Abstract
The likelihood of suffering a bone fracture is not solely predicated on areal bone mineral density. As people age, there are numerous changes to the skeleton occurring at multiple length scales (from millimeters to submicron scales) that reduce the ability of bone to resist fracture. Herein is a review of the current knowledge about the role of the extracellular matrix (ECM) in this resistance, with emphasis on engineering principles that characterize fracture resistance beyond bone strength to include bone toughness and fracture toughness. These measurements of the capacity to dissipate energy and to resist crack propagation during failure precipitously decline with age. An age-related loss in collagen integrity is strongly associated with decreases in these mechanical properties. One potential cause for this deleterious change in the ECM is an increase in advanced glycation end-products, which accumulate with aging through non-enzymatic collagen crosslinking. Potential regulators and diagnostic tools of the ECM with respect to fracture resistance are also discussed.
Keywords: Bone Quality, Toughness, Strength, Fracture Toughness, Collagen, Structure, Architecture, Microdamage, Porosity, Biomechanics, Fracture Risk, Advanced Glycation End-products, Osteoporosis, Diabetes, Aging, Finite Element Analysis, Non-collagenous proteins, Mineral, Raman Spectroscopy, Fourier Transform Infrared, Transforming growth factor beta, Crosslinking, Computed-tomography, Matrix metalloproteinase, Neurofibromatosis type 1
Introduction
The risk of suffering a bone fracture increases with age, and in addition to post-menopausal osteoporosis, certain diseases such as diabetes [1] and chronic kidney disease (CDK) [2] also increase fracture risk. While an age-related or diabetes-related propensity to fall can certainly increase the chance of breaking a bone [3], the skeleton does undergo deleterious changes with aging and disease onset, and these changes reduce the ability of bone to resist fracture. One well recognized change is a loss of bone mass, and as such, osteoporosis therapies are designed to prevent bone loss (e.g., bisphosphonates) or promote bone gain (e.g., intermittent parathyroid hormone). Moreover, assessment of bone loss as a T-score or Z-score derived from dual-energy X-ray absorptiometry (DXA) are used clinically to assess fracture risk.
Despite the effectiveness of anti-resorptive therapy [4] and bone anabolic therapy [5] in increasing DXA-derived areal bone mineral density (aBMD) and more importantly reducing the incidence of fracture, certain individuals still sustain fractures with treatment. In addition, long-term bisphosphonate use has been associated with atypical, sub-trochanteric fractures, though no causal link has been established to date [6]. Thus, fractures are not solely the result of low bone mass or density, and treatment-related reductions in fracture incidence are not solely explained by changes in aBMD [7].
As further evidence supporting the notion that bone mass is not the sole determinant of fracture resistance, aBMD is not a particularly accurate predictor of an individual’s risk of fracture, with many fractures occurring in subjects with T-scores below −2.5 (the number of standard deviation below or above young adult normal mean aBMD) [8]. In effect, the age-related increase in fracture risk is independent of the age-related decrease in BMD [8, 9]. That is, a 70 year old is at a much greater risk of breaking a bone than a 50 year old with the same BMD, an observation first published in the 1980’s [10]. In addition to aging, the diabetes-associated increased risk of bone fracture is disproportionate to differences in aBMD between diabetics and age-matched non-diabetics [11]. Type 2 diabetes (T2DM) is not necessarily associated with lower BMD [12], and, in several studies, aBMD was actually found to be higher in T2DM patients [11, 13] than in age-matched, non-diabetics. This inability of aBMD to predict individual fracture risk has various origins, but one indication is that aging and certain diseases affect bone in ways that are invisible to DXA-derived measures of bone mass (e.g., collagen integrity [14]). Capturing the importance of non-BMD factors, bone quality was defined in a NIH conference as “the sum total of characteristics of the bone that influence the bone’s resistance to fracture” [15]. Those characteristics span the multiple length scales, from millimeters to submicrons, comprising the hierarchical organization of bone and include the extracellular matrix (ECM).
Besides bone mass, cortical bone structure and trabecular bone architecture are determinants of fracture resistance. Avoiding the DXA limitations of acquiring measurements from a 2D projection, quantitative, X-ray computed-tomography (QCT) provides both structural parameters and volumetric BMD (in equivalent density of a hydroxyapatite phantom). High resolution, peripheral CT (HR-pQCT) can provide additional architectural parameters such as trabecular thickness and connectivity density [16]. With regards to cortical bone structure, moment of inertia and cross-sectional area correlate with whole bone strength as determined by biomechanical testing of cadaveric femurs and radii in simulated fall configurations (Fig 1) [17, 18]. In clinical studies involving QCT, patients with a femoral neck or trochanteric fracture had a smaller cortical cross-sectional area and lower moment of inertia at the neck than age-matched non-fracture patients [19], indicating decreased bone strength. The importance of bone structure to fracture resistance has been further demonstrated in recent studies that used the finite element method (FEM) to predict bone strength from patients’ CT scan of the hip and found strength was strongly associated with fracture risk [20, 21••]. In addition, QCT-FEM assessment of vertebral body strength had a greater ability of discriminating vertebral fractures than areal BMD or volumetric BMD in a cross-sectional study of post-menopausal women [22]. Nonetheless, given that fractures can arise from fatigue loading, and that the fracture process involves the growth of cracks through the bone tissue, bone strength is not likely the sole contributor to a fracture event. Fracture resistance of bone also depends on the inherent quality of the ECM as described herein.
Figure 1.
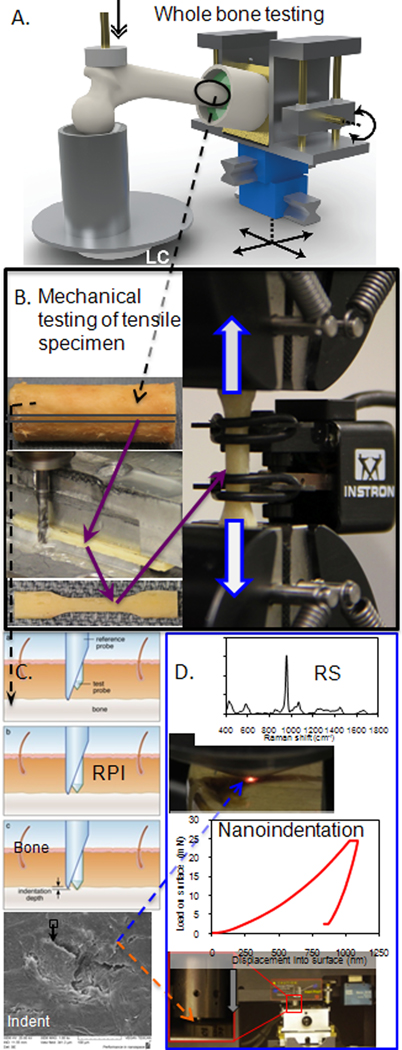
To fully characterize the contributors to the fracture resistance of bone, the tissue is tested at multiple length scales, which involves analyses at progressively smaller length-scales A. macro-structure (> 10 mm), testing of a femur in a side-ways fall configuration to whole bone strength; B. sub-macro-structure (2–5 mm), tensile mechanical testing of uniform cortical bone strips to quantify apparent material propeties; C. micro-structure (150–350 μm), reference point indentation (RPI) to characterize in situ or in vivo damage resistance of the ECM, as shown in the scanning electron micrograph in the lower portion of the panel; D. sub-micro-structure (0.5–25 μm), Raman spectroscopy (RS) to assess compositional properties of the matrix such as mineral to collagen ratio and crystallinity and nanoindentation to acquire complimentary properties of modulus and hardness
Age-related changes in bone toughness
From the 1960s to the present, studies involving the mechanical testing of fresh frozen, cadaveric bone have identified numerous age-related determinants of bone’s resistance to fracture (Table 1). In these cadaveric studies, bone samples are milled or cored to produce specimens with uniform geometry at the length scale of several millimeters (Fig 1) [23], which is 10–20 times greater than the size of an osteon or trabecula. With respect to cortical bone, aging affects the ability of bone to dissipate energy during failure (bone toughness) to a greater extent than it does the material strength [24–27]. Basically, older bone is brittle while younger bone is ductile, and this age-related change is primarily due to a loss in the ability of aged bone to deform or stretch beyond the point at which damage begins to form in the ECM (i.e., yield point or proportional limit) [27].The exact mechanism causing a decrease in bone toughness with age still requires further investigation. However, it is known that the organic phase of the ECM, namely type 1 collagen, is primarily responsible for bone toughness. For example, in mechanical testing studies of cadaveric bone, an age-related decrease in the stability of collagen correlated with bone toughness [26] as did an age-related decrease in collagen content [27]. In addition, manipulations to the collagen, such as thermal-induced collagen denaturation [28], formalin fixation [29], and high energy, gamma or X-ray irradiation [30, 31], reduce the toughness of bone without necessarily affecting its material strength.
Table 1.
Age-related changes in bone at different length scales have been associated with changes in fracture resistance
| Length Scale (μm) | Bone tissue characteristic | Material Property | Ref | ||
|---|---|---|---|---|---|
| 100 | Osteons per area | ↑ | Tensile strength | ↓ | [23] |
| 100 | Percentage osteons | ↑ | Resistance to crack propagation | ↓ | [34] |
| 10–50 | Porosity | ↑ | Post-yield toughness | ↓ | [25] |
| 10–50 | Porosity | ↑ | Resistance to crack propagation | ↓ | [35] |
| 1–5 | Microdamage | ↑ | Resistance to crack propagation | ↓ | [33] |
| 0.1 | Collagen integrity | ↓ | Toughness | ↓ | [26] |
| 0.1 | Collagen crosslinks | ↑ | Fracture toughness | ↓ | [45] |
| 0.1 | Collagen content | ↓ | Post-yield energy dissipation | ↓ | [27] |
Fracture toughness as a measurement of fracture resistance
In engineering mechanics, strength is not the only material property guiding design. Since all materials have flaws or void spaces, however small, there is potential for a crack to form during the service life of the material. If the crack reaches a critical size, failure of the material is inevitable. Therefore, there are mechanical tests that characterize the ability of a material to resist crack initiation and crack propagation (a.k.a. fracture toughness) in addition to those that measure strength (yield or peak stress) and toughness (area under the stress-vs.-strain cure). Whether measured by the critical stress intensity factor [32, 33] or the strain energy release rate [34, 35], fracture toughness of human bone decreases during aging (Table 1). Moreover, healthy bone tissue possesses the ability to demand greater energy to continue propagating a crack as the crack grows in length (i.e., R-curve behavior) [36, 37], and this behavior is lost or reduced with aging [38, 39].
The determinants of fracture toughness are rather complex, spanning multiple length scales (Table 1), and as such, clinical surrogates of fracture toughness are not obvious. With respect to mechanism, Vashishth and co-workers [40, 41] observed the formation of microscopic cracks within the ECM of bone – bovine, antler, and human – occupying ‘process zones’ ahead and in the wake of a propagating crack. Ritchie and co-workers [42, 43•] further observed unbroken ‘ligaments’ of bone tissue along the path of the propagating crack. As explained by Ritchie, these processes contribute to toughening, with an intrinsic mechanism of formation of the frontal microdamage zone and extrinsic mechanisms of crack bridging within the ECM as well as crack deflection around osteons [44]. The exact changes in the ECM affecting these mechanisms remain to be fully elucidated, but age-related increases in non-enzymatic collagen crosslinking are implicated [43•, 45, 46].
The aforementioned observations of toughening mechanisms were made through analyses of ex vivo bone samples using non-clinical instruments such as scanning electron microscopy and synchrotron micro-CT (SR-μCT). Perhaps the closest clinical measurement of bone’s resistance to crack propagation is the indentation distance increase (IDI) that is quantified using reference point indentation (RPI). Unlike microindentation or nanoindentation in which a hard tip (e.g., diamond) penetrates the material such that depth, force, and contact area are recorded to quantify hardness and modulus, RPI utilizes a reference probe (akin to a hypodermic needle) and a test probe that slides through the reference probe indenting the bone tissue (Fig 1) [47]. Moreover, RPI is designed for clinical use. Specifically, the RPI reference probe engages the patient’s bone under the periosteum of the tibial mid-shaft; the test probe performs 20 cycles of micro-indents into the tissue (50–100 μm in diameter x 100–200 μm in depth) in force control; and then, among other properties, the instrument records IDI, the relative distance that the test probe travels into the ECM of bone. During indentation, microdamage forms below the indenter (Fig 1), and presumably, bone matrix with less resistance to microdamage formation allows the test probe to penetrate deeper than bone matrix with high resistance. Recently, the IDI for 27 fracture patients was observed to be 47% greater than the IDI for 8 age-matched patients (p=0.008) in the same hospital for non-fracture reasons [48••]. Using cadaveric bone from several donors, IDI was found to be inversely proportional to crack growth toughness [48••], thus linking local indents to apparent-level fracture toughness.
Formation of fatigue microdamage in the extracellular matrix
Although the bone’s ECM dissipates energy through the generation of microdamage as a means to improve its fracture toughness, bone must still minimize the accumulation of fatigue-induced microdamage in non-cracked regions. When subjected to repeated (i.e., cyclic) loading over time at stresses well below the yield strength of bone, the bone matrix accumulates linear microcracks and diffuse patches of nanocracks. Among other functions, bone remodeling serves to remove this fatigue-induced microdamage [49, 50]. Nonetheless, the degree of microdamage in bone increases with age [51, 52], suggesting age-related changes in bone’s ECM favors microdamage formation. Certainly, engineering measurements of fatigue properties such as fatigue life (number of cycles to failure, Nf) and fatigue strength (y-intercept on an applied stress vs. Nf semi-log curve) of cadaveric bone decrease with age [53, 54]. The changes in the ECM that lead to such declines are not fully understood, but fatigue microdamage is known to accumulate between osteons in the interstitial sites [55], and interstitial sites (i.e., remnants of remodeled osteons) have an older tissue age with higher mineral-to-collagen ratio [56] and greater concentrations of mature collagen crosslinks [57]. Moreover, the number of lacunae in the ECM and the degree of mineralization in peri-lucanar space has been observed to decrease and increase, respectively, with age [58•]. Also, greater microdamage has been associated with a reduction in osteocyte lacunar density [59]. Thus, a ‘brittling’ of the ECM with human and tissue age lowers the tissue strain surrounding a crack, allowing it to propagate through micro-structural barriers like cement lines, as recently observed for human cortical bone [60].
Organic contribution to fracture resistance: collagen, AGEs, and NCPs
As previously discussed, Type 1 collagen, which comprises 90% of the organic matrix, is the primary determinant of bone toughness. It is critical to overall fracture resistance, even though increasing mineral content is still the primary focus of drug discovery for fracture prevention. The classic example of collagen’s importance in bone is osteogenesis imperfecta (OI), known of course as the brittle bone disease, in which mutations in the gene encoding collagen strands (col1a1) lead to various grades of severity in the loss of fracture resistance. OI mice have bones with less post-yield deformation (i.e., more brittle) than do wild-type mice [61, 62], and there is greater microdamage formation with altered morphology in OI bones [63]. Beyond this defect in collagen that causes other changes in the matrix (e.g., crystallinity), crosslinking is also important. For example, disrupting enzymatic collagen crosslinking by treating rats with a lysyl oxidase inhibitor reduces bone strength without affecting mineralization [64].
Increases in advanced glycation end-products (AGEs) perhaps have garnered the greatest attention as a potential change in the organic matrix causing a decrease in fracture resistance of bone. Formed through a non-enzymatic reaction process involving sugar, these crosslinks of collagen increase in concentration with age and diabetes, even though bone turns over or remodels. Age-related increases in pentosidine, a quantifiable biomarker of AGEs, are associated with a decrease in post-yield energy dissipation and fracture toughness of bone [27, 45]. Clinically, serum or urine levels of pentosidine have been associated with higher incidence of fracture in post-menopausal women [65, 66••] and subjects with T2DM [67, 68]. The concentration of pentosidine is higher in rodents with diabetes relative to control bone [69], and given enough time for diabetes to progress in rodents, bones become brittle [70]. Although a mechanism is not fully delineated, one possibility is that AGEs stiffen the collagen matrix such that the fibrils dissipate less energy, allowing microdamage to more readily form and propagate through the ECM.
Recently, attention has also been given to non-collagenous proteins (NCPs) as contributors to the fracture resistance of bone. Initially, small-scale mechanics using atomic force microscopy demonstrated that phosphorylated proteins can bridge neighboring mineralized collagen fibrils forming so-called sacrificial bonds resisting fibril separation [71]. Subsequently, osteopontin was one NCP observed to dissipate energy through this mechanism [72]. Indeed, mice lacking osteopontin (OPN−/− mice) have bones with 30% lower fracture toughness compared to wild-type bones. However, it should be noted that this difference may not solely be due to the role of OPN as a sacrificial bonding agent since OPN is involved in mineralization and OPN−/− bones exhibited greater tissue heterogeneity than OPN+/+ bones [73]. Interestingly, osteonal tissue has higher amounts of OPN and osteocalcin than does interstitial tissue [74], and as previously mentioned, interstitial sites are where microcracks are most often observed in cortical bone.
The role of water in fracture resistance
As an essential component of the ECM, water is bound to both collagen and mineral phases via hydrogen-hydrogen bonding. As the amount of water removal from human cortical bone increases with an increase in drying temperature (24°C to 50 °C to 70 °C), the toughness of bone significantly decreases, suggesting loosely and tightly bound water contribute to fracture resistance [75]. Using solid-state nuclear magnetic resonance (NMR), a structural layer of water was observed between the collagen and mineral phases of bone [76] and could act as another sacrificial layer protecting collagen fibrils from shear forces [76]. Measurements of bound water using 1H NMR spectroscopy have been correlated with the mechanical properties of human cortical bone [77, 78], and if successfully translated to clinical imaging (magnetic resonance imaging), bound water could potentially be an important indicator of fracture resistance.
Spectroscopic characterization of bone tissue with respect to fracture resistance
Fourier Transform Infrared (FTIR) and Raman Spectroscopy (RS) are two complementary optical techniques widely used to gain insight into the biophysical nature of bone’s ECM as it relates to fracture resistance. In brief, when light impinges on chemical bonds, energy is gained or lost depending on the light wavelength and molecular vibrations of the chemical moieties in the tissue. The intensity spectrum of collected light (Fig 1) corresponds to the biochemical distribution of the bone matrix. FTIR indirectly measures differential absorption of light by anti-symmetric vibrations through the attenuation of transmitted infrared wavelengths. Due to water absorption, FTIR usually requires thin, dehydrated ex-vivo samples [79]. RS utilizes direct reflectance measures of differential scattering off symmetric vibrations, shifted relative to the input wavelength. Despite limited spectral sensitivity relative to its FTIR counterpart and added complications from concurrent fluorescence, RS with its reflectance design has clinical potential as a noninvasive instrument for bone matrix measures in situ and even diagnostic capability through skin [80, 81].
While the potential for optical spectroscopy to assess fracture resistance clinically is nascent, many studies into the ECM of bone have already contributed to our understanding of fracture resistance. Investigations utilizing optical spectroscopies have demonstrated: FTIR sensitivity to many processes involved in matrix mineralization [82]; Raman sensitivity to damage and defects in the bone matrix [83]; and Raman spectral changes in the ECM related to age-related changes in the mechanical properties of cortical bone [84, 85]. Building upon established Raman markers of bone quality [86], we recently demonstrated consistent Raman measurements of compositional differences between osteonal and interstitial tissue from human cortical bone across bone orientation and processing conditions [56].
Recently,there have been efforts to determine whether spectroscopy can assess fracture risk and treatment-related changes to the ECM. Expanding upon evidence for a spectroscopic profile of osteoporosis[87], Boskey and co-workers analyzed iliac crest biopsies with FTIR. They correlated matrix turnover rates of osteoporotic bone with relative tissue homogeneity and specifically demonstrated globally decreased mineral to matrix ratio and increased crystal size in tissue from patients with osteoporosis [88]. In another FTIR study, Gourion-Arsiquaudet al. [89] showed that fragility fractures were associated with locally increased mineral content, carbonate substitution and collagen crosslinking. Interestingly, carbonate substitution provided the greatest difference between fracture and non-fracture patients. Moreover, the associations of the FTIR-derived properties of mature to immature collagen crosslinking ratio and mineral to collagen ratio with fracture risk were independent of aBMD. Further insight into the effects of bisphosphonates (BisP) on bone matrix was provided by, Gourion-Arsiquaud et al.. They examined the tibia of beagles after 1 year BisP treatment and found increased mineral content and increased collagen cross-linking maturity that was also more homogenous than bone from untreated controls [90]. Then, in an FTIR analysis of iliac crest samples obtained from patients enrolled in the Fracture Prevention Trial, Paschalis et al. showed a spectral profile of teriparatide (recombinant human PTH) treated bone that displayed lower matrix mineralization, crystallinity, and relatively fewer mature collagen crosslinks, all factors consistent with indicators of younger bone matrix [91]. Spectral profiles of bone ECM provide a powerful resource for the investigation of treatment effects and mechanistic studies in ways that go beyond mineral content.
To begin establishing a profile for fracture risk assessment by RS, McCreadie et al. analyzed both iliac crest biopsies and proximal femurs from donors with and without an osteoporotic fracture [92]. In the case of the femoral head samples, the mineralization was greater for fracture patient than for non-fracture controls. Analysis of the cortical bone of the iliac crest showed that carbonate substitution (a mineral lattice change that occurs with tissue aging) was also greater for the fracture group than for the non-fracture group. Taken together, these spectroscopic studies suggest that osteoporosis causes the ECM to become more homogenous and exhibit accelerated aging. As technology advances toward noninvasive instrumentation, spectroscopy could provide clinical evaluation of bone matrix quality.
Potential regulators of bone tissue quality
There are several factors that affect the fracture resistance of bone through their influence on the formation of the ECM. One such factor is transforming growth factor beta (TGF-β), an abundant protein sequestered in the bone matrix that regulates bone cell recruitment and differentiation upon being activated. Examining the bones of several mouse models of inhibited or augmented TGF-β signaling by X-ray tomography, RS, and micro- and macro- mechanical testing, Balooch et al. showed that bone matrix quality and fracture resistance decreased as TGF-β activity increased [93]. In subsequent research, TGF-β repression of Runx2 was found to modulate bone ECM properties such that that the repression-induced decrease in modulus and hardness of cochlear bones caused deafness in a model of cleidocranial dysplasia (Runx2 +/−) [94]. Given the ability of TGF-β to regulate tissue mineralization and fracture resistance, several pre-clinical studies investigated the effect of inhibiting TGF-β. When given to young mice, a small molecule inhibitor of TGF-β type I receptor kinase activity (SD 208) increased trabecular bone volume and vertebral body failure load by increasing osteoblast differentiation and reducing osteoclast differentiation [95]. In another mouse study, an anti-TGF-β antibody, directed at all three isoforms of TGF-β ligand, also increased trabecular bone volume fraction and mineralization [96]. In addition, this antibody-mediated inhibition of TGF-β increased the estimated bending strength of the femur mid-shaft as assessed by three point bending tests. At the tissue level, TGF-β inhibition increased the mineral to matrix ratio and modulus, as assessed by RS and nanoindentation, respectively.
Besides TGF-β, enzymes that process collagen such as the matrix metalloproteinases (MMPs) may have specific roles in the maintenance of the bone ECM, thereby regulating fracture resistance. Our study of cortical and trabecular bone from wild-type, Mmp2−/−, and Mmp9−/− mice indicated differential effects on the biochemical, structural, and biomechanical properties of the bone across multiple length scales [97•]. Specifically, the loss of MMP-2 caused a decrease in strength with no effect on toughness, whereas the loss of MMP-9 did not affect strength but caused the bones to become brittle. These MMPs are both a gelatinase. However, among the bone cells, MMP-2 is expressed by osteoblasts and MMP-9 is primarily expressed in osteoclasts, indicating that each cell type likely plays a unique role in fracture resistance.
A significant number of rare congenital defects involve connective tissues and can subsequently provide a wealth of knowledge about the development and remodeling of bone ECM. Recent investigations have shown this through Neurofibromatosis type 1. An osteoblast-specific deletion of NF1, which normally functions to inhibit RAS-ERK signaling in multiple cell lineages, using Cre-LOX technology caused hypomineralization in intact bones [98] as well as in fracture calluses, which were also found to be weaker than those of age-matched wild-type mice [99]. Loss of NF1 in osteochondroprogenitor cells also leads to hypomineralization as well as skeletal dysplasia with a phenotype similar to the human disease [100].
Conclusions
Beyond mineral density and content, the integrity of the extracellular matrix is an important contributor to the fracture resistance of bone. The ECM is especially organized to prevent the initiation and propagation of microdamage. With aging though, changes occur in the ECM that cause bone to become brittle. Thus, the age-related increase in fracture risk may be thought of as problem of bone brittleness, not just poor bone strength. A lack of bone brittleness requires a well-organized collagen phase of the ECM dissipating energy as strains exceed the elastic limit of bone. This organization can be disrupted by disease and aging, and such disruption may include increases in non-enzymatic collagen crosslinks. Recent efforts are providing new insight into the factors regulating the compositional and biomechanical properties of the ECM. By viewing bone as an organ organized in a precise hierarchical fashion with a dynamic extracellular matrix, novel insights are possible for establishing the mechanisms by which diseases and aging affect fracture resistance, and ultimately uncovering new treatment and diagnostic strategies for the prevention of bone fractures.
Acknowledgements
The authors thank Adam Horch and Matthew Murry for assistance in generating the image of biomechanical test of the proximal femur and Professor Paul Hansma for the image of reference point indentation.
Footnotes
Disclosures Conflicts of interest: J.S. Nyman: has received grant support from National Science Foundation, Department of Defense, Veterans Affairs; and has a patent (planned, pending or issued) for Vanderbilt University and the Department of Veterans Affairs for U.S. Provisional Application No. 61/369,248: System and Method for Determining Mechanical Properties of Bone Structures; A.J. Makowski: none.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- [1].Janghorbani M, Van Dam RM, Willett WC, Hu FB: Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007, 166(5): 495–505. [DOI] [PubMed] [Google Scholar]
- [2].Nickolas TL, Leonard MB, Shane E: Chronic kidney disease and bone fracture: a growing concern. Kidney International 2008, 74(6): 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stevens JA, Olson S: Reducing falls and resulting hip fractures among older women. Home Care Provider 2000, 5(4): 134–139; quiz 140–131. [DOI] [PubMed] [Google Scholar]
- [4].Watts NB: Treatment of osteoporosis with bisphosphonates. Rheum Dis Clin North Am 2001, 27(1): 197–214. [DOI] [PubMed] [Google Scholar]
- [5].Neer RM, Arnaud CD, Zanchetta JR, et al. : Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001, 344(19): 1434–1441. [DOI] [PubMed] [Google Scholar]
- [6].Shane E, Burr D, Ebeling PR, et al. : Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2010, 25(11): 2267–2294. [DOI] [PubMed] [Google Scholar]
- [7].Chen P, Miller PD, Delmas PD, et al. : Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res 2006, 21(11): 1785–1790. [DOI] [PubMed] [Google Scholar]
- [8].Kanis JA, Johnell O, Oden A, et al. : Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int 2001, 12(12): 989–995. [DOI] [PubMed] [Google Scholar]
- [9].Siris ES, Brenneman SK, Barrett-Connor E, et al. : The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50–99: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 2006, 17(4): 565–574. [DOI] [PubMed] [Google Scholar]
- [10].Hui SL, Slemenda CW, Johnston CC, Jr.: Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 1988, 81(6): 1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].de L, II, van der Klift M, de Laet CE, et al. : Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int 2005, 16(12): 1713–1720. [DOI] [PubMed] [Google Scholar]
- [12].Hampson G, Evans C, Petitt RJ, et al. : Bone mineral density, collagen type 1 alpha 1 genotypes and bone turnover in premenopausal women with diabetes mellitus. Diabetologia 1998, 41(11): 1314–1320. [DOI] [PubMed] [Google Scholar]
- [13].Vestergaard P: Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int 2007, 18(4): 427–444. [DOI] [PubMed] [Google Scholar]
- [14].Zioupos P: Ageing human bone: factors affecting its biomechanical properties and the role of collagen. J Biomater Appl 2001, 15(3): 187–229. [DOI] [PubMed] [Google Scholar]
- [15].Fyhrie DP: Summary--Measuring “bone quality”. J Musculoskelet Neuronal Interact 2005, 5(4): 318–320. [PubMed] [Google Scholar]
- [16].Nickolas TL, Stein E, Cohen A, et al. : Bone mass and microarchitecture in CKD patients with fracture. J AmSoc Nephrol 2010, 21(8): 1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cheng XG, Lowet G, Boonen S, et al. : Assessment of the strength of proximal femur in vitro: relationship to femoral bone mineral density and femoral geometry. Bone 1997, 20(3): 213–218. [DOI] [PubMed] [Google Scholar]
- [18].Lochmuller EM, Lill CA, Kuhn V, et al. : Radius bone strength in bending, compression, and falling and its correlation with clinical densitometry at multiple sites. J Bone Miner Res 2002, 17(9): 1629–1638. [DOI] [PubMed] [Google Scholar]
- [19].Ito M, Wakao N, Hida T, et al. : Analysis of hip geometry by clinical CT for the assessment of hip fracture risk in elderly Japanese women. Bone 2010, 46(2): 453–457. [DOI] [PubMed] [Google Scholar]
- [20].Orwoll ES, Marshall LM, Nielson CM, et al. : Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res 2009, 24(3): 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]••.Amin S, Kopperdhal DL, Melton LJ 3rd, et al. : Association of hip strength estimates by finite-element analysis with fractures in women and men. J Bone Miner Res 2011, 26(7): 1593–1600.Accounting for the contribution of bone structure to hip strength, finite element analysis of computed-tomgoraphy scans acquired from 580 subjects could predict osteoporotic fractures with a predicted strength of < 3000 N being the critical threshold for skeletal fragility.
- [22].Imai K, Ohnishi I, Matsumoto T, et al. : Assessment of vertebral fracture risk and therapeutic effects of alendronate in postmenopausal women using a quantitative computed tomography-based nonlinear finite element method. Osteoporos Int 2009, 20(5): 801–810. [DOI] [PubMed] [Google Scholar]
- [23].Evans FG: Mechanical properties and histology of cortical bone from younger and older men. Anat Rec 1976, 185(1): 1–11. [DOI] [PubMed] [Google Scholar]
- [24].Burstein AH, Reilly DT, Martens M: Aging of bone tissue: mechanical properties. J Bone Joint Surg Am 1976, 58(1): 82–86. [PubMed] [Google Scholar]
- [25].McCalden RW, McGeough JA, Barker MB, Court-Brown CM: Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am 1993, 75(8): 1193–1205. [DOI] [PubMed] [Google Scholar]
- [26].Zioupos P, Currey JD, Hamer AJ: The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res 1999, 45(2): 108–116. [DOI] [PubMed] [Google Scholar]
- [27].Nyman JS, Roy A, Tyler JH, et al. : Age-related factors affecting the postyield energy dissipation of human cortical bone. J Orthop Res 2007, 25(5): 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang X, Bank RA, TeKoppele JM, Agrawal CM: The role of collagen in determining bone mechanical properties. J Orthop Res 2001, 19(6): 1021–1026. [DOI] [PubMed] [Google Scholar]
- [29].Stefan U, Michael B, Werner S: Effects of three different preservation methods on the mechanical properties of human and bovine cortical bone. Bone 2010, 47(6): 1048–1053. [DOI] [PubMed] [Google Scholar]
- [30].Currey JD, Foreman J, Laketic I, et al. : Effects of ionizing radiation on the mechanical properties of human bone. J Orthop Res 1997, 15(1): 111–117. [DOI] [PubMed] [Google Scholar]
- [31].Barth HD, Launey ME, Macdowell AA, et al. : On the effect of X-ray irradiation on the deformation and fracture behavior of human cortical bone. Bone 2010, 46(6): 1475–1485. [DOI] [PubMed] [Google Scholar]
- [32].Zioupos P, Currey JD: Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone 1998, 22(1): 57–66. [DOI] [PubMed] [Google Scholar]
- [33].Zioupos P: Accumulation of in-vivo fatigue microdamage and its relation to biomechanical properties in ageing human cortical bone. JMicrosc 2001, 201(2): 270–278. [PubMed] [Google Scholar]
- [34].Yeni YN, Brown CU, Wang Z, Norman TL: The influence of bone morphology on fracture toughness of the human femur and tibia. Bone 1997, 21(5): 453–459. [DOI] [PubMed] [Google Scholar]
- [35].Yeni YN, Brown CU, Norman TL: Influence of bone composition and apparent density on fracture toughness of the human femur and tibia. Bone 1998, 22(1): 79–84. [DOI] [PubMed] [Google Scholar]
- [36].Malik CL, Stover SM, Martin RB, Gibeling JC: Equine cortical bone exhibits rising R-curve fracture mechanics. Journal of biomechanics 2003, 36(2): 191–198. [DOI] [PubMed] [Google Scholar]
- [37].Nalla RK, Kruzic JJ, Kinney JH, Ritchie RO: Mechanistic aspects of fracture and R-curve behavior in human cortical bone. Biomaterials 2005, 26(2): 217–231. [DOI] [PubMed] [Google Scholar]
- [38].Nalla RK, Kruzic JJ, Kinney JH, Ritchie RO: Effect of aging on the toughness of human cortical bone: evaluation by R-curves. Bone 2004, 35(6): 1240–1246. [DOI] [PubMed] [Google Scholar]
- [39].Koester KJ, Barth HD, Ritchie RO: Effect of aging on the transverse toughness of human cortical bone: Evaluation by R-curves. Journal of the Mechanical Behavior of Biomedical Materials 2011, 4(7): 1504–1513. [DOI] [PubMed] [Google Scholar]
- [40].Vashishth D, Behiri JC, Bonfield W: Crack growth resistance in cortical bone: concept of microcrack toughening. J Biomech 1997, 30(8): 763–769. [DOI] [PubMed] [Google Scholar]
- [41].Vashishth D, Tanner KE, Bonfield W: Experimental validation of a microcracking-based toughening mechanism for cortical bone. J Biomech 2003, 36(1): 121–124. [DOI] [PubMed] [Google Scholar]
- [42].Nalla RK, Kinney JH, Ritchie RO: Mechanistic fracture criteria for the failure of human cortical bone. Nat Mater 2003, 2(3): 164–168. [DOI] [PubMed] [Google Scholar]
- [43]•.Zimmermann EA, Schaible E, Bale H, et al. : Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci U S A 2011, 108(35): 14416–14421.Analzying contributors from the sub-microstructure to fracture toughness of bone by X-ray diffraction, this study provides evidence that non-enzymatic collagen crosslinking lowers fibril strain, thereby reducing resistance to crack propagation.
- [44].Ritchie RO: How does human bone resist fracture? Annals of the New York Academy of Sciences 2010, 1192(72–80. [DOI] [PubMed] [Google Scholar]
- [45].Wang X, Shen X, Li X, Agrawal CM: Age-related changes in the collagen network and toughness of bone. Bone 2002, 31(1): 1–7. [DOI] [PubMed] [Google Scholar]
- [46].Tang SY, Vashishth D: The relative contributions of non-enzymatic glycation and cortical porosity on the fracture toughness of aging bone. J Biomech 2011, 44(2): 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hansma P, Turner P, Drake B, et al. : The bone diagnostic instrument II: indentation distance increase. The Review of scientific instruments 2008, 79(6): 064303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]••.Diez-Perez A, Guerri R, Nogues X, et al. : Microindentation for in vivo measurement of bone tissue mechanical properties in humans. J Bone Miner Res 2010, 25(8): 1877–1885.In a small cohort, a direct measurement of the resistance to micro-indentation by bone tissue (five separate 200 micron spots on the tibia mid-shaft) was found to discriminate those with an osteoporotic fracture from age-matched subjects without a fracture.
- [49].Mori S, Burr DB: Increased intracortical remodeling following fatigue damage. Bone 1993, 14(2): 103–109. [DOI] [PubMed] [Google Scholar]
- [50].Verborgt O, Gibson GJ, Schaffler MB: Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 2000, 15(1): 60–67. [DOI] [PubMed] [Google Scholar]
- [51].Schaffler MB, Choi K, Milgrom C: Aging and matrix microdamage accumulation in human compact bone. Bone 1995, 17(6): 521–525. [DOI] [PubMed] [Google Scholar]
- [52].Mori S, Harruff R, Ambrosius W, Burr DB: Trabecular bone volume and microdamage accumulation in the femoral heads of women with and without femoral neck fractures. Bone 1997, 21(6): 521–526. [DOI] [PubMed] [Google Scholar]
- [53].Diab T, Sit S, Kim D, et al. : Age-dependent fatigue behaviour of human cortical bone. Eur J Morphol 2005, 42(1–2): 53–59. [DOI] [PubMed] [Google Scholar]
- [54].Zioupos P, Gresle M, Winwood K: Fatigue strength of human cortical bone: Age, physical, and material heterogeneity effects. J Biomed Mater Res A 2007. [DOI] [PubMed] [Google Scholar]
- [55].Norman TL, Wang Z: Microdamage of human cortical bone: incidence and morphology in long bones. Bone 1997, 20(4): 375–379. [DOI] [PubMed] [Google Scholar]
- [56].Nyman JS, Makowski AJ, Patil CA, et al. : Measuring Differences in Compositional Properties of Bone Tissue by Confocal Raman Spectroscopy. Calcif Tissue Int 2011, 89(2): 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nyman JS, Roy A, Acuna RL, et al. : Age-related effect on the concentration of collagen crosslinks in human osteonal and interstitial bone tissue. Bone 2006, 39(6): 1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]•.Busse B, Djonic D, Milovanovic P, et al. : Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 2010, 9(6): 1065–1075.Using quantitative backscattered electron imaging and histomorphometry, this study found that the number of osteocyte lacunae decreased in the femoral cortex with age, and the peri-lucanar tissue tended to be hypermineralized in the bone of elderly donors.
- [59].Vashishth D, Verborgt O, Divine G, et al. : Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone 2000, 26(4): 375–380. [DOI] [PubMed] [Google Scholar]
- [60].Chan KS, Chan CK, Nicolella DP: Relating crack-tip deformation to mineralization and fracture resistance in human femur cortical bone. Bone 2009, 45(3): 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Miller E, Delos D, Baldini T, et al. : Abnormal mineral-matrix interactions are a significant contributor to fragility in oim/oim bone. Calcif Tissue Int 2007, 81(3): 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Uveges TE, Kozloff KM, Ty JM, et al. : Alendronate treatment of the brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J Bone Miner Res 2009, 24(5): 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dong XN, Zoghi M, Ran Q, Wang X: Collagen mutation causes changes of the microdamage morphology in bone of an OI mouse model. Bone 2010, 47(6): 1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Oxlund H, Barckman M, Ortoft G, Andreassen TT: Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone 1995, 17(4 Suppl): 365S-371S. [DOI] [PubMed] [Google Scholar]
- [65].Shiraki M, Kuroda T, Tanaka S, et al. : Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab 2008, 26(1): 93–100. [DOI] [PubMed] [Google Scholar]
- [66]••.Gineyts E, Munoz F, Bertholon C, et al. : Urinary levels of pentosidine and the risk of fracture in postmenopausal women: the OFELY study. Osteoporos Int 2009. 21(2): 243–50.In a cohort of French post-menopausal women (OFELY), the incidence of fracture was higher for those with urinary pentosidine levels in the highest quartile, but pentosidine was not an independent risk factor for hip and vertebral fracture with respect to age.
- [67].Schwartz AV, Garnero P, Hillier TA, et al. : Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab 2009, 94(7): 2380–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yamamoto M, Yamaguchi T, Yamauchi M, et al. : Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2008, 93(3): 1013–1019. [DOI] [PubMed] [Google Scholar]
- [69].Silva MJ, Brodt MD, Lynch MA, et al. : Type 1 diabetes in young rats leads to progressive trabecular bone loss, cessation of cortical bone growth, and diminished whole bone strength and fatigue life. J Bone Miner Res 2009, 24(9): 1618–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nyman JS, Even JL, Jo CH, et al. : Increasing duration of type 1 diabetes perturbs the strength-structure relationship and increases brittleness of bone. Bone 2011, 48(4): 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fantner GE, Hassenkam T, Kindt JH, et al. : Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater 2005, 4(8): 612–616. [DOI] [PubMed] [Google Scholar]
- [72].Fantner GE, Adams J, Turner P, et al. : Nanoscale ion mediated networks in bone: osteopontin can repeatedly dissipate large amounts of energy. Nano Lett 2007, 7(8): 2491–2498. [DOI] [PubMed] [Google Scholar]
- [73].Thurner PJ, Chen CG, Ionova-Martin S, et al. : Osteopontin deficiency increases bone fragility but preserves bone mass. Bone 2010, 46(6): 1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sroga GE, Karim L, Colon W, Vashishth D: Biochemical characterization of major bone-matrix proteins using nanoscale-size bone samples and proteomics methodology. Mol Cell Proteomics 2011, 10(9): M110 006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Nyman JS, Roy A, Shen X, et al. : The influence of water removal on the strength and toughness of cortical bone. J Biomech 2006, 39(5): 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wilson EE, Awonusi A, Morris MD, et al. : Three structural roles for water in bone observed by solid-state NMR. Biophys J 2006, 90(10): 3722–3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nyman JS, Ni Q, Nicolella DP, Wang X: Measurements of mobile and bound water by nuclear magnetic resonance correlate with mechanical properties of bone. Bone 2008, 42(1): 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Horch RA, Gochberg DF, Nyman JS, Does MD: Non-invasive predictors of human cortical bone mechanical properties: T(2)-discriminated H NMR compared with high resolution X-ray. PloS One 2011, 6(1): e16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Boskey AL: Assessment of bone mineral and matrix using backscatter electron imaging and FTIR imaging. CurrOsteoporos Rep 2006, 4(2): 71–75. [DOI] [PubMed] [Google Scholar]
- [80].Draper ER, Morris MD, Camacho NP, et al. : Novel assessment of bone using time-resolved transcutaneous Raman spectroscopy. J Bone Miner Res 2005, 20(11): 1968–1972. [DOI] [PubMed] [Google Scholar]
- [81].Schulmerich MV, Cole JH, Kreider JM, et al. : Transcutaneous Raman spectroscopy of murine bone in vivo. Appl Spectrosc 2009, 63(3): 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Boskey AL, Moore DJ, Amling M, et al. : Infrared analysis of the mineral and matrix in bones of osteonectin-null mice and their wildtype controls. J Bone Miner Res 2003, 18(6): 1005–1011. [DOI] [PubMed] [Google Scholar]
- [83].Timlin JA, Carden A, Morris MD, et al. : Raman spectroscopic imaging markers for fatigue-related microdamage in bovine bone. Anal Chem 2000, 72(10): 2229–2236. [DOI] [PubMed] [Google Scholar]
- [84].Yerramshetty JS, Akkus O: The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone 2008, 42(3): 476–482. [DOI] [PubMed] [Google Scholar]
- [85].Ager JW, Nalla RK, Breeden KL, Ritchie RO: Deep-ultraviolet Raman spectroscopy study of the effect of aging on human cortical bone. J Biomed Opt 2005, 10(3): 034012. [DOI] [PubMed] [Google Scholar]
- [86].Morris MD, Mandair GS: Raman assessment of bone quality. Clin Orthop 2011, 469(8): 2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Paschalis EP, Betts F, DiCarlo E, et al. : FTIR microspectroscopic analysis of human iliac crest biopsies from untreated osteoporotic bone. Calcif Tissue Int 1997, 61(6): 487–492. [DOI] [PubMed] [Google Scholar]
- [88].Boskey AL, Dicarlo E, Paschalis E, et al. : Comparison of mineral quality and quantity in iliac crest biopsies from high- and low-turnover osteoporosis: an FT-IR microspectroscopic investigation. Osteoporos Int 2005, 16(12): 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gourion-Arsiquaud S, Faibish D, Myers E, et al. : Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res 2009, 24(9): 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gourion-Arsiquaud S, Allen MR, Burr DB, et al. : Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone 2010, 46(3): 666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Paschalis EP, Glass EV, Donley DW, Eriksen EF: Bone mineral and collagen quality in iliac crest biopsies of patients given teriparatide: new results from the fracture prevention trial. J Clin Endocrinol Metab 2005, 90(8): 4644–4649. [DOI] [PubMed] [Google Scholar]
- [92].McCreadie BR, Morris MD, Chen TC, et al. : Bone tissue compositional differences in women with and without osteoporotic fracture. Bone 2006, 39(6): 1190–1195. [DOI] [PubMed] [Google Scholar]
- [93].Balooch G, Balooch M, Nalla RK, et al. : TGF-beta regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci U S A 2005, 102(52): 18813–18818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chang JL, Brauer DS, Johnson J, et al. : Tissue-specific calibration of extracellular matrix material properties by transforming growth factor-beta and Runx2 in bone is required for hearing. EMBO reports 2010, 11(10): 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mohammad KS, Chen CG, Balooch G, et al. : Pharmacologic inhibition of the tgf-Beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS ONE 2009, 4(4): e5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Edwards JR, Nyman JS, Lwin ST, et al. : Inhibition of TGF-beta signaling by 1D11 antibody treatment increases bone mass and quality in vivo. J Bone Miner Res 2010, 25(11): 2419–2426. [DOI] [PubMed] [Google Scholar]
- [97]•.Nyman JS, Lynch CC, Perrien DS, et al. : Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. J Bone Miner Res 2011, 26(6): 1252–1260.In an assessment of bones from wild-type and genetic knock-out mice, this study found that the loss of matrix metalleoproteinase (MMP-) 2 decreased bone strength, whereas the loss of MMP-9 decreased bone toughness, thereby suggesting that matrix processing proteins are important to the fracture resistance of bone.
- [98].Elefteriou F, Benson MD, Sowa H, et al. : ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab 2006, 4(6): 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wang W, Nyman JS, Moss HE, et al. : Local low-dose lovastatin delivery improves the bone-healing defect caused by Nf1 loss of function in osteoblasts. J Bone Miner Res 2010, 25(7): 1658–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wang W, Nyman JS, Ono K, et al. : Mice lacking Nf1 in osteochondroprogenitor cells display skeletal dysplasia similar to patients with neurofibromatosis type I. Hum Mol Genet 2011, 20(20): 3910–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]


