Significance
Phosphorus (P) is essential for life. As the fifth-most-abundant element in living cells, P is required for the synthesis of an array of biological molecules including (d)NTPs, nucleic acids, and membranes. Organisms typically acquire environmental P as inorganic phosphate (Pi). While essential for growth and viability, excess intracellular Pi is toxic for both bacteria and eukaryotes. Using the bacterium Salmonella enterica serovar Typhimurium as a model, we establish that Pi cytotoxicity is manifested following its assimilation into adenosine triphosphate (ATP), which acts as a chelating agent for Mg2+ and other cations. Our findings identify physiological processes disrupted by excessive Pi and how bacteria tune P assimilation to cytoplasmic Mg2+ levels.
Keywords: phosphate toxicity, ATP, magnesium, MgtC, Salmonella
Abstract
Phosphorus (P) is an essential component of core biological molecules. In bacteria, P is acquired mainly as inorganic orthophosphate (Pi) and assimilated into adenosine triphosphate (ATP) in the cytoplasm. Although P is essential, excess cytosolic Pi hinders growth. We now report that bacteria limit Pi uptake to avoid disruption of Mg2+-dependent processes that result, in part, from Mg2+ chelation by ATP. We establish that the MgtC protein inhibits uptake of the ATP precursor Pi when Salmonella enterica serovar Typhimurium experiences cytoplasmic Mg2+ starvation. This response prevents ATP accumulation and overproduction of ribosomal RNA that together ultimately hinder bacterial growth and result in loss of viability. Even when cytoplasmic Mg2+ is not limiting, excessive Pi uptake increases ATP synthesis, depletes free cytoplasmic Mg2+, inhibits protein synthesis, and hinders growth. Our results provide a framework to understand the molecular basis for Pi toxicity. Furthermore, they suggest a regulatory logic that governs P assimilation based on its intimate connection to cytoplasmic Mg2+ homeostasis.
As a component of a large number of biological molecules, phosphorus (P) is required for key biological functions. These include the formation of cellular boundaries, the storage and transfer of chemical energy, the integration and propagation of information in signal transduction pathways, and the storage, transmission, and expression of genetic information. Bacteria take up P mainly as inorganic phosphate (PO4−3; Pi), which is then assimilated in the cytoplasm via its incorporation into adenosine triphosphate (ATP). ATP functions as the main cellular P-carrier molecule, mediating both the transfer of Pi among biological molecules and the release of chemical energy to power energy-dependent processes (1). Cells must tightly regulate Pi acquisition and utilization because P assimilation is essential but excessive cytoplasmic Pi is toxic (2–9). Here, we elucidate how bacteria coordinate Pi acquisition and consumption to prevent the deleterious effects of unbalanced Pi metabolism.
Following assimilation, the negative charges from Pi groups in biomolecules are neutralized by positively charged ionic species present in the cytoplasm. As such, the majority of cytoplasmic ATP exists as a salt with positively charged magnesium (Mg2+), the most abundant divalent cation in living cells. Therefore, the ATP:Mg2+ salt, rather than the ATP anion, is the substrate for most ATP-dependent enzymatic reactions (10, 11). In enteric bacteria, ATP stimulates transcription of ribosomal RNA (rRNA) genes (12, 13). The synthesis and activity of ribosomes consume the majority of the ATP in rapidly growing cells (14). During ribosome biogenesis, the negative charges from Pi groups in the rRNA backbone chelate large amounts of Mg2+ ions (14, 15). This process reduces electrostatic repulsion among Pi groups in the rRNA backbone, enabling the folding and assembly of functional ribosomes (15, 16). Thus, ATP and rRNA constitute the largest cytoplasmic reservoirs of Pi and Mg2+ (12, 14–20). Given this inherent connection between Pi and Mg2+, we wondered if the cytotoxic effects of excessive Pi uptake result from Pi assimilation into ATP and subsequent disruption of Mg2+ dependent processes.
In the gram-negative bacterium Salmonella enterica serovar Typhimurium (Salmonella), prolonged growth in limiting Mg2+ induces a Mg2+ starvation response mediated by the MgtA, MgtB and MgtC proteins (21–24). MgtA and MgtB are high-affinity, ATP-dependent Mg2+ importers that increase the Mg2+ concentration in the cytoplasm (25, 26). MgtC decreases intracellular ATP levels (27), thereby reducing rRNA synthesis, which lowers the steady-state amounts of ribosomes and slows down the translation rate (28). This response reduces the concentration of assimilated P and, consequently, the quantity of Mg2+ required as a counter ion, allowing the remaining cytoplasmic Mg2+ to stabilize existing ribosomes and to maintain other vital cellular processes that exhibit a strict dependence on Mg2+ (21, 28, 29).
MgtC promotes Salmonella survival inside mammalian macrophages and the establishment of systemic infections (30, 31). In macrophages, MgtC inhibits the activity of Salmonella’s own F1Fo ATP synthase, the enzyme catalyzing ATP production via oxidative phosphorylation (32, 33). MgtC appears to operate by distinct mechanisms inside macrophages versus low-Mg2+ media because an MgtC variant has been identified that fails to promote intramacrophage survival while enabling growth and viability under cytoplasmic Mg2+ starvation (34). In this paper, we reveal that Pi uptake must be tightly controlled to maintain cytoplasmic Mg2+ homeostasis. We establish that during cytoplasmic Mg2+ starvation, MgtC lowers ATP levels by inhibiting Pi uptake, thus limiting an ATP precursor instead of interfering with its enzymatic generation (Fig. 1). MgtC hinders the activity of the main Pi uptake system in Salmonella and likely other bacterial species. Counterintuitively, limiting exogenous Pi availability rescues translation, promotes growth, and restores viability to an mgtC null mutant. Excess Pi is toxic even at physiological concentrations of cytoplasmic Mg2+ due to its incorporation into ATP and subsequent disruption of Mg2+-dependent processes. Our findings provide a conceptual framework to understand the underlying basis of Pi cytotoxicity observed in bacteria and eukaryotes (3–9, 35–38). Moreover, we uncover the regulatory logic by which cells control of P assimilation.
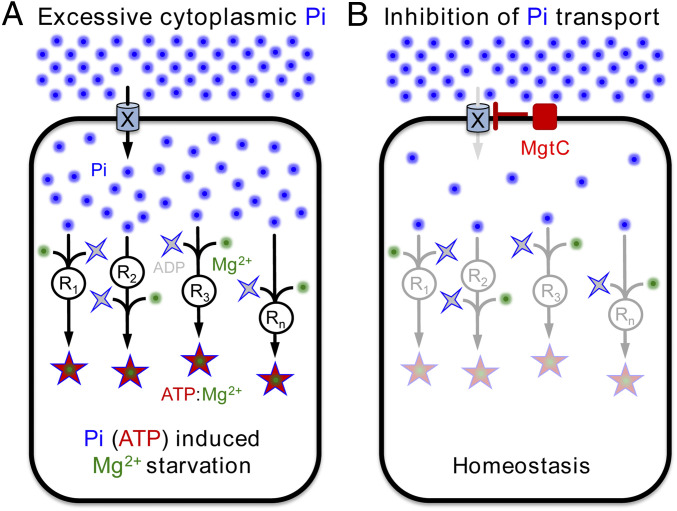
Fig. 1.
Model depicting how Salmonella enterica prevents Pi cytotoxicity. (A) Excessive Pi is imported into the cytoplasm via an inner membrane transport system (denoted by an X). Pi is assimilated by the cells through reactions that synthesize ATP (R1, R2, R3, Rn). Excessive ATP chelates free cytoplasmic Mg2+, thereby inducing cytoplasmic Mg2+ starvation. (B) Cells express the MgtC membrane protein to inhibit Pi uptake via X. The reduction in the availability of cytoplasmic Pi simultaneously slows all ATP-generating reactions in the cell, freeing Mg2+ ions and restoring homeostasis.
Results
The MgtC Protein Limits ATP Accumulation during Low Cytoplasmic Mg2+ Stress by an F1Fo Synthase-Independent Mechanism.
ATP exists as a Mg2+ salt in living cells (11, 14). Therefore, when cells face limiting cytoplasmic Mg2+ concentrations, they reduce ATP amounts to free Mg2+ ions so they can be used for other cellular processes, such as ribosome assembly (28, 29). In macrophages, MgtC inhibits Salmonella’s own F1Fo ATP synthase, the enzyme responsible for ATP synthesis via oxidative phosphorylation (32). Notably, an mgtC atpB double mutant—lacking both MgtC and the AtpB subunit of the ATP synthase—was reported to harbor similar ATP amounts than an atpB single mutant under cytoplasmic Mg2+ starvation conditions (32). This result led to the notion that MgtC prevents a nonphysiological rise in ATP levels by inhibiting the F1Fo ATP synthase also under cytoplasmic Mg2+ starvation (32, 39, 40). Below, we present the result of experiments that reexamine the interpretation of the aforementioned results.
The F1Fo ATP synthase uses the proton motive force generated by the respiratory electron transport chain to synthesize ATP from adenosine diphosphate and Pi (41, 42). Consequently, an mgtC atpB double mutant (or any other strain lacking a functional ATP synthase) relies exclusively on fermentative pathways to produce ATP via substrate-level phosphorylation (43). However, the low ATP amounts observed in the mgtC atpB double mutant were obtained during growth on medium containing glycerol as the carbon source (32). Given that the glycerol fermentation is extremely inefficient (44, 45), we reasoned that the low ATP amounts in the mgtC atpB double mutant could simply reflect an inability to efficiently ferment glycerol.
To test this notion, we compared ATP amounts in wild-type, mgtC, atpB, and mgtC atpB isogenic strains grown in minimal medium containing readily fermentable glucose as the carbon source and low (10 µM) Mg2+ to induce cytoplasmic Mg2+ starvation (22, 23). As hypothesized, mgtC and mgtC atpB strains had 42- and 29-fold higher ATP amounts relative to their mgtC+ isogenic counterparts, respectively (Fig. 2A), after 5 h of growth, when cytoplasmic Mg2+ becomes limiting (28). Hence, atpB inactivation does not abrogate the intracellular ATP accumulation resulting from mgtC inactivation provided that bacteria are fed glucose as carbon source.
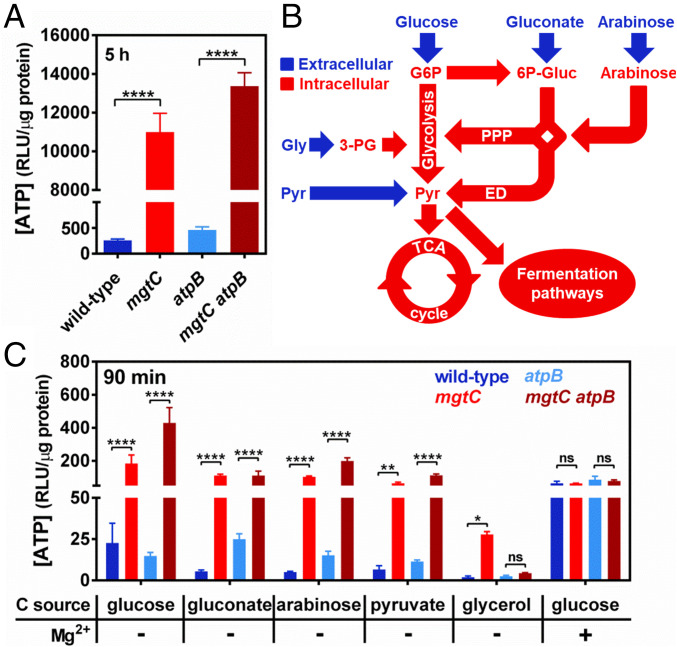
Fig. 2.
F1Fo synthase-independent ATP accumulation in an mgtC mutant during cytoplasmic Mg2+ starvation. (A) Intracellular ATP levels in wild-type (14028s), mgtC (EL4), atpB (MP24), or mgtC atpB (MP25) Salmonella following 5 h of growth. Cells were grown in MOPS medium containing 10 μM MgCl2 and 2 mM K2HPO4. (B) Schematic representation of carbon flow through the central metabolic pathways in bacteria with key extracellular (blue) and intracellular (red) metabolites. G6P, glucose-6-phosphate; 6P-Gluc, 6-Phosphogluconate; Gly, glycerol; Pyr, pyruvate; PPP, pentose phosphate pathway; ED, Entner–Doudoroff pathway. (C) Intracellular ATP levels in wild-type (14028s), mgtC (EL4), atpB (MP24), or mgtC atpB (MP25) Salmonella. Cells were grown in MOPS medium containing 10 mM MgCl2, 2 mM K2HPO4, and 25 mM glucose until OD600 ∼0.4, washed thrice, and grown for additional 90 min in MOPS medium supplemented with the indicated carbon source (25 mM glucose, 25 mM sodium gluconate, 30 mM l-arabinose, 50 mM sodium pyruvate, or 50 mM glycerol) and containing 2 mM K2HPO4 and either 0 (−) or 10 (+) mM MgCl2. Means ± SDs of three independent experiments are shown. *P < 0.05, **P < 0.01, ****P < 0.0001; ns, no significant difference. (A) Two-tailed t test; (C) two-way ANOVA with Tukey’s correction.
To further test our hypothesis, we compared ATP amounts among the four strains at 90 min following a nutritional downshift from medium containing high (10 mM) Mg2+ and glucose, to media lacking Mg2+ and containing one of various carbon sources that are metabolized via distinct pathways (Fig. 2B). During cytoplasmic Mg2+ starvation, the mgtC strain had higher ATP amounts than the wild-type strain regardless of the carbon source (Fig. 2C). By contrast, the mgtC atpB strain had higher ATP amounts than the atpB single mutant during growth on readily fermentable carbon sources (glucose, gluconate, arabinose, or pyruvate) but was unable to do so during growth on inefficiently fermentable glycerol (Fig. 2 B and C). As a control, all the strains tested displayed similar ATP amounts when resuspended in high Mg2+ glucose-containing medium (Fig. 2C). Taken together, these results indicate that the low ATP levels previously observed in an mgtC atpB strain experiencing cytoplasmic Mg2+ starvation (32) are caused by an inability to efficiently ferment glycerol as opposed to an inability to inhibit the F1Fo ATP synthase. Furthermore, these results indicate that MgtC inhibits multiple ATP-generating reactions in the cell, not just the one that is carried out by the F1Fo complex.
MgtC Inhibits Pi Acquisition Thereby Limiting ATP Synthesis during Cytoplasmic Mg2+ Starvation.
Cellular ATP can be synthesized by several catabolic reactions (46–48). How, then, can MgtC control the activity of multiple ATP-producing enzymatic reactions? We reasoned that regardless of the identity of the enzymes catalyzing ATP formation, the overall rate of ATP synthesis in the cell could be restricted by the availability of substrates. MgtC could, therefore, function by inhibiting the synthesis or acquisition of an ATP precursor. Interestingly, at the onset of cytoplasmic Mg2+ starvation, a temporary shortage in the concentration of free cytoplasmic Mg2+ destabilizes the bacterial ribosomal subunits (28). The resulting decrease in translation efficiency reduces ATP consumption and, consequently, the recycling of Pi from ATP. This lowers the concentration of cytoplasmic Pi, transiently activating the cytoplasmic Pi-starvation sensing two-component system composed of the PhoB and PhoR proteins (PhoB/PhoR) (17). Three lines of evidence led us to hypothesize that MgtC prevents ATP synthesis by limiting Pi influx into the cell. First, PhoB/PhoR activation is hampered in an mgtC mutant (17), indicating that this strain experiences excess cytoplasmic Pi. Second, mgtC and mgtC atpB strains have higher intracellular steady-state Pi amounts than wild-type and atpB Salmonella strains (Fig. 3A), respectively. Thus, MgtC prevents Pi accumulation in an atpB-independent fashion. And third, when Mg2+ and Pi are abundant, heterologous expression of the mgtC gene activates PhoB/PhoR, further suggesting that MgtC causes a shortage in cytoplasmic Pi (SI Appendix, Fig. S1) (17).
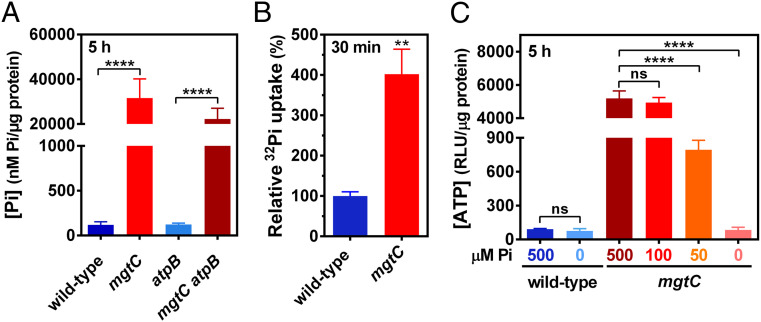
Fig. 3.
MgtC-dependent inhibition of Pi transport and assimilation into ATP during cytoplasmic Mg2+ starvation. (A) Total intracellular Pi in wild-type (14028s), mgtC (EL4), atpB (MP24), or mgtC atpB (MP25) Salmonella following 5 h of growth in MOPS medium containing 10 μM MgCl2 and 2 mM K2HPO4. (B) Relative radioactive orthophosphate (32Pi) uptake in wild-type (14028s) or mgtC (EL4) cells. Bacteria were grown in MOPS medium containing 10 μM MgCl2 and 500 μM K2HPO4 during 3 h before the addition of 32Pi to the cultures. Levels of 32Pi accumulated in cells were determined after 30 min of labeling by liquid scintillation counting, as described in Materials and Methods. 32Pi uptake values were normalized against the wild-type strain. (C) Intracellular ATP levels in wild-type (14028s) or mgtC (EL4) Salmonella following 5 h of growth in MOPS medium containing 10 μM MgCl2 and the indicated concentration of K2HPO4 (μM Pi). Means ± SDs of at least three independent experiments are shown. **P < 0.01, ****P < 0.0001; ns, no significant difference. (A–C) Two-tailed t tests.
To test our hypothesis, we measured transport of radiolabeled Pi (32Pi) following MgtC expression from its native chromosomal location in response to cytoplasmic Mg2+ starvation. We established that mgtC cells accumulated four times more radioactivity than the isogenic wild-type strain after 30 min of growth in the presence of 32Pi (Fig. 3B). This result suggests that the increased steady-state intracellular Pi levels observed for mgtC and mgtC atpB mutants (Fig. 3A) arises from increased uptake of extracellular Pi rather than to increased Pi release from intracellular sources. Note that all 32Pi transport assays were performed in the presence of cold Pi, at a molar ratio of 1:25 (32Pi:Pi), to prevent expression of the PstSCAB Pi transporter resulting from a lack of Pi in the growth medium (1). Therefore, this assay underestimates Pi influx into cells (see Discussion and Materials and Methods).
If MgtC restricts Pi import to the cytoplasm and Pi is required for ATP synthesis, a decrease in Pi availability in the growth medium should correct the high ATP amounts of the mgtC mutant. To test this prediction, we measured ATP amounts in wild-type and mgtC cells grown in minimal media with low Mg2+ and decreasing concentrations of exogenous Pi. Note that bacteria are able to grow in medium lacking exogenous Pi due to the residual Pi present in the mixture of casamino acids supplemented to the culture medium (see Materials and Methods). Strikingly, a decrease in exogenous Pi from 500 to 0 µM lowered ATP amounts in the mgtC mutant to wild-type levels (Fig. 3C). By contrast, ATP amounts remained invariably low in the wild-type strain (Fig. 3C). Importantly, in the absence of exogenous Pi, wild-type and mgtC strains had similar ATP amounts (Fig. 3C). Taken together, these results indicate that MgtC controls ATP synthesis by limiting Pi uptake.
MgtC Inhibits a Noncanonical Pi Transport System.
Salmonella encodes two bona fide Pi import systems: pitA and pstSCAB (Fig. 4A). While PitA functions as a metal:phosphate (M:Pi)/proton symporter (1, 49–51), PstSCAB is a high affinity, ATP-dependent Pi transporter (Fig. 4A) (1, 8, 51, 52). In addition to pitA and pstSCAB, Salmonella also harbors an yjbB homolog, which encodes a sodium/phosphate symporter. Whereas YjbB promotes Pi export (53), we made the cautious assumption that YjbB may also import Pi (Fig. 4A).
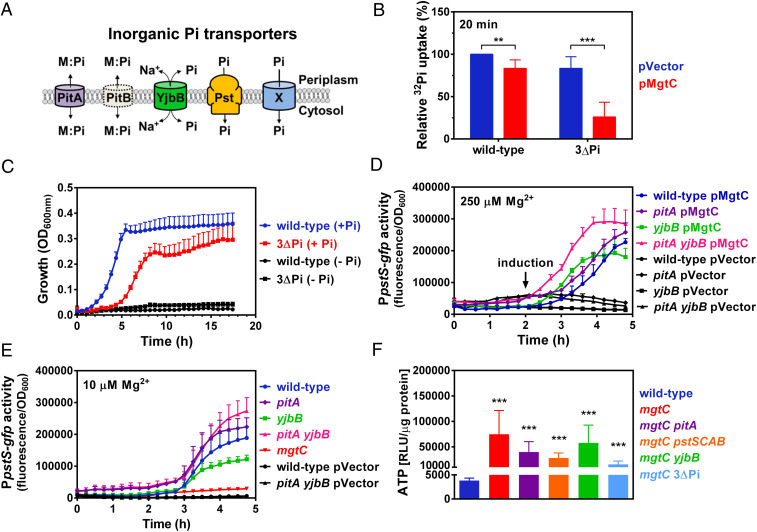
Fig. 4.
A noncanonical Pi transport system is inhibited by MgtC. (A) Schematic representation of inorganic Pi transporters harbored by Salmonella enterica and Escherichia coli. Note that PitB (gray, dashed outline) is absent from Salmonella. M:Pi, metal-phosphate complex; X, inferred, uncharacterized Pi transporter inhibited by MgtC. (B) Relative 32Pi uptake in wild-type (14028s) or ∆3Pi (RB39) carrying either pVector (pUHE-21) or pMgtC (pUHE-MgtC). Bacteria were grown in MOPS medium containing 250 μM MgCl2 and 500 μM K2HPO4 until OD600 ∼0.2. Cultures were then propagated for 15 min in the presence of 750 μM IPTG prior to the addition of 32Pi. Transport of 32Pi was allowed to take place for 20 min. Intracellular 32Pi accumulation was determined by liquid scintillation counting, as described in Materials and Methods. 32Pi uptake values were normalized against the wild-type pVector strain. **P < 0.01, ***P < 0.001, unpaired two-tailed t test. (C) Growth curve of wild-type (14028s) or ∆3Pi (RB39) Salmonella. Cells were grown in MOPS medium containing 10 mM MgCl2 and either 0 (−Pi) or 500 (+Pi) μM of K2HPO4. (D) Fluorescence from wild-type (14028s), pitA (MP1251), yjbB (MP1252), and pitA yjbB (MP1479p) Salmonella carrying pPpstS-GFPc and either pVector or pMgtC. Cells were grown in MOPS medium containing 250 μM MgCl2 and 500 μM K2HPO4. A total of 250 μM of IPTG were added after 2 h of growth. (E) Fluorescence from wild-type (14028s), pitA (MP1251), yjbB (MP1252), pitA yjbB (MP1479p), and mgtC (EL4) Salmonella carrying pPpstS-GFPc or pVector (the promoterless GFP plasmid pGFPc). Cells were grown in MOPS medium containing 10 μM MgCl2 and 500 μM K2HPO4. (F) Intracellular ATP levels in wild-type (14028s), mgtC (EL4), mgtC pitA (MP1254), mgtC pstSCAB (MP1720), mgtC yjbB (MP1255), or mgtC ∆3Pi (RB43) Salmonella. Cells were grown in MOPS medium containing 10 mM Mg and 2 mM 2-AP OD600 ∼0.4. Cells were subsequently washed thrice with MOPS medium lacking MgCl2 and any P source and grown for additional 90 min in MOPS medium lacking Mg2+ and containing 2 mM K2HPO4. ***P < 0.001, unpaired two-tailed t tests against the wild-type strain. For all graphs (B–F), means ± SDs of at least three independent experiments are shown.
To test MgtC’s role on inhibition of PitA, PstSCAB, or YjbB, we measured 32Pi uptake following ectopic MgtC expression in wild-type and isogenic pitA pstSCAB yjbB triple mutant (3∆Pi) Salmonella. 32Pi uptake was slightly (17%) lower in wild-type Salmonella expressing MgtC than in those with the vector control (Fig. 4B). By contrast, MgtC reduced Pi uptake by 70% in the 3∆Pi background relative to the vector control (Fig. 4B). Because MgtC decreases Pi influx in a pitA-, pstSCAB-, and yjbB- independent manner, we reasoned that there must be an additional Pi influx system. In support of this notion, the 3∆Pi mutant grew using Pi as the sole P source (Fig. 4C), albeit with a longer lag and to a lower final optical density unit (OD600) than the wild-type strain (Fig. 4C).
Two additional lines of evidence indicate that MgtC inhibits a noncanonical Pi transporter. First, by decreasing cytoplasmic Pi amounts (Figs. 3 and 4B), heterologous MgtC expression elicits a dose-dependent activation of PhoB/PhoR that results in transcription of the PhoB-activated pstSCAB operon (SI Appendix, Fig. S1) (17). Hence, removal of the Pi transporter targeted by MgtC should result in MgtC-irresponsive constitutively high pstS expression because MgtC would no longer impact PhoB/PhoR activation. We determined that MgtC expression (either heterologously in medium containing 250 µM Mg2+ or natively in medium containing 10 µM Mg2+) induced pstS transcription in pitA, yjbB, and pitA yjbB mutants (Fig. 4 D and E). We did not examine the pstSCAB mutant because the PstSCAB transporter participates in the regulation of the PhoB/PhoR two-component system through a physical interaction (54). Consequently, inactivation of the transporter through mutations in pst genes leads to PhoB/PhoR hyperactivation, effectively preventing signal transduction (1, 3, 54, 55). In addition, we reasoned that it would be highly unlikely for cells to harbor a regulatory circuit whereby MgtC inhibition of PstSCAB transporter activity would promote PstSCAB expression (SI Appendix, Fig. S1) (17), requiring more MgtC protein, ad infinitum.
And second, deleting the MgtC-inhibited Pi transporter should correct the exacerbated ATP accumulation of the mgtC mutant (Fig. 3C). However, mutations in either pitA, pstSCAB, or yjbB or a combined deletion of all these genes did not lower ATP amounts in the mgtC mutant (Fig. 4F). Note that due to the longer lag time of 3ΔPi mutant strains growing with Pi as the sole P source (Fig. 4C), we cultured all tested strains in minimal medium containing 2-aminoethylphosphonic acid [2-AP] as the P source and subsequently subjected them to a nutritional downshift to medium supplemented with 2 mM Pi and lacking Mg2+ for 90 min prior to ATP quantification. Because 2-AP is imported into the cytoplasm through an organic P system (PhnSTUV) prior to removal of Pi, this allows cells to bypass the need for inorganic P source and grow at the same rate. In sum, these results indicate that MgtC inhibits Pi uptake by targeting an unidentified Pi transporter.
Phosphate Limitation Rescues the Translation and Growth Defects of an mgtC Mutant Experiencing Cytoplasmic Mg2+ Starvation.
An mgtC mutant experiencing cytoplasmic Mg2+ starvation exhibits ribosomal assembly defects, inefficient translation, growth arrest, and a loss of viability (21, 28). This is presumably due to Mg2+ chelation by excess ATP molecules because the mgtC mutant phenotypes are corrected by promoting ATP hydrolysis (28, 29). Given that MgtC lowers ATP synthesis by inhibiting Pi acquisition (Figs. 3 A and C and 4B), these phenotypes should also be rescued by limiting Pi access.
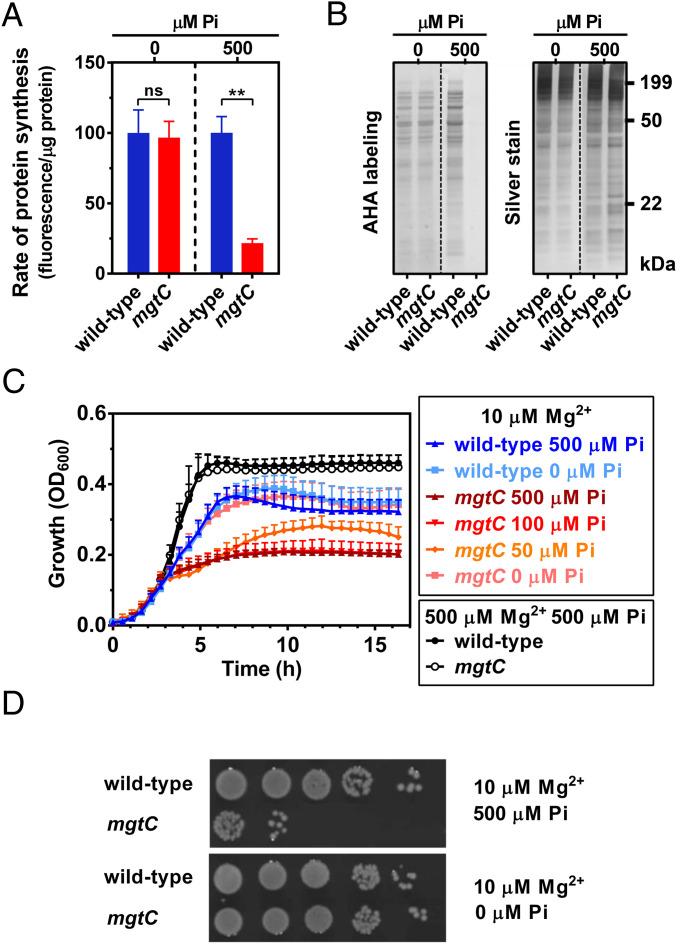
As predicted, wild-type and mgtC strains showed similar translation rates in the absence of exogenous Pi (Fig. 5 A and B). By contrast, during growth in 500 µM Pi, the translation rate of the mgtC mutant was 4.7-fold lower than that of the wild-type strain (Fig. 5 A and B). Moreover, decreasing exogenous Pi in the growth medium from 500 to 0 µM progressively increased growth of the mgtC mutant (Fig. 5C). Remarkably, in the absence of exogenous Pi, the mgtC mutant displayed growth yield and kinetics indistinguishable from that of the wild-type strain (Fig. 5C). As expected, growth of wild-type and mgtC strains was indistinguishable in medium containing 500 µM Pi when the Mg2+ concentration was raised from 10 to 500 µM (Fig. 5C), thereby preventing Mg2+ starvation. Furthermore, the loss of viability of the mgtC mutant (21) in medium containing 500 µM Pi was suppressed by not adding Pi (Fig. 5D). The mutant maintained the same number of colony-forming units (CFU) per OD600 as the wild-type strain. Altogether, these results establish that MgtC prevents the toxic effects of excessive Pi uptake.
Fig. 5.
Effect of phosphate limitation on the translation rate, growth, and viability of an mgtC mutant during cytosolic Mg2+ starvation. (A) Quantification and (B) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the rate of protein synthesis (l-azidohomoalanine [AHA] labeling) of wild-type or mgtC (EL4) Salmonella. Cells were grown in MOPS medium containing 10 mM MgCl2 and 2 mM K2HPO4 until OD600 ∼0.4. Cells were subsequently washed thrice with MOPS medium lacking MgCl2, K2HPO4, and amino acids and grown for additional 90 min in MOPS medium lacking methionine and containing 10 μM MgCl2 plus the indicated concentration of K2HPO4 (μM Pi). Means ± SDs of four independent experiments are shown. **P < 0.01, unpaired two-tailed t test. ns, no significant difference. The gel is representative of four independent experiments. Samples from 0 and 500 μM Pi were resolved and imaged from different gels (indicated by dashed lines). (C) Growth curve of wild-type (14028s) or mgtC (EL4) Salmonella. Cells were grown in MOPS medium containing the indicated concentrations of MgCl2 and K2HPO4. Means ± SDs of three independent experiments are shown. (D) Viable cell count of wild-type (14028s) or mgtC (EL4) Salmonella following 16 h of growth in MOPS medium containing 10 μM MgCl2 and 0 or 500 μM K2HPO4. Cell suspensions were normalized to the same OD600 and diluted, and 5 μL was spotted on plates. Images were taken after incubation of plates at 37 °C for 18 h and are representative of three independent experiments.
Excess Pi Is Toxic Even under High Mg2+ Conditions.
Excessive Pi uptake hinders bacterial growth even when Mg2+ is not limiting and cells are not anticipated to experience cytoplasmic Mg2+ starvation (3, 9). For instance, mutations that increase PstSCAB activity or expression cause heightened Pi uptake and growth inhibition in Escherichia coli (1–3, 5). The data presented in the previous sections suggest that the growth inhibition resulting from PstSCAB overexpression results from excessive Pi incorporation into ATP and subsequent chelation of Mg2+, that, in turn, give rise to elevated ATP levels, inhibition of translation and growth, and induction of MgtC expression (21, 27, 28). Because these phenotypes are observed during cytoplasmic Mg2+ starvation, they may be corrected by increasing the availability of free cytoplasmic Mg2+ (e.g., through the provision of excess Mg2+ in the growth medium) or the reversal of Pi assimilation (e.g., through enzymatic hydrolysis of ATP) (21, 28, 29).
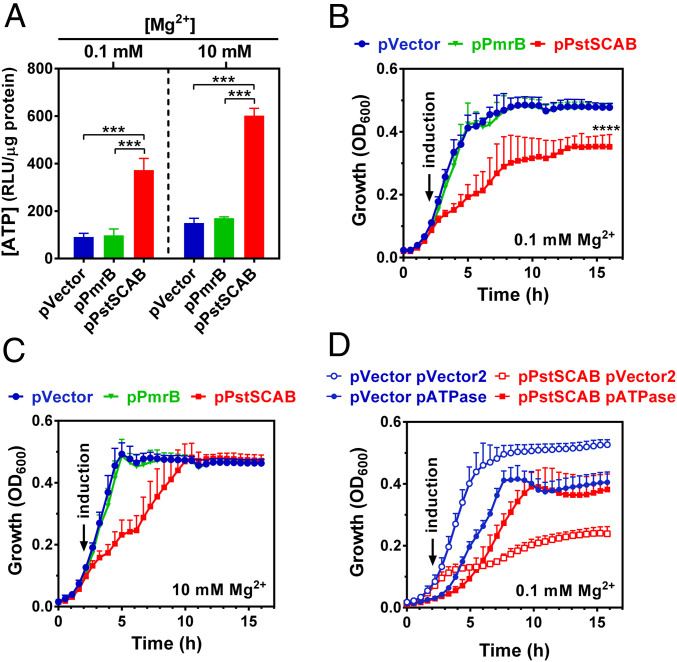
First, we determined the phenotypic consequences of ectopic PstSCAB expression in medium containing high (10 mM) Pi and intermediate (0.1 mM) Mg2+ concentrations, two conditions that do not typically result in cytoplasmic Mg2+ starvation (22, 23) and, thus, MgtC expression (SI Appendix, Fig. S2). We established that ATP amounts were four times higher in PstSCAB-expressing bacteria than in control strains harboring either an empty vector or a plasmid expressing the inner membrane protein PmrB (Fig. 6A). Notably, the PstSCAB-dependent increase in ATP amounts is accompanied by reductions in growth rate and growth yield, phenotypes not observed in the control strains (Fig. 6B).
Fig. 6.
Effect of PstSCAB on growth, P assimilation, and Mg2+ homeostasis. (A) Intracellular ATP levels of cultures depicted in B and C. Measurements were conducted at 5 h of growth. (B, C) Growth curves of wild-type (14028s) Salmonella carrying pVector (pUHE-21), pPstSCAB (pUHE-PstSCAB), or pPmrB (pUHE-PmrB) in MOPS medium containing 0.1 (B) and 10 mM MgCl2 (C). (D) Growth curve of wild-type (14028s) Salmonella carrying either pVector or pPstSCAB and either pVector2 (pBbB2K-GFP) or pATPase (pBbB2K-AtpAGD). In all experiments, cells were grown in MOPS medium containing 10 mM K2HPO4 and the indicated MgCl2 concentration. Ectopic protein expressions were induced by adding 250 μM IPTG to the cultures after 2 h of growth. Means ± SDs of three independent experiments are shown. (A, B) ***P < 0.001, ****P < 0.0001, unpaired two-tailed t test against pVector.
And second, the growth defect resulting from PstSCAB expression is caused by an increase in ATP amounts resulting in chelation of free Mg2+. This is because PstSCAB expression caused only a minor reduction in growth rate, enabling cells to reach the same growth yields as the control strains when the Mg2+ concentration was increased 100-fold (to 10 mM) (Fig. 6C). Importantly, Mg2+ rescued growth of the PstSCAB-expressing strain even though ATP amounts remained fourfold higher than those of the control strains harboring the empty vector or the PmrB-expressing plasmid (Fig. 6A). In addition, a plasmid-encoded soluble subunit of the ATPase (17) corrected the growth defect caused by PstSCAB expression (Fig. 6D) whereas the vector control did not (Fig. 6D). Taken together, these results indicate that excess Pi imported into the cytoplasm is rapidly assimilated into ATP, causing a decrease in the levels of free cytoplasmic Mg2+ and inhibiting growth.
Excessive Pi Uptake Impairs Translation and Promotes MgtC Expression during Growth in High Mg2+.
If excessive Pi uptake leads to physiological conditions resembling cytoplasmic Mg2+ starvation (Fig. 6 A–D) (21, 28, 29), then increased Pi uptake resulting from PstSCAB expression should also decrease translation rates. Furthermore, because a reduction in translation rates caused by cytoplasmic Mg2+ starvation promotes mgtC transcription (22, 23, 27, 28, 56), excessive Pi uptake should also promote MgtC expression. We tested these predictions by measuring temporal fluorescence changes in wild-type strains harboring both a plasmid-borne gfp transcriptional fusion to the promoter and leader regions of mgtC, and either the empty vector, the pPmrB plasmid, or the pPstSCAB plasmid.
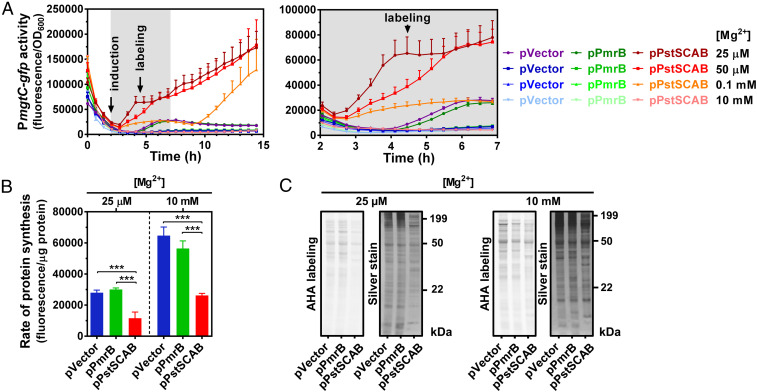
Fluorescence from mgtC-gfp increased 1 h following PstSCAB induction (Fig. 7A). This increase was absent or delayed in the control strains harboring the empty vector or expressing PmrB (Fig. 7A). Note that during growth in 25 µM Mg2+, the control strains display a minor increase in fluorescence after 4 h of growth. This small increase in fluorescence results from late onset of cytoplasmic Mg2+, which is delayed when compared to cells grown at 10 µM Mg2+ (SI Appendix, Fig. S2) (17, 22, 23, 57). The effect of PstSCAB expression on the time and fluorescence levels of the mgtC-gfp strain was inversely related to the availability of Mg2+ in the growth medium. That is, fluorescence resulting from PstSCAB expression in cultures grown in 25 µM Mg2+ were higher than those grown in 50 µM Mg2+, which, in turn, were higher than those grown in 0.1 mM Mg2+ (Fig. 7A). Bacteria grown in 0.1 mM Mg2+ displayed a relatively mild increase in mgtC-gfp activity 1 h after PstSCAB expression, and their fluorescence levels rose rapidly at 7.5 h post induction (Fig. 7A). The delayed mgtC induction likely reflects the time at which bacteria no longer neutralize the excess of intracellular Pi incorporated into ATP because they exhausted the Mg2+ available in the growth medium and, consequently, are unable to efficiently stabilize their ribosomes (28). We conclude that increased ATP production resulting from excessive Pi assimilation disrupts translation by sequestering free Mg2+ ions.
Fig. 7.
Effect of PstSCAB expression on mgtC transcription and cellular translation rates during growth under conditions of moderate and high Mg2+. (A) Fluorescence from wild-type (14028s) Salmonella carrying transcriptional reporter pPmgtC-GFPc and either pVector (pUHE-21), pPstSCAB (pUHE-PstSCAB), or pPmrB (pUHE-PmrB). Full time course (Left) and inset between 2 and 7 h (Right) of the experiments are shown. (B) Quantification and (C) SDS-PAGE analysis of the rate of protein synthesis (l-azidohomoalanine [AHA] labeling) of wild-type (14028s) Salmonella carrying either pVector, pPmrB, or pPstSCAB. In all experiments, cells were grown in MOPS medium containing 10 mM K2HPO4 and the indicated concentration of MgCl2. For AHA labeling, bacteria were cultured in MOPS medium lacking methionine (see Materials and Methods). A total of 250 μM IPTG was added to the cultures following 2 h of growth. AHA was incorporated to the cultures at 4.5 h. Means ± SDs of three independent experiments are shown. Gels are representative of three independent experiments. ***P < 0.001, unpaired two-tailed t test against pVector.
To determine whether excessive Pi uptake compromises ribosome activity, we measured translation rates in the same strain set. During growth in medium containing 25 µM Mg2+, PstSCAB expression caused a twofold reduction in translation rates relative to control strains (Fig. 7 B and C). Notably, PstSCAB expression also caused a relative reduction in translation rates during growth in medium containing a 400-fold excess (10 mM) of Mg2+ (Fig. 7 B and C). However, at 10 mM Mg2+, translation rates were approximately twofold higher across all strains (Fig. 7 B and C). Taken together, these results indicate that the rapid assimilation of too much imported Pi reduces the availability of free cytoplasmic Mg2+, thereby lowering translation efficiency and promoting MgtC expression.
Discussion
We have identified a physiological connection between Pi assimilation and Mg2+ homeostasis whereby cells exert tight control over Pi uptake to avoid uncontrollable ATP synthesis and depletion of free cytoplasmic Mg2+ (Fig. 1). We establish that the Salmonella MgtC protein lowers ATP amounts by inhibiting Pi uptake (Figs. 3 A–C and 4B), thereby limiting the availability of an ATP precursor rather than interfering with the enzymatic catalysis of ATP-generating reactions per se (Figs. 2 A–C and 3 A–C). MgtC hinders the activity of a yet unidentified transporter that functions as the main Pi uptake system in Salmonella (Fig. 4 A–F). This inhibitory activity could take place through a number of different mechanisms. These include 1) a direct physical interaction with the transporter and 2) indirectly through interaction(s) with cellular component(s) that inhibit the Pi transporter activity, synthesis, or degradation. Furthermore, even when cytoplasmic Mg2+ is not limiting, excessive Pi uptake leads to increased ATP synthesis, which reduces free cytoplasmic Mg2+, impairing translation and inhibiting growth (Figs. 6 A–D, 7 A–C and 8).
Fig. 8.
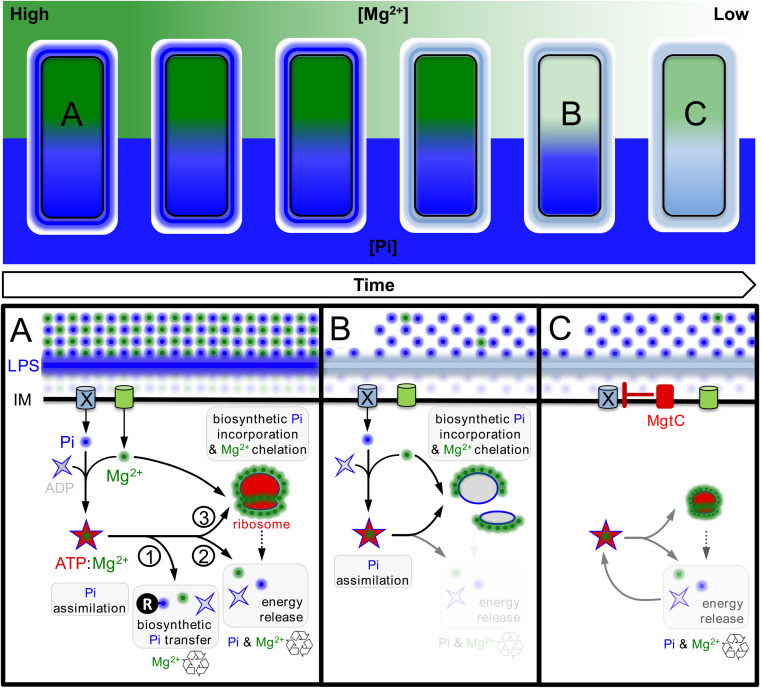
Model illustrating how limitation of phosphate assimilation maintains cytoplasmic Mg2+ homeostasis in Salmonella enterica. (Top) Overview of the temporal adaptation of S. enterica to Mg2+ starvation. Mg2+ (green) and Pi (blue) concentrations in the environment, lipopolysaccharide (LPS), and cytoplasm are depicted as gradients with dark colors denoting high concentration and light colors representing low concentrations. (Bottom) Schematic depicting molecular events and responses underlying the adaptation. (A) During homeostasis, Pi is imported into the cytoplasm through dedicated inner membrane (IM) transport systems (X [unknown transporter] and PitA). Cells assimilate imported Pi through the synthesis of ATP, which exists as a salt with positively charged Mg2+ (ATP:Mg2+). ATP:Mg2+ 1) mediates the transfer of Pi among biological molecules, 2) powers energy-dependent enzymatic reactions, and 3) promotes ribosome biogenesis (1). ATP hydrolysis for the release of energy recycles Pi and Mg2+, whereas the biosynthetic transfer of Pi typically recycles cytoplasmic Mg2+. (B) After consuming the Mg2+ present in the environment, cells eventually experience a shortage in cytoplasmic Mg2+ levels (22, 23, 57). Insufficient cytoplasmic Mg2+ impairs ribosomal subunit assembly, lowering translation efficiency (28). This reduces the consumption of ATP by translation reactions and, consequently, decreases the recycling of Mg2+ and Pi from ATP:Mg2+ (17). (C) Salmonella enterica expresses the MgtC membrane protein as a homeostatic response to cytoplasmic Mg2+ starvation. MgtC inhibits Pi uptake through an unknown transporter (X), thereby preventing assimilation Pi into ATP. As the levels of ATP and ribosomes decrease, free Mg2+ ions necessary for core processes such as translation are recovered, increasing the efficiency of protein synthesis and the recycling of Mg2+ and Pi from ATP:Mg2+. The density and size of cartoons represent the concentrations of Mg2+, Pi, and ribosomes.
The Physiological Connection between Pi and Mg2+ Homeostasis.
The capacity of MgtC to maintain physiological ATP levels during cytoplasmic Mg2+ starvation had been so far ascribed to its inhibitory effect on Salmonella’s F1Fo ATP synthase (32). Here, we demonstrate that this inaccurate conclusion resulted from an experimental setting arising from the propagation of atpB strains in a poorly fermentable carbon source (Fig. 2 A–C). Whereas the function of MgtC in macrophages is associated with the activity of the F1Fo ATP synthase (32), the specific role of this protein within this biological context is not well understood. Cytoplasmic Mg2+ starvation can trigger MgtC expression by impairing the activity of bacterial ribosomes (Fig. 7) (27, 28, 40). However, MgtC promotes Salmonella survival irrespective of whether macrophages can restrict the access of bacteria to Mg2+ (30, 58, 59). This suggests that a macrophage-induced stress, other than Mg2+ starvation, impairs Salmonella translation, mimicking a physiological signal experienced during cytoplasmic Mg2+ starvation. That is, when inside macrophages, Salmonella may “sense” cytoplasmic Mg2+ starvation even though Mg2+ may not be limiting in the subcellular compartment harboring Salmonella (60).
MgtC inhibits Pi uptake independently of all known Pi importers (PitA and PstSCAB) as well as the Pi exporter YjbB. When heterologously expressed in E. coli, MgtC activates the PhoB/PhoR system (SI Appendix, Fig. S3). In addition to PstSCAB and PitA, E. coli harbors PitB, a Pi importer absent from Salmonella (Fig. 4A) (1, 51, 61). Notably, E. coli strains containing mutations in all of these three Pi transport systems are still able to grow on minimal medium with Pi as the only P source (51, 62). This indicates that E. coli encodes an additional Pi transporter(s) that may be targeted by MgtC in Salmonella. Given that MgtC sequelogs promote growth in low Mg2+ in a large number of distantly related species (34, 63–69), the Pi transporter targeted by MgtC is likely to be widespread in bacteria.
If MgtC inhibits the activity of a single Pi importer, what then prevents Pi uptake by other transport systems? PhoB promotes transcription of the high affinity PstSCAB Pi importer in response to a decrease in cytoplasmic Pi. During the initial stages of cytoplasmic Mg2+ starvation, the ribosomal subunits are unable to assemble efficiently (28). This leads to a decrease in translation efficiency and a concomitant reduction in ATP hydrolysis and free cytoplasmic Pi, which triggers pstSCAB transcription (17). Whereas MgtC inhibits the main Pi transporter, expression of MgtA and MgtB results in an influx of Mg2+ into the cytoplasm (21–26). Both responses restore ribosomal subunit assembly thereby increasing translation efficiency (Fig. 8). ATP consumption during translation (most notably the charging of transfer RNAs and the synthesis of guanosine triphosphate, which is subsequently used by elongation factors) (28) replenishes intracellular Pi, which represses pstSCAB transcription by inactivating PhoB/PhoR (17).
The availability of the Pi:Zn2+ salt regulates pitA transcription (70). In addition, PitA is posttranscriptionally repressed during Mg2+ starvation in E. coli. This repression is orchestrated by the Mg2+-sensing PhoP/PhoQ two-component system (71), which, in Salmonella, activates transcription of mgtCB and mgtA (21). Because growth in low Mg2+ decreases pitA mRNA levels in both Salmonella and Yersinia pestis (72, 73), inhibition of PitA expression appears to be a common feature of the Mg2+ starvation response in enteric bacteria. Given that PitA transports M:Pi salts, a reduction in PitA abundance may hinder uptake of other metal cations, such as zinc, that can readily replace scarce Mg2+ in enzymatic reactions (74–76). In this context, it is interesting to note that the Pho84 Pi transporter of Saccharomyces cerevisiae can promote metal toxicity by importing M:Pi salts (77–80).
If excessive ATP is toxic during cytoplasmic Mg2+ starvation, why, then, does MgtC function by hindering Pi uptake and not by directly inhibiting ATP-generating enzyme(s)? Depending on the growth condition, the production of ATP via the oxidation of carbon can occur via several distinct pathways, each involving dozens of enzymes (48). While we can conceive that a single protein may have the capacity to directly inhibit a myriad of distinct enzymes, evolution has likely provided cells with a more parsimonious solution for this problem. Because Pi is an ATP precursor, limitation of Pi uptake enables cells to hinder all ATP generating reactions independently from the metabolic pathway(s) used to oxidize the available carbon source(s) (Figs. 2B and 7). Hence, the inhibition of Pi uptake by MgtC allows Salmonella to lower ATP synthesis, eliciting a physiological response to a lethal depletion of cytoplasmic Mg2+, whether carbon is being metabolized via classic glycolysis, the Entner–Doudoroff, the Pentose Phosphate, the Tricarboxylic Acid Cycle, or any other energy-generating pathway (Figs. 2B and 8).
On the Activation of PhoR by MgtC.
Choi et al. reported that MgtC promotes PstSCAB expression and Pi uptake due to PhoB/PhoR activation resulting from a direct physical interaction between the MgtC and PhoR proteins (81). Our data provide independent pieces of evidence demonstrating that MgtC actually inhibits Pi uptake, which helps maintain cellular viability during cytoplasmic Mg2+ starvation.
First, the proposal that MgtC furthers Pi uptake (81) is incompatible with the fact that Pi is acutely toxic to cells experiencing cytoplasmic Mg2+ starvation (Fig. 5 C and D) and that MgtC is produced in response to cytoplasmic Mg2+ starvation (23, 24). Second, during cytoplasmic Mg2+ starvation, MgtC expression follows PhoB/PhoR activation and pstSCAB transcription, and MgtC production results in inactivation of PhoB/PhoR (SI Appendix, Fig. S4 A and B) (17). Third, this temporal chain of events is supported by a disruption in protein synthesis due to excessive Pi exacerbating cytoplasmic Mg2+ starvation and by influx of extracellular Pi via PstSCAB inducing mgtC expression (Figs. 6 A–D and 7 A–C). And fourth, substitution of the PhoR leucine 421 by an alanine was reported to disrupt PhoR interaction with MgtC, preventing PhoB/PhoR activation in cytoplasmic Mg2+ starvation (81). However, wild-type and phoRL421A strains display similar pstS transcriptional kinetics in response to ectopic or chromosomal MgtC expression (SI Appendix, Fig. S5). Notably, cytoplasmic Mg2+ starvation also disrupts translation homeostasis in E. coli (28), thereby inducing a shortage in free cytoplasmic Pi and triggering PhoB/PhoR activation (SI Appendix, Fig. S6) (17), even though E. coli lacks MgtC.
Pi Toxicity and the Control of P Assimilation.
Mutations that increase Pi uptake via the PstSCAB transport system inhibit growth in a wide range of bacterial species (1–9). However, the underlying molecular basis for this inhibition has remained elusive. That MgtC promotes growth and viability during cytoplasmic Mg2+ starvation (21, 23) by inhibiting Pi uptake (Figs. 3 A and B and 4B) and, consequently, ATP synthesis (Fig. 3C) (29) prompted us to examine the physiological basis of Pi toxicity observed in the context of the aforementioned mutations. We established that increased Pi transport via PstSCAB causes a rise in ATP concentrations (Fig. 6A). Increased ATP disrupts the pools of free cytoplasmic Mg2+ (Fig. 7A), thereby inhibiting translation (Fig. 7 B and C), growth (Fig. 6B), and triggering MgtC expression when the Mg2+ concentration in the growth medium is sufficiently high to silence its expression (Fig. 7A) (22, 23, 27). Our results both establish that the toxic effects of excessive Pi are manifested following its assimilation into ATP and shed light into the underlying causes of Pi toxicity by revealing a logic for cellular control of P assimilation. The rapid synthesis of ATP (Fig. 6 A–C), and ATP-derived highly charged Pi anions-containing molecules, such as rRNA and poly-Pi (28, 82, 83), depletes the pools of free cytoplasmic Mg2+. Therefore, when biosynthetic precursors are abundant, but Mg2+ is limiting, cells adjust Pi uptake as an effective way to inhibit ATP-generating reactions (Fig. 1).
Materials and Methods
Bacterial Strains, Plasmid Constructs, Primers, and Growth Conditions.
The bacterial strains and plasmids used in this study are listed in SI Appendix, Table S1, and oligonucleotide sequences are presented in SI Appendix, Table S2. Single gene knockouts and deletions were carried out as described (84). Mutations generated via this method were subsequently moved into clean genetic backgrounds via phage P22-mediated transduction as described (85). For chromosomal point mutations, detailed strain construction is described below. Bacterial strains used in recombination and transduction experiments were grown in Luria–Bertani (LB) medium at 30 °C or 37 °C (84–86). When required, the LB medium was supplemented with ampicillin (100 μg/mL), chloramphenicol (20 μg/mL), kanamycin (50 μg/mL), and/or l-arabinose (0.2% wt/vol).
Unless stated otherwise, physiological experiments with bacteria were carried out at 37 °C with shaking at 250 rpm in MOPS medium (87) lacking CaCl2 (to avoid repression of the PhoP/PhoQ system) (88) and supplemented with 0.1% (wt/vol) bacto casamino acids (BD Difco), 25 mM glucose, and the indicated amounts of MgCl2 and K2HPO4. Experiments were conducted as follows: After overnight (∼16 to 20 h) growth in MOPS medium containing 10 mM MgCl2 and 2 mM K2HPO4, cells were washed three times in medium lacking Mg2+ and Pi, inoculated (1:100) in fresh medium containing the indicated concentrations of MgCl2 and K2HPO4, and propagated for the corresponding amount of time. It should be noted that at a concentration of 0.1% (wt/vol) bacto casamino acids (BD Difco), the medium already contains ∼163 μM Pi. During physiological experiments, selection of plasmids was accomplished by the addition of ampicillin at 100 μg/mL (overnight growth) or 30 μg/mL (experimental condition), chloramphenicol at 20 μg/mL (overnight growth) or 10 μg/mL (experimental condition), and/or kanamycin at 50 μg/mL (overnight growth) or 20 μg/mL (experimental condition). Unless specified otherwise, heterologous expression of proteins was achieved by treatment of cultures with 250 µM (pMgtC, pPstSCAB) isopropyl β-D-1-thiogalactopyranoside (IPTG). ATPase expression from pBbB2k-AtpAGD was attained without the addition of the inductor.
Construction of Plasmids Used in This Study.
Phusion High-Fidelity DNA Polymerase (New England Biolabs) was used in PCR to amplify DNA inserts. These DNA inserts were cloned into linearized plasmids (17, 27, 89–91) using NEBuilder HiFi DNA Assembly Cloning Kit (New England BioLabs). A detailed construction of plasmids is described in the SI Appendix.
Construction of phoRL421A Strain.
Chromosomal point mutation was generated as described (84, 86). A detailed procedure is described in the SI Appendix.
Estimation of Intracellular ATP.
Intracellular ATP was estimated as described (17). A detailed description of this method is found in the SI Appendix.
Estimation of Intracellular Pi.
Total Pi in the samples was estimated from crude cell extracts using the molybdenum blue method (17, 92). A detailed description of this method is found in the SI Appendix.
Phosphate Transport Assay.
Wild-type (14028s) or mgtC (EL4) Salmonella were grown in MOPS medium containing 10 μM MgCl2 and 500 μM K2HPO4 during 3 h. Wild-type (14028s) or ∆3Pi (RB39) cells harboring either pVector or pMgtC were grown in MOPS containing 250 μM MgCl2 and 500 μM K2HPO4 until OD600 ∼0.2, at which point, MgtC expression was induced for 15 min with the addition of 750 μM IPTG. To assay the transport of Pi, 20 μCi of radioactive Pi solution (10 μL from a 2 mCi K2H32PO4 at a concentration of 2 mM K2H32PO4, PerkinElmer catalog number NEX055) was added to 1 mL cell suspension. At the indicated time points, 50 μL of each sample was submitted to rapid filtration through 0.45 μm mixed cellulose ester membrane filters (Whatman) with an applied vacuum. The filters were washed three times with 1 mL PBS buffer and subsequently soaked in 5 mL scintillation fluid (Research Products International). The amount of radioactivity taken up by the cells was determined with a scintillation counter (Triathler multilabel tester, HIDEX) using the 32P-window and by counting each vial for 20 s. Radioactive counts per minute were normalized by protein content using a Rapid Gold BCA Protein Assay Kit (Pierce). 32Pi uptake of each sample was normalized against the corresponding control in each independent experiment.
Monitoring Bacterial Growth and Gene Expression via Fluorescence.
Bacterial growth and fluorescence measurements were performed using SpectraMax i3x plate reader (Molecular Devices). The detailed protocols are described in the SI Appendix.
l-azidohomoalanine Protein Labeling and Quantification.
In vivo labeling of proteins and estimation of translation rates were performed as described (26), with minor modifications highlighted in the SI Appendix.
Image Acquisition, Analysis, and Manipulation.
Plates, gel, and membrane images were acquired using an Amersham Imager 600 (GE Healthcare Life Sciences). ImageJ software (93) was used to crop the edges and adjust the brightness and contrast of the images. These modifications were simultaneously performed across the entire set images to be shown.
Supplementary Material
Acknowledgments
M.H.P. is supported by Grant AI148774 from the NIH and funds from The Pennsylvania State University College of Medicine. E.A.G. is supported by Grant AI49561 from the NIH.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021370118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
Change History
March 16, 2021: The author line has been updated.
References
- 1.Wanner B. L., “Phosphorus assimilation and control of the phosphate regulon” in Escherichia Coli and Salmonella: Cellular and Molecular Biology, Neidhardt F. C., et al., Eds. (American Society for Microbiology, ed. 2, 1996), pp. 1357–1381. [Google Scholar]
- 2.Webb D. C., Rosenberg H., Cox G. B., Mutational analysis of the Escherichia coli phosphate-specific transport system, a member of the traffic ATPase (or ABC) family of membrane transporters. A role for proline residues in transmembrane helices. J. Biol. Chem. 267, 24661–24668 (1992). [PubMed] [Google Scholar]
- 3.Steed P. M., Wanner B. L., Use of the rep technique for allele replacement to construct mutants with deletions of the pstSCAB-phoU operon: Evidence of a new role for the PhoU protein in the phosphate regulon. J. Bacteriol. 175, 6797–6809 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morohoshi T., et al., Accumulation of inorganic polyphosphate in phoU mutants of Escherichia coli and Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol. 68, 4107–4110 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice C. D., Pollard J. E., Lewis Z. T., McCleary W. R., Employment of a promoter-swapping technique shows that PhoU modulates the activity of the PstSCAB2 ABC transporter in Escherichia coli. Appl. Environ. Microbiol. 75, 573–582 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Almeida L. G., Ortiz J. H., Schneider R. P., Spira B., phoU inactivation in Pseudomonas aeruginosa enhances accumulation of ppGpp and polyphosphate. Appl. Environ. Microbiol. 81, 3006–3015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubin E. A., Henry J. T., Fiebig A., Crosson S., Laub M. T., Identification of the PhoB regulon and role of PhoU in the phosphate starvation response of Caulobacter crescentus. J. Bacteriol. 198, 187–200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J. J., Sinha D., Wayne K. J., Winkler M. E., Physiological roles of the dual phosphate transporter systems in low and high phosphate conditions and in capsule maintenance of Streptococcus pneumoniae D39. Front. Cell. Infect. Microbiol. 6, 63 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.diCenzo G. C., Sharthiya H., Nanda A., Zamani M., Finan T. M., PhoU allows rapid adaptation to high phosphate concentrations by modulating PstSCAB transport rate in Sinorhizobium meliloti. J. Bacteriol. 199, 1–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire M. E., Cowan J. A., Magnesium chemistry and biochemistry. Biometals 15, 203–210 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Storer A. C., Cornish-Bowden A., Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem. J. 159, 1–5 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider D. A., Gaal T., Gourse R. L., NTP-sensing by rRNA promoters in Escherichia coli is direct. Proc. Natl. Acad. Sci. U.S.A. 99, 8602–8607 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray H. D., Schneider D. A., Gourse R. L., Control of rRNA expression by small molecules is dynamic and nonredundant. Mol. Cell 12, 125–134 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Pontes M. H., Sevostyanova A., Groisman E. A., When too much ATP is bad for protein synthesis. J. Mol. Biol. 427, 2586–2594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein D. J., Moore P. B., Steitz T. A., The contribution of metal ions to the structural stability of the large ribosomal subunit. RNA 10, 1366–1379 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gesteland R. F., Unfolding of Escherichia coli ribosomes by removal of magnesium. J. Mol. Biol. 18, 356–371 (1966). [DOI] [PubMed] [Google Scholar]
- 17.Pontes M. H., Groisman E. A., Protein synthesis controls phosphate homeostasis. Genes Dev. 32, 79–92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillooly J. F., et al., The metabolic basis of whole-organism RNA and phosphorus content. Proc. Natl. Acad. Sci. U.S.A. 102, 11923–11927 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elser J. J., et al., Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 6, 936–943 (2003). [Google Scholar]
- 20.Bremer H., Dennis P. P., Modulation of chemical composition and other parameters of the cell at different exponential growth rates. Ecosal Plus, 3 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Soncini F. C., García Véscovi E., Solomon F., Groisman E. A., Molecular basis of the magnesium deprivation response in Salmonella typhimurium: Identification of PhoP-regulated genes. J. Bacteriol. 178, 5092–5099 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cromie M. J., Shi Y., Latifi T., Groisman E. A., An RNA sensor for intracellular Mg2+. Cell 125, 71–84 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Spinelli S. V., Pontel L. B., García Véscovi E., Soncini F. C., Regulation of magnesium homeostasis in Salmonella: Mg2+ targets the mgtA transcript for degradation by RNase E. FEMS Microbiol. Lett. 280, 226–234 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Groisman E. A., et al., Bacterial Mg2+ homeostasis, transport, and virulence. Annu. Rev. Genet. 47, 625–646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snavely M. D., Miller C. G., Maguire M. E., The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J. Biol. Chem. 266, 815–823 (1991). [PubMed] [Google Scholar]
- 26.Tao T., Snavely M. D., Farr S. G., Maguire M. E., Magnesium transport in Salmonella typhimurium: mgtA encodes a P-type ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J. Bacteriol. 177, 2654–2662 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee E. J., Groisman E. A., Control of a Salmonella virulence locus by an ATP-sensing leader messenger RNA. Nature 486, 271–275 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pontes M. H., Yeom J., Groisman E. A., Reducing ribosome biosynthesis promotes translation during low Mg2+stress. Mol. Cell 64, 480–492 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pontes M. H., Lee E. J., Choi J., Groisman E. A., Salmonella promotes virulence by repressing cellulose production. Proc. Natl. Acad. Sci. U.S.A. 112, 5183–5188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanc-Potard A. B., Groisman E. A., The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 16, 5376–5385 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eriksson S., Lucchini S., Thompson A., Rhen M., Hinton J. C., Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47, 103–118 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Lee E. J., Pontes M. H., Groisman E. A., A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium’s own F1Fo ATP synthase. Cell 154, 146–156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okuno D., Iino R., Noji H., Rotation and structure of FoF1-ATP synthase. J. Biochem. 149, 655–664 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Rang C., et al., Dual role of the MgtC virulence factor in host and non-host environments. Mol. Microbiol. 63, 605–622 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Aung K., et al., pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol. 141, 1000–1011 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen T. T., et al., Mitochondrial oxidative stress mediates high-phosphate-induced secretory defects and apoptosis in insulin-secreting cells. Am. J. Physiol. Endocrinol. Metab. 308, E933–E941 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Luan M., et al., Vacuolar phosphate transporters contribute to systemic phosphate homeostasis vital for reproductive development in Arabidopsis. Plant Physiol. 179, 640–655 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Razzaque M. S., Phosphate toxicity: New insights into an old problem. Clin. Sci. (Lond.) 120, 91–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park M., Nam D., Kweon D.-H., Shin D., ATP reduction by MgtC and Mg2+ homeostasis by MgtA and MgtB enables Salmonella to accumulate RpoS upon low cytoplasmic Mg2+ stress. Mol. Microbiol. 110, 283–295 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Park M., Kim H., Nam D., Kweon D.-H., Shin D., The mgtCBR mRNA leader secures growth of Salmonella in both host and non-host environments. Front. Microbiol. 10, 2831 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senior A. E., The proton-translocating ATPase of Escherichia coli. Annu. Rev. Biophys. Biophys. Chem. 19, 7–41 (1990). [DOI] [PubMed] [Google Scholar]
- 42.Harold F. M., Maloney P. C., “Energy transduction by ion currents” in Escherichia Coli and Salmonella: Cellular and Molecular Biology, Neidhardt F. C., et al., Eds. (American Society for Microbiology, ed. 2, 1996), pp. 283–306. [Google Scholar]
- 43.Butlin J. D., Cox G. B., Gibson F., Oxidative phosphorylation in Escherichia coli K-12: The genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim. Biophys. Acta 292, 366–375 (1973). [DOI] [PubMed] [Google Scholar]
- 44.Murarka A., Dharmadi Y., Yazdani S. S., Gonzalez R., Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl. Environ. Microbiol. 74, 1124–1135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richter K., Gescher J., Accelerated glycerol fermentation in Escherichia coli using methanogenic formate consumption. Bioresour. Technol. 162, 389–391 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Marr A. G., Growth rate of Escherichia coli. Microbiol. Rev. 55, 316–333 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell J. B., Cook G. M., Energetics of bacterial growth: Balance of anabolic and catabolic reactions. Microbiol. Rev. 59, 48–62 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madigan M. T., Martinko J. M., Bender K. S., Buckley D. H., Stahl D. A., “Microbial metabolism” in Brock Biology of Microorganisms (Pearson, ed. 14, 2015), pp. 73–105. [Google Scholar]
- 49.Rosenberg H., Gerdes R. G., Chegwidden K., Two systems for the uptake of phosphate in Escherichia coli. J. Bacteriol. 131, 505–511 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Veen H. W., Abee T., Kortstee G. J., Konings W. N., Zehnder A. J., Translocation of metal phosphate via the phosphate inorganic transport system of Escherichia coli. Biochemistry 33, 1766–1770 (1994). [DOI] [PubMed] [Google Scholar]
- 51.Harris R. M., Webb D. C., Howitt S. M., Cox G. B., Characterization of PitA and PitB from Escherichia coli. J. Bacteriol. 183, 5008–5014 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cox G. B., Webb D., Rosenberg H., Specific amino acid residues in both the PstB and PstC proteins are required for phosphate transport by the Escherichia coli Pst system. J. Bacteriol. 171, 1531–1534 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Motomura K., et al., Overproduction of YjbB reduces the level of polyphosphate in Escherichia coli: A hypothetical role of YjbB in phosphate export and polyphosphate accumulation. FEMS Microbiol. Lett. 320, 25–32 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Gardner S. G., Johns K. D., Tanner R., McCleary W. R., The PhoU protein from Escherichia coli interacts with PhoR, PstB, and metals to form a phosphate-signaling complex at the membrane. J. Bacteriol. 196, 1741–1752 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wanner B. L., Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J. Mol. Biol. 191, 39–58 (1986). [DOI] [PubMed] [Google Scholar]
- 56.Sevostyanova A., Groisman E. A., An RNA motif advances transcription by preventing Rho-dependent termination. Proc. Natl. Acad. Sci. U.S.A. 112, E6835–E6843 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cromie M. J., Groisman E. A., Promoter and riboswitch control of the Mg2+ transporter MgtA from Salmonella enterica. J. Bacteriol. 192, 604–607 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cunrath O., Bumann D., Host resistance factor SLC11A1 restricts Salmonella growth through magnesium deprivation. Science 366, 995–999 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Yeom J., Shao Y., Groisman E. A., Small proteins regulate Salmonella survival inside macrophages by controlling degradation of a magnesium transporter. Proc. Natl. Acad. Sci. U.S.A. 117, 20235–20243 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blanc-Potard A. B., Groisman E. A., How pathogens feel and overcome magnesium limitation when in host tissues. Trends Microbiol. 29, 98–106 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffer S. M., Schoondermark P., van Veen H. W., Tommassen J., Activation by gene amplification of pitB, encoding a third phosphate transporter of Escherichia coli K-12. J. Bacteriol. 183, 4659–4663 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffer S. M., Tommassen J., The phosphate-binding protein of Escherichia coli is not essential for P(i)-regulated expression of the pho regulon. J. Bacteriol. 183, 5768–5771 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buchmeier N., et al., A parallel intraphagosomal survival strategy shared by mycobacterium tuberculosis and Salmonella enterica. Mol. Microbiol. 35, 1375–1382 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Lavigne J. P., O’callaghan D., Blanc-Potard A. B., Requirement of MgtC for Brucella suis intramacrophage growth: A potential mechanism shared by Salmonella enterica and Mycobacterium tuberculosis for adaptation to a low-Mg2+ environment. Infect. Immun. 73, 3160–3163 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maloney K. E., Valvano M. A., The mgtC gene of Burkholderia cenocepacia is required for growth under magnesium limitation conditions and intracellular survival in macrophages. Infect. Immun. 74, 5477–5486 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belon C., Gannoun-Zaki L., Lutfalla G., Kremer L., Blanc-Potard A. B., Mycobacterium marinum MgtC plays a role in phagocytosis but is dispensable for intracellular multiplication. PLoS One 9, e116052 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belon C., et al., A macrophage subversion factor is shared by intracellular and extracellular pathogens. PLoS Pathog. 11, e1004969 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le Moigne V., et al., MgtC as a host-induced factor and vaccine candidate against Mycobacterium abscessus infection. Infect. Immun. 84, 2895–2903 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cafiero J. H., Lamberti Y. A., Surmann K., Vecerek B., Rodriguez M. E., A Bordetella pertussis MgtC homolog plays a role in the intracellular survival. PLoS One 13, e0203204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson R. J., et al., Expression of the PitA phosphate/metal transporter of Escherichia coli is responsive to zinc and inorganic phosphate levels. FEMS Microbiol. Lett. 289, 219–224 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Yin X., et al., The small protein MgtS and small RNA MgrR modulate the PitA phosphate symporter to boost intracellular magnesium levels. Mol. Microbiol. 111, 131–144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srikumar S., et al., RNA-seq brings new insights to the intra-macrophage transcriptome of Salmonella Typhimurium. PLoS Pathog. 11, e1005262 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez J. C., et al., Evolution of a bacterial regulon controlling virulence and Mg2+ homeostasis. PLoS Genet. 5, e1000428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beard S. J., et al., Evidence for the transport of zinc(II) ions via the pit inorganic phosphate transport system in Escherichia coli. FEMS Microbiol. Lett. 184, 231–235 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Dudev T., Lim C., Principles governing Mg, Ca, and Zn binding and selectivity in proteins. Chem. Rev. 103, 773–788 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Foster A. W., Osman D., Robinson N. J., Metal preferences and metallation. J. Biol. Chem. 289, 28095–28103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen L. T., Ajua-Alemanji M., Culotta V. C., The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J. Biol. Chem. 278, 42036–42040 (2003). [DOI] [PubMed] [Google Scholar]
- 78.Rosenfeld L., et al., The effect of phosphate accumulation on metal ion homeostasis in Saccharomyces cerevisiae. J. Biol. Inorg. Chem. 15, 1051–1062 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenfeld L., Culotta V. C., Phosphate disruption and metal toxicity in Saccharomyces cerevisiae: Effects of RAD23 and the histone chaperone HPC2. Biochem. Biophys. Res. Commun. 418, 414–419 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ofiteru A. M., et al., Overexpression of the PHO84 gene causes heavy metal accumulation and induces Ire1p-dependent unfolded protein response in Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 94, 425–435 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Choi S., et al., The Salmonella virulence protein MgtC promotes phosphate uptake inside macrophages. Nat. Commun. 10, 3326 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rudat A. K., Pokhrel A., Green T. J., Gray M. J., Mutations in Escherichia coli polyphosphate kinase that lead to dramatically increased in vivo polyphosphate levels. J. Bacteriol. 200, 10.1128/JB.00697-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y., et al., The composition and implications of polyphosphate-metal in enhanced biological phosphorus removal systems. Environ. Sci. Technol. 53, 1536–1544 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Datta S., Costantino N., Court D. L., A set of recombineering plasmids for gram-negative bacteria. Gene 379, 109–115 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Davis R., Bolstein D., Roth J., Advanced Bacterial Genetics (Cold Spring Harbor Lab Press, Cold Spring Harbor, NY, 1980). [Google Scholar]
- 86.Khetrapal V., et al., A set of powerful negative selection systems for unmodified Enterobacteriaceae. Nucleic Acids Res. 43, e83 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neidhardt F. C., Bloch P. L., Smith D. F., Culture medium for enterobacteria. J. Bacteriol. 119, 736–747 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.García Véscovi E., Soncini F. C., Groisman E. A., Mg2+ as an extracellular signal: Environmental regulation of Salmonella virulence. Cell 84, 165–174 (1996). [DOI] [PubMed] [Google Scholar]
- 89.Soncini F. C., García Véscov E., Groisman E. A., Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J. Bacteriol. 177, 4364–4371 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee T. S., et al., BglBrick vectors and datasheets: A synthetic biology platform for gene expression. J. Biol. Eng. 5, 12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang A. C., Cohen S. N., Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134, 1141–1156 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kanno S., et al., Performance and limitations of phosphate quantification: Guidelines for plant biologists. Plant Cell Physiol. 57, 690–706 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Schneider C. A., Rasband W. S., Eliceiri K. W., NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.