ABSTRACT
Introduction
Low back pain (LBP) is common in warfighters. Noninvasive interventions are necessary to expedite return-to-function. Soft tissue manipulation, for example, massage, is a method used to treat LBP. Instrument-assisted soft tissue manipulation (IASTM) uses a rigid device to mobilize the tissue. This study explored the effects of IASTM on pain, function, and biomarkers.
Methods
Sprague-Dawley rats (n = 44) were randomized to groups (n = 6/grp): (A) cage control; (B) 3 days (3d) postinjury (inj), untreated; (C) 3d inj, < 30-minute post-IASTM treatment; (D) 3d inj, 2 hours (2h) post-IASTM; (E) 14 days (14d) inj, untreated; (F) 14d inj, < 30-minute post-IASTM; and (G) 14d inj, 2h post-IASTM. Researchers induced unilateral LBP in Sprague-Dawley rats using complete Freund’s adjuvant injection. Conscious rodents received IASTM for 5 min/session once at 3 days or 3×/week × 2weeks (6× total) over 14 days. Biomarker plasma levels were determined in all groups, while behavioral outcomes were assessed in two groups, D and G, at three time points: before injury, pre-, and post-IASTM treatment. Circulating mesenchymal stem cell levels were assessed using flow cytometry and cytokine plasma levels assayed.
Results
The back pressure pain threshold (PPT) lowered bilaterally at 3 days postinjury (P < .05), suggesting increased pain sensitivity. IASTM treatment lowered PPT more on the injured side (15.8%; P < 0.05). At 14 days, back PPT remained lower but similar side to side. At 3 days, paw PPT increased 34.6% in the contralateral rear limb following treatment (P < .01). Grip strength did not vary significantly. Gait coupling patterns improved significantly (P < .05). Circulating mesenchymal stem cell levels altered significantly postinjury but not with treatment. Neuropeptide Y plasma levels increased significantly at 3 days, 2h post-IASTM (53.2%) (P < .05). Interleukin-6 and tumor necrosis factor-alpha did not vary significantly. At 14 days, regulated on activation, normal T cell expressed and secreted decreased significantly <30-minute post-IASTM (96.1%, P < .002), while IL-10 trended upward at 2h (53.1%; P = .86).
Conclusions
LBP increased pain sensitivity and diminished function. IASTM treatment increased pain sensitization acutely in the back but significantly reduced pain sensitivity in the contralateral rear paw. Findings suggest IASTM may positively influence pain modulation and inflammation while improving gait patterns. Soft tissue manipulation may be beneficial as a conservative treatment option for LBP.
INTRODUCTION
Neuromusculoskeletal conditions are common, and low back pain (LBP) is the leading cause of disability in the United States.1 Active duty military personnel and veterans report LBP more frequently than the general population, and LBP is one of the most prevalent musculoskeletal regions of chronic pain in this population.2–4 Noninvasive and nonpharmacological interventions are needed to address the pain and functional limitations associated with LBP and other neuromusculoskeletal pain conditions.5,6 Soft tissue manipulation, for example, massage, is a type of manual therapy used by clinicians to address LBP.7 Soft tissue manipulation is a type of mechanotherapy that introduces a mechanical stimulus to the tissue to positively influence tissue healing and repair.8 However, the underlying mechanisms for soft tissue manual therapy are not well understood or optimized.9
Instrument-assisted soft tissue manipulation (IASTM) uses rigid devices to deliver the manipulating force to the targeted tissue through the intact surface of the body. Initial IASTM animal and clinical research have demonstrated positive outcomes, including improved biomechanical properties, tissue organization, and increased blood flow. Possible angiogenesis in injured IASTM-treated rat knee ligaments as compared to contralateral untreated injured controls has also been demonstrated.10
A potential benefit of massage-based modalities is decreased inflammation.11 Other forms of mechanotherapy, such as whole-body platform vibration training and exercise, have shown anti-inflammatory effects, along with angiogenesis, and increased mesenchymal stem cell (MSC) circulation levels.12,13 Interestingly, higher circulating MSC levels and activity are associated with decreased inflammation and enhanced angiogenesis.13
This preliminary study sought to explore the effects of IASTM on select biomarkers, pain, and behavior in conscious rodents with chemically induced chronic LBP. This research tested the hypothesis that IASTM would attenuate inflammation, increase MSC circulation levels, mitigate pain, and improve functional outcomes. The intent was to provide important insight into the underlying mechanisms of soft tissue manual therapy.
METHODS
Ethics Statement
The examiners conducted all procedures according to the Indiana University Institutional Animal Care and Use Committee and national standards.
Animal Subjects
Experiments used adult male Sprague-Dawley rats (n = 44) (307.4 ± 7.3 g; 11 to 16 weeks old). All animals were acclimated to their surroundings in solid-bottom cages for a minimum of 3 days before handling, under standard lighting and temperature conditions with food and water ad libitum, and housed throughout the entire study at the Indiana University Laboratory Animal Resource Center in the Stark Neurosciences Research Institute (Indianapolis, IN). Examiners began acclimating animals to handling 5 minutes at a time for 3 days to minimize animal stress during behavioral testing and IASTM treatment. Following this initial period of handling, animals were introduced to a swaddling handhold and behavioral testing procedures while incentivized with food for three more days. Under the direction of the facility’s veterinarian, examiners trained conscious animals to tolerate swaddling during IASTM delivery to the low back. During the swaddling handhold, a research assistant wrapped animals with a soft cloth starting above the thoracolumbar region while leaving an opening at the cephalic end for exposure of the animal’s nose. The research assistant then gently secured one hand around the cloth while holding the proximal tail with the other hand (Fig. 1A). Animals were weighed weekly using a scale accurate to 0.1 g.
FIGURE 1.
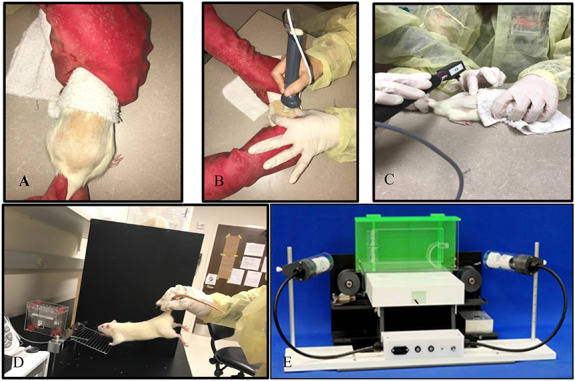
Instrument-assisted soft tissue manipulation treatment (IASTM) and functional testing. (A) Conscious rats were held using a light restraint technique, while (B) the examiner utilized a rigid device to apply IASTM to the low back. (C) An electronic Von Frey monofilament was used to determine pain pressure threshold; (D) a TSE Grip Strength Meter for forepaw grip strength testing; and (E) a TreadScan 3.0 for gait pattern analysis.
To investigate the immediate and cumulative effects of IASTM treatment, animal group assignment was attuned to specific time periods for intervention and sampling. Animals were randomized to the following groups (n = 6/grp): (A) cage control; for the immediate effects: (B) 3 days (3d) postinjured (inj), untreated, (C) 3d inj, < 30-minute post-IASTM treatment, and (D) 3d inj, 2 hours (2h) post-IASTM; and for the cumulative effects: (E) 14 days (14d) inj, untreated, (F) 14d inj, <30-minute post-IASTM, and (G) 14d inj, 2h post-IASTM.
Injury
The investigators induced chronic inflammatory LBP in rats using a validated method previously described in the literature with a slight modification, that is, a skin incision was not used before injection of the chemical irritant.14,15 Two weeks of inflammation in a rodent model is considered chronic.14,15 Complete Freund’s adjuvant (CFA) was injected (50 µL) unilaterally into the same side (right) of the lumbar region while animals were under isoflurane anesthesia (3% for initial knockdown in a plastic container and 1.5% at 1.5 L/min via a nose cone during the injection). Rodents’ dorsal spines were shaved before injection and then as needed from the mid-scapular to thoracolumbar region to reduce irritation from rubbing of the hair during IASTM treatment. The intrafascial injection was administered into the thoracolumbar fascia by pushing a 27-gauge cannula through the skin, 3-mm lateral to the spinous processes, at the L4-L5 vertebral level.14 A skin incision was not made before injection to minimize soft tissue damage and avoid a wound that could impede intervention. Because the subcutaneous tissue in rats’ lumbar region is loose, the skin can be pushed about 2 cm cranially to make the injections. As a result, the skin perforation did not cover the injection site or interfere with intervention.
Intervention
A research assistant swaddled and held the conscious animals throughout all treatment sessions. A single examiner, trained and experienced in IASTM, administered treatment to all subjects. The examiner used an IASTM device designed for treating small areas to manipulate the injured tissue (GT3, Graston Technique, IN).16 The treatment edge on the device is an appropriate size to manipulate soft tissue in the thoracolumbar region in rats. The examiner applied curvilinear, longitudinal, and cross-fiber strokes from below the inferior angle of the scapula to above the iliac crest, starting lighter and moving deeper as tolerated (Fig. 1B). Treatment lasted 5 min/session at a pressure within subject tolerance, for example, no withdraw response, no fur pigmentation/discoloration, or vocalization. In other testing, a force-sensing device determined the average force applied to uninjured tissue on the backs of the rats was 2.46N ± 0.42N (i.e., 0.55 ± 0.09 lbs).17 Examiners administered a single IASTM treatment at 3 days postinjury to determine the acute effects of soft tissue manipulation intervention, and applied multiple IASTM sessions (3sessions/week × 2weeks, 6 sessions total) over 14 days to consider the cumulative effects. Animals ambulated ad libitum after intervention.
Data Collection Time Points
To assess biomarker outcomes, examiners collected blood samples upon death according to the group assignments. Samples were collected from cage controls for baseline measures. To determine acute effects, samples were collected at 3d postinjury; at 3d, <30 minutes immediately post-IASTM treatment; and at 3d, 2h post-IASTM treatment. To consider longer-term effects, samples were collected during later time points at 14d postinjury; at 14d, <30-minute post-IASTM treatment and; at 14d, 2h post-treatment. To assess pain and behavioral outcomes, animals from two of the groups, the 3d injured, 2h post-IASTM treatment group (D) and the 14d injured, 2h post-IASTM treatment group (G) were tested at three time points: preinjury, pre-IASTM treatment, and post-IASTM treatment.
Pain and Behavior Testing
The IU Neuroscience Center Animal Behavioral Core (ABC) research lab coordinator trained examiners on all equipment, procedures, and standardized protocols. Animals were treated and tested at the same time of the day. Before testing, animals were acclimated to the behavioral testing room for a minimum of 10 minutes.
Pain
An animal’s pressure pain threshold (PPT) is the minimum pressure that elicits an aversive reaction, such as withdraw, vocalization, or hyperpigmentation. A decreased PPT corresponds to heightened pain sensitivity to a mechanical stimulus. Conversely, a higher PPT indicates less sensitivity.18 The PPT determination was based on the ABC’s standardized protocol and manufacturer’s guidelines. Examiners used an electronic von Frey anesthesiometer (IITC 2390 series, Life Science Instruments, Woodland Hills, CA, USA) to apply pressure at increasing intensity for five trials using a blunt tip (3.46 mm2) for mechanical stimulation at a targeted site (Fig. 1C). A blunt tip is thought to activate nociceptors from deeper tissues while nociceptors in the skin are either not or only marginally excited. PPT results are measured in grams.19 PPT measurements sites included the thoracolumbar injection site and contralateral noninjected site, as well as on the plantar surface of the ipsilateral and contralateral paws. For PPT testing of the low back, examiners held animals in the swaddled position as described previously. For PPT testing of the hind limbs, investigators placed animals in clear bins on a grated surface that allowed access to surface area on the rear paws.
Grip Strength
Examiners used a TSE Grip Strength Meter System to determine the grasping grip strength of the animals using the “2-paw-measurement” (forepaw) method based on the ABC’s standardized protocol and manufacturer’s manual (Fig. 1D). Grip strength in rats is tested by triggering the animal’s grasp reflex, that is, getting the rat to hold onto a bar with both front paws and suspending it by its tail while the examiner pulls gently until it releases its grip. Ponds is the unit of measure. Results are normalized to the rat’s weight (ponds/gram). The animals completed up to 15 trials (2 to 3s/trial with 10s rest between trials). Testing stopped once four criterion trials (rat grips with both front paws) were obtained and then averaged.20
Gait Testing
Examiners analyzed the rodents’ gait pattern behavior using the TreadScan 3.0 and BCamCap program (Clever Sys, Inc.) video capture system based on the ABC’s standardized protocol and manufacturer’s protocol (Fig. 1E). Animals walked within the TreadScan 3.0 for 2 minutes or until normal gait without signs of distressed were displayed, whichever came sooner. Stride length, time and width, swing and stance time, and step pattern and regularity served as parameters to assess gait behavior, patterns, and coordination.21 The ABC’s experienced research lab coordinator performed the gait analysis.
Biomarker Analysis
Circulating Stem/Progenitor Cells
Researchers deeply anesthetized rats and drew whole blood samples by cardiac puncture (7 to 10 mL), using ethylenediaminetetraacetic acid as an anticoagulant. Blood samples were collected at the same time of day since circulating stem/progenitor cell levels are known to fluctuate with circadian rhythms.22 Peripheral blood mononuclear cells were isolated by density gradient centrifugation using lymphocyte mammal (Cedarlane), according to the manufacturer’s instructions. The samples were stained for MSCs and analyzed by flow cytometric analysis with the BD LSRII housed in the Indiana University Simon Cancer Center Flow Cytometry facilities using an established protocol.23 Data were acquired uncompensated, exported as FCS 3.0 files, and analyzed using FlowJo software.
Circulating Cytokines
Researchers stored plasma and tissue samples at −80°C until consumed in assay tests. Quantitative analysis of inflammatory markers Regulated on Activation Normal T cell Expressed and Secreted (RANTES), interleukin-6 (IL-6), and tumor necrosis factor-alpha and anti-inflammatory (interleukin-10 [IL-10]) cytokines from blood plasma samples was performed on a MagPix instrument from Luminex using multiplex technology.24 The MagPix had current calibration and verification reports at the time of assay. Neuropeptide Y (NPY), a marker associated with pain modulation, was analyzed using an enzyme immunoassay kit following the manufacturer’s instructions (#EIA-ProNPY, RayBiotech, GA, USA). Duplicate samples from each subject were run simultaneously.
Data Analysis
Data analysis used repeated measures ANOVA with Bonferroni adjustment for multiple comparisons and paired t-tests as appropriate via the IBM SPSS Statistics Package 25.0. The level of significance was set at P < .05.
RESULTS
Pain/Behavior Outcomes
Back and Paw PPT
At 3 days postinjury, the back PPT lowered by 30.1% on the injured side (right) (P < .05) and 36.3% on the contralateral uninjured side (left) as compared to preinjury testing (P < .05), suggesting that injury alone increased acute pain sensitivity bilaterally. IASTM treatment lowered the back PPT from postinjury levels bilaterally, but not significantly. Nonetheless, the PPT remained significantly lower following treatment compared to preinjury levels; 46.3% lower on the injured side (P < .000) and 37.0% on the contralateral uninjured side (P < .007). However, a significant difference was found in the back PPT following IASTM treatment when compared side to side, with the injured side being 15.8% lower (P < .04) than the uninjured side. At 14 days, back PPT remained significantly decreased on both sides compared to preinjury levels (P < .001); however, IASTM treatment did not lower the PPT as it had acutely, and no side-to-side differences were detected.
Paw PPT increased 34.6% in the contralateral rear paw, opposite to the side of back injury, at 3 days postinjury following IASTM-treatment as compared to preinjury levels (P < .01), and was significantly higher than the ipsilateral paw PPT (P < .05), suggesting a distal crossover treatment effect in attenuating pain sensitivity. At 14 days, the contralateral paw PPT again increased in response to treatment, that is, lowered pain sensitivity, approaching significance (P = .08). The PPT remained lower on the ipsilateral paw, but a significant side-to-side difference no longer existed. Ipsilateral paw PPT increased somewhat following IASTM-treatment at 3 and 14 days, but not significantly. Back and paw PPT findings are summarized in Fig. 2A-D.
FIGURE 2.
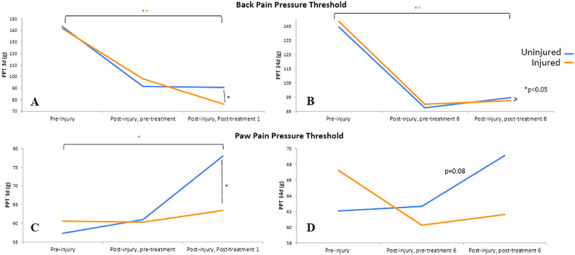
Back and paw pressure pain threshold (PPT). An Electronic Von Frey monofilament was used to test back and paw PPT. (A) 3 days postinjury, a significant drop in back PPT occurred bilaterally and lowered further on the injured side post-treatment. (B) Back PPT was also significantly lower on both sides at 14 days postinjury compared to preinjury, but a side-to-side difference no longer existed following treatment. Paw PPT increased significantly at (C) 3 days and (D) approached a significant increase at 14 days postinjury after treatment on the side contralateral to the injured side of the back.
Grip Strength
Grip strength did not vary significantly with high variability. At 3 days, however, grip strength decreased by 12% after injury and then increased by 18% following treatment to near preinjury levels, suggesting some functional improvement with intervention (Fig. 3A). Significant changes in grip strength were not detected at 14 days (not depicted).
FIGURE 3.
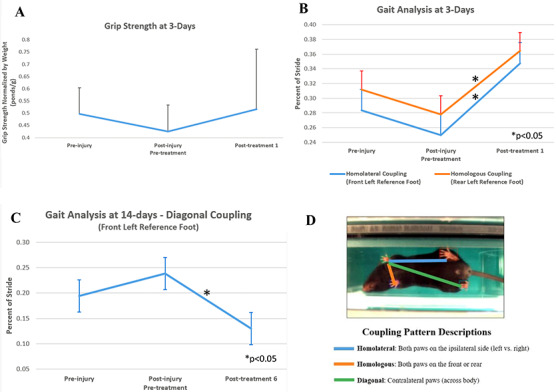
Behavioral outcomes. (A) Grip strength did not vary significantly, but returned to near preinjury levels following treatment in the 3-day injured 2h post-IASTM group. (B) Gait was analyzed using the TreadScan 3.0. Homolateral and homologous coupling patterns significantly improved, with reference to the front left and rear left paw, respectively, in the 3-day injured 2h post-IASTM group following a single treatment. Homolateral coupling represents the coordination of front and rear foot on the same side. Homologous coupling indicates the coordination between the left and right foot on the front or rear girdle. (C) Diagonal coupling was also significantly different, with reference to the front left paw, in the 14-day injured 2h post-IASTM group after 6 treatments. Diagonal coupling is the timing and coordination between a front and diagonally opposite rear foot. (D) Coupling patterns are depicted, including homolateral (front to rear paw on same side), homologous (front to opposite front paw), and diagonal coupling (front to opposite rear paw).
Gait Pattern
At 3 days, examiners observed significant improvements in gait to near preinjury levels immediately following one treatment, for homolateral and homologous coupling patterns with reference to the front left and rear left, respectively (P < .02) (Fig. 3B). The homolateral coupling parameter is the fraction of the stride of a reference foot, when the foot on the same side starts its stride. It represents the coordination of front and rear foot placement on the same side.21 In this case, it reflects how much of stride the front left limb had completed before the initiation of stride of the rear left limb. Homologous coupling is the fraction of stride reference foot when the same foot (front or rear) on the opposite side starts stride. It represents the coordination and timing between the left and right foot of the front or rear girdle.21 In this case, it reflects how much of stride the rear left foot had completed before initiation of stride on the rear right limb.
At 14 days, after repeated IASTM treatment, diagonal coupling improved to near preinjury levels with all limbs as a reference point, but a significant difference was seen in diagonal coupling with respect to the front left paw (P < .001) (Fig. 3C). Diagonal coupling is the fraction of the reference stride when the diagonal stride starts. It represents the synchronous timing and coordination between a front foot and diagonally opposite rear foot.21 In this case it reflects how much of stride the front left foot had completed before initiation of stride on the rear right limb on the same side as the injured back. No other gait parameters exhibited significant changes, including stance time, stride time or length, inter-step distance, or velocity. Fig. 3D illustrates coupling patterns definitions.
Biomarker Outcomes
Visual observation during or immediately following IASTM-treatment revealed transient hyperemia in the treated area that was not present before intervention (Fig. 4A). Dissection of the animals’ backs revealed that untreated injured tissue was generally more adherent at 14 days than treated injured tissue, with gross improvements seen in tissue quality with treatment. A representative image of a soft tissue adhesion observed at the site of injection in an injured, untreated animal is depicted in Fig. 4B. Tissue adherence could be associated with complete Freund’s adjuvant inflammation or damage from the injection.
FIGURE 4.
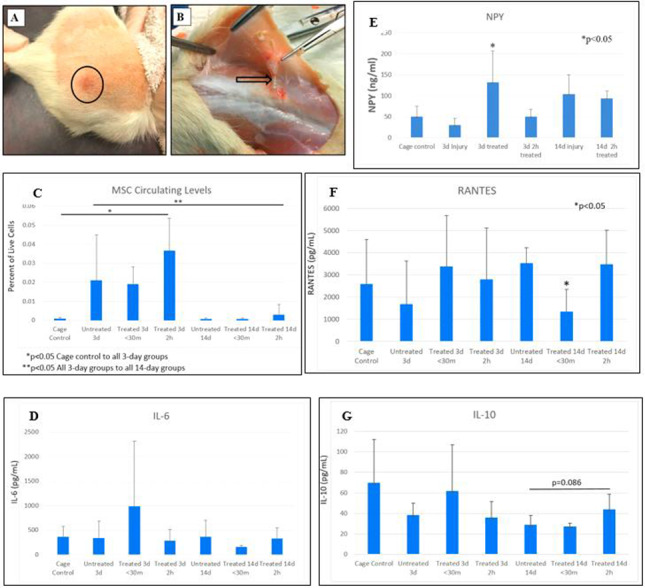
Observations and cytokine level outcomes. (A) A period of transient hyperemia observed superficially in the treated area immediately following intervention. (B) A representative image of soft tissue adhesion (arrow tip) observed at the injection site in an injured, untreated rat at 14 days. (C) Mesenchymal stem cell circulating levels varied significantly from cage controls in injured animals at 3 days. (D) Alterations in IL-6 were insignificant. (E) Neuropeptide Y (NPY) levels increased significantly post-IASTM treatment at 3 days postinjury. (F) At 14 days, regulated on activation normal T cell expressed and secreted (RANTES) significantly decreased at <30 minutes, and (G) IL-10 increased 2h after treatment.
Circulating Stem/Progenitor Cell Levels
At 3 days, circulating MSC levels varied significantly from cage controls compared to all injured groups (P < .05), but not significantly between injured, untreated, and IASTM-treated animals. Circulating MSCs were highest at 2h post-IASTM treatment but did not reach significance.
At 14 days, circulating MSC levels returned to near cage control levels, dropping significantly in all 14-day groups compared to each of the 3-day groups (P < .05). Once again, MSC levels did not vary significantly between the injured groups, whether treated or untreated. MSC levels were again highest at the 2h post-IASTM treatment time point, but not significantly. Findings are depicted in Fig. 4C.
Circulating Cytokines Levels
At 3 days, IL-6 increased at <30-minute post-IASTM treatment and declined at 2h after treatment but did not reach the level of significance with high variability (Fig. 4D). Changes in tumor necrosis factor-alpha levels were not detected (not depicted). NPY increased 3-fold (314.6%) in injured animals immediately post-IASTM treatment at 3 days (P < .05) as compared to injured untreated animals, then dropped at 2h post-IASTM treatment. NPY levels remained elevated at 14 days, but not significantly (Fig. 4E). At 14 days, circulating RANTES levels varied significantly, decreasing by 96.1% at <30-minute treatment (P < .002) and rising again to untreated levels at 2h post-treatment (Fig. 4F). At 14 days, IL-10 levels increased 53.1% 2h post-IASTM treatment with a trend toward significance (P = .086) as compared to untreated animals (Fig. 4G).
DISCUSSION
This preliminary study explored the effects of soft tissue manipulation on pain sensitivity, function, and selected biomarkers in conscious rodents with chemically induced unilateral LBP. The back PPT initially decreased on both sides and lowered even more on the IASTM-treated injured side at 3 days postinjury, suggesting increased pain sensitivity with injury and possibly treatment. After 14 days, the difference between the sides of the back equalized with repeated IASTM applications, although never returned to preinjury levels. Unexpectedly, rear paw PPT improved in the hind limb contralateral to the side of back injury, suggesting a possible positive crossover effect distally in response to treatment. Grip strength did not vary significantly. Gait coupling patterns improved to near preinjury values at 3 and 14 days. MSC circulating levels varied significantly in response to acute injury, but not in response to treatment. Pain modulating and inflammatory biomarkers varied significantly with treatment. Soft tissue manipulation may help to attenuate inflammation, promote pain modulation, and improve gait function. Findings have positive implications for military personnel and civilians with LBP.
This research presents a novel method of delivering soft tissue manipulation/massage in conscious rodents with LBP, using an established experimental rodent model for chemically induced chronic inflammation. It is important to emphasize the animals’ level of consciousness, since soft tissue manual therapy is typically performed on conscious humans in the clinic, and sentient awareness of the manipulating stimulus may influence results. Investigators used IASTM to deliver a targeted force to mobilize the tissue in the injured low back region. This model represents a replicable method for translational research exploring the underlying mechanisms of soft tissue manual therapy.
The method used to assess the threshold for pain in response to pressure (i.e., PPT) is an indicator of mechanical hyperalgesia. A lower PPT is associated with heightened pain sensitivity.18,25 This study found the animals’ sensitivity to pain in the back increased with injury and worsened to some degree with treatment acutely. This appears to mirror what occurs in the clinic, as patients with acute injury may report increased tenderness in the affected region post-IASTM treatment.26 After repeated IASTM treatments, the animals’ heightened pain sensitivity diminished to some degree and equalized between sides, although remained significantly higher when compared to preinjury levels.
Interestingly, at 3 days, paw PPT significantly increased distally on the opposite limb and did not lower on the ipsilateral limb, suggesting a distal crossover effect of decreased pain sensitivity may occur in response to treatment. Crossover effects have been demonstrated in other preclinical studies investigating the effects of mimetic massage on muscle atrophy in sarcopenia and stroke models.27,28 The underlying mechanisms and potential ability of soft tissue manual therapy to positively affect remote sites of pain and healing requires additional investigation. Furthermore, studies need to determine optimal IASTM treatment dose frequencies and dose pressures for restoring pain sensitivity to uninjured levels.
The method for testing grip strength, that is, getting the rat to hold onto a bar with both front paws while the examiner gently pulls through its tail, imparts a stretch or traction to the rodent’s spine.29 In this study, grip strength decreased after injury but increased after treatment, suggesting some functional improvement with soft tissue manual therapy. However, these findings did not reach the level of significance as a result of high subject variability. Further testing using larger sample sizes is needed.
Gait assessment found improved coupling patterns following IASTM-treatment to near preinjury levels, reflecting better motion patterns, timing, and trunk elongation. Homolateral coupling on the side opposite the injured back decreased acutely following injury, but significantly increased immediately following treatment, signaling increased contralateral trunk lengthening and coordination between the front and rear limbs. This represents a potential positive crossover effect from the side of soft tissue manipulation. Homologous coupling also significantly improved, signifying improved timing and coordination in pelvic girdle and hind limb motion in response to treatment. Diagonal coupling improved with all limbs after repeated treatments, but reached significance with respect to the front limb opposite the side of back injury, representing improved timing and coordination during reciprocal gait.
Circulating stem cell levels have been shown to increase with other forms of mechanotherapy.12,13 Enhanced healing, decreased neuropathic pain, and anti-inflammation have been linked to increased circulating stem cell levels.30 In this study, an acute increase in MSC cell circulation levels appeared to exist in response to injury at 3 days, with IASTM treatment having limited effect. MSC circulation levels did increase at 2h post-IASTM treatment, but the effect did not reach significance. In the longer term, at 14 days, circulating MSC levels returned to baseline levels, and once again, MSC levels increased at 2h post-IASTM treatment but not significantly. The inability to detect a significant treatment effect at 2h post-IASTM may be a result of high variability in a small sample size. Future studies should include a larger sample size and consider additional stem/progenitor cell populations.
Soft tissue manual therapy may have an anti-inflammatory effect in the longer term, as suggested by a significant decrease in RANTES levels immediately following IASTM-treatment and a trend in increased IL-10 levels. RANTES is a pro-inflammatory cytokine.31 IL-10 promotes anti-inflammation.32 Elevated NPY levels suggest IASTM intervention may help to modulate pain. NPY is a small amino acid that has many functions, one being pain modulation.33 Findings could have positive implications for the use of manual therapy in LBP and other inflammatory conditions and warrants additional exploration. This is noteworthy since inflammation is an underlying or associated cause in several major disease processes, including cardiovascular disease, arthritis, and cancer.34
Limitations
The experiments used only male rodents. The focus on male rodents aimed to reduce subject variability since at least one of the outcome measures, circulating stem/progenitor cell levels, is known to vary with biological sex.35 Nonetheless, females must be considered in future studies. This study used chemically induced chronic inflammatory LBP in rodents, but exploration in other conditions and humans is needed.
Future Directions
Subsequent studies could consider preclinical models of LBP with larger sample sizes. Future studies should explore tissue-level effects and the dose-pressure and dose-frequency response of soft tissue manual therapy on LBP outcomes. Sampling at different time points beyond 2h post-treatment may prove informative. The impact of this noninvasive modality on pain, function, and biomarker outcomes requires consideration in human populations.
CONCLUSIONS
This study explored the effects of a form of soft tissue manual therapy, IASTM, using a chronic inflammatory LBP model in rats. Results indicate IASTM may help to modulate pain, attenuate chronic inflammation, and improve functional gait. LBP lowered PPTs, sensitizing subjects to painful stimuli. Interestingly, pain sensitivity decreased in the hind limb opposite the side of back injury subsequent to IASTM treatment, suggesting this intervention may have positive, distal crossover effects. Of importance, conscious animals were the recipients of IASTM in this study, which may enhance the translation of findings. Findings suggest soft tissue manual therapy may be useful as a conservative treatment option for LBP in warfighters and other populations.
ACKNOWLEDGMENTS
Ryan Ball and Josh Roy, Indiana University, for undergraduate research assistance. Dr. Gwendolyn Sowa, University of Pittsburgh, for her and her lab’s assistance and training in analysis of mechanosensitive biomarkers. Dr. Xiao-Ming Xu, Indiana University, for use of his lab space, research assistants, and equipment during this project
Contributor Information
M Terry Loghmani, Department of Physical Therapy, School of Health and Human Sciences, Indiana University, Indianapolis, IN 46202, USA.
Carolyn Tobin, Department of Physical Therapy, School of Health and Human Sciences, Indiana University, Indianapolis, IN 46202, USA.
Colleen Quigley, Department of Physical Therapy, School of Health and Human Sciences, Indiana University, Indianapolis, IN 46202, USA.
Alanna Fennimore, Department of Physical Therapy, School of Health and Human Sciences, Indiana University, Indianapolis, IN 46202, USA.
FUNDING
Supported in part by the National Institute of Child Health and Human Development (NICHD). University of Pittsburgh Alliance for Regenerative Rehabilitation Research Training (AR3T) grant and research sabbatical (PTE Federal Award No. 5P2CHD086843-03); Indiana University Purdue University Indianapolis (IUPUI) Release Time for Research grant; IUPUI FORCES grant; IUPUI Life Health Sciences Internship program; IUPUI Undergraduate Research Opportunities Program, and PI start-up fund.
REFERENCES
- 1. Hoy DG, Smith E, Cross M, et al. : Reflecting on the global burden of musculoskeletal conditions: lessons learnt from the global burden of disease 2010 study and the next steps forward. Ann Rheum Dis 2015; 74(1): 4-7. [DOI] [PubMed] [Google Scholar]
- 2. Knox J, Orchowski J, Scher DL, Owens BD, Burks R, Belmont PJ: The incidence of low back pain in active duty United States military service members. Spine 2011; 36(18): 1492-500. [DOI] [PubMed] [Google Scholar]
- 3. Roy TC: Diagnoses and mechanisms of musculoskeletal injuries in an infantry brigade combat team deployed to Afghanistan evaluated by the brigade physical therapist. Mil Med 2011; 176(8): 903-8. [DOI] [PubMed] [Google Scholar]
- 4. Goulet JL, Kerns RD, Bair M, et al. : The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain 2016; 157(8): 1696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Institutes of Health Department of Health and Human Services : NIH-wide strategic plan Fiscal Years 2016-20. National Health Interview Survey 2012. Available at https://www.nih.gov/sites/default/files/about-nih/strategic-plan-fy2016-2020-508.pdf; accessedMarch 20, 2020.
- 6. American Physical Therapy Association Beyond opioids: how physical therapy can transform pain management to improve health. APTA White Paper 2018. Available at https://www.apta.org/uploadedFiles/APTAorg/Advocacy/Federal/Legislative_Issues/Opioid/APTAOpioidWhitePaper.pdf; accessed March 20, 2020.
- 7. Kumar S, Beaton K, Hughes T: The effectiveness of massage therapy for the treatment of nonspecific low back pain: a systematic review of systematic reviews. Int J Gen Med 2013; 6: 733-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson WR, Scott A, Loghmani MT, Ward SR, Warden SJ: Understanding mechanobiology: physical therapists as a force in mechanotherapy and musculoskeletal regenerative rehabilitation. Phys Ther 2016; 96(4): 560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Field T: Massage therapy research review. Complement Ther Clin Pract 2014; 20(4): 224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loghmani MT, Warden SJ: Instrument-assisted cross fiber massage increases tissue perfusion and alters microvascular morphology in the vicinity of healing knee ligaments. BMC Complement Altern Med 2013; 13(240): 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crane JD, Ogborn DI, Cupido C, et al. : Massage attenuates inflammatory signaling after exercise-induced muscle damage. Sci Transl Med 2012; 4(119): 1-8. [DOI] [PubMed] [Google Scholar]
- 12. Jawed Y, Beli E, March K, Kaleth A, Loghmani MT: Whole-body vibration training increases stem/progenitor cell circulation levels and may attenuate inflammation. Mil Med 2020; 185(Suppl 1): 404-12. doi: 10.1093/milmed/usz247 [DOI] [PubMed] [Google Scholar]
- 13. Boppart MD, De Lisio M, Witkowski S: Exercise and stem cells. Prog Mol Biol Transl Sci 2015; 135: 423-56. [DOI] [PubMed] [Google Scholar]
- 14. Hoheisel U, Mense S: Inflammation of the thoracolumbar fascia excites and sensitizes rat dorsal horn neurons. Eur J Pain 2015; 19(3): 419-28. [DOI] [PubMed] [Google Scholar]
- 15. Hoheisel U, Reuter R, Fernandes de Freitas M, Treede R-D, Mense S: Injection of nerve growth factor into a low back muscle induces long-lasting latent hypersensitivity in rat dorsal horn neurons. Pain 2013; 154(10): 1953-60. [DOI] [PubMed] [Google Scholar]
- 16. Graston Technique: Available at https://www.grastontechnique.com; accessed March 18, 2020.
- 17. Al Otaibi AM, Chien S, Loghmani MT, Anwar S: Force and motion sensing for instrument-assisted soft tissue manipulation device. J Med Devices 2017; 11(3). doi: 10.1115/1.4036654 [DOI] [Google Scholar]
- 18. Den Bandt L, Paulis WD, Beckwee D, Ickmans K, Nijs J, Voogt L: Pain mechanisms in low back pain: a systematic review with meta-analysis of mechanical quantitative sensory testing outcomes in people with nonspecific low back pain. J Orthop Sports Phys Ther 2019; 49(10): 698-715. [DOI] [PubMed] [Google Scholar]
- 19. IITC Life Science : Electronic Von Frey Monofilament. Available at http://www.iitcinc.com/Product%20pages/Analgesia/vonfrey.html; accessed March 20, 2020.
- 20. TSE Systems : TSE Grip Strength Meter. Available at https://www.tse-systems.com/product-details/grip-strength-meter; accessed March 20, 2020.
- 21. CleverSys, Inc .: TreadScan™ 3.0 User’s Guide. Available at https://www.cleversysinc.com/CleverSysInc/cis_products/treadscan; accessed March 18, 2020.
- 22. Mendez-Ferrer S, Lucas D, Battista M, Frenette PS: Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008; 452: 442-7. [DOI] [PubMed] [Google Scholar]
- 23. Estes ML, Mund JA, Mead L, et al. : Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry A 2010; 77A(9): 831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luminex : MAGPIX Multiplexing. Available at https://www.luminexcorp.com/magpix; accessed March 18, 2020.
- 25. Walton D, MacDermid J, Nielson W, Teasell R, Chiasson M, Brown L: Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J Orthop Sports Phys Ther 2011; 41(9): 644-50. [DOI] [PubMed] [Google Scholar]
- 26. Vardiman JP, Siedlik J, Herda T et al. : Instrument-assisted soft tissue mobilization: effects on the properties of human plantar flexors. Int J Sports Med 2014; 36(3): 197-203. [DOI] [PubMed] [Google Scholar]
- 27. Miller BF, Hamilton KL, Majeed ZR, et al. : Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol 2018; 596 (1): 83-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sen CK, Khanna S, Harris H, et al. : Robot-assisted mechanical therapy attenuates stroke-induced limb skeletal muscle injury. FASEB J 2017; 31(3): 927-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corey SM, Vizzard MA, Bouffard NA, Badger GJ, Langevin HM, Baccei ML: Stretching of the back improves gait, mechanical sensitivity and connective tissue inflammation in a rodent model. PLoS One 2012; 7(1): 29831. doi: 10.1371/journal.pone.0029831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ennis WJ, Sui A, Bartholomew A: Stem cells and healing: impact on inflammation. Adv Wound Care 2013; 2(7): 369-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van den Berg R, Jongbloed EM, de Schepper EIT, Bierma-Zeinstra SMA, Koes BW, Luijsterburg PAJ: The association between pro-inflammatory biomarkers and nonspecific low back pain: a systematic review. Spine J 2018; 18(11): 2140-51. [DOI] [PubMed] [Google Scholar]
- 32. Cabral-Santos C, de Lima Junior E, Fernandes I, et al. : Interleukin-10 responses from acute exercise in healthy subjects: a systematic review. J Cell Physiol 2018; 234(7): 9956-65. doi: 10.1002/jcp.27920 [DOI] [PubMed] [Google Scholar]
- 33. Diaz-delCastillo M, Woldbye DPD, Heegaard AM: Neuropeptide Y and its involvement in chronic pain. Neuroscience 2018; 387: 162-9. [DOI] [PubMed] [Google Scholar]
- 34. Guo H, Callaway JB, Ting JP-Y: Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015; 21(7): 677-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aksu AE, Rubin JP, Dudas JR, et al. : Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg 2008; 60(3): 306-22. [DOI] [PubMed] [Google Scholar]


