ABSTRACT
Introduction
The current study was designed to test the potential role of recombinant human MG53 (rhMG53) protein on protecting against alkaline-induced corneal injury in mice.
Materials and Methods
A round filter paper with 2-mm diameter was soaked in 1 mol/L of NaOH solution. The mouse alkaline injury was generated by placing the filter paper directly on the cornea for 30 seconds and washed with 30-mL saline; 10 µL of rhMG53 solution (20 µg/mL) or saline control was topically administrated on the mouse corneas (twice per day for 10 days). Re-epithelialization was measured by fluorescein staining and imaged by a slit lamp equipped with a digital camera. Clinical neovascularization and opacity scores were measured every day after injury. Ten days after injury, mice were sacrificed and corneas were dissected out for flat mount staining of CD31 for neovascularization.
Results
MG53 was present in both dog aqueous humor and human tears. mg53-/- corneas were more susceptible to alkaline-induced corneal injury. Topical treatment of rhMG53 improved re-epithelialization, suppressed neovascularization, and fibrosis induced by alkaline injury.
Conclusions
rhMG53 may be an effective means to treat corneal wounding.
INTRODUCTION
Corneal injuries commonly occur in the battlefield because of exposure to shell fragments and grenades, blast trauma from improvised explosive devices, chemical weapons (mustard agent), or high intensity lasers.1,2 Whether the cause of these injuries are traumatic or pathologic (i.e., corneal infection), the resulting sequela are often loss of transparency because of corneal fibrosis. Facilitating repair of the corneal epithelium and preventing scar formation and vascularization will have beneficial effects in treating ocular injuries and reducing significant visual impairment.
The cornea is one of the most densely innervated structures in the body, making corneal wounds exceptionally painful. Delays in repair increase the risk of corneal scarring and vision loss. The standard treatment of corneal wounds includes maximizing preservative-free topical lubricants, minimizing evaporative tear loss, applying antibiotics and tetracycline, protecting the surface with a bandage soft contact lens, and performing surgery. The choice of treatments depends on the severity of injury and corneal involvement. However, even in combination, these measures often lack efficacy.3–5 The failure of standard therapies and difficulty in formulating strategies to prevent corneal ulcers has triggered interest in developing drugs/interventions based on specific biological mechanisms. Unfortunately, there are no FDA-approved biologic treatments that facilitate corneal wound healing.
Our previous studies have shown that MG53, a member of tripartite motif (TRIM) family proteins, plays an important role in protecting against multiple organ injuries, including skeletal muscle, lung, heart, kidney, brain, and skin.6–12 We found that application of recombinant human MG53 (rhMG53) protein could promote tissue repair and regeneration and inhibit fibrotic remodeling associated with tissue injuries. In light of these studies, we recently published a study to explore the potential role of MG53 in corneal wound healing.13 We found that mg53-/- corneas are susceptible to alkaline-induced injury, and when treated with rhMG53 protein, mg53-/- corneas recovered better than those treated with saline as control.
In the present study, we performed alkaline-induced corneal injury with wild-type mice. We found that consistent with the observations in mg53-/- animals, topical application of rhMG53 could facilitate re-epithelialization of injured corneas. Furthermore, neovascularization and fibrosis associated with corneal injury were also suppressed by rhMG53 treatment. Our study demonstrated that topical administration of rhMG53 could effectively promote corneal healing in wild-type mice. Thus, rhMG53 might be a potential therapeutic to treat human corneal wounds in both military and civilian settings.
METHODS
rhMG53 Protein Production and Quality Control
Escherichia coli fermentation was used to produce high-purity (>97% purity) rhMG53 protein.7 Each batch of rhMG53 was tested with established microglass bead injury assay to ensure its activity for plasma membrane repair.7,14
Sample Collection
The canine aqueous humor collection is approved by The Ohio State University Institutional Animal Care and Use Committee (IACUC). To evaluate expression of MG53 in the media surrounding the cornea, aqueous humor was collected from normal canine cadaveric globes immediately following enucleation. All samples were immediately frozen at −80°C until analysis.
A total of 6 human volunteers were enrolled (age >18 years). This study was approved by The Ohio State University Institutional Review Board. Participants signed an informed consent document following the tenets of the Declaration of Helsinki. Nonreflex tears were collected from the inferior tear prism using 5-μL Drummond glass microcapillary tubes. All samples were immediately frozen at −80°C until analysis.
Western Blot
Dog aqueous humor and human tear samples were separated by sodium dodecyl sulfate-Polyacrylamide Gel Electrophoresis. Proteins were electro transferred to polyvinylidene difluoride membranes with a transfer device (Bio-Rad, Hercules, CA). The membranes were washed with phosphate-buffered saline/tween (phosphate-buffered saline + 0.5% Tween-20), blocked with 5% nonfat milk in phosphate-buffered saline/tween for 1 hour, and incubated with MG53 primary antibody overnight at 4°C on an orbital shaker. Anti-rabbit Immunoglobulin G (IgG) horseradish peroxidase conjugated secondary antibody was applied at 1:5000 dilution and incubated for 2 hours with shaking at room temperature. Blots were visualized with an enhanced chemoluminescence plus kit (Pierce, Rockford, IL).
Animal and In Vivo Corneal Wound Healing Mouse Model
All animal care and usage followed National Institute of Health guidelines and were in accordance with the Association for research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Mouse studies received Institutional Care and Use Committee approval by The Ohio State University. For corneal wound healing model, injury was induced under anesthesia and all animals received topical and systemic analgesics for at least 72 hours after injury for pain management.
C57BL/6J wild-type mice were obtained from The Jackson Laboratory. A filter paper disc (2-mm diameter) soaked with 1 mol/L of NaOH was placed on the axial cornea for 30 seconds to induce injury; 10 µL of rhMG53 (20 µg/mL) or saline solution were topically applied to corneas (twice per day, for 10 days). After application of solutions, the mice were put into an isoflurane filled gas chamber for 10 minutes before transferred back to the cages for recovery.
The clinical opacity and vascularization scores were determined by using a handheld slit lamp (Kowa, SL-17, Nagoya, Japan), using a modified Hackett-McDonald scoring system. Ten days postalkaline injury, the mice were sacrificed and eyes underwent analyses. In order to obtain details regarding the responses to rhMG53 or saline treatment, globes were fixed either for horizontal sectioning or flat mount staining.
Immunohistochemistry
Enucleated globes were fixed in 4% paraformaldehyde (PFA) overnight at 4°C. After fixation, samples were washed with 70% ethanol. Washed samples were processed and embedded in paraffin. Paraffin sections (5 μm) were cut and stained with Hematoxylin-Eosin.
Immunofluorescent staining of CD31 was performed using flat mount corneas following previously published studies.13,15 Briefly, enucleated globes were fixed in 4% PFA for 1 hour at 4°C. Corneas were carefully dissected out (Leica, Stereo Zoom 4 Microscope) and placed back in 4% PFA for fixation overnight at 4°C. Subsequently, corneas were washed in Phosphate Buffered Saline (PBS) and incubated in blocking buffer (0.5% Triton X-100 and 5% goat serum in PBS) for at least 2 hours at room temperature to permeabilize the tissue and prevent nonspecific binding of the primary antibody. Anti-CD31 (BD Biosciences, Cat. No. 550274) antibody in blocking buffer was applied to the tissue and incubated at 4°C overnight. After 6 washes (1 hour per wash at room temperature) with washing buffer (0.5% Triton X-100 in PBS), a secondary antibody in blocking buffer was applied to the corneas and incubated overnight at 4°C. Tissues were washed with PBS (three times, 1 hour/wash) at room temperature. Corneas were transferred into a dissection plate and four incisions from periphery towards the center of the corneas were made to facilitate flat mount for imaging.
Statistical Analysis
All data are expressed as mean ± standard error of the mean. Groups were compared by Student’s t-test and analysis of variance for repeated measures. A value of P < 0.05 was considered significant.
RESULTS
MG53 Is Present in Aqueous Humor and Tear Film
MG53 (TRIM72) belongs to tripartite motif (TRIM) protein family. Human MG53 protein has 477 amino acids with four TRIM signature domains (RING, B-box, Coiled-coil and SPRY) (Fig. 1A). To evaluate the potential role of MG53 in corneal physiology, we performed Western blot analysis and found that MG53 protein can be detected in both canine aqueous humor (Fig. 1B) and human tear film (Fig. 1C). This observation suggested that first, MG53 might play a role in ocular physiology. Thus, application of rhMG53 is less likely to cause adverse effects because of the endogenous presence of MG53 in body fluid directly contacting the cornea.
FIGURE 1.
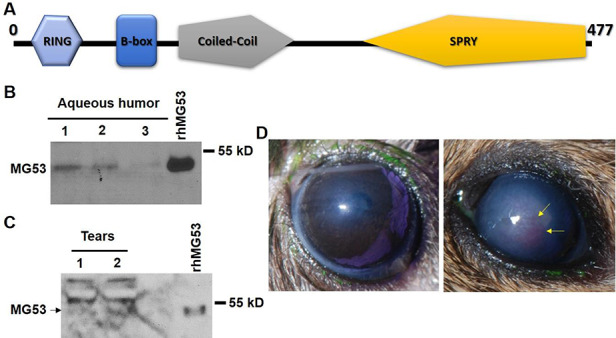
MG53 is present in aqueous humor and tears; genetic ablation of MG53 leads to compromised corneal wound healing. (A) MG53 is a member of TRIM family proteins with RING, B-box, Coiled-coil, and SPRY domains. Endogenous MG53 can be detected in canine aqueous humor (B) and human tear (pointed by the arrow) (C) samples. Bands above 55 kDa in (C) are nonspecific protein recognized by the antibody. (D) After alkaline-induced corneal injury, mg53-/- cornea developed higher level of opacity and vascular invasion as compared to those in wild-type cornea.
mg53-/- Corneas Are Susceptible to NaOH-Induced Injury
We then performed NaOH-induced corneal injury in both mg53-/- and wild-type littermate mice. We found that wild-type corneas developed less vascular invasion and opacity than mg53-/- corneas following alkaline injury, which is consistent with our previous observation.13 This observation further suggested that MG53 plays a critical role in corneal wound healing (Fig. 1D).
rhMG53 Promotes Corneal Wound Healing in Wild-Type Mice
Our previous studies have shown that rhMG53 treatment could improve the compromised healing capacity in mg53-/- mice. However, in order to develop rhMG53 as a potential therapy for human corneal wound, we have to treat wild-type corneas to determine whether application of rhMG53 is still effective when the animals have endogenous MG53.
Intact corneal epithelial is not permeable for fluorescein, a green chemical dye. Thus, increased fluorescein staining on cornea indicates damage in corneal epithelium. As shown in Fig. 2A, treatment of rhMG53 promoted re-epithelialization as evidenced by fluorescein staining (47.1% ± 29.8% in control group versus 14.9% ± 4.2% in rhMG53-treated group at day 3 after injury, P < 0.05). Furthermore, rhMG53 treatment group showed consistently lower opacity score (Fig. 2B), as evidenced by more transparent appearance of the cornea (lower panels). Similar observation was made for vascular invasion following alkaline injury. As shown in Fig. 2C, clinical neovascularization score in rhMG53 was lower in the rhMG53-treated group. Ten days after the injury, corneas were dissected and stained with CD31, a blood vessel marker. Clearly, the pronounced vascular invasion in injured corneas was reduced by rhMG53 treatment (Fig. 2C). Taken together, the present study provides evidence that suggests potential protective effects of rhMG53 in corneal injury in wild-type mice.
FIGURE 2.
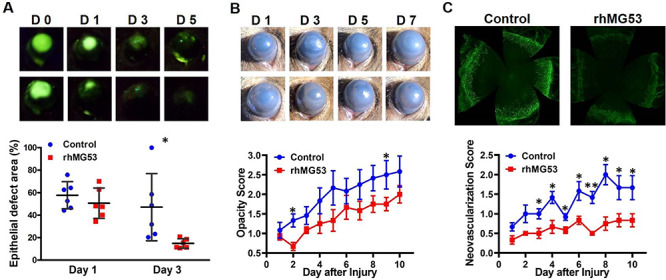
rhMG53 treatment promotes re-epithelialization and suppresses fibrosis and neovascularization after corneal wound in wild-type mice. (A) Representative images of fluorescein staining of corneas at indicated time points (upper panels) and quantification of fluorescein intensity by ImageJ software. (B) Representative macro photos of injured corneas at indicated time points (upper panels) and opacity scores (lower panel). (C) Flat mount confocal images of CD31 signal in injured corneas from saline control group and rhMG53-treated group (upper panels). Clinical opacity scores after alkaline injury were summarized (lower panel). (n = 6 per group. *P < 0.05; **P < 0.01).
DISCUSSION
Ocular trauma has been shown as one of the major causes of morbidity and mortality of military members; however, the effective treatments are largely limited. After injury, cornea, as the window of the eye, unlike skin, requires quick healing without excessive fibrosis and neovascularization.16,17 A series of studies from our group and other groups have established that rhMG53 could promote tissue healing while inhibiting fibrosis and inflammation.6–10,12,13,18 Thus, in the present study, we designed experiments to test whether rhMG53 treatment could promote corneal wound healing while inhibiting fibrotic remodeling and vascular invasion that impair vision. Indeed, our data demonstrated that treatment of rhMG53 could facilitate re-epithelialization after alkaline-induced corneal injury. More importantly, rhMG53 could maintain corneal transparency by limiting fibrosis and vascular invasion associated with corneal injury. Thus, our finding suggests that rhMG53 might be an effective means to treat corneal injury.
Of course, more studies are required for development of rhMG53 as a therapeutic for human treatment. For example, although alkaline injury is a major form of corneal injury, mechanical ocular injury is also common in battlefields. Determining the effectiveness of rhMG53 in treating mechanical corneal injuries will be critical for developing rhMG53 for military applications. In addition, although we have shown rhMG53 treatment could inhibit corneal fibrosis via the Transforming growth factor beta (TGF-β) pathway, more experiments are required to determine the molecular mechanisms underlying MG53 mediated inhibition of neovascularization.13 Especially, the role of each domain of the TRIM motif (RING, B-box, coiled-coil) and SPRY domain in MG53 should be investigated in future studies. More importantly, in the present study, we administrated rhMG53 in saline solution, which greatly limited time for rhMG53 treatment. To overcome this limitation, more studies will be planned to develop an rhMG53 delivery approach that maintains rhMG53 on the ocular surface for a relatively long time without washout by tear or degradation. For this purpose, a nontoxic and transparent hydrogel might be a better carrier for rhMG53 delivery on ocular surface. Together with hydrogel, a standard protein carrier, such as albumin, should be used in the future experiments, which can serve as a better control to show the contribution of hydrogel itself to wound healing.
Corneal wound healing is a complex process. In the current study, we have demonstrated the role of rhMG53 in relatively short term (10 days) after injury. A long-term study will help us understand the role of rhMG53 on chronic healing process. It will also be critical to test whether the protective role of rhMG53 applies to other injurious conditions, such as corneal transplantation. Finally, development of a protocol to generate Good manufacturing practices (GMP) -level rhMG53 is also critical for human trials. All of these studies require collaborations among scientists and physicians with complimentary expertise and strong funding support.
CONCLUSION
The current study suggests that MG53 plays a critical role in ocular physiology, and application of rhMG53 protein could serve as a potential therapeutic to treat ocular trauma in both military and civilian applications.
Contributor Information
Owen Guo, Dublin Jerome High School, Dublin, OH 43016, USA; Department of Surgery, Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA.
Brent Ju, Upper Arlington High School, Upper Arlington, OH 43221, USA; Department of Surgery, Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA.
McKinley H Shawver, Department of Surgery, Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA.
Bingchuan Geng, MD, Department of Surgery, Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA.
Siqi Wei, Department of Surgery, Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA.
Terriah Early, Department of Surgery, Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA.
Frank Yi, BS, Department of Surgery, Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA.
Tao Tan, MD, PhD, Department of Surgery, Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA; TRIM-edicine, Inc, Columbus, OH 43212, USA.
Heather L Chandler, PhD, College of Optometry, The Ohio State University, Columbus, OH 43210, USA.
Jianjie Ma, PhD, Department of Surgery, Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA; Dublin Jerome High School, Dublin, OH 43016, USA.
Hua Zhu, PhD, Department of Surgery, Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA; Dublin Jerome High School, Dublin, OH 43016, USA.
FUNDING
This study was supported by a grant from U.S. Department of Defense (W81XWH1810787) and grants from National Institutes of Health (EY030621, GM123887).
CONFLICT OF INTEREST
JM and TT have an equity interest in TRIM-edicine, Inc., which develops MG53 for treatment of human diseases. Patents on the use of MG53 are held by Rutgers University and The Ohio State University.
REFERENCES
- 1. Ari AB: Eye injuries on the battlefields of Iraq and Afghanistan: public health implications. Optometry - J Am Optometric Assoc 2006; 77(7): 329-39. [DOI] [PubMed] [Google Scholar]
- 2. Hilber D, Mitchener TA, Stout J, Hatch B, Canham-Chervak M: Eye injury surveillance in the U.S. department of defense, 1996-2005. Am J Prev Med 2010; 38(1S): S78-85. [DOI] [PubMed] [Google Scholar]
- 3. Tuli SS, Schultz GS, DM D: Science and strategy for preventing and managing corneal ulceration. Ocul Surf 2007; 5(1): 23-39. [DOI] [PubMed] [Google Scholar]
- 4. Nishida T: Translational research in corneal epithelial wound healing. Eye Contact Lens 2010; 36(5): 300-04. [DOI] [PubMed] [Google Scholar]
- 5. Abdelkader H, Patel DV, CNJ M, RG A: New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Exp Ophthalmol 2011; 39(3): 259-70. [DOI] [PubMed] [Google Scholar]
- 6. Zhu H, Hou J, Roe JL, et al. : Amelioration of ischemia-reperfusion-induced muscle injury by the recombinant human MG53 protein. Muscle Nerve 2015; 52(5): 852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weisleder N, Takizawa N, Lin P, et al. : Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med 2012; 4(139): 139ra185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia Y, Chen K, Lin P, et al. : Treatment of acute lung injury by targeting MG53-mediated cell membrane repair. Nat Commun 2014; 5: 4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu J, Zhu H, Zheng Y, et al. : Cardioprotection of recombinant human MG53 protein in a porcine model of ischemia and reperfusion injury. J Mol Cell Cardiol 2015; 80: 10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duann P, Li H, Lin P, et al. : MG53-mediated cell membrane repair protects against acute kidney injury. Sci Transl Med 2015; 7(279): 279ra236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yao Y, Zhang B, Zhu H, et al. : MG53 permeates through blood-brain barrier to protect ischemic brain injury. Oncotarget 2016; 7(16): 22474-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li H, Duann P, Lin PH, et al. : Modulation of wound healing and scar formation by MG53 protein-mediated cell membrane repair. J Biol Chem 2015; 290(40): 24592-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chandler HL, Tan T, Yang C, et al. : MG53 promotes corneal wound healing and mitigates fibrotic remodeling in rodents. Commun Biol 2019; 2: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu H, Lin P, De G, et al. : Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair. J Biol Chem 2011; 286(15): 12820-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson C, Zhou Q, Wang S: An alkali-burn injury model of corneal neovascularization in the mouse. J Vis Exp 2014; 86. doi: 10.3791/51159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pieramici DJ, Sternberg P Jr, Aaberg TM Sr, et al. : A system for classifying mechanical injuries of the eye (globe). The ocular trauma classification group. Am J Ophthalmol 1997; 123(6): 820-31. [DOI] [PubMed] [Google Scholar]
- 17. Mader TH, Aragones JV, Chandler AC, et al. : Ocular and ocular adnexal injuries treated by united states military ophthalmologists during operations desert shield and desert storm. Ophthalmology 1993; 100(10): 1462-7. [DOI] [PubMed] [Google Scholar]
- 18. Gushchina LV, Bhattacharya S, McElhanon KE, et al. : Treatment with recombinant human MG53 protein increases membrane integrity in a mouse model of limb girdle muscular dystrophy 2b. Mol Ther 2017; 25(10): 2360-71. [DOI] [PMC free article] [PubMed] [Google Scholar]


