Abstract
Background
For patients with diabetic foot ulcers, offloading is one crucial aspect of treatment and aims to redistribute pressure away from the ulcer site. In addition to offloading strategies, patients are often advised to reduce their activity levels. Consequently, patients may avoid exercise altogether. However, it has been suggested that exercise induces an increase in vasodilation and tissue blood flow, which may potentially facilitate ulcer healing. The aim of this systematic review was to determine whether exercise improves healing of diabetic foot ulcers.
Review
We conducted a systematic search of MEDLINE, CINAHL and EMBASE between July 6, 2009 and July 6, 2019 using the key terms and subject headings diabetes, diabetic foot, physical activity, exercise, resistance training and wound healing. Randomised controlled trials were included in this review.
Three randomised controlled trials (139 participants) were included in this systematic review. All studies incorporated a form of non-weight bearing exercise as the intervention over a 12-week period. One study conducted the intervention in a supervised setting, while two studies conducted the intervention in an unsupervised setting. Two studies found greater improvement in percentage wound size reduction in the intervention group compared with the control group, with one of these studies achieving statistically significant findings (p < 0.05). The results of the third study demonstrated statistically significant findings for total wound size reduction (p < 0.05), however results were analysed within each treatment group and not between groups.
Conclusion
This systematic review found there is insufficient evidence to conclusively support non-weight bearing exercise as an intervention to improve healing of diabetic foot ulcers. Regardless, the results demonstrate some degree of wound size reduction and there were no negative consequences of the intervention for the participants. Given the potential benefits of exercise on patient health and wellbeing, non-weight bearing exercise should be encouraged as part of the management plan for treatment of diabetic foot ulcers. Further research is required to better understand the relationship between exercise and healing of diabetic foot ulcers.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13047-021-00456-w.
Keywords: Diabetic foot ulcer, Exercise, Physical therapy, Wound healing
Background
Diabetic foot ulcers (DFUs) are a serious and devastating complication of diabetes, affecting 26 million people worldwide annually [1]. People with diabetes have an approximate 25% lifetime risk of developing a foot ulcer compared to those without diabetes [2–4], and prevalence has been reported at 4–10% of the diabetic population [5, 6]. DFUs develop following injury, usually in the presence of peripheral neuropathy, ischaemia or both [2, 7]. The initial ulcer may be precipitated by acute, chronic repetitive or continuously applied mechanical stress, or thermal trauma [7]. Approximately 50% of DFUs occur on the plantar aspect of the foot [8] and if not treated appropriately, can progress into chronic and non-healing ulcers [2]. DFUs are a recognised risk factor for poor health outcomes, including major limb amputation [9–11], and are also associated with a financial burden to the health care system due to extensive healing times [12], reduced quality of life and an increased rate of mortality [13].
Management of DFUs include treatment of foot infection, appropriate dressing plans with regular sharp debridement of nonviable tissue, revascularisation (if indicated), and pressure offloading [14]. Offloading is one crucial aspect of treatment and aims to redistribute pressure away from the ulcer site [1], thereby, reducing further tissue trauma and facilitating the wound healing process [15]. This can be achieved via an offloading device, such as a total contact cast (TCC) or a controlled ankle motion (CAM) walker [1]. In addition to offloading strategies, patients are often advised to reduce their activity levels [16–18]. Consequently, patients may avoid exercise altogether [19]. However, exercise is important for overall heath and may reduce the risks of developing cardiovascular diseases [20]. In relation to the diabetic population specifically, inactivity may lead to diabetes macrovascular and microvascular complications, including ischaemic heart disease, cerebrovascular disease, peripheral vascular diease, retinopathy, nephropathy and peripheral neuropathy [21].
Inactivity is one modifiable risk factor for developing diabetes macrovascular and microvascular complications [21]. The Action in Diabetes and Vascular Disease Preterax and Diamicron MR Controlled Evaluation (ADVANCE) randomised controlled trial (RCT) study [22] reports a strong association between moderate and rigorous physical activity with a reduced incidence of cardiovascular events, microvascular complications, as well as all-cause mortality in participants with type 2 diabetes. However, this RCT did not include participants with DFUs and literature regarding the association between physical activity and vascular complications is limited [23].
The mechanism of exercise on healing of DFUs is not well investigated. In the diabetic population, hyperglycaemia inhibits nitric oxide (NO) synthesis, affecting insulin resistance and reducing the vasodilator response in blood vessels [24]. A meta-analysis by Qiu et al. [25] suggests that exercise induces an increase in blood flow, leading to an increase in NO synthesis and reducing oxidative stress in persons with type 2 diabetes. The combination of vasodilation and increase in tissue blood flow may potentially facilitate ulcer healing [24–27].
The International Working Group on the Diabetic Foot (IWGDF) guidelines [28] support various forms of foot-related exercises, such as strengthening and stretching, to improve modifiable risk factors for incidence of foot ulceration [29–38]. These exercises aim to improve plantar pressure distribution, neuropathy symptoms, reduced foot sensation and foot-ankle joint mobility [29–38]. However, where there are pre-ulcerative lesions or active ulceration, it is recommended weight bearing or foot-related exercises should be avoided [1].
To our knowledge, there is currently no systematic review investigating the effect of exercise and healing of DFUs. A systematic review published by Matos et al. [39] explores physical activity and exercise on diabetic foot related outcomes. However, the outcomes of interest were not specific to wound healing. A second systematic review published by Aagaard et al. [40] investigates the benefits and harms of exercise and DFUs. This study examines exercise and quality of life and adverse events and outcomes of exercise in relation to DFUs. Though this paper does not analyse the effects of exercise and wound healing, it highlights the need for further well-conducted RCTs to guide rehabilitation, including exercise in a semi-supervised and supervised setting [40].
The purpose of this review was to systematically identify, critique and evaluate literature investigating the effect of exercise and healing of DFUs. The primary outcome measure was wound size reduction. The secondary outcome measures were adherence to exercise, complications and adverse events.
Method
Registration
This systematic review was prospectively registered on PROSPERO (Registration No. 147487).
Eligibility criteria
Study design
To ensure the highest quality of evidence, only RCTs were included in this review.
Population
The RCTs could be conducted in any setting, including, but not limited to, hospital, private clinics, community settings, or within the participant’s home setting. Participants had to be 18 years and over, diagnosed with either type 1 or type 2 diabetes mellitus, have an active foot ulcer and attending a diabetic foot service.
Intervention
The intervention included any form of physical activity that was prescribed and measured by a health professional or member of the research team. This included provision of a prescribed exercise sheet.
Comparator
The comparator of interest was usual care.
Outcome
The primary outcome of interest was wound size reduction (measured in %, cm2 or cm). The secondary outcomes of interest were adherence to exercise, complications and adverse events.
Information sources
Electronic database searches were conducted in MEDLINE, CINAHL and EMBASE between July 6, 2009 and July 6, 2019 using the key terms and subject headings diabetes mellitus, diabetes, foot disease, diabetic foot, physical activity, exercise therapy, exercise, resistance training, physical fitness, physical therapy, aerobic exercise, exercise therapy, wound, foot ulcer, pressure ulcer, foot ulcer and wound healing. The searches were performed on the three selected electronic databases as they are commonly used databases, and most relevant to the subject of DFUs. A 10-year time period was applied to the search strategy to ensure currency of literature. The search strategy for MEDLINE is shown in Additional file 1.
The reference lists of included studies were checked and citation tracking was performed using Google Scholar to further identify relevant articles for inclusion. Searches were performed again in December 2020 to ensure any new citations were identified and assessed prior to submission.
Study selection
The titles and abstracts of records identified in the search strategy were independently screened by two reviewers (MT and MH) based on the inclusion and exclusion criteria. Full text articles were obtained for articles where a decision could not be made to include or exclude. Any disparities were discussed until consensus was reached.
Data extraction
A customised tool was created and utilised to extract data from included studies. Information extracted included author, population, setting, details of randomisation, description of exercise intervention, frequency or intensity of intervention, duration of intervention, delivery mode of intervention, description of control and interventions groups, outcome measurements for control and intervention groups, and results of analysis. The customised tool is shown in Additional file 2.
One reviewer performed data extraction (MT), while a second reviewer (MH) confirmed the extracted data. Any disparities were discussed until consensus was reached.
Risk of bias in individual studies
Methodological quality of included studies was assessed using the Physiotherapy Evidence Database (PEDro) Scale. This scale consists of 11 items and has been shown to have fair to good reliability [41]. Item 1 pertains to external validity and is not used to calculate the PEDro score (as outlined in the PEDro guideline). Each criterion is given a score of 1 or 0, with a maximum achievable score of 10. Studies with a score equal to or greater than seven indicates high methodological quality, a score between four and six (inclusive) indicates moderate methodological quality, and a score equal to or below three indicates low methodological quality [41]. Studies are critiqued based on the following characteristics:
Random allocation to groups;
Allocation concealment;
Similar baseline characteristics, regarding the most important prognostic indicator;
Blinding of subjects, assessors and therapists;
Measurement of at least one key outcome obtained for more than 85% of the subjects initially allocated to groups;
All subjects received the treatment or control condition as allocated, or where this was not the case, data for at least one key outcome was analysed by ‘intention to treat’;
Between group statistical comparisons reported for at least one key outcome;
Provision of point measures and measures of variability for at least one key outcome [42].
Two reviewers (MT and MH) independently applied the PEDro scale to the included studies. Any discrepancies were discussed until consensus was reached.
Summary measures
All studies were analysed descriptively and incorporated mean, median and standard deviation (SD).
Results
Study selection
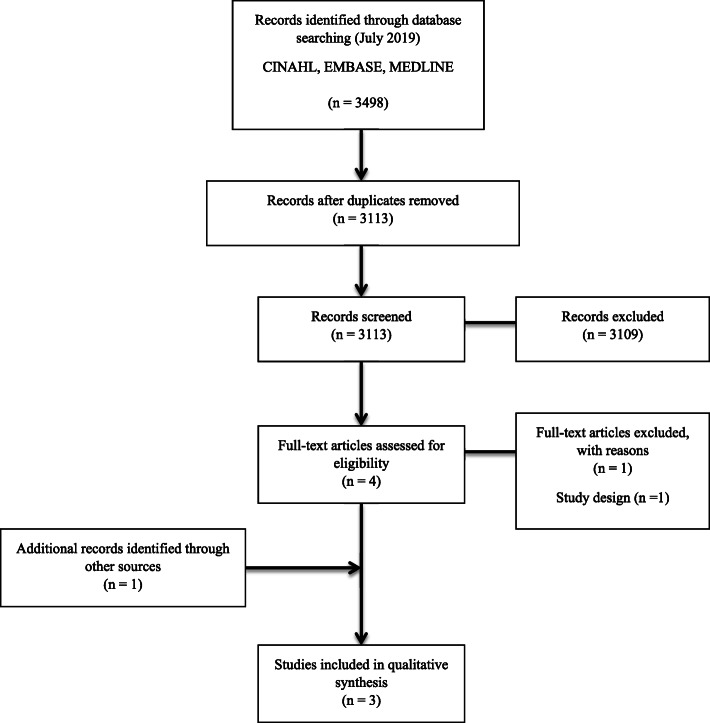
The results of the search process are shown in Fig. 1. The initial search strategy yielded 3498 results across the three databases. Following the removal of duplications and screening of titles and abstracts, four studies were identified for full text review. On review of the full text articles, two studies met the inclusion criteria. Citation tracking was performed on the two included studies, identifying one additional study for inclusion. Therefore, a total of three studies were included in this review.
Fig. 1.
Flow diagram presenting the process undertaken to identify eligible studies
Study characteristics
Three RCTs (139 participants), of which one was a pilot study [43], were included in this review. There were 71 participants allocated to the intervention groups and 68 participants allocated to the control groups. The duration for all study interventions was 12 weeks. The mean age of participants in the intervention groups were 61.90 [43], 61.03 [44], and 69.06 [45]. The mean age of participants in the control groups were 74.25 [43], 65.76 [44], and 68.50 [45]. The age range of all participants was 41 to 94 years. Characteristics of included studies are presented in Table 1. A detailed table of the extracted data (including individual study results) is available in Additional file 3.
Table 1.
Study characteristics of included studies
| Author | Participants/Age (Years) | Mean Diabetes Duration (Years) | Presence of Neuropathy | Previous Ulceration | BMI (Kg/m2) | Presence of Co-Morbidities | ||
|---|---|---|---|---|---|---|---|---|
| Intervention Group (IG) | Control Group (CG) | Male | ||||||
| Flahr [43] |
10 Mean age = 61.9 Age range = 49–74 |
8 Mean age = 74.25 Age range = 54–94 |
67% | Not reported | 50% of participants reported to have 100% loss of sensorya | Not reported | Not reported | IG = 6b CG = 3c |
| Eraydin and Avsar [44] |
30 Mean age = 61.03 ± 9.97 Age range = 41–80 |
30 Mean age = 65.76 ± 8.57 Age range = 49–80 |
62% | IG = 16.23 ± 8.57 CG = 17.46 ± 8.79 | Not reported | 70% | IG = 31.36 ± 7.62 CG = 28.58 ± 4.66 | Not reported |
| Joseph et al. [45] |
31 Mean age = 69.06 ± 4.79 Age range = Not reported |
30 Mean age: 68.50 ± 5.01 Age range = Not reported |
51% | IG = 21.77 ± 7.77 CG = 18.73 ± 7.16 | Not reported | Not reported | IG = 27.66 ± 5.44 CG = 22.96 ± 3.23 | Not reported |
| Author | Setting | Description of Intervention | Frequency/Duration of Intervention/Delivery Mode of Intervention | Outcome Measures | Primary Outcome | Secondary Outcome | ||
|
Wound Measurements IG = Intervention Group CG = Control Group |
Adherence to Exercise in Intervention Group/Complication and Adverse Events | |||||||
| Flahr [43] | Home | Non-weight bearing exercises including ankle inversion, eversion, flexion and extension - 4 in total. |
10 times each, twice daily 12 weeks Education session in clinic. Provision of written material. Unsupervised exercise |
Percentage wound size reduction by participant, self-reported number of days of exercise frequency |
Final wound measurement and percentage wound size reduction after 12 weeks: IG1 = 0.22 cm2 (− 88%); CG1 = 0.79 cm2 (+ 25%) IG2 = Withdrew (− 59%); CG2 = 0.49 cm2 (+ 14%) IG3 = 0.09 cm2 (− 67%); CG3 = 0.14 cm2 (− 88%) IG4 = Closed (− 100%); CG4 = Closed (− 100%) IG5 = 0.12 cm2 (− 25%); CG5 = 9.18 cm2 (+ 2%) IG6 = Closed (− 100%); C6G = Closed (− 100%) IG7 = 0.05 cm2 (− 69%); CG7 = Withdrew IG8 = 0.09 cm2 (− 67%); CG8 = Closed (− 100%) IG9 = Closed (− 100%); CG9 = 0.06 cm2 (− 95%) IG10 = 2.36 cm2 (− 131%) (p = 0.70) |
1 time/day = 1 (10.0%) 2 times/day = 2 (20.0%) 3 times/day = 1 (10.0%) 2 times every 3rd day = 1 (10.0%) Stopped after 8 weeks = 2 (20.0%) Didn’t exercise = 1 (10.0%) Unknown = 2 (20.0%) 1 participant in IG withdrew due to Osteomyelitis |
||
| Eraydin and Avsar [44] | Home |
Non-weight bearing foot exercises to be completed seated: plantar flexion, dorsiflexion, inversion, eversion, circumduction and plantar dorsiflexion of toes - 18 in total. Exercises to be completed standing once wounds healed. |
10 repeats, twice daily 12 weeks 20–30 min education session in clinic. Provision of written material. Unsupervised exercise |
Mean DFU area, DFU total depth, self-reported exercise frequency |
Baseline and final measurements: Distribution of DFU Area Averages (SD): IG: 12.63 cm2 (14.43) IG: 3.29 cm2 (3.80) (p = 0.00)d CG: 24.67 cm2 (20.70) CG: 18.52 cm2 (21.49) (p = 0.00) d Distribution of DFU Total Depth (SD): IG: 0.56 cm (0.85) IG: 0.28 cm (0.38) (p = 0.01) d CG: 0.61 cm (0.84) CG: 0.80 cm (1.26) (p = 0.37) d |
0–30 days = 8 (26.7%) 31–60 days = 15 (50.0%) 61–90 days = 7 (23.3%) |
||
| Joseph et al. [45] | Exercise clinic | Participants rode on a bicycle ergometer with foot interaction kept constant with standard gym pedal and specialised offloading insole padding to relieve pressure to ulcer. |
3 times per week at exercise clinic 12 weeks Participants encouraged to increase their exercise time by 5 mins each 2 weeks until they reach 50 mins at the 9th week, which was maintained until the end of the program. Supervised exercise |
Percentage wound size reduction |
Percentage Wound Size Reduction after 12 weeks (SD): IG: 94.08% (18.50) CG: 54.76% (17.19) (p < 0.05) |
Not reported | ||
aData break down not available
b 30% had arthritis, 10% had a history of cerebral vascular incident, 10% had a history of back surgery, 10% reported a history of a herniated disc
c 38.5% had arthritis
dIntragroup comparison
All three studies incorporated a form of non-weight bearing exercise as the intervention [43–45]. Two studies [43, 44] investigated the effect of prescribed non-weight bearing exercise in the home setting, while one study [45] investigated the effect of supervised non-weight bearing aerobic exercise at a clinic.
For the two studies that prescribed non-weight bearing exercises in the home setting, exercises were performed ten times, twice daily and consisted of different exercise protocols [43, 44]. The exercises required participants to be in a seated position and included plantar flexion, dorsiflexion, inversion, eversion and circumduction of the foot, and plantar and dorsiflexion of the toes. Both studies required participants to keep an exercise log [43, 44]. In contrast, the supervised non-weight bearing aerobic exercises were performed three times weekly, for up to 50 min per session [45]. In this study, participants attended an exercise clinic where they were required to ride a bicycle ergometer, while using an insole pad to offload the DFU during exercise [45]. All participants in the control groups received usual care.
All three studies measured wound healing [43–45]. Two studies measured percentage wound size reduction [43, 45], and one study measured total wound size reduction (cm2) as well as wound depth (cm) [44]. Two studies reported participants’ self-adherence to exercise [43, 44].
As the studies differed in the way they prescribed exercises and measured the outcomes of interest, the results could not be pooled in a meta-analysis.
Risk of bias within studies
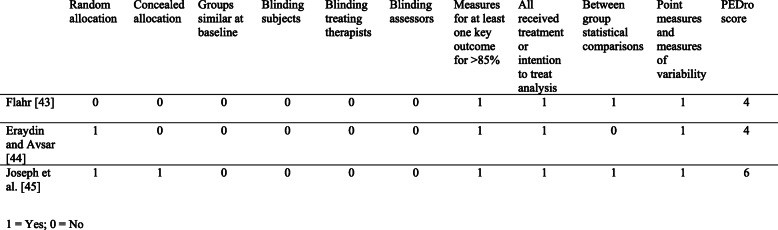
The quality assessment scores obtained ranged between four and six, indicating moderate methodological quality of included studies. As it is not possible to blind both the participant and treating therapist, studies in this review were not able to achieve a score greater than eight out of ten on the PEDro scale. The PEDro scale scoring of the included studies is shown in Fig. 2.
Fig. 2.
PEDro scale scoring
Percentage reduction of wound size
Two studies [43, 45] measured percentage reduction of wound size.
One study [43] found no significant difference in percentage wound size reduction between both study arms (p = 0.70). In this study, 90% of the intervention group (n = 9) experienced a reduction in wound size of 26 to 100% after 12 weeks, compared to the control group where 33% of participants (n = 3) experienced 31% increase in wound size.
Another study [45] reported significantly greater percentage reduction in wound size in the intervention group compared to the control groups. In this study, the mean percentage wound size reduction after 12 weeks was 94.08% (SD, 18.50) and 54.76% (SD, 17.19) in the intervention and control groups respectively (p < 0.05).
Total wound size reduction
One study [44] measured total wound size reduction (cm2). The results of this study were reported within groups only and did not compare results between both study arms. In this study, both intervention and control groups had significantly reduced wound areas between baseline and 12 weeks (p < 0.05). The mean total wound size improved from 12.63cm2 (SD, 14.43) to 3.29cm2 (SD, 3.80) in the intervention group (p = 0.00), compared with an improvement of 24.67cm2 (SD, 20.70) to 18.52cm2 (SD, 21.49) in the control group (p = 0.00) [44].
Total wound depth
One study [44] measured total wound depth and conducted intragroup comparisons. This study reported a significant difference in wound depth for the intervention group, but not the control group. The DFU total depth in the intervention group reduced from 0.56 cm (SD, 0.85) to 0.28 cm (SD, 0.38) (p = 0.01) compared with the control group, which increased from 0.61 cm (SD, 0.84) to 0.80 cm (SD, 1.26) (p = 0.37) [44].
Adherence to exercise
Two studies measured participants’ self reported adherence to exercise [43, 44]. One study reported 20% of participants exercising two times per day, 20% of participants ceasing exercise after 8 weeks, 20% of participants not reporting frequency, 10% of participants exercising one time per day, 10% of participants exercising two times every third day, 10% not exercising at all and 10% exercising three times per day [43].
A second study reported 26.7% of participants exercising between 0 and 30 days, 50% of participants exercising between 31 and 60 days and 23.3% of participants exercising between 61 and 90 days [44].
A third study did not measure adherence to exercise, as the intervention was conducted in a supervised setting [45].
Complication and adverse events
One adverse event was recorded across the three studies in which a participant from the intervention group experienced wound deterioration due to osteomyelitis [43].
Discussion
All three studies included in this review utilised non-weight bearing exercises as the intervention. While the search strategy was designed to capture studies utilising all forms of exercise (i.e. weight bearing and non-weight bearing), this result may be due to weight bearing exercise being considered detrimental to healing of DFUs [1, 17].
This review found a mixture of positive and inconclusive results to support non-weight bearing exercise as an intervention to improve healing of DFUs. One of the included studies [43] was conducted in the form of a pilot study. The small sample sizes of the study may have affected the reliability of the study’s results, and therefore, the results of this study should be interpreted with caution. While a second study [44] achieved statistically significant results with respect to total wound size reduction, these findings should also be interpreted with caution as the results were analysed within each treatment group and not between the treatment groups. A third study reported statistically significant percentage reduction of wound sizes [45]. There were no reported deaths and no reported minor or major amputations as a result of the exercise intervention. There was one adverse event recorded in which a participant in the intervention group experienced wound deterioration due to developing osteomyelitis [43], but this was not deemed related to the prescribed intervention.
The results of this systematic review support supervised exercise programs in preference to unsupervised exercise programs completed in the home setting. While unsupervised exercise programs in the home setting are beneficial in that they are low cost, accessible, safe and easy to implement [46], adherence may potentially be an issue and is influenced by multiple factors such as age, motivation, believing in its benefits, follow ups and the complexity of exercises prescribed [47]. Two studies [43, 44] measured self-reported adherence to non-weight bearing exercise in the home setting in the form of an exercise log, where participants were required to record the type and number of exercises completed. This review found that participants were more likely to experience issues with maintaining accurate records relating to their adherence to exercise when participating in an unsupervised exercise program [43, 44], which is similar to the findings of a study by Anar [47]. A large component of an unsupervised exercise program relies on the participant’s self-motivation, which may be variable [48], their ability to perform the exercise independently as well as accurately reporting exercise frequency [49].
While unsupervised home exercises may be more accessible for patients, this review found that adherence and outcomes were more favourable when exercises were performed in a supervised setting. Exercise conducted in a supervised setting enables a structured program, promotes motivation through visual feedback and encourages participants to achieve the minimum required dose of exercise [50]. However, supervised exercise programs also present with barriers including difficulty in the initial set up of a program, costs, availability of classes and limits on the number of participants allowed to engage per class [51].
Currently, patients presenting with DFUs are discouraged from weight bearing activity in order to minimise plantar pressure to the ulcer site [1, 17]. Of concern is that all types of exercise may then be avoided, despite the fact that exercise has numerous benefits for people with diabetes, including improvement of blood sugar control and lipid profile [52–54]. The findings of this systematic review suggest that non-weight bearing exercises may be safely utilised as part of the management and treatment plan for patients with DFUs and may potentially be beneficial for wound healing [43–45]. Non-weight bearing exercise programs should be designed by a dedicated health professional and DFUs should be closely monitored.
To our knowledge, this is the first systematic review to investigate the effects of exercise and healing of DFUs. The strengths of this review include a rigorous inclusion and exclusion criteria, search strategy, and the included studies were systematically selected, reviewed and assessed by two independent reviewers using standardised methods.
Limitations of this review include the small number of studies, small sample sizes, different participant characteristics at baseline, and moderate quality of studies. Furthermore, the prescribed exercises were dissimilar and the outcomes of interests were measured differently for each study. As a result, the data could not be pooled in a meta-analysis. The search strategy involved three electronic databases and only English text studies were considered, potentially omitting further RCTs that could have been included in the review. Expanding this systematic review to include all study designs, and not just RCTs, may have resulted in a larger number of included trials. However, the overall quality of included studies may have been negatively impacted.
Conclusion
There is insufficient evidence from this systematic review to conclusively support exercise as an intervention to improve healing of DFUs. Regardless, the results demonstrate some degree of wound size reduction and there were no negative consequences of the intervention for the participants. Given the potential benefits of exercise on patient health and wellbeing, non-weight bearing exercise should be encouraged as part of the management plan for treatment of DFUs. Further research is required to better understand the relationship between exercise and healing of DFUs. Further high quality RCTs with larger sample sizes and conducted in a supervised environment are required in order to determine the effects of exercise on healing of DFUs. Additionally, the exercise protocol (including the type and frequency) that should be prescribed for these patients remains unclear and requires further investigation.
Supplementary Information
Acknowledgements
The authors would like to acknowledge Sarah Dallimore for their feedback throughout the review process.
Abbreviations
- DFU
Diabetic foot ulcer
- PEDro
Physiotherapy evidence database
- RCT
Randomised controlled trial
- SD
Standard deviation
Authors’ contributions
MT conceived the study idea and completed the search strategy. MT and MH were involved in the processes of article selection, data extraction, quality assessment and data analysis. Both authors drafted the manuscript and approved of the final text prior to submission.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article (and its additional file).
Ethics approval and consent to participate
Not applicable.
Consent for publication
The results presented in this paper have not been published previously in whole or part.
Competing interests
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Morica M. Tran, Email: morica.tran@easternhealth.org.au
Melanie N. Haley, Email: melanie.haley@easternhealth.org.au
References
- 1.Bus SA, Armstrong DG, Gooday C, Jarl G, Caravaggi C, Viswanathan V, Lazzarini PA. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36. [DOI] [PubMed]
- 2.Haji Zaine N, Burns J, Vicaretti M, Fletcher J, Begg L, Hitos K. Characteristics of diabetic foot ulcers in Western Sydney, Autralia. J Foot Ankle Res. 2014;7:39. doi: 10.1186/s13047-014-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Working Group on the Diabetic Foot. Epidemiology of Diabetic Foot Infections in a Population Based Cohort. Presentation presented at; 2003; Noordwijkerhout, the Netherlands.
- 4.Lavery L, Armstrong D, Wunderlich R, Tredwell J, Boulton A. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care. 2003;26:1435–1438. doi: 10.2337/diacare.26.5.1435. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49:106–116. doi: 10.1080/07853890.2016.1231932. [DOI] [PubMed] [Google Scholar]
- 6.Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3:4. doi: 10.1007/s13300-012-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanagh PR, Lipsky BA, Bradbury AW, Botek G. Treatment for diabetic foot ulcers. Lancet. 2005;366:1725–1735. doi: 10.1016/S0140-6736(05)67699-4. [DOI] [PubMed] [Google Scholar]
- 8.Bus SA, Haspels R, Busch-Westbroek TE. Evaluation and optimisation of therapeutic footwear for neuropathic diabetic foot patients using in-shoe plantar pressure analysis. Diabetes Care. 2011;34:1595–1600. doi: 10.2337/dc10-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namgoong S, Jung S, Han S, Jeong S, Dhong E, Kim W. Risk factors for major amputation in hospitalised diabetic foot patients. Int Wound J. 2015;13:13–19. doi: 10.1111/iwj.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weledji E, Fokam P. Treatment of the diabetic foot – to amputate or not? BMC Surg. 2014;14:83. doi: 10.1186/1471-2482-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SY, Kim TH, Choi JY, Kwon YJ, Choi DH, Kim KC, Kim MJ, Hwang HK, Lee KB. Predictors for amputation in patients with diabetic foot wound. Vasc Spec Int. 2018;34:109–116. doi: 10.5758/vsi.2018.34.4.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghav A, Khan Z, Labala R, Ahmad J, Noor S, Mishra B. Financial burden of diabetic foot ulcers to world: a progressive topic to discuss always. Ther Adv Endocrinol Metab. 2017;9:29–31. doi: 10.1177/2042018817744513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chammas N, Hill R, Edmonds M. Increased mortality in diabetic foot ulcer patients: the significance of ulcer type. J Diabetes Res. 2016;2016:1–7. doi: 10.1155/2016/2879809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayman G, Vas P, Dhatariya K, Driver V, Hartemann A, Londahl M, Piaggesi A, Apelqvist J, Attinger C, Game F. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36. [DOI] [PubMed]
- 15.Mcintosh C, Halford G. The importance of effective offloading and footwear for the diabetic foot " diabetic foot ulcers are frequently associated with adverse outcomes, including limb and/or life threatening infection and lower extremity amputation. ". Wounds Essentials. 2014;9:21–28. [Google Scholar]
- 16.Kruse I. Evaluation and treatment of diabetic foot ulcers. Clin Diabetes. 2006;24:91–93. doi: 10.2337/diaclin.24.2.91. [DOI] [Google Scholar]
- 17.Frykberg RG, Banks J. Management of Diabetic Foot Ulcers: a review. Fed Pract. 2016;33:16–23. [PMC free article] [PubMed] [Google Scholar]
- 18.Bergin SM, Gurr JM, Allard BP, Holland EL, Horsley MW, Kamp MC, Lazzarini PA, Nube VL, Sinha AK, Warnocl JT, Alford JB, Wraight PR. Australian diabetes foot network: management of diabetes-related foot ulceration — a clinical update. Med J Aust. 2012;197:226–229. doi: 10.5694/mja11.10347. [DOI] [PubMed] [Google Scholar]
- 19.Crews RT, Schneider KL, Yalla SV, Reeves ND, Vileikyte L. Physiological and psychological challenges of increasing physical activity and exercise in patients at risk of diabetic foot ulcers: a critical review. Diabetes Metab Res Rev. 2016;32:791–804. doi: 10.1002/dmrr.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papatheodorou K, Banach M, Bekiari E, Rizzo M, Edmonds M. Complications of Diabetes. J Diabetes Res 2017. 2018;11:3086167. doi: 10.1155/2018/3086167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heller SR. A summary of the ADVANCE trial. Diabetes Care. 2009;32:S357–S361. doi: 10.2337/dc09-S339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blomster JI, Chow CK, Zoungas S, Woodward M, Patel A, Poulter NR, Marre M, Harrap S, Chalmers J, Hillis GS. The influence of physical activity on vascular complications and mortality in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:1008–1012. doi: 10.1111/dom.12122. [DOI] [PubMed] [Google Scholar]
- 24.Bøtker H, Møller N. ON NO—the continuing story of nitric oxide, diabetes, and cardiovascular disease. Diabetes. 2013;62:2645–2647. doi: 10.2337/db13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu S, Cai X, Yin H, Sun Z, Zügel M, Steinacker JM, Schumann U. Exercise training and endothelial function in patients with type 2 diabetes: a meta-analysis. Cardiovasc Diabetol. 2018;17:64. doi: 10.1186/s12933-018-0711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korthuis RJ. Skeletal muscle circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2011. [PubMed] [Google Scholar]
- 27.Castilla DM, Liu ZJ, Velazquez OC. Oxygen: implications for wound healing. Adv Wound Care (New Rochelle) 2012;1:225–230. doi: 10.1089/wound.2011.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bus SA, Lavery LA, Monteiro-Soares M, Rasmussen A, Raspovic A, Sacco ICN, Van Netten JJ. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update) Diab Metab Res Rev. 2020;36:e3269. doi: 10.1002/dmrr.3269. [DOI] [PubMed] [Google Scholar]
- 29.Sartor CD, Hasue RH, Cacciari LP, Butugan MK, Watari R, Pássaro AC, Giacomozzi C, Sacco ICN. Effects of strengthening, stretching and functional training on foot function in patients with diabetic neuropathy: results of a randomized controlled trial. BMC Musculoskelet Disord. 2014;15:137. doi: 10.1186/1471-2474-15-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melai T, Schaper NC, IJzerman TH, de Lange TLH, Willems PJB, Lima Passos V, Lieverse AG, Meijer K, Savelberg HHCM. Lower leg muscle strengthening does not redistribute plantar load in diabetic polyneuropathy: a randomised controlled trial. J Foot Ankle Res. 2013;6:41. doi: 10.1186/1757-1146-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pataky Z, De León RD, Allet L, Golay A, Assal M, Assal JP, Hauert CA. Biofeedback for foot offloading in diabetic patients with peripheral neuropathy. Diabet Med. 2009;27:61–64. doi: 10.1111/j.1464-5491.2009.02875.x. [DOI] [PubMed] [Google Scholar]
- 32.York R, Perell-Gerson K, Barr M, Durham J, Roper J. Motor learning of a gait pattern to reduce forefoot plantar pressures in individuals with diabetic peripheral neuropathy. PM R. 2009;1:434–441. doi: 10.1016/j.pmrj.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 33.De León RD, Allet L, Golay A, Philippe J, Assal JP, Hauert C, Pataky Z. Biofeedback can reduce foot pressure to a safe level and without causing new at-risk zones in patients with diabetes and peripheral neuropathy. Diabetes Metab Res Rev. 2013;29:139–144. doi: 10.1002/dmrr.2366. [DOI] [PubMed] [Google Scholar]
- 34.Cerrahoglu L, Koşan U, Sirin T, Ulusoy A. Range of motion and plantar pressure evaluation for the effects of self-care foot exercises on diabetic patients with and without neuropathy. J Am Podiatr Med Assoc. 2016;106:189–200. doi: 10.7547/14-095. [DOI] [PubMed] [Google Scholar]
- 35.Goldsmith J, Lidtke R, Shott S. The effects of range-of-motion therapy on the plantar pressures of patients with diabetes mellitus. J Am Podiatr Med Assoc. 2002;92:483–490. doi: 10.7547/87507315-92-9-483. [DOI] [PubMed] [Google Scholar]
- 36.Kanchanasamut W, Pensri P. Effects of weight-bearing exercise on a mini-trampoline on foot mobility, plantar pressure and sensation of diabetic neuropathic feet; a preliminary study. Diabet Foot Ankle. 2017;8:1287239. doi: 10.1080/2000625X.2017.1287239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iunes D, Rocha C, Borges N, Marcon C, Pereira V, Carvalho L. Self-care associated with home exercises in patients with type 2 diabetes mellitus. PLoS One. 2014;9:e114151. doi: 10.1371/journal.pone.0114151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fayed EE, Badr NM, Mahmoud S, Hakim SA. Exercise therapy improves plantar pressure distribution in patients with diabetic peripheral neuropathy. Int J Pharm Tech Res. 2016;9:151–159. [Google Scholar]
- 39.Matos M, Mendes R, Silva A, Sousa N. Physical activity and exercise on diabetic foot related outcomes: a systematic review. Diabetes Res Clin Pract. 2018;139:81–90. doi: 10.1016/j.diabres.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Aagaard T, Moeini S, Skou S, Madsen U, Brorson S. Benefits and harms of exercise therapy for patients with diabetic foot ulcers: a systematic review. Int J Low Extrem Wounds. 2020;00:1–15. doi: 10.1177/1534734620954066. [DOI] [PubMed] [Google Scholar]
- 41.Maher C, Sherrington C, Herbert R, Moseley A, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 42.De Morton N. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Australian Journal of Physiotherapy. 2009;55:129–133.43 . [DOI] [PubMed]
- 43.Flahr D. The effect of nonweight-bearing exercise and protocol adherence on diabetic foot ulcer healing: a pilot study. Ostomy Wound Manage. 2010;56:40–50. [PubMed] [Google Scholar]
- 44.Eraydin Ş, Avşar G. The effect of foot exercises on wound healing in type 2 diabetic patients with a foot ulcer. J Wound Ostomy Continence Nurs. 2018;45:123–130. doi: 10.1097/WON.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 45.Joseph N, Goddy C, Okoye G, Egwuonwu A, Ezeukwu A. Effect of Twelve Weeks Supervised Aerobic Exercise on Ulcer Healing and Changes in Selected Biochemical Profiles of Diabetic Foot Ulcer Subjects. International Journal of Diabetes Research. 2014;3:41–48.
- 46.Anar S. The effectiveness of home-based exercise programs for low back pain patients. J Phys Ther Sci. 2016;28:2727–2730. doi: 10.1589/jpts.28.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Creasy S, Rogers R, Davis K, Gibbs B, Kershaw E, Jakicic J. Effects of supervised and unsupervised physical activity programmes for weight loss. Obes Sci Pract. 2017;3:143–152. doi: 10.1002/osp4.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shigaki C, Kruse RL, Mehr D, Sheldon KM. Bin Ge, Moore C, Lemaster J. motivation and diabetes self-management. Chronic Illn. 2010;6:202–214. doi: 10.1177/1742395310375630. [DOI] [PubMed] [Google Scholar]
- 49.Lacroix A, Kressig RW, Muehlbauer T, Gschwind YJ, Pfenninger B, Bruegger O, Granacher U. Effects of a supervised versus an unsupervised combined balance and strength training program on balance and muscle power in healthy older adults: a randomized controlled trial. Gerontology. 2016;62:275–288. doi: 10.1159/000442087. [DOI] [PubMed] [Google Scholar]
- 50.Fennell C, Peroutky P, Glickman EL. Effects of supervised training compared to unsupervised training on physical activity, muscular endurance, and cardiovascular parameters. MOJ Orthopedics & Rheumatology. 2016;5:00184. doi: 10.15406/mojor.2016.05.00184. [DOI] [Google Scholar]
- 51.Murgitroyd E, Fraser S, Hebson A, Lewis D. Implementation of a supervised exercise therapy programme. The Annals of The Royal College of Surgeons of England. 2019;101:7–13. doi: 10.1308/rcsann.2018.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–2696. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas D, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3:CD002968. doi: 10.1002/14651858.CD002968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article (and its additional file).