and it is unknown whether to recommend that they remain on long-term high-dose aspirin. Certainly, it would be undesirable to have a patient without AERD continue on a treatment that has potential adverse effects without confirmation of true benefit.
An alternative approach to the patient with no symptoms during desensitization would be to have them return later for a full 1-dose 325 mg oral aspirin confirmatory challenge in a closely monitored and equipped outpatient setting with 3 hours of observation (Fig 1). Aspirin must be washed out for 2 weeks before rechallenge, and antihistamines, LTMDs, cromolyn, and ketotifen are held for 7 days before this confirmatory challenge. Oral steroids are either discontinued or weaned down to the lowest dose needed to control the patient’s asthma. Once this occurs, patients who react to the open full-dose challenge are confirmed to have had a silent desensitization, whereas those who do not react (double-negatives) are truly not patients with AERD and will not obtain benefit from aspirin therapy nor do they need to be labeled as “NSAID allergic.”139
APPROACH TO DIFFICULT DESENSITIZATIONS
Aspirin desensitization is widely regarded as safe. Yet, more difficult scenarios need to be prepared for in all situations. It is clear that the reaction to aspirin is systemic.4,49,55,116 In a large series of 167 consecutive desensitizations, 23 (13.7%) were classified as severe83 including having a gastrointestinal reaction, a decline in FEV1 more than 30%, and/or requiring injectable epinephrine or 3 or more doses of β2-agonist. Such patients can have reactions longer than anticipated and require a higher degree of nursing and physician attention during the desensitization.
In patients with a gastrointestinal reaction, the severity of these symptoms often eclipses the respiratory symptoms. Unfortunately, gastrointestinal reactions are not easy to abort. Treatment with histamine 1 receptor and histamine 2 receptor antagonists, ondansetron, and IV fluids should be considered. In a study of 45 patients with AERD undergoing aspirin desensitization or challenge, the addition of misoprostol during the procedure was not shown to reduce the intensity of overall symptoms.140 However, it is still possible that misoprostol may provide some clinical benefit in a select patient population and could be considered under certain circumstances but the side effects must be discussed with the patient before initiation. Injectable epinephrine may be required and should be considered in the treatment of moderate to severe gastrointestinal anaphylaxis.141 Of note, in a small study, recent endoscopic sinus surgery appears to lessen the severity of gastrointestinal symptoms during desensitization.29 In patients with significant reactions, using a slower paced protocol could also successfully achieve full desensitization.
Another subgroup of severe reactors manifests prominent laryngeal symptoms. The presentation includes shortness of breath, but stridor can frequently be heard over the pharynx. These patients may or may not have significant drops in FEV1 and concomitant lower airway involvement. Although short-acting β2-agonists can relieve bronchospasm, the addition of racemic epinephrine nebulization can abort the laryngeal symptoms.
Acute pancreatitis has been reported in several situations as an ensuing complication during or immediately following aspirin desensitization. 142,143 This does present a problem in the acute setting of delineating a typical gastrointestinal reaction from the rare evolving pancreatitis. Yet, given the cases reported in the literature, in any patient with persistent epigastric pain or symptoms refractory to standard therapy, pancreatitis should be considered. The mechanism for this phenomenon is unknown.
NEED OF FURTHER RESEARCH
Although the clinical utility of ATAD in patients with AERD has been appreciated for decades, there remain many unanswered questions especially in regard to the mechanisms by which daily treatment with aspirin, but not other COX-1 inhibitors, provides clinical benefit. In addition, there are no clinically validated metrics that predict in advance which patients with AERD will respond best to ATAD. Furthermore, subphenotypes of AERD have been described,144 and further work is necessary to determine whether a particular phenotype (or endotype) may preferentially respond to ATAD. Finally, in the era of novel biologics being developed for asthma and CRSwNP, consensus is still needed among physicians as to when to initiate these therapeutics versus ATAD in the management of patients with AERD.
Conclusions
Since it was first described in the early 1980s, ATAD has held an unusual status in the field of allergy. It may be considered too risky to perform in some practices and at times has been controversial and obscure. It is unequivocal that patients with AERD represent some of the most severe asthmatic and nasal polyp sufferers. Both blinded and longitudinal studies consistently show benefit of ATAD, and there is a wide breadth of data and clinical experience on the safety of both the desensitization and the maintenance aspirin therapy. Although not all patients with AERD will respond, ATAD has the potential to improve upper and lower respiratory tract symptoms, prevent or delay sinus surgery, and enhance quality of life in most affected patients. Selecting the appropriate patient for aspirin desensitization/maintenance therapy is essential, and diagnostic aspirin challenges to confirm the diagnosis of AERD should be considered in patients with an uncertain clinical history. In addition, ATAD can be a highly economical treatment option when compared with both frequent sinus surgeries and the new array of biologics that are now available for nasal polyposis and severe type 2 asthma. In conclusion, aspirin desensitization followed by maintenance aspirin therapy is a unique treatment option that should be considered in all eligible patients with AERD as a means to improve clinical outcomes and delay or prevent future sinus surgery.
AAAAI Position Statements, Work Group Reports, and Systematic Reviews are not to be considered to reflect current AAAAI standards or policy after five years from the date of publication. The statement below is not to be construed as dictating an exclusive course of action nor is it intended to replace the medical judgment of healthcare professionals. The unique circumstances of individual patients and environments are to be taken into account in any diagnosis and treatment plan. The statement reflects clinical and scientific advances as of the date of publication and is subject to change.
For reference only.
Acknowledgments
Disclosure of potential conflict of interest: W. W. Stevens served on scientific advisory boards for GlaxoSmithKline, Genentech, and Bristol Myers Squibb. E. Jerschow has served on scientific advisory boards for GlaxoSmithKline, Sanofi/Regeneron, and Novartis/Genentech; is a consultant for GlaxoSmithKline, received a research grant from AstraZeneca and Cumberland; and is a National Board of Medical Examiners/United States Medical Licensing Exam committee member. A. P. Baptist reports grant support from AstraZeneca and Novartis. J. V. Bosso has served on scientific advisory boards for GlaxoSmithKline, Sanofi/Regeneron, Novartis, AstraZeneca, and Optinose. K. M. Buchheit has served on scientific advisory boards for Regeneron, Genentech, AstraZeneca, and GlaxoSmithKline. K. N. Cahill has served on scientific advisory boards for Novartis, Regeneron, Teva, GlaxoSmithKline, and Blueprint Medicines. S. H. Cho served on an advisory board for ALK. J. M. Levy has served on scientific advisory boards for AstraZeneca and Regeneron. T. M. Laidlaw has served on scientific advisory boards for GlaxoSmithKline, Sanofi-Genzyme, Optinose, and Regeneron. A. A. White served on speakers bureau for AstraZeneca, Regeneron/Sanofi, and Optinose; on advisory boards for Genentech, Regeneron, and Optinose; received research support from AstraZeneca, and is on the board of directors for the Western Society of Allergy, Asthma, and Immunology. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations used
- AERD
Aspirin-exacerbated respiratory disease
- ATAD
Aspirin therapy after desensitization
- COX
Cycloxgenase
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- CysLT
Cysteinyl leukotriene
- FESS
Functional endoscopic sinus surgery
- ICS
Inhaled corticosteroid
- IV
Intravenous
- LTMD
Leukotriene-modifying drug
- LTRA
Leukotriene receptor antagonist
- PGD2
Prostaglandin D2
- PGE2
Prostaglandin E2
- PPI
Proton-pump inhibitor
- NSAID
Nonsteroidal anti-inflammatory drug
- 5-LO
5-lipoxygenase
REFERENCES
- 1.Roca-Ferrer J, Garcia-Garcia FJ, Pereda J, Perez-Gonzalez M, Pujols L, Alobid I, et al. Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. J Allergy Clin Immunol 2011;128:66–72.e1. [DOI] [PubMed] [Google Scholar]
- 2.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis 1991;143:1025–9. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Novo CA, Watelet JB, Claeys C, Van Cauwenberge P, Bachert C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol 2005;115:1189–96. [DOI] [PubMed] [Google Scholar]
- 4.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2015;135:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol 2015;135:676–81.e1. [DOI] [PubMed] [Google Scholar]
- 6.Stevens WW, Peters AT, Hirsch AG, Nordberg CM, Schwartz BS, Mercer DG, et al. Clinical characteristics of patients with chronic rhinosinusitis with nasal polyps, asthma, and aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract 2017;5:1061–70.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahill KN, Johns CB, Cui J, Wickner P, Bates DW, Laidlaw TM, et al. Automated identification of an aspirin-exacerbated respiratory disease cohort. J Allergy Clin Immunol 2017;139:819–25.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters AT, Spector S, Hsu J, Hamilos DL, Baroody FM, Chandra RK, et al. Diagnosis and management of rhinosinusitis: a practice parameter update. Ann Allergy Asthma Immunol 2014;113:347–85. [DOI] [PubMed] [Google Scholar]
- 9.Bachert C, Hellings PW, Mullol J, Naclerio RM, Chao J, Amin N, et al. Dupilumab improves patient-reported outcomes in patients with chronic rhinosinusitis with nasal polyps and comorbid asthma. J Allergy Clin Immunol Pract 2019;7: 2447–9.e2. [DOI] [PubMed] [Google Scholar]
- 10.Mendelsohn D, Jeremic G, Wright ED, Rotenberg BW. Revision rates after endoscopic sinus surgery: a recurrence analysis. Ann Otol Rhinol Laryngol 2011;120: 162–6. [DOI] [PubMed] [Google Scholar]
- 11.Young J, Frenkiel S, Tewfik MA, Mouadeb DA. Long-term outcome analysis of endoscopic sinus surgery for chronic sinusitis. Am J Rhinol 2007;21:743–7. [DOI] [PubMed] [Google Scholar]
- 12.Berges-Gimeno MP, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2003;111:180–6. [DOI] [PubMed] [Google Scholar]
- 13.Havel M, Ertl L, Braunschweig F, Markmann S, Leunig A, Gamarra F, et al. Sinonasal outcome under aspirin desensitization following functional endoscopic sinus surgery in patients with aspirin triad. Eur Arch Otorhinolaryngol 2013;270:571–8. [DOI] [PubMed] [Google Scholar]
- 14.Walters KM, Waldram JD, Woessner KM, White AA. Long-term clinical outcomes of aspirin desensitization with continuous daily aspirin therapy in aspirin-exacerbated respiratory disease. Am J Rhinol Allergy 2018;32:280–6. [DOI] [PubMed] [Google Scholar]
- 15.White AA, Stevenson DD. Aspirin-exacerbated respiratory disease. N Engl J Med 2018;379:2281–2. [DOI] [PubMed] [Google Scholar]
- 16.Becker RC, Burns M, Gore JM, Spencer FA, Ball SP, French W, et al. Early assessment and in-hospital management of patients with acute myocardial infarction at increased risk for adverse outcomes: a nationwide perspective of current clinical practice. The National Registry of Myocardial Infarction (NRMI-2) Participants. Am Heart J 1998;135:786–96. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski ML, Agache I, Bavbek S, Bakirtas A, Blanca M, Bochenek G, et al. Diagnosis and management of NSAID-exacerbated respiratory disease (N-ERD)—a EAACI position paper. Allergy 2019;74:28–39. [DOI] [PubMed] [Google Scholar]
- 18.Widal F, Abraimi P, Lermoyex J. Anaphylaxie et idiosyncrasie. Presse Med 1922;30:189–93. [Google Scholar]
- 19.Klion A Widal on the aspirin triad and induction of tolerance. Allergy Asthma Proc 1993;14:371–2. [Google Scholar]
- 20.Treasure hunt: pertinent excerpts from past literature. J Asthma 1987;24:297–300.3327855 [Google Scholar]
- 21.Zeiss CR, Lockey RF. Refractory period to aspirin in a patient with aspirin-induced asthma. J Allergy Clin Immunol 1976;57:440–8. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson DD, Simon RA, Mathison DA. Aspirin-sensitive asthma: tolerance to aspirin after positive oral aspirin challenges. J Allergy Clin Immunol 1980;66:82–8. [DOI] [PubMed] [Google Scholar]
- 23.Lumry WR, Curd JG, Zeiger RS, Pleskow WW, Stevenson DD. Aspirin-sensitive rhinosinusitis: the clinical syndrome and effects of aspirin administration. J Allergy Clin Immunol 1983;71:580–7. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson DD, Hankammer MA, Mathison DA, Christiansen SC, Simon RA. Aspirin desensitization treatment of aspirin-sensitive patients with rhinosinusitis-asthma: long-term outcomes. J Allergy Clin Immunol 1996;98:751–8. [DOI] [PubMed] [Google Scholar]
- 25.Berges-Gimeno MP, Simon RA, Stevenson DD. Early effects of aspirin desensitization treatment in asthmatic patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 2003;90:338–41. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Simon RA, Stevenson DD. Selection of aspirin dosages for aspirin desensitization treatment in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2007;119:157–64. [DOI] [PubMed] [Google Scholar]
- 27.Cho KS, Soudry E, Psaltis AJ, Nadeau KC, McGhee SA, Nayak JV, et al. Long-term sinonasal outcomes of aspirin desensitization in aspirin exacerbated respiratory disease. Otolaryngol Head Neck Surg 2014;151:575–81. [DOI] [PubMed] [Google Scholar]
- 28.Adappa ND, Ranasinghe VJ, Trope M, Brooks SG, Glicksman JT, Parasher AK, et al. Outcomes after complete endoscopic sinus surgery and aspirin desensitization in aspirin-exacerbated respiratory disease. Int Forum Allergy Rhinol 2018;8:49–53. [DOI] [PubMed] [Google Scholar]
- 29.Shah S, Ponduri A, Pelletier T, Ren Z, Keskin T, Roizen G, et al. Endoscopic sinus surgery improves aspirin treatment response in AERD patients. Int Forum Allergy Rhinol 2019;9:1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweet JM, Stevenson DD, Simon RA, Mathison DA. Long-term effects of aspirin desensitization–treatment for aspirin-sensitive rhinosinusitis-asthma. J Allergy Clin Immunol 1990;85:59–65. [DOI] [PubMed] [Google Scholar]
- 31.Rozsasi A, Polzehl D, Deutschle T, Smith E, Wiesmiller K, Riechelmann H, et al. Long-term treatment with aspirin desensitization: a prospective clinical trial comparing 100 and 300 mg aspirin daily. Allergy 2008;63:1228–34. [DOI] [PubMed] [Google Scholar]
- 32.Comert S, Celebioglu E, Yucel T, Erdogan T, Karakaya G, Onerci M, et al. Aspirin 300 mg/day is effective for treating aspirin-exacerbated respiratory disease. Allergy 2013;68:1443–51. [DOI] [PubMed] [Google Scholar]
- 33.Jerschow E, Edin ML, Pelletier T, Abuzeid WM, Akbar NA, Gibber M, et al. Plasma 15-hydroxyeicosatetraenoic acid predicts treatment outcomes in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract 2017;5:998–1007.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMains KC, Kountakis SE. Medical and surgical considerations in patients with Samter’s triad. Am J Rhinol 2006;20:573–6. [DOI] [PubMed] [Google Scholar]
- 35.Levy JM, Rudmik L, Peters AT, Wise SK, Rotenberg BW, Smith TL. Contemporary management of chronic rhinosinusitis with nasal polyposis in aspirin-exacerbated respiratory disease: an evidence-based review with recommendations. Int Forum Allergy Rhinol 2016;6:1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson DD, Pleskow WW, Simon RA, Mathison DA, Lumry WR, Schatz M, et al. Aspirin-sensitive rhinosinusitis asthma: a double-blind crossover study of treatment with aspirin. J Allergy Clin Immunol 1984;73:500–7. [DOI] [PubMed] [Google Scholar]
- 37.Swierczynska-Krepa M, Sanak M, Bochenek G, Strek P, Cmiel A, Gielicz A, et al. Aspirin desensitization in patients with aspirin-induced and aspirin-tolerant asthma: a double-blind study. J Allergy Clin Immunol 2014;134:883–90. [DOI] [PubMed] [Google Scholar]
- 38.Esmaeilzadeh H, Nabavi M, Aryan Z, Arshi S, Bemanian MH, Fallahpour M, et al. Aspirin desensitization for patients with aspirin-exacerbated respiratory disease: a randomized double-blind placebo-controlled trial. Clin Immunol 2015;160:349–57. [DOI] [PubMed] [Google Scholar]
- 39.Fruth K, Pogorzelski B, Schmidtmann I, Springer J, Fennan N, Fraessdorf N, et al. Low-dose aspirin desensitization in individuals with aspirin-exacerbated respiratory disease. Allergy 2013;68:659–65. [DOI] [PubMed] [Google Scholar]
- 40.Mortazavi N, Esmaeilzadeh H, Abbasinazari M, Babaie D, Alyasin S, Nabavizadeh H, et al. Clinical and immunological efficacy of aspirin desensitization in nasal polyp patients with aspirin-exacerbated respiratory disease. Iran J Pharm Res 2017;16:1639–47. [PMC free article] [PubMed] [Google Scholar]
- 41.Chu DK, Lee DJ, Lee KM, Schunemann HJ, Szczeklik W, Lee JM. Benefits and harms of aspirin desensitization for aspirin-exacerbated respiratory disease: a systematic review and meta-analysis. Int Forum Allergy Rhinol 2019;9:1409–19. [DOI] [PubMed] [Google Scholar]
- 42.Nasser SM, Patel M, Bell GS, Lee TH. The effect of aspirin desensitization on urinary leukotriene E4 concentrations in aspirin-sensitive asthma. Am J Respir Crit Care Med 1995;151:1326–30. [DOI] [PubMed] [Google Scholar]
- 43.Bobolea I, Del Pozo V, Sanz V, Cabanas R, Fiandor A, Alfonso-Carrillo C, et al. Aspirin desensitization in aspirin-exacerbated respiratory disease: new insights into the molecular mechanisms. Respir Med 2018;143:39–41. [DOI] [PubMed] [Google Scholar]
- 44.Cahill KN, Cui J, Kothari P, Murphy K, Raby BA, Singer J, et al. Unique effect of aspirin therapy on biomarkers in aspirin-exacerbated respiratory disease: a prospective trial. Am J Respir Crit Care Med 2019;200:704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med 2002;347:1493–9. [DOI] [PubMed] [Google Scholar]
- 46.Arm JP, O’Hickey SP, Spur BW, Lee TH. Airway responsiveness to histamine and leukotriene E4 in subjects with aspirin-induced asthma. Am Rev Respir Dis 1989;140:148–53. [DOI] [PubMed] [Google Scholar]
- 47.Kanaoka Y, Maekawa A, Austen KF. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem 2013;288:10967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christie PE, Schmitz-Schumann M, Spur BW, Lee TH. Airway responsiveness to leukotriene C4 (LTC4), leukotriene E4 (LTE4) and histamine in aspirin-sensitive asthmatic subjects. Eur Respir J 1993;6:1468–73. [PubMed] [Google Scholar]
- 49.Daffern PJ, Muilenburg D, Hugli TE, Stevenson DD. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of respiratory responses. J Allergy Clin Immunol 1999;104:559–64. [DOI] [PubMed] [Google Scholar]
- 50.Cianferoni A, Schroeder JT, Kim J, Schmidt JW, Lichtenstein LM, Georas SN, et al. Selective inhibition of interleukin-4 gene expression in human T cells by aspirin. Blood 2001;97:1742–9. [DOI] [PubMed] [Google Scholar]
- 51.Steinke JW, Culp JA, Kropf E, Borish L. Modulation by aspirin of nuclear phospho-signal transducer and activator of transcription 6 expression: possible role in therapeutic benefit associated with aspirin desensitization. J Allergy Clin Immunol 2009;124:724–30.e4. [DOI] [PubMed] [Google Scholar]
- 52.Katial RK, Strand M, Prasertsuntarasai T, Leung R, Zheng W, Alam R. The effect of aspirin desensitization on novel biomarkers in aspirin-exacerbated respiratory diseases. J Allergy Clin Immunol 2010;126:738–44. [DOI] [PubMed] [Google Scholar]
- 53.Steinke JW, Payne SC, Borish L. Interleukin-4 in the generation of the AERD phenotype: implications for molecular mechanisms driving therapeutic benefit of aspirin desensitization. J Allergy (Cairo) 2012;2012:182090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsieh FH, Lam BK, Penrose JF, Austen KF, Boyce JA. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C(4) synthase expression by interleukin 4. J Exp Med 2001;193:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cahill KN, Murphy K, Singer J, Israel E, Boyce JA, Laidlaw TM. Plasma tryptase elevation during aspirin-induced reactions in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2019;143:799–803.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng X, Ramsden MK, Negri J, Baker MG, Payne SC, Borish L, et al. Eosinophil production of prostaglandin D2 in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2016;138:1089–97.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto M, Okano M, Fujiwara T, Kariya S, Higaki T, Nagatsuka H, et al. Expression and characterization of PGD2 receptors in chronic rhinosinusitis: modulation of DP and CRTH2 by PGD2. Int Arch Allergy Immunol 2009;148:127–36. [DOI] [PubMed] [Google Scholar]
- 58.Laidlaw TM, Boyce JA. Aspirin-exacerbated respiratory disease-new prime suspects. N Engl J Med 2016;374:484–8. [DOI] [PubMed] [Google Scholar]
- 59.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha, 11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol 2003;111:743–9. [DOI] [PubMed] [Google Scholar]
- 60.Laidlaw TM, Cutler AJ, Kidder MS, Liu T, Cardet JC, Chhay H, et al. Prostaglandin E2 resistance in granulocytes from patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2014;133:1692–701.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machado-Carvalho L, Martin M, Torres R, Gabasa M, Alobid I, Mullol J, et al. Low E-prostanoid 2 receptor levels and deficient induction of the IL-1beta/IL-1type I receptor/COX-2 pathway: vicious circle in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2016;137:99–107.e7. [DOI] [PubMed] [Google Scholar]
- 62.Machado-Carvalho L, Torres R, Perez-Gonzalez M, Alobid I, Mullol J, Pujols L, et al. Altered expression and signalling of EP2 receptor in nasal polyps of AERD patients: role in inflammation and remodelling. Rhinology 2016;54:254–65. [DOI] [PubMed] [Google Scholar]
- 63.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood 2012;119:3790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J 2000;16:432–6. [DOI] [PubMed] [Google Scholar]
- 65.Samter M, Beers RF Jr. Intolerance to aspirin: clinical studies and consideration of its pathogenesis. Ann Intern Med 1968;68:975–83. [DOI] [PubMed] [Google Scholar]
- 66.Israel E, Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Shapiro J, et al. The pivotal role of 5-lipoxygenase products in the reaction of aspirin-sensitive asthmatics to aspirin. Am Rev Respir Dis 1993;148:1447–51. [DOI] [PubMed] [Google Scholar]
- 67.Stevenson DD, Simon RA, Mathison DA, Christiansen SC. Montelukast is only partially effective in inhibiting aspirin responses in aspirin-sensitive asthmatics. Ann Allergy Asthma Immunol 2000;85:477–82. [DOI] [PubMed] [Google Scholar]
- 68.Feldman JM, Zeigler AE, Nelson K, Morales-Raveendran E, Pelletier T, Roizen G, et al. Depression symptoms and quality of life among individuals with aspirin-exacerbated respiratory disease. J Asthma 2019;56:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee-Sarwar K, Johns C, Laidlaw TM, Cahill KN. Tolerance of daily low-dose aspirin does not preclude aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract 2015;3:449–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jerschow E, Edin ML, Chi Y, Hurst B, Abuzeid WM, Akbar NA, et al. Sinus surgery is associated with a decrease in aspirin-induced reaction severity in patients with aspirin exacerbated respiratory disease. J Allergy Clin Immunol Pract 2019;7:1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang GX, Palumbo ML, Singer JI, Cahill KN, Laidlaw TM. Sinus surgery improves lower respiratory tract reactivity during aspirin desensitization for AERD. J Allergy Clin Immunol Pract 2019;7:1647–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017;377:613–22. [DOI] [PubMed] [Google Scholar]
- 73.Baschat AA, Dewberry D, Seravalli V, Miller JL, Block-Abraham D, Blitzer MG. Maternal blood-pressure trends throughout pregnancy and development of pre-eclampsia in women receiving first-trimester aspirin prophylaxis. Ultrasound Obstet Gynecol 2018;52:728–33. [DOI] [PubMed] [Google Scholar]
- 74.James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv 2008;63:49–57. [DOI] [PubMed] [Google Scholar]
- 75.Heymann MA, Rudolph AM, Silverman NH. Closure of the ductus arteriosus in premature infants by inhibition of prostaglandin synthesis. N Engl J Med 1976;295:530–3. [DOI] [PubMed] [Google Scholar]
- 76.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012;107:345–60, quiz 61. [DOI] [PubMed] [Google Scholar]
- 77.Eid RC, Palumbo ML, Laidlaw TM, Buchheit KM, Cahill KN. A retrospective analysis of esophageal eosinophilia in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract 2019;7:1338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Belay ED, Bresee JS, Holman RC, Khan AS, Shahriari A, Schonberger LB. Reye’s syndrome in the United States from 1981 through 1997. N Engl J Med 1999;340:1377–82. [DOI] [PubMed] [Google Scholar]
- 79.Chen JR, Buchmiller BL, Khan DA. An hourly dose-escalation desensitization protocol for aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract 2015;3:926–31.e1. [DOI] [PubMed] [Google Scholar]
- 80.Dursun AB, Woessner KA, Simon RA, Karasoy D, Stevenson DD. Predicting outcomes of oral aspirin challenges in patients with asthma, nasal polyps, and chronic sinusitis. Ann Allergy Asthma Immunol 2008;100:420–5. [DOI] [PubMed] [Google Scholar]
- 81.Hope AP, Woessner KA, Simon RA, Stevenson DD. Rational approach to aspirin dosing during oral challenges and desensitization of patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2009;123:406–10. [DOI] [PubMed] [Google Scholar]
- 82.Miller B, Mirakian R, Gane S, Larco J, Sannah AA, Darby Y, et al. Nasal lysine aspirin challenge in the diagnosis of aspirin-exacerbated respiratory disease: asthma and rhinitis. Clin Exp Allergy 2013;43:874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waldram J, Walters K, Simon R, Woessner K, Waalen J, White A. Safety and outcomes of aspirin desensitization for aspirin-exacerbated respiratory disease: a single-center study. J Allergy Clin Immunol 2018;141:250–6. [DOI] [PubMed] [Google Scholar]
- 84.Shaker M, Lobb A, Jenkins P, O’Rourke D, Takemoto SK, Sheth S, et al. An economic analysis of aspirin desensitization in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2008;121:81–7. [DOI] [PubMed] [Google Scholar]
- 85.Anderson WC III, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol 2019;122:367–72. [DOI] [PubMed] [Google Scholar]
- 86.White AA, Stevenson DD, Simon RA. The blocking effect of essential controller medications during aspirin challenges in patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 2005;95:330–5. [DOI] [PubMed] [Google Scholar]
- 87.Lee DK, Haggart K, Robb FM, Lipworth BJ. Montelukast protects against nasal lysine-aspirin challenge in patients with aspirin-induced asthma. Eur Respir J 2004;24:226–30. [DOI] [PubMed] [Google Scholar]
- 88.Berges-Gimeno MP, Simon RA, Stevenson DD. The effect of leukotriene-modifier drugs on aspirin-induced asthma and rhinitis reactions. Clin Exp Allergy 2002;32:1491–6. [DOI] [PubMed] [Google Scholar]
- 89.Pauls JD, Simon RA, Daffern PJ, Stevenson DD. Lack of effect of the 5-lipoxygenase inhibitor zileuton in blocking oral aspirin challenges in aspirin-sensitive asthmatics. Ann Allergy Asthma Immunol 2000;85:40–5. [DOI] [PubMed] [Google Scholar]
- 90.Waldram JD, Simon RA. Performing aspirin desensitization in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am 2016;36:693–703. [DOI] [PubMed] [Google Scholar]
- 91.Szczeklik A, Serwonska M. Inhibition of idiosyncratic reactions to aspirin in asthmatic patients by clemastine. Thorax 1979;34:654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeGregorio GA, Singer J, Cahill KN, Laidlaw T. A 1-day, 90-minute aspirin challenge and desensitization protocol in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract 2019;7:1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lang DM, Aronica MA, Maierson ES, Wang XF, Vasas DC, Hazen SL. Omalizumab can inhibit respiratory reaction during aspirin desensitization. Ann Allergy Asthma Immunol 2018;121:98–104. [DOI] [PubMed] [Google Scholar]
- 94.Hayashi H, Mitsui C, Nakatani E, Fukutomi Y, Kajiwara K, Watai K, et al. Omalizumab reduces cysteinyl leukotriene and 9alpha, 11beta-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2016;137:1585–7.e4. [DOI] [PubMed] [Google Scholar]
- 95.Phillips-Angles E, Barranco P, Lluch-Bernal M, Dominguez-Ortega J, Lopez-Carrasco V, Quirce S. Aspirin tolerance in patients with nonsteroidal anti-inflammatory drug-exacerbated respiratory disease following treatment with omalizumab. J Allergy Clin Immunol Pract 2017;5:842–5. [DOI] [PubMed] [Google Scholar]
- 96.DeConde AS, Suh JD, Mace JC, Alt JA, Smith TL. Outcomes of complete vs targeted approaches to endoscopic sinus surgery. Int Forum Allergy Rhinol 2015;5: 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morrissey DK, Bassiouni A, Psaltis AJ, Naidoo Y, Wormald PJ. Outcomes of modified endoscopic Lothrop in aspirin-exacerbated respiratory disease with nasal polyposis. Int Forum Allergy Rhinol 2016;6:820–5. [DOI] [PubMed] [Google Scholar]
- 98.Naidoo Y, Bassiouni A, Keen M, Wormald PJ. Risk factors and outcomes for primary, revision, and modified Lothrop (Draf III) frontal sinus surgery. Int Forum Allergy Rhinol 2013;3:412–7. [DOI] [PubMed] [Google Scholar]
- 99.Pelletier T, Roizen G, Ren Z, Hudes G, Rosenstreich D, Jerschow E. Comparable safety of 2 aspirin desensitization protocols for aspirin exacerbated respiratory disease. J Allergy Clin Immunol Pract 2019;7:1319–21. [DOI] [PubMed] [Google Scholar]
- 100.Nassiri M, Babina M, Dolle S, Edenharter G, Rueff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol 2015;135:491–9. [DOI] [PubMed] [Google Scholar]
- 101.White JL, Greger KC, Lee S, Kahoud RJ, Li JT, Lohse CM, et al. Patients taking beta-blockers do not require increased doses of epinephrine for anaphylaxis. J Allergy Clin Immunol Pract 2018;6:1553–8.e1. [DOI] [PubMed] [Google Scholar]
- 102.Nizankowska-Mogilnicka E, Bochenek G, Mastalerz L, Swierczynska M, Picado C, Scadding G, et al. EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy 2007;62:1111–8. [DOI] [PubMed] [Google Scholar]
- 103.Macy E, Bernstein JA, Castells MC, Gawchik SM, Lee TH, Settipane RA, et al. Aspirin challenge and desensitization for aspirin-exacerbated respiratory disease: a practice paper. Ann Allergy Asthma Immunol 2007;98:172–4. [DOI] [PubMed] [Google Scholar]
- 104.Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma, & Immunology, American College of Allergy, Asthma, & Immunology, Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010;105:259–73. [DOI] [PubMed] [Google Scholar]
- 105.Lee RU, White AA, Ding D, Dursun AB, Woessner KM, Simon RA, et al. Use of intranasal ketorolac and modified oral aspirin challenge for desensitization of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 2010;105:130–5. [DOI] [PubMed] [Google Scholar]
- 106.Stevenson DD, Simon RA. Selection of patients for aspirin desensitization treatment. J Allergy Clin Immunol 2006;118:801–4. [DOI] [PubMed] [Google Scholar]
- 107.White A, Bigby T, Stevenson D. Intranasal ketorolac challenge for the diagnosis of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 2006;97:190–5. [DOI] [PubMed] [Google Scholar]
- 108.Patriarca G, Bellioni P, Nucera E, Schiavino D, Papa G, Schinco G, et al. Intranasal treatment with lysine acetylsalicylate in patients with nasal polyposis. Ann Allergy 1991;67:588–92. [PubMed] [Google Scholar]
- 109.Klimek L, Pfaar O. Aspirin intolerance: does desensitization alter the course of the disease? Immunol Allergy Clin North Am 2009;29:669–75. [DOI] [PubMed] [Google Scholar]
- 110.Melillo G, Balzano G, Bianco S, Dahlen B, Godard P, Kowalsky ML, et al. Report of the INTERASMA Working Group on Standardization of Inhalation Provocation Tests in Aspirin-induced Asthma. Oral and inhalation provocation tests for the diagnosis of aspirin-induced asthma. Allergy 2001;56:899–911. [DOI] [PubMed] [Google Scholar]
- 111.Mita H, Higashi N, Taniguchi M, Higashi A, Akiyama K. Increase in urinary leukotriene B4 glucuronide concentration in patients with aspirin-intolerant asthma after intravenous aspirin challenge. Clin Exp Allergy 2004;34:1262–9. [DOI] [PubMed] [Google Scholar]
- 112.Feldman M, Cryer B. Aspirin absorption rates and platelet inhibition times with 325-mg buffered aspirin tablets (chewed or swallowed intact) and with buffered aspirin solution. Am J Cardiol 1999;84:404–9. [DOI] [PubMed] [Google Scholar]
- 113.Schuler CFt, Baldwin JL, Baptist AP. Frequency and severity of reactions to a 325-mg aspirin dose during desensitization. Ann Allergy Asthma Immunol 2017;118:333–8.e1. [DOI] [PubMed] [Google Scholar]
- 114.Laidlaw TM, Cahill KN, Cardet JC, Murphy K, Cui J, Dioneda B, et al. A trial of type 12 purinergic (P2Y12) receptor inhibition with prasugrel identifies a potentially distinct endotype of patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2019;143:316–24.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.White A, Ludington E, Mehra P, Stevenson DD, Simon RA. Effect of leukotriene modifier drugs on the safety of oral aspirin challenges. Ann Allergy Asthma Immunol 2006;97:688–93. [DOI] [PubMed] [Google Scholar]
- 116.Bosso JV, Schwartz LB, Stevenson DD. Tryptase and histamine release during aspirin-induced respiratory reactions. J Allergy Clin Immunol 1991;88:830–7. [DOI] [PubMed] [Google Scholar]
- 117.Jerschow E, Ren Z, Hudes G, Sanak M, Morales E, Schuster V, et al. Utility of low-dose oral aspirin challenges for diagnosis of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 2016;116:321–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kowalski ML, Grzelewska-Rzymowska I, Rozniecki J, Szmidt M. Aspirin tolerance induced in aspirin-sensitive asthmatics. Allergy 1984;39:171–8. [DOI] [PubMed] [Google Scholar]
- 119.Szmidt M, Wasiak W. The influence of misoprostol (synthetic analogue of prostaglandin E1) on aspirin-induced bronchoconstriction in aspirin-sensitive asthma. J Investig Allergol Clin Immunol 1996;6:121–5. [PubMed] [Google Scholar]
- 120.Basomba A, Romar A, Pelaez A, Villalmanzo IG, Campos A. The effect of sodium cromoglycate in preventing aspirin induced bronchospasm. Clin Allergy 1976;6:269–75. [DOI] [PubMed] [Google Scholar]
- 121.Williams AN, Simon RA, Woessner KM, Stevenson DD. The relationship between historical aspirin-induced asthma and severity of asthma induced during oral aspirin challenges. J Allergy Clin Immunol 2007;120:273–7. [DOI] [PubMed] [Google Scholar]
- 122.Pleskow WW, Stevenson DD, Mathison DA, Simon RA, Schatz M, Zeiger RS. Aspirin desensitization in aspirin-sensitive asthmatic patients: clinical manifestations and characterization of the refractory period. J Allergy Clin Immunol 1982;69:11–9. [DOI] [PubMed] [Google Scholar]
- 123.Laidlaw TM, Cahill KN. Current knowledge and management of hypersensitivity to aspirin and NSAIDs. J Allergy Clin Immunol Pract 2017;5:537–45. [DOI] [PubMed] [Google Scholar]
- 124.Do T, Canty E, Bajaj P, Ishmael F, Craig T. Long-term assessment of aspirin desensitization shows successful bridging with non-aspirin nonsteroidal anti-inflammatory drugs for procedures. Allergy Asthma Proc 2019;40:311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ta V, White AA. Survey-defined patient experiences with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract 2015;3:711–8. [DOI] [PubMed] [Google Scholar]
- 126.Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. Risk factors for NSAID-associated upper GI clinical events in a long-term prospective study of 34 701 arthritis patients. Aliment Pharmacol Ther 2010;32:1240–8. [DOI] [PubMed] [Google Scholar]
- 127.Lanza FL, Chan FK, Quigley EM. Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 2009;104:728–38. [DOI] [PubMed] [Google Scholar]
- 128.Graham DY, Agrawal NM, Campbell DR, Haber MM, Collis C, Lukasik NL, et al. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med 2002;162:169–75. [DOI] [PubMed] [Google Scholar]
- 129.Ekstrom P, Carling L, Wetterhus S, Wingren PE, Anker-Hansen O, Lundegardh G, et al. Prevention of peptic ulcer and dyspeptic symptoms with omeprazole in patients receiving continuous non-steroidal anti-inflammatory drug therapy. A Nordic multicentre study. Scand J Gastroenterol 1996;31:753–8. [DOI] [PubMed] [Google Scholar]
- 130.Lin KJ, Hernandez-Diaz S, Garcia Rodriguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology 2011;141:71–9. [DOI] [PubMed] [Google Scholar]
- 131.Baker TW, Quinn JM. Aspirin therapy in aspirin-exacerbated respiratory disease: a risk-benefit analysis for the practicing allergist. Allergy Asthma Proc 2011;32: 335–40. [DOI] [PubMed] [Google Scholar]
- 132.Whitlock EP, Burda BU, Williams SB, Guirguis-Blake JM, Evans CV. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164: 826–35. [DOI] [PubMed] [Google Scholar]
- 133.Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Selak V, Kerr A, Poppe K, Wu B, Harwood M, Grey C, et al. Annual risk of major bleeding among persons without cardiovascular disease not receiving antiplatelet therapy. JAMA 2018;319:2507–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke 2005;36:1801–7. [DOI] [PubMed] [Google Scholar]
- 136.Garcia-Rodriguez LA, Gaist D, Morton J, Cookson C, Gonzalez-Perez A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology 2013;81:566–74. [DOI] [PubMed] [Google Scholar]
- 137.Shah NH, Schneider TR, DeFaria Yeh D, Cahill KN, Laidlaw TM. Eosinophilia-associated coronary artery vasospasm in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract 2016;4:1215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pelletier T, Tamayev R, Iammatteo M, Nautsch D, Hudes G, Lukin D, et al. Eosinophilic esophagitis as possible complication of aspirin treatment in patient with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 2017;118:120–2. [DOI] [PubMed] [Google Scholar]
- 139.White AA, Bosso JV, Stevenson DD. The clinical dilemma of “silent desensitization” in aspirin-exacerbated respiratory disease. Allergy Asthma Proc 2013;34: 378–82. [DOI] [PubMed] [Google Scholar]
- 140.Walters KM, Simon RA, Woessner KM, Wineinger NE, White AA. Effect of misoprostol on patients with aspirin-exacerbated respiratory disease undergoing aspirin challenge and desensitization. Ann Allergy Asthma Immunol 2017;119: 71–6. [DOI] [PubMed] [Google Scholar]
- 141.Sheikh A, Shehata YA, Brown SG, Simons FE. Adrenaline (epinephrine) for the treatment of anaphylaxis with and without shock. Cochrane Database Syst Rev 2008;CD006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hoyte FC, Weber RW, Katial RK. Pancreatitis as a novel complication of aspirin therapy in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2012;129:1684–6. [DOI] [PubMed] [Google Scholar]
- 143.Durrani SR, Kelly JT. Pancreatitis as a complication of aspirin desensitization for aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2013;131: 244–6. [DOI] [PubMed] [Google Scholar]
- 144.Bochenek G, Kuschill-Dziurda J, Szafraniec K, Plutecka H, Szczeklik A, Nizankowska-Mogilnicka E. Certain subphenotypes of aspirin-exacerbated respiratory disease distinguished by latent class analysis. J Allergy Clin Immunol 2014;133:98–103. e1–6. [DOI] [PubMed] [Google Scholar]


