Significance
The mechanisms that promote the transition from chronic phase to blast crisis (BC) in chronic myeloid leukemia (CML) patients are not fully described. Given the loss of miR-15/16 loci is critical in the development of myeloid and lymphocytic leukemia, here we evaluated the correlation between the expression of miR-15a/16 and miR-15b/16 with the transition of CML from chronic phase to BC. A significant reduction of miR-15a, miR-15b, and miR-16 expression and an overexpression of their targets, such as Bmi-1, ROR1, and Bcl-2, can describe the progression from chronic phase to BC. This study suggests that targeting different oncogenes activated by the same genetic/epigenetic alteration could represent an important advancement in the treatment of BC CML.
Keywords: miR-15/16 cluster, chronic myeloid leukemia, blast crisis
Abstract
Despite advances that have improved the treatment of chronic myeloid leukemia (CML) patients in chronic phase, the mechanisms of the transition from chronic phase CML to blast crisis (BC) are not fully understood. Considering the key role of miR-15/16 loci in the pathogenesis of myeloid and lymphocytic leukemia, here we aimed to correlate the expression of miR-15a/16 and miR-15b/16 to progression of CML from chronic phase to BC. We analyzed the expression of the two miR-15/16 clusters in 17 CML patients in chronic phase and 22 patients in BC and in 11 paired chronic phase and BC CML patients. BC CMLs show a significant reduction of the expression of miR-15a/-15b/16 compared to CMLs in chronic phase. Moreover, BC CMLs showed an overexpression of miR-15/16 direct targets such as Bmi-1, ROR1, and Bcl-2 compared to CMLs in chronic phase. This study highlights the loss of both miR-15/16 clusters as a potential oncogenic driver in the transition from chronic phase to BC in CML patients.
Chronic myelogenous leukemia (CML) is a malignant disorder characterized by an increase in myeloid cells that maintains their ability to differentiate (1). It represents almost 20% of all leukemias in adults and with appropriate targeted therapy has an indolent course (2). CML proceeds in three phases: chronic, accelerated, and blastic (blast crisis, BC) (3).
Untreated CMLs progress to an accelerated phase and then to a BC, which is essentially incurable (4). At present, mechanisms responsible for the progression of chronic phase CML to BC are not fully understood, although several additional cytogenetic abnormalities, such as trisomy 8, trisomy 19, isochromosome17, and double Philadelphia chromosomes, have been observed in BC CML samples (5).
MicroRNAs are negative regulators of gene expression by binding to the 3′ untranslated region (UTR) of their mRNA targets (6, 7). We have observed the loss of miR-15/16–1 in the great majority of chronic lymphocytic leukemia (CLL) (∼80%). Such loss of expression is due, for the most part, to a deletion of the miR-15/16 locus at chromosome 13q14 and/or epigenetic silencing (8, 9). Knockout of the miR-15a/16–1 locus in mice also results in delayed-onset CLL (10). We have also knocked miR-15b/16–2, another locus of the miR-15/16 family, and we observed that the knockout (KO) mice developed CLL a little earlier and with higher penetrance than the miR-15a/16–1 KO mice (11). We subsequently generated KO mice for both loci: miR-15a/16–1 and miR-15b/16–2 (12). Interestingly, we identified that 77% of the double-KO mice developed acute myeloid leukemia (AML), while the remaining 23% developed a B-cell lymphoma (13). In addition, ∼30%, of myelodysplastic syndromes (MDSs) transform into AML (14). Thus, we investigated MDS, MDS transforming into AML, AML derived from MDS, and two large cohorts of patients with AML. We discovered that loss of expression of both loci of miR-15/16 occurred in the MDS transforming into AML and in a large fraction of AMLs (13). These losses resulted in overexpression of at least two targets of miR-15/16, BCL2, a driver oncogene, and ROR1, a potential oncogene encoding an embryonic surface antigen (15, 16). Thus loss of miR-15/16 plays an important role not only in the pathogenesis of CLL, but also in the development and progression of a fraction of AMLs and the transformation of MDS into AML.
Since more than 30 yrs after the discovery of the breakpoint cluster region-Abl tyrosine kinase (BCR/ABL) chimeric oncogene in CML we still do not know the cause of progression of CML to BC, here we investigate whether BC progression could be due to the loss of expression of both loci of miR-15/16.
Results and Discussion
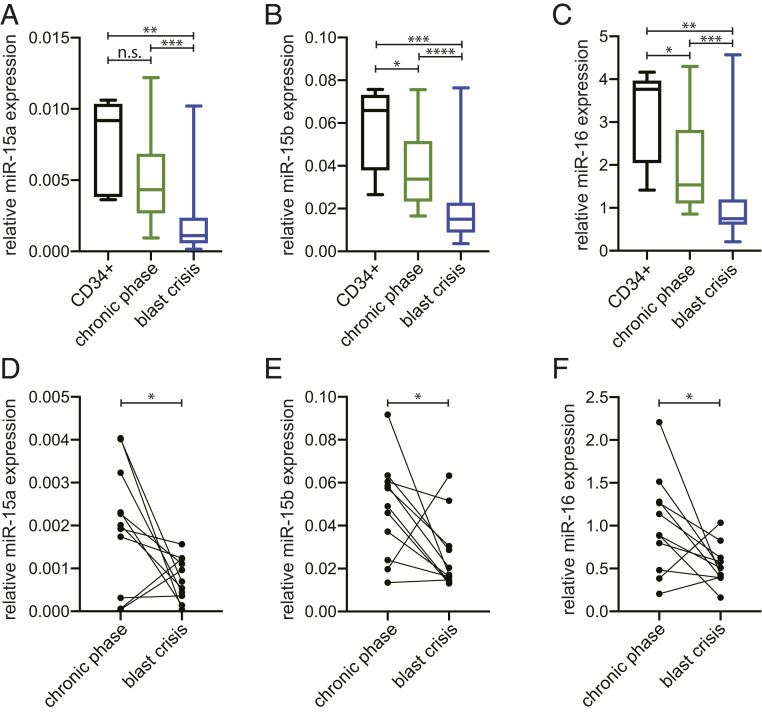
We first examined the expression of miR-15a that maps at 13q14, miR-15b that maps at 3q25, and miR-16 that maps at 13q14 and 3q25 (miR-16–1 and miR-16–2 are identical) in CML patients in chronic phase and BC (Table 1). Normal CD34+ bone marrow cells were used as a control. As shown in Fig. 1 chronic-phase CMLs expressed less miR-15a (Fig. 1A), miR-15b (Fig. 1B), and miR-16 (Fig. 1C) compared to normal CD34+ control cells. BC CML cells expressed statistically significantly lower levels of all three microRNAs compared to normal CD34+ cells and to chronic-phase CML cells (Fig. 1 A–C). The comparison was also carried out in cells from paired chronic phase and BC CML samples (Table 2). Significant decreases of all three microRNAs expression was observed in cells from BC compared to those from chronic phase (Fig. 1 D–F). These decreases were found in nine out of these 11 pairs for miR-15a (Fig. 1D), miR-15b (Fig. 1E), and miR-16 (Fig. 1F). Interestingly, among these 11 paired samples, two cases from the same patients exhibited very low microRNAs levels in chronic phase, but increased in BC.
Table 1.
Characteristics of CML patients included in this study
| Value | |
| Demographic features | |
| Gender, n (%) | |
| Male | 29 (74) |
| Female | 10 (26) |
| Clinical features | |
| Age at diagnosis, median ± SD, y | 50 ± 13.5 |
| Disease phase, n (%) | |
| CP | 17 (44) |
| BC | 22 (56) |
| Initial white cell count, mean ± SD, 109/L | 93 ± 118.3 |
| Initial blasts count, mean ± SD, 109/L | 5.6 ± 25.3 |
SD, standard deviation.
Fig. 1.
MiR-15/16 cluster expression in CML patients. MicroRNAs expression by qRT-PCR in CD34+ healthy controls and CML patients’ cells in CP (n = 17) and in BC (n = 22). Box plots of miR-15a (statistical analysis for all groups Kruskal–Wallis P value = 0.0005431) (A), miR-15b (statistical analysis for all groups Kruskal–Wallis P value = 8.292 × 10−5) (B), miR-16 (statistical analysis for all groups Kruskal–Wallis P value = 0.0003215) (C). (D–F) MicroRNAs expression by qRT-PCR of paired CML patients’ cells (n = 11) in CP and BC. Bipartite graph of miR-15a (one-way Wilcoxon signed-rank P value = 0.021) (D), miR-15b (one-way Wilcoxon signed-rank P value = 0.02686) (E), miR-16 (one-way Wilcoxon signed-rank P value = 0.01611) (F). n.s., not significant. *, 0.01< P value ≤ 0.05; **, 0.001 < P value ≤ 0.01; ***, 0.0001 < P value ≤ 0.001; ****P value ≤ 0.0001.
Table 2.
Characteristics of paired CML patients included in this study
| Value | |
| Demographic features | |
| Gender, n (%) | |
| Male | 3 (27) |
| Female | 8 (73) |
| Clinical features | |
| Age at diagnosis, median ± SD, y | 45 ± 17.3 |
| Initial white cell count, mean ± SD, 109/L | 20.5 ± 197.7 |
| Initial blasts count, mean ± SD, 109/L | 2.5 ± 26 |
SD, standard deviation.
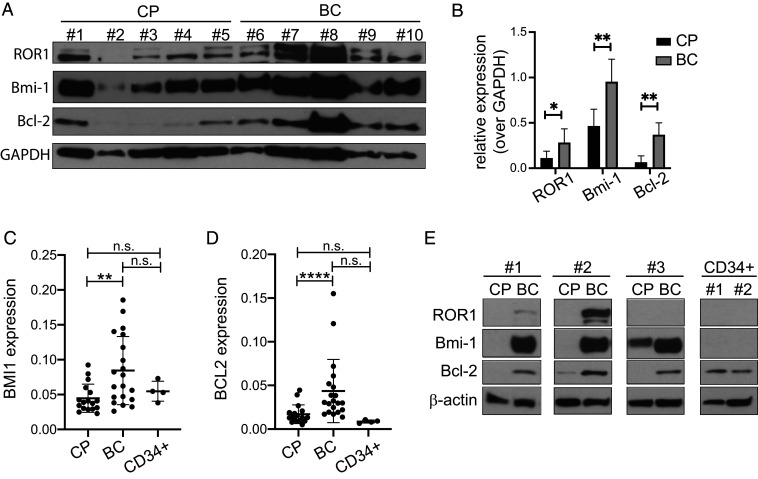
We next determined protein and transcript expression levels of Bcl-2, ROR1, and Bmi-1, all known targets of miR-15/16 (17–19) in five cases of CML in chronic phase and five cases of CML in BC. The expression of all three proteins was more elevated in the five cases of BC compared to the CMLs in chronic phase (with the exception of case 1) as shown by Western blot in Fig. 2A and by its relative quantification (Fig. 2B). A possible explanation of the outlier behavior of case 1 probably lies in the associated clinical information: the cells were collected just 3 mo before clinical disease transformation in BC; thus, this CML case was most likely already progressing to BC and protein expression accordingly modulated. Moreover, we determined BMI1 and BCL2 mRNA levels in chronic phase (CP), BC CML patients, and in CD34+ cells from healthy donors. BMI1 and BCL2 expression levels were significantly higher in BC patients compared to CP patients (Fig. 2 C and D). No statistically significant differences for BMI1 and BCL2 expression levels were observed in the comparison between CP/BC and C34+ cells from healthy donors. We then measured the protein levels of Bcl-2, Bmi-1, and ROR1 in cells from three paired patients in CP and BC and from CD34+ cells from two healthy donors. As shown in Fig. 2E, the expression of ROR1 (undetectable in patient 3 and in CD34+ cells), Bmi-1 (undetectable in CD34+ cells), and Bcl-2 markedly increased when the disease progressed, supporting the finding that progression of CML from chronic to BC is accompanied by higher expression of oncogenic targets of miR-15/16. Moderate to high levels of Bmi-1 were previously detected in some AML patients, especially in M0 subtype of myeloid leukemia (20). Interestingly, it has been reported that accelerated and BC CML cells express higher levels of Bmi-1 than CP (21).
Fig. 2.
MiR-15/16 targets expression in CML patients. (A) Immunoblotting of ROR1, Bmi-1, and Bcl-2 performed on five CML patients’ cells in CP and five CMLs cells in BC. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a normalizer. (B) Densitometry relative quantification of ROR1, Bmi-1, and Bcl-2 expression respect to GAPDH loading control. CML patients’ cells in CP and in BC were grouped. One-way Wilcoxon rank-sum test was applied. (C and D) Gene expression by qRT-PCR in CML patients’ cells in CP (n = 17), in BC (n = 20) and CD34+ cells from healthy donors (n = 4). Box plots expression of BMI1 (statistical analysis for all groups Kruskal–Wallis P value = 0.00027) (C) and BCL2 (statistical analysis for all groups Kruskal–Wallis P value = 4.37 × 10−5) (D). (E) Western blot analysis of ROR1, Bmi-1, and Bcl-2 performed on three paired CML patients’ cells in CP, BC, and CD34+ from two healthy donors. β-actin was used as a normalizer. *, 0.01 < P value ≤ 0.05; **, 0.001 < P value ≤ 0.01; ***, 0.0001 < P value ≤ 0.001; ****P value ≤ 0.0001.
MiR-15/16 target not only BCL2, but also several other oncogenes known to be involved in human and mouse malignancies including BMI1 and MYB, that have been shown to be elevated in CML (21–24) and AML (20, 25–28). We have shown that a presumptive oncogene ROR1, which is expressed in most CLLs concordantly with BCL2, is also a target of miR-15/16 (19). Interestingly the levels of Bmi-1 in BC CMLs appeared to be higher than in CMLs in CP (22).
From these results we can infer that in most cases of progression from CP into BC CML miR-15/16 are progressively down-regulated, resulting in overexpression of Bcl-2, ROR1, and Bmi-1, three established oncogenes that promote increased survival and proliferation. Of note, in a minority of two cases, the mechanism of the progression does not seem to involve the enhanced expression of Bcl-2, Bmi-1, and ROR1 since we found miR-15/16 increased rather than decreased. The mechanism that describes the loss of miR-15/16 is not fully understood and needs future studies. Our hypothesis strongly suggests that the deregulation of miR-15/16 expression in the progression from CP to BC is due to deletions and/or methylation of miRNAs promoters as we previously observed in AML patients samples. Despite this limitation on a bigger picture our data suggest that treatment of BC CML should involve the concurrent targeting of these different oncogenes. In fact, it is possible that targeting just one of these proteins, like in the case of treatment of CLL with venetolax, will not be sufficient to kill most or all the malignant cells (29–31) leaving an opportunity for the rise of resistance. Thus, targeting two, three, or more oncogenes activated by the same genetic/epigenetic alteration has the considerable advantage of tackling the resistance problem, since unlikely the leukemic cells will develop resistance to multiple drugs at the same time.
Materials and Methods
Human Tissue Samples.
CML unpaired samples were obtained from Princess Margaret Cancer Centre in Toronto, Canada and from MD Anderson Cancer Center in Houston, TX. A total of 39 samples was collected from 22 patients in CP and from 17 patients in BC (Table 1). Paired CML samples were obtained from MD Anderson Cancer Center and The Ohio State University. A total of 22 samples was collected from 11 patients: first set (blood sample or bone marrow aspirates) was collected in CP and second set was collected from the same patient in BC (Table 2). Samples were separated by using Ficoll-Hypaque and viable cells were frozen and stored in liquid nitrogen. This study was carried out under the protocols approved by the Institutional Review Boards of The Ohio State University, the Princess Margaret Cancer Center, and MD Anderson Cancer Center. All samples and clinical data were deidentified. CD34+ from healthy donors were used as the controls.
RNA and Quantitative Real-Time PCR.
Total RNA from CML samples was isolated using TRIzol (Invitrogen), following the provided instructions. For quantitative real-time PCR (qRT-PCR), TaqMan miRNA assays from ThermoFisher (miR-15a#000389, miR-15b#000390, miR-16#000391) were used to detect mature miRNAs. qRT-PCR was performed as described by Lovat et al. (13). RNU44 (ThermoFisher TaqMan Assay 00194) and RNU48 (ThermoFisher TaqMan Assay 001006) were used as normalizers for CML samples.
Western Blot Analysis.
Due to the low number of cells available per patient sample, especially in BC phase, CML patients’ protein were precipitated after RNA extraction with TRIzol (Invitrogen) following the protocol described in ref. 32. After the last wash with 100% ethanol alcohol (EtOH), the protein pellet was resuspended in 500 µl of 1:1 solution of 1% sodium dodecyl sulfate (SDS) and 8 M urea in Tris⋅HCl 1 M pH 8.0 followed by five cycles of 15-s sonication and 30 s on ice incubation to solubilize the pellet. Then, the sample was concentrated through Amicon column and protein lysate was separated on Criterion Tris⋅HCl 4–20% precast gel (BioRad) and transferred onto a nitrocellulose membrane (HybondC, Amersham). Anti-Bcl-2 (Cell Signaling Technologies), anti-Bmi-1, and anti-ROR1 (Abclonal), anti-GAPDH (Genetex), and anti-β-actin (Sigma-Aldrich) were used to incubate the membrane.
Statistical Analysis.
One-way Wilcoxon rank-sum test was used for each unpaired pairwise analysis shown in Figs. 1 A–C and 2 C and D, while one-way Wilcoxon signed-rank test was applied for each paired analysis shown in Fig. 1 D–F. For both tests we used the wilcox.test function from the stats R Package (R version 3.5.1). Kruskal–Wallis rank-sum test was employed for each multivariate analysis present in Figs. 1 A–C and 2 C and D, particularly using the kruskal.test function from the stats R package.
Acknowledgments
This work was supported by NIH/NCI Grant NIH R35 CA197706 to C.M.C. This work was supported in part by The Ohio State University Comprehensive Cancer Center (OSU CCC) and NIH Grant P30 CA16058. We thank the Genomic Shared Resource at OSU CCC for conducting the qRT-PCR analysis.
Footnotes
The authors declare no competing interest.
Data Availability
All study data are included in the article.
References
- 1.Faderl S., et al., The biology of chronic myeloid leukemia. N. Engl. J. Med. 341, 164–172 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H. M., et al., Chronic myelogenous leukemia in blast crisis. Analysis of 242 patients. Am. J. Med. 83, 445–454 (1987). [DOI] [PubMed] [Google Scholar]
- 3.Sawyers C. L., Chronic myeloid leukemia. N. Engl. J. Med. 340, 1330–1340 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Radich J. P., The Biology of CML blast crisis. Hematology Am. Soc. Hematol. Educ. Program 2007, 384–391 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Wang W., et al., Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood 127, 2742–2750 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel D. P., MicroRNAs: Target recognition and regulatory functions. Cell 136, 215–233 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croce C. M., Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 10, 704–714 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calin G. A., et al., Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. U.S.A. 99, 15524–15529 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calin G. A., et al., A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 353, 1793–1801 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Klein U., et al., The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 17, 28–40 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Lovat F., et al., miR-15b/16-2 deletion promotes B-cell malignancies. Proc. Natl. Acad. Sci. U.S.A. 112, 11636–11641 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovat F., et al., Knockout of both miR-15/16 loci induces acute myeloid leukemia. Proc. Natl. Acad. Sci. U.S.A. 115, 13069–13074 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovat F., et al., Combined loss of function of two different loci of miR-15/16 drives the pathogenesis of acute myeloid leukemia. Proc. Natl. Acad. Sci. U.S.A. 117, 12332–12340 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adès L., Itzykson R., Fenaux P., Myelodysplastic syndromes. Lancet 383, 2239–2252 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Baskar S., et al., Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin. Cancer Res. 14, 396–404 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Daneshmanesh A. H., et al., Ror1, a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. Int. J. Cancer 123, 1190–1195 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya R., et al., MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res. 69, 9090–9095 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimmino A., et al., miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. U.S.A. 102, 13944–13949 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rassenti L. Z., et al., MicroRNA dysregulation to identify therapeutic target combinations for chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. U.S.A. 114, 10731–10736 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawa M., et al., BMI-1 is highly expressed in M0-subtype acute myeloid leukemia. Int. J. Hematol. 82, 42–47 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Saudy N. S., et al., BMI1 gene expression in myeloid leukemias and its impact on prognosis. Blood Cells Mol. Dis. 53, 194–198 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Mohty M., Yong A. S., Szydlo R. M., Apperley J. F., Melo J. V., The polycomb group BMI1 gene is a molecular marker for predicting prognosis of chronic myeloid leukemia. Blood 110, 380–383 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Corradini F., et al., Enhanced proliferative potential of hematopoietic cells expressing degradation-resistant c-Myb mutants. J. Biol. Chem. 280, 30254–30262 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Lidonnici M. R., Corradini F., Waldron T., Bender T. P., Calabretta B., Requirement of c-Myb for p210(BCR/ABL)-dependent transformation of hematopoietic progenitors and leukemogenesis. Blood 111, 4771–4779 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chowdhury M., et al., Expression of Polycomb-group (PcG) protein BMI-1 predicts prognosis in patients with acute myeloid leukemia. Leukemia 21, 1116–1122 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Rizo A., et al., Repression of BMI1 in normal and leukemic human CD34(+) cells impairs self-renewal and induces apoptosis. Blood 114, 1498–1505 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Anfossi G., Gewirtz A. M., Calabretta B., An oligomer complementary to c-myb-encoded mRNA inhibits proliferation of human myeloid leukemia cell lines. Proc. Natl. Acad. Sci. U.S.A. 86, 3379–3383 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelicci P. G., Lanfrancone L., Brathwaite M. D., Wolman S. R., Dalla-Favera R., Amplification of the c-myb oncogene in a case of human acute myelogenous leukemia. Science 224, 1117–1121 (1984). [DOI] [PubMed] [Google Scholar]
- 29.Carter B. Z., et al., Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci. Transl. Med. 8, 355ra117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts A. W., et al., Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 311–322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souers A. J., et al., ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Simões A. E. S., et al., Efficient recovery of proteins from multiple source samples after TRIzol(®) or TRIzol(®)LS RNA extraction and long-term storage. BMC Genomics 14, 181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article.