Significance
Multiple sclerosis (MS) can transition from a relapsing-remitting form (RRMS) into a chronic progressive form (secondary progressive MS, SPMS). SPMS pathogenesis remains poorly understood with diagnosis based entirely on retrospective clinical monitoring. We show that T helper cells expressing the transcription factor Eomes (Eomes+ Th cells) were significantly increased in peripheral blood from SPMS patients versus healthy subjects or RRMS and other progressive MS patients. Moreover, Eomes+ Th cells expressing granzyme B were found infiltrating brain tissues in SPMS autopsy samples, providing support for a pathogenic role. Analysis of clinical status on follow-up indicated the value of measuring Eomes+ Th cells for SPMS diagnosis and prognostic monitoring. This study may contribute to future development of biomarker-assisted “precision medicine” for MS.
Keywords: multiple sclerosis, secondary progressive multiple sclerosis, autoimmunity, Eomes+ Th cells, biomarkers
Abstract
Multiple sclerosis (MS), a putative autoimmune disease of the central nervous system (CNS), commonly presents as relapsing-remitting MS (RRMS), characterized by recurrent episodes of peripheral disabling symptoms resulting from inflammatory CNS damage. Many RRMS patients transition to a chronic disease course with progressive neurological dysfunctions (secondary progressive MS, SPMS), with the progression rate varying between patients and over time. SPMS pathogenesis is now linked to immune-cell–mediated processes, although the mechanisms driving SPMS transition and progression remain elusive, and SPMS lacks biomarkers and effective treatments. We report the crucial involvement of cytotoxic CD4+ T cells expressing Eomes (Eomes+ Th cells) in SPMS pathogenesis—a Th cell subset previously identified in a mouse model of late/chronic autoimmune CNS inflammation. Few Eomes+ Th cells circulate in RRMS patient peripheral blood (n = 44), primary progressive MS (PPMS) patients (n = 25), or healthy controls (n = 42), but Eomes+ Th cells were significantly increased in SPMS (n = 105, P < 0.0001). Strikingly, lymphocytes isolated from SPMS autopsy brain samples revealed CD4+ T cells infiltrating CNS that coexpressed Eomes and the cytotoxic molecule granzyme B. In particular, the Eomes+ Th cell levels were increased in SPMS patients in progressive disease phases versus SPMS patients without current disability increases (P < 0.0001). Moreover, Eomes level acted as a biomarker to predict SPMS patients at risk of disease worsening with over 80% accuracy (ROC-AUC = 0.8276). Overall, our results indicate that granzyme B-expressing Eomes+ T helper cells are involved in the pathogenesis of SPMS, with significant implications for SPMS biomarkers and therapeutic targets.
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS) that manifests with a variety of disabling neurological symptoms, resulting from localized CNS damage in inflammatory lesions (1, 2). Results of genome wide association studies combined with the efficacy of drugs targeting immune components indicate that MS is an autoimmune disease driven by autoreactive T cells (3–5). Patients with relapsing-remitting MS (RRMS) suffer from recurrent exacerbations of neurological signs, including motor and sensory disturbances, followed by a remission period that may last for months to years. Current treatment regimens for RRMS offer relatively effective control of the disease and prevent relapses to varying degrees.
A proportion of patients with RRMS develop a progressive form of MS referred to as secondary progressive MS (SPMS) (6–8). Transition to the SPMS stage is accompanied by continuous deterioration of activities required for normal daily life, such as the ability to walk, and often manifests with cognitive impairment due to brain atrophy. In early studies, SPMS disease progression was attributed solely to neurodegenerative mechanisms because the rate of disability progression appeared to be very similar across SPMS patients (9, 10). However, there is a growing appreciation from clinical practice that disease trajectories are not constant but changeable in patients with SPMS, questioning the neurodegenerative model (11, 12). Furthermore, recent clinical trials have showed a significant efficacy of therapies targeting lymphocytes in SPMS (13, 14), highlighting the importance of active immune processes in this disease condition. Moreover, SPMS development has been directly linked with functional alterations in a T cell subset (15). Thus, an immune-cell–mediated process is implicated in the pathogenesis of SPMS rather than neurodegeneration per se, giving new hope that understanding the role of T cells in SPMS development could lead to the identification of key cellular and molecular components that may serve as potential therapeutic targets or useful biomarkers. Notably, the diagnosis of SPMS currently requires retrospective evaluation of medical records or prospective follow-up for many months to ascertain the continuous progression of neurological dysfunction. Thus, the development of biomarker-assisted diagnostic methods is a critical requirement for making an early diagnosis of SPMS.
We previously revealed that CD4+ T helper cells expressing the transcription factor Eomes (Eomes+ Th cells) play a crucial role in the development of chronic neuroinflammation in a MOG35–55 peptide-induced model of experimental autoimmune encephalomyelitis (EAE) (16). This EAE model rapidly manifests with acute neurological symptoms mediated by NR4A2-dependent Th17 cells (17, 18) but quickly resolves into a chronic form in which Eomes+ Th cells play a pathogenic role. While Eomes expression by cytotoxic CD8+ T cells or natural killer cells is widely acknowledged (19, 20), we revealed that Eomes+ Th cells involved in EAE are cytotoxic T cells capable of producing granzyme B (16), and accordingly, blocking Eomes or granzyme B expression lead to the suppression of chronic EAE.
A tight relationship between Eomes and neuroinflammation is also suggested in human diseases. Notably, an association between Eomes polymorphism and MS was revealed by genome-wide association studies (21, 22). Our prior analysis showed an increase of Eomes+ CD4+ T cells in the peripheral blood and cerebrospinal fluid from a small number of patients with SPMS (16). Recently, an expansion of similar cytotoxic CD4+ T cells has been documented in the tissue or blood samples from rheumatoid arthritis (23) and MS (24, 25), further supporting the role of Eomes+ Th cells in autoimmune inflammatory processes. Of note, in our EAE model, Eomes+ CD4+ T cells appear to be generated in the CNS inflammatory lesions via in situ priming or epitope spreading (26). However, information available in human disease is fragmentary and does not either support or exclude the scenario that is postulated in rodent EAE.
In the present study, we measured the frequency (%) of Eomes+ Th cells/CD4+ T cells in the peripheral blood from 66 patients with SPMS (105 samples), 39 with RRMS (44 samples), and 25 with primary progressive MS (PPMS) as compared with 42 healthy controls (HC). First, an elevation of Eomes+ Th cells was confirmed in over 50% of all patients with SPMS, whereas such an elevation was seen only in a few patients with RRMS, one patient with PPMS and one healthy subject. These results indicate a significant link of Eomes+ Th cells with SPMS. By applying mathematical modeling to evaluate Eomes-expressing Th cell distribution, we next showed that SPMS patients could be divided into groups that were “Eomes-hi” or Eomes-lo” at the time of sampling. Intriguingly, “Eomes-hi” status was significantly associated with SPMS patients who were in the progressive phase, as judged by subsequent increases of disability. Analysis of individual data showed that “Eomes-hi” or “Eomes-lo” status is not always fixed but interchangeable, which presumably result from changes in inflammatory disease activity.
We here reveal that a major proportion of Eomes+ Th cells derived from the peripheral blood of SPMS patients express granzyme B and an increase of granzyme B-secreting CD4+ T cells in SPMS blood samples largely corresponds to Eomes+ Th cells. Finally, we explored if Eomes+granzyme B+ Th cells might be present in brain autopsy samples. To approach this question, we used flow cytometry to analyze lymphocytes isolated from unfixed, unfrozen, postmortem CNS tissues, which showed the predominance of Eomes+granzyme B+ Th cells infiltrating brain tissue in SPMS autopsy samples, which allows us to postulate that Eomes+ Th cells may play a pathogenic role in the development of SPMS.
Materials and Methods
Selection of Patients and Controls.
The Ethics Committee of the National Center of Neurology and Psychiatry (NCNP) on human experimentation approved the study according to Declaration of Helsinki guidelines, and written informed consent was obtained from all participants. The subjects donated blood samples for flow cytometric measurement, and cohorts are listed in Table 1.
Table 1.
Clinical characteristics of patients and healthy subjects who participated in this study
| Individual samples | Unique patients | HC | |||||
| RRMS | SPMS | PPMS | RRMS | SPMS | PPMS | ||
| Number | 44 | 105 | 25 | 39 | 66 | 14 | 42 |
| Male (%) | 11(25%) | 34(32%) | 11(44%) | 10 (26%) | 23 (35%) | 5 (33%) | 18 (43%) |
| Female (%) | 33(75%) | 71(67%) | 14(56%) | 29 (74%) | 43 (65%) | 10 (67%) | 24 (57%) |
| Age ± SD (range) | 39.2 ± 8.0 (21 to 55) | 45.5 ± 8.6 (24 to 67) | 46.8 ± 10.0 (27 to 67) | 38.8 ± 8.1 (21 to 55) | 45.0 ± 8.9 (24 to 67) | 44.1 ± 9.5 (27 to 60) | 37.6 ± 15.9 (19 to 63) |
| Onset age ± SD (range) | 29.8 ± 9.1 (11 to 53) | 30.8 ± 8.4 (13 to 54) | 36.72 ± 8.0 (22 to 52) | 29.5 ± 9.5 (11 to 53) | 31.5 ± 8.6 (13 to 54) | 36.1 ± 7.9 (22 to 52) | NA |
| Disease duration ± SD (range) | 10.9 ± 7.5 (0 to 38) | 14.9 ± 6.7 (1 to 38) | 10.3 ± 7.2 (0 to 25) | 10.9 ± 1.2 (0 to 38) | 13.7 ± 6.9 (1 to 38) | 8.3 ± 5.9 (0 to 20) | NA |
| ARR ± SD (range) | 0.3 ± 0.7 (0 to 3) | 0.34 ± 0.62 (0 to 3) | 0.44 ± 0.92 (0 to 4) | 0.9 ± 1.0 (0 to 3) | 0.40 ± 0.66 (0 to 3) | 0.5 ± 2.1 (0 to 4) | NA |
| EDSS ± SD | 2.4 ± 1.8 | 6.0 ± 1.4 | 5.2 ± 1.7 | 0.3 ± 0.8 | 5.8 ± 1.5 | 4.8 ± 1.1 | NA |
| ΔEDSS ± SD | 0.3 ± 0.7 | 0.4 ± 1.0 | ND | 0.9 ± 1.0 | 1.1 ± 1.6 | ND | NA |
| Progressive:stationary | 4:29 | 22:54 | 16:9 | 4:25 | 12:32 | 9:6 | NA |
| Eomes% ± SD (range) | 6.1 ± 3.5 (0.9 to 3.5) | 15.2 ± 14 (0.4 to 78.5) | 6.9 ± 6.7 (1.2 to 30.4) | 6.3 ± 3.5 (0.9 to 15) | 17.8 ± 15.9 (0.5 to 78.5) | 7.8 ± 4.7 (2.7 to 13.6) | 5.2 ± 3.5 (0.5 to 14) |
Postmortem tissue samples from four autopsy cases (SPMS, neuromyelitis optica, and Parkinson’s Disease [PD]) were obtained through the NCNP Brain Bank. Cerebral and cerebellar hemispheres as well as brainstem were dissected in the sagittal plane at the time of autopsy. Some areas were fixed in 4% paraformaldehyde and embedded in paraffin. Serial sections (6 µm thick) were immunostained with anti-CD3 (Novocastra) or anti-CD4 (DAKO) with an I-View DAB Universal Kit (Roche) and counterstained with hematoxylin. Unfixed tissue samples were weighed and disassociated using a gentleMACS system (Miltenyi Biotech) to obtain a single cell suspension before immune cell examination and enumeration by flow cytometry.
Standard clinical criteria (11) indicate SPMS diagnosis for patients whose neurological disabilities get steadily worse for more than 6 mo despite a past diagnosis of RRMS. Attending clinicians, who were blinded from flow cytometry data, scored Expanded Disability Status Scale (EDSS) and Annualized Relapse Rate (ARR). In the present study, “in remission” was defined as a clinically stable state lasting for more than 30 d; “At relapse” was determined as the period within 30 d after the initiation of neurological exacerbation. We defined “progressive” as a clinical state with an increased annual change in EDSS score (Δ-EDSS ≥0.5) and “stationary” as a state without any changes in EDSS score (Δ-EDSS ≤0). Detailed clinical characteristics of the enrolled MS patients and HC are shown in Tables 1 and 2.
Table 2.
Treatment information (number of patients per group)
| Treatment | RRMS total 44 | SPMS total 105 |
| PSL | 16 (36%) | 63 (60%) |
| Other immunosuppressive* | 3 (7%) | 40 (38%) |
| No PSL or other Immunosuppressive* | 28 (63%) | 30 (29%) |
| IFN-β | 11 (25%) | 19 (18%) |
| GA | 7 (16%) | 14 (13%) |
| Fingolimod | 2 (5%) | 16 (15%) |
| NTZ | 0 (0%) | 6 (6%) |
| Dimethyl fumarate | 0 (0%) | 2 (2%) |
Numbers of RRMS and SPMS patients under different treatment regimens by sample number for the cohort studies.
Non-PSL immunosuppressives include azathioprine, methotrexate, cyclophosphamide, and bucillamine.
Blood Collection and PBMC Purification.
BMC were separated from donated blood samples on a Ficoll gradient (Ficoll Paque Plus, GE Healthcare) after centrifugation at 400 g for 30 min. Serum was collected and centrifuged to remove cells, and cell-free serum aliquots were frozen and stored at −80 °C until assessment. Control serum was provided by NCNP Biobank, a member of the National Center Biobank Network. Cytokine concentrations in samples were measured by a Luminex assay (R&D Systems) and acquired on a Bioplex 200 (BioRad. Neurofilament light chain (NFl) was measured in serum using the Simoa system, the corresponding reagent kit, and standards (all Quanterix) using a commercial service (Cold Spring Biotech).
Flow Cytometry.
PBMC were resuspended in phosphate-buffered saline with 5% fetal calf serum and stained with fluorochrome-conjugated antibodies against surface antigens (BioLegend). Cells were then fixed with an intracellular Transcription Factor Staining kit (eBioscience) according to manufacturers instructions. Cells were then intracellularly stained with fluorochrome-conjugated anti-Eomes antibody (eBioscience) in permeabilization buffer. Flow cytometry data were acquired using a FACSCanto II (BD Cytometry systems) with a FACSDiva software and analyzed using FlowJo software (Treestar). For examination of Th cells, PBMC were first gated through a doublet gate and a size/scatter gate, before selecting T cells based on CD3 and CD4 expression. Typical gating is shown in SI Appendix, Fig. S1A.
Statistical Analyses.
Data were analyzed using Prism software (GraphPad) and R statistical packages (a language and environment for statistical computing. R Foundation for Statistical Computing https://www.R-project.org/). Data are presented as individual points with mean values ± SD. Statistical testing was performed by a Mann–Whitney U test for comparing two sets of nonparametric data, a Kruskal–Wallis test with Dunn’s correction for multiple comparison for comparing more than two related groups, and D’Agostino–Pearson omnibus normality tests for normal distribution, and correlation tests were performed by the Spearman method. Odds ratios and receiver operator curve (ROC) curves for biomarker assessment were carried out in Prism. Biomarker significance was measured with a χ2 test. Details of statistical approaches in the R environment and packages used are cited in SI Appendix, Supplementary Methods.
Results
A Proportion of SPMS Patients Show Increased Frequencies of Eomes-Expressing Th Cells.
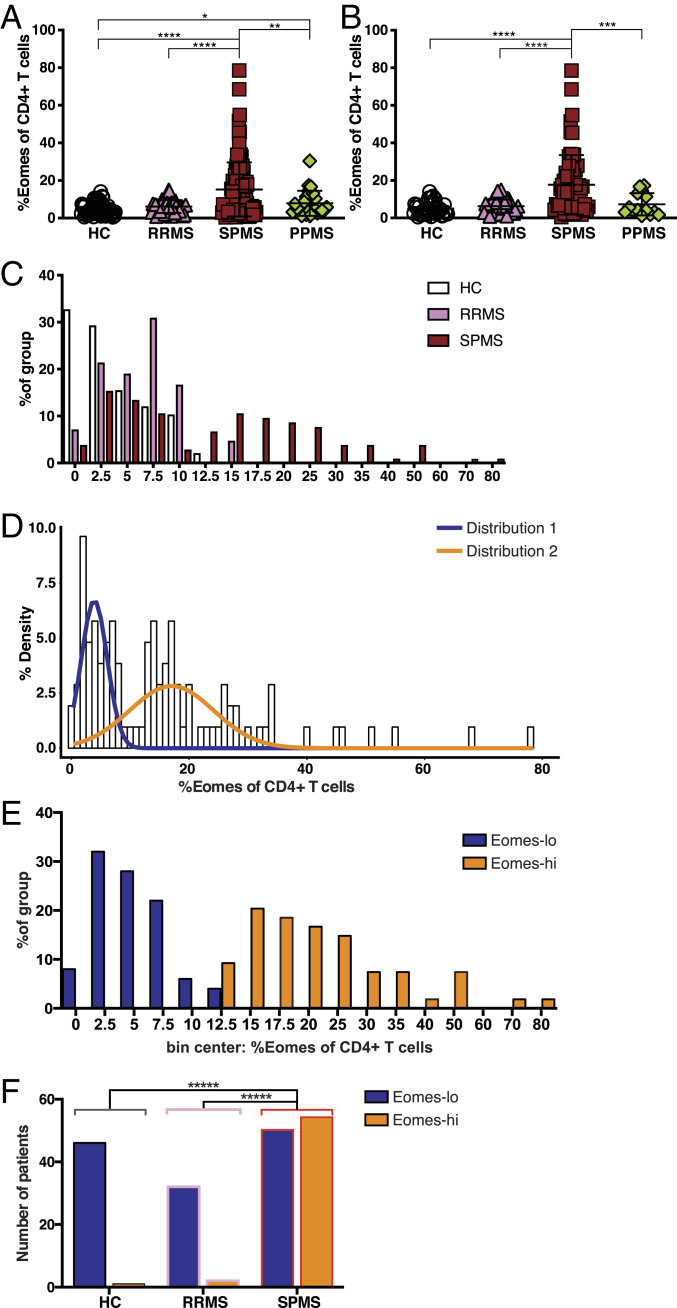
Peripheral blood from 30 human HC subjects showed that CD3+ T cells contained a population of Eomes+ Th cells (SI Appendix, Fig. S1 A and B). We confirmed that there was no significant difference between HC and RRMS (44 samples) with regard to Eomes+ Th cells proportion among CD4+ T cells (5.2 ± 3.5% for HC and 6.3 ± 3.5% for RRMS [mean ± SD]; Fig. 1A and Table 1). In contrast, a large proportion of the 105 samples obtained from SPMS patients showed significant increases in this population. As this study included data from 27 patients sampled at multiple times, to avoid any potential bias toward outlier patients, we reassessed the dataset with repeated samples excluded (Fig. 1B). The increase in Eomes+ Th cells remained extant after exclusion of the repeated samples. Further to dividing Th cells into Eomes+/− based on staining, the increase was also apparent when Eomes level was considered across the CD4+ T cell populations as a whole, but the Eomes+ Th cells expressed similar levels of Eomes across the groups (SI Appendix, Fig. S1C).
Fig. 1.
High proportions of Eomes+ Th cells are associated with SPMS. (A) Proportion of Eomes+ cells among CD4+ Th cells. Data from all the samples are shown. *P < 0.05, **P < 0.01, ****P < 0.0001, based on one-tailed Mann–Whitney U test. (B) Proportion of Eomes+ cells among CD4+ Th cells. When data were obtained at multiple times, only data from the first samples are included here. ***P < 0.001, ****P < 0.0001, based on one-tailed Mann–Whitney U test. (C) Distribution of HC, RRMS, and SPMS samples split into bins, based on % of Eomes+ cells among CD4+ T cells. (D) Histogram of Gaussian mixture modeling of Eomes+ Th cells in SPMS patients according to a binomial distribution. (E) Distribution of the Eomes proportion (Eomes+ cells among CD4+ Th cells) for SPMS patients split into Eomes-lo (<13%) and Eomes-hi (≥13%). (F) Histogram for number of subjects in each patient/control group split by Eomes-hi/Eomes-lo status based on a 13% cutoff. ****P < 0.0001, two-tailed χ2 test with Yates’ correction. SPMS versus RRMS OR = 17.28; SPMS versus HC OR = 49.68; HC Eomes-lo (n = 46), HC Eomes-hi (n = 1); RRMS Eomes-lo (n = 32), RRMS Eomes-hi (n = 2); and SPMS Eomes-lo (n = 50), SPMS Eomes-hi (n = 54).
Although the clinical resemblance between PPMS and SPMS is widely appreciated in the progression of disability independent of relapse, only a single patient with PPMS in the present cohort showed an increased frequency of Eomes+ Th cells (Fig. 1A and Table 1). This result suggests that brain inflammation triggered by relapses might be a prerequisite for the induction of Eomes+ Th cells. Independent of the disease group, Eomes+ Th cells had down-regulated CCR7 (SI Appendix, Fig. S1D), indicating an effector/memory T cell phenotype. Most Eomes+ Th cells in SPMS expressed CD45RO, and the majority of the cells had also down-regulated CD27, indicating highly differentiated T effector memory subsets (SI Appendix, Fig. S1E). However, Eomes+ Th cells from HCs, although few in number, were found spread across more diverse subsets, mostly stem-cell–like memory T cells and transitional memory T cells, with a lower proportion of T effector memory cells. In contrast to CD4+ T cells, a large proportion of CD8+ T cells expressed Eomes, and this proportion did not differ between patient groups (SI Appendix, Fig. S1F).
Next, we examined the distribution of Eomes+ Th cell populations in HC and MS patients. While HC and RRMS patients were normally distributed in this analysis, SPMS patients were not (Fig. 1C, D’Agostino–Pearson omnibus normality tests, alpha 0.05). Mathematical modeling using Gaussian mixture models indicated that Eomes+ Th cell levels in SPMS patients formed a binomial distribution with overlapping normal distributions (Fig. 1D). Further, this model was used to calculate a cutoff value for distinguishing patient samples that were distributed in the subpopulation with a higher proportion of Eomes-expressing Th cells. Due to the predicted overlap of distributions, a 99% contribution of the upper distribution was used, generating a cutoff value of 13%. Thus, SPMS patients were divided into two types according to this modeling: Eomes proportions ≥13% were used to segregate patients into those with “Eomes-hi” status and those with “Eomes-lo” status (Eomes proportions <13%). A caveat to this model is that this conservative approach will miss some patients that should form part of the upper distribution; however, few patients with normal Eomes levels will be included in the Eomes-hi distribution. Indeed, Eomes-lo SPMS patients were normally distributed and did not significantly differ from HC and RRMS patients (Fig. 1E, D’Agostino–Pearson omnibus normality tests, alpha 0.05; Student’s t test, P > 0.05). Similar cutoffs for a normally distributed Eomes-lo population were also obtained by K-mean clustering and Jenks natural breaks optimization approaches (SI Appendix, Fig. S2 A–D). SPMS patients with Eomes-hi status also had a normal distribution with a greatly increased population mean value compared with HC, RRMS, and Eomes-lo SPMS. These distribution data indicate that circulating Eomes+ Th cells are actually increased in a subpopulation of SPMS patients. As similar T cells expressing Eomes have a cytotoxic potential (16, 27), we suspected that Eomes+ Th cells might represent a genuine pathogenic cell population driving inflammatory damage in SPMS.
Currently, a transition from RRMS to SPMS in individual patients can be diagnosed after the patients have already entered the SPMS stage for many months. Therefore, there is an unmet need for a biomarker to discern patients who have recently developed SPMS. As most patients with Eomes-hi status were found among SPMS patients, we next evaluated the potential value of the Eomes-expressing Th cell proportion as a biomarker to distinguish SPMS from RRMS patients. ROC analysis (SI Appendix, Fig. S2E) indicated a significant result (AUC = 0.699, 95% CI, 0.616 to 0.781). Using the cutoff value indicated by mathematical modeling (13% Eomes+ Th cells), almost all subjects with Eomes-hi status had a diagnosis of SPMS (false discovery rate [FDR] = 0.041). While the SPMS group had significantly more subjects with Eomes-hi status compared with the other groups (Fig. 1F; P < 0.0001, two-tailed χ2 tests), approximately half of SPMS patients were Eomes-lo at the time of sampling. Thus, although this simple blood test correctly identified a population of SPMS patients, the sensitivity at this cutoff was 50.96%, giving a high rate of false negatives.
Eomes-hi Phenotype Is Linked to Disease Progression in SPMS Patients.
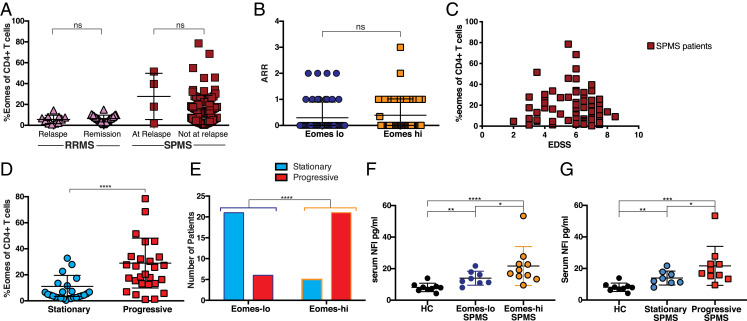
As experiments in a chronic EAE model indicated Eomes+ Th cells as potentially pathogenic (16), and Eomes+ Th cells are increased in a proportion of SPMS patients, we addressed if these cells might have an active role in disease processes. To this end, we examined if any relationship was apparent between the Eomes+ Th cell proportion and clinically defined disease activity in SPMS patients. Prior studies have indicated that SPMS may be associated with short periods of acute relapses (28, 29), based on the criteria used for RRMS (12, 30), although the long-term effect of such relapses on SPMS progression has been shown to be minimal (31). Therefore, we were curious to know if the level of Eomes+ Th cells changed during relapse in RRMS or SPMS. However, no significant increase or decrease in the Eomes+ Th cell proportion was observed during relapse in either RRMS or SPMS patients (Fig. 2A), as compared with nonrelapse samples. We next compared the ARR for SPMS patients with Eomes-hi or Eomes-lo status. The mean ARR for our SPMS patients was 0.349 on the whole (SD 0.6175, Table 1), which agrees with the reported ARR for large numbers of SPMS patients (11). We observed no significant difference in ARR between patient groups based on Eomes status (Fig. 2B).
Fig. 2.
Relationship between Eomes+ Th cell proportions and current disease status. (A) Current relapse versus nonrelapse at sampling time. Shown are percentages of Eomes-expressing cells among the CD4+CD3+ PBMC for RRMS or SPMS patients at relapse or nonrelapse state. Not significant (ns) (P = 0.234), two-tailed Mann–Whitney U test; RRMS at relapse (n = 11); RRMS during remission (n = 30); SPMS at relapse (n = 4); and SPMS at nonrelapse state (n = 100). (B) ARR for individual samples from SPMS patients with Eomes-lo and Eomes-hi status. ns (P = 0.232), two-tailed Mann–Whitney U test; and Eomes-lo (n = 50), Eomes-hi (n = 54). (C) EDSS and proportions of Eomes+ Th cells among CD4+ Th cells in individual SPMS patients. Spearman correlation r = −0.07 and P = 0.50. (D) Proportion of Eomes+ Th cells among CD4+ T cells from SPMS patients (Table 2). After exclusion of patients with an initial EDSS of seven or higher before sampling, the samples were categorized as being from progressive or stationary status based on clinical assessment after sampling, which was validated by EDSS ****P < 0.0001 Mann–Whitney U test. (E) Proportion of SPMS patients in SI Appendix, Table S1 grouped by Eomes-lo or -hi based on a 13% cutoff with progressive status ****P < 0.0001 χ2 test, OR = 15.2; and Eomes-lo (n = 27), Eomes-hi (n = 31). (F) Serum levels of NFl. Comparison of HC, Eomes-lo SPMS, and Eomes-hi SPMS. *P < 0.05, **P < 0.01, ****P < 0.0001. (G) Serum levels of NFl. Comparison of HC, SPMS at stationary phase (stationary SPMS), and SPMS at progressive phase (progressive SPMS). *P < 0.05, **P < 0.01, ***P < 0.001.
To assess current disability status for MS, experts commonly use EDSS, a measure for neurological dysfunction based on clinical assessment (32). Although there was no correlation between EDSS and Eomes+ Th cell proportions in SPMS (Fig. 2C, Spearman correlation r = −0.07 and P = 0.50), patients with the highest Eomes+ Th cell proportions were clustered between EDSS 4 to 6. Next, we used annual change in EDSS score (Δ-EDSS) as a measure of ongoing disease activity. Detailed clinical characteristics of the enrolled MS patients and HC are shown in Tables 1 and 2. In SPMS between assessments (33), patients were operationally divided based on clinical state into “stationary” (Δ-EDSS ≤ 0) and “progressive” (Δ-EDSS ≥ 0.5). EDSS is a nonlinear scale with much greater increases in disabilities required to increase the score at higher levels, so unsurprisingly patients with higher EDSS scores tended to show lower increases in EDSS (SI Appendix, Fig. S3A). Therefore, in common with other studies depending on clinical evaluation of SPMS patients (13, 34), we excluded patients with an initial EDSS score of seven or higher from further progressive/stationary analysis. In addition, we ensured that the samples examined were carefully controlled regarding PBMC sampling time and EDSS score (SI Appendix, Fig. S3B). In this cohort (described in SI Appendix, Table S1), patients who were judged as with Eomes-hi status were at higher risk for entering progressive phase thereafter, with regard to the EDSS changes and clinical evaluation (Fig. 2D; P < 0.0001 Mann–Whitney U test). Furthermore, more than 81% of the SPMS population with Eomes-hi status was judged as during progressive phase by a retrospective analysis at 6 to 12 mo after measuring the Eomes, whereas only 22% of the Eomes-lo group was in the progressive phase (Fig. 2E). Of note, the progressive disease state was confirmed in the SPMS patients showing Eomes-hi status with an accuracy of 80% (positive predictive value [PPV] of 0.813, a negative predictive value [NPV] of 0.778, and an FDR of 0.188); the proportion of progressive cases among SPMS patients with Eomes-hi status was significantly greater than those that were Eomes-lo (Fig. 2E, P < 0.0001 χ2 test with Yates’ correction, odds ratio [OR] = 15.2, Eomes-lo n = 27, Eomes-hi n = 32, cutoff 13%). There was a weak correlation between Δ-EDSS and Eomes proportion (Two-tailed Spearman correlation r = 0.304 and P = 0.0251; SI Appendix, Fig. S3C), suggesting that those with the highest Eomes levels at sampling might suffer the highest increase of disability following sampling.
To further confirm the link between the SPMS progression and the level of circulating Eomes+ Th cells, we compared brain volumes based on MRI calculations between SPMS patients that were in Eomes-hi or Eomes-lo status when sampled. Total brain volume and the volumes of white matter and gray matter were individually normalized to intracranial space volume. All SPMS patients had significantly reduced brain volume and white matter volume compared with age-matched HC. SPMS patients recorded as having a stage of Eomes-hi had a statistically significant reduction in gray matter volume (SI Appendix, Table S2). Gray matter atrophy is characteristic for SPMS and correlates with an increased severity in disability (35). Although our imaging data were consistent with the hypothesis that high numbers of circulating Eomes+ Th cells are associated with more progression in SPMS, this single time-point analysis was too underpowered to draw a conclusion. Equally, those patients identified with low levels of Eomes+ Th cells when sampled may have also undergone stages of Eomes-hi, and it is more likely brain atrophy could be linked to the duration of Eomes-hi responses rather than the current status.
The increasing concentrations of NFl in cerebrospinal fluid (CSF) and serum has been associated with ongoing CNS damage in RRMS (36, 37). Further, a decrease in NFl serum levels may correlate with reductions in disease activity in RRMS resulting from therapeutic intervention (38). These works encourage the use of NFl levels as a marker to evaluate current CNS pathology in SPMS. Subsequently, we found that serum levels of NFl were significantly increased in SPMS patients compared with HC subjects. Moreover, the NFl levels were significantly higher in SPMS with Eomes-hi versus Eomes-lo status (Fig. 2F) and in SPMS during progressive state versus stationary phase (Fig. 2G). These findings are supportive of our premise that more active and destructive pathology manifests in the CNS of the Eomes-hi SPMS group.
Eomes-hi as a Biomarker for SPMS Patients in a Progressive State.
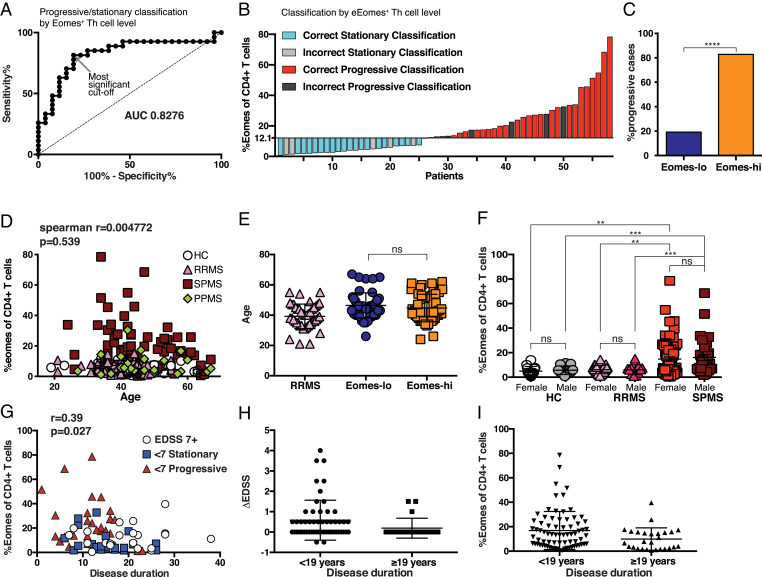
Selection of the cutoff level to identify patients in a progressive state is critical when we evaluate the value of %Eomes Th cells as a potential biomarker. In fact, the proportion of progressive patients who were identified among the Eomes-hi group varies with cutoff level (SI Appendix, Fig. S4A). Therefore, we performed an ROC curve analysis to evaluate %Eomes Th cells as a potential biomarker. However, as such analysis is only reliable for a normally distributed biomarker (39), we transformed %Eomes with a Log2 function. The resulting Log2-Eomes distribution was confirmed as normal using a D’Agostino–Pearson omnibus normality test (alpha 0.05). ROC curve analysis of the transformed data showing sensitivity/specificity of Log2 Eomes as a predictor of progression over a range of cutoffs indicated that this measure was a successful biomarker (Fig. 3A; AUC = 0.8276, P < 0.0001). We recalculated the cutoff value using Fisher’s exact tests on Log2 Eomes to determine the most significant cutoff value as a biomarker for progressive disease (40). These analyses indicated a 12.1% Eomes cutoff (indicated by a gray arrow in Fig. 3A), a figure reassuringly similar to that predicted by mathematical modeling as forming a cutoff for those patients with elevated %Eomes+ Th cells from those with normal levels. As shown in SI Appendix, Fig. S4B, this cutoff value corresponds with the highest accuracy as a biomarker to segregate patients into progressive state (defined by Δ-EDSS) or stationary/slowly progressive state. Using this cutoff, a waterfall plot was generated to depict the effectiveness of Eomes proportion as a diagnostic tool (Fig. 3B). This cutoff gives a PPV = 0.81 and NPV = 0.83 (P = 3.7 × 10−6, two-tailed Fisher’s exact test), and using this cutoff, 80% of SPMS patients with Eomes-hi status were in a progressive stage (Fig. 3C). These data support the potential value of the Eomes measurement to identify SPMS patients and judge who is at risk for further clinical worsening.
Fig. 3.
Eomes-hi as a biomarker for SPMS patients in a progressive state. (A) ROC plot of progressive/stationary prediction by sensitivity versus 1-specficity for Log2 transformed %Eomes+ of Th cells for SPMS patients (SI Appendix, Table S1); the gray arrow shows most significant cutoff value at Eomes = 12.1. (B) Waterfall plot of predictive classification of progressive/stationary status in SPMS patients using cutoff Log2-Eomes = 3.683 (Eomes = 12.83). (C) Proportion of progressive cases among Eomes-lo and Eomes-hi populations based on a cutoff of 12.1%. ****P < 0.0001, Fisher’s exact test, OR = 21.8 (Eomes-hi n = 31, Eomes-lo n = 26). Percentage of Eomes-expressing cells among the CD4+CD3+ was calculated for individual samples in each patient group as listed in Table 1. (D) The subjects’ ages were compared with Eomes+ proportions for correlation (Spearman correlation r=−0.0037 and P = 0.65) and (E) compared between SPMS patients with the Eomes proportion below 13% (Eomes-lo) or above 13% (Eomes-hi). (F) Percentage of Eomes-expressing cells among the CD4+CD3+ for each patient group split into male and female subjects (**P < 0.001, ***P < 0.0001, ns two-tailed Mann–Whitney U test). (G) Eomes+ cell proportion among CD4+CD3+ T cells corresponding to disease duration of SPMS patients. Data are grouped by the EDSS level into SPMS “Stationary below EDSS 7” (<7 Stationary), SPMS “Progressive below EDSS 7” (<7 Progressive), or SPMS with “EDSS of 7 or above” (EDSS 7+). (H) Eomes proportion among Th cells for SPMS patients split by disease duration (over 19 y history, n = 55 or under 19 y history, n = 21); P = 0.0180 Mann–Whitney U test. (I) The change in EDSS over 1 y (Δ-EDSS) for SPMS patients split by disease duration (over 19 y history, n = 55 or under 19 y history, n = 21); P = 0.0294 Mann–Whitney U test.
Patient Age and Sex Do Not Influence Eomes+ Th Cells.
Eomes expression has been demonstrated in exhausted T cells that may accumulate with age (41, 42). Although SPMS patients in this cohort did not significantly differ from other groups in age (Table 1), we tested to see if the difference in Eomes expression could be explained by age differences. There was no correlation between Eomes proportion and age in any group or for all samples (Fig. 3D, Spearman correlation r = −0.0037 and P = 0.65). In addition, using a 13% cutoff, SPMS patients with either Eomes-lo status or Eomes-hi status did not differ in age characteristics (Fig. 3E). Furthermore, sex ratio differences between groups could not explain the Eomes data (Fig. 3F). We also analyzed a possible relationship between the Eomes level and disease duration. Since patients with longer disease duration tended to show an increased Eomes level, data were reexamined after separating the patients into stationary state, progressive state (SI Appendix, Table S1 criteria), and those with an EDSS of seven or above (Fig. 3G). There was no correlation between the Eomes level and disease duration in any group or for the data as a whole. In addition, it was noted that patients with long disease duration (above 19 y history) had lower levels of Eomes+ Th cells (Fig. 3H). As these patients also had significantly lower Δ-EDSS than the whole SPMS cohort (Fig. 3I), it is conceivable that this group of SPMS patients forms a separate patient population that is not actively driven by Eomes+ Th cells.
Relationship between Eomes+ Th Cells and Treatment.
Next, we explored possible links between particular treatments and the level of circulating Eomes+ Th cells. As no approved drugs were available, a proportion of SPMS patients in the NCNP hospital have received treatment with the corticosteroid prednisolone (PSL). As such, the proportion of patients treated in this manner is higher in SPMS than RRMS (SI Appendix, Table S1). It is important to rule out an effect of steroid treatment on the induction of Eomes+ Th cells in SPMS over RRMS and HC; no increase in Eomes+ Th cells was observed in PSL treated patients (SI Appendix, Fig. S5A). In addition, treatment with other immunosuppressive drugs was not associated with Eomes+ Th cell levels (SI Appendix, Fig. S5 B and C) nor glatiramer acetate (GA) (SI Appendix, Fig. S5D). Therefore, despite the disparity in treatment between groups, we can rule out the effects of these drug treatments as causing the differences in the Eomes+ Th cell level.
Of interest, SPMS patients treated with Natalizumab (NTZ) were mostly Eomes-lo (anti–alpha-4 integrin; SI Appendix, Fig. S5E), although this analysis was underpowered to reveal a significant difference between the groups. Moreover, interferon-beta (IFN-β)–treated SPMS patients had significantly lower Eomes Th cell proportions, compared with other treatments (SI Appendix, Fig. S5F, Mann–Whitney U test P < 0.05).
Differential Inflammatory Phenotypes Are Associated with Eomes-hi and Eomes-lo SPMS.
It is conceivable that differences in Eomes+ Th cell levels in individual patients could reflect the differential state of disease activity, given the correlation between the Eomes proportion and serum NFl, a marker for current CNS pathology (Fig. 2 F and G). To evaluate this possibility, we measured the serum levels of 60 cytokines, chemokines, and other potential biomarkers in samples from SPMS patients with Eomes-hi or Eomes-lo status, compared with RRMS patients and HCs. Clustering analysis indicated that the Eomes-hi group had a distinct cytokine profile (SI Appendix, Fig. S6A). For example, SPMS patients that were in Eomes-hi status had significantly higher serum IL-7 and lower CD27, a biomarker associated with conventional immune activation, as compared with Eomes-lo SPMS patients (SI Appendix, Fig. S6B). Similar differences were observed between samples from patients with progressive versus stationary SPMS (SI Appendix, Fig. S6C).
Kinetic Analysis of Eomes+ Th Cells in Individual Patients.
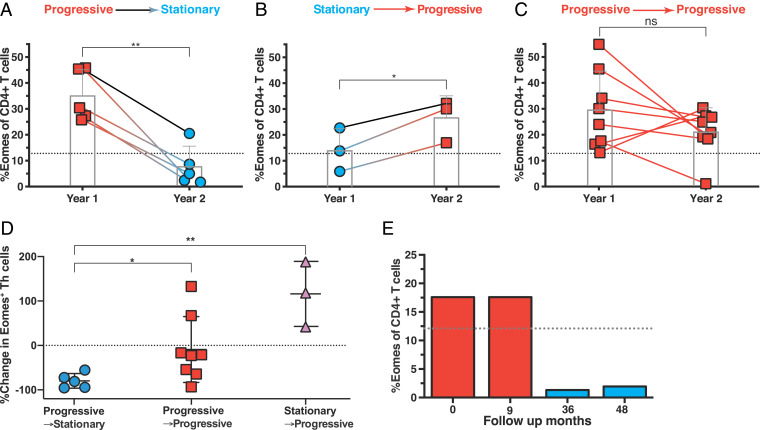
Results of serum cytokine/chemokine profiling, NFl measurement, and clinical assessment all indicated a link between an Eomes-hi phenotype and a progressive state of SPMS. We further evaluated if the Eomes+ Th level changed dynamically within individual patients in correspondence with disease status and reassessed 16 SPMS patients, in whom both the Eomes+ Th level and the annual Δ-EDSS during year one of this study and in year two were recorded. The patients were subdivided into three groups based on the changes in annual Δ-EDSS (Fig. 4 A–C), which either decreased (Progressive → Stationary; n = 5), increased (Stationary → Progressive; n = 3), or sustained progressive status (Progressive → Progressive; n = 8). For “Progressive → Stationary” patients, the clinical improvement was accompanied with a reduction of %Eomes+ CD4+ T cells in all cases (Fig. 4A), whereas an increase of %Eomes+ Th cells was observed in “Stationary → Progressive” patients (Fig. 4B). Although the changes observed in “Progressive → Progressive” patients who experienced further disease deterioration were diverse, seven out of eight remained Eomes-hi, and there was no significant difference in the Eomes level over time (Fig. 4C). Furthermore, measuring the change in Eomes+ Th cells between year one and year two revealed groups that differed significantly with each other (Fig. 4D). However, this retrospective analysis of annual disability changes is not fine grained to account for the exact period of inflammatory damage that leads to observable disease burden changes, and the Eomes+ Th cell level may change with rapid dynamics.
Fig. 4.
Changes in the Eomes proportion in individual patients. We retrospectively analyzed the data for the Eomes proportion (Eomes+ Th cells among CD4+ T cells) in 16 SPMS patients, from whom blood samples were obtained at two to four time points. (A) Eomes+ Th cell level for SPMS patients with the clinical status of progressive phase and Eomes-hi in year one which entered the stationary phase in year two (Progressive → Stationary, n = 5), possibly because they received active treatment with NTZ or corticosteroids. (B) Eomes+ Th cell level for SPMS patients in a clinically stationary phase on first sampling were found to have entered a progressive phase in year two (Stationary → Progressive, n = 8); Eomes level was assessed early in each annual period. (C) Eomes+ Th cell level for SPMS patients with the clinical status of progressive phase and Eomes-hi in year one that remained in progressive status (Progressive → Progressive, n = 8) (*P < 0.05, **P < 0.01, two-tailed, paired Student’s t test). (D) Changes in the Eomes proportion in Progressive → Stationary (A, n = 5), Progressive → Progressive (B, n = 8), and Stationary → Progressive (C, n = 3) groups (*P < 0.05, **P < 0.05, two-tailed, unpaired Student’s t test). (E) Changes in Eomes+ Th cells in a representative patient with SPMS. In this female patient with SPMS, first (0 mo) and second (9 mo) samples were obtained during the progressive phase, whereas the other samples (36 and 48 samples) were analyzed during a stationary phase (see also the text).
We show a representative case, in whom disease activity and Eomes levels had reduced in parallel after changing the therapy. The patient was diagnosed as RRMS at the age of 19. After several clinical relapses under IFN-β therapy, her neurological disabilities accumulated gradually along with progression of global parenchymal atrophy, consistent with SPMS. After the age of 39, Eomes status was evaluated twice during the first year, which showed that the patient had an Eomes-hi phenotype. However, on follow-up, progression of neurological disabilities had ceased, possibly owing to treatment with drugs, including corticosteroids. Coincident with the stationary status, the Eomes level was greatly reduced (Fig. 4E).
Increased Granzyme B Production by Th Cells in SPMS.
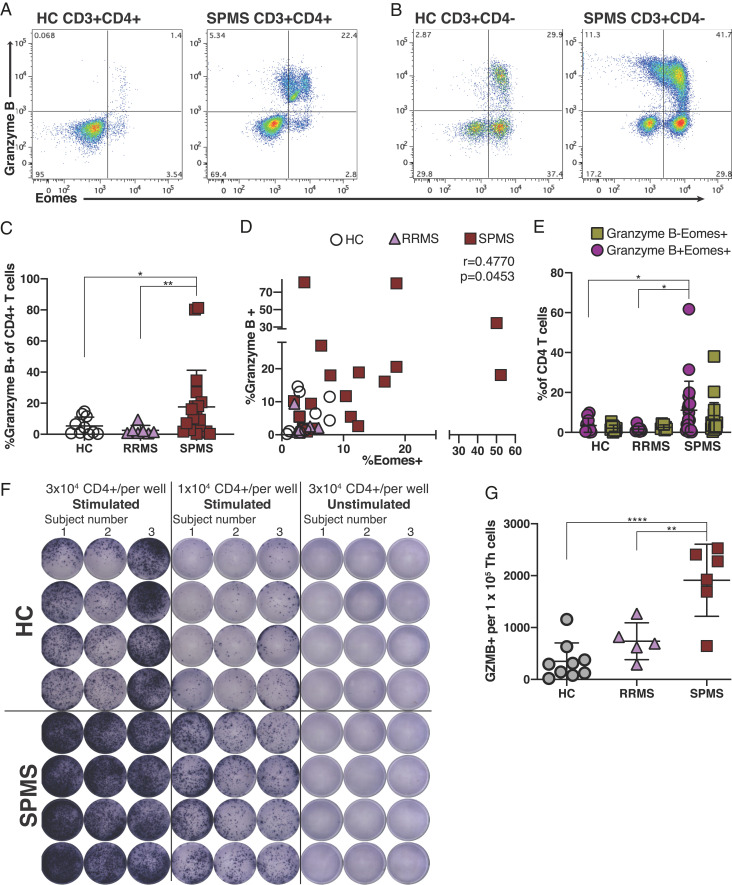
Reminiscent of cytotoxic CD8+ T cells, Eomes+ Th cells involved in chronic EAE are shown to release granules containing granzyme B upon stimulation (16). Inspired by a previous study on a rodent neurodegeneration model mediated by granzyme B (43), we considered a model by which cytotoxic Eomes+ Th cells could directly destroy neuronal tissue in a granzyme B-dependent manner. Flow cytometric analysis showed expression of granzyme B in a high proportion of Eomes+ Th cells in SPMS (Fig. 5 A and B), with around half of CD4− T cells also expressing granzyme B. Granzyme B-expressing Th cells were significantly increased in SPMS patients, as compared with HC or RRMS, in parallel with the increase of Eomes+ Th cells (Fig. 5C). Consistently, Eomes+ Th cell proportions correlated with that of granzyme B+ Th cells (Fig. 5D). Although granzyme B is not expressed by all Eomes+ Th cells in SPMS, increases in Th cells coexpressing Eomes and granzyme B was specifically associated with SPMS (Fig. 5E), hinting that this population might be the population most associated with pathogenicity.
Fig. 5.
Eomes+ Th cells produce granzyme B in SPMS patients. (A–E) Flow cytometric analysis. A representative flow cytometric intracellular staining for Eomes and granzyme B. PBMC samples were derived from HC and SPMS, and CD3+CD4+ Th cells (A) and CD3+CD4− T cells were analyzed (B). (C) Proportion of granzyme B+ cells among CD4+ Th cells in HC (n = 10), RRMS (n = 7), and SPMS (n = 18). (D) Correlation of %granzyme B+ and %Eomes+ cells among Th cells from patient sample groups in C (r2 = 0.4779, P = 0.0453, Spearman rank test). (E) Proportions (%) of Eomes+ granzyme B− and Eomes+ granzyme B+ cells among CD4+ T cells in HC (n = 10), RRMS (n = 7), and SPMS (n = 18) ELISpot assay data. (F) Untouched CD4+ Th cells were separated from PBMC from three HC (Top) and three SPMS subjects. The cells from each sample were seeded at the indicated cell numbers (HC top wells; SPMS bottom wells) and stimulated or left unstimulated for 72 h. (G) Granzyme B production was quantified in quadruplicate for number of spots and granzyme B-spot formation per 100,000 seeded CD4+ Th cells for each patient group was plotted (HC, n = 9; RRMS, n = 5; and SPMS, n = 6). Each data point is an average of four wells from an individual subject, and the mean ± SD is shown for each patient group. *P < 0.05, **P < 0.01, ****P < 0.0001.
In parallel, we tested the ability of peripheral blood CD4+ T cells to secrete granzyme B by enzyme-linked immune absorbent spot (ELISpot) assay; untouched Th populations were prepared from fresh whole blood before polyclonal stimulation and enumeration of the frequency of granzyme B producers. We observed robust GZMB production from circulating Th cells from SPMS patients, with fewer producers from HC Th cells (Fig. 5F). Counting Th-cell–secreting granzyme B revealed a significantly higher proportion of Th cells primed to make a granzyme B in SPMS versus HC or RRMS (Fig. 5G). Given that Eomes+ Th cells are the most important source of granzyme B among CD4+ T cells (Fig. 5A), these data indicate that Eomes+ Th cells could play a pathogenic role that depends on a granzyme B-mediated cytotoxic mechanism.
Eomes+ Th Cells Infiltrate CNS Tissue in SPMS.
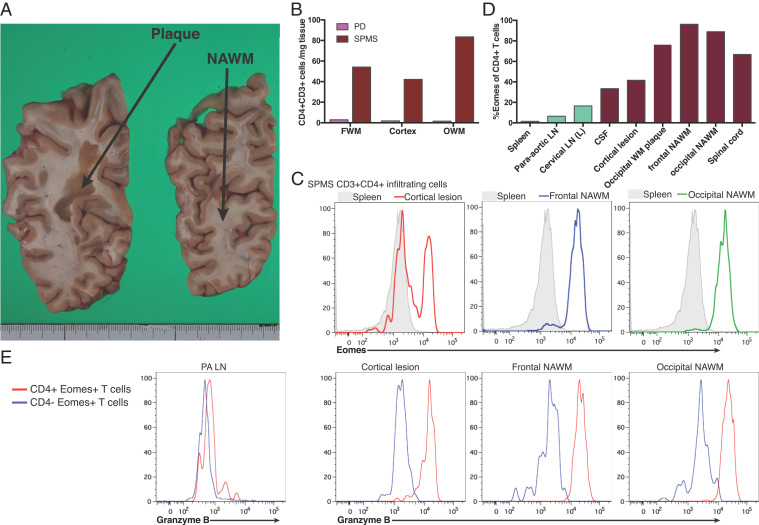
Although we have demonstrated increased Eomes+ Th cells in peripheral blood and CSF of SPMS patients [Fig. 1 and Raveney et al. (16)], the ability of Eomes+ Th cells to infiltrate into the CNS tissues as a requirement to generate pathogenic potential remained unknown. To answer this key question, CNS tissue samples from a patient with SPMS were examined shortly after death. Histopathological analysis revealed no active lesion with ongoing demyelination events in the CNS tissue, although numerous old plaques were observed (Fig. 6A). Although Th cells did not infiltrate control brain tissue (SI Appendix, Fig. S7A), infiltrating CD3+ T cells were identified in normal appearing white matter (NAWM) of the SPMS brain by immunocytochemistry (SI Appendix, Fig. S7B). These scattered T cells contained both CD4+ and CD8+ T cells (SI Appendix, Fig. S7 C and D). CD3+ T cells that were CD4 negative were only rarely observed in the lesion tissue around the vessels (SI Appendix, Fig. S7 E–G).
Fig. 6.
Analysis of Eomes+granzyme B+ CD4+ T cells in the CNS tissue samples. Postmortem samples of the CNS tissue from a 51 y-old female patient with SPMS and a 88-y-old male patient with PD associated with psychiatric manifestations were analyzed in detail. There were no infectious elements or tumor in these patients at autopsy. (A) An image of 8 mm sliced serial coronal sections including calcarine sulcus from right cerebrum of the SPMS patient. For examination, we obtained samples from the gelatinous large plaque in the parieto-occipital white matter (OWM) (left slice) and the adjacent NAWM in the occipital lobe (right slice). (B) Numbers of infiltrating CD3+CD4+ T cells were calculated and normalized per mg weight of each tissue sample for frontal white matter, cortex, or OWM from PD and SPMS. In the analysis of SPMS, white matter samples were derived from “normal appearing” tissue and presence of lesion tissue was confirmed in cortical samples. (C, D) Eomes expression in infiltrating CD3+CD4+ T cells from SPMS tissue samples. Not only CNS samples, but para-aortic lymph nodes (PA LN), cervical lymph nodes, and CSF samples were obtained from the same patient for flow cytometer analysis. Representative plots shown in C demonstrate that CD4+ T cells from cortical lesion contain Eomes+ cells, whereas those frontal and occipital NAWM (Frontal NAWM, and Occipital NAWM) are mostly Eomes positive, as compared with splenic CD3+CD4+ T cells (gray filled). (D) Proportion of Eomes-expressing cells among CD4+ T cells derived from each CNS sample and secondary lymphoid samples. (E) Granzyme B expression in CD4+Eomes+ T cells versus CD4−Eomes+ T cells from PA LN and CNS samples.
To more accurately assess cell numbers in tissues and T cell phenotypes, we used flow cytometry, a method frequently employed to analyze CNS-infiltrating T cells in murine EAE (16). CNS tissue samples were dissociated mechanically and enzymatically, and the resulting single cell suspensions were stained for surface and intracellular molecules. Infiltrating cells were enumerated and normalized per mg of tissue sample. Whereas very few CD4+ Th cells were observed in the control PD brain tissue, CNS tissue samples from the SPMS subject had significantly higher numbers of Th cells (Fig. 6B and SI Appendix, Fig. S7H). These cell numbers were at a comparable level per tissue weight to those observed for T cells infiltrating CNS tissue during chronic mouse EAE (16) (SI Appendix, Fig. S7I). Moreover, compared with Th cells from the secondary lymphoid tissue, a much greater proportion of CNS-infiltrating Th cells in the SPMS subject expressed high levels of Eomes (Fig. 6 C and D). In particular, Th cells isolated from frontal and occipital NAWM lesions were mostly Eomes positive. When CNS autopsy samples from two neuromyelitis optica (NMO) cases were examined, Eomes+ Th cells were absent, or present at much lower levels (SI Appendix, Fig. S7 J and K), although significant Th cell infiltration was observed. Strikingly, in addition to Eomes, infiltrating Th cells in the SPMS CNS tissue were also positive for granzyme B (Fig. 6E). These observations from the postmortem tissues strongly link the Eomes+ Th cells to the pathogenesis of SPMS.
Discussion
Transition from RRMS to SPMS is a critical event as the two disease forms differ substantially in clinical course and responsiveness to therapy. Despite this, key cellular and molecular elements in SPMS pathogenesis have not been sufficiently characterized; thus, SPMS lacks both useful biomarkers and a rational treatment strategy. In this study, we provide conclusive evidence that Eomes+ Th cells are involved in SPMS, offering the possibility of new biomarkers and treatments for SPMS. Eomes+ Th cells are significantly increased in the peripheral blood of SPMS compared with RRMS, and 81.3% of progressive phase SPMS samples and 22.3% stationary phase SPMS samples were classified as Eomes-hi status, based on a 13% cutoff from mathematical modeling (Fig. 1A). In contrast, an Eomes-hi phenotype was only rarely observed in RRMS (2/44; 5%), PPMS (1/25; 4.0%), or HC samples (1/47; 2%). A well-known biomarker for RRMS, NFl, was significantly increased in the sera of Eomes-hi as compared with Eomes-lo status of SPMS patients (Fig. 2F). However, as NFl is also increased in RRMS, NFl is not a useful biomarker for distinguishing SPMS from RRMS.
We have also demonstrated that serum cytokine/chemokine profiles in SPMS patients with Eomes-hi status differed from those that were Eomes-lo (SI Appendix, Fig. S6), potentially indicating differing immune mechanisms at play in these groups. Critically, subjects with a Eomes-hi phenotype could change into Eomes-lo concurrent with a decline in disease activity (Fig. 4). Thus, we postulate that the Eomes+ Th cell number can be regarded as a biomarker reflecting the inflammatory activity in SPMS. With regard to SPMS pathogenesis, it can also be argued that Eomes+ Th cells may play an active role in driving disease progression. In support of this hypothesis, we showed that circulating Eomes+ Th cells have the potential to produce granzyme B, a neurotoxic mediator. Moreover, we confirmed that Eomes+ Th cells and granzyme B were present in the CNS tissue from a patient with SPMS by flow cytometric analysis of autopsy brains (Fig. 6). Notably, similar small numbers of Eomes+ Th cells could be found in the brain parenchyma from a mouse chronic EAE model (SI Appendix, Fig. S7I). In the mouse brain, Eomes+ T cells play a critical role in chronic neuroinflammation (16); therefore, the presence of Eomes+ granzyme B+ T cells in SPMS autopsy samples has significant implications. CD4+ T cells were virtually absent from control PD samples (Fig. 6B); whereas autopsy samples from one of the two NMO patients contained lymphocytes expressing Eomes in restricted areas (SI Appendix, Fig. S7 J and K).
Recent research has highlighted the presence of CD8+ T cells, including tissue-resident memory (TRM) T cells, among the brain-infiltrating T cells in MS (44). Histopathologic examination of tissue sections has identified CD8+ subsets which tend to form clusters around lesion sites (45), and much further research into CNS-infiltrating T cells in MS has focused on these CD8+ T cells. However, SPMS is characterized by diffuse CNS damage and atrophy, and thus this standard technique may not be sensitive enough to discern scattered populations of Th cells. Interestingly, although cytotoxic-like Eomes+ Th cells share many features with cytotoxic CD8+ T cells, TRM cells have been reported to down-regulate Eomes (46, 47). Furthermore, a report indicates that brain-infiltrating CD8+ T cells lack cytotoxic markers in progressive MS (48); thus, any association between TRM cells and pathogenicity remains unclear. Flow cytometric studies of MS brain support our finding that a significant number of CD4+ Th cells also infiltrate CNS tissue in progressive MS (48–50), but these cells have not been extensively characterized nor their pathogenic potential appraised until now.
Using an ELISpot assay, we also showed granzyme B production by peripheral blood CD4+ T cells was enhanced in SPMS as compared with RRMS (Fig. 5 F and G). Significant results obtained by this relatively simple standard assay are striking, as many similar attempts to characterize the immunological characteristics of SPMS have been unsuccessful as single parameter approaches. Based on the clarity of the results, granzyme B is implicated as a key driver in the pathogenesis of SPMS. Although some granzyme B production was generated by Eomes-negative CD4+ T cells, Eomes+ Th cells are a major source of granzyme B in these patient Th-cell populations. At present, the relationship between the Eomes-positive and -negative populations producing granzyme B requires further study.
Evidence for the role of immune mechanisms in SPMS has accumulated in recent years. In particular, the efficacy of anti-α4 integrin antibody NTZ and sphingosine-1-phosphate receptor antagonist Siponimod in SPMS strongly supports the role of T cells in SPMS (13, 34, 51). Beneficial effects of IFN-β have also been suggested in a clinical trial (52–54), although it was limited to some SPMS cases who were at higher risk of progression (55, 56). Interestingly, treatment with NTZ or IFN-β was linked to low Eomes+ Th cells in our study (SI Appendix, Fig. S5), giving a tantalizing possibility that such treatments may be effective at targeting Eomes+ Th cells producing granzyme B. Despite the wide gamut of treatments approved, effectiveness of each treatment in individual cases of MS is unpredictable in general (57). Assuming that the immunopathogenesis of MS varies among individual patients and between stages or phases of disease, experts anticipate a future personalized medicine approach to MS management. The data presented in this study are very supportive of the value and feasibility of a personalized medicine approach for SPMS. We speculate that the measurement of Eomes+ Th cells, or related biomarkers, will not only provide basic information for the pathogenesis of SPMS but could also provide targets for future clinical studies and clinical trials for the development of new drugs.
It is important to note that study of SPMS is complicated by the diversity regarding clinical manifestations, disease duration, and past medical treatments. Moreover, limitations to this study include bias for patient populations (patients recruited from a single center in Japan) and brain infiltrating cell analysis conducted only in a single patient with SPMS and three control disease patients. Future studies should be carried out to confirm the relevance of our findings in the context of multicenter collaborative studies, which may consider a more fine-grained approach using wearable devices to examine disease progressive status.
Supplementary Material
Acknowledgments
We would like to thank C. Tomi, A. Takeo, and H. Yamaguchi for excellent technical assistance, Dr. A. Kimura for critical review, and express our appreciation for all our volunteer blood donors and hospital staff. Some serum samples were provided by the NCNP Biobank, a member of the National Center Biobank Network. B.J.E.R. is a recipient of fellowships from Japanese Multiple Sclerosis Society, the Kato Memorial Trust for Nambyo Research, and a Japan Society for the Promotion of Science KAKENHI Grant (No. 17K16135). D.T. received funding from the Intramural Research Grant (27-6) for Neurological and Psychiatric Disorders (NCNP). T.Y. received funding from the Practical Research Projects Grant for Rare/Intractable Disease (Japan Agency for Medical Research and Development, Grant No. 17ek0109097, 17ek0109155).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021818118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Pierson E., Simmons S. B., Castelli L., Goverman J. M., Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunol. Rev. 248, 205–215 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reich D. S., Lucchinetti C. F., Calabresi P. A., Multiple sclerosis. N. Engl. J. Med. 378, 169–180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashinskaya V. V., Kulakova O. G., Boyko A. N., Favorov A. V., Favorova O. O., A review of genome-wide association studies for multiple sclerosis: Classical and hypothesis-driven approaches. Hum. Genet. 134, 1143–1162 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Beecham A. H.et al.; International Multiple Sclerosis Genetics Consortium (IMSGC); Wellcome Trust Case Control Consortium 2 (WTCCC2); International IBD Genetics Consortium (IIBDGC) , Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 45, 1353–1360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin R., Sospedra M., Rosito M., Engelhardt B., Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur. J. Immunol. 46, 2078–2090 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Rovaris M., et al., Secondary progressive multiple sclerosis: Current knowledge and future challenges. Lancet Neurol. 5, 343–354 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Compston A., Coles A., Multiple sclerosis. Lancet 372, 1502–1517 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Katz Sand I., Krieger S., Farrell C., Miller A. E., Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult. Scler. 20, 1654–1657 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Leray E., et al., Evidence for a two-stage disability progression in multiple sclerosis. Brain 133, 1900–1913 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trapp B. D., Nave K. A., Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 31, 247–269 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Lorscheider J.et al.; MSBase Study Group , Defining secondary progressive multiple sclerosis. Brain 139, 2395–2405 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Lublin F. D., et al., Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 83, 278–286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kappos L.et al.; EXPAND Clinical Investigators , Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 391, 1263–1273 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Chaudhry B. Z., Cohen J. A., Conway D. S., Sphingosine 1-phosphate receptor modulators for the treatment of multiple sclerosis. Neurotherapeutics 14, 859–873 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadowaki A., Saga R., Lin Y., Sato W., Yamamura T., Gut microbiota-dependent CCR9+CD4+ T cells are altered in secondary progressive multiple sclerosis. Brain 142, 916–931 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raveney B. J., et al., Eomesodermin-expressing T-helper cells are essential for chronic neuroinflammation. Nat. Commun. 6, 8437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raveney B. J., Oki S., Yamamura T., Nuclear receptor NR4A2 orchestrates Th17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS One 8, e56595 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doi Y., et al., Orphan nuclear receptor NR4A2 expressed in T cells from multiple sclerosis mediates production of inflammatory cytokines. Proc. Natl. Acad. Sci. U.S.A. 105, 8381–8386 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Intlekofer A. M., et al., Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Pearce E. L., et al., Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302, 1041–1043 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Patsopoulos N. A.et al.; Bayer Pharma MS Genetics Working Group; Steering Committees of Studies Evaluating IFNβ-1b and a CCR1-Antagonist; ANZgene Consortium; GeneMSA; International Multiple Sclerosis Genetics Consortium , Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Ann. Neurol. 70, 897–912 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S., et al., Variant of EOMES associated with increasing risk in Chinese patients with relapsing-remitting multiple sclerosis. Chin. Med. J. (Engl.) 131, 643–647 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chemin K., et al., EOMES-positive CD4+ T cells are increased in PTPN22 (1858T) risk allele carriers. Eur. J. Immunol. 48, 655–669 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Peeters L. M., et al., Cytotoxic CD4+ T cells drive multiple sclerosis progression. Front. Immunol. 8, 1160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafflick D., et al., Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat. Commun. 11, 247 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C., et al., Extrapituitary prolactin promotes generation of Eomes-positive helper T cells mediating neuroinflammation. Proc. Natl. Acad. Sci. U.S.A. 116, 21131–21139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeuchi A., Saito T., CD4 CTL, a cytotoxic subset of CD4+ T cells, their differentiation and function. Front. Immunol. 8, 194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Confavreux C., Vukusic S., Moreau T., Adeleine P., Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med. 343, 1430–1438 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Lublin F. D., Reingold S. C.; National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis , Defining the clinical course of multiple sclerosis: Results of an international survey. Neurology 46, 907–911 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Thompson A. J., et al., Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 17, 162–173 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Tremlett H., Yousefi M., Devonshire V., Rieckmann P., Zhao Y.; UBC Neurologists , Impact of multiple sclerosis relapses on progression diminishes with time. Neurology 73, 1616–1623 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtzke J. F., Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 33, 1444–1452 (1983). [DOI] [PubMed] [Google Scholar]
- 33.Jacques F. H., Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 84, 963 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Kapoor R.et al.; ASCEND investigators , Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): A phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 17, 405–415 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Roosendaal S. D., et al., Grey matter volume in a large cohort of MS patients: Relation to MRI parameters and disability. Mult. Scler. 17, 1098–1106 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Lycke J. N., Karlsson J. E., Andersen O., Rosengren L. E., Neurofilament protein in cerebrospinal fluid: A potential marker of activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 64, 402–404 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Disanto G.et al.; Swiss Multiple Sclerosis Cohort Study Group , Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 81, 857–870 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siller N., et al., Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult. Scler. 25, 678–686 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Grund B., Sabin C., Analysis of biomarker data: Logs, odds ratios, and receiver operating characteristic curves. Curr. Opin. HIV AIDS 5, 473–479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budczies J., et al., Cutoff finder: A comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One 7, e51862 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wherry E. J., Kurachi M., Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee K. A., et al., Characterization of age-associated exhausted CD8+ T cells defined by increased expression of Tim-3 and PD-1. Aging Cell 15, 291–300 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang T., et al., Granzyme B-induced neurotoxicity is mediated via activation of PAR-1 receptor and Kv1.3 channel. PLoS One 7, e43950 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machado-Santos J., et al., The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 141, 2066–2082 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lassmann H., Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front. Immunol. 9, 3116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackay L. K., et al., T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43, 1101–1111 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Wakim L. M., et al., The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol. 189, 3462–3471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fransen N. L., et al., Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 143, 1714–1730 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Smolders J., et al., Tissue-resident memory T cells populate the human brain. Nat. Commun. 9, 4593 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Nierop G. P., et al., Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol. 134, 383–401 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller D. H.et al.; International Natalizumab Multiple Sclerosis Trial Group , A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348, 15–23 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Romme Christensen J., et al., Natalizumab in progressive MS: Results of an open-label, phase 2A, proof-of-concept trial. Neurology 82, 1499–1507 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Cadavid D., Jurgensen S., Lee S., Impact of natalizumab on ambulatory improvement in secondary progressive and disabled relapsing-remitting multiple sclerosis. PLoS One 8, e53297 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giovannoni G., Natalizumab improves walking and upper-limb disability compared with placebo in patients with secondary progressive multiple sclerosis: An integrated, post hoc area under the outcome-time curve analysis from the ASCEND trial. ECTRIMS Online Library, 202484 (2017). [Google Scholar]
- 55.Kappos L.et al.; European (EU-SPMS) Interferon beta-1b in Secondary Progressive Multiple Sclerosis Trial Steering Committee and Independent Advisory Board; North American (NA-SPMS) Interferon beta-1b in Secondary Progressive Multiple Sclerosis Trial Steering Committee and Independent Advisory Board , Interferon beta-1b in secondary progressive MS: A combined analysis of the two trials. Neurology 63, 1779–1787 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MS (SPECTRIMS) Study Group , Randomized controlled trial of interferon- beta-1a in secondary progressive MS: Clinical results. Neurology 56, 1496–1504 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Kalincik T.et al.; MSBase Study Group , Towards personalized therapy for multiple sclerosis: Prediction of individual treatment response. Brain 140, 2426–2443 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.