Haddix and Rasband discuss work from Wang et al. describing how neuromuscular synapse elimination influences myelination.
Abstract
In this issue, Wang et al. (2021. J. Cell Biol. https://doi.org/10.1083/jcb.201911114) describe a phenomenon in which neuromuscular junction synapse elimination triggers myelination of terminal motor axon branches. They propose a mechanism initiated by synaptic pruning that depends on synaptic activity, cytoskeletal maturation, and the associated anterograde transport of trophic factors including Neuregulin 1-III.
Neuromuscular junctions (NMJs) are a favorite model system to study the development, maintenance, and function of neuronal synapses because of their accessibility, size, and simplicity. Although many synaptic mechanisms discovered at the peripheral NMJ have provided important insights into synaptic mechanisms in the central nervous system (CNS), the phenomena of synapse elimination and refinement remain poorly understood in both. In the peripheral nervous system (PNS), synapse elimination is an essential developmental step that removes redundant presynaptic inputs to the muscle fiber. In addition, peripheral motor axon terminals must become myelinated to facilitate rapid and synchronized acetylcholine release to the muscle fiber. However, whether these two essential events during PNS development are coordinately regulated remains unknown.
The immature rodent NMJ is first innervated by many axons which are then removed until the synapse reaches a dually innervated state (1). These two axons then further compete for synaptic territory, leaving one “winner” that eventually occupies the motor endplate by the end of the second postnatal week. To determine the relationship between synapse elimination and myelination, Wang et al. (2) used the formation of paranodal junctions between axons and Schwann cells as a surrogate for myelination and then determined whether axons that occupied NMJs in a singly or dually innervated state were more or less likely to be myelinated. They found that when the NMJ is dually innervated, myelination of the terminal axon branch is inhibited; neither synaptic occupancy of the competing axons nor axon diameter influenced myelination. However, once synapse elimination at the NMJ is complete, i.e., a single axon terminal innervates the motor endplate, the winner branch becomes myelinated. Thus, synapse elimination precedes myelination of the terminal axon branch, and competition between dually innervated NMJs restricts myelination.
What mechanisms regulate the coordinated maturation of the motor neuron, Schwann cell, and muscle circuit? Since previous studies showed that synapse elimination at the NMJ depends on muscle activity (3), Wang et al. (2) inhibited synapse elimination by blocking acetylcholine receptors with α-bungarotoxin (α-Btx). This inhibition of motor endplate and muscle activity increased not only the number of dually innervated NMJs, but also significantly decreased myelination of terminal axon branches of singly innervated NMJs. Thus, neuromuscular activity must induce retrograde signaling mechanisms that promote not only synapse elimination but also myelination.
During synapse elimination, the microtubule cytoskeleton of retracting axons is degraded and reduced (4). In contrast, axons that singly innervate NMJs have a higher microtubule content. α-Btx–dependent block of neuromuscular transmission reduced microtubule content in axons that singly innervate NMJs. Thus, α-Btx treatment simultaneously reduces both microtubule content and myelination.
To determine if a mature microtubule-based cytoskeleton is causally related to myelination, Wang et al. used spastin knockout (spastinKO) mice to artificially stabilize microtubules. Although spastinKO mice had delayed axon branch removal, stabilization of the microtubule cytoskeleton increased myelination of axons that dually innervated NMJs. Thus, the brake that synaptic competition normally places on terminal branch myelination can be overcome by increasing the mass and maturity of the microtubule cytoskeleton.
How does axonal microtubule stability influence terminal axon myelination? Microtubules participate in the anterograde and retrograde transport of diverse cargoes including mitochondria and growth factors. To determine if anterograde axonal transport promotes myelination of axons that singly innervate NMJs, Wang et al. used a dominant-negative mutant of kinesin-1 heavy chain which binds cargo, but lacks the protein’s motor domain, thereby impairing transport. After confirming transport inhibition by tracking impaired movement of the β1 subunit of voltage gated sodium channels, they found that myelination and node of Ranvier formation were significantly delayed in singly innervated NMJs expressing the dominant negative kinesin. Taken together, these results suggest that synapse elimination promotes maturation of the microtubule cytoskeleton which allows more efficient delivery of promyelinating signals to the terminal branch.
What could these promyelinating signals be? One obvious candidate is Neuregulin 1 type III (Nrg1-III), which has long been known to promote myelination of peripheral nervous system axons (5). Consistent with this idea, conditional deletion of Nrg1-III dramatically reduced the number of myelinated axon terminals that singly innervate NMJs but did not alter the number of dually innervated NMJs. In contrast, overexpression of Nrg1-III in a transgenic mouse removed the competition-dependent block on myelination resulting in more myelination of both dually and singly innervating axon terminals. In these same transgenic Nrg1-III mice, among those NMJs that were singly innervated, their corresponding axons had higher levels of Nrg1-III. Remarkably, even in these same transgenic overexpressers, inhibition of muscle activity reduced the amount of Nrg1-III found on singly innervated axons, consistent with the observed impairment of the microtubule-based cytoskeleton after α-Btx treatment. ERK1/2 and AKT are downstream effectors of Nrg1-III in Schwann cells and implicated in the myelination pathway. Immunostaining of Schwann cells ensheathing singly innervating axon terminals revealed higher levels of pERK and pAKT.
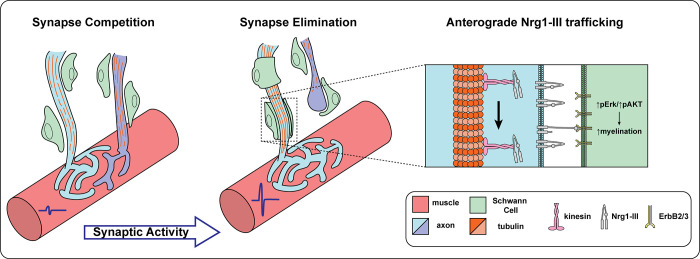
Taken together, the experiments performed by Wang et al. (2) suggest that as multiple axons actively compete for synaptic dominance at the NMJ, the myelination of their terminal branches is delayed. Upon synapse elimination, neuromuscular activity promotes a retrograde signal that increases maturity of the microtubule cytoskeleton. Maturation of the microtubule-based cytoskeleton facilitates the transport of promyelinating signals like Nrg1-III which, when presented to Schwann cells, results in myelination of the “winner” terminal axon branch of a singly innervated NMJ (Fig. 1).
Figure 1.
Synapse elimination promotes myelination of terminal motor axon branches. During early development, NMJs are innervated by multiple axons that compete for endplate territory. During this time, the terminal branches of the axons are not myelinated, and the tubulin cytoskeletal network remains immature. Synaptic activity induces elimination of redundant connections, which leads the winner axon’s microtubule-based cytoskeleton to mature and increase, while the microtubule cytoskeleton is degraded in the retracting axon. The maturity of the cytoskeleton allows for kinesin dependent anterograde transport of Neuregulin 1-III, which then initiates a promyelination signaling cascade via AKT and ERK activation.
To the best of our knowledge, this is the first demonstration of plasticity of myelination downstream of activity and synapse refinement in the peripheral motor nervous system. Many studies in the CNS demonstrate that de novo myelination occurs in response to neuronal activity and learning paradigms (6, 7), although the mechanisms responsible remain unknown. Thus, synapse refinement and elimination-dependent myelination may be a paradigm to uncover mechanisms of learning- and activity-dependent myelination in the CNS. Functionally, the addition of myelin to the terminal motor axon branch promotes efficient neurotransmitter release through faster action potential propagation, improved metabolic support of the axon, and more efficient depolarization of the presynaptic terminal by clustered Na+ channels at the terminal heminode (8). Whether any or all of these benefits also exist in the CNS remains unknown.
This is also the first demonstration of postsynaptic activity driving myelination of a presynaptic axon. Although it is clear that a retrograde signal from the muscle promotes the further maturation and subsequent myelination of the terminal axon, the identity of this cue is unknown. One interesting candidate for a muscle-derived competition and axonal maturation cue is the neurotrophin brain-derived neurotrophic factor (BDNF), which is released during muscle activity (9). Consistent with this idea, BDNF promotes axon maturation by stimulating both actin polymerization and microtubule assembly (10). It will be interesting to test the role of trophic factors in activity-dependent synapse elimination and subsequent myelination in both the CNS and PNS.
In conclusion, Wang et al. (2) is an excellent addition to a growing body of research that demonstrates how neuronal activity promotes and modulates myelination. Furthermore, it stands as another example of how using simple model systems, such as the NMJ, may provide insights and have important implications for much more complicated biological systems.
Acknowledgments
This work was supported by National Institutes of Health grants AR074988 and NS044916.
The authors declare no competing financial interests.
References
- 1.Tapia, J.C., et al. 2012. Neuron. 10.1016/j.neuron.2012.04.017 [DOI] [Google Scholar]
- 2.Wang, M., et al. 2021. J. Cell Biol. 10.1083/jcb.201911114 [DOI] [Google Scholar]
- 3.Buffelli, M., et al. 2003. Nature. 10.1038/nature01844 [DOI] [Google Scholar]
- 4.Brill, M.S., et al. 2016. Neuron. 10.1016/j.neuron.2016.09.049 [DOI] [Google Scholar]
- 5.Taveggia, C., et al. 2005. Neuron. 10.1016/j.neuron.2005.08.017 [DOI] [Google Scholar]
- 6.Gibson, E.M., et al. 2014. Science. 10.1126/science.1252304 [DOI] [Google Scholar]
- 7.McKenzie, I.A., et al. 2014. Science. 10.1126/science.1254960 [DOI] [Google Scholar]
- 8.Saab, A.S., et al. 2013. Curr. Opin. Neurobiol. 10.1016/j.conb.2013.09.008 [DOI] [Google Scholar]
- 9.Hurtado, E., et al. 2017. Front. Mol. Neurosci. 10.3389/fnmol.2017.00147 [DOI] [Google Scholar]
- 10.Namekata, K., et al. 2012. J. Neurosci. 10.1523/JNEUROSCI.4884-11.2012 [DOI] [Google Scholar]