Abstract
Background:
Anxiety in bipolar disorder (BD) exacerbates emotion dysregulation and reduces treatment response. We recently conducted a pilot trial of transdiagnostic CBT to target anxiety and emotion dysregulation in BD adjunctive to pharmacotherapy. Reductions in depression and anxiety symptoms were significantly predicted by baseline levels of neuroticism and perceived affective control, as well as changes over time in emotion regulation skills. The present study investigates mechanism of treatment response by examining the relationship between baseline emotion regulation-related neural circuitry and trial outcomes.
Methods:
Nineteen patients completed baseline resting state fMRI scans prior to treatment randomization. Functional connectivity between the anterior insula (AI) and regions in the salience network (SN), default mode network (DMN), and executive control network (ECN) were examined as predictors of baseline and treatment-related changes in emotion regulation.
Results:
Greater improvements in emotion regulation were predicted by weaker right dorsal AI – right ventrolateral prefrontal cortex (VLPFC; SN) and stronger bilateral dorsal AI – bilateral amygdala functional connectivity. Baseline neuroticism was negatively correlated with right dorsal AI- inferior parietal lobule (ECN) functional connectivity, and baseline deficits in perceived affective control were positively associated with ventral AI – bilateral dACC (SN) connectivity.
Limitations:
Small sample limits interpretability of treatment-specific effects.
Conclusion:
Baseline functional connectivity of emotion-regulation related neural circuitry significantly predicted change in emotion regulation-related dimensions associated with anxiety and depression symptom reduction. Future studies are needed to determine if employing methods such as neuromodulation to rehabilitate relevant neural circuitry may improve ultimate treatment outcomes of transdiagnostic CBT for BD and anxiety.
Keywords: Bipolar disorder, anxiety disorders, emotion regulation, anterior insula, functional connectivity networks
Introduction
Bipolar disorder (BD) is a chronic and debilitating disorder associated with a range of negative outcomes, including impaired social and occupational functioning, substance dependence, and suicidality. These poor outcomes are exacerbated by the presence of comorbid anxiety, which affects over a third of all patients presenting for treatment and as high as 86% of patients over the course of a lifetime (Merikangas et al., 2007; Simon et al., 2004). Comorbid anxiety in BD is associated with a range of poorer outcomes and prognoses, including greater disorder chronicity, greater disorder severity, greater functional impairment, and increased suicidality (Deckersbach et al., 2014; Goldberg and Fawcett, 2012; Lee and Dunner, 2008; Otto et al., 2006). Developing viable treatments that target not only mood symptoms but also anxiety symptoms in BD is of paramount importance towards improving outcomes for these patients.
Treatments that specifically focus on improving emotion regulation skills are particularly needed for the treatment of BD and anxiety. BD is characterized by both emotion lability and the inability to adaptively manage or regulate emotional experiences, and this dysregulation has been shown to be further intensified by anxiety symptoms (Heissler et al., 2014). Several researchers have identified a link between chronic emotion dysregulation in BD and impulsive and self-destructive behaviors, interpersonal problems, disruptions in work productivity, and increased suicidality (Kessler et al., 2006; Muhtadie et al., 2014; Phillips and Vieta, 2007; Samalin et al., 2016; Swann et al., 2009; Van Rheenen et al., 2015; Wolkenstein et al., 2014). Thus, developing viable treatments that focus on targeting and improving emotion regulation skills may also be particularly beneficial for individuals with BD.
We recently tested the application of a transdiagnostic cognitive behavioral therapy (CBT) to the treatment of euthymic BD and comorbid anxiety using the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders (UP; (Ellard et al., 2017). The UP is grounded in emotion science and affective neuroscience and was developed specifically to target maladaptive emotion processing as a transdiagnostic process that contributes to both mood and anxiety symptoms, with the aim to increase adaptive emotion regulation skills (Barlow et al., 2010a; Barlow et al., 2010b; Ellard et al., 2010). Treatment with the UP adjunctive to pharmacotherapy treatment as usual (UP+TAU) was compared to TAU alone. Results from this trial found significantly greater linear reductions in both anxiety and depression in the UP+TAU group relative to TAU alone. In addition to symptom-based outcomes, we collected outcomes on dimensions related to emotion dysregulation, including perceived control of emotions, difficulties with emotion regulation skills, and trait neuroticism. UP-related changes in anxiety and depression were negatively associated with baseline levels of neuroticism, perceived control of emotions, and difficulties in emotion regulation. In addition, UP-related changes in anxiety symptoms were positively correlated with improvements in perceived affective control and emotion regulation skills, whereas UP-related changes in depression symptoms were positively correlated with changes in neuroticism.
These outcomes are important because, whereas the overall outcomes from this pilot study were encouraging, not everyone treated with the UP benefitted. Those patients with elevated trait neuroticism, less perceived control of emotions, and greater deficits in baseline emotion regulation skills fared less well than those with baseline scores on these dimensions closer to a normative range. In addition, the degree to which perceived control of emotions and emotion regulation skills changed as a result of treatment was significantly related to reduction in anxiety and depression symptoms. Therefore, the results from this pilot trial suggest both baseline integrity in emotion regulation ability and the malleability of emotion regulation skills portend ultimate treatment gains using a transdiagnostic CBT approach in BD.
Existing studies of healthy individuals implicate a distributed neurocircuitry underlying successful emotion regulation. Specifically, converging evidence shows the ventrolateral prefrontal cortex (VLPFC), medial PFC (mPFC), anterior cingulate cortex (ACC), anterior insula and amygdala form a distributed network supporting adaptive emotion regulation. (Kohn et al., 2014; Phillips et al., 2003; Phillips et al., 2008) Within this circuitry, amygdala and anterior insula serve to signal salience and sensory information (Phillips et al., 2003), whereas the VLPFC, ACC, and mPFC integrate and regulate this information according to cognitive or situational demands (Cabeza and Nyberg, 2000; Ghashghaei et al., 2007). Increased VLPFC activation coupled with decreased amygdala activation has been found in studies of emotion regulation (Ochsner et al., 2002), motivation and behavioral inhibition (Aron, 2007), suggesting the VLPFC plays a particularly key role in maintaining emotional homeostasis for healthy, adaptive functioning. Studies of both implicit and explicit emotion regulation have identified the VLPFC as playing a pivotal role in successful regulation (Ochsner et al., 2002; Tupak et al., 2014). Additionally, whereas there has been less of a focus on the role of the anterior insula in emotion regulation studies than other regions such as the amygdala, VLPFC or DLPFC, this region has been recently highlighted for its role in flexible, adaptive switching between neural networks supporting executive control, salience processing, or default-mode processing (Sridharan et al., 2008). The VLPFC, in conjunction with the anterior insula has been shown to form a fronto-insular network that plays a key role in regulating the intensity of emotional responses (Sridharan et al., 2008).
Neuroimaging studies of individuals with BD consistently point to hypoactivation of bilateral VLPFC coupled with hyperactivation of limbic structures in the context of emotion processing, as well as aberrant amygdala-VLPFC functional connectivity (Chase and Phillips, 2016; Dima et al., 2013; Foland-Ross et al., 2012; Kanske et al., 2015; Morris et al., 2012; Phillips and Swartz, 2014; Pompei et al., 2011; Townsend et al., 2013). For example, relative to healthy controls, decreased VLPFC activation and corresponding increased amygdala activation has been demonstrated during emotion regulation using cognitive reappraisal in euthymic BD patients (Kanske et al., 2015). Decreased VLPFC activation, and corresponding increased amygdala activation, has also been demonstrated during performance on emotional Stroop and affect labeling tasks (Dima et al., 2013; Lagopoulos and Malhi, 2007; Malhi et al., 2005; Pompei et al., 2011). Several studies have shown increased functional connectivity between amygdala and VLPFC during emotion regulation and at rest, suggesting inefficient downregulation of amygdala by VLPFC (Chase and Phillips, 2016; Townsend et al., 2013). This suggests euthymic BD is associated with a functional disruption in a key neural pathway by which the VLPFC exerts regulatory control over amygdala responses. Thus, impaired recruitment of VLPFC and disrupted functional connectivity between VLPFC and the broader neurocircuitry supporting emotion regulation may play a key role in the severe emotion dysregulation seen in bipolar disorder.
In the current study, we sought to examine the association between baseline functional connectivity of emotion regulation related neurocircuitry and transdiagnostic CBT outcomes in order to better understand specific factors that might influence the ability for BD patients to ultimately benefit from this approach to treatment. Based on outcomes from our pilot trial, we wished to understand the relationship between the intrinsic functional connectivity of neural networks supporting adaptive emotion regulation and baseline levels of neuroticism, perceived affective control, and difficulties with emotion regulation. In addition, we examined intrinsic neural functional connectivity at baseline as a predictor of changes in these dimensions. We focused our functional connectivity analyses on neural networks implicated in the regulation of emotion and cognition; specifically, we took a seed-to-target approach, using the anterior insula as our seed region, and target regions from the executive control (ECN), default mode (DMN), and salience networks (SN; (Seeley et al., 2007; Yeo et al., 2011). We selected the anterior insula as the seed region as this region has been identified as a key neural hub implicated in flexible switching between processing modes (external, goal directed processing via ECN; internally focused processing via the DMN, and salience processing via SN), representing an important component of adaptive emotion regulation (Aldao et al., 2015; Goulden et al., 2014; Menon and Uddin, 2010; Sridharan et al., 2008). Thus, functional connectivity of broader functional neurocircuitry with the anterior insula represents an important neural pathway through which adaptive emotion regulation is supported. In addition, given the role of salience and reward processing in dysregulation in BD, we examined functional connectivity of the anterior insula to salience (amygdala) and reward processing regions (nucleus accumbens, ventral tegmental area). Given the existing literature discussed above, we were particularly interested in the relationship between insula-VLPFC functional connectivity and treatment outcomes.
Methods
Participants
Participants were recruited from the Massachusetts General Hospital (MGH) Dauten Family Center for Bipolar Treatment Innovation. The MGH Institutional Review Board approved the study protocol and participants provided written informed consent prior to the initiation of any study procedure. Psychiatric diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (SCID-IV;(First et al., 1997) and mood episode severity was assessed using the Hamilton Depression Rating Scale (HAM-D;(Hamilton, 1960) and Young Mania Rating Scale (Young et al., 1978). Eligible participants met criteria for bipolar disorder, did not meet criteria for a major depressive or manic episode at study entry (HAM-D-17 ≤16 and YMRS ≤12), and met criteria for at least one of the following anxiety disorders: generalized anxiety disorder (n = 19), panic disorder (n = 14), or social phobia (n = 18). All participants were required to have at least 3 months of stability on their current dosage(s) of medication(s), and were asked to maintain this dosage throughout the study period. Stable maintenance pharmacotherapy was defined as maximum tolerated dosages according to Texas Implementation of Medication Algorithm (Suppes et al., 2005), as prescribed by a psychiatrist. Individuals were excluded if they endorsed current suicidal ideation; history of seizure disorder, brain injury, or neurological disease; met criteria for a psychotic disorder; reported psychotic symptoms; met criteria for a substance use disorder within the past 12 months, or had received electroconvulsive therapy (ECT) within the six months preceding study enrollment. All eligible participants were offered participation in resting state fMRI scans at baseline, prior to randomization. Participation in the fMRI study was optional and not a condition for participating in the treatment trial. Nineteen patients agreed to take part in the adjunctive resting state fMRI study and are included in the present analyses. Participants completed baseline fMRI scans, and were randomly assigned to either continued psychopharmacological treatment as usual (TAU, n=8) or TAU plus 18 weekly sessions of the UP (UP+TAU, n=11). Table 1 shows baseline characteristics of these participants.
Table 1.
Study Demographics.
| Variable | Whole Sample N = 19 | UP + TAU n = 11 | TAU n = 8 | Sig. P | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age | 44.05 (15.42) | 43.08 (13.84) | 44.25 (14.27) | 0.49 | |
| N (%) | n (%) | n (%) | |||
| Gender | |||||
| Male | 10 (52.6) | 5 (45.5) | 5 (62.5) | 0.55 | |
| Female | 9 (47.4) | 6 (54.5) | 3 (37.5) | ||
| Race | 0.55 | ||||
| White | 16 (84.2) | 10 (90.9) | 6 (75.0) | ||
| Black | 2 (10.5) | 1 (9.1) | 1 (12.5) | ||
| Other or unreported | 1 (5.2) | 0 (0.0) | 1 (12.5) | ||
| Ethnicity | 0.90 | ||||
| Hispanic/Latino | 1 (5.26) | 1 (9.1) | 1 (12.5) | ||
| Not Hispanic/Latino | 16 (84.2) | 9 (81.8) | 7 (87.5) | ||
| Unreported | 2 (5.3) | 1 (9.1) | 0 (0.0) | ||
| Education | 0.84 | ||||
| Graduate School | 5 (26.3) | 3 (27.3) | 2 (25.0) | ||
| College Graduate | 8 (42.1) | 5 (45.5) | 3 (37.5) | ||
| Partial College | 5 (26.3) | 2 (18.2) | 3 (37.5) | ||
| High School Graduate | 1 (5.3) | 1 (9.1) | 0 (0.0) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Medication Load* | |||||
| Mood Stabilizers | 1.68 (1.13) | 1.76 (1.05) | 1.57 (1.30) | 0.74 | |
| Antidepressants | 1.04 (1.01) | 0.94 (1.17) | 1.17 (0.63) | 0.64 | |
| Anxiolytics/Hypnotics | 0.44 (0.52) | 0.54 (0.63) | 0.30 (0.28) | 0.34 | |
| Baseline Symlptoms | |||||
| HAM-D | 11.00 (6.38) | 9.45 (6.76) | 13.13 (5.52) | 0.23 | |
| HAM-A | 14.63 (7.34) | 12.91 (7.83) | 17.00 (6.30) | 0.24 | |
| YMRS | 3.37 (3.11) | 3.45 (2.59) | 3.25 (3.92) | 0.89 | |
| ACS | 4.28 (0.48) | 4.26 (0.45) | 4.32 (0.53) | 0.80 | |
| DERS | 2.78 (0.56) | 2.79 (0.25) | 2.77 (0.64) | 0.94 | |
| NEO-N | 5.73 (1.45) | 5.64 (1.12) | 5.89 (1.89) | 0.73 | |
Note: HAM-D = Hamilton Depression Rating Scale. HAM-A = Hamilton Anxiety Rating Scale. YMRS = Young Mania Rating Scale. ACS = Affective Control Scale. DERS = Difficulties in Emotion Regulation Scale. NEO-N = NEO Five Factor Inventory – Neuroticism.
Means and standard deviations of standardized medication load metric, calculated by dividing individual patient’s medication dosages by the World Health Organization (WHO) Defined Daily Dose for that medication (ATC/DDD; https://www.whocc.no/).
Emotion regulation measures
Affective Control Scale (ACS;(Williams et al., 1997).
The ACS is a 42-item self-report measure designed to assess perceived controllability of emotions and fear of loss of control when experiencing strong affective states. ACS subscales expand on the construct of fear of fear, including fear of anxiety, fear of depression, fear of anger, and fear of strong positive affective states. Higher scores indicate less perceived control of emotions. The ACS has demonstrated acceptable internal consistency, test-retest reliability, and concurrent and divergent validity (Berg et al., 1998; Williams et al., 1997).
Difficulties in Emotion Regulation Scale (DERS;(Gratz and Roemer, 2004).
The DERS is a 36-item, self-report measure developed to assess clinically relevant difficulties in emotion regulation, including 1) nonacceptance of emotional responses (Nonacceptance), 2) difficulties engaging in goal-directed behaviors (Goals), 3) impulse control difficulties (Impulse), 4) lack of emotional awareness (Awareness), 5) limited access to emotion regulation strategies (Strategies), 6) and lack of emotional clarity (Clarity). Higher scores indicate greater difficulties with emotion regulation. The DERS has demonstrated adequate internal consistency and adequate test-retest reliability (Fowler et al., 2014; Gratz and Roemer, 2004).
NEO Five-Factor Inventory (NEO-FFI;(Costa and McCrea, 1992).
The NEO-FFI was developed to assess five domains of personality: (1) neuroticism (NEO-N), the tendency to experience negative emotions in response to stressors; (2) extraversion (NEO-E), the tendency towards increased sociability, positive emotionality, and general activity; (3) openness to experience (NEO-O), the tendency towards curiosity versus conservativeness; (4) agreeableness (NEO-A), the tendency towards altruistic and cooperative behavior; and (5) conscientiousness (NEO-C), the tendency towards thoughtful and deliberative planning and organization. The NEO-FFI contains 60 items rated on a 5-point Likert-type scale (1 = strongly disagree to 5 = strongly agree). Scores are used to derive five separate domain scores (12 items per domain), with higher scores indicating greater tendency towards that specific personality dimension. Each of the five domains of the NEO FFI has been found to possess adequate internal consistency and temporal stability (Costa and McCrea, 1992; Robins et al., 2001). For the current study, we focused on the dimension of Neuroticism, as increased trait neuroticism has been linked to both increased emotion dysregulation and increased anxiety and mood-related psychopathology (Barlow et al., 2014).
MRI acquisition
Subjects were scanned using a 3-Tesla Siemens Skyra MRI scanner. Structural data was acquired with an anatomical T1-weighted multi-echo magnetization prepared rapid gradient-echo sequence with parameters: Repetition time (TR) = 2530ms, echo time (TE) = 1.69ms, inversion time (TI) = 1100ms, flip angle = 7.0°, number of excitations = 1, slice thickness = 1mm, field of view (FoV) = 256mm, in-plane resolution = 1.0 × 1.0mm, and a matrix of 256 × 256. Resting-state BOLD data was collected with a whole-brain echoplanar imaging sequence with the following parameters: TR = 3000ms, TE = 30ms, flip angle = 85°, slice thickness = 3.0mm, in-plane resolution = 3.0 × 3.0mm, FoV = 216mm. Subjects were instructed to keep eyes open and try to stay focused passively on a fixation cross displayed behind them during the resting-state scan (white cross on black background).
MRI Data preprocessing
MRI preprocessing and first level analyses were carried out with a combination of tools from FSL v5.0.4 (FMRIB, Oxford, UK) and SPM2 (Wellcome Department of Cognitive Neurology, London, UK) using in-house scripts as previously described in Van Dijk et al. (Van Dijk et al., 2010). First four volumes were dropped to allow for T1 equilibration effects. Slice-time correction was done using SPM2. Six degree-of-freedom rigid body translation and rotation were used to correct for head motion using FSL. Spatial normalization to the Montreal Neurological Institute (MNI) atlas space, resampled to 2mm isotropic voxels spatially smoothed using a 6mm full-width half-maximum (FWHM) Gaussian kernel, band-pass filtered between 0.01 and 0.08 Hz. Sources of spurious variance and their first temporal derivatives were removed with whole brain linear regression and the residual BOLD time-courses were retained for functional connectivity analysis.
Anatomical Mask Definition
Bilateral dorsal AI seed regions of interest (ROI) were defined using masks from the Kelly et al. (Kelly et al., 2012) three-cluster solution (http://fcon_1000.projects.nitrc.org; Figure 1A). Selection of target ROIs from the ECN, SN, and DMN were defined using max voxel locations as described in Seeley et al. (Seeley et al., 2007). Target nodes for the ECN included bilateral posterior parietal cortex (in a region of inferior parietal lobule, IPL), and bilateral dorsolateral prefrontal cortex (DLPFC). Target nodes for the DMN included bilateral posterior cingulate cortex and bilateral vmPFC. Target nodes for the SN included bilateral VLPFC and bilateral dorsal anterior cingulate (see Figure 1B, which includes MNI coordinates). Bilateral amygdala, nucleus accumbens and ventral tegmental area (vta) ROIs were defined using masks provided by the Anatomical Automatic Labeling tool in the Wake Forest University PickAtlas (Maldjian et al., 2004; Maldjian et al., 2003; Tzourio-Mazoyer et al., 2002). Spherical masks were created around identified coordinates with a 10mm diameter for cortical ROIs, and a 5mm diameter for amygdala ROIs, using the MarsBar SPM toolbox.
Figure 1.

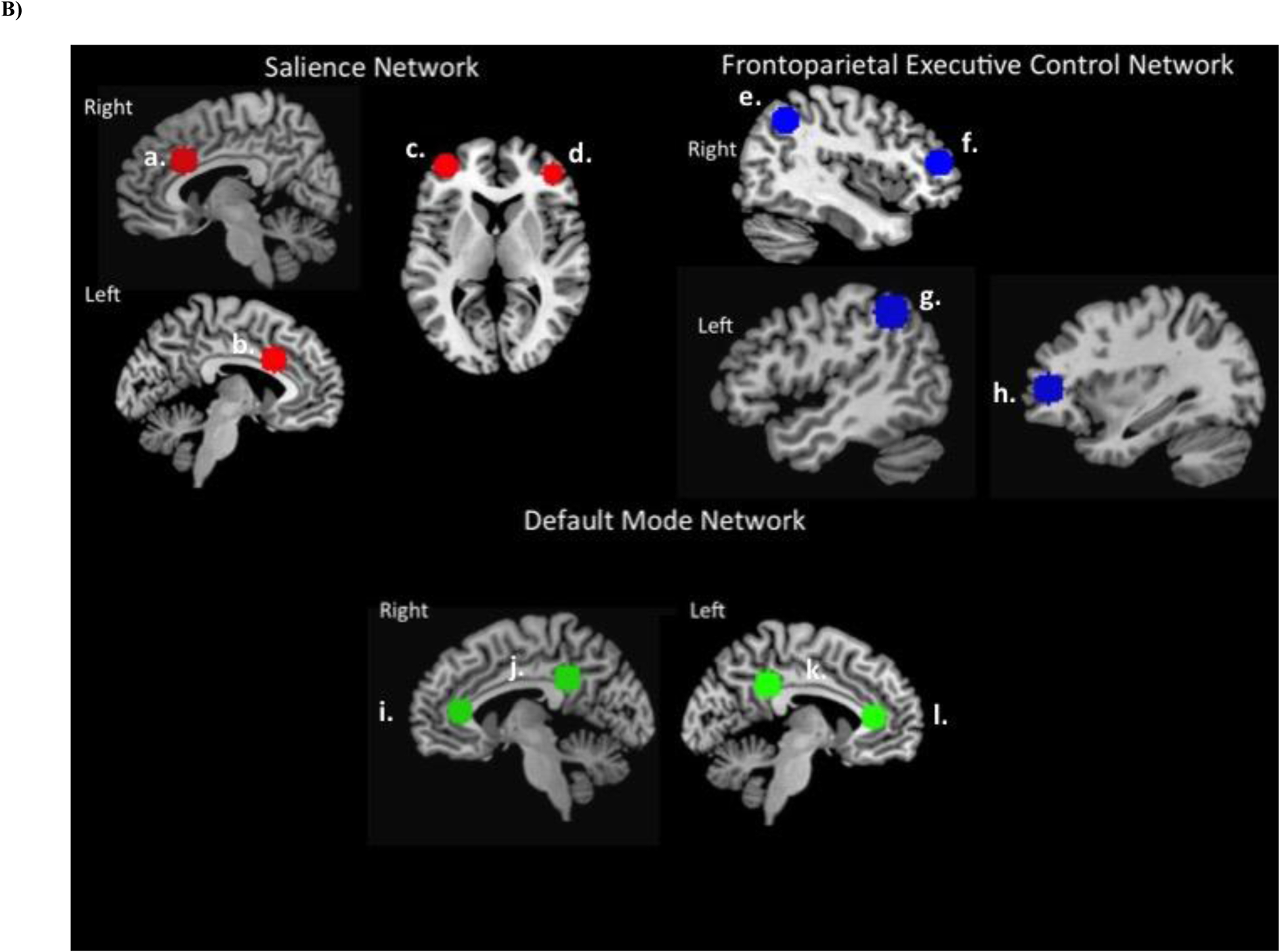
Functional network ROI locations. A) Anterior insula ROIs. Masked clusters include bilateral 2mm spherical masks from dorsal and ventral mid anterior insula, derived from Kelly et al. (2012). Masks available for download at http://fcon_1000.projects.nitrc.org. B) Functional network target regions. ROI masks derived from the Wake Forest University Pickatlas (WFU PickAtlas) Automatic Anatomical Labeling tool using 10mm spherical ROIs. Center of spheres selected from functional network max voxel locations specified in Seeley et al. (2007), listed as MNI coordinates (x, y, z). Salience network (SN): (a) right dACC (6, 22, 30); (b) left dACC (−6, 18, 30); (c) left VLPFC/DLPFC (−38, 52, 10); (d) right VLPFC (42, 46, 0); Frontoparietal executive control network (ECN): (e) right lateral parietal (38, −56, 44), (f) right DLPFC (46, 46, 14); (g) left inferior parietal lobule (−48, −48, 48); (h) left DLPFC (−34, 46, 10). Default mode network (DMN): (i) right vmPFC (2, 36, 10); (j) right posterior cingulate cortex (7, −43, 33); (k) left posterior cingulate cortex (−7, −43, 33); (l) left vmPFC (−2, 36, 10)
Medication Load
To control for baseline effects of medications, a standardized medication load metric was calculated for each individual by dividing the patient’s medication dosages by the World Health Organization (WHO) Defined Daily Dose for that medication (ATC/DDD; https://www.whocc.no/). Medications were then classified into three classes (mood stabilizers, antidepressants, anxiolytics/hypnotics). Medication load values for each medication class were then entered separately into regression models.
Statistical Analyses
Resting-State fMRI Analyses.
Connectivity maps were obtained at the individual subject level for bilateral dorsal and ventral AI seed regions by averaging the signal across all voxels in the ROI and calculating Pearson’s product moment correlation between the mean ROI time-series and the time-series from each whole brain acquired voxel. Correlation maps were converted to z-maps using Fisher’s r-to-z transformation. Mean Fisher’s z-transformed values from target ROI masks were extracted using MarsBar and imported into SPSS (IBM, version 24.0) for analysis.
Relationship between functional connectivity and emotion regulation dimensions.
To examine the relationship between treatment-relevant emotion regulation dimensions and intrinsic functional connectivity at baseline, a series of bivariate Pearson’s correlations were conducted between z-transformed functional connectivity values and baseline measures of perceived control (ACS), emotion regulation skills (DERS), and neuroticism (NEO-N). We employed bootstrap methods to control for multiple comparisons (10,000 iterations, resample and replace). Bootstrap-derived 95% confidence intervals (CI) are reported for Pearson’s r coefficients.
To examine neural predictors of treatment-related change in emotion regulation dimensions, linear regressions were conducted with z-transformed connectivity values as the independent variable and the slope of change in ACS, DERS, and NEO-N entered as the dependent variables. Slope of change was calculated using the following formula:
In addition, gender, age, and medication load were entered as independent variables of no interest. Given the small sample size, linear regressions were run using the entire sample. We again employed bootstrap methods to all regression analyses to control for multiple comparisons (10,000 iterations, resample and replace). Bootstrap-derived 95% confidence intervals (CI) are reported for unstandardized B coefficients. As an exploratory analysis, significant results from these linear regressions were repeated in each treatment group separately to explore treatment-related relationships between emotion regulation-related neurocircuitry and emotion regulation outcomes.
Results
At study entry, treatment groups did not differ in gender, race, ethnicity, age, education, or medication load (Table 1). Treatment groups did not significantly differ on any clinical measure at baseline (p’s >.05).
Functional connectivity and baseline emotion regulation.
Baseline levels of neuroticism (NEO-N) were significantly negatively correlated with functional connectivity between anterior insula and the executive control network; specifically, right dorsal anterior insula – left IPL (r = −.44, p = .05, Bootstrap 95% CI (Pearson’s r): −.69, −.02, Figure 2A).
Figure 2.
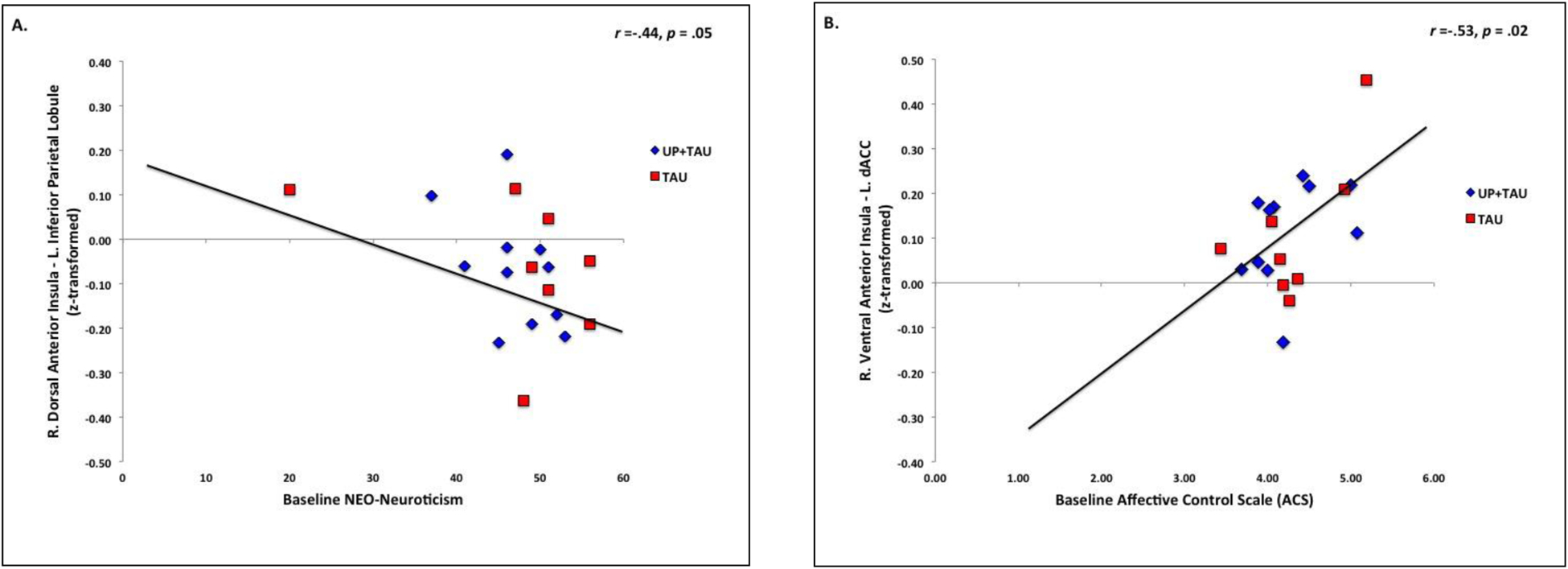
Relationship (Pearson’s r) between A) baseline levels of neuroticism and right dorsal AI – left IPL functional connectivity; and B) baseline levels of perceived affective control and right ventral AI – right dACC functional connectivity. Functional connectivity values reflect r-to-z-transformed scores. Higher NEO-Neuroticism scores reflect higher levels of neuroticism. Higher ACS scores reflect greater deficits in perceived affective control.
Baseline levels of affective control (ACS) were significantly correlated with functional connectivity between anterior insula and the salience network. Specifically, baseline deficits in affective control were positively associated with right ventral anterior insula – left dACC functional connectivity (r = .53, p = .02, Bootstrap 95% CI (Pearson’s r): .13, .78, Figure 2B), and negatively correlated with right ventral anterior insula – right VLPFC functional connectivity, but this finding did not survive corrections (r = −.46, p = .05; Bootstrap 95% CI (Pearson’s r): −.93, .37).
No significant correlations were found between baseline emotion regulation skills (DERS) and functional connectivity between anterior insula and salience, default mode or executive control network regions.
Functional connectivity as a predictor of change in affective control (ACS).
Greater reductions over time in ACS scores across all patients were significantly predicted by weaker baseline functional connectivity between anterior insula and the salience network; specifically, left anterior insula – right VLPFC functional connectivity (left ventral anterior insula - right VLPFC: ß = 1.12, t = 5.34, p = .001, Bootstrap 95% CI (B-value): 16.15, 66.20; Figure 3A; left dorsal anterior insula - right VLPFC: ß = .74, t = 3.77, p = .005, Bootstrap 95% CI (B-value): 1.81, 35.27). Significantly weaker functional connectivity between right dorsal anterior insula and right VLPFC was also found, but this did not survive corrections (ß = .85, t = 3.63, p = .007, Bootstrap 95% CI (B-value): −3.73, 27.93; Figure 3B).
Figure 3.
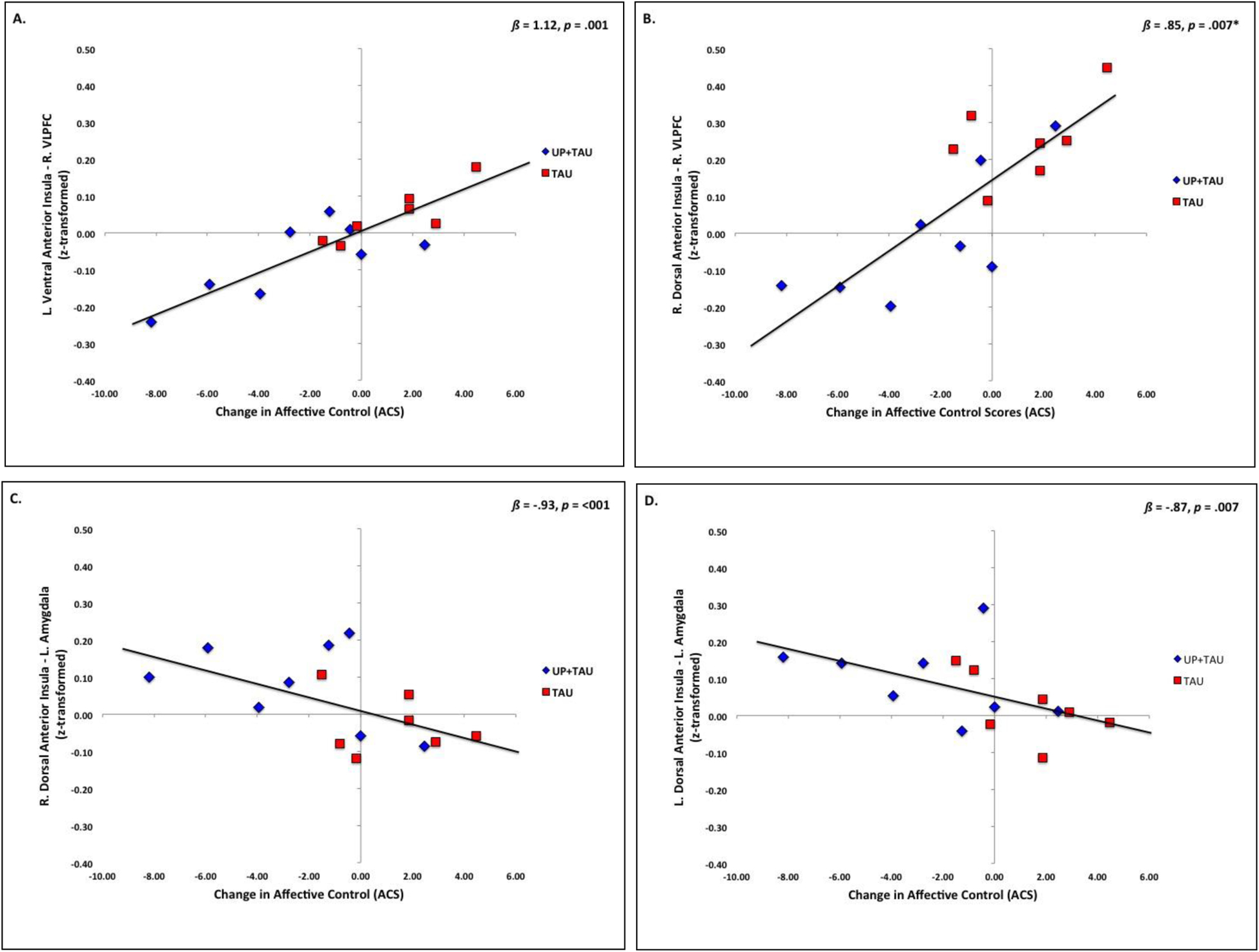
Relationship (Pearson’s r) between slope of change (slope b) in perceived affective control (ACS scores) and baseline functional connectivity between A) left ventral anterior insula – right VLPFC; B) right dorsal anterior insula – right VLPFC; C) right dorsal anterior insula – left amygdala; and D) left dorsal anterior insula – left amygdala. Functional connectivity values reflect r-to-z-transformed scores. Change slope scores represent the slope of change in ACS scores over 6 monthly assessments. Negative change score values represent linear reductions in ACS scores over time. Higher ACS scores reflect greater deficits in perceived affective control. Displayed ß and p-values represent results of linear regression models controlling for age, gender, and medication load. *Result shown is uncorrected; did not survive corrections for multiple comparisons.
Greater reductions in time in ACS scores were also significantly predicted by stronger bilateral dorsal anterior insula – bilateral amygdala functional connectivity (left dorsal anterior insula - left amygdala: ß = −.87, t = −3.60, p = .007, Bootstrap 95% CI: −64.03, −4.23, Figure 3C; left dorsal anterior insula - right amygdala: ß = −.78, t = −3.33, p = .01; Bootstrap 95% CI: −48.69, −3.08; right dorsal anterior insula - left amygdala: ß = −.93, t = −5.76, p = <.001, Bootstrap 95% CI: −46.75, −12.52, Figure 3D), with the strongest effect in right dorsal anterior insula – left amygdala functional connectivity. Right dorsal anterior insula – right amygdala functional connectivity also significantly predicted changes in ACS, but this result did not survive corrections for multiple comparisons (ß = −.66, t = −3.03, p = .02, Bootstrap 95% CI: −54.59, 0.33). Anterior insula functional connectivity to executive control network, default mode network, or reward processing regions was not a significant predictor of changes in affective control.
Looking within treatment groups, reductions in ACS scores were significantly predicted by weaker right dorsal anterior insula -right VLPFC functional connectivity in the UP+TAU group (right dorsal anterior insula - right VLPFC: ß = .75, t = 2.82, p = .05, Bootstrap 95% CI: 6.03, 30.42); but not the TAU group (right dorsal anterior insula - right VLPFC: ß = .51, t = 1.31, p = .25, Bootstrap 95% CI: −15.31, 18.27). Weaker left ventral anterior insula - right VLPFC functional connectivity was a significant predictor of ACS score reductions for both groups (UP+TAU: ß =.76, t = 2.89, p = .03, Bootstrap 95% CI: 4.39, 45.77; TAU: ß =.87, t = 3.99, p = .01, Bootstrap 95% CI: 14.60, 41.15).
Stronger right dorsal anterior insula - left amygdala functional connectivity was a significant predictor of reductions in ACS scores for the UP+TAU group (ß = −1.46, t = −6.69, p = .003, Bootstrap 95% CI: −97.40, −30.17), but not TAU (ß = −.48, t = −0.97, p = .40, Bootstrap 95% CI: −25.63, 23.63). By contrast, stronger left dorsal anterior insula - bilateral amygdala functional connectivity was a significant predictor of reductions in ACS scores for the TAU group (left dorsal anterior insula - left amygdala: ß = −.80, t = −3.73, p = .03, Bootstrap 95% CI: −34.89, −2.74); left dorsal anterior insula - right amygdala: ß = −.97, t = −6.16, p = .009, Bootstrap 95% CI: −30.72, −9.79), but not the UP+TAU group (left dorsal anterior insula - left amygdala: ß = −.76, t = −2.13, p = .10, Bootstrap 95% CI: −56.64, 7.520; left dorsal anterior insula - right amygdala: ß = −.53, t = −1.40, p = .24; Bootstrap 95% CI: −53.18, 17.58).
Functional connectivity as a predictor of change in emotion regulation skills (DERS).
Greater changes over time in emotion regulation skills (DERS) across all patients were significantly predicted by weaker baseline functional connectivity between anterior insula and the salience network, but results did not survive bootstrap corrections (left ventral AI – right VLPFC: ß = 0.83, t = 2.22, p = .05, Bootstrap 95% CI: −34.63, 70.94). Reductions over time in DERS scores across all patients were also significantly predicted by weaker baseline functional connectivity between anterior insula and reward processing regions, but these results also did not survive corrections (left ventral AI – right NAcc: ß = 0.70, t = 2.62, p = .03; Bootstrap 95% CI: −2.44, 5.88; left ventral AI – left vta: ß = −0.68, t = −2.43, p = .04, Bootstrap 95% CI: −28.64, 5.68, Figure 7B). Anterior insula functional connectivity to executive control or default mode network regions was not a significant predictor of changes in emotion regulation skills.
Discussion
In the current study, we sought to understand the relationship between the intrinsic functioning of neural networks supporting adaptive regulation and treatment outcomes of a transdiagnostic CBT treatment for bipolar disorder and comorbid anxiety. Specifically, based upon trial outcomes reported elsewhere (Ellard et al., 2017), we examined the relationship between baseline levels of neuroticism, perceived emotion control, and emotion regulation ability and the intrinsic functional connectivity of emotion regulation neurocircuitry. Baseline levels of neuroticism and perceived affective control, two factors significantly related to symptom improvement following transdiagnostic CBT, were significantly associated with functional connectivity between the anterior insula and executive control and salience network regions respectively. Specifically, higher baseline neuroticism was associated with weaker anterior insula-IPL (ECN) functional connectivity, whereas stronger anterior insula-dACC (SN) functional connectivity was associated with lower perceived affective control. Further, weaker anterior insula-VLPFC (SN) functional connectivity predicted greater CBT-related improvements in affective control. The anterior insula is a region implicated in mapping the visceral experience of emotions to higher cognitive processes and switching between salience processing and attentional control (Craig, 2009, 2011; Menon and Uddin, 2010), and is thought to play a key role in the switching between internally-focused (DMN), saliency-focused (SN), and externally-focused or goal-oriented (ECN) modes of information processing (Goulden et al., 2014; Sridharan et al., 2008). The ability to flexibly switch between processing modes is key to adaptive emotion regulation (Aldao et al., 2015; Barrett et al., 2016; Thayer and Lane, 2000). Increased correlated activation between the anterior insula and regions of the DMN, SN or ECN at rest may represent greater reliance upon one functional network over another. In the current study, those BD patients showing lesser anterior insula – salience network functional connectivity and greater anterior insula – executive control network functional connectivity were better able to benefit from transdiagnostic CBT, perhaps suggesting less reliance upon saliency processing and greater reliance upon goal-oriented processing with implications for successful treatment engagement and benefit.
The findings here implicating VLPFC functional connectivity in affective control outcomes align with existing data discussed above highlighting the role of the VLPFC both in adaptive emotion regulation in healthy controls and dysregulation in BD. The VLPFC has direct efferent anatomical connections to the amygdala, and afferent connections from the amygdala through anterior insula, situating this structure in a key position to integrate emotional and cognitive information and instantiate emotion regulation (Kohn et al., 2014). Indeed, studies in healthy individuals have shown the right VLPFC, in conjunction with the anterior insula, forms a fronto-insular network that is crucial for adaptively switching between salience and executive control network processing, and plays a key role in regulating the intensity of emotional responses (Sridharan et al., 2008). By contrast, several studies in bipolar patients have shown inefficient VLPFC downregulation of amygdala responses to emotion-related stimuli (Cerullo et al., 2012; Dima et al., 2013; Foland et al., 2008; Horacek et al., 2015; Kanske et al., 2015; Pompei et al., 2011; Townsend et al., 2013), and increased functional connectivity between bilateral VLPFC and amygdala at rest (Chai et al., 2011; Chepenik et al., 2010; Torrisi et al., 2013). These findings differentiate BD patients from healthy controls (for a recent review see Chase & Phillips, 2016). In the current study both stronger functional connectivity between bilateral dorsal anterior insula and left amygdala and weaker functional connectivity between bilateral AI and right VLPFC were found to predict greater improvements in perceived emotion control over time, and this effect was most pronounced in the group treated with transdiagnostic CBT. Thus, changes in perceived affective control may be related to more efficient affective regulation along a VLPFC-anterior insula-amygdala pathway. Further, data from the current study suggest the more efficient this pathway is functioning at baseline, the greater the chance for transdiagnostic CBT to be beneficial for patients with BD and anxiety. This may in part be due to greater ability to engage cognitive control mechanisms needed to support emotion regulation and goal-directed behavior. Future studies examining the effective connectivity between these regions both during rest and during performance on emotion regulation tasks would help to clarify the causal relationship between these regions and adaptive regulation, and the influence of these regions on successful engagement in transdiagnostic CBT.
Stronger right ventral anterior insula functional connectivity to dACC was also associated with lower baseline endorsement of perceived affective control, which in turn predicted worse outcomes to transdiagnostic CBT. As another key node in the salience network (Seeley et al., 2007), the dACC has been implicated as an important region for emotion regulation (Ochsner et al., 2002; Ochsner et al., 2004), and has been implicated in conflict monitoring between emotional signals and cognitive demands (see (Etkin et al., 2011) for a review). Functional and structural abnormalities of both dACC and anterior insula have recently been identified as a specific transdiagnostic marker of pathology in a large meta-analysis (Goodkind et al., 2015). Using data from nearly 200 studies and over 7,000 individuals with a range of diagnoses including schizophrenia, bipolar disorder, depression, obsessive compulsive disorder, generalized anxiety and addiction, Goodkind et al. (2015) found converging grey matter loss and deviations in functional recruitment across all diagnoses in the dACC and bilateral anterior insula. In the current study, increased functional coupling of these two salience processing regions was significantly associated with greater impairment in perceived affective control with detrimental impacts on CBT treatment outcomes, further implicating these regions not only in pathology but also treatment efficacy.
Functional connectivity between the anterior insula and left IPL was significantly related to baseline levels of neuroticism, which in turn predicted poorer depression and anxiety symptom outcomes following transdiagnostic CBT. Neuroticism is defined as a trait tendency towards more frequent and intense experiences of negative emotions. The IPL represents a key node in the frontoparietal control network, and has been identified as an important region in an integrative multi-network system implicated in emotion-cognition integration (Cromheeke and Mueller, 2014; Wager et al., 2008; Wang et al., 2017). The left IPL has also been identified as part of a dorsal attention network responsible for voluntarily reorienting attention and behavior in response to relevant interoceptive stimuli in order to align with ongoing goal-directed needs, and plays an important role in regulating the endogenous stimuli-driven ventral attention network (Corbetta et al., 2008; Wang et al., 2017). Thus, weaker regulatory recruitment of the left IPL may be related to the increased intensity and frequency of negative emotions associated with a neurotic temperament in BD. Recent work from our lab found aberrant right dorsal anterior insula - left IPL functional connectivity differentiated bipolar depression from unipolar depression and healthy controls (Ellard et al., 2018). Further, decreased IPL recruitment and weaker anterior insula -IPL functional connectivity was found in bipolar patients relative to healthy controls during performance on a negatively-valenced cognitive-affective interference task (Ellard et al., under review). Here, weaker functional connectivity between anterior insula and IPL was associated with greater baseline levels of trait neuroticism. This suggests anterior insula – IPL functional connectivity may be another key pathway by which adaptive emotion processing may be compromised in BD, and may additionally affect the ability for BD patients to benefit from transdiagnostic CBT.
There are several limitations to the interpretation of these results. First and foremost, the present study includes a modest sample compromising statistical power. In addition, given the small sample size, specific treatment related effects should be treated as exploratory. Replication in future studies with larger sample sizes would be required to lend full credence and confidence to these results. Second, all participants included in this study were being treated with medications at study entry. Although we attempted to control for medication effects in our analyses, we cannot fully rule out the influence of medication on the results presented here. Third, all of the analyses presented here are cross-sectional; therefore, we do not have a true estimation of long-term impacts of intrinsic functional connectivity on emotion regulation-related variables and treatment outcome. Fourth, we elected for a more conservative approach to the examination of the neural circuitry supporting emotion regulation by taking a seed-to-target region of interest approach. Examining these results at the whole-brain level would potentially provide more nuanced results.
Conclusion
Treatment of BD with comorbid anxiety using a transdiagnostic, emotion focused CBT approach proved efficacious overall, but many patients still did not benefit from the treatment (Ellard et al., 2017). Here, we examined the relationship between emotion regulation-related variables and the intrinsic functional neurocircuitry supporting emotion regulation in order to better understand the relationship of emotion regulation-related neurocircuitry to treatment outcomes. The functional connectivity between anterior insula and key regions in executive control and salience networks implicated in adaptive emotion regulation was significantly related to both baseline emotion processing-related dimensions of neuroticism and perceived emotion control and to the magnitude of treatment related change in these dimensions. Those patients who exhibited greater baseline normative integrity in these neural circuits supporting adaptive emotion regulation showed greater improvement following transdiagnostic CBT. Results from this study shed light on potential targets for improving emotion dysregulation in BD with comorbid anxiety using a transdiagnostic CBT approach, and provide important information on potential therapeutic mechanisms of change, which might be exploited toward better treatment gains through novel interventions such as non-invasive neuromodulation as an adjunctive treatment strategy to transdiagnostic CBT. Future clinical studies will allow us to more precisely measure the relationship between neural networks supporting emotion regulation in BD with anxiety, and the potential for treatment-related gains using transdiagnostic CBT.
Highlights.
Examines neural predictors of a transdiagnostic CBT approach to treating anxiety in bipolar disorder (Unified Protocol for Transdiagnostic Treatment of Emotional Disorders).
Baseline functional connectivity of neurocircuitry supporting emotion regulation was associated with pre-treatment neuroticism and perceived control of emotions, two dimensions of emotion regulation that predicted treatment-related anxiety and depression symptom reduction.
Baseline functional connectivity of neurocircuitry supporting emotion regulation was a significant predictor of treatment-related improvements in emotion regulation skills.
Baseline functioning of emotion-regulation related neurocircuitry has a significant impact on treatment outcomes for patients with bipolar disorder and comorbid anxiety.
Funding Source
Dr. Ellard gratefully acknowledges support from the National Institutes of Health National Institute of Neurological Disorders and Stroke (NIH NINDS) Training Program in Recovery and Restoration of CNS Health and Function (T32 NS100663–01) and a NIH Postdoctoral National Research Service Award (F32 MH098490) from the National Institute of Mental Health. This work was additionally funded in part by the Dauten Family Center for Bipolar Treatment Innovation, Boston, MA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldao A, Sheppes G, Gross JJ, 2015. Emotion regulation flexibility. Cognitive Therapy and Research 39, 263–278. [Google Scholar]
- Aron AR, 2007. The neural basis of inhibition in cognitive control. Neuroscientist 13, 214–228. [DOI] [PubMed] [Google Scholar]
- Barlow D, Farchione T, Fairholme C, Ellard K, Boisseau C, Allen L, May J, 2010a. Unified protocol for transdiagnostic treatment of emotional disorders: Therapist guide: Oxford University Press. USA. [Google Scholar]
- Barlow DH, Ellard K, Fairholme C, Farchione T, Boisseau C, Allen L, Ehrenreich-May J, 2010b. The unified protocol for transdiagnostic treatment of emotional disorders: Client workbook. New York: Oxford University Press. Accessed on April 24, 2014. [Google Scholar]
- Barlow DH, Ellard KK, Sauer-Zavala S, Bullis JR, Carl JR, 2014. The Origins of Neuroticism. Perspect Psychol Sci 9, 481–496. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, Hamilton P, 2016. An active inference theory of allostasis and interoception in depression. Philos Trans R Soc Lond B Biol Sci 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg CZ, Shapiro N, Chambless DL, Ahrens AH, 1998. Are emotions frightening? II: An analogue study of fear of emotion, interpersonal conflict, and panic onset. Behaviour Research and Therapy 36, 3–15. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L, 2000. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12, 1–47. [DOI] [PubMed] [Google Scholar]
- Cerullo MA, Fleck DE, Eliassen JC, Smith MS, DelBello MP, Adler CM, Strakowski SM, 2012. A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disord 14, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, Cohen BM, Ongur D, 2011. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology 36, 2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Phillips ML, 2016. Elucidating neural network functional connectivity abnormalities in bipolar disorder: toward a harmonized methodological approach. Biol Psychiatry Cogn Neurosci Neuroimaging 1, 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepenik LG, Raffo M, Hampson M, Lacadie C, Wang F, Jones MM, Pittman B, Skudlarski P, Blumberg HP, 2010. Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res 182, 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL, 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrea RR, 1992. Revised neo personality inventory (neo pi-r) and neo five-factor inventory (neo-ffi). Psychological Assessment Resources. [Google Scholar]
- Craig AD, 2009. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2011. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci 1225, 72–82. [DOI] [PubMed] [Google Scholar]
- Cromheeke S, Mueller SC, 2014. Probing emotional influences on cognitive control: an ALE meta-analysis of cognition emotion interactions. Brain Structure and Function 219, 995–1008. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Peters AT, Sylvia L, Urdahl A, Magalhaes PV, Otto MW, Frank E, Miklowitz DJ, Berk M, Kinrys G, Nierenberg A, 2014. Do comorbid anxiety disorders moderate the effects of psychotherapy for bipolar disorder? Results from STEP-BD. Am J Psychiatry 171, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D, Jogia J, Collier D, Vassos E, Burdick KE, Frangou S, 2013. Independent modulation of engagement and connectivity of the facial network during affect processing by CACNA1C and ANK3 risk genes for bipolar disorder. JAMA Psychiatry 70, 1303–1311. [DOI] [PubMed] [Google Scholar]
- Ellard KK, Bernstein EE, Hearing C, Baek JH, Sylvia LG, Nierenberg AA, Barlow DH, Deckersbach T, 2017. Transdiagnostic treatment of bipolar disorder and comorbid anxiety using the Unified Protocol for Emotional Disorders: A pilot feasibility and acceptability trial. J Affect Disord 219, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard KK, Fairholme CP, Boisseau CL, Farchione TJ, Barlow DH, 2010. Unified protocol for the transdiagnostic treatment of emotional disorders: Protocol development and initial outcome data. Cognitive and Behavioral Practice 17, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard KK, Gosai AK, Felicione JM, Peters AT, Shea CV, Sylvia LG, Nierenberg AA, Dougherty DD, Deckersbach T, under review. Deficits in frontoparietal activation and anterior insula functional connectivity during regulation of cognitive-affective interference in bipolar disorder. Bipolar Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard KK, Zimmerman JP, Kaur N, Van Dijk KRA, Roffman JL, Nierenberg AA, Dougherty DD, Deckersbach T, Camprodon JA, 2018. Functional Connectivity Between Anterior Insula and Key Nodes of Frontoparietal Executive Control and Salience Networks Distinguish Bipolar Depression From Unipolar Depression and Healthy Control Subjects. Biol Psychiatry Cogn Neurosci Neuroimaging 3, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1997. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV), Clinician Version. American Psychiatric Association, Washington, D.C. [Google Scholar]
- Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM, 2008. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res 162, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Bookheimer SY, Lieberman MD, Sugar CA, Townsend JD, Fischer J, Torrisi S, Penfold C, Madsen SK, Thompson PM, Altshuler LL, 2012. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage 59, 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JC, Charak R, Elhai JD, Allen JG, Frueh BC, Oldham JM, 2014. Construct validity and factor structure of the difficulties in emotion regulation scale among adults with severe mental illness. Journal of psychiatric research 58, 175–180. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H, 2007. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34, 905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D, Fawcett J, 2012. The importance of anxiety in both major depression and bipolar disorder. Depress Anxiety 29, 471–478. [DOI] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A, 2015. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG, 2014. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage 99, 180–190. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Roemer L, 2004. Multidemensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment 26, 41–45. [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissler J, Kanske P, Schonfelder S, Wessa M, 2014. Inefficiency of emotion regulation as vulnerability marker for bipolar disorder: evidence from healthy individuals with hypomanic personality. J Affect Disord 152–154, 83–90. [DOI] [PubMed] [Google Scholar]
- Horacek J, Mikolas P, Tintera J, Novak T, Palenicek T, Brunovsky M, Hoschl C, Alda M, 2015. Sad mood induction has an opposite effect on amygdala response to emotional stimuli in euthymic patients with bipolar disorder and healthy controls. J Psychiatry Neurosci 40, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Schonfelder S, Forneck J, Wessa M, 2015. Impaired regulation of emotion: neural correlates of reappraisal and distraction in bipolar disorder and unaffected relatives. Transl Psychiatry 5, e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP, 2012. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage 61, 1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RM, Jin R, Merikangas KR, Simon GE, Wang PS, 2006. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. Am J Psychiatry 163, 1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U, 2014. Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. Neuroimage 87, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagopoulos J, Malhi GS, 2007. A functional magnetic resonance imaging study of emotional Stroop in euthymic bipolar disorder. Neuroreport 18, 1583–1587. [DOI] [PubMed] [Google Scholar]
- Lee JH, Dunner DL, 2008. The effect of anxiety disorder comorbidity on treatment resistant bipolar disorders. Depress Anxiety 25, 91–97. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH, 2004. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21, 450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R, 2005. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord 7 Suppl 5, 58–69. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ, 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC, 2007. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64, 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Sparks A, Mitchell PB, Weickert CS, Green MJ, 2012. Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Transl Psychiatry 2, e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhtadie L, Johnson SL, Carver CS, Gotlib IH, Ketter TA, 2014. A profile approach to impulsivity in bipolar disorder: the key role of strong emotions. Acta Psychiatr Scand 129, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD, 2002. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14, 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ, 2004. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 23, 483–499. [DOI] [PubMed] [Google Scholar]
- Otto MW, Simon NM, Wisniewski SR, Miklowitz DJ, Kogan JN, Reilly-Harrington NA, Frank E, Nierenberg AA, Marangell LB, Sagduyu K, Weiss RD, Miyahara S, Thas ME, Sachs GS, Pollack MH, Investigators S-B, 2006. Prospective 12-month course of bipolar disorder in out-patients with and without comorbid anxiety disorders. Br J Psychiatry 189, 20–25. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R, 2003. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54, 504–514. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC, 2008. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13, 829, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA, 2014. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry 171, 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Vieta E, 2007. Identifying functional neuroimaging biomarkers of bipolar disorder: toward DSM-V. Schizophr Bull 33, 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompei F, Dima D, Rubia K, Kumari V, Frangou S, 2011. Dissociable functional connectivity changes during the Stroop task relating to risk, resilience and disease expression in bipolar disorder. Neuroimage 57, 576–582. [DOI] [PubMed] [Google Scholar]
- Robins RW, Fraley RC, Roberts BW, Trzesniewski KH, 2001. A longitudinal study of personality change in young adulthood. Journal of personality 69, 617–640. [DOI] [PubMed] [Google Scholar]
- Samalin L, de Chazeron I, Vieta E, Bellivier F, Llorca PM, 2016. Residual symptoms and specific functional impairments in euthymic patients with bipolar disorder. Bipolar Disord 18, 164–173. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Otto MW, Wisniewski SR, Fossey M, Sagduyu K, Frank E, Sachs GS, Nierenberg AA, Thase ME, Pollack MH, 2004. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry 161, 2222–2229. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V, 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 105, 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppes T, Dennehy EB, Hirschfeld RM, Altshuler LL, Bowden CL, Calabrese JR, Crismon ML, Ketter TA, Sachs GS, Swann AC, Texas Consensus Conference Panel on Medication Treatment of Bipolar, D., 2005. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry 66, 870–886. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG, 2009. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disord 11, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2000. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 61, 201–216. [DOI] [PubMed] [Google Scholar]
- Torrisi S, Moody TD, Vizueta N, Thomason ME, Monti MM, Townsend JD, Bookheimer SY, Altshuler LL, 2013. Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar Disord 15, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL, 2013. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry 73, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupak SV, Dresler T, Guhn A, Ehlis A-C, Fallgatter AJ, Pauli P, Herrmann MJ, 2014. Implicit emotion regulation in the presence of threat: neural and autonomic correlates. Neuroimage 85, 372–379. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL, 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103, 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rheenen TE, Murray G, Rossell SL, 2015. Emotion regulation in bipolar disorder: profile and utility in predicting trait mania and depression propensity. Psychiatry Res 225, 425–432. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN, 2008. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie S, Guo X, Becker B, Fox PT, Eickhoff SB, Jiang T, 2017. Correspondent Functional Topography of the Human Left Inferior Parietal Lobule at Rest and Under Task Revealed Using Resting-State fMRI and Coactivation Based Parcellation. Hum Brain Mapp 38, 1659–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KE, Chambless DL, Aherns A, 1997. Are emotions frightening? An extension of the fear of fear construct. Behavior Research and Therapy 33, 579–583. [DOI] [PubMed] [Google Scholar]
- Wolkenstein L, Zwick JC, Hautzinger M, Joormann J, 2014. Cognitive emotion regulation in euthymic bipolar disorder. J Affect Disord 160, 92–97. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D, 1978. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]


