Abstract
There are minimal data regarding the prevalence of cancer in patients with coronavirus disease 2019 (COVID-19), as well as the incidence of severe illness and rate of mortality in COVID-19 patients with cancer. PubMed, Embase, Cochrane Library, and Web of Science were systematically searched, from database inception to July 15, 2020, for studies of patients with COVID-19 that included information regarding comorbid cancer. In total, 109 eligible global studies were included in this systematic review. Ninety studies with 94,845 COVID-19 patients, among which 4106 exhibited comorbid cancer, were included in the meta-analysis regarding prevalence of comorbid cancer. Twenty-three studies with 71,969 COVID-19 patients, among which 4351 with comorbid cancer had severe illness or death, were included in the meta-analysis. The overall prevalence of cancer among COVID-19 patients was 0.07 (95% CI 0.05–0.09). The cancer prevalence in COVID-19 patients was higher in Europe (0.22, 95% CI 0.17–0.28) than in the Asia-Pacific region (0.04, 95% CI 0.03–0.06) or North America (0.05, 95% CI 0.04–0.06). The cancer prevalence in COVID-19 patients aged >60 years was 0.10 (95% CI 0.07–0.14), while the prevalence among patients aged ≤60 years was 0.05 (95% CI 0.03–0.06). The pooled prevalence of severe illness among COVID-19 patients with cancer was 0.34 (95% CI 0.26–0.42) and the pooled mortality rate of COVID-19 patients with cancer was 0.20 (95% CI 0.16–0.25). Pooled incidences of severe illness among COVID-19 patients with cancer from Asia Pacific, Europe, and North America were 0.38 (95% CI 0.24–0.52), 0.39 (95% CI 0.25–0.53), and 0.26 (95% CI 0.20–0.31), respectively; pooled mortality rates from the Asia-Pacific region, Europe, and North America were 0.17 (95% CI 0.10–0.24), 0.26 (95% CI 0.18–0.35), and 0.19 (95% CI 0.13–0.25), respectively.
Keywords: Covid-19, Cancer, Clinical characteristics, Epidemiology
1. Introduction
Globally, more than 18.5 million confirmed cases of coronavirus disease 2019 (COVID-19) have been reported (https://www.worldometers.info/coronavirus). As infection has become widespread, concern has grown regarding the influence of COVID-19 on patients with cancer. The results of previous studies suggest that patients with a history of active malignancy might have greater risks of COVID-19 onset, COVID-19-related complications, and worse prognosis [1,2]. These risks are partly related to the following factors [3]: 1) a systemic immunosuppressive state caused by malignancy and anticancer treatments, such as chemotherapy, surgery, or immunomodulatory drugs (e.g., programmed death 1 [PD-1]/programmed death-ligand 1 [PD-L1] inhibitors) [[4], [5], [6]]; 2) older ages and major comorbidities including cancer, which increase the risks of COVID-19-related morbidity and mortality [7]; and 3) greater contact with the healthcare system through provider visits for anticancer therapy, monitoring, preventive care, and supportive care [3]. In February 2020, Liang et al. published a nationwide analysis of cancer patients with COVID-19 in China [8]. Eighteen (1%) of 1590 COVID-19 patients had a history of cancer, higher than the prevalence of cancer in the overall Chinese population (285.83 per 100000 people [0.29%], based on 2015 cancer epidemiology statistics [9]). There have been some clinical trials investigating the relationships between COVID-19 and cancers. Dai et al. performed a multicenter study including 105 patients with cancer and 536 age-matched noncancer patients confirmed with COVID-19, finding that patients with cancer appear more vulnerable to COVID-19 and COVID-19 patients with cancer had higher risks in all severe outcomes [2]. Further, patients with hematologic cancer, lung cancer, or with metastatic cancer (stage IV) had the highest frequency of severe events [2]. Patients with nonmetastatic cancer experienced similar frequencies of severe conditions to those observed in patients without cancer [2]. In a multi-centre, two-arm, parallel-group, triple-blind, phase 2–3 randomised controlled trial, Allahyari et al. investigated the effect of hydroxychloroquine on the prevention of COVID-19 in cancer patients being treated [10]. The primary end point is to investigate the incidence of COVID-19 in patients being treated for their cancer over a 2-month period. The trial began on April 14, 2020 and recruitment is still ongoing [10].
The clinical and prognostic characteristics of cancer patients with COVID-19 have also been explored in recent studies. In March 2020, Zhang et al. carried out multivariate analyses on 28 cancer patients with COVID-19 from three hospitals in Wuhan, China; they showed that cancer patients exhibited deteriorating conditions and poor outcomes because of COVID-19 [11]. On behalf of the COVID-19 and Cancer Consortium, Kuderer et al. reported significant associations between 30-day all-cause mortality and multiple factors (older age, male sex, prior history of smoking, number of comorbidities, and receipt of azithromycin plus hydroxychloroquine) [3]. However, the previous studies have been restricted by small sample sizes, geographic regions, and a lack of generalisability to the overall population of COVID-19 patients with cancer. In addition, limited clinical information and considerable heterogeneity regarding the course of the disease lead to a lack of clarity concerning the epidemiological characteristics, clinical characteristics, and treatment principles of cancer patients with COVID-19. There is an urgent need to investigate the following points: 1) the estimated prevalence of cancer among COVID-19 patients, as a function of various factors; 2) whether cancer patients with COVID-19 have distinct clinical courses and worse outcomes, compared with COVID-19 patients without cancer; and 3) whether there are geographic variations in the severe illness and mortality rate among cancer patients with COVID-19. Thus, we conducted a systematic review and meta-analysis of observational studies concerning cancer patients with COVID-19. Our findings will aid in the management of cancer patients with COVID-19.
2. Methods
This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. Two reviewers (K.X. and Q.Y.) independently undertook the literature search, assessment for eligibility, data extraction, and qualitative assessment. Inconsistencies were reviewed by a third reviewer (L.Z.) and resolved by consensus. The research protocol was registered and approved in PROSPERO (registration #CRD42020196014).
2.1. Data sources and searches
A comprehensive literature search of bibliographic databases was conducted to identify all relevant articles. To identify studies regarding COVID-19 in cancer patients, PubMed, Embase, Cochrane Library, and Web of Science were searched from the inception of each database to July 15, 2020. Additionally, abstracts and presentations of all major conference proceedings were reviewed. Key/relevant MeSH terms and keywords used in this study were as follows: “2019-ncov,” “novel coronavirus,” “COVID-19,” “SARS-CoV-2,” “new coronavirus,” “coronavirus disease 2019,” “cancer,” “tumor,” “malignancy,” and “neoplasm.” The specific literature search strategies are shown in Table S1. Reference lists were also reviewed in a snowball sampling technique to identify additional studies. Two investigators (X.K. and Y.Q.) independently screened the titles and abstracts of identified articles. Major conflicts were resolved by a third researcher (L.Z.). The full texts of identified studies were further reviewed by two independent reviewers (J.W. and Y.F.). The search was again extended by review of references of articles included in the final selection.
2.2. Study selection and data extraction
Published studies containing epidemiological and clinical data of cancer patients with COVID-19 were identified without geographic restrictions. Eligibility criteria were as follows: 1) studies reporting data regarding COVID-19 confirmed patients, survivors, and COVID-19-related death; 2) studies containing epidemiological or clinicopathological data regarding cancer patients with COVID-19; and 3) studies limited to humans. Exclusion criteria were as follows: 1) letters, reviews, conference proceedings, commentaries, quality of life studies, cost-effectiveness analyses, and publications in which the cancer data or COVID-19 data could not be ascertained; and 2) duplicate studies from the same population or database (only the most recent study was included in the analysis). Two investigators (K.X. and Q.Y.) independently reviewed the list of retrieved articles to choose potentially relevant articles; disagreements were discussed and resolved by consensus with a third investigator (L.Z.). Both reviewers also independently extracted data from all studies; discrepancies were resolved by consensus with the third investigator (L.Z.). The following information was extracted from each publication: publication details, data collection period, sample size, sex, age, study design, data used for patient characterisation, clinical definition of COVID-19 used in the study, hospital where patients were treated, patients’ basic vital signs, symptoms and signs, race, smoking status, obesity status, number of comorbidities, interval between cancer diagnosis and hospitalisation, cancer histotype, tumour stage, treatments used for both cancer and COVID-19, laboratory results, severity staging, and prognosis.
2.3. Endpoint setting and stratification strategy
The primary outcome was the prevalence of cancer among COVID-19 patients. This prevalence was defined as the number of COVID-19 patients with cancer divided by the total number of COVID-19 patients in the study. Secondary outcomes included the severe illness incidence and mortality rate among cancer patients with COVID-19. The stratification strategy for subgroup analysis of cancer prevalence in COVID-19 patients was as follows:
-
1)
by continent: patient populations were stratified into three groups: Europe, the Asia-Pacific region, and North America.
-
2)
by country: patient populations were stratified into two groups: China and other countries.
-
3)
by age: patients were stratified into two groups: mean age >60 years and mean age ≤60 years.
-
4)
by sample size: studies were stratified into two groups: sample size ≤100 and sample size >100.
-
5)
by study design: studies were stratified into two groups: cohort studies and non-cohort studies (e.g., case-series, case-control, and cross-sectional studies).
To investigate severe illness incidence and mortality rate among COVID-19 patients with cancer, a subgroup analysis was also performed with stratification by continent.
2.4. Data synthesis and analysis
Statistical heterogeneity among studies was evaluated using Cochran's Q test and the I2 statistic; I2 (% residual variation due to heterogeneity) values of 25%, 50%, and 75% were considered to represent low, moderate, and high heterogeneity, respectively [13]. For I2 values > 50%, a random-effects model was applied. For I2 values < 50%, a fixed effects model was applied [14]. If substantial heterogeneity was detected, meta-regression analyses were performed to determine the proportion of between-study variance explained by participant characteristics and study characteristics. The pooled prevalence of cancer comorbidity among COVID-19 patients in different studies and corresponding 95% confidence intervals (CIs) were used to estimate cancer prevalence among COVID-19 patients, as well as the proportions of cancer patients with severe illness and death from COVID-19. Both Metan and Metaprop approaches were used to generate pooled prevalences. Metaprop built further on the Metan procedure, which allowed computation of 95% CIs using the score statistic and the exact binomial method; this approach incorporated the Freeman-Tukey double arcsine transformation of proportions [15]. Meta-analysis results using Metaprop are shown in the main text; results obtained using the Metan approach are shown in the supplementary material.
2.5. Assessment for study quality and risk of publication bias
Articles included in the single-arm prevalence meta-analysis were analysed for risk of bias by using guidelines from the Agency for Healthcare Research and Quality (AHRQ) (https://www.ncbi.nlm.nih.gov/books/NBK35156), which can be tailored for the assessment of cross‐sectional studies [16]. This methodological quality assessment tool uses an 11-item checklist and is recommended for the assessment of cross-sectional studies. An item was scored “0” if the answer was “NO” or “UNCLEAR,” and “1” if the answer was “YES.” Article quality was assessed as follows: low quality = 0–3; moderate quality = 4–7; high quality = 8–11.
The Newcastle-Ottawa Scale (NOS) quality assessment tool was used to evaluate the quality of the selected comparative studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). This tool measured the key aspects of the methodology in selected studies with regard to design quality and risk of bias estimates based on three design criteria: 1) selection of study participants; 2) comparability of study groups; 3) assessment of outcome and exposure with a star system (maximum of nine stars) [17]. Studies with a score of 7–9 stars were considered to have a low risk of bias, studies with a score of 4–6 stars were considered to have a medium risk of bias, and studies with a score of ≤3 were considered to have a high risk of bias.
Publication bias was estimated using Begg's funnel plot, in which the SE of the prevalence of each study was plotted against its prevalence; the corresponding Begg's test was performed to test for small-study effects [18]. All reported P-values are two-sided. A P-value <0.05 was considered to indicate statistical significance. All analyses were performed using Stata version 14.2 (StataCorp, College Station, TX, USA) [19,20].
2.6. Role of the funding sources
The funders have no conflicts of interest. The funders of the study had no roles in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors have full access to all data in this study and had final responsibility concerning the decision to submit for publication.
3. Results
3.1. Characteristics of the included studies
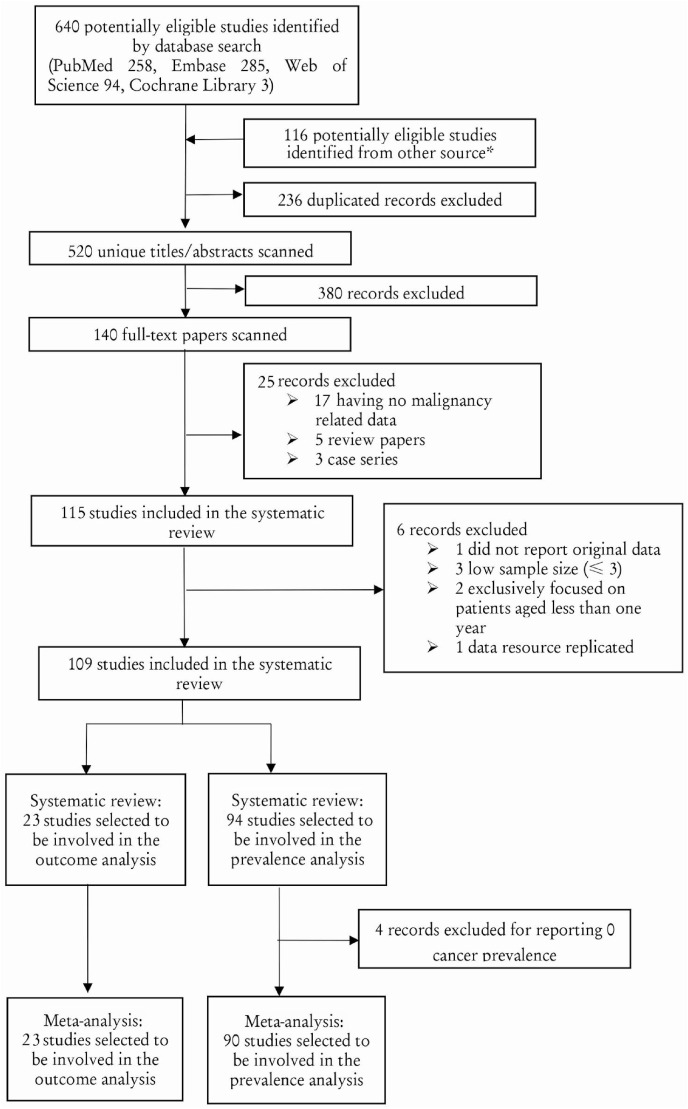
Fig. 1 shows the study selection flowchart. After screening and eligibility assessment, we included a total of 109 studies (79 retrospective cohort studies, 22 case-series studies, five case-control studies, and three cross-sectional studies) in the final quantitative and qualitative syntheses of evidence. Among the 109 studies, 90 reported the number of patients with comorbid cancer among COVID-19 patients. These studies were published between December 2019 and June 2020; they contained information regarding 94,845 COVID-19 patients, among which 4106 exhibited comorbid cancer (Table 1 ). Among the 109 studies, 23 included 71,969 COVID-19 patients, among which 4351 patients with comorbid cancer exhibited severe illness or death during the study period. These studies provided detailed clinical outcomes information concerning the COVID-19 patients with cancer, including severe illness incidence and mortality rate (Table 2 ).
Fig. 1.
Study Eligibility Flowchart. * Because many COVID-19 patients with comorbid cancer-related data were only reported in tables, we searched PubMed using a “COVID-19” search strategy, scanning and extracting studies with “data in tables.”
Table 1.
Summary of characteristics of included studies of COVID-19 patients with comorbid cancer.
| First author | Country | Continent | Study Design | Data Collection | Confirmation of COVID-19 | COVID-19 Patients | Age (years) | Male Percentage | Cancer |
|---|---|---|---|---|---|---|---|---|---|
| Beyrouti R [26] | UK | Europe | Case Series | Medical Records | RT-PCR | 6 | 61.0–85.0 | 0.83 | 2 |
| Cai Q [27] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 298 | 47.5 ± 3.0–61.0 | 0.49 | 4 |
| Chen J [28] | China | Asia Pacific | Retrospective Cohort | EHR | Laboratory confirmed | 249 | 51.0 (36.0–64.0) | 0.51 | 1 |
| Chen L [29] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 29 | 56.0 (26.0–79.0) | 0.72 | 1 |
| Chen N [30] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 99 | 55.5 ± 13.1 | 0.68 | 1 |
| Chen T [31] | China | Asia Pacific | Case Series | EHR | RT-PCR | 274 | 62.0 (44.0–70.0) | 0.62 | 7 |
| Cheng Y [32] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 701 | 63.0 (50.0–71.0) | 0.52 | 32 |
| Conversano A [32] | Italy | Europe | Retrospective Cohort | Medical Records | RT-PCR | 191 | 63.4 ± 14.9 | 0.69 | 29 |
| Dai M [33] | China | Asia Pacific | Retrospective Cohort | EHR | WHO Guideline | 641 | 64.0 ± 14.0 | 0.55 | 105 |
| Deng G [34] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 44672 | NA | 0.51 | 107 |
| Du RH [35] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 109 | 70.7 ± 10.9 | 0.68 | 8 |
| Du Y [36] | China | Asia Pacific | Retrospective Cohort | EHR | PCR | 85 | 65.8 ± 14.2 | 0.73 | 6 |
| Duanmu Y [37] | USA | North America | Cross-Sectional Study | EHR | PCR | 100 | 45.0 (32.0–65.0) | 0.56 | 3 |
| Feng Y [38] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 476 | 53.0 (40.0–64.0) | 0.57 | 57 |
| Fernández-Ruiz M [39] | Spain | Europe | Case Series | Medical Records | RT-PCR | 18 | 71.0 ± 12.8 | 0.78 | 4 |
| Grasselli G [40] | Italy | Europe | Case Series | EHR | RT-PCR | 1591 | 63.0 (56.0–70.0) | 0.82 | 81 |
| Grillet F [41] | France | Europe | Retrospective Cohort | Medical Records | RT-PCR | 100 | 66.0 ± 13.0 | 0.7 | 20 |
| Guan WJ [42] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 1590 | 49.0 ± 16.0 | 0.57 | 130 |
| Guan WJ [42] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 1099 | 47.0 (35.0–58.0) | 0.58 | 10 |
| Guo W [43] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 174 | 59 .0 (49.0–67.0) | 0.44 | 21 |
| He Y [32] | China | Asia Pacific | Retrospective Cohort | Medical Records | Not specified | 65 | 51.0 (27.0–68.0) | 0.48 | 1 |
| Hou W [44] | China | Asia Pacific | Retrospective Cohort | EHR | PCR | 101 | 50.9 ± 20.1 | 0.44 | 5 |
| Hu L [45] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 323 | 61.0 (23.0–91.0) | 0.51 | 5 |
| Huang C [46] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 41 | 49.0 (41.0–58.0) | 0.73 | 1 |
| Ihle-Hansen H [47] | Norway | Europe | Retrospective Cohort | Medical Records | RT-PCR | 43 | 67.8 | 0.67 | 5 |
| Inciardi R M [48] | Italy | Europe | Retrospective Cohort | Medical Records | RT-PCR | 99 | 68 .0 ± 12 .0 | 0.85 | 33 |
| Ji M [49] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 101 | 51.0 (37.0–61.0) | 0.48 | 12 |
| Jin X [50] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 651 | 46.1 ± 14.2 | NA | 0 |
| KCDC [51] | Korea | Asia Pacific | Cross-Sectional Study | Government reports | NA | 54 | 75.5 (66.0–80.0) | 0.61 | 13 |
| Kalligeros M [52] | USA | North America | Retrospective Cohort | EHR | RT-PCR | 103 | 60.0 (52.0–70.0) | 0.61 | 9 |
| Li J [53] | China | Asia Pacific | Case Series | Medical Records | RT-PCR | 1178 | 55.5 (38.0–67.0) | 0.46 | 32 |
| Li K [32,54] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 78 | 44.6 | 0.49 | 9 |
| Li X [55] | China | Asia Pacific | Retrospective Cohort | EHR | WHO Guideline | 548 | 60.0 (48.0–69.0) | 0.51 | 24 |
| Liang W [5] | China | Asia Pacific | Retrospective Cohort | EHR | Laboratory confirmed | 1590 | NA | NA | 18 |
| Lin L [56] | China | Asia Pacific | Case-Control Study | Medical Records | RT-PCR | 95 | 45.3 | 0.47 | 5 |
| Liu K [57] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 137 | 57.0 (20.0–83.0) | 0.45 | 2 |
| Liu W [58] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 78 | 38.0 (33.0–57.0) | 0.64 | 4 |
| Liu Y [59] | China | Asia Pacific | Case Series | Medical Records | Laboratory confirmed | 12 | NA | 0.66 | 0 |
| Lovell N [60] | UK | Europe | Case Series | Medical Records | Not specified | 101 | 82.0 (72.0–89.0) | NA | 25 |
| Luong-Nguyen M [61] | France | Europe | Retrospective Cohort | Medical Records | Not specified | 15 | 62.0 (35.0–68.0) | 0.6 | 8 |
| Malard F [62] | France | Europe | Retrospective Cohort | Medical Records | PCR | 25 | 72 | 0.68 | 20 |
| Mancia G [63] | Italy | Europe | Case-Control Study | Medical Records | RT-PCR | 6272 | 68.0 ± 13.0 | 0.63 | 1091 |
| Mao L [64] | China | Asia Pacific | Case Series | EHR | RT-PCR | 214 | 52.7 ± 15.5 | 0.41 | 13 |
| Meng Y [65] | China | Asia Pacific | Retrospective Cohort | Medical Records | Not specified | 168 | 67.0 ± 15.0 | 0.52 | 1 |
| Million M [66] | France | Europe | Retrospective Cohort | EHR | RT-PCR | 1061 | 43.6 | 0.46 | 161 |
| Miyashita H [1] | USA | North America | Retrospective Cohort | EHR | RT-PCR | 5688 | NA | NA | 334 |
| Molina JM [67] | France | Europe | Retrospective Cohort | Medical Records | PCR | 11 | 58.7 | 0.64 | 5 |
| Montopoli M [68] | Italy | Europe | Case-Control Study | EHR | WHO Guideline | 9280 | NA | 0.49 | 786 |
| Myers LC [69] | USA | North America | Retrospective Cohort | Medical Records | PCR | 377 | 61.0 (50.0–73.0) | 0.56 | 18 |
| Nair V [70] | USA | North America | Case Series | EHR | RT-PCR | 10 | 57.0 (47.0–67.0) | 0.6 | 0 |
| O'Reilly GM [71] | Australia | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 240 | 60.0 ± 21.0 | 0.55 | 1 |
| Pan L [72] | China | Asia Pacific | Cross-Sectional Study | EHR | RT-PCR | 204 | 52.9 ± 16.0 | 0.55 | 13 |
| Pereira MR [73] | USA | North America | Case Series | Medical Records | RT-PCR | 90 | 57.0 (46.0–68.0) | 0.59 | 3 |
| Qi X [74] | China | Asia Pacific | Retrospective Cohort | Medical Records | Not specified | 70 | 41.0 (27.5–50.0); | 0.72 | 2 |
| Qin C [75] | China | Asia Pacific | Retrospective Cohort | EHR | WHO Guideline | 452 | 58.0 (22.0–95.0) | 0.52 | 54 |
| Safiya Richardson [76] | USA | North America | Case Series | EHR | PCR | 5700 | 63.0 (52.0–75.0) | 0.6 | 320 |
| Shi H [77] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 81 | 49.5 ± 11.0 | 0.52 | 4 |
| Shi S [78] | China | Asia Pacific | Retrospective Cohort | Medical Records | WHO Guideline | 416 | 64.0 (21.0–95.0) | 0.49 | 9 |
| Shi Y [79] | China | Asia Pacific | Retrospective Cohort | EHR | WHO Guideline | 487 | 46.0 ± 19.0 | 0.53 | 5 |
| Stroppa EM [80] | Italy | Europe | Retrospective Cohort | EHR | RT-PCR | 56 | 71.64 (50–84) | 0.8 | 25 |
| Sun B [81] | China | Asia Pacific | Retrospective Cohort | Medical Records | Not specified | 38 | 58.0 (49.0–69.5) | 91 | 4 |
| Sun H [82] | USA | North America | Case Series | EHR | PCR | 30 | 84.5 (71.0–97.0) | 0.47 | 7 |
| Sun Y [83] | Singapore | Singapore | Case-Control Study | EHR | PCR | 788 | 34 | 0.49 | 94 |
| Wan S [84] | China | Asia Pacific | Case Series | EHR | RT-PCR | 135 | 47.0 (36.0–5.0) | 0.53 | 4 |
| Wang B [85] | China | Asia Pacific | Case Series | Medical Records | RT-PCR | 26 | 5.0–72.0 | 0.42 | 1 |
| Wang D [86] | China | Asia Pacific | Case Series | EHR | RT-PCR | 138 | 56.0 (42.0–68.0) | 0.54 | 10 |
| Wang K [87] | China | Asia Pacific | Case Series | Medical Records | RT-PCR | 144 | 53 | 0.51 | 1 |
| Wang L [88] | China | Asia Pacific | Case Series | EHR | PCR | 26 | 42.0 (33.5–53.3) | 0.42 | 1 |
| Wang L [89] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 116 | 54.0 (38.0–69.0) | 0.58 | 12 |
| Wang L [90] | China | Asia Pacific | Retrospective Cohort | EHR | Laboratory confirmed | 339 | 71.0 ± 8.0 | NA | 15 |
| Wang Y [91] | China | Asia Pacific | Retrospective Cohort | Medical Records | WHO Guideline | 46 | NA | 0.57 | 2 |
| Wang Z [92] | China | Asia Pacific | Case Series | EHR | RT-PCR | 69 | 42.0 (35.0–62.0) | 0.46 | 4 |
| Wang, K. [93] | China | Asia Pacific | Retrospective Cohort | EHR | Not specified | 114 | 53.0 (23.0–78.0) | 0.51 | 1 |
| Wu C [94] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 201 | 51.0 (43.0–60.0) | 0.64 | 1 |
| Wu J [95] | China | Asia Pacific | Retrospective Cohort | EHR | WHO Guideline | 280 | 43.1 ± 19.0 | 0.54 | 33 |
| Xie J [96] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 140 | 60.0 (47.0–68.0) | 0.51 | 5 |
| Xu X [97] | China | Asia Pacific | Case Series | EHR | RT-PCR | 90 | 50.0 (18.0–86.0) | 0.43 | 2 |
| Yang F [98] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 92 | 69.8 (30.0–97.0) | 0.58 | 4 |
| Yang F [99] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 1575 | 63 (34–98) | 0.54 | 52 |
| Yang X [100] | China | Asia Pacific | Retrospective Cohort | EHR | Laboratory confirmed | 52 | 59.7 (13.3) | 0.67 | 2 |
| Yao Q [101] | China | Asia Pacific | Retrospective Cohort | Medical Records | Not specified | 108 | 52.0 (37.0–58.0) | 0.4 | 2 |
| Yasukawa K [102] | USA | North America | Retrospective Cohort | Medical Records | Not specified | 50 | 62.0 (22.0–78.0) | 0.58 | 4 |
| Yin L [103] | China | Asia Pacific | Case-Control Study | EHR | RT-PCR | 45 | 52.4 | 0.56 | 3 |
| Yu Q [104] | China | Asia Pacific | Retrospective Cohort | Medical Records | Laboratory confirmed | 625 | 46.9 ± 15.4 | 0.53 | 6 |
| Yuan M [105] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 27 | 60.0 (47.0–69.0) | 0.44 | 1 |
| Zhang J [106] | China | Asia Pacific | Retrospective Cohort | Medical Records | RT-PCR | 290 | NA | 0.53 | 35 |
| Zhang J [107] | China | Asia Pacific | Retrospective Cohort | Medical Records | Laboratory confirmed | 111 | 38.0 (32.0–57.0) | 0.41 | 8 |
| Zhang L [108] | China | Asia Pacific | Retrospective Cohort | EHR | Laboratory confirmed | 343 | 62.0 (48.0–69.0) | 0.5 | 41 |
| Zhang R [109] | China | Asia Pacific | Case Series | EHR | RT-PCR | 120 | 45.4 ± 15.6 | 0.36 | 7 |
| Zhang X [110] | China | Asia Pacific | Retrospective Cohort | EHR | WHO Guideline | 645 | 46.7 ± 13.8 | 51.50% | 0 |
| Zhou F [111] | China | Asia Pacific | Retrospective Cohort | EHR | RT-PCR | 191 | 56.0 (18.0–87.0) | 0.62 | 2 |
| Zhou Z [112] | China | Asia Pacific | Retrospective Cohort | EHR | WHO Guideline | 254 | 50.6 (5.0–87.0) | 0.45 | 30 |
| Zhu W [113] | China | Asia Pacific | Retrospective Cohort | EHR | PCR | 32 | 46.0 (35.0–52.0) | 0.47 | 2 |
| Ziehr DR [114] | USA | North America | Retrospective Cohort | EHR | Not specified | 66 | 58.0 (23.0–87.0) | 0.65 | 5 |
Note: COVID-19: Defined in accordance with WHO Guidelines [118]; Age (years): Mean ± SD or Median (range).
Table 2.
Summary of characteristics of included studies of COVID-19 patients with cancer and clinical outcomes.
| First author | Country | Study Design | Data Collection | COVID-19 Confirmation | COVID-19 Patients | Age (year) | Male Percentage | Cancer Patients | Severe illness with cancer | Severe illness without cancer | Death with cancer | Death without cancer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dai M [33] | China | Retrospective Cohort | EHR | WHO guideline | 641 | 64 | 0.55 | 105 | 35 | 75 | 12 | 27 |
| Guan WJ [42] | China | Retrospective Cohort | EHR | RT-PCR | 1590 | 49 | 0.57 | 130 | 9 | 245 | 3 | 47 |
| He W [115] | China | Retrospective Cohort | EHR | CT scan | 13 | 35 | 0.54 | 13 | 4 | NA | 8 | NA |
| Liang W [8] | China | Retrospective Cohort | EHR | Laboratory confirmed | 1590 | 63.1 | NA | 18 | 7 | 124 | NA | NA |
| Luo J [116] | USA | Retrospective Cohort | EHR | RT-PCR | 69 | 69 (31–91) | 0.48 | 69 | 24 | NA | 16 | NA |
| Ma J [117] | China | Retrospective Cohort | EHR | RT-PCR | 37 | 62 | 0.541 | 37 | 20 | NA | 5 | NA |
| Martín-MoroF [118] | Spain | Retrospective Cohort | EHR | RT-PCR | 34 | 72.5 (35–94) | 0.56 | 34 | NA | NA | 11 | NA |
| Mehta V [119] | USA | Retrospective Cohort | EHR | RT-PCR | 218 | 69(10–92) | NA | 218 | 45 | NA | 61 | NA |
| Miyashita H [120] | USA | Retrospective Cohort | EHR | RT-PCR | 5688 | NA | NA | 334 | NA | NA | 37 | 518 |
| Montopoli M [68] | Italy | Case-Control Study | EHR | WHO guideline | 4532 | NA | 0.49 | 430 | 121 | NA | 75 | 312 |
| Nicole M Kuderer [121] | USA, Canada and Spain | Retrospective Cohort | EHR | Laboratory confirmed | 928 | 66 (57–76) | 0.5 | 928 | 242 | NA | 121 | NA |
| Rafi Kabarriti NPB [122] | USA | Retrospective Cohort | EHR | RT-PCR | 107 | 70 (30–95) | 0.5 | 107 | NA | NA | 24 | NA |
| Stroppa EM [80] | Italy | Retrospective Cohort | EHR | RT-PCR | 56 | 71 (50–84). | 0.8 | 25 | 12 | NA | 9 | NA |
| T de Rojas [123] | Spain | Case Series | EHR | PCR | 15 | 11(1–19) | 0.93 | 15 | NA | NA | NA | NA |
| Wei X [124] | China | Retrospective Cohort | EHR | RT-PCR | 252 | 64.8 ± 13.3 | 0.52 | 252 | 121 | NA | NA | NA |
| Yang F [99] | China | Retrospective Cohort | EHR | RT-PCR | 1575 | 63 (34–98) | 0.54 | 52 | 19 | NA | 11 | NA |
| Yang KY [125] | China | Retrospective Cohort | EHR | RT-PCR | 8161 | 63 (14–96) | 0.47 | 205 | 52 | NA | 40 | NA |
| Yu J [126] | China | Case Series | EHR | WHO guideline | 12 | 66 | 0.83 | 12 | 3 | NA | 3 | NA |
| Zhang L [11] | China | Retrospective Cohort | EHR | RT-PCR | 28 | 65 | 0.61 | 28 | 15 | NA | 8 | NA |
| Lee LY [127] | UK | Retrospective Cohort | EHR | RT-PCR | 800 | 69 (59–76) | 0.56 | 800 | 360 | NA | 226 | NA |
| Garassino MC [128] | Italy/Spain/France/Switzerland/Netherlands/USA/UK/China | Retrospective Cohort | EHR | RT-PCR; Symptom or radiologically suspected COVID-19. | 200 | 68.0 (61.8–75.0) | 0.70 | 200 | 13 | NA | 66 | NA |
Note: COVID-19: Defined in accordance with WHO Guidelines [118]; Age (years): Mean ± SD or Median (range).
3.2. Prevalence of cancer among COVID-19 patients
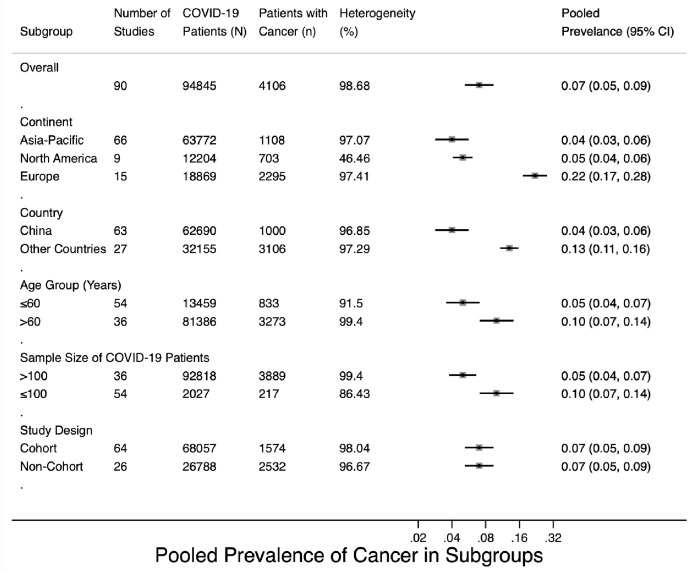
Pooled prevalence of cancer in different subgroups were shown in Fig. 2 . The overall cancer prevalence among the entire COVID-19 patient cohort was 0.07 (95% CI 0.05–0.09, weights determined using random-effects analysis model, Fig. S1). For subgroup analysis, patient populations were from Europe (14 studies in total, including six from Italy, four from France, two from the United Kingdom (UK), one from Spain, and one from Norway), the Asia-Pacific region (72 studies in total, including 70 from China, one from Korea, and one from Australia), and North America (eight studies, all from the United States of America). There were 36 studies with patients aged >60 years; for the other 54 studies, patients were aged ≤60 years. Subgroup analysis revealed that the prevalence of COVID-19 was higher among cancer patients in Europe (prevalence rate 0.22, 95% CI 0.17–0.28) than among cancer patients in the Asia-Pacific region (0.04, 95% CI 0.03–0.06) or North America (0.05, 95% CI 0.04–0.06) (Fig. S2). When stratified by country, the prevalence of COVID-19 among cancer patients was 0.04 (95% CI 0.03–0.06) in China; this rate was lower than in other countries (0.13, 95% CI 0.11–0.16) (Fig. S3). The prevalence of COVID-19 was higher among cancer patients aged >60 years (0.10, 95% CI 0.07–0.14) than among patients aged ≤60 years (0.05, 95% CI 0.03–0.06) (Fig. S4). The prevalence was slightly higher in studies with a sample size ≤100 (0.10, 95% CI 0.07–0.14) than in studies with a sample size >100 (0.05, 95% CI 0.04–0.07) (Fig. S5). As shown in Fig. S6, the pooled prevalence in cohort studies was estimated to be 0.07 (95% CI 0.05–0.09), similar to the estimated prevalence in non-cohort studies (0.07, 95% CI 0.05–0.09). The results obtained from the Metan method (Figs. S7A–F in supplementary material) were similar to results obtained from the Metaprop method.
Fig. 2.
Pooled prevalence of cancer in subgroups.
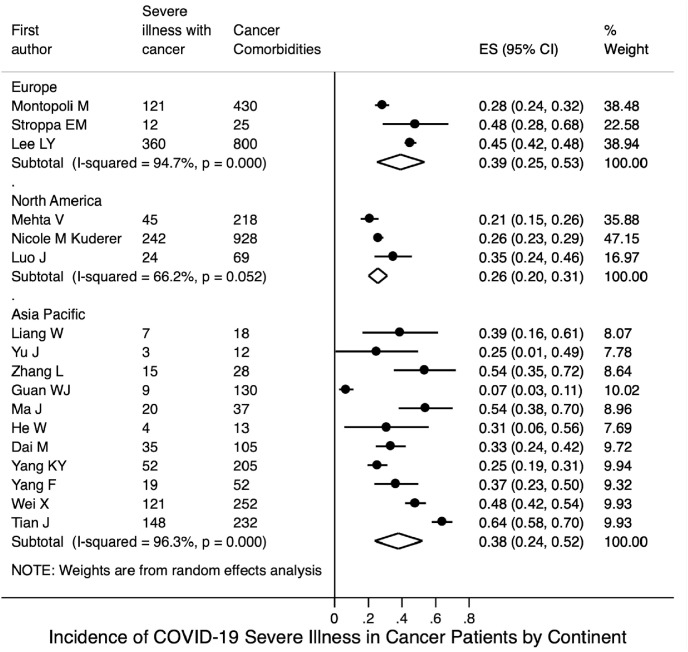
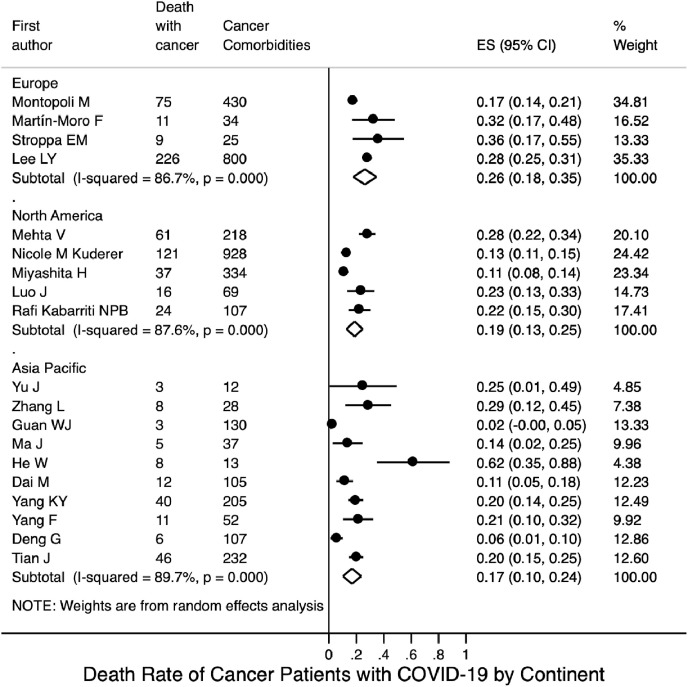
3.3. Severe illness incidence and mortality rate in COVID-19 patients with cancer
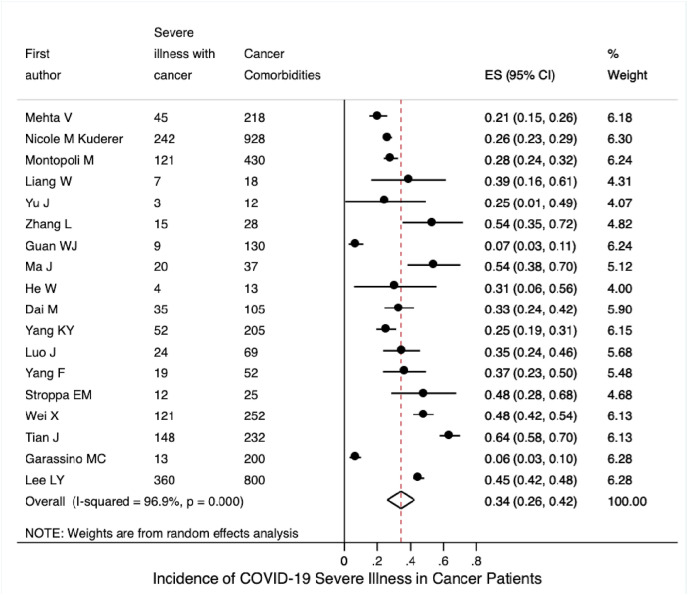
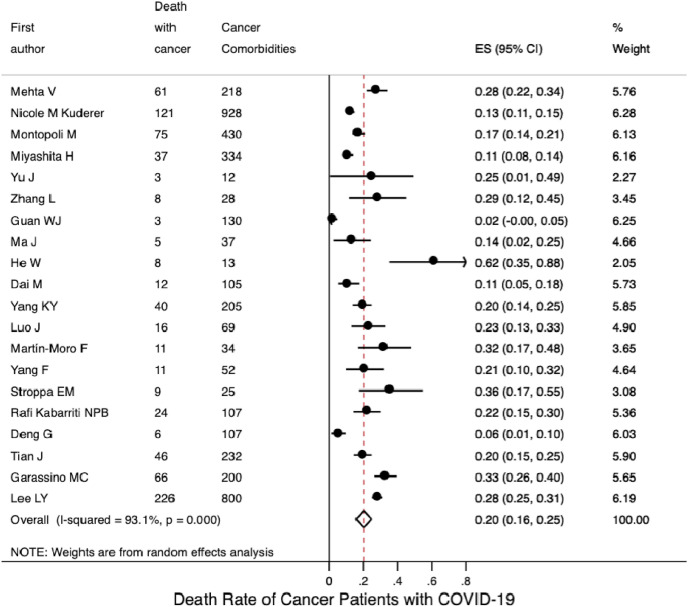
Based on the included 18 studies with available data, the pooled incidence of severe illness in COVID-19 patients with cancer was 0.34 (95% CI 0.26–0.42; Fig. 3 A); based on four studies with available data, the pooled incidence of severe illness in COVID-19 patients without cancer was 0.14 (95% CI 0.08–0.20; Fig. S8A). Seventeen studies reported mortality data for COVID-19 patients with cancer. Their mortalities were synthesised using a random-effects model; the pooled mortality rate was 0.20 (95% CI 0.16–0.25; Fig. 4 A). Based on the six included studies with available mortality data concerning COVID-19 patients without cancer, the pooled mortality rate was estimated to be 0.05 (95% CI 0.03–0.08; Fig. S8B).
Fig. 3(A).
Incidence of severe illness among COVID-19 patients with cancer.
Fig. 4(A).
Mortality rate of COVID-19 patients with cancer.
Subgroup analysis by continent, on the basis of the 10 studies with available data, the pooled incidence of severe illness in COVID-19 patients with cancer from the Asia-Pacific region was 0.38 (95% CI 0.24–0.52); this was similar to the pooled incidence in patients from Europe, based on three studies (0.39, 95% CI 0.25–0.53; Fig. 3B), but was higher than the pooled incidence in patients from North America, based on three studies (0.26, 95% CI 0.20–0.31). Fig. 4 B shows that the pooled mortality rates of COVID-19 patients with cancer were 0.17 in the Asia-Pacific region (95% CI 0.10–0.24; 10 studies), 0.26 in Europe (95% CI 0.18–0.35; four studies), and 0.19 in North America (95% CI 0.13–0.25; five studies).
Fig. 3(B).
Incidence of severe illness among COVID-19 patients with cancer stratified by continent.
Fig. 4(B).
Mortality rate of COVID-19 patients with cancer stratified by continent.
3.4. Heterogeneity and meta-regression
Forest plots of cancer prevalence among COVID-19 patients (Figs. S1–S6) reveal high heterogeneity, with an overall I2 of 98.68% (P < 0.001). Table 3 shows the results of meta-regression for the included 90 studies regarding cancer prevalence in COVID-19 patients. The between-study variance could be explained by the estimated differences with continent as a statistically significant variable (I2 = 62.37%, τ2 = 0.0015, and P = 0.04), and with country as a statistically significant variable (I2 = 46.74%, τ2 = 0.0011, and P < 0.001). Age group (I2 = 70.49%, τ2 = 0.0016, and P = 0.20), study design (I2 = 57.22%, τ2 = 0.0015, and P = 0.35), and sample size (I2 = 70.17%, τ2 = 0.0015, and P = 0.075) could not explain the heterogeneity.
Table 3.
Summary of meta-regression results for the 90 included studies.
| Regression Variables | Number of Studies | Random Effect Pooled Prevalence (95% CI) | I2 (%) | τ2 | P-value |
|---|---|---|---|---|---|
| 1) Continent | |||||
| Asia-Pacific | 66 | 0.04 (0.03–0.06) | 62.37 | 0.0015 | 0.04* |
| Europe | 15 | 0.22(0.17–0.28) | |||
| North America | 9 | 0.05 (0.04–0.06) | |||
| 2) Country | |||||
| China | 63 | 0.04 (0.03–0.06) | 46.74 | 0.0011 | <0.001* |
| Other Countries | 27 | 0.13 (0.11–0.16) | |||
| 3) Age Group (Years) | |||||
| ≤60 | 54 | 0.05 (0.03–0.06) | 70.49 | 0.0016 | 0.2 |
| >60 | 36 | 0.10 (0.07–0.14) | |||
| 4) Study Design | |||||
| Cohort | 64 | 0.07 (0.05–0.09) | 57.22 | 0.0015 | 0.35 |
| Non-Cohort | 26 | 0.07 (0.05–0.09) | |||
| 5) Sample Size | |||||
| ≤100 | 54 | 0.10 (0.07–0.14) | 70.17 | 0.0015 | 0.075 |
| >100 | 36 | 0.05 (0.04–0.07) | |||
Note: I2, percent residual variation due to heterogeneity; τ2, residual maximum likelihood estimates of between-study variance; CI, confidence interval.
3.5. Qualitative assessment and publication bias
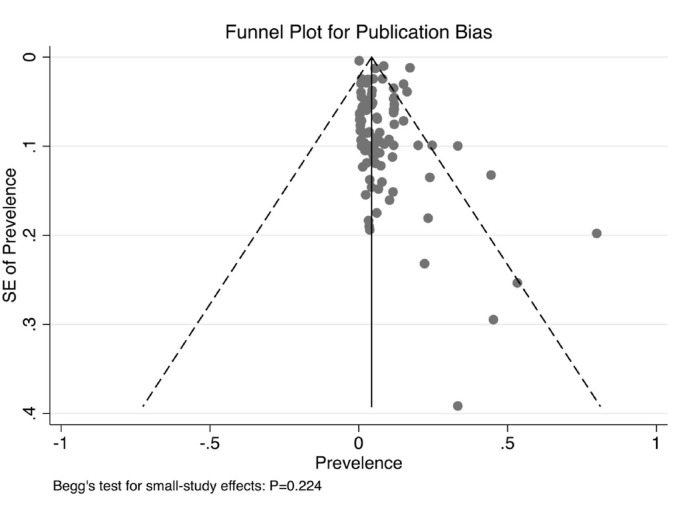
Selected single-arm studies were evaluated for quality in accordance with AHRQ guidelines. As indicated in Table S2, of the 94 studies, 48 (score 4–7; 51.06%) showed a moderate risk of bias and 46 (score 0–3; 48.94%) showed a low risk of bias. The NOS tool was used to conduct a qualitative assessment of the selected studies to review their quality and detect possible bias. As shown in Table S3, of the 23 studies, one exhibited a low risk of bias (7–9 stars; 4.35%), 19 studies exhibited a moderate risk of bias (4–6 stars; 78.26%), one exhibited a high risk of bias (≤3 stars; 4.35%), and two could not be assessed because they were case series studies. No significant publication bias was observed in this meta-analysis (P = 0.224; funnel plot shown in Fig. 5 ).
Fig. 5.
Funnel plot with pseudo 95% confidence limits and Begg's test.
4. Discussion
This systematic review and meta-analysis of 109 global studies is the most comprehensive meta-analysis to pool formally published studies concerning the prevalence of cancer in patients with COVID-19, as well as the incidence of severe illness and rate of mortality in cancer patients with COVID-19. Our findings revealed that the overall pooled prevalence of cancer in patients with COVID-19 in these studies was 0.07, higher than the prevalence of 0.02 (95% CI, 0.02–0.03) published by Aakash et al. in April 2020 [21]. In the Asia-Pacific region, the prevalence was slightly lower (0.04) than in North America (0.06), whereas the prevalence was significantly higher in Europe (0.22). Moreover, studies from China showed a lower overall prevalence (0.04), compared with studies from other countries (0.10). Statistical analyses from 2015 [9] revealed that the prevalence of cancer in the overall Chinese population was 285.83 per 100 000 people (0.29%), which is much lower than the prevalence of cancer in COVID-19 patients in China.
In May 2020, a pre-print of a meta-analysis by Venkatesulu et al., which included 31 studies concerning outcomes in cancer patients affected by COVID-19, demonstrated that cancer patients with COVID-19 had a higher likelihood of death (odds ratio [OR] 2.54) and that cancer patients were more likely to be intubated. They also showed that cancer patients with COVID-19 were older than the normal population and had higher rates of comorbidities. However, that study lacked pooled prevalences by some key variables, such as age and geographic region. Here, we conducted multiple subgroup analyses of cancer prevalence by continent, country, mean patient age, sample size, and study design, which yielded more detailed information; the findings also provided pooled prevalence data regarding cancer morbidity among patients with COVID-19. Notably, we found that age was associated with the prevalence of cancer in COVID-19 patients. Cancer prevalence among COVID-19 patients also differed across geographic regions, presumably for the following reasons:
-
1)
In response to the COVID-19 pandemic, many countries implemented policies such as screening of healthcare staff and patients with respect to COVID-19 symptoms and travel history, placement of restrictions on employee business travel, establishment of COVID-19 hotline and response teams, engagement in telehealth and online meetings, education of staff to effectively use personal protective equipment, and minimisation of admissions and follow-up visits [[21], [132]]. Both prevalence and clinical outcomes might be affected by national or regional public health and epidemic prevention policies, which may also partially account for differences across geographic regions. Situations in Asian countries (e.g., China, South Korea, and Singapore) may partly be due to the government's policy, which included aggressive action involving the use of social-distancing measures to slow disease spread, as well as extensive testing and isolation of infected people to reduce the potential for transmission. This strategy helped the countries to contain the outbreak. However, several countries in Europe and North America required considerably longer intervals to address the spread of COVID-19. Some evidence suggests that patients with multiple morbidities (e.g., cancer) were more susceptible to COVID-19 and more likely to experience severe illness and worse outcomes. Therefore, in locations where the disease has not been well-controlled for an extended interval, inadequate public health measures (e.g., absence of mask use and premature large-scale gatherings) might cause cancer patients in these areas to experience a greater risk of COVID-19.
-
2)
The subtypes of COVID-19 that are prevalent on different continents may exhibit considerable differences in terms of molecular structure, virulence, and invasiveness. In a phylogenetic network analysis of 160 COVID-19 genomes, Forster et al. found three central variants distinguished by amino acid alterations, which they regarded as subtypes A, B, and C [22]. The A and C subtypes were significantly more prevalent mainly in Europeans and Americans. In contrast, the B subtype was most common in East Asia; its ancestral genome appears not to have spread outside East Asia prior to mutation into derived B subtypes, which indicates founder effects or immunological/environmental resistance against this subtype outside of Asia [22].
-
3)
There are disparities in COVID-19 susceptibility and clinical outcomes across ethnic backgrounds. There has been debate concerning the extent to which the effects of COVID-19 differ among ethnic groups; thus far, relevant studies have explored the impact of ethnicity on COVID-19 mortality and morbidity. For instance, Santorelli et al. analysed the mortality rates in 1276 inpatients in Bradford with test results for COVID-19 across ethnic groups [23]. The age-adjusted risk of dying from COVID-19 was slightly lower in South Asian patients than in British patients (risk ratio [RR] 0.87; 95% CI 0.41–1.84) [23]. Similarly, Public Health England (PHE) reported disparities concerning the risks and outcomes of COVID-19 [24]. After adjustments for sex, age, and region, people from a Black, Asian, and Minority Ethnic (BAME) background had a higher risk of death from COVID-19 than British people; however, following adjustments for comorbidities, there were no differences in COVID-19 mortality among ethnic groups [24]. Few studies have compared the prevalence or incidence rates of COVID-19 among ethnic groups. In May 2020, Niedzwiedz et al. linked participants in the UK Biobank [25] to COVID-19 testing data from PHE. Of 392,116 participants in the cohort, 2658 were tested for COVID-19; 948 had positive test results. The incidences of COVID-19 were higher in Irish (RR 1.42; 95% CI 1.00–2.03), South Asian (RR 2.42; 95% CI 1.75–3.36), and Black (RR 3.35; 95% CI 2.48–4.53) individuals, compared with British individuals [25]. Our meta-analysis indicated that Caucasian patients with cancer were more likely to be infected with COVID-19, compared with Asian patients with cancer, which contrasted with the findings of previous epidemiological studies.
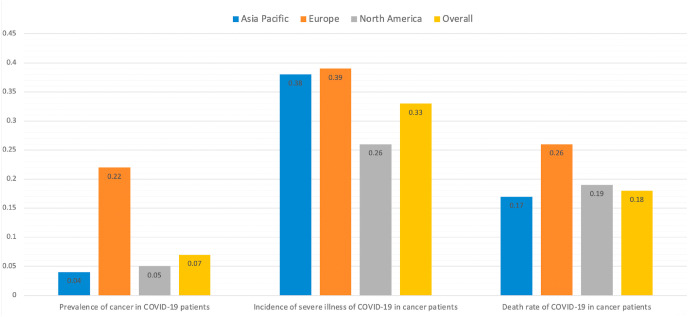
In May 2020, Kuderer et al. published a cohort study based on 928 cancer patients with COVID-19 [3]. They found that independent factors associated with mortality included age, male sex, smoking status, number of comorbidities, Eastern Cooperative Oncology Group performance status of ≥2, active cancer, and treatment with azithromycin plus hydroxychloroquine. In our study, because data regarding potential risk factors associated with mortality were limited among the included studies, we did not assess associations of clinical outcome with potential prognostic variables mentioned above, a considerable limitation of this study. However, a novel aspect and strength of the present meta-analysis is that we performed subgroup analysis by continent to assess the severe illness incidence and mortality rate among COVID-19 patients with cancer. There were some notable findings (Fig. 6 ): 1) European COVID-19 patients had both the highest cancer prevalence (0.22) and highest cancer patient mortality (0.26); 2) North American COVID-19 patients had a cancer prevalence similar to that of patients from the Asia-Pacific region, but had the lowest severe illness incidence among cancer patients (0.26); 3) compared with patients from the Asia-Pacific region, European COVID-19 patients had a much higher cancer prevalence, whereas the incidences of severe illness among cancer patients were similar in these two groups (Asia-Pacific region, 0.38; Europe, 0.39); and 4) compared with patients from the Asia-Pacific region, North American COVID-19 patients had a much lower severe illness incidence among cancer patients (0.26), whereas cancer prevalence and cancer patient mortality were similar. Overall, European COVID-19 patients were most likely to both develop cancer and experience cancer progression to severe illness and death. Although COVID-19 patients in the Asia-Pacific region had the lowest cancer prevalence, their severe illness incidence was similar to that of European patients. Finally, a continent-stratified analysis of severe illness incidence and mortality rate among COVID-19 patients with cancer is shown in Figs. S8A–B; given that the included studies were limited, subgroup analysis of patients without cancer was not performed. Another strength of this meta-analysis is that it descriptively demonstrated the severe illness incidence and mortality rate in COVID-19 patients with and without cancer. Cancer patients typically exhibit systemic immunosuppressive states caused by the malignancy itself and anticancer treatments (e.g., chemotherapy, surgery, or immunomodulatory drugs such as PD-1/PD-L1 inhibitors) [4,6,8]; thus, their ability to resist the virulence and invasiveness of the infection is considerably weaker. In addition, these patients are often older and exhibit one or more major comorbidities, such that they are at increased risk for COVID-19-related mortality [7].
Fig. 6.
Prevalence, severe illness incidence, and mortality rate of COVID-19 patients with cancer, stratified by continent.
Our systematic review and meta-analysis had several limitations: 1) Heterogeneity was observed in the included studies, both for the estimation of prevalence and for the analyses of severe illness and mortality. We minimised the influence of heterogeneity by using a random-effects model; we also performed exploratory subgroup analyses by continent, country, age, sample size, and study design. Furthermore, we used both Metan and Metaprop approaches to test the robustness of the results. 2) This study might have been limited by the retrospective nature of most of the included studies. To minimise possible inaccuracies, we conducted subgroup analysis by study design, separately pooling the prevalences of cohort and non-cohort studies (e.g., case-series, case-control, and cross-sectional studies). 3) The definitions of “severe illness” were not uniform and differed among the included studies, which might lead to heterogeneities to some extent. Generally, severe illness referred to a composite of severe illness requiring mechanical ventilation, admission to an ICU, admission to hospital, or a combination of these; mechanical ventilation; admission to an ICU; admission to hospital; and need for supplemental oxygen during the course of COVID-19. 4) Most included studies did not indicate a specific time period for the mortality rate; hence, the objectivity of the mortality comparison might have been influenced by reporting bias due to the lack of conformity concerning the time interval. In addition, longer-term follow-up and larger sample sizes are needed to understand the epidemiological and clinical characteristics of cancer patients more completely during the course of COVID-19 [[133], [134]]. 5) When performing continent-stratified analysis for severe illness incidence and mortality rate, the included studies for each subgroup were limited due to data availability. 6) Although we performed a thorough assessment of literature quality, illustrating that the problem of bias was not serious and within the acceptable range, we acknowledge the likely effect of selection bias in the primary studies included in this meta-analysis, especially given that many of the studies use convenience sampling. 7) The datalock used was July 15 2020. Given the pace at which COVID-19 data is being published, we will update our analysis in a timely fashion.
5. Conclusions
Taken together with previously published results, our meta-analysis provides a comprehensive picture of the epidemiological and clinical characteristics of cancer patients with COVID-19. The estimated cancer prevalence among COVID-19 patients was 0.07 (95% CI 0.05–0.09); this prevalence increased with age. The prevalence was much higher in Europe than in the Asia-Pacific region or North America. COVID-19 patients with cancer were at risk of more severe illness and a higher mortality rate. These findings concerning cancer patients with COVID-19 reinforce important considerations for clinical care and emphasise the urgent need for more data with longer-term follow-up, larger sample sizes, and more detailed sociodemographic and clinicopathological variables. In the future, with the availability of additional data, it will be important to investigate differences across more sociodemographic and clinicopathological features (e.g., sex, race, smoking status, symptoms and signs, cancer type, laboratory results, and tumour stage) in COVID-19 patients with and without cancer.
Funding
This work was supported by the Natural Science Foundation of China (81872160), the Natural Science Foundation of China (82072940), the China National Key R&D (or Research and Development) Program (2020AAA0105000, 2020AAA0105004), the Beijing Municipal Natural Science Foundation (Key Project) (7191009), the Beijing Municipal Natural Science Foundation (7204293), the Special Research Fund for Central Universities, Peking Union Medical College (3332019053), the Beijing Hope Run Special Fund of Cancer Foundation of China (LC2019B03), the Beijing Hope Run Special Fund of Cancer Foundation of China (LC2019L07), the Beijing Hope Run Special Fund of Cancer Foundation of China (LC2020L01), and the Golden Bridge Project Seed Fund of Beijing Association for Science and Technology (ZZ20004). The Hong Kong Polytechnic University (1-BE58), and the International Institute of Spatial Lifecourse Epidemiology (ISLE).
Author contributions
Conceptualization, Yu Jiang, Lin Zhang, Xiangyi Kong, Yi Fang, Jing Wang; Data Collection, Yihang Qi, Lin Zhang, Xiangyi Kong; Methodology, Junjie Huang, Yang Zhao, Xuzhen Qin, Zhihong Qi, Adejare (Jay) Atanda, Lei Zhang, Peng Jia; Supervision, Yu Jiang, Asieh Golozar; Writing - original draft, Xiangyi Kong, Lin Zhang, Yihang Qi; Writing - review & editing, Asieh Golozar, Adejare (Jay) Atanda, Lei Zhang, Xiangyi Kong, Lin Zhang. All authors have read and agreed to the published version of the manuscript.
Ethical approval
The analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College institutional review board provided a waiver (exemption) for approval.
Declaration of competing interest
The authors declare that they have no conflicts of interests.
Acknowledgments
We thank Ryan Chastain-Gross, Ph.D., from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.canlet.2021.02.012.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Miyashita H., Mikami T., Chopra N., Yamada T., Chernyavsky S., Rizk D., Cruz C. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann. Oncol. : Off. J. Eur. Soc. Med. Oncol. 2020;31:1088–1089. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., Zhang Z., You H., Wu M., Zheng Q., Xiong Y., Xiong H., Wang C., Chen C., Xiong F., Zhang Y., Peng Y., Ge S., Zhen B., Yu T., Wang L., Wang H., Liu Y., Chen Y., Mei J., Gao X., Li Z., Gan L., He C., Li Z., Shi Y., Qi Y., Yang J., Tenen D.G., Chai L., Mucci L.A., Santillana M., Cai H. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Canc. Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.Y., Desai A., de Lima Lopes G., Grivas P., Painter C.A., Peters S., Thompson M.A., Bakouny Z., Batist G., Bekaii-Saab T., Ma Bilen, Bouganim N., Larroya M.B., Castellano D., Del Prete S.A., Doroshow D.B., Egan P.C., Elkrief A., Farmakiotis D., Flora D., Galsky M.D., Glover M.J., Griffiths E.A., Gulati A.P., Gupta S., Hafez N., Halfdanarson T.R., Hawley J.E., Hsu E., Kasi A., Khaki A.R., Lemmon C.A., Lewis C., Logan B., Masters T., McKay R.R., Mesa R.A., Morgans A.K., Mulcahy M.F., Panagiotou O.A., Peddi P., Pennell N.A., Reynolds K., Rosen L.R., Rosovsky R., Salazar M., Schmidt A., Shah S.A., Shaya J.A., Steinharter J., Stockerl-Goldstein K.E., Subbiah S., Vinh D.C., Wehbe F.H., Weissmann L.B., Wu J.T., Wulff-Burchfield E., Xie Z., Yeh A., Yu P.P., Zhou A.Y., Zubiri L., Mishra S., Lyman G.H., Rini B.I., Warner J.L. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet (London, England) 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sica A., Massarotti M. Myeloid suppressor cells in cancer and autoimmunity. J. Autoimmun. 2017;85:117–125. doi: 10.1016/j.jaut.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., Li C., Ai Q., Lu W., Liang H., Li S., He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China, the Lancet. Oncology. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blimark C., Holmberg E., Mellqvist U.H., Landgren O., Björkholm M., Hultcrantz M., Kjellander C., Turesson I., Kristinsson S.Y. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100:107–113. doi: 10.3324/haematol.2014.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, february 12-march 16, 2020. Morb. Mortal. Wkly. Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang W.H., Guan W.J., Chen R.C., Wang W., Li J.F., Xu K., Li C.C., Ai Q., Lu W.X., Liang H.R., Li S.Y., He J.X. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng R.S., Sun K.X., Zhang S.W., Zeng H.M., Zou X.N., Chen R., Gu X.Y., Wei W.W., He J. Report of cancer epidemiology in China, 2015. Chin. J. Oncol. 2019;41:19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Allahyari A., Rahimi H., Khadem-Rezaiyan M., Mozaheb Z., Seddigh-Shamsi M., Bary A., Kamandi M., Azimi S.A., HasanAbadi S.E., Noferesti A., Shariatmaghani S.S., Rafatpanah H., Khatami S., Imani A.J., Mortazi H., Nodeh M.M. Effect of hydroxychloroquine on COVID-19 prevention in cancer patients undergoing treatment: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:575. doi: 10.1186/s13063-020-04485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., Jia P., Guan H.Q., Peng L., Chen Y., Peng P., Zhang P., Chu Q., Shen Q., Wang Y., Xu S.Y., Zhao J.P., Zhou M. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. : Off. J. Eur. Soc. Med. Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Fisher D.J., Carpenter J.R., Morris T.P., Freeman S.C., Tierney J.F. Meta-analytical methods to identify who benefits most from treatments: daft, deluded, or deft approach? BMJ (Clin. Res. ed.) 2017;356:j573. doi: 10.1136/bmj.j573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Archiv. Publ. Health = Archiv. belges de sante publique. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viswanathan Meera. agency for healthcare research & quality; 2013. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures Further Development of the RTI Item Bank. [PubMed] [Google Scholar]
- 17.Zhang L., Zou H., Zhao Y., Hu C., Atanda A., Qin X., Jia P., Jiang Y., Qi Z. Association between blood circulating vitamin D and colorectal cancer risk in Asian countries: a systematic review and dose-response meta-analysis. BMJ open. 2019;9 doi: 10.1136/bmjopen-2019-030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L., Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74:785–794. doi: 10.1111/biom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balduzzi Sara, Rücker Gerta, Guido Schwarzer. How to perform a meta-analysis with R: a practical tutorial. Evid. Base Ment. Health. 2019;22 doi: 10.1136/ebmental-2019-300117. ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahlke Jeffrey A., Wiernik Brenton M. Psychmeta : an R package for psychometric meta-analysis. Appl. Psychol. Meas. 2018;43 doi: 10.1177/0146621618795933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai Aakash, Sachdeva Sonali, Parekh Tarang, Desai Rupak. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Global Oncol. 2020;6:557–559. doi: 10.1200/GO.20.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. U. S. A. 2020;117:9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santorelli G., Sheldon T., West J., Cartwright C., Wright J. COVID-19 in-patient hospital mortality by ethnicity. Wellcome Open Res. 2020;5:86. doi: 10.12688/wellcomeopenres.15913.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravi K. Ethnic disparities in COVID-19 mortality: are comorbidities to blame? Lancet (London, England) 2020;396:22. doi: 10.1016/S0140-6736(20)31423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer L.J. UK Biobank: bank on it. Lancet (London, England) 2007;369:1980–1982. doi: 10.1016/S0140-6736(07)60924-6. [DOI] [PubMed] [Google Scholar]
- 26.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., Humphries F., Jäger H.R., Losseff N.A., Perry R.J., Shah S., Simister R.J., Turner D., Chandratheva A., Werring D.J. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatr. 2020:1–4. doi: 10.1136/jnnp-2020-323586. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai Qingxian, Huang Deliang, Ou Pengcheng, Hong Yu, Zhu Zhibin, Zhang Xia, Su Yinan, Ma Zhenghua, Zhang Yiming, Li Zhiwei, He Qing, Fu Yang, Liu Lei, Chen Jun. 2020. COVID-19 in a Designated Infectious Diseases HospitalOutside Hubei Province. China. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T., Li F., Xu Q., Zhang Y., Xu S., Song Z., Zeng Y., Shen Y., Shi Y., Zhu T., Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J., Deng Y., Wei S. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia], chung-hua chieh Ho Ho hu hsi tsa chih Chinese. J. Tuberculosis Respir. Dis. 2020;43:203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clin. Res. ed.) 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain A., Sharma G., Thakur K., Raza K., Shivhare U.S., Ghoshal G., Katare O.P. Beta-carotene-Encapsulated solid lipid nanoparticles (BC-SLNs) as promising vehicle for cancer: an investigative assessment. AAPS PharmSciTech. 2019;20:100. doi: 10.1208/s12249-019-1301-7. [DOI] [PubMed] [Google Scholar]
- 33.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., Zhang Z., You H., Wu M., Zheng Q., Xiong Y., Xiong H., Wang C., Chen C., Xiong F., Zhang Y., Peng Y., Ge S., Zhen B., Yu T., Wang L., Wang H., Liu Y., Chen Y., Mei J., Gao X., Li Z., Gan L., He C., Li Z., Shi Y., Qi Y., Yang J., Tenen D.G., Chai L., Mucci L.A., Santillana M., Cai H. Cancer discovery; 2020. Patients with Cancer Appear More Vulnerable to SARS-COV-2: a Multi-Center Study during the COVID-19 Outbreak. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng G., Yin M., Chen X., Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care. 2020;24:179. doi: 10.1186/s13054-020-02902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du R.H., Liu L.M., Yin W., Wang W., Guan L.L., Yuan M.L., Li Y.L., Hu Y., Li X.Y., Sun B., Peng P., Shi H.Z. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in wuhan, China. Annals Am. Thoracic Soc. 2020;17:839–846. doi: 10.1513/AnnalsATS.202003-225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P., Li T., Cao F., Chang C., Hu Q., Jin Y., Xu G. Clinical features of 85 fatal cases of COVID-19 from wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. 32242738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duanmu Youyou, Ian P., Brown William R. Gibb, Singh Jessica, Matheson Loretta W., Blomkalns Andra L., Govindarajan Prasanthi. Characteristics of emergency department patients with COVID-19 at a single site in northern California: clinical observations and public health implications. Acad. Emerg. Med. 2020;27:505–509. doi: 10.1111/acem.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun Feng, Yun Ling, Bai Tao, Xie Yusang, Huang Jie, Li Jian, Xiong Weining, Yang Dexiang, Chen Rong, Lu Fangying, Lu Yunfei, Liu Xuhui, Chen Yuqing, Li Xin, Li Yong, Summah Hanssa, Lin Huihuang, Yan Jiayang, Zhou Min, Qu Jieming. COVID-19 with different severity: a multi-center study of clinical features. Am. J. Respir. Crit. Care Med. 2020:201. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández-Ruiz M., Andrés A., Loinaz C., Delgado J.F., López-Medrano F., San Juan R., González E., Polanco N., Folgueira M.D., Lalueza A., Lumbreras C., Aguado J.M. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am. J. Transplant. : Off. J. Am. Soc. Transplant. Am. Soc.Transplant Surg. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grasselli Giacomo, Zangrillo Alberto, Alberto Zanella, Antonelli Massimo, Cabrini Luca, Castelli Antonio, Cereda Danilo, Coluccello Antonio, Foti Giuseppe, Fumagalli Roberto, Iotti Giorgio, Latronico Nicola, Lorini Luca, Merler Stefano, Natalini Giuseppe, Piatti Alessandra, Vito Ranieri Marco, Scandroglio Anna Mara, Storti Enrico, Cecconi Maurizio, Pesenti Antonio. For the COVID-19 lombardy ICU network, baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. J. Am. Med. Assoc. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;2963:201544. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., Ou C.Q., Li L., Chen P.Y., Sang L., Wang W., Li J.F., Li C.C., Ou L.M., Cheng B., Xiong S., Ni Z.Y., Xiang J., Hu Y., Liu L., Shan H., Lei C.L., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Cheng L.L., Ye F., Li S.Y., Zheng J.P., Zhang N.F., Zhong N.S., He J.X. The European respiratory journal; 2020. Comorbidity and its Impact on 1590 Patients with Covid-19 in China: A Nationwide Analysis; p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., Qin R., Wang H., Shen Y., Du K., Zhao L., Fan H., Luo S., Hu D. Diabetes/metabolism research and reviews; 2020. Diabetes Is a Risk Factor for the Progression and Prognosis of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou W., Zhang W., Jin R., Liang L., Xu B., Hu Z. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infect. Dis. (London, England) 2020;52:498–505. doi: 10.1080/23744235.2020.1759817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu L., Chen S., Fu Y., Gao Z., Long H., Wang J.M., Ren H.W., Zuo Y., Li H., Wang J., Xu Q.B., Yu W.X., Liu J., Shao C., Hao J.J., Wang C.Z., Ma Y., Wang Z., Yanagihara R., Deng Y. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America; 2020. Risk Factors Associated with Clinical Outcomes in 323 COVID-19 Hospitalized Patients in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ihle-Hansen H., Berge T., Tveita A., Rønning E.J., Ernø P.E., Andersen E.L., Wang C.H., Tveit A., Myrstad M. 2020. COVID-19: Symptoms, Course of Illness and Use of Clinical Scoring Systems for the First 42 Patients Admitted to a Norwegian Local Hospital, Tidsskrift for Den Norske Laegeforening : Tidsskrift for Praktisk Medicin; p. 140. ny raekke. [DOI] [PubMed] [Google Scholar]
- 48.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D., Italia L., Zaccone G., Tedino C., Fabbricatore D., Curnis A., Faggiano P., Gorga E., Lombardi C.M., Milesi G., Vizzardi E., Volpini M., Nodari S., Specchia C., Maroldi R., Bezzi M., Metra M. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji M., Yuan L., Shen W., Lv J., Li Y., Li M., Lu X., Hu L., Dong W. Characteristics of disease progress in patients with coronavirus disease. Wuhan, China, Epidemiol. Infect. 2019;148:e94. doi: 10.1017/S0950268820000977. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin Xi, Lian Jiang-Shan, Hu Jian-Hua, Gao Jianguo, Zheng Lin, Zhang Yi-Min, Shao-Rui Hao, Jia Hong-Yu, Cai Huan, Zhang Xiao-Li, Guo-Dong Yu, Xu Kai-Jin, Wang Xiao-Yan, Gu Jue-Qing, Zhang Shan-Yan, Ye Chan-Yuan, Jin Ci-Liang, Lu Ying-Feng, Xia Yu, Xiao-Peng Yu, Huang Jian-Rong, Xu Kang-Li, Ni Qin, Yu Cheng-Bo, Zhu Biao, Li Yong-Tao, Liu Jun, Zhao Hong, Zhang Xuan, Liang Yu, Guo Yong-Zheng, Su Jun-Wei, Tao Jing-Jing, Lang Guan-Jing, Wu Xiao-Xin, Wu Wen-Rui, Qv Ting-Ting, Xiang Dai-Rong, Yi Ping, Ding Shi, Chen Yanfei, Ren Yue, Qiu Yun-Qing, Li Lan-Juan, Sheng Jifang, Yang Yida. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention Analysis on 54 mortality cases of coronavirus disease 2019 in the Republic of Korea from january 19 to march 10, 2020. J. Kor. Med. Sci. 2020;35:e132. doi: 10.3346/jkms.2020.35.e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A., Mylonakis E. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28:1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in wuhan, China. JAMA Cardiol. 2020;5(7):825–830. doi: 10.1001/jamacardio.2020.1624. 32324209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li K., Fang Y., Li W., Pan C., Qin P., Zhong Y., Liu X., Huang M., Liao Y., Li S. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur. Radiol. Mar. 2020;25:1–10. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z., Zhang C., Yue J., Zhang Z., Renz H., Liu X., Xie J., Xie M., Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020:12. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin L., Jiang X., Zhang Z., Huang S., Zhang Z., Fang Z., Gu Z., Gao L., Shi H., Mai L., Liu Y., Lin X., Lai R., Yan Z., Li X., Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 57.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., Xiao W., Wang Y.N., Zhong M.H., Li C.H., Li G.C., Liu H.G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W., Tao Z.W., Wang L., Yuan M.L., Liu K., Zhou L., Wei S., Deng Y., Liu J., Liu H.G., Yang M., Hu Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury, Science China. Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lovell N., Maddocks M., Etkind S.N., Taylor K., Carey I., Vora V., Marsh L., Higginson I.J., Prentice W., Edmonds P., Sleeman K.E. Characteristics, symptom management, and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J. Pain Symptom Manag. 2020;60:e77–e81. doi: 10.1016/j.jpainsymman.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luong-Nguyen M., Hermand H., Abdalla S., Cabrit N., Hobeika C., Brouquet A., Goéré D., Sauvanet A. Nosocomial infection with SARS-cov-2 within departments of digestive surgery. J. Visceral Surg. 2020;157:S13–S18. doi: 10.1016/j.jviscsurg.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malard F., Genthon A., Brissot E., van de Wyngaert Z., Marjanovic Z., Ikhlef S., Banet A., Lapusan S., Sestilli S., Corre E., Paviglianiti A., Adaeva R., M 'Hammedi-Bouzina F., Labopin M., Legrand O., Dulery R., Mohty M. Bone marrow transplantation; 2020. COVID-19 Outcomes in Patients with Hematologic Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of covid-19. N. Engl. J. Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. 32275288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng Y., Wu P., Lu W., Liu K., Ma K., Huang L., Cai J., Zhang H., Qin Y., Sun H., Ding W., Gui L., Wu P. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Million M., Lagier J.C., Gautret P., Colson P., Fournier P.E., Amrane S., Hocquart M., Mailhe M., Esteves-Vieira V., Doudier B., Aubry C., Correard F., Giraud-Gatineau A., Roussel Y., Berenger C., Cassir N., Seng P., Zandotti C., Dhiver C., Ravaux I., Tomei C., Eldin C., Tissot-Dupont H., Honoré S., Stein A., Jacquier A., Deharo J.C., Chabrière E., Levasseur A., Fenollar F., Rolain J.M., Obadia Y., Brouqui P., Drancourt M., La Scola B., Parola P., Raoult D. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Trav. Med. Infect. Dis. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Maladies Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V., Carbone G.M., Cavalli A., Pagano F., Ragazzi E., Prayer-Galetti T., Alimonti A. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann. Oncol. : Off. J. Eur. Soc. Med. Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. 32387456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Myers Laura, Stephen Parodi, Liu Vincent. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. J. Am. Med. Assoc. 2020;323 doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nair Vinay, Jandovitz Nicholas, Hirsch Jamie S., Nair Gayatri, Molmenti Ernesto P. COVID in kidney transplant recipients. Am. J. Transplant. 2020;20(7):1941–1943. doi: 10.1111/ajt.15967. 32233067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Reilly G.M., Mitchell R.D., Rajiv P., Wu J., Brennecke H., Brichko L., Noonan M.P., Hiller R., Mitra B., Luckhoff C., Paton A., Smit V., Santamaria M.J., Cameron P.A. EMA; 2020. Epidemiology and Clinical Features of Emergency Department Patients with Suspected COVID-19: Initial Results from the COVID-19 Emergency Department Quality Improvement Project (COVED-1), Emergency Medicine Australasia. [DOI] [PubMed] [Google Scholar]
- 72.Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J., Li P., Hu B., Wang J., Hu C., Jin Y., Niu X., Ping R., Du Y., Li T., Xu G., Hu Q., Tu L. Clinical characteristics of COVID-19 patients with digestive symptoms in hubei, China: a descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pereira M.R., Mohan S., Cohen D.J., Husain S.A., Dube G.K., Ratner L.E., Arcasoy S., Aversa M.M., Benvenuto L.J., Dadhania D.M., Kapur S., Dove L.M., Brown R.S., Rosenblatt R.E., Samstein B., Uriel N., Farr M.A., Satlin M., Small C.B., Walsh T.J., Kodiyanplakkal R.P., Miko B.A., Aaron J.G., Tsapepas D.S., Emond J.C., Verna E.C. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am. J. Transplant. : Off. J. Am. Soc. Transplant. Am. Soc.Transplant Surg. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qi Xiaolong, Liu Chuan, Jiang Zicheng, Gu Ye, Zhang Guo, Shao Chuxiao, Yue Hongmei, Chen Zhenhuai, Ma Baoyi, Liu Dengxiang, Zhang Lin, Wang Jitao, Xu Dan, Lei Junqiang, Li Xun, Huang Huihong, Wang Yan, Liu Hongyan, Yang Jie, Dong Jiahong. Multicenter analysis of clinical characteristics and outcome of COVID-19 patients with liver injury. J. Hepatol. 2020;73(2):455–458. doi: 10.1016/j.jhep.2020.04.010. 32305291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America; 2020. Dysregulation of Immune Response in Patients with COVID-19 in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richardson Safiya, Hirsch Jamie S., Narasimhan Mangala, Crawford James M. Thomas McGinn, karina W. Davidson, and the northwell COVID-19 research Consortium, presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J. Am. Med. Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study, the Lancet. Infect. Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. 32211816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. 1st. Vol. 24. Critical Care; 2020. Host Susceptibility to Severe COVID-19 and Establishment of a Host Risk Score: Findings of 487 Cases outside Wuhan; p. 108. 32188484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stroppa Elisa, Toscani Ilaria, Citterio Chiara, Anselmi Elisa, Elena Zaffignani, Codeluppi Mauro, Cavanna Luigi. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy) Future Oncol. 2020;16(20):1425–1432. doi: 10.2217/fon-2020-0369. 32403946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun B., Feng Y., Mo X., Zheng P., Wang Q., Li P., Peng P., Liu X., Chen Z., Huang H., Zhang F., Luo W., Niu X., Hu P., Wang L., Peng H., Huang Z., Feng L., Li F., Zhang F., Li F., Zhong N., Chen L. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microb. Infect. 2020;9:940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun H., Lee J., Meyer B.J., Myers E.L., Nishikawa M.S., Tischler J.L., Blinderman C.D. Characteristics and palliative care needs of COVID-19 patients receiving comfort-directed care. J. Am. Geriatr. Soc. 2020;68:1162–1164. doi: 10.1111/jgs.16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun Yinxiaohe, Koh Vanessa, Kalisvar Marimuthu, Tek Ng Oon, Young Barnaby, Shawn Vasoo, Chan Monica, Lee Vernon J.M., De Partha P., Timothy Barkham. Clinical Infectious Diseases; 2020. Epidemiological and Clinical Predictors of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., Lang C., Huang D., Sun Q., Xiong Y., Huang X., Lv J., Luo Y., Shen L., Yang H., Huang G., Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. 32198776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang B., Wang L., Kong X., Geng J., Xiao D., Ma C., Jiang X.M., Wang P.H. Long-term coexistence of SARS-CoV-2 with antibody response in COVID-19 patients. J. Med. Virol. 2020;92(9):1684–1689. doi: 10.1002/jmv.25946. 32343415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. 32031570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang K., Kang S., Tian R., Zhang X., Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID-19) in the Xiaogan area. Clin. Radiol. 2020;75:341–347. doi: 10.1016/j.crad.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang L., Duan Y., Zhang W., Liang J., Xu J., Zhang Y., Wu C., Xu Y., Li H. Epidemiologic and clinical characteristics of 26 cases of COVID-19 arising from patient-to-patient transmission in liaocheng, China. Clin. Epidemiol. 2020;12:387–391. doi: 10.2147/CLEP.S249903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Luwen, Li Xun, Chen Hui, Yan Shaonan, Dong Li, Li Yan, Gong Zuojiong. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from wuhan, China. Am. J. Nephrol. 2020;51 doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L., He W., Yu X., Hu D., Bao M., Liu H., Zhou J., Jiang H. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y., Jiang W., He Q., Wang C., Wang B., Zhou P., Dong N., Tong Q. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct. Targeted Ther. 2020;5:57. doi: 10.1038/s41392-020-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in wuhan, China, clinical infectious diseases. Off. Publ. Infect. Dis. Soc. Am. 2020;71(15):769–777. doi: 10.1093/cid/ciaa272. 32176772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang K., Kang S., Tian R., Zhang X., Zhang X., Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID-19) in the Xiaogan area. Clin. Radiol. 2020;75:341–347. doi: 10.1016/j.crad.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Inter. Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. 32167524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J., Wang D., Liu C., Meng Y., Cui L., Yu J., Cao H., Li L. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J. Intern. Med. 2020;288:128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 96.Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G., Kara T., Somers V.K. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin. Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu X.G., Zhang H.L., Zhang Q., Dong J., Huang Y., Tong D.W. Porcine epidemic diarrhea virus M protein blocks cell cycle progression at S-phase and its subcellular localization in the porcine intestinal epithelial cells. Acta Virol. 2015;59:265–275. doi: 10.4149/av_2015_03_265. [DOI] [PubMed] [Google Scholar]
- 98.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Analysis of 92 deceased patients with COVID-19. J. Med. Virol. 2020;92(11):2511–2515. doi: 10.1002/jmv.25891. 32293741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Analysis of 92 deceased patients with COVID-19. J. Med. Virol. 2020;92(11):2511–2515. doi: 10.1002/jmv.25891. 32293741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. 32105632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yao Q., Wang P., Wang X., Qie G., Meng M., Tong X., Bai X., Ding M., Liu W., Liu K., Chu Y. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol. Arch. Intern. Med. 2020;130:390–399. doi: 10.20452/pamw.15312. [DOI] [PubMed] [Google Scholar]
- 102.Yasukawa K., Minami T. Point-of-Care lung ultrasound findings in patients with COVID-19 pneumonia. Am. J. Trop. Med. Hyg. 2020;102:1198–1202. doi: 10.4269/ajtmh.20-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yin L., Mou H., Shao J., Zhu Y., Pang X., Yang J., Zhang J., Shi W., Yu S., Wang H. Correlation between Heart fatty acid binding protein and severe COVID-19: a case-control study. PloS One. 2020;15 doi: 10.1371/journal.pone.0231687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu Q., Wang Y., Huang S., Liu S., Zhou Z., Zhang S., Zhao Z., Yu Y., Yang Y., Ju S. Multicenter cohort study demonstrates more consolidation in upper lungs on initial CT increases the risk of adverse clinical outcome in COVID-19 patients. Theranostics. 2020;10:5641–5648. doi: 10.7150/thno.46465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PloS One. 2020;15 doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang J.J., Cao Y.Y., Dong X., Wang B.C., Liao M.Y., Lin J., Yan Y.Q., Akdis C.A., Gao Y.D. 2020. Distinct Characteristics of COVID-19 Patients with Initial rRT-PCR-Positive and rRT-PCR-Negative Results for SARS-CoV-2. Allergy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang J., Yu M., Tong S., Liu L.Y., Tang L.V. Predictive factors for disease progression in hospitalized patients with coronavirus disease 2019 in Wuhan, China. J. Clin. Virol. 2020;127:104392. doi: 10.1016/j.jcv.2020.104392. the official publication of the Pan American Society for Clinical Virology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemostasis : JTH. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang R., Ouyang H., Fu L., Wang S., Han J., Huang K., Jia M., Song Q., Fu Z. European radiology; 2020. CT Features of SARS-CoV-2 Pneumonia According to Clinical Presentation: a Retrospective Analysis of 120 Consecutive Patients from Wuhan City. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang X., Cai H., Hu J., Lian J., Gu J., Zhang S., Ye C., Lu Y., Jin C., Yu G., Jia H., Zhang Y., Sheng J., Li L., Yang Y. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int. J. Infect. Dis. : IJID : Off. Publ. Int. Soc. Infect. Dis. 2020;94:81–87. doi: 10.1016/j.ijid.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou Zili, Zhao Ning, Shu Yan, Han Shengbo, Shu Xiaogang. Gastroenterology; 2020. Effect of Gastrointestinal Symptoms on Patients Infected with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu W., Xie K., Lu H., Xu L., Zhou S., Fang S. Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. J. Med. Virol. 2020;92(9):1525–1532. doi: 10.1002/jmv.25763. 32167181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ziehr D.R., Alladina J., Petri C.R., Maley J.H., Moskowitz A., Medoff B.D., Hibbert K.A., Thompson B.T., Hardin C.C. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am. J. Respir. Crit. Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.He W., Chen L., Chen L., Yuan G., Fang Y., Chen W., Wu D., Liang B., Lu X., Ma Y., Li L., Wang H., Chen Z., Li Q., Gale R.P. Leukemia; 2020. COVID-19 in Persons with Haematological Cancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jia Luo, Rizvi Hira, Egger Jacklynn, Preeshagul Isabel, Wolchok Jedd, Hellmann Matthew. Cancer Discovery; 2020. Impact of PD-1 Blockade on Severity of COVID-19 in Patients with Lung Cancers. CD-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma J., Yin J., Qian Y., Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center's retrospective study. J. Infect. 2020;14:14. doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martín-Moro Fernando, Juan Marquet, Piris Miguel, Berta M Michael, Sáez Adolfo J., Corona Magdalena, Jiménez Carlos, Astibia Beatriz, García Irene, Rodríguez Eulalia, García-Hoz Carlota, Fortún-Abete Jesús. Pilar Herrera, Javier López-Jiménez, Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br. J. Haematol. 2020;190:e16–e20. doi: 10.1111/bjh.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mehta Vikas, Goel Sanjay, Kabarriti Rafi, Cole Daniel, Verma Amit. Cancer Discovery; 2020. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miyashita H., Mikami T., Chopra N., Yamada T., Chernyavsky S., Rizk D., Cruz C. Annals of oncology : official journal of the European Society for Medical Oncology; 2020. Do Patients with Cancer Have a Poorer Prognosis of COVID-19? an Experience in New York City. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.Y., Desai A., de Lima Lopes G., Grivas P., Painter C.A., Peters S., Thompson M.A., Bakouny Z., Batist G., Bekaii-Saab T., Bilen M.A., Bouganim N., Larroya M.B., Castellano D., Del Prete S.A., Doroshow D.B., Egan P.C., Elkrief A., Farmakiotis D., Flora D., Galsky M.D., Glover M.J., Griffiths E.A., Gulati A.P., Gupta S., Hafez N., Halfdanarson T.R., Hawley J.E., Hsu E., Kasi A., Khaki A.R., Lemmon C.A., Lewis C., Logan B., Masters T., McKay R.R., Mesa R.A., Morgans A.K., Mulcahy M.F., Panagiotou O.A., Peddi P., Pennell N.A., Reynolds K., Rosen L.R., Rosovsky R., Salazar M., Schmidt A., Shah S.A., Shaya J.A., Steinharter J., Stockerl-Goldstein K.E., Subbiah S., Vinh D.C., Wehbe F.H., Weissmann L.B., Wu J.T., Wulff-Burchfield E., Xie Z., Yeh A., Yu P.P., Zhou A.Y., Zubiri L., Mishra S., Lyman G.H., Rini B.I., Warner J.L. Lancet; London, England): 2020. Clinical Impact of COVID-19 on Patients with Cancer (CCC19): a Cohort Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kabarriti Rafi, Brodin N. Patrik, Maron Maxim I., Tomé Wolfgang A., Ohri Nitin. Extent of prior lung irradiation and mortality in COVID-19 patients with a cancer history. Adv. Radiat. Oncol. 2020;5(4):707–710. doi: 10.1016/j.adro.2020.04.028. 32775778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Rojas T., Pérez-Martínez A., Cela E., Baragaño M., Galán V., Mata C., Peretó A., Madero L. COVID-19 infection in children and adolescents with cancer in Madrid. Pediatr. Blood Canc. 2020;67 doi: 10.1002/pbc.28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wei X., Su J., Yang K., Wei J., Wan H., Cao X., Tan W., Wang H. Elevations of serum cancer biomarkers correlate with severity of COVID-19. J. Med. Virol. 2020;92(10):2036–2041. doi: 10.1002/jmv.25957. 32347972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang K., Sheng Y., Huang C., Jin Y., Xiong N., Jiang K., Lu H., Liu J., Yang J., Dong Y., Pan D., Shu C., Li J., Wei J., Huang Y., Peng L., Wu M., Zhang R., Wu B., Li Y., Cai L., Li G., Zhang T., Wu G. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study, the Lancet. Oncology. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in wuhan, China. JAMA Oncol. 2020;6(7):1108–1110. doi: 10.1001/jamaoncol.2020.0980. 32211820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee L.Y., Cazier J.B., Angelis V., Arnold R., Bisht V., Campton N.A., Chackathayil J., Cheng V.W., Curley H.M., Fittall M.W., Freeman-Mills L., Gennatas S., Goel A., Hartley S., Hughes D.J., Kerr D., Lee A.J., Lee R.J., McGrath S.E., Middleton C.P., Murugaesu N., Newsom-Davis T., Okines A.F., Olsson-Brown A.C., Palles C., Pan Y., Pettengell R., Powles T., Protheroe E.A., Purshouse K., Sharma-Oates A., Sivakumar S., Smith A.J., Starkey T., Turnbull C.D., Várnai C., Yousaf N., Kerr R., Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet (London, England) 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garassino M.C., Whisenant J.G., Huang L.C., Trama A., Torri V., Agustoni F., Baena J., Banna G., Berardi R., Bettini A.C., Bria E., Brighenti M., Cadranel J., De Toma A., Chini C., Cortellini A., Felip E., Finocchiaro G., Garrido P., Genova C., Giusti R., Gregorc V., Grossi F., Grosso F., Intagliata S., La Verde N., Liu S.V., Mazieres J., Mercadante E., Michielin O., Minuti G., Moro-Sibilot D., Pasello G., Passaro A., Scotti V., Solli P., Stroppa E., Tiseo M., Viscardi G., Voltolini L., Wu Y.L., Zai S., Pancaldi V., Dingemans A.M., Van Meerbeeck J., Barlesi F., Wakelee H., Peters S., Horn L. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang S., Yu W., Jia P. Telemedicine: A promising approach for diabetes management - Where is the evidence. J. Diabetes Complicat. 2021;35(2):107802. doi: 10.1016/j.jdiacomp.2020.107802. [DOI] [PubMed] [Google Scholar]
- 133.Jia P. Spatial lifecourse epidemiology. Lancet Planet. Health. 2019;3(2):e57–e59. doi: 10.1016/S2542-5196(18)30245-6. [DOI] [PubMed] [Google Scholar]
- 134.Jia P. Understanding the epidemic course in order to improve epidemic forecasting. GeoHealth. 2020;4(10) doi: 10.1029/2020GH000303. e2020GH000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.