Significance
Nav1.1 is a major Nav subtype in the brain. Up to 900 nonsense and missense mutations in SCN1A, the coding gene for Nav1.1, have been identified in patients with epilepsy syndromes. Here, we report the cryo-EM structure of the human Nav1.1–β4 complex. Comparative structural analysis of hundreds of missense disease mutations in Nav1.1 and Nav1.5, whose structure is reported in the accompanying paper, reveals 70 loci that are common in these two channels. Several clusters, defined as the mutational hotspots, are identified and generally classified as the structural mutations and functional mutations. Our comparative structural analyses establish a framework for structure–function relationship dissection of Nav disease variants and will facilitate development of precision medicine for various sodium channelopathies.
Keywords: Nav1.1, epileptic seizure, Dravet syndrome, fast inactivation, cryo-EM structure
Abstract
Among the nine subtypes of human voltage-gated sodium (Nav) channels, the brain and cardiac isoforms, Nav1.1 and Nav1.5, each carry more than 400 missense mutations respectively associated with epilepsy and cardiac disorders. High-resolution structures are required for structure–function relationship dissection of the disease variants. We report the cryo-EM structures of the full-length human Nav1.1–β4 complex at 3.3 Å resolution here and the Nav1.5-E1784K variant in the accompanying paper. Up to 341 and 261 disease-related missense mutations in Nav1.1 and Nav1.5, respectively, are resolved. Comparative structural analysis reveals several clusters of disease mutations that are common to both Nav1.1 and Nav1.5. Among these, the majority of mutations on the extracellular loops above the pore domain and the supporting segments for the selectivity filter may impair structural integrity, while those on the pore domain and the voltage-sensing domains mostly interfere with electromechanical coupling and fast inactivation. Our systematic structural delineation of these mutations provides important insight into their pathogenic mechanism, which will facilitate the development of precise therapeutic interventions against various sodium channelopathies.
The nine subtypes of human voltage-gated sodium (Nav) channels are responsible for the initiation and transmission of electrical impulses in different tissues: Nav1.1 to Nav1.3 and Nav1.6 mainly function in the central nervous system, Nav1.7 to Nav1.9 are mostly distributed in the peripheral nervous system, Nav1.4 is specialized in skeletal muscle, and Nav1.5 is the primary cardiac isoform (1–4). Abnormalities of these channels, hinging on their tissue specificity, are associated with a broad spectrum of channelopathies. To date, more than 1,000 disease mutations have been identified in the primary sequence of Nav channels, among which Nav1.1 and Nav1.5 each host more than 400 missense mutations (5–8).
Nav1.1 is encoded by SCN1A, which may have the largest number of epilepsy-related mutations. Up to 900 SCN1A mutations, more than half of which result in truncations (9), have been identified in epilepsy syndromes with different severities. Nonsense and hundreds of missense mutations of SCN1A are found in 70 to 80% of patients with Dravet syndrome, which is also known as the severe myoclonic epilepsy of infancy (10–13) (SI Appendix, Table S1). Several dozen missense mutations are associated with generalized epilepsy with febrile seizures plus and intractable childhood epilepsy with generalized tonic-clonic seizures (10) (SI Appendix, Table S2). Although most of the Nav1.1 disease mutations lead to loss of function to different degrees, some represent gain of function. In most cases, the pathogenic mechanism remains elusive.
A brief summary of Nav1.5 pathophysiology is presented in the companion paper (14). Mechanistic understanding of the sodium channelopathies entails high-resolution structures of human Nav channels. In the past 4 y, we have reported the cryoelectron microscopy (cryo-EM) structures, at resolutions ranging between 2.6 and 4.0 Å, of Nav channels from insect (NavPaS), electric eel (EeNav1.4), and finally human, including Nav1.2, Nav1.4, Nav1.5, and Nav1.7, in the presence of multiple modulators, such as β1 and β2 subunits, peptide toxins, and small-molecule toxins tetrodotoxin and saxitoxin (15–21). Structures of a truncated rat Nav1.5 were recently reported (22). All structurally resolved eukaryotic Nav channels except for NavPaS exhibit similar conformations of potentially inactivated state.
Notwithstanding these advances, high-resolution structures of human Nav1.1 and Nav1.5 wild-type and representative disease variants are necessary to provide accurate templates to directly map the disease mutations and to facilitate drug discovery. Furthermore, as these two channels harbor 80% of all identified mutations related to sodium channelopathies, a comparative analysis of their structures may reveal potential mutational hotspots, offering invaluable insight into the function and disease mechanism of Nav channels.
Here we present the cryo-EM structure of human Nav1.1 associated with a modulating auxiliary subunit β4. In the accompanying paper, we report the structure of human Nav1.5 that carries a common disease variant E1784K. Comparative structural analyses have revealed several clusters of disease mutations that are common to both Nav1.1 and Nav1.5.
Results
Cryo-EM Structure of the Human Nav1.1–β4 Complex.
Nav1.1 was reported to be subject to regulation by all four β subunits, β1 to β4 (23, 24). Epilepsy-associated mutations have also been found on SCN1B, the gene encoding β1 (25). Considering that we have already obtained the structures of other human Nav channels in complex with β1, β2, or both (16, 18–21), we attempted to resolve the structure of human Nav1.1 in the presence of the remaining two β isoforms. Electrophysiological characterizations showed reduced Nav1.1 conductance in the presence of either β3 or β4, while the activation and inactivation properties remained nearly unchanged (SI Appendix, Fig. S1 and Table S3). Protein expression, purification, and cryo-EM analysis was carried out following our previously reported protocol with minor modifications (18) (SI Appendix, Fig. S2). Briefly, we transiently coexpressed full-length human Nav1.1 with both β3 and β4 subunits in HEK293F cells. A three-dimensional (3D) reconstruction was obtained at an overall resolution of 3.3 Å out of 133,127 selected particles using cryoSPARC (26) (Fig. 1A and SI Appendix, Figs. S2 and S3 and Table S4).
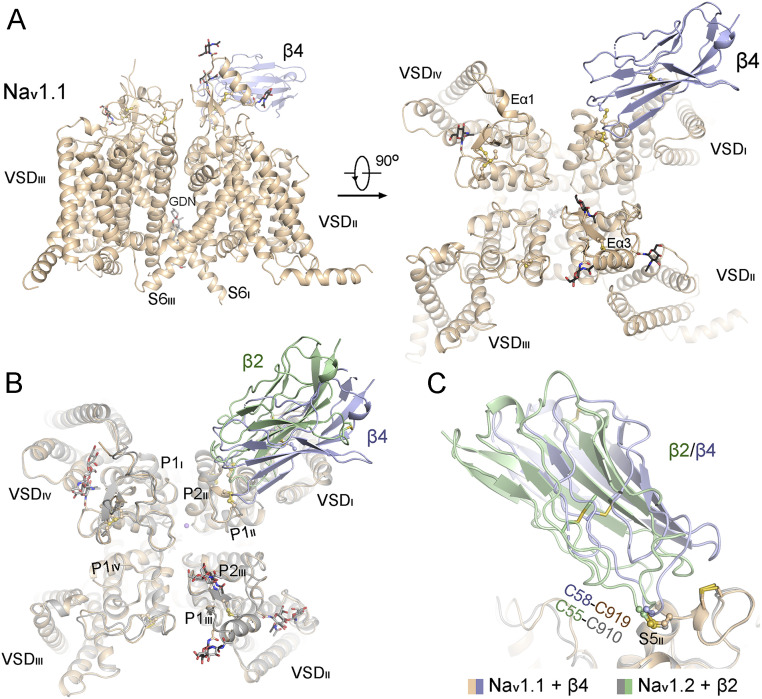
Fig. 1.
Structural determination of full-length human Nav1.1 in the presence of the β4 subunit. (A) Overall structure of the human Nav1.1–β4 complex. A side and an extracellular view are shown. The sugar moieties and an intracellular gate-penetrating GDN molecule are shown as sticks. The moderate resolution of the density for β4 does not support accurate model building. The crystal structure of β4 (PDB code 4MZ2) is docked into the density as a rigid body. (B) The structure of Nav1.1 is nearly identical to that of Nav1.2 (PDB code 6J8E). The complex structures of Nav1.1–β4 and Nav1.2–β2 can be superimposed with an rmsd of 0.68 Å over 1,040 Cα atoms. (C) Both β2 and β4 interact with the α subunit of Nav channels through a disulfide bond with a conserved Cys residue, Cys919 on Nav1.1 and Cys910 on Nav1.2. All structure figures were prepared in PyMol (59).
The map supported assignment of 1,138 side chains of Nav1.1, with four sugar moieties on the extracellular loops (ECLs) and one glyco-diosgenin (GDN), which was the detergent used for protein purification, penetrating the intracellular gate as observed in the structures of Nav1.2 and Nav1.4 (16, 18, 19) (SI Appendix, Table S4). However, there was no visible density corresponding to β3, and the β4 subunit was resolved as a blob (SI Appendix, Fig. S2C), only allowing for rigid-body docking of the crystal structure (Protein Data Bank [PDB] code 4MZ2).
The overall structure of the Nav1.1–β4 complex is nearly identical to that of the Nav1.2–β2 complex (PDB code 6J8E), having an rmsd of 0.68 Å over 1,040 aligned Cα atoms in the α subunits, with the four voltage-sensing domains (VSDs) up and similar pore radii (Fig. 1B and SI Appendix, Fig. S4). β2 and β4 share sequence similarity of 52%. In the docked model, Cys58 of β4 is placed adjacent to Cys919 in Nav1.1, reminiscent of the disulfide bond between Nav1.2-Cys910 and β2-Cys55 (Fig. 1C). The low resolutions of β2 and β4 in the respective electron microscope (EM) maps suggest the lack of specific interactions between these two β subunits and the α subunits other than the covalent linkage.
Structural Overview of Disease Mutations in Nav1.1 and Nav1.5.
Next, we set out to examine the disease-associated missense mutations on the structures of Nav1.1 and Nav1.5 (14) (Fig. 2 and SI Appendix, Tables S1 and S2). Although ∼420 disease-related missense mutations are found in each channel, their domain distributions are different. In Nav1.1, up to 341 mutations can be mapped to the extracellular and transmembrane regions, whereas, in Nav1.5, only 261 mutations are resolved, with the remaining 171 on the invisible intracellular segments (Fig. 2 and SI Appendix, Tables S1 and S2).
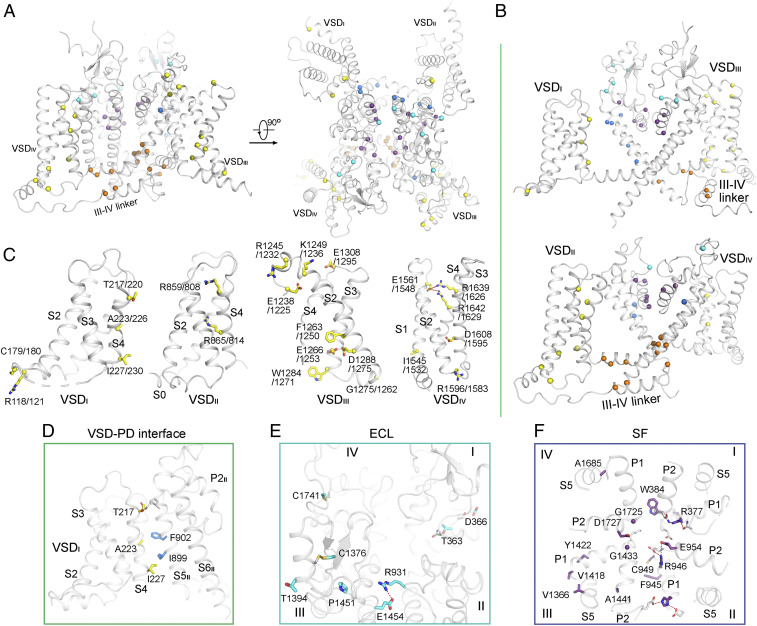
Fig. 2.
Structural mapping of disease-associated missense mutations in Nav1.1 and Nav1.5. The missense mutations in Nav1.1 (A) and Nav1.5 (B) that are associated with sodium channelopathies are mapped to the structures presented here and in the companion paper (14). The diagonal repeats in each channel are shown in the top and bottom rows. The Cα atoms of these residues are shown as spheres and colored by functional entities indicated below the structures. In this context, the ECLs refer to those above the PD, the SF includes the P1 and P2 helices and the parts of the S5 segments that directly interact with the pore helices, the PD extends to the S4-S5 segments, and the FI segments refer to the Ile/Phe/Met (IFM) motif-containing III-IV linker and surrounding residues.
At first glance, these mutations span almost the entire resolved structures. To dissect the hundreds of residues, we group them based on the following structural and functional entities: the ECLs, the selectivity filter (SF) and supporting segments, the pore domain (PD), the VSDs, and the segments related to fast inactivation (FI; Fig. 2).
It is not surprising that the four VSDs harbor the largest number of mutations in both channels, 101 on Nav1.1 and 83 on Nav1.5. However, it is unexpected that the ECL is also enriched of mutations: 57 and 45 on Nav1.1 and Nav1.5, respectively. Of particular note, many of these affected residues are not involved in posttranslational modifications or interaction with the β subunits because they are not exposed on the surface (Fig. 2). Below the ECL, Nav1.1 has 55 mutations mapped to the P1-SF-P2 segments, while Nav1.5 has only 31. In addition, 17 and 4 disease-related residues on the extracellular halves of the S5 and S6 segments directly interact with P1 and P2, also contributing to the stabilization of the SF, making the SF-related mutations increase to 71 in Nav1.1 and 35 in Nav1.5 (Fig. 2).
Common Disease Mutations in Nav1.1 and Nav1.5.
It is impractical to examine each and every resolved mutation locus in one manuscript. We therefore focus on the common loci on Nav1.1 and Nav1.5 that may help reveal potential hotspots of mutations in sodium channelopathies. A total of 66 common loci in both channels are identified to be associated with diseases (Fig. 3 A and B and SI Appendix, Table S5). Note that multiple mutations that affect the same residue will be counted as one locus, while the number of related disease mutations is even larger. Among these common sites, 8 are on the ECL, 14 on the SF region, 9 on PD, 22 on VSDs, and 13 on the FI (Fig. 3B and SI Appendix, Table S5).
Fig. 3.
Structural mapping of the disease-related missense mutations that are common to Nav1.1 and Nav1.5. (A) Mapping of the disease-associated missense mutations common to Nav1.1 and Nav1.5 on the structure of Nav1.1. (B) The diagonal repeats in Nav1.1 are shown in the top and bottom rows. (C) Distribution of the common disease mutations on the VSDs. The residue numbers of Nav1.1 and Nav1.5 are labeled before and after the slash, respectively. The Cα atoms and side chains of the related residues are shown as spheres and sticks, respectively, and colored by structural entities defined in Fig. 2. (D) A cluster of common disease-related residues mapped to the interface between VSDI and S5II. (E and F) The majority of common disease-related residues in ECL (E) and SF (F) may be involved in structural integrity. In E, a tilted extracellular view is shown. In F, an extracellular view of the SF and the supporting segments is shown. The common disease residues are shown as sticks and labeled conforming to their sequence number in Nav1.1. The polar interactions are indicated by the red dashed line. The color scheme in this figure is the same as defined in Fig. 2.
In the following, we will focus on several representative clusters of common mutations for structure–function relationship analysis. To simplify illustration, residue number conforms to the sequence of Nav1.1 if not otherwise indicated.
VSDs are, in general, the most extensively characterized functional entities in voltage-gated ion channels. Alteration of VSD residues, particularly the gating charge (GC) residues and those that facilitate the voltage-dependent motion of GCs, mostly leads to shift of activation or/and inactivation curves. Distribution of disease mutation in these four VSDs are asymmetric: VSDIII harbors the largest number of common disease mutations that involve the charge transfer center residues (27) and a number of polar residues that coordinate the GC residues; common disease mutations of GC residues are also observed in VSDII and VSDIV, but none of the common mutations on VSDI involves GC or GC-coordinating residues (Fig. 3C). Interestingly, our comparative structural analysis reveals a mutational hotspot that is mapped to the interface between VSDI and S5II of the PD (Fig. 3D).
Thr217, Ala223, and Ile227 on VSDI are aligned on the PD-facing side of S4I, with the latter two interacting with Phe902 and Ile899 on S5II through van der Waals contacts (Fig. 3D). This arrangement immediately suggests their participation in the electromechanical coupling, which is corroborated by reported functional characterizations. F902C of Nav1.1, a mutation found in Dravet syndrome, completely abolished conduction, likely owing to loss of coupling with VSDI (28). Interestingly, mutations of the three corresponding VSDI residues in Nav1.5, T220I, A226V, and I230T all reduced the current by half (29–31), suggesting similar functional roles of these residues in coupling to the PD. Supporting the significance of this interface, a disease-related mutation in the homotetrameric Kv1.2 (UniProt ID P16389), L298F, is also mapped on the interface of VSD and PD (32).
Among the eight ECL common mutation sites, six are engaged in intradomain packing. Thr363 and Asp366 on repeat I participate in the local interaction network with surrounding polar residues. Arg931 on repeat II and Glu1454 on repeat III form a salt bridge. Cys1376 and Cys1761 each form a disulfide bond with their respective neighboring Cys residues in repeats III and IV (Fig. 3E). Their structural roles suggest that the pathogenic mechanism of many ECL mutations may be related to proper folding and trafficking of the channels. Supporting this notion, two Brugada syndrome-associated mutations, Nav1.5-D356N and R878C (equivalent of Nav1.1-Asp366 and Arg931, respectively), and a familial sick sinus syndrome mutation, Nav1.5-R878H, all abolished Na+ current (29, 33, 34).
Replacement of Nav1.1-Thr363 by Pro/Arg is related to Dravet syndrome, and the corresponding Nav1.5-T353I is found in Brugada syndrome. Nav1.5-T353I resulted in 50% reduction of the Na+ current and left shift of the inactivation curve (35). The L5I loop, where Thr363/353 is localized, is adjacent to the S1-S2 loop of VSDIV. Replacement of Thr with the hydrophobic Ile/Pro or a bulkier basic residue, Arg, may disrupt the local conformation, hence changing the electrophysiological properties of the channel indirectly through VSDIV, whose motion is critical for inactivation.
The SF represents an important hotspot for disease mutations (Fig. 3F). Glu954 on P2II is part of the “DEE” Na+ coordination site in the SF vestibule (17). Replacing this acidic residue by Lys, as found in Dravet syndrome, directly disrupts cation attraction. Asp1727 on P2IV may also help attract cation to the SF entrance. D1714G, a Brugada syndrome–causing mutation in Nav1.5, reduced Na+ current by 80% (36). Apart from these two loci, all other SF mutations appear to impair structural stability of this functional entity (Fig. 3F). Some structure-disrupting mutations in this region of Nav1.5 may be lethal, accounting for the smaller number of SF mutations in Nav1.5 than Nav1.1 (Fig. 2 A and B).
A Mutational Hotspot Related to FI.
Finally, the segments involved in FI represents a major hotspot for disease mutations that constitute two clusters, one concerning the IFM motif and its receptor site and the other mapped to the interface between the III-IV linker and the S4-S5IV segment (Fig. 4A). These distributions are consistent with the established knowledge that depolarization of VSDIV initiates FI and the IFM motif is the critical executor for FI (37–39). As the structures of Nav1.1 and Nav1.5 likely represent an inactivated state, we reasoned that mutations that destabilize the present conformation may disfavor inactivation, corresponding to a right shift of the steady-state inactivation curve, whereas substitutions that favor the current conformational state lead to a left shift of the inactivation curve.
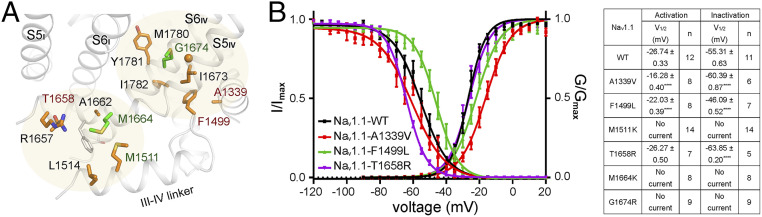
Fig. 4.
Structure–function analysis of FI-related common disease mutations. (A) Two clusters of common disease mutations related to FI. The disease mutations whose electrophysiological characterizations are presented in B are labeled brown. The mutants that had no detectable Na+ current are labeled dark green. Met1664 and Met1780, whose corresponding disease mutations are identified in at least three human Nav channels, are colored green. (B) Electrophysiological characterization of Nav1.1 variants containing mutations at the FI region. (Left) Voltage-dependent activation and inactivation curves. G/Gmax and I/Imax represent normalized conductance and ion current. (Right) A brief summary of the electrophysiological parameters. All data points are presented as mean ± SEM, and n values indicate the number of independent cells. Further details are provided in Materials and Methods and SI Appendix, Table S3.
Replacement of Phe1499 in Nav1.1, the most critical residue in the IFM motif, by Leu, may compromise the interaction between the IFM motif and the hydrophobic receptor cavity. Indeed, it was reported that Nav1.1-F1499L and Nav1.5-F1486L both resulted in evident right shift of the inactivation curve without major impact on activation (40, 41). The same results were obtained in our electrophysiological characterizations (Fig. 4B and SI Appendix, Table S3). Ala1339 on the S4-S5III is a major constituent of the IFM receptor site (Fig. 4A). A1339V, a relative minor alteration that may increase the affinity for IFM, caused right shift of the activation curve and slight left shift of the inactivation curve. The right shift of activation may be due to the altered coupling between S4-S5III and S6IV.
Thr1658 is positioned on the S4-S5IV, next to the two basic residues, Lys1654 and Arg1657, which represent extended GCs for S4IV. Its mutation to Arg may lower the depolarization threshold for the outward motion of S4IV, hence favoring inactivation. Indeed, the inactivation curve of Nav1.1-T1658R shifted toward the negative potential, while the activation curve remained unchanged (Fig. 4 A and B). Among our tested mutations, M1511K, M1664K, and G1674R all led to undetectable Na+ current, preventing accurate mechanistic dissection (Fig. 4 A and B).
Discussion
Nav1.1 and Nav1.5 carry the majority of disease-causing mutations among all Nav channels. Structure-based functional dissection of hundreds of mutations may shed light on the understanding of the pathogenic mechanism. Our analyses suggest that disease-associated mutations can be, in general, classified into structural mutations and functional mutations. The former affect protein folding, trafficking, and interaction with cofactors, while the latter change electrophysiological properties, including conductance and activation/inactivation kinetics. Two distinct conformational states have been obtained for eukaryotic Nav channels, NavPaS and others, affording the opportunity to examine the mobility of different segments (Movie S1).
The disease-related residues that remain relatively stable between different conformations are likely to play a structural role, while those that undergo displacement may mainly be involved in electromechanical coupling for activation and inactivation. Our comparative structural analyses show that most of the common mutations mapped to the ECL and SF may affect the structural stability by disrupting local interaction network, while many of those in VSDs, PD, and FI are prone to functional variations.
It is noted that the general distribution pattern of disease mutations may be related to the unique physiological functions of the mutation-carrying channels. For instance, the larger number of Nav1.5 mutations on the intracellular segments is consistent with the critical modulation of Nav1.5 function by multiple cytosolic factors such as CaM and ankyrin G (42–46). Nav1.1 has more structural mutations than Nav1.5, particularly at the ECL and SF region. It is possible that similar mutations in Nav1.5, which is the primary cardiac form, may lead to embryonic death and hence not counted as disease mutations.
A comprehensive understanding of the disease mutations requires investigation at different levels with multiple approaches. Our structure–function analysis presented here serves as a starting point toward a more precise and more systematic dissection of the disease mutations irrespective of the Nav subtypes, which will facilitate development of precise therapeutic interventions against sodium channelopathies.
Materials and Methods
Transient Expression of Human Nav1.1-β3-β4.
The cDNAs for human Nav1.1 (UniProt P35498), β3 (UniProt Q9NY72), and β4 (UniProt Q8IWT1) were codon-optimized and synthesized by a commercial source (BGI). They were separately cloned into the pCAG vector (47) with twin Strep-tag and FLAG tag in tandem at the amino terminus of Nav1.1 and no affinity tag for β3 and β4. HEK293F cells (Invitrogen) were cultured in SMM 293T-I medium (Sino Biological) under 5% CO2 in a Multitron-Pro shaker (Infors; 130 rpm) at 37 °C and transfected with plasmids when the cell density reached 1.5 × 106 cells per milliliter. Approximately 2.5 mg plasmids (1.5 mg for Nav1.1 plus 0.5 mg for β3 and β4 each) were preincubated with 4 mg 25-kDa linear polyethylenimines (Polysciences) in 25 mL fresh medium for 15 to 30 min and added into 1 L cell culture. Transfected cells were cultured for 48 h before harvesting.
Protein Purification.
The protocol for protein purification was nearly identical to that for the human Nav1.4–β1 complex (18). Cells (28 L) cotransfected with Nav1.1–β3-β4 were harvested by centrifugation at 800 × g and resuspended in lysis buffer containing 25 mM Tris⋅HCl (pH 7.5) and 150 mM NaCl. The suspension was supplemented with protease inhibitor mixture containing 2 mM phenylmethylsulfonyl fluoride, aprotinin (3.9 μg/mL), pepstatin (2.1 μg/mL), and leupeptin (15 μg/mL) and sonicated to homogenize the agglomerated cells. After incubation at 4 °C for 3 h with 1% (wt/vol) n-dodecyl-β-D-maltopyranoside (Anatrace) and 0.1% (wt/vol) cholesteryl hemisuccinate Tris salt (Anatrace), the cell lysate was ultracentrifuged at 20,000 × g for 45 min, and the supernatant was applied to anti-Flag M2 affinity gel (Sigma) by gravity at 4 °C. Then, W buffer containing 25 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 0.06% GDN (Anatrace), and protease inhibitor mixture was used in the following procedures. After rinsing the resin four times, the target proteins were eluted with W buffer plus 200 μg/mL FLAG peptide (Sigma). The eluent was then applied to Strep-Tactin Sepharose (IBA), and the purification protocol was similar to the previous steps except the elution buffer, which was W buffer plus 2.5 mM D-desthiobiotin (IBA). The eluent was added with phenytoin sodium (Selleck) to a final concentration of ∼200 μM, protease inhibitor mixture, and additional GDN to reach 0.12% and incubated at 4 °C for 2 h. Then, it was concentrated using a 100-kDa–cutoff Centricon (Millipore) and further purified by gel filtration with a Superose-6 column (GE Healthcare) in W buffer plus 200 μM phenytoin sodium. The presence of target proteins was confirmed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and mass spectrometry. The peak fractions of the gel filtration were pooled and the proteins were concentrated to ∼1 mg/mL for cryo-EM analysis.
Whole-Cell Electrophysiology.
HEK293T cells (Invitrogen) were cultured in Dulbecco’s modified Eagle medium (BI) supplemented with 4.5 mg/mL glucose and 10% fetal bovine serum (BI). Cells for subsequent patch-clamp recordings were plated onto glass coverslips and were transiently cotransfected using Lipofectamine 2000 (Invitrogen) with the expression plasmids and an enhanced green fluorescent protein-encoding plasmid. Cells with green fluorescence were selected for patch-clamp recording at 18 to 36 h after transfection. All experiments were performed at room temperature. No further authentication was performed for the commercially available cell line. Mycoplasma contamination was not tested.
The whole-cell Na+ currents were recorded using an EPC10-USB amplifier with Patchmaster software v2*90.2 (HEKA Elektronic), filtered at 3 kHz (low-pass Bessel filter), and sampled at 50 kHz. The borosilicate pipettes (Sutter Instrument) had a resistance of 2 to 4 MΩ, and the electrodes were filled with the internal solution composed of (in millimoles) 105 CsF, 40 CsCl, 10 NaCl, 10 EGTA, 10 Hepes, pH 7.4, with CsOH. The bath solutions contained (in millimoles): 140 NaCl, 4 KCl, 10 Hepes, 10 D-glucose, 1 MgCl2, 1.5 CaCl2, pH 7.4, with NaOH. Data were analyzed using Origin (OriginLab) and GraphPad Prism (GraphPad Software).
The voltage dependence of ion current (I-V) was analyzed using a protocol consisting of steps from a holding potential of −120 mV for 200 ms to voltages ranging from −90 to +80 mV for 50 ms in 5-mV increments. The linear component of leaky currents and capacitive transients were subtracted using the P/4 procedure. In the activation and conductance density calculation, we used the equation G = I/(V− Vr), where Vr (the reversal potential) represents the voltage at which the current is zero. For the activation curves, conductance (G) was normalized and plotted against the voltage from −90 mV to +20 mV. To obtain the conductance density curves, G was divided by the capacitance (C) and plotted against the voltage from −90 mV to +20 mV. For voltage dependence of inactivation, cells were clamped at a holding potential of −90 mV and were applied to step prepulses from −120 mV to +20 mV for 1,000 ms with an increment of 5 mV. Then, the Na+ current was recorded at the test pulse of 0 mV for 50 ms. The peak currents under the test pulses were normalized and plotted against the prepulse voltage. Activation and inactivation curves were fit to a Boltzmann function to obtain V1/2 and slope values. Time course of inactivation data from the peak current at 0 mV was fitted to a single exponential equation: y = A1 exp(−x/τinac) + y0, where A1 was the relative fraction of current inactivation, τinac was the time constant, x was the time, and y0 was the amplitude of the steady-state component.
All data points are presented as mean ± SEM, and n is the number of experimental cells from which recordings were obtained. Statistical significance was assessed using an unpaired t test with Welch’s correction, one-way ANOVA, and extra sum-of-squares F test.
Cryo-EM Data Acquisition.
For cryo-EM sample preparation and data collection, the same machines, software, and protocols were used as previously described (18). Aliquots of 3.5 μL freshly purified Nav1.1–β3-β4 were placed on glow-discharged holey carbon grids (Quantifoil Au 300 mesh, R1.2/1.3). Grids were blotted for 2.5 s or 3.0 s and plunge-frozen in liquid ethane cooled by liquid nitrogen with Vitrobot Mark IV (Thermo Fisher). Electron micrographs were acquired on a Titan Krios electron microscope (Thermo Fisher) operating at 300 kV and equipped with Cs corrector, Gatan K3 Summit detector, and GIF Quantum energy filter with a slit width of 20 eV. A total of 6,750 movie stacks were automatically collected using AutoEMation (48) and a preset defocus range from −1.8 µm to −1.5 µm in superresolution mode at a nominal magnification of 64,000×. Each stack was exposed for 2.56 s with 0.08 s per frame, resulting in 32 frames per stack. The total dose rate was 48 e−/Å2 for each stack. The stacks were motion corrected with MotionCor2 (49) and binned twofold, resulting in 1.0979 Å per pixel. Dose weighting was performed (50). The defocus values were estimated using Gctf (51).
Image Processing.
A diagram of data processing is presented in SI Appendix, Fig. S2. A total of 6,704 micrographs were manually selected from 6,750 micrographs, and a total of 3,160,451 particles were automatically picked using topaz (52). These particles were applied to two-dimensional (2D) and 3D classifications in cryoSPARC (26) After 2D classification, 325,348 good particles were identified and subsequently applied to homogeneous refinement. Then, one additional round of heterogeneous refinement was performed, during which the particles were classified into four classes and the good class was selected, resulting in a dataset of 133,127 particles. The selected particles were subject to the uniform refinement and nonuniform refinement procedure in cryoSPARC, finally yielding a 3D map with an overall resolution of 3.3 Å. The resolution was estimated with the gold-standard Fourier shell correlation 0.143 criterion (53) with high-resolution noise substitution (54).
Model Building and Structure Refinement.
For the model building and structure refinement, the same software was used, and the process was similar as before with minor modifications (18). Sequences for the nine human Nav channels were aligned using ClustalW. Model building was performed based on the 3.3-Å reconstruction map for Nav1.1. The coordinates of human Nav1.2 (PDB accession 6J8E) were fitted into the EM map by CHIMERA (55). The residues of human Nav1.2 were mutated to the corresponding ones in human Nav1.1 in COOT (56), and each residue was manually checked. The chemical properties of amino acids were taken into consideration during model building. There was no density corresponding to β3. The crystal structure of β4 (PDB accession 4MZ2) was docked into the corresponding density as a rigid body. In total, 1,138 residues in Nav1.1 were assigned with side chains, and 4 sugar moieties and 1 GDN were built. The N-terminal 115 residues, ECL (residues 283 to 311), intracellular I-II linker (residues 440 to 748), II-III linker (residues 997 to 1,200), and C-terminal sequences after Glu1795 were not modeled due to the lack of corresponding densities.
Structure refinement was performed using the phenix.real_space_refine application in PHENIX (57) real space with secondary structure and geometry restraints. Overfitting of the overall model was monitored by refining the model in one of the two independent maps from the gold-standard refinement approach and testing the refined model against the other map (58). Statistics of the map reconstruction and model refinement are provided in SI Appendix, Table S4.
Supplementary Material
Acknowledgments
We thank Xiaomin Li (Tsinghua University) for technical support during EM image acquisition. We thank the Tsinghua University Branch of the China National Center for Protein Sciences (Beijing) for providing the cryo-EM facility support. We thank the computational facility support from the Bio-Computing Platform of Tsinghua University Branch of the China National Center for Protein Sciences (Beijing). This work was funded by the National Key R&D Program (Grant 2016YFA0500402 to X.P.) from the Ministry of Science and Technology of China and the Beijing Nova Program (Grant Z191100001119127) from the Beijing Municipal Science and Technology Commission. N.Y. is supported by the Shirley M. Tilghman endowed professorship from Princeton University.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100066118/-/DCSupplemental.
Data Availability
Density map and model data have been deposited in the Electron Microscopy Data Bank and PDB (accession nos. 30851 and 7DTD).
References
- 1.Hodgkin A. L., Huxley A. F., Resting and action potentials in single nerve fibres. J. Physiol. 104, 176–195 (1945). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgkin A. L., Huxley A. F., A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hille B., Ion Channels of Excitable Membranes (Sinauer Associates, Sunderland, MA, ed. 3, 2001), p. 814. [Google Scholar]
- 4.Ahern C. A., Payandeh J., Bosmans F., Chanda B., The hitchhiker’s guide to the voltage-gated sodium channel galaxy. J. Gen. Physiol. 147, 1–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catterall W. A., Sodium channels, inherited epilepsy, and antiepileptic drugs. Annu. Rev. Pharmacol. Toxicol. 54, 317–338 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Bagal S. K., Marron B. E., Owen R. M., Storer R. I., Swain N. A., Voltage gated sodium channels as drug discovery targets. Channels (Austin) 9, 360–366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubinstein M., et al., Association of rare missense variants in the second intracellular loop of NaV1.7 sodium channels with familial autism. Mol. Psychiatry 23, 231–239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W., Liu M., Yan S. F., Yan N., Structure-based assessment of disease-related mutations in human voltage-gated sodium channels. Protein Cell 8, 401–438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohmori I., Kahlig K. M., Rhodes T. H., Wang D. W., A. L. George, Jr, Nonfunctional SCN1A is common in severe myoclonic epilepsy of infancy. Epilepsia 47, 1636–1642 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Harkin L. A.et al.; Infantile Epileptic Encephalopathy Referral Consortium , The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain 130, 843–852 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Selmer K. K., et al., Parental SCN1A mutation mosaicism in familial Dravet syndrome. Clin. Genet. 76, 398–403 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Marini C., et al., The genetics of Dravet syndrome. Epilepsia 52, 24–29 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Escayg A., et al., A novel SCN1A mutation associated with generalized epilepsy with febrile seizures plus–And prevalence of variants in patients with epilepsy. Am. J. Hum. Genet. 68, 866–873 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z., et al., Structure of human Nav1.5 reveals the fast inactivation-related segments as a mutational hotspot for the long QT syndrome. Proc. Natl. Acad. Sci. U.S.A., 10.1073/pnas.2100069118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen H., et al., Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 355, eaal4326 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Yan Z., et al., Structure of the Nav1.4-β1 complex from electric eel. Cell 170, 470–482.e11 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Shen H., et al., Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Science 362, eaau2596 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Pan X., et al., Structure of the human voltage-gated sodium channel Nav1.4 in complex with β1. Science 362, eaau2486 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Pan X., et al., Molecular basis for pore blockade of human Na+ channel Nav1.2 by the μ-conotoxin KIIIA. Science 363, 1309–1313 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Shen H., Liu D., Wu K., Lei J., Yan N., Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science 363, 1303–1308 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Li Z., et al., Structural basis for pore blockade of the human cardiac sodium channel Nav1.5 by tetrodotoxin and quinidine. bioRxiv [Preprint]. 10.1101/2019.12.30.890681 (December 30, 2019). [DOI]
- 22.Jiang D., et al., Structure of the cardiac sodium channel. Cell 180, 122–134.e10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rusconi R., et al., Modulatory proteins can rescue a trafficking defective epileptogenic Nav1.1 Na+ channel mutant. J. Neurosci. 27, 11037–11046 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aman T. K., et al., Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels. J. Neurosci. 29, 2027–2042 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace R. H., et al., Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat. Genet. 19, 366–370 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Punjani A., Rubinstein J. L., Fleet D. J., Brubaker M. A., cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Tao X., Lee A., Limapichat W., Dougherty D. A., MacKinnon R., A gating charge transfer center in voltage sensors. Science 328, 67–73 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes T. H., Lossin C., Vanoye C. G., Wang D. W., A. L. George, Jr, Noninactivating voltage-gated sodium channels in severe myoclonic epilepsy of infancy. Proc. Natl. Acad. Sci. U.S.A. 101, 11147–11152 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gui J., et al., Multiple loss-of-function mechanisms contribute to SCN5A-related familial sick sinus syndrome. PLoS One 5, e10985 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma D., et al., Identification of an INa-dependent and Ito-mediated proarrhythmic mechanism in cardiomyocytes derived from pluripotent stem cells of a Brugada syndrome patient. Sci. Rep. 8, 11246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veerman C. C., et al., Switch from fetal to adult SCN5A isoform in human induced pluripotent stem cell-derived cardiomyocytes unmasks the cellular phenotype of a conduction disease-causing mutation. J. Am. Heart Assoc. 6, e005135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syrbe S.et al.; EuroEPINOMICS RES consortium , De novo loss- or gain-of-function mutations in KCNA2 cause epileptic encephalopathy. Nat. Genet. 47, 393–399 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makiyama T., et al., High risk for bradyarrhythmic complications in patients with Brugada syndrome caused by SCN5A gene mutations. J. Am. Coll. Cardiol. 46, 2100–2106 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Kapplinger J. D., et al., Enhanced classification of Brugada syndrome-associated and long-QT syndrome-associated genetic variants in the SCN5A-encoded Na(v)1.5 cardiac sodium channel. Circ. Cardiovasc. Genet. 8, 582–595 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfahnl A. E., et al., A sodium channel pore mutation causing Brugada syndrome. Heart Rhythm 4, 46–53 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amin A. S., Verkerk A. O., Bhuiyan Z. A., Wilde A. A. M., Tan H. L., Novel Brugada syndrome-causing mutation in ion-conducting pore of cardiac Na+ channel does not affect ion selectivity properties. Acta Physiol. Scand. 185, 291–301 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Capes D. L., Goldschen-Ohm M. P., Arcisio-Miranda M., Bezanilla F., Chanda B., Domain IV voltage-sensor movement is both sufficient and rate limiting for fast inactivation in sodium channels. J. Gen. Physiol. 142, 101–112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stühmer W., et al., Structural parts involved in activation and inactivation of the sodium channel. Nature 339, 597–603 (1989). [DOI] [PubMed] [Google Scholar]
- 39.West J. W., et al., A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc. Natl. Acad. Sci. U.S.A. 89, 10910–10914 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbieri R., Bertelli S., Pusch M., Gavazzo P., Late sodium current blocker GS967 inhibits persistent currents induced by familial hemiplegic migraine type 3 mutations of the SCN1A gene. J. Headache Pain 20, 107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D. W., et al., Cardiac sodium channel dysfunction in sudden infant death syndrome. Circulation 115, 368–376 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Mohler P. J., et al., Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc. Natl. Acad. Sci. U.S.A. 101, 17533–17538 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abriel H., Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J. Mol. Cell. Cardiol. 48, 2–11 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Kim J., et al., Calmodulin mediates Ca2+ sensitivity of sodium channels. J. Biol. Chem. 279, 45004–45012 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Tan H. L., et al., A calcium sensor in the sodium channel modulates cardiac excitability. Nature 415, 442–447 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Allouis M., et al., 14-3-3 is a regulator of the cardiac voltage-gated sodium channel Nav1.5. Circ. Res. 98, 1538–1546 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Matsuda T., Cepko C. L., Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. U.S.A. 101, 16–22 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lei J., Frank J., Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope. J. Struct. Biol. 150, 69–80 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Zheng S. Q., et al., MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grant T., Grigorieff N., Measuring the optimal exposure for single particle cryo-EM using a 2.6 Å reconstruction of rotavirus VP6. eLife 4, e06980 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang K., Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimanius D., Forsberg B. O., Scheres S. H., Lindahl E., Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. eLife 5, e18722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenthal P. B., Henderson R., Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Chen S., et al., High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pettersen E. F., et al., UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams P. D., et al., PHENIX: A comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amunts A., et al., Structure of the yeast mitochondrial large ribosomal subunit. Science 343, 1485–1489 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeLano W. L., The PyMOL Molecular Graphics System on World Wide Web (Schrödinger, New York, NY, 2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Density map and model data have been deposited in the Electron Microscopy Data Bank and PDB (accession nos. 30851 and 7DTD).