Abstract
Recognition of invading pathogens by the innate immune system is essential to initiate antimicrobial responses and trigger adaptive immunity. This is largely mediated by an array of pattern‐recognition receptor families that are essential for recognizing conserved molecular motifs characteristic of pathogenic microbes. One such family is the Toll‐like receptors (TLRs). Activation of TLRs induces production of pro‐inflammatory cytokines and type I interferons: the former triggers the synthesis of inflammatory mediators which cause fever, pain and other inflammation, and the latter mediates antiviral responses. Over the past decade, significant progress has been made in structural elucidation of TLRs in higher eukaryotes. The TLR structures with and without agonist and antagonist have been revealed by X‐ray crystallography and cryo‐electron microscopy studies, demonstrating the activated dimer formation induced by the agonistic ligand and the inhibition mechanism of the antagonistic ligand. Intracellular assembled structures and the TLR‐chaperone complex are also reported. As the structural understanding of TLRs becomes better integrated with biochemical and immunological studies, a more comprehensive picture of their architectural and functional properties will emerge. This review summarizes recent advances in structural biological and mechanistic studies on TLRs.
Keywords: endolysosome, innate immunity, Myddosome, TIR, toll‐like receptor
1. INTRODUCTION
Toll‐like receptors (TLRs) are pattern‐recognition receptors of the innate immune system that are activated when conserved molecular signatures on microbial or host molecules [pathogen‐ and danger‐associated molecular patterns (PAMPs and DAMPs, respectively)] are detected. 1 Mammalian TLRs were named due to their similarity to the Toll protein from Drosophila, which was originally identified in fly embryonic development. 2 However, Toll was subsequently shown to be critical for Drosophila immune defense against fungi and bacteria via induction of pathways homologous with those activating the mammalian transcription factor NF‐κB. 3 , 4 Activation of TLRs triggers signaling cascades that induce the generation of pro‐inflammatory cytokines such as IL‐1, IL‐6 and TNF‐α, and type I interferons (IFNs) (Figure 1a). Released cytokines mediate the induction of additional inflammatory cytokines and the enzymes that synthesize inflammatory mediators. 5 Cyclooxygenase‐2 and type II phospholipase A2 are the well‐known enzymes which are induced by IL‐1 and activate the prostaglandin production cascade. Interferons initiate a signalling cascade, which leads to the induction of more than 300 IFN‐stimulated genes. 6 Some IFN‐inducible proteins have broad antiviral effects. For example, 2′ ‐5′ oligoadenylate synthases are activated by viral dsRNA and produce 2′ ‐5′ oligoadenylates, which in turn activate RNase L, mediating RNA degradation. Another well‐studied IFN‐induced antiviral effector is PKR, a member of the eukaryotic initiation factor 2α (eIF2α) kinase family. Activation of PKR by dsRNA leads to eIF2α phosphorylation with a consequent blockade of translation of most cellular and viral mRNAs.
FIGURE 1.
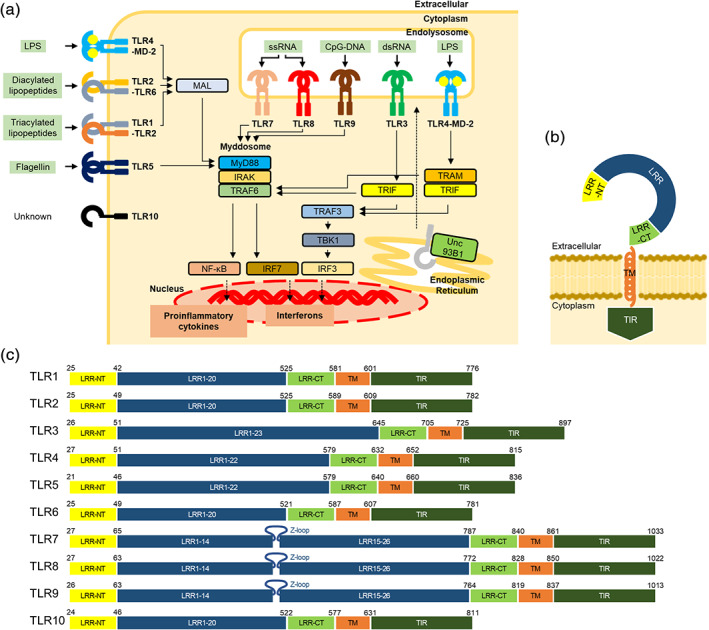
TLR signaling and domain organization of TLRs. (a) Schematic illustration of TLR signaling pathway and downstream effector molecules. The ligand of each TLR is shown on the side, respectively. Nucleic‐acids sensing TLRs are trafficked from the endoplasmic reticulum to the endolysosome by Unc93B1. TLR signaling is initiated by the ligand recognition, followed by the interaction with adaptor proteins, such as MyD88, MAL, TRIF, and TRAM. Finally, the downstream signaling induces the expression of inflammatory cytokines or type I interferons. (b) Schematic representation of full‐length TLR. Extracellular domain is composed of the N‐terminal (LRR‐NT), LRRs and the C‐terminal (LRR‐CT) regions, followed by a single transmembrane segment (TM) and an intracellular TIR domain (TIR). (c) Schematic representation of full‐length human TLR1‐10. The color scheme is the same as that used in (b). LRRs are numbered. TLR7‐9 contain a characteristic Z‐loop between LRR14 and LRR15. Residues are numbered based on reported structures and amino‐acid sequence alignment information, 48 respectively. Residues of TLR10 are numbered based on UniProt
TLRs activation and downstream signaling need to be tightly regulated (Figure 1a), as dysregulation of TLR signaling is associated with a number of disorders including cancer, allergy, autoimmunity, inflammatory bowel disease and atherosclerosis. 7 Therefore, TLRs are promising targets for immune modulation strategies, since they have both known modulators and proven therapeutic potential.
Structural information, combined with extensive biochemical and immunological data accumulated over decades, enables us to reveal the mechanisms by which proteins regulate their molecular functions, and to deepen structural and functional understanding of the Toll‐like receptors. In this review, we combine the recent data and our new understandings from structural studies to discuss the working mechanism of TLRs.
1.1. TLR families and TLR signaling pathway
To date, 10 TLRs have been identified in humans (TLR1–10). 8 Although the set of human TLRs might constitute an immunological redundancy for recognizing PAMP ligands, the 10 TLRs in humans are functionally partitioned into two subgroups depending on their cellular localization and ligands sensed. One group is composed of TLR1, TLR2, TLR4, TLR5, TLR6 and poorly characterized TLR10, which are localized to cell membranes and predominantly recognize bacterial components such as lipids, lipoproteins, and proteins. The other group is composed of TLR3, TLR7, TLR8, and TLR9, which are localized to endolysosomes, where they recognize microbial nucleic acids. 9
TLRs possess an N‐terminal extracellular ligand binding domain composed of leucine‐rich repeat (LRR), a single transmembrane (TM) segment, and a C‐terminal Toll/interleukin‐1 receptor (TIR) homology domain 10 , 11 (Figure 1b,c). The LRR domain is responsible for ligand recognition, while the cytoplasmic TIR domain initiates downstream signaling cascades by interacting with their adaptor proteins such as myeloid differentiation response protein 88 (MyD88), MyD88 adaptor‐like (MAL), TIR‐domain‐containing adapter‐inducing interferon‐β (TRIF), and TRIF‐related adaptor molecule (TRAM). 12 The downstream cascade activates transcription factors such as NF‐κBs and interferon regulatory factors (IRFs), leading to production of proinflammatory cytokines and type I interferons. 9
In induction of inflammatory responses, TLRs transduce signals via 2 major pathways dependent primarily on the adaptors MyD88 or TRIF. 13 , 14 On sensing PAMPs or DAMPs, TLRs dimerize and reorient their TIR domains, which allow docking of the TIR containing proteins, MyD88 and MAL. MAL is a bridging adaptor and it interacts with MyD88 through TIR‐TIR interaction. In addition to the TIR domain, MyD88 contains a death domain that facilitates its interaction with interleukin‐1 receptor (IL‐1R)‐associated kinase 4 (IRAK4), which can both auto‐phosphorylate and trans‐phosphorylate IRAK2/1. 15 This inter‐domain interaction results in a large multimeric molecule, referred to as myddosome, 16 the phosphorylation of which leads to the activation and dimerization of tumor necrosis factor receptor associated factor 6 (TRAF6). The collection of these activities promotes inflammation and host defense.
TRIF dependent signaling is a separate arm of TLR signaling perpetuated only by TLR3 and TLR4, where TRIF interacts with TRAF3 and TRAF6. Upon detection of dimerized TLR4 in endolysosomes, TRAM is thought to interact with TRIF and promote TRAF3‐dependent activation of the kinase TBK1. TBK1 then drives the induction of IFN and interferon‐stimulated gene expression. 17 , 18 , 19
1.2. General structural architecture of extracellular domain of TLRs
The first reported crystal structure of the extracellular domain of TLR (TLR‐ECD) was the unliganded form of TLR3. 20 , 21 This structure provided the general structural architecture of the extracellular domain of TLR.
TLR‐ECD shares a common structural framework adopting horseshoe‐shaped structures built from leucine‐rich repeat (LRR) motifs (Figure 1b). The N‐ and C‐terminal ends of the TLR‐ECD are capped with the LRR‐NT and LRR‐CT motifs. The LRR‐NTs are disulfide‐linked β‐hairpins, whereas the LRR‐CTs are globular structures that contain two α helices and are stabilized by two disulfide bonds.
The TLR7 family (TLR7, TLR8, and TLR9) comprise approximately 800 amino acids and 26 LRR modules, which is the largest number of LRRs among the human TLRs identified to date (Figure 1c). The TLR7 family contain a characteristic insertion loop (Z‐loop) of approximately 30–40 amino acids between LRR14 and LRR15 and the proteolytic processing of Z‐loop in the endolysosomes is required for generation of functional, mature receptors. 22 , 23 , 24 , 25 , 26 After the cleavage, the N‐ and C‐terminal halves of ECD associate with each other, and both fragments are involved in ligand binding. 27 , 28
1.3. Agonist‐induced activated structures of TLRs
Similar to unliganded TLR3, the crystal structure of unliganded TLR9 ECD is reported in a monomeric form. 29 In contrast, TLR8 possesses a distinct feature: the unliganded TLR8 ECD crystallizes as a dimeric structure and exists as a dimer in solution. 28 It is still uncertain whether the authentic apo‐forms of TLRs are monomeric or dimeric. A study of full‐length TLR9 in cell suggested that TLR9 exists as a pre‐formed homodimer, 30 though structural work of TLR9 ECD shows a monomer. 29 The discrepancy can be overcome by the structural study using TLRs embedded in the membrane, reflecting the physiological condition.
To date, the structures of the homologs of all human TLRs (TLR1–9) in agonist‐induced activated forms are reported, except for poorly characterized TLR10 (Figure 2, Table 1), and their ligand recognition mechanisms are extensively reviewed. 31 , 32 , 33 , 34 It is widely accepted that the ligand (agonist)‐induced dimerization of TLR‐ECDs results in the coordinate dimerization of the cytosolic TIR domain, promoting the recruitment of adaptor proteins that facilitate signal transduction. TLR dimers in the activated form are typically “m” shaped, where the C‐terminal regions of the two TLR protomers are positioned in close proximity. Recent studies of how TLRs 7, 8, and 9 detect their nucleic acid ligands have illustrated more complicated mechanism. In brief, they recognize degradation products of nucleic acids at two distinct ligand‐binding sites and the ligands synergistically activate the TLR7 family. Moreover, the Z‐loop cleavage relieves the steric hindrance, by which the ligand‐induced oligomerization is hampered.
FIGURE 2.

Agonist‐induced activated structures of TLRs. The representative activated dimer structures of TLR1‐TLR2‐Pam3CSK4 (PDB ID: 2Z7X), TLR2‐Diprovocim (PDB ID: 6NIG), TLR2‐TLR6‐Pam2CSK4 (PDB ID: 3A79), TLR3‐dsRNA (PDB ID: 3CIY), TLR4‐MD‐2‐LPS (PDB ID: 3FXI), TLR5‐FliC (PDB ID: 3 V47), TLR7‐guanosine‐polyU (PDB ID: 5GMF), TLR8‐ORN06 (PDB ID: 4R07), and TLR9‐DNA1668 (PDB ID: 3WPC). The color scheme is the same as that used in Figure 1b. The agonists are shown in orange. The gray regions in the TLR1‐TLR2‐Pam3CSK4, TLR2‐Diprovocim, TLR2‐TLR6‐Pam2CSK4, and TLR5‐FliC structures indicate the non‐TLR portions. All structural figures were generated using the PyMOL program 70
TABLE 1.
Structures of agonist/antagonist‐bound or apo‐forms of TLRs
| TLRs | Species | Agonist/antagonist | Method | Resolution | PDB ID; comments | References |
|---|---|---|---|---|---|---|
| Apo form | ||||||
| TLR1 | Human | ‐ | X‐ray | 2.3 Å | 6NIH | 51 |
| TLR3 | Human | ‐ | X‐ray | 2.1, 2.4 Å | 1ZIW, 2A0Z | 20, 21 |
| ‐ | Cryo‐EM | 3.4 Å | 7C76; complex with Unc93B1 | 48 | ||
| Mouse | X‐ray | 2.7 Å | 3CIG | 52 | ||
| TLR4 | Mouse | ‐ | X‐ray | 2.8 Å | 2Z64; complex with MD‐2 | 35 |
| TLR7 | Human | ‐ | Cryo‐EM | 4.2 Å | 7CYN; complex with Unc93B1 | 48 |
| TLR8 | Human | ‐ | X‐ray | 2.3 Å | 3W3G | 28 |
| ‐ | X‐ray | 2.6 Å | 5HDH; Z‐loop uncleaved | 53 | ||
| TLR9 | Horse | ‐ | X‐ray | 2.4 Å | 3WPB | 29 |
| Mouse | ‐ | X‐ray | 2.0 Å | 3WPF | 29 | |
| Agonist‐bound form | ||||||
| TLR1‐TLR2 | Human | Pam3CSK4 | X‐ray | 2.1 Å | 2Z7X | 54 |
| TLR2 | Human | Diprovocim | X‐ray | 2.4 Å | 6NIG | 51 |
| TLR2‐TLR6 | Mouse | Pam2CSK4 | X‐ray | 2.9 Å | 3A79 | 55 |
| TLR3 | Mouse | dsRNA (46 mer) | X‐ray | 3.4 Å | 3CIY | 52 |
| TLR4 | Human | LPS Ra | X‐ray | 3.1 Å | 3FXI; complex with MD‐2 | 56 |
| Mouse | LPS re | X‐ray | 2.5 Å | 3VQ2; complex with MD‐2 | 36 | |
| Lipid IVa | X‐ray | 2.7 Å | 3VQ1; complex with MD‐2 | 36 | ||
| Lipid A | X‐ray | 2.7 Å | 5IJD; complex with MD‐2 | 57 | ||
| Neoseptin‐3 | X‐ray | 2.6 Å | 5IJC; complex with MD‐2 | 57 | ||
| TLR5 | Zebrafish | FliC | X‐ray | 2.5 Å | 3 V47 | 58 |
| TLR7 | Monkey | Guanosine, polyU | X‐ray | 2.5 Å | 5GMF | 59 |
| Loxoribine, polyU | X‐ray | 2.6 Å | 5GMG | 59 | ||
| R848 | X‐ray | 2.2 Å | 5GMH | 59 | ||
| Chemical ligands | X‐ray | 2.6–2.8 Å | 6LVY, 6LVX, 6LVZ | 39 | ||
| TLR8 | Human | Chemical ligands | X‐ray | 1.8–2.7 Å | 3W3J, 3W3K, 3W3L, 3W3M, 3W3N, 3WN4, 4QBZ, 4QC0, 4R6A, 5AWA, 5AWB, 5AWC, 5AWD, 5AZ5 | 28, 60, 61, 62, 63, 64 |
| ssRNA a | X‐ray | 2.0‐2.6 Å | 4R07, 4R08, 4R09 | 65 | ||
| Uridine | X‐ray | 1.9 Å | 4R0A | 65 | ||
| TLR9 | Bovine | DNA1668 (12 mer) | X‐ray | 2.4 Å | 3WPE | 29 |
| Horse | DNA1668 (12 mer) | X‐ray | 1.6 Å | 3WPC | 29 | |
| Two DNAs b | X‐ray | 1.8–2.7 Å | 5Y3J, 5Y3K, 5Y3L, 5Y3M, 5ZLN | 66 | ||
| Antagonist‐bound form | ||||||
| TLR4 | Human | Eritoran | X‐ray | 2.7 Å | 2Z65; complex with MD‐2 | 35 |
| TLR7 | Monkey | Chemical ligands | X‐ray | 2.6 Å | 6LW0 c | 39 |
| Cryo‐EM | 2.8 Å | 6LW1 | 39 | |||
| TLR8 | Human | Chemical ligands | X‐ray | 2.4–2.9 Å | 5WYX, 5WYZ, 5Z14, 5Z15, 6KYA, 6TY5, 6V9U | 37, 38, 67, 68, 69 |
| TLR9 | Horse | iDNA4084 (12 mer) | X‐ray | 2.8 Å | 3WPD | 29 |
| Mouse | iDNA4084 (12 mer) | X‐ray | 2.3 Å | 3WPG, 3WPH | 29 | |
| iDNA_super (18 mer) | X‐ray | 2.3 Å | 3WPI | 29 | ||
ORN06 (20 mer), ssRNA40 (20 mer), phophorothiated ORN06 (20 mer); Degradation products of ssRNA (uridine and a short oligonucleotide) are observed in the density map.
CpG DNA (10 mer) and 6 mer DNA.
Closed form.
1.4. Antagonist‐bound inactivated structures of TLRs
While the wealth of structural information has improved our understanding of activated forms of TLRs, only a few structures of the inactivated forms bound to antagonist have been reported (Table 1).
The first report is the structure of TLR4 antagonist, Eritoran (or E5564), in complex with TLR4–MD‐2 35 (Figure 3a). Eritoran is a synthetic molecule derived from the lipid A component of the non‐pathogenic LPS of Rhodobacter sphaeroides. It is a high affinity antagonist and has useful therapeutic activity against severe sepsis. All four lipid chains of Eritoran are completely submerged inside the pocket and cannot provide a hydrophobic dimerization surface that can be used for interaction with TLR4. Therefore Eritoran cannot induce dimerization necessary for the activation. Lipid IVa, a precursor form of LPS, acts as an antagonist of human TLR4–MD‐2, but exhibits weak agonistic activity for the mouse TLR4–MD‐2 complex. The structure of the lipid IVa bound to mouse TLR4–MD‐2 complex 36 provides a clear explanation as to why this molecule activates mouse but not human TLR4. The lipid IVa adopts a very different configuration when compared to the existing structure of human MD‐2 and lipid IVa, one which is closely similar to that of the active human complex. However, the hydrophobic pocket of MD‐2 is not fully occupied due to less acyl chains, probably resulting in a weak agonistic activity.
FIGURE 3.
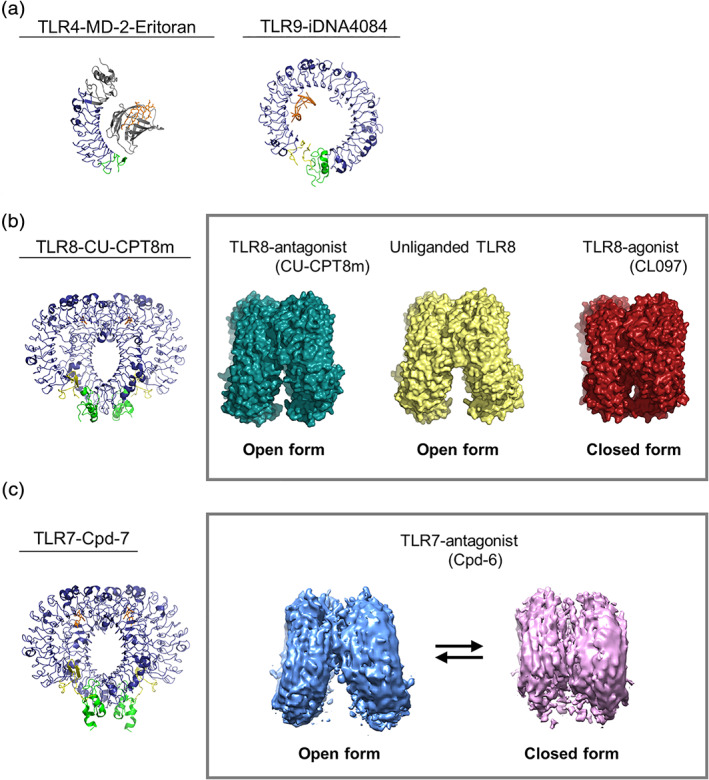
Antagonist‐bound inactivated structures of TLRs. (a) The structures of TLR4‐MD‐2‐Eritoran (PDB ID: 2Z65) and TLR9‐iDNA4084 (PDB ID: 3WPG). The antagonists are shown in orange. The gray region in the TLR4‐MD‐2‐Eritoran structure indicates the non‐TLR portion. (b) The structure of TLR8‐CU‐CPT8m (PDB ID: 5WYX) (left) and the density maps of TLR8‐antagonist (CU‐CPT8m) (PDB ID: 5WYX), unliganded TLR8 (PDB ID: 3W3G), and TLR8‐agonist (CL097) (PDB ID: 3W3J) (right). The antagonists in the structure are shown in orange. (c) The structure of TLR7‐Cpd‐7 (PDB ID: 6LW1) (left) and the density maps of TLR7‐antagonist (Cpd‐6) in open and closed forms (EMDB ID: EMD‐30000, EMD‐1000) (right). The antagonists are shown in orange. The color scheme of all the structures is the same as that used in Figure 1(b). Structural figures were generated using the PyMOL 70 or UCSF Chimera program 71
The TLR9 structure with its antagonist inhibitory DNA (iDNA) was subsequently reported 29 (Figure 3a). In the TLR9‐iDNA complex, iDNA forms stem‐loop structures that fit snugly into the interior of the ring structure of TLR9 with a stronger binding affinity for TLR9 compared to CpG‐containing agonists. Therefore, a partial overlap between the binding interfaces of CpG‐DNA and iDNA would account for the antagonistic effect of iDNA.
Recently, complex structures of TLR8‐antagonists were reported 37 , 38 (Figure 3b). Unlike other TLRs, TLR8 forms homodimers even before binding ligands and the overall structure of the unliganded dimeric structure is essentially the same as that of TLR8 with its antagonists (Figure 3b, right). Both structures adopt a C2‐symmetric open‐form conformation formed by face to face interaction of two TLR8 protomers and the antagonists were bound at two hydrophobic pockets, sandwiched at the interface of two protomers. Antagonist binding did not induce significant structural changes except for the local loop regions. This is in sharp contrast to the large structural reorganization induced by agonist binding. It should be noted that it is not possible for the antagonist and agonist binding pockets to co‐exist. The antagonist binding pocket is only formed in the preformed dimer in the unliganded state, whereas the agonist binding pocket is only formed in the active dimer. Consistent with this, isothermal titration calorimetry experiments showed that the agonists like R848 were unable to bind to TLR8 in the presence of the antagonists. The preceding occupation of the pockets effectively excludes the intrusion of agonists onto the interface of the TLR8 dimer. Of interest, TLR8 utilizes the common platform for both agonist and antagonist binding on one side of the interface, while the other side is completely different due to a conformational reorganization. The antagonists fix and stabilize the inactivated dimer state almost equivalent to the unliganded dimer state.
Most recently, the cryo‐electron microscopy (cryo‐EM) structure of TLR7‐antagonist complex was reported 39 (Figure 3c). Similar to TLR8, TLR7 exhibits an open‐form conformation harboring separated C‐terminus of the protomers, but inactivated dimers of TLR7 more separated from each other compared to those of TLR8. The antagonist binding pocket of TLR7 is essentially the same as that of TLR8, but is more spacious, while that of TLR8 is more compact. In this study, TLR7 dynamics underlying antagonism was reported. The cryo‐EM study of TLR7 in complex with weak antagonist (Cpd‐6) yielded two major types of TLR7 dimer (Figure 3c, right): one was the closed form which was also observed in the crystal structure, and the other was the open form. In contrast, strong antagonist induced only the open form of TLR7. Combining crystallography and cryo‐EM revealed the structural equilibrium between the open (inactive) and closed (active) conformations that underlie TLR7 antagonism.
1.5. Structural study of intracellular TIR domain, death domain and Myddosome
Structures of many TIR domains from both receptors and adaptors are available and have been well reviewed. 33 , 40 TIRs are generally similar to each other in that all of them include a central five‐stranded β‐sheet surrounded by five α‐helices (Figure 4). The highly conserved BB loop of the TIR domain, “so called as it” connects the second 𝛽 strand (𝛽B) to the second α‐helix (αB), plays an important role in signaling. Due to the weak interactions, active oligomeric structures remain unknown. Recent work 41 demonstrates that the TIR domains of the adaptor proteins MAL and MyD88 are able to form large spontaneous filaments in solution. Likewise, the TIR domain of TLR4 was also able to form cofilaments with MyD88 TIRs, suggesting that a smaller filament likely facilitates TIR‐induced TLR signaling.
FIGURE 4.
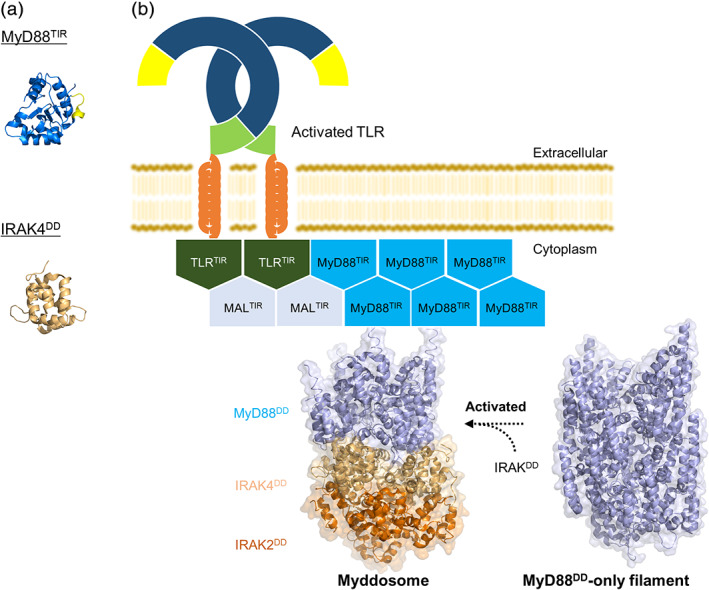
Intracellular assembled structures. (a) The structures of MyD88TIR (PDB ID: 4EO7) and IRAK4DD (PDB ID: 2A9I). BB loop of MyD88TIR is shown in yellow. (b) Model of TIR‐ and DD‐induced TLR signaling. The crystal structures of Myddosome (PDB ID: 3MOP) and MyD88DD‐only filament (PDB ID: 6I3N) are shown. MyD88DD‐only filaments can form Myddosome with IRAK4DD and IRAK2DD upon the ligand binding to TLRs. All structural figures were generated using the PyMOL program 70
Association of TIR domains is thought to provide a stable platform for MyD88 binding and oligomerization. MyD88 comprises an N‐terminal death domain (DD), homologous to that found in death receptors like FAS, and a C‐terminal TIR domain linked by an interdomain. DD domain serves to recruit a family of kinases known as IRAKs, forming a Myddosome. This is composed of a ring of six MyD88DDs that serve as a seed for assembly of a ring of four IRAK4DDs and ultimately four IRAK2DDs in a helix 16 (Figure 4b). All assemblies rely on three distinct types of interactions between DDs, allowing sequential assembly of a large complex. Because the crystal structure of Myddosome was assembled in the absence of the MyD88 TIR domain and of TLRs, it is unclear whether all MyD88 molecules in the Myddosome are bound to a TLR or IL‐1R through their TIR domain or whether the clustering of two TIR domains is enough to allow stable DD association that seeds binding of additional MyD88DDs and assembly of a full Myddosome.
Most recently, cryo‐EM structure of large helical “MyD88DD‐only” filaments is reported 42 (Figure 4b). Its molecular architecture is reminiscent of Myddosome containing MyD88DD and IRAKDD: MyD88DD‐only filament retains a similar 6:4 DD arrangement to that observed in the MyD88DD/IRAKDD. This structural work suggests that “pre‐formed Myddosomes” may exist independently of membrane‐associated receptor or adaptor TIR protein interactions, allowing us to explain the high sensitivity and fast response times even when the concentration of activating ligands is low.
1.6. Compartmentalization of nucleic‐acids sensing TLRs and solute carrier transporter
Compartmentalization of nucleic‐acids sensing TLRs in the endolysosome limits their activation by self‐derived nucleic acids and reduces the possibility of autoimmune reactions. The chaperone Unc93B1 is indispensable for the trafficking of TLRs from the endoplasmic reticulum (ER) to the endolysosome. 43 , 44 Unc93B1 binds endosomal TLRs in the ER and somehow facilitates their proper folding. Once folded, Unc93B1 remains bound to its client TLRs and is necessary to maintain TLR stability after synthesis. 45 Upon delivery to endolysosomes, TLR7 and Unc93B1 remain associated and Unc93B1 functions to restrict the signaling activities of TLR7. This restriction of signaling is mediated by the cytosolic protein syntenin‐1, which acts as a negative‐regulator of TLR7‐ Unc93B1 in TLR7 activation, by transporting them to the intralumenal vesicles of multivesicular bodies, where TLR7 signaling terminates. 46 In the context of TLR9 signaling, Unc93B1 has a different post‐trafficking function. TLR9 must be released from Unc93B1 in order to bind CpG DNA and promote downstream signaling events. 47 Structural studies of the TLR‐Unc93B1 complex reveal that Unc93B1 harbors a major facilitator superfamily fold and TLR TM helix interacts mainly with the Unc93B1 TM3 in an antiparallel fashion, forming extensive hydrophobic interactions 48 (Figure 5). It is unknown that Unc93B1 functions as a classic transporter but the structures provide the first example of an MFS‐like protein that associates with another protein via transmembrane protein–protein interactions.
FIGURE 5.
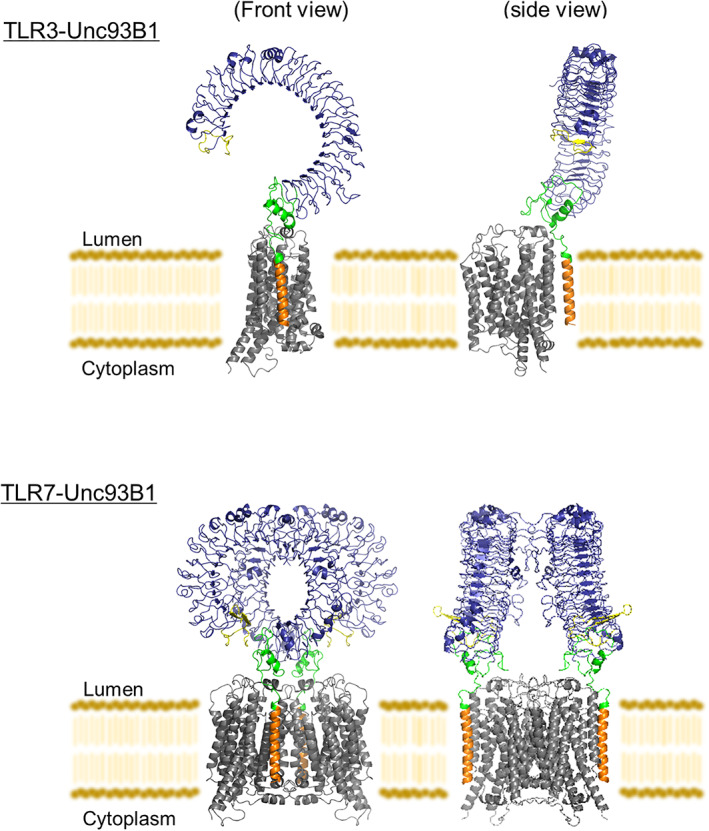
Structures of TLR‐Unc93B1. The structures of TLR3‐Unc93B1 (PDB ID: 7C76) and TLR7‐Unc93B1 (PDB ID: 7CYN). The color scheme of TLRs is the same as that used in Figure 1b. Unc93B1 is shown in gray. All structural figures were generated using the PyMOL program 70
Immune cells possess a multilayered system for controlling the endosomal/lysosomal environment, involving not only the proton concentration but also the concentrations of other ions and substances. Solute carriers (SLCs) form the largest group of membrane transporter proteins and play a critical role in cellular and organismal metabolism by mediating exchanges between the environment and the intracellular milieu as well as between subcellular organelles. For example, SLC15A4, proton‐coupled histidine/oligopeptide transporter, is required for TLR7 and TLR9‐mediated type I interferon productions possible by endolysosomal pH regulation. 49 Recently, SLC15A4 is reported to form signaling complex with TASL for endosomal TLR7‐9 responses and IRF5 activation. 50 The endosome/lysosome compartments play crucial roles in immune cell functions as signalling platforms, of which environments are strictly regulated. This role is mostly conducted by SLCs, but the recent work suggests that SLCs also function as signaling hubs.
2. CONCLUSIONS
Recent years have seen remarkable progress in structural biological studies on TLRs, opening new avenues for TLR research. The structures of the TLRs complexes with their physiological ligands as well as with synthetic ligands provide clues as to how these TLRs participate in immune responses. This will facilitate the development of novel therapeutic agents that either block or stimulate TLR activities. However, most structural information is limited to an extracellular or intracellular domain, or its complex with the ligand, restricting our understanding of the mechanism by which TLRs are regulated. Moreover, little structural knowledge is available regarding higher‐order signaling complexes, such as Myddosome and inflammasome, which are formed to execute signal transduction and signal amplification in most innate immunity pathways. Structural information of full‐length TLRs embedded in lipid and the large assembly complex including adaptor proteins has been long awaited, because it will provide a comprehensive view of pattern recognition, TLR activation and TLR signaling in the natural environment. Though these structural studies face technical problems such as protein production and structure determination, recent remarkable progresses in the protein expression systems, crystallization of the membrane proteins and rapidly developing cryo‐EM techniques would be expected to overcome these difficulties.
AUTHOR CONTRIBUTIONS
Jinta Asami: Visualization; writing‐original draft. Toshiyuki Shimizu: Writing‐original draft; writing‐review and editing.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
This work was supported by a Grant‐in‐Aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (T.S.); Core Research for Evolutional Science and Technology (CREST) (T.S.).
Asami J, Shimizu T. Structural and functional understanding of the toll‐like receptors. Protein Science. 2021;30:761–772. 10.1002/pro.4043
REFERENCES
- 1. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 2. Anderson KV, Bokla L, Nusslein‐Volhard C. Establishment of dorsal‐ventral polarity in the drosophila embryo: The induction of polarity by the toll gene product. Cell. 1985;42:791–798. [DOI] [PubMed] [Google Scholar]
- 3. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/toll/cactus controls the potent antifungal response in drosophila adults. Cell. 1996;86:973–983. [DOI] [PubMed] [Google Scholar]
- 4. Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila toll is activated by gram‐positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001;414:756–759. [DOI] [PubMed] [Google Scholar]
- 5. Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. [DOI] [PubMed] [Google Scholar]
- 6. Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. [DOI] [PubMed] [Google Scholar]
- 7. Hennessy EJ, Parker AE, O'Neill LA. Targeting toll‐like receptors: Emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. [DOI] [PubMed] [Google Scholar]
- 8. Barreiro LB, Ben‐Ali M, Quach H, et al. Evolutionary dynamics of human toll‐like receptors and their different contributions to host defense. PLoS Genet. 2009;5:e1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawai T, Akira S. The role of pattern‐recognition receptors in innate immunity: Update on toll‐like receptors. Nat Immunol. 2010;11:373–384. [DOI] [PubMed] [Google Scholar]
- 10. Bell JK, Mullen GED, Leifer CA, Mazzoni A, Davies DR, Segal DM. Leucine‐rich repeats and pathogen recognition in toll‐like receptors. Trends Immunol. 2003;24:528–533. [DOI] [PubMed] [Google Scholar]
- 11. Matsushima N, Tanaka T, Enkhbayar P, et al. Comparative sequence analysis of leucine‐rich repeats (LRRs) within vertebrate toll‐like receptors. BMC Genomics. 2007;8:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Neill LA, Bowie AG. The family of five: TIR‐domain‐containing adaptors in toll‐like receptor signalling. Nat Rev Immunol. 2007;7:353–364. [DOI] [PubMed] [Google Scholar]
- 13. Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88‐deficient mice to endotoxin. Immunity. 1999;11:115–122. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88‐independent toll‐like receptor signaling pathway. Science. 2003;301:640–643. [DOI] [PubMed] [Google Scholar]
- 15. Janssens S, Beyaert R. Functional diversity and regulation of different interleukin‐1 receptor‐associated kinase (Irak) family members. Mol Cell. 2003;11:293–302. [DOI] [PubMed] [Google Scholar]
- 16. Lin SC, Lo YC, Wu H. Helical assembly in the MyD88‐IRAK4‐IRAK2 complex in TLR/IL‐1R signalling. Nature. 2010;465:885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fitzgerald KA, McWhirter SM, Faia KL, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. [DOI] [PubMed] [Google Scholar]
- 18. McWhirter SM, Fitzgerald, KA , Rosains, J , Rowe, DC , Golenbock, DT , & Maniatis, T . IFN‐regulatory factor 3‐dependent gene expression is defective in Tbk1‐deficient mouse embryonic fibroblasts. Proc Natl Acad Sci U S A. 2004;101:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharma S, tenOever B, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK‐related pathway. Science. 2003;300:1148–1151. [DOI] [PubMed] [Google Scholar]
- 20. Bell JK, Botos I, Hall PR, et al. The molecular structure of the toll‐like receptor 3 ligand‐binding domain. Proc Natl Acad Sci U S A. 2005;102:10976–10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choe J, Kelker MS, Wilson IA. Crystal structure of human toll‐like receptor 3 (TLR3) ectodomain. Science. 2005;309:581–585. [DOI] [PubMed] [Google Scholar]
- 22. Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by toll‐like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011;208:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ewald SE, Lee BL, Lau L, et al. The ectodomain of toll‐like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishii N, Funami K, Tatematsu M, Seya T, Matsumoto M. Endosomal localization of TLR8 confers distinctive proteolytic processing on human myeloid cells. J Immunol. 2014;193:5118–5128. [DOI] [PubMed] [Google Scholar]
- 25. Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of toll‐like receptor 9. Nat Immunol. 2008;9:1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sepulveda FE, Maschalidi S, Colisson R, et al. Critical role for asparagine endopeptidase in endocytic toll‐like receptor signaling in dendritic cells. Immunity. 2009;31:737–748. [DOI] [PubMed] [Google Scholar]
- 27. Onji M, Kanno A, Saitoh SI, et al. An essential role for the N‐terminal fragment of toll‐like receptor 9 in DNA sensing. Nat Commun. 2013;4:1949. [DOI] [PubMed] [Google Scholar]
- 28. Tanji H, Ohto U, Shibata T, Miyake K, Shimizu T. Structural reorganization of the toll‐like receptor 8 dimer induced by agonistic ligands. Science. 2013;339:1426–1429. [DOI] [PubMed] [Google Scholar]
- 29. Ohto U, Shibata T, Tanji H, et al. Structural basis of CpG and inhibitory DNA recognition by toll‐like receptor 9. Nature. 2015;520:702–705. [DOI] [PubMed] [Google Scholar]
- 30. Latz E, Verma A, Visintin A et al. Ligand‐induced conformational changes allosterically activate toll‐like receptor 9. Nat Immunol. 2007;8:772–779. [DOI] [PubMed] [Google Scholar]
- 31. Kang JY, Lee JO. Structural biology of the toll‐like receptor family. Annu Rev Biochem. 2011;80:917–941. [DOI] [PubMed] [Google Scholar]
- 32. Shimizu T. Structural insights into ligand recognition and regulation of nucleic acid‐sensing toll‐like receptors. Curr Opin Struct Biol. 2017;47:52–59. [DOI] [PubMed] [Google Scholar]
- 33. Yin Q, Fu TM, Li J, Wu H. Structural biology of innate immunity. Annu Rev Immunol. 2015;33:393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Z, Ohto U, Shimizu T. Toward a structural understanding of nucleic acid‐sensing toll‐like receptors in the innate immune system. FEBS Lett. 2017;591:3167–3181. [DOI] [PubMed] [Google Scholar]
- 35. Kim HM, Park BS, Kim JI, et al. Crystal structure of the TLR4‐MD‐2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. [DOI] [PubMed] [Google Scholar]
- 36. Ohto U, Fukase K, Miyake K, Shimizu T. Structural basis of species‐specific endotoxin sensing by innate immune receptor TLR4/MD‐2. Proc Natl Acad Sci U S A. 2012;7421‐7426(2012):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu Z, Tanji H, Jiang S, et al. Small‐molecule TLR8 antagonists via structure‐based rational design. Cell Chem Biol. 2018;25:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang S, Hu Z, Tanji H, et al. Small‐molecule inhibition of TLR8 through stabilization of its resting state. Nat Chem Biol. 2018;14:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tojo S, Zhang Z, Matsui H, et al. Structural analysis reveals TLR7 dynamics underlying antagonism. Nat Commun. 2020;11:5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nimma S, Ve T, Williams SJ, Kobe B. Towards the structure of the TIR‐domain signalosome. Curr Opin Struct Biol. 2017;43:122–130. [DOI] [PubMed] [Google Scholar]
- 41. Ve T, Vajjhala PR, Hedger A, et al. Structural basis of TIR‐domain‐assembly formation in MAL‐ and MyD88‐dependent TLR4 signaling. Nat Struct Mol Biol. 2017;24:743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moncrieffe MC, Bollschweiler D, Li B, et al. MyD88 death‐domain oligomerization determines myddosome architecture: Implications for toll‐like receptor signaling. Structure. 2020;28:281–289.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide‐sensing toll‐like receptors to endolysosomes. Nature. 2008;452:234–238. [DOI] [PubMed] [Google Scholar]
- 44. Lee BL, Moon JE, Shu JH, et al. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife. 2013;2:e00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pelka K, Bertheloot D, Reimer E, et al. The chaperone UNC93B1 regulates toll‐like receptor stability independently of endosomal TLR transport. Immunity. 2018;48:911–922.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Majer O, Liu B, Kreuk LSM, Krogan N, Barton GM. UNC93B1 recruits syntenin‐1 to dampen TLR7 signalling and prevent autoimmunity. Nature. 2019;575:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Majer O, Liu B, Woo BJ, Kreuk LSM, van Dis E, Barton GM. Release from UNC93B1 reinforces the compartmentalized activation of select TLRs. Nature. 2019;575:371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishida H, Asami J, Zhang Z. Cryo‐EM structures of toll‐like receptors in complex with UNC93B1. Nat Struct Mol Biol, 2021;28:173–180. [DOI] [PubMed] [Google Scholar]
- 49. Kobayashi T, Shimabukuro‐Demoto S, Yoshida‐Sugitani R, et al. The histidine transporter SLC15A4 coordinates mTOR‐dependent inflammatory responses and pathogenic antibody production. Immunity. 2014;41:375–388. [DOI] [PubMed] [Google Scholar]
- 50.Heinz LX, Lee J, Kapoor U. TASL is the SLC15A4‐associated adaptor for IRF5 activation by TLR7‐9. Nature. 2020;581:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Su L, Wang Y, Wang J, et al. Structural basis of TLR2/TLR1 activation by the synthetic agonist diprovocim. J Med Chem. 2019;62:2938–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu L, Botos I, Wang Y, et al. Structural basis of toll‐like receptor 3 signaling with double‐stranded RNA. Science. 2008;320:379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanji H, Ohto U, Motoi Y, Shibata T, Miyake K, Shimizu T. Autoinhibition and relief mechanism by the proteolytic processing of toll‐like receptor 8. Proc Natl Acad Sci U S A. 2016;113:3012–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jin MS, Kim SE, Heo JY, et al. Crystal structure of the TLR1‐TLR2 heterodimer induced by binding of a tri‐acylated lipopeptide. Cell. 2007;130:1071–1082. [DOI] [PubMed] [Google Scholar]
- 55. Kang JY, Nan X, Jin MS, et al. Recognition of lipopeptide patterns by toll‐like receptor 2‐toll‐like receptor 6 heterodimer. Immunity. 2009;31:873–884. [DOI] [PubMed] [Google Scholar]
- 56. Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4‐MD‐2 complex. Nature. 2009;458:1191–1195. [DOI] [PubMed] [Google Scholar]
- 57. Wang Y, Su L, Morin MD, et al. TLR4/MD‐2 activation by a synthetic agonist with no similarity to LPS. Proc Natl Acad Sci U S A. 2016;113:E884–E893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yoon SI, Kurnasov O, Natarajan V, et al. Structural basis of TLR5‐flagellin recognition and signaling. Science. 2012;335:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Z, Ohto U, Shibata T, et al. Structural analysis reveals that toll‐like receptor 7 is a dual receptor for guanosine and single‐stranded RNA. Immunity. 2016;45:737–748. [DOI] [PubMed] [Google Scholar]
- 60. Beesu M, Caruso G, Salyer ACD, et al. Structure‐based design of human TLR8‐specific agonists with augmented potency and adjuvanticity. J Med Chem. 2015;58:7833–7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Beesu M, Caruso G, Salyer ACD, et al. Identification of a human toll‐like receptor (TLR) 8‐specific agonist and a functional pan‐TLR inhibitor in 2‐aminoimidazoles. J Med Chem. 2016;59:3311–3330. [DOI] [PubMed] [Google Scholar]
- 62. Ganapathi L, Haren SV, Dowling DJ, et al. The imidazoquinoline toll‐like receptor‐7/8 agonist hybrid‐2 potently induces cytokine production by human newborn and adult leukocytes. PLoS One. 2015;e0134640:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kokatla HP, Sil D, Tanji H, et al. Structure‐based design of novel human toll‐like receptor 8 agonists. ChemMedChem. 2014;9:719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yoo E, Salunke DB, Sil D, et al. Determinants of activity at human toll‐like receptors 7 and 8: Quantitative structure–activity relationship (QSAR) of diverse heterocyclic scaffolds. J Med Chem. 2014;57:7955–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tanji H, Ohto U, Shibata T, et al. Toll‐like receptor 8 senses degradation products of single‐stranded RNA. Nat Struct Mol Biol. 2015;22:109–115. [DOI] [PubMed] [Google Scholar]
- 66. Ohto U, Ishida H, Shibata T, Sato R, Miyake K, Shimizu T. Toll‐like receptor 9 contains two DNA binding sites that function cooperatively to promote receptor dimerization and activation. Immunity. 2018;48:649–658. [DOI] [PubMed] [Google Scholar]
- 67. Jiang S, Tanji H, Yin K, et al. Rationally designed small‐molecule inhibitors targeting an unconventional pocket on the TLR8 protein‐protein interface. J Med Chem. 2020;63:4117–4132. [DOI] [PubMed] [Google Scholar]
- 68. Knoepfel T, Nimsgern P, Jacquier S, et al. Target‐based identification and optimization of 5‐indazol‐5‐yl pyridones as toll‐like receptor 7 and 8 antagonists using a biochemical TLR8 antagonist competition assay. J Med Chem. 2020;63:8276–8295. [DOI] [PubMed] [Google Scholar]
- 69. Mussari CP, Dodd DS, Sreekantha RK, et al. Discovery of potent and orally bioavailable small molecule antagonists of toll‐like receptors 7/8/9 (TLR7/8/9). ACS Med Chem Lett. 2020;11:1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. DeLano WL. An open‐source molecular graphics tool. CCP4 Newslett Protein Cryst. 2002;40:82–92. [Google Scholar]
- 71. Pettersen EF, Goddard TD, Huang CC, et al. UCSF chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. [DOI] [PubMed] [Google Scholar]


