Significance
Retinopathy is the leading cause of blindness, and development of effective therapy is urgently needed. Here, we defined an unprecedented subgroup of microglia that is responsible for causing retinopathy under hypoxia. Mechanistic studies demonstrated the signaling pathway of hypoxia-induced necroptosis of retinal microglia, i.e., the hypoxia–RIP1–RIP3–MLKL signaling axis, triggered an explosive release of FGF2, which in its turn to induce retinal neovascularization. Simultaneous targeting of necroptosis–FGF2 pathway and VEGF produces synergistic effects for treating retinopathy. On the basis of our findings, we propose a concept of necroptotic microglia-induced retinal angiogenesis and highlight a combination therapy for effective treatment of retinopathy.
Keywords: necroptosis, microglia, FGF2, RIP, retinal angiogenesis
Abstract
Retinal neovascularization is a leading cause of severe visual loss in humans, and molecular mechanisms of microglial activation-driven angiogenesis remain unknown. Using single-cell RNA sequencing, we identified a subpopulation of microglia named sMG2, which highly expressed necroptosis-related genes Rip3 and Mlkl. Genetic and pharmacological loss of function demonstrated that hypoxia-induced microglial activation committed to necroptosis through the RIP1/RIP3-mediated pathway. Specific deletion of Rip3 gene in microglia markedly decreased retinal neovascularization. Furthermore, hypoxia induced explosive release of abundant FGF2 in microglia through RIP3-mediated necroptosis. Importantly, blocking signaling components of the microglia necropotosis–FGF2 axis largely ablated retinal angiogenesis and combination therapy with simultaneously blocking VEGF produced synergistic antiangiogenic effects. Together, our data demonstrate that targeting the microglia necroptosis axis is an antiangiogenesis therapy for retinal neovascular diseases.
Retinal vascular diseases including retinopathy of prematurity (ROP), diabetic retinopathy (DR), and age-related macular degeneration (AMD) are characterized by growing irregular and leaky microvessels, which often lead to a catastrophic loss of vision. Targeting pathological neovascularization offers an attractive approach for treating retinal diseases (1, 2). VEGF-A is a well-characterized angiogenic factor in promoting angiogenesis, and anti-VEGF drugs have been successfully used for treating AMD and DR in the clinic. However, the therapeutic efficacy is generally limited and novel, and alternative therapeutic modalities are urgently needed. Microglia, a specialized type of immune cell resident in central nervous system, are necessary for shaping developmental angiogenesis in the retina and are responsible for irregular sprouting and growth of the vasculature during pathological insults (3–5). The molecular mechanisms underlying microglia-mediated retinal neovascularization are largely unknown.
Microglia act as a surveillance guard to maintain the homeostasis of retinal microenvironment. They become immediately activated in response to pathological insults such as ischemia, hypoxia, and injury stress (6, 7). It is believed that activated immune cells often experience cellular death to eliminate overactive immune responses and alleviate an inflammatory condition (8, 9). The discovery of necroptosis has challenged this classic view of activation-induced apoptosis and provides a new regulatory mechanism of immune responses (10, 11). Necroptosis is a tightly regulated process involving a complex formation between receptor-interacting protein-3 with -1 (RIP3 with RIP1), followed by initiating a cascade effect of inflammation and tissue damages (12–14). Therefore, necroptosis distinguishes itself from the unregulated necrosis and programmed apoptosis by triggering a controllable and programmed proinflammatory process (10, 13–15). Necroptosis is mainly reported to occur in macrophages (MFs) and contributes to many physiological and pathological processes. Recently, evidence has shown microglial necroptosis in the brain is a crucial process of regulating neuroinflammation (16). To date, hypoxia/ischemia-induced microglial necroptosis in retinal angiogenesis remains completely uncharacterized.
In this study, we employed an unbiased approach of microglial single-cell RNA-sequencing (scRNA-seq) analysis and a clinically relevant mouse model of hypoxia-driven retinal angiogenesis, the oxygen-induced retinopathy (OIR) model (17), to investigate the role of microglial necroptosis and the underlying mechanism in retinal angiogenesis by both genetic and pharmacological loss-of-function assays. We discovered that in response to hypoxia an emerging subpopulation of microglia-committed cell death is the primary biology process for causing retinal angiogenesis. A regulated mechanism of explosive release of FGF2 from hypoxia-induced necroptotic microglia was responsible for retinal neovascularization. We provided evidence showing several therapeutic targets for potential treatment of retinal angiogenic diseases, including the RIP1 and RIP3-mediated microglia necroptosis and necroptotic microglia-released FGF2 in stimulation of retinal angiogenesis. Based on our findings, we propose an effective combination therapy by simultaneous targeting of the necroptosis- and VEGF-triggered signaling.
Results
Defining an OIR-Specific Subpopulation of Microglia and the Necroptotic Pathway by scRNA-Seq Analysis.
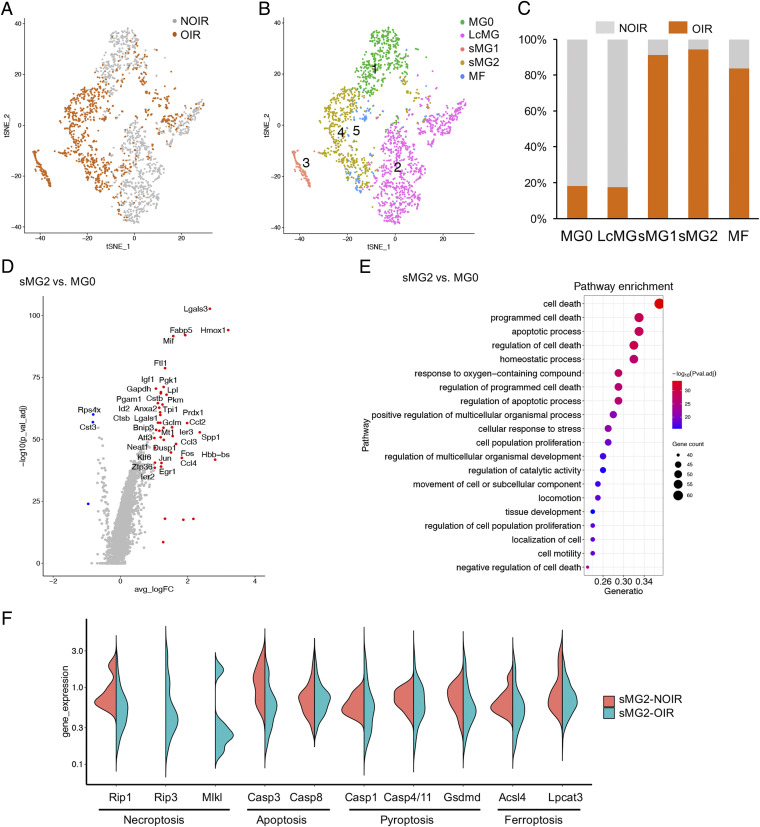
To identify the full repertoire of microglia molecular states during hypoxia-induced angiogenesis, we performed single-cell RNA profiling on retinal microglia using the 10X Genomics platform. A mouse OIR model that recapitulated clinical retinopathy was developed. CD11b-positive cells were enriched by magnetic bead selection from pooled retinae of NOIR or OIR mice (n = 6). Approximately, 17,701 sequenced cells met quality control metrics (SI Appendix, Fig. S1 A and B). After unsupervised clustering by Seurat package, we selected clusters of microglia that expressed marker genes such as Aif1, Trem2, Csf1r, etc. (n = 2,678 cells) for downstream analysis (SI Appendix, Fig. S1C). It gave rise to five distinct clusters (Fig. 1 A–C). The majority of microglia in the NOIR control sample formed a well-defined homeostatic MG0 cluster and a large cluster of microglia (LcMG). Both clusters showed high expression of Cx3cr1, Tmem119, Siglech, P2ry12, and other microglia signature genes (SI Appendix, Fig. S2A), with corresponding low expression of myeloid cell markers, including Mrc1, CD163, and Ccr2 (SI Appendix, Fig. S2D). Particularly, compared with LcMG, the expression profile of cytokine/chemokines or their receptors such as Il6ra, Il1a, Icam1, Ccl3, Ccr5, and Lgals3bp was reduced in the MG0 cluster (SI Appendix, Fig. S2B), indicating that MG0 was probably in a resting homeostatic state without activation. Clusters of MFs including monocyte-derived MFs and perivascular MFs came mostly from OIR mice and were enriched for myelomonocytic genes, including Cfp, Ifitm3, Ccr2, Mrc1, and CD163 (SI Appendix, Fig. S2 C and D). Of note, we observed two small microglia clusters (sMG1 and sMG2) that were found almost exclusively in OIR (Fig. 1 A–C). The sMG1 expressed Snca, Ube2l6, etc., which were involved in neuroinflammation (SI Appendix, Fig. S2E). The sMG2 cluster presented with enriched Mif, Hmox1, Prdx1, Igf1, Lpl, and Fabp5, which have recently been shown to be up-regulated in degenerative disease-associated microglia (SI Appendix, Fig. S2E). The emerging sMG2 in OIR was particularly distinct from the steady state of MG0, and closer examination of the profiles and key marker genes of sMG2 to MG0 revealed significant expression enrichment of Lgals3, Hmox1, Mif, Ccl3, Ccl2, Igf1, etc., genes (Fig. 1D and SI Appendix, Table S1). Gene set enrichment analysis (Gene Ontology [GO]) of sMG2-specific genes revealed significant involvement in cell death (Fig. 1E). Since sMG1 was involved in nervous system process, eye development, and related biology process (SI Appendix, Fig. S3), we focused on sMG2 in OIR. To clarify the form of cell death in sMG2, we adopted and violin plotted the feature genes of well-established forms and several new modes of cell death, including necroptosis, apoptosis, pyroptosis, and ferroptosis. Interestingly, we found Rip3 and Mlkl, two characterized necroptotic genes, were specifically enriched in sMG2 in OIR retina (Fig. 1F), while the feature genes of apoptosis, pyroptosis, and ferroptosis did not show any statistic differences in sMG2 between NOIR and OIR, indicating sMG2 mainly experienced necroptosis in OIR. These findings demonstrate that the OIR retina is featured with an emerging necroptosis-sensitive sMG2 cells.
Fig. 1.
Single-cell RNA-seq uncovers a distinct subtype of necroptotic microglia in a retinal angiogenesis model. (A and B) t-SNE plot of the 2,678 single microglial cells depicting the separation of five clusters in NOIR and OIR. (C) Bar graphs showed sample components of each cluster in B. (D) Volcano plot showed the fold change of genes (log2 scale) and significance (−log10 scale) between sMG2 and MG0. Up-regulated genes were indicated by a red dot and down-regulated genes in blue. P values were determined by Mann–Whitney U test with false discovery rate correction. (E) Gene Ontology (GO) analysis showing enrichment of GO terms in sMG2. (F) The expression of necroptotic genes like Rip3 and Mlkl was exclusively enriched in sMG2 from OIR.
Specific RIP1/3-Necroptosis Occurs in a Subpopulation of Activated Microglia and Is Required for Retinal Angiogenesis.
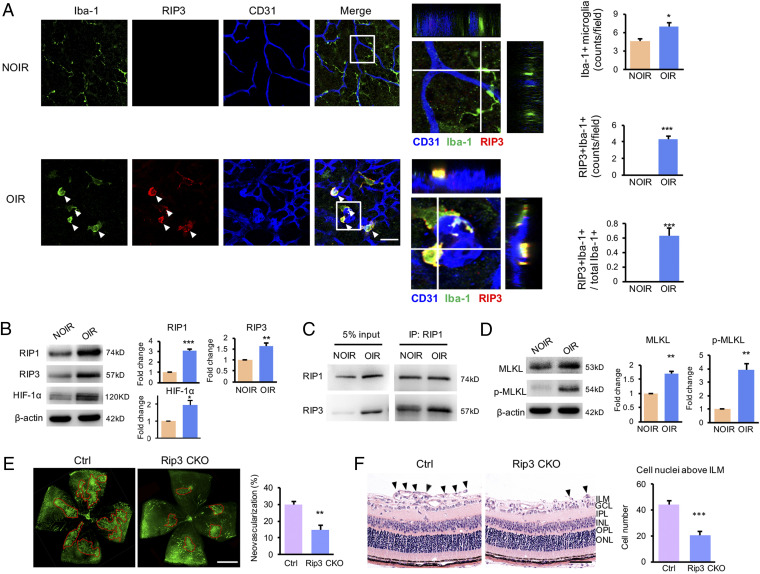
As sMG2 was specifically associated with OIR-necroptosis, we first validated these cells by immunohistochemistry in OIR retina. In the nonischemic control retina, only a limited number of Iba1+ microglia cells, which exhibited a ramified-resting state, were detectable. In contrast, Iba1+ microglial signals were significantly increased in the OIR retina, and particularly, many microglia were characterized by RIP3- or RIP1-positive signals, which were lacking in the NOIR retina (Fig. 2A and SI Appendix, Fig. S4), suggesting a microglial population in OIR, but not NOIR, experienced necroptosis. In addition to the increases of absolute numbers of RIP1+Iba-1+ or RIP3+Iba-1+ cells, the ratios of these cells to total Iba-1+ microglial counts were also markedly increased in OIR retinae (Fig. 2A and SI Appendix, Fig. S4), indicating most microglia cells in OIR undertook necroptosis upon ischemia insults. Besides, most of RIP1+Iba-1+ or RIP3+Iba-1+ microglia, probably sMG2 cells in OIR, were localized around the CD31+ vascular tufts and characterized by an activated morphological phenotype with enlarged cell somas and thick and short lamellipodia (Fig. 2A and SI Appendix, Fig. S4). In the cryosections, the RIP1+Iba-1+ or RIP3+Iba-1+ microglia were increased and distributed along the superficial retinal layer (SI Appendix, Fig. S5 A and B), where the retinal angiogenesis occurred prominently, indicating the sMG2 cells may aggregate and contribute to retinal neovascularization.
Fig. 2.
An activated subpopulation of microglia experienced necroptosis in response to hypoxia and promoted retinal neovascularization. (A) In the NOIR retina, the Iba-1+ microglia adopted a resting state without RIP3 expression and featured small cell bodies with thin and long processes. In contrast, the Iba-1+ microglia increased in the OIR retina and most cells expressed RIP3. The three-dimensional images confirmed the costaining of Iba-1 and RIP3. These RIP3+Iba-1+ microglia (white arrowheads) were found around the vascular tufts, characterized by enlarged cell somas with thick and short lamellipodia. The statistics showed the percentage of RIP3+Iba-1+ cells to total Iba-1+ microglial cells were about 60%. n = 3 retinae, 6 images/retina. (B) Both necroptotic proteins (RIP1 and RIP3) were increased in OIR retinae. HIF-1α, the important transcriptional factor under hypoxic condition, was also up-regulated in the OIR retina. (C) The immunoprecipitation data showed the binding of RIP3 and RIP1 was highly up-regulated in OIR retina. (D) A significant elevation of MLKL and p-MLKL expression was observed in retina from OIR. (E) Representative images of IB4 staining of retinal whole mounts in OIR mice. Rip3 conditional deficient in CX3CR1+ microglia (Rip3 CKO) significantly reduced the area of neovascular tufts compared with Rip3fl/flCre− controls. n = 6 retinae. (F) Neovascular cells anterior to the ILM (black arrowheads) were markedly reduced in the Rip3-CKO OIR mice. n = 6 eyes, 10 sections/eye. GCL, ganglion cell layer; ILM, internal limiting membrane; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer. (Scale bars: 50 µm in A and F; 1 mm in E.) *P < 0.05; **P < 0.01; ***P < 0.001.
We then analyzed expression levels of necroptotic proteins in OIR by immunoblot. As expected, RIP1 and RIP3 proteins were markedly elevated in the retina from OIR mice relative to the non-OIR control (Fig. 2B). Coimmunoprecipitation experiments showed a physical interaction between RIP1 and RIP3 in OIR retina (Fig. 2C). The expression level of MLKL, a critical RIP3 downstream component and the effector of necroptosis, was also elevated. Interestingly, the phosphorylated MLKL in its active form was exclusively detected in the OIR retina (Fig. 2D). These findings confirmed a possible involvement of the RIP1/3/MLKL-mediated necroptosis in retinal neovascularization. HIF-1α, an important transcriptional factor in response to hypoxia, was also up-regulated in the OIR retina and might be involved in necroptosis (Fig. 2B). In order to distinguish necroptosis from apoptosis, we analyzed caspase-8 and caspase-3, two crucial components in initiating and executing cellular apoptosis, and the cleaved caspase-8 and -3 were almost undetectable in both NOIR and OIR retina (SI Appendix, Fig. S6 A and B). The level of cFLIP, an inhibitor of caspase-8 activation, was increased in the OIR retina (SI Appendix, Fig. S6C), indicating the possibility of apoptosis inhibition in OIR. These findings suggested functional involvement of caspase-8– and caspase-3–mediated cellular apoptosis be excluded in regulating retinal neovascularization.
To elucidate the role of necroptotic microglia in retinal angiogenesis, we generated a conditional knockout (CKO) mouse strain to selectively delete Rip3 gene in microglia cells using the Rip3fl/fl-Cx3cr1-CRE system (Rip3fl/fl;Cx3cr1-CRE mice). Compared to control littermates, OIR in Rip3fl/fl;Cx3cr1-CRE mice (Rip3 CKO) showed marked reduction of retinal neovascularization and neovascular cells anterior to internal limiting membrane (ILM), the hallmarks of angiogenesis in OIR (Fig. 2 E and F). These genetic data provide evidence that microglial Rip3-mediated necroptosis specifically contributes to the development of retinopathy. To exclude the involvement of other cells in necroptosis in OIR, double immunohistochemical staining showed GFAP+ cells lacked RIP1 or RIP3 expression (SI Appendix, Fig. S7A), indicating necroptosis did not occur in astrocytes/Müller cells. Similarly, RIP1- or RIP3-positive signals were not colocalized with βIII-tubulin (RGCs) (SI Appendix, Figs. S7B and S8A), Map-2 (neurons) (SI Appendix, Fig. S7C), Rhodopsin and Opsin (photoreceptors) (SI Appendix, Fig. S8 B and C), and PKC-α (bipolar cells) (SI Appendix, Fig. S7D). Together, OIR-induced necroptosis occurs specifically in microglia cells.
Hypoxia Triggers Microglia Necroptosis In Vitro.
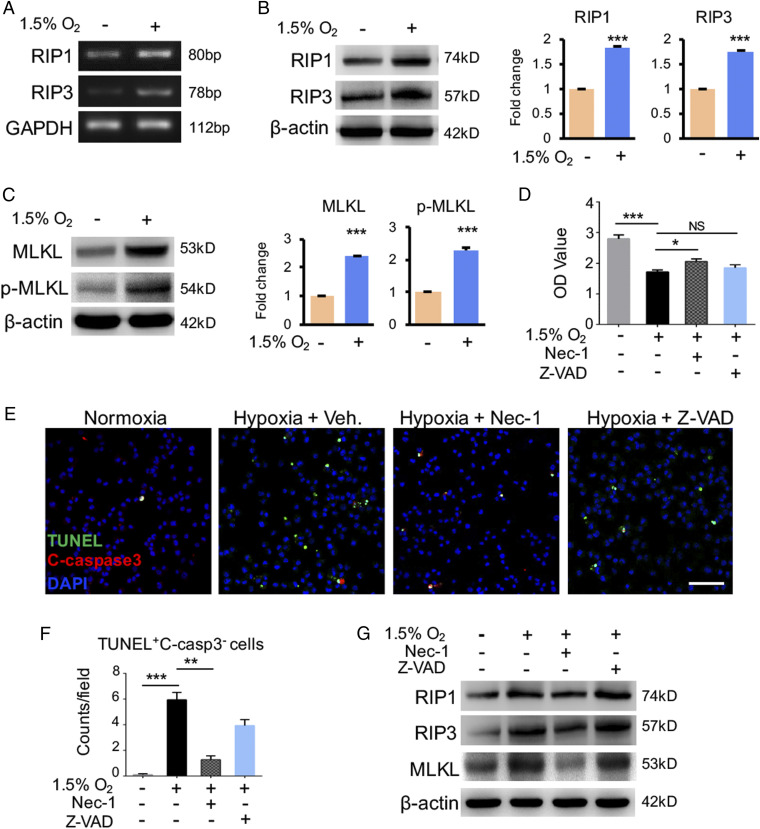
To gain mechanistic insights underlying microglial necroptosis in OIR, we studied the impact of hypoxia on necroptosis in vitro. Primary mouse microglia cells and BV2 microglia cell line were cultured under a 1.5% oxygen condition. Intriguingly, hypoxia significantly induced RIP1 and RIP3 expression levels (Fig. 3 A and B), suggesting that RIP1 and RIP3 were probably hypoxia-inducible genes. Notably, the MLKL protein level, especially the p-MLKL, was markedly up-regulated under the hypoxic condition (Fig. 3C). A CCK8 assay showed hypoxia significantly jeopardized microglia cell viability (Fig. 3D). Treatment of hypoxia-exposed microglia cells with Nec-1, a selective allosteric inhibitor of RIP1, rescued cell survival to some extent. In contrast, treatment with a pan caspase inhibitor, zVAD-fmk, targeting apoptosis pathways had no significant impact on cell viability (Fig. 3D). Consistently, an increased population of TUNEL+caspase-3− necroptotic cells were found in the hypoxia-treated primary microglia and Nec-1 treatment significantly counteracted the necroptosis (Fig. 3 E and F). The up-regulation of necroptotic machinery including RIP1, RIP3, and MLKL in hypoxia-exposed microglia was reduced by Nec-1 but not zVAD-fmk (Fig. 3G). These findings provide mechanistic insights into the hypoxia-induced microglial necroptosis.
Fig. 3.
Hypoxia-induced necroptosis in cultured microglia. (A and B) The microglia were cultured under hypoxic condition (1.5% O2, 5% CO2) for 24 h with condition of (21% O2, 5% CO2) as normoxia control. The expression of RIP1 and RIP3 at RNA (A) and protein level (B) was elevated in hypoxic group. Both MLKL and p-MLKL (C) were also increased in microglia cultured under hypoxic condition. n = 3. (D) CCK8 assay revealed that the survival of microglia cells was reduced after hypoxia insults compared to normoxia; however, the pretreatment of Nec-1 abolished this reduction. In the condition of Z-VAD pretreatment, no significant difference was found in comparison to hypoxia group without any pretreatment. n = 3. (E and F) Representative images and statistical analysis of TUNEL assay combined with cleaved caspase-3 staining in cultured microglia cells. A prominent elevation of TUNEL+C-casp3− necroptotic cells in the hypoxic group, which was suppressed by Nec-1 treatment. (Scale bars: 100 µm.) n = 3 cultures, 4 images/culture. (G) Western blot for the necroptotic machineries (RIP1, RIP3, and MLKL) in primary microglia cells stimulated with Nec-1, Z-VAD, or no administration. Hypoxia insults induce expression of these genes, which would be suppressed by Nec-1 but not Z-VAD. n = 3. (Scale bars: 50 µm in E.) *P < 0.05; **P < 0.01; ***P < 0.001; NS, no significance.
Release of FGF2 by Microglial Necroptosis.
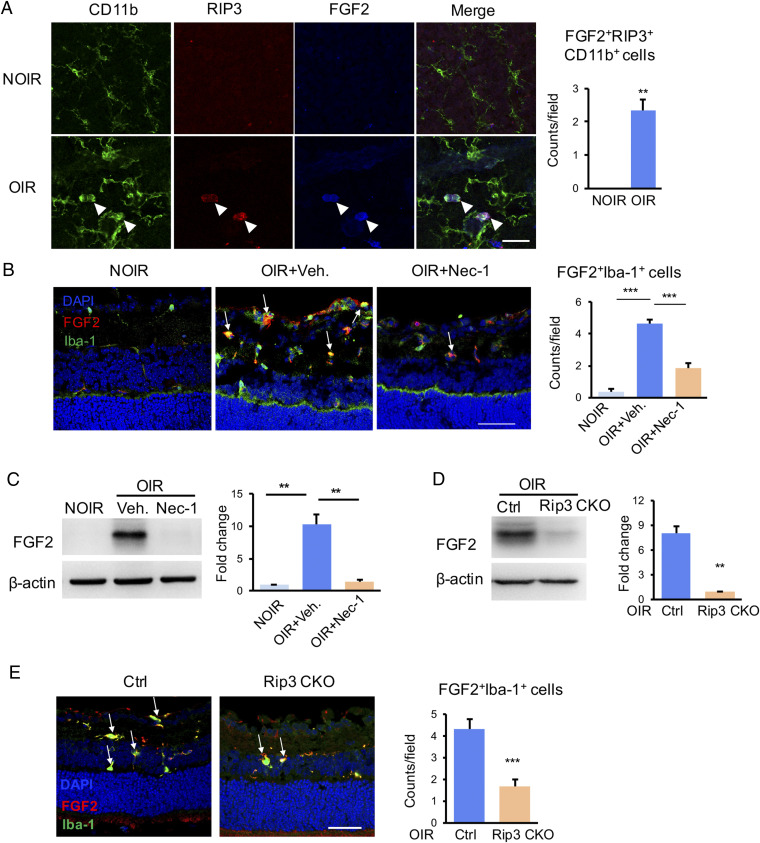
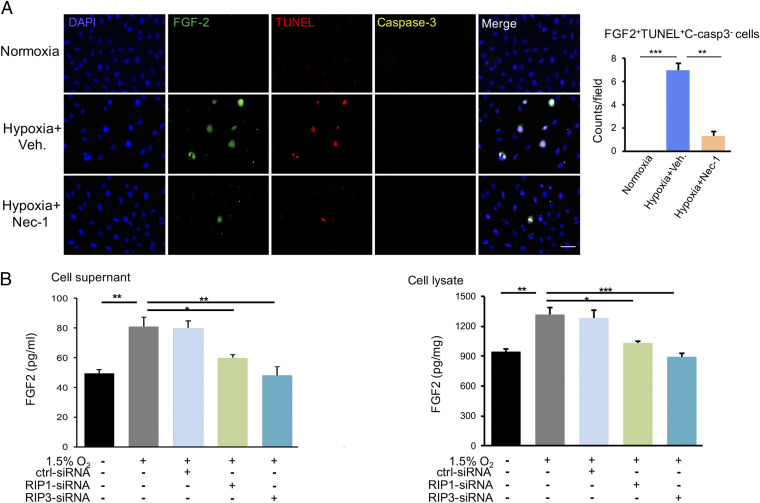
We next investigated molecular players in causing retinal angiogenesis through the mechanism of necroptosis. A qPCR-based microarray assay was employed to define angiogenic molecules. Among all angiogenic factors, FGF2 was the most up-regulated gene in the OIR (SI Appendix, Fig. S9A). Nec-1 treatment impaired FGF2 expression and the production of some of the other cytokines including VEGF (SI Appendix, Fig. S9B). Multiple cytokines assays showed the protein level of FGF2 also increased in the OIR retina, which was obliterated in Nec1-treated or Rip3-CKO OIR mice (SI Appendix, Fig. S9C). Interestingly, FGF2 expression was colocalized with the RIP3+CD11b+ necroptotic microglia in OIR, indicating microglia might be the primary cell type for production of FGF2 (Fig. 4A). Importantly, the number of FGF2+Iba-1+ microglial subpopulation was markedly reduced by Nec-1 (Fig. 4B). The FGF2 protein expression was completely eliminated in the Nec-1–treated OIR retina (Fig. 4C). Consistently, Rip3-CKO OIR mice presented with less FGF-2+Iba-1+ microglia (Fig. 4E) and marked reduction of FGF2 production (Fig. 4D) compared with Rip3fl/fl;Cx3cr1Cre− OIR controls. In vitro, FGF2 was observed to be expressed in TUNEL+C-caspase-3− necroptotic microglia and Nec-1 reduced the numbers of FGF2+TUNEL+C-caspase-3− cells (Fig. 5A). These findings demonstrate that FGF2 production is dependent on the RIP1/RIP3-mediated microglial necroptosis.
Fig. 4.
Improved FGF2 production in necroptotic microglia in vivo. (A) Immunofluorescence staining in retinal whole mounts revealed strong staining of FGF2 in RIP3+CD11b+ necroptotic microglia from OIR retina (white arrowheads), while lack of FGF2 and RIP3 expression in the NOIR control. n = 3 retinae, 6 images/retina. (B) The FGF2+Iba-1+ microglia were increased in OIR, which was abrogated by Nec-1. n = 3 eyes, 6 sections/eye. (C) The Western blot result showed that the elevated FGF2 in OIR were inhibited significantly in the Nec-1–treated ones. n = 6 retinae. (D) The FGF2 expression was markedly suppressed in the OIR retina from Rip3 CKO mice in comparison to controls. n = 6 retinae. (E) The FGF2+Iba-1+ microglia were also highly reduced in Rip3 CKO-OIR retina. n = 3 eyes, 6 sections/eye. (Scale bars: 50 µm in A, B, and E.) **P < 0.01; ***P < 0.001.
Fig. 5.
Hypoxia-induced microglia necroptosis released FGF2 in vitro. (A) In vitro, the TUNEL+C-caspase-3− necroptotic microglia showed a strong labeling of FGF2 under the hypoxic condition, whereas Nec-1 could reduce the counts of FGF2+TUNEL+C-caspase-3− cells. n = 3 cultures, 4 images/culture. (B) The supernatant and cellular lysate of cultured microglia were collected and detected the protein amounts of FGF2 by ELISA. The FGF2 protein level was elevated in the conditioned medium of hypoxia-exposed microglial cells, which was suppressed by blockade of RIP1 and RIP3 using siRNA. Consistently, the FGF2 level in cellular lysis showed a similar trend. (Scale bars: 50 µm in A.) *P < 0.05; **P < 0.01; ***P < 0.001.
FGF2 is known as a signal-less nonsecreted angiogenic factor and its release from cells remains an enigma. Several possible mechanisms and pathways have been proposed, including pore opening in cell membrane, membrane vesicles shedding, and other alternative pathways (18, 19). It is highly plausible that the necroptotic process promotes release of intracellular FGF2 to the extracellular space, where the FGF receptors are located. To investigate this possibility, we performed in vitro experiments using a microglia cell culture system. Expectedly, an elevated level of FGF2 protein was detected in the conditioned medium of hypoxia-exposed N9 microglial cells. Blockade of RIP1 and RIP3 by small interfering RNA (siRNA) ablated the elevated FGF2 levels in the cultured medium (Fig. 5B). In the cellular lysates, FGF2 was also increased in hypoxia-exposed microglial cells, which was abrogated with siRNA specific for RIP1 and RIP3 (Fig. 5B). Thus, these data indicated that the RIP1-RIP3–mediated necroptosis induced the production and release of FGF2 from microglia.
Synergistic Inhibition of Retinal Angiogenesis by Anti-Necroptosis and Anti-VEGF Combination Therapy.
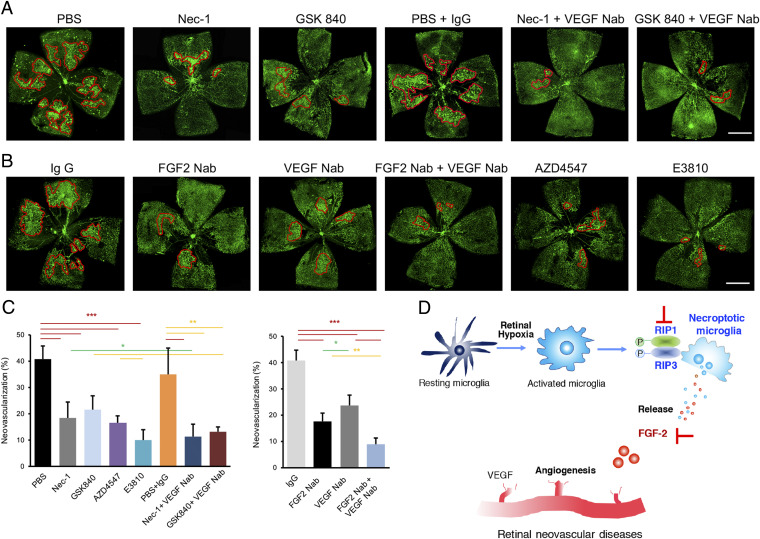
Knowing the essential proangiogenic mechanism of necroptotic microglia-triggered release of FGF2 in OIR, we further evaluated the efficacy of antiangiogenic therapy by targeting this signaling pathway. To achieve this goal, we employed an array of inhibitors that blocked the necroptosis–FGF2 axis in OIR mice, including inhibitors for RIP1 (Nec-1) and RIP3 (GSK840), a neutralizing antibody (Nab) for FGF2, or an inhibitor for FGFR tyrosine kinase (AZD4547). As expected, intravitreal administration of these agents individually inhibited retinal neovascularization (Fig. 6 A–C). The newly formed irregular and primitive vasculatures were significantly ablated (Fig. 6 A–C), indicating blocking RIP1–RIP3–FGF2/FGFR signaling provides a therapeutic paradigm for treating retinal neovascular diseases.
Fig. 6.
Combined inhibition of necroptosis–FGF2 signaling and VEGF signaling ameliorated pathologic angiogenesis in OIR. (A) Single intravitreal injection of inhibitors for RIP1 or RIP3 (Nec-1 or GSK840, respectively) could suppress the angiogenesis development in OIR. In addition, the OIR retina present little angiogenic tufts after combined treatment of Nec-1 or GSK840 and VEGF Nab. (B) Single intravitreal injection of FGF2 neutralizing antibody (Nab) suppressed the formation of angiogenic tufts in OIR, while the neovascularization area in the VEGF Nab group was also reduced compared with IgG injection. In particular, combination therapy with FGF2 Nab and VEGF Nab presented with the least degree of retinal angiogenesis. Similarly, blocking FGF2 pathway by AZD4547, a potent inhibitor of FGFRs, ameliorated the pathologic angiogenesis, while E3810, a dual inhibitor of VEGFR and FGFR, exerted the most potent effect of antiangiogenesis. (C) The statistics of neovascularization percentage in A and B. n = 6 retinae. (Scale bars: 1 mm.) *P < 0.05 (green); **P < 0.01 (yellow); ***P < 0.001 (red). (D) Schematic drawing showing microglia necroptosis-mediated angiogenesis in retinal neovascular diseases. Hypoxia in retina activated the resting microglia and induced RIP1/3-mediated necroptosis in activated microglia. The necroptotic microglia could produce and release amounts of FGF2, which synergized with VEGF promoted angiogenesis in the retina, contributing to the development of retinal neovascular diseases. Blockade of RIP1/3-mediated necroptosis and inhibition of FGF2/VEGF pathway could be potential antiangiogenesis therapies for retinal neovascular diseases.
Since VEGF is another hypoxia-induced proangiogenic factor for retinal angiogenesis and anti-VEGF agents are routinely used for treating eye disease, we explored the combination therapy by simultaneous targeting microglial necroptosis–FGF2 and VEGF signaling pathways. As showed in the Fig. 6 A–C, the combination therapies of necroptosis blockade by Nec-1 or GSK840 and VEGF blockade significantly inhibited neovascularization in comparison to single treatment. In addition, combination of FGF2 Nab and VEGF Nab also remarkably suppressed angiogenesis in OIR (Fig. 6 B and C). Similarly, treatment with a multikinase inhibitor for FGFR and VEGFR, namely E3810, produced a comparably potent antiangiogenic effect in the OIR (Fig. 6 B and C). These findings provide evidence that simultaneous targeting of the necroptosis–FGF2/FGFR and VEGF–VEGFR signaling pathways produces synergistic antiangiogenic effect in the ischemia/hypoxia-induced retinopathy.
Discussion
Although microglia are now recognized as functional modulators of retinal diseases, the extent of their heterogeneity and functional significance in the retina in response to hypoxia remains elusive. In this study, we have identified a previously unprecedent subpopulation of microglia that are specifically exemplified under OIR. This OIR-specific subpopulation of microglia induces retinal neovascularization through necroptosis, which is a mechanism to release proangiogenic factors such as FGF2. These necroptotic microglia migrate and reside around superficial retinal angiogenesis and blocking necroptosis ablates pathological angiogenesis. Based on these findings, we propose a therapeutic paradigm for treating retinal vascular diseases by targeting microglial necroptosis.
In this work, we describe the biological effect of “the life after death.” Necroptosis, a highly regulated process, like a “suicide” activation, would initiate the microglia-mediated angiogenesis. As showed in a schematic diagram (Fig. 6D), retinal microglia are activated and undergo necroptosis in response to hypoxia insults through activation of RIP1–RIP3 signaling. The necroptotic microglia subsequently release FGF2, which synergizes with VEGF in promoting retinal angiogenesis. The hypoxia as a trigger for retinal angiogenesis has several significant impacts on the retina, including the following: 1) hypoxia induces VEGF expression; 2) hypoxia induces microglial necroptosis; 3) hypoxia elevates FGF2 expression in necroptotic microglia; and 4) hypoxia induces FGF2 release. The broad biological impacts of hypoxia on retinal angiogenesis suggest multitargeted approaches are needed for effectively blocking retinal neovascularization. Here, we have provided mechanistic insights on retinal neovascularization and identified therapeutic targets for potential treatment of retinopathy.
Our study uncovers a mechanism of necroptosis-mediated FGF2 release. This study describes an FGF2-dependent mechanism that mediates microglial necroptosis-induced retinal angiogenesis. These are unexpected and surprising findings because hypoxia-induced VEGF expression has been considered to be the key factor to induce retinal angiogenesis (20, 21). Along this orthodoxical view, therapeutic agents targeting VEGF and its signaling pathway have been developed for treating retinal angiogenesis diseases (2, 22). Indeed, anti-VEGF drugs show remarkably clinical benefits in AMD, DR, and ROP patients (2, 22–24). However, the anti-VEGF drugs often become resistant and produce limited clinical benefits in a majority of retinopathy patients (25). Thus, there is an urgent need for additive therapy to anti-VEGF treatment for retinal angiogenic diseases. Unlike VEGF, which is mainly engaged in endothelial cell (EC) migration and vascular permeability, FGF2 acts as an endothelial cell proliferative factor and displays more potent angiogenic effect (26, 27). FGF2 not only promotes EC proliferation and EC organization into tube-like structures, but also is required for submacular fibrosis in the wet AMD pathogenesis. Recently, RBM-007, an anti-FGF2 aptamer, has been investigated for tolerability in wet AMD patients in a phase 1/2a clinical study. It holds promise as an additive or alternative therapy to anti-VEGF treatments for wet AMD.
FGF2 expression has been reported to be up-regulated in response to hypoxia in vivo and in vitro. Chronic hypoxia induced the expression of FGF2 and its receptor in cerebral astrocytes (28). In hypoxia-stimulated endothelial cells, the HIF-1α–FGF2 amplification pathway was required for cellular survival (29). Some studies further showed that HIF-1α and FGF2 could reciprocally regulate their expression by an IRES-dependent mechanism (30). In this study, we have found that FGF2 expression is more abundant in microglial cells in the hypoxic retina. Moreover, hypoxia in vitro and in vivo further elevates FGF2 expression levels in microglial cells, which is concomitant to the death commitment. Blockade of necroptosis by Rip1/3 knockdown down-regulated FGF2 expression and release, indicating the possibility that FGF-2 expression level depended on RIP1/3. Although FGF2 has been reported as a potent angiogenic factor for long, the anti-FGF2 drugs for treating retinal neovascular disease in human patients linger. One of the possible reasons is probably due to its intrinsic and enigmatic feature as a growth factor. Unlike most other growth factors, FGF2 lacks a secretory signal peptide and is a cytosolic protein (31). It is less likely to be actively secreted from its biosynthetic cells and its release is an enigma (18, 19, 31, 32). Upon necroptosis, a high amount of FGF2 is explosively released from microglia locally to induce robust retinal angiogenesis. This mechanism of sudden release of FGF2 at high level would cause severe functional consequence of retinal damage by triggering robust angiogenesis. Thus, preventing and blocking microglial necroptosis is crucial for treating retinal diseases. Necroptosis and apoptosis are elementary immune cell death mechanisms that operate in various immune responses. In the vasculature, apoptosis has been considered to be required for developmental vessel regression, while we found it is necroptosis that mainly contributes to retinal angiogenesis in OIR.
In conclusion, this study revealed that the necroptosis phenotype of microglia may represent a key feature of pathological angiogenesis in retina. Our data provide several unexpected mechanistic insights, including hypoxia-induced microglial cell necroptosis; necroptosis-induced FGF2 release; and FGF2-induced retinal angiogenesis. Based on our findings, it is reasonable to speculate that targeting the RIP1/RIP3 necroptosis and FGF2–FGFR signaling pathways would provide a therapeutic approach for treating ischemic/hypoxia-associated retinal disease. In particular, combinative blockade of necroptosis pathway and inhibition of VEGF pathway produces an additive and perhaps synergistic effects for treating angiogenic retinopathy. This therapeutic concept warrants future clinical validation.
Materials and Methods
Mice and OIR Model.
All animals were kept in a specific pathogen-free facility in Animal Laboratories of Zhongshan Ophthalmic Center and the experiments were approved by the Institutional Animal Care and Use Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University. OIR model was established according to a previously described method in SI Appendix, SI Materials and Methods (17).
Cell Culture, Transfection, CCK8 Assays, and Immunofluorescence Staining.
Detailed descriptions of primary microglia cell and cell line cultures, transfection, CCK8 assays, and immunofluorescence staining procedures can be found in SI Appendix, SI Materials and Methods. The primary microglia cells were isolated and cultured as described previously (6).
Quantification of Neovascularization in the Whole-Mounted and Sectional Retinas.
Detailed descriptions of neovascularization quantification procedures can be found in SI Appendix, SI Materials and Methods.
scRNA-Seq.
Detailed descriptions of tissue harvesting, single-cell preparation, library preparation, data analysis, particular clustering analysis, and GO term analysis can be found in SI Appendix, SI Materials and Methods and Table S1.
Western Blotting, Real-Time PCR, ELISA, and Array Analysis.
Detailed descriptions of Western blotting, real-time PCR, ELISA, and other array procedures (33) can be found in SI Appendix, SI Materials and Methods and Table S2.
Statistics.
Data quantification were performed blindly and presented as mean ± SE of measurement (SEM). Data were analyzed statistically using one-way ANOVA with Bonferroni’s post hoc test for comparisons of three and more groups or two-tailed Student’s t test for two group comparisons. To assess significance, a value of P < 0.05 was considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001). The sample sizes and P values are indicated in the figure legends.
Supplementary Material
Acknowledgments
This work was supported by National Key R&D Program of China (2018YFA0108300); National Natural Science Foundation of China (81630022 and 81700825); Natural Science Foundation of Guangdong Province, China (2019B151502006); and Science and Technology Program of Guangzhou, China (201906010076 and 201707020006).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. J.L.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2023290118/-/DCSupplemental.
Data Availability
The single-cell RNA sequencing data have been deposited in the Gene Expression Omnibus (GEO) database (accession no. GSE152928). All other study data are included in the article and supporting information.
References
- 1.Zhang K., Zhang L., Weinreb R. N., Ophthalmic drug discovery: Novel targets and mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug Discov. 11, 541–559 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Wells J. A.et al.; Diabetic Retinopathy Clinical Research Network , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 372, 1193–1203 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlstetter M., et al., Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 45, 30–57 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Reemst K., Noctor S. C., Lucassen P. J., Hol E. M., The indispensable roles of microglia and astrocytes during brain development. Front. Hum. Neurosci. 10, 566 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold T., Betsholtz C., The importance of microglia in the development of the vasculature in the central nervous system. Vasc. Cell 5, 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yun S. P., et al., Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 24, 931–938 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liddelow S. A., et al., Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pender M. P., Activation-induced apoptosis of autoreactive and alloreactive T lymphocytes in the target organ as a major mechanism of tolerance. Immunol. Cell Biol. 77, 216–223 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Pender M. P., Rist M. J., Apoptosis of inflammatory cells in immune control of the nervous system: Role of glia. Glia 36, 137–144 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Pasparakis M., Vandenabeele P., Necroptosis and its role in inflammation. Nature 517, 311–320 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Wallach D., Kang T. B., Dillon C. P., Green D. R., Programmed necrosis in inflammation: Toward identification of the effector molecules. Science 352, aaf2154 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Ofengeim D., et al., Activation of necroptosis in multiple sclerosis. Cell Rep. 10, 1836–1849 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., et al., The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng L., Jin W., Wang X., RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc. Natl. Acad. Sci. U.S.A. 112, 11007–11012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linkermann A., Green D. R., Necroptosis. N. Engl. J. Med. 370, 455–465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd A. F., et al., Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci. 22, 1046–1052 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connor K. M., et al., Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 4, 1565–1573 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taverna S., et al., Shedding of membrane vesicles mediates fibroblast growth factor-2 release from cells. J. Biol. Chem. 278, 51911–51919 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Rhoads D. N., Eskin S. G., McIntire L. V., Fluid flow releases fibroblast growth factor-2 from human aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 20, 416–421 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Aiello L. P., et al., Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 331, 1480–1487 (1994). [DOI] [PubMed] [Google Scholar]
- 21.Sidman R. L., et al., The peptidomimetic Vasotide targets two retinal VEGF receptors and reduces pathological angiogenesis in murine and nonhuman primate models of retinal disease. Sci. Transl. Med. 7, 309ra165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mintz-Hittner H. A., Kennedy K. A., Chuang A. Z.; BEAT-ROP Cooperative Group , Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 364, 603–615 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaffe G. J., et al., Differential response to anti-VEGF regimens in age-related macular degeneration patients with early persistent retinal fluid. Ophthalmology 123, 1856–1864 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Rao P., et al., Real-world vision in age-related macular degeneration patients treated with single anti-VEGF drug type for 1 year in the IRIS registry. Ophthalmology 125, 522–528 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Yang S., Zhao J., Sun X., Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Devel. Ther. 10, 1857–1867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao R., et al., Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ. Res. 94, 664–670 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Cross M. J., Claesson-Welsh L., FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 22, 201–207 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Flores C., Stewart J., Salmaso N., Zhang Y., Boksa P., Astrocytic basic fibroblast growth factor expression in dopaminergic regions after perinatal anoxia. Biol. Psychiatry 52, 362–370 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Calvani M., Rapisarda A., Uranchimeg B., Shoemaker R. H., Melillo G., Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood 107, 2705–2712 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conte C., et al., FGF2 translationally induced by hypoxia is involved in negative and positive feedback loops with HIF-1alpha. PLoS One 3, e3078 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steringer J. P., et al., Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-dependent oligomerization of fibroblast growth factor 2 (FGF2) triggers the formation of a lipidic membrane pore implicated in unconventional secretion. J. Biol. Chem. 287, 27659–27669 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steringer J. P., Nickel W., The molecular mechanism underlying unconventional secretion of fibroblast growth factor 2 from tumour cells. Biol. Cell 109, 375–380 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Huang Z., et al., Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ. 25, 180–189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The single-cell RNA sequencing data have been deposited in the Gene Expression Omnibus (GEO) database (accession no. GSE152928). All other study data are included in the article and supporting information.