Key Points
Anakinra improved 28-day survival rate in patients with COVID-19–associated ARDS.
Anakinra was well tolerated, with no increase in infection-related adverse events.
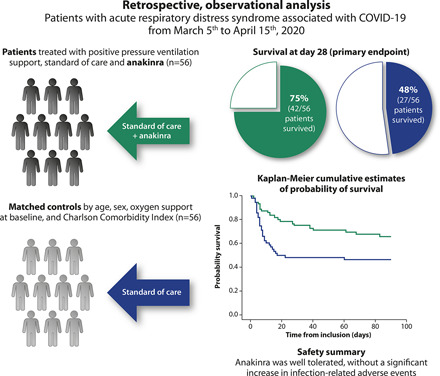
Visual Abstract
Abstract
The IL-1 receptor antagonist, anakinra, may represent a therapeutic option for acute respiratory distress syndrome (ARDS) associated with coronavirus disease 2019 (COVID-19). In this study, COVID-19 ARDS patients admitted to the Azienda Socio Sanitaria Territoriale of Lecco, Italy, between March 5th to April 15th, 2020, and who had received anakinra off-label were retrospectively evaluated and compared with a cohort of matched controls who did not receive immunomodulatory treatment. The primary end point was survival at day 28. The population consisted of 112 patients (56 treated with anakinra and 56 controls). Survival at day 28 was obtained in 69 patients (61.6%) and was significantly higher in anakinra-treated patients than in the controls (75.0 versus 48.2%, p = 0.007). When stratified by continuous positive airway pressure support at baseline, anakinra-treated patients’ survival was also significant compared with the controls (p = 0.008). Univariate analysis identified anakinra usage (odds ratio, 3.2; 95% confidence interval, 1.47–7.17) as a significant survival predictor. This was not supported by multivariate modeling. The rate of infectious-related adverse events was similar between groups. In conclusion, anakinra improved overall survival and invasive ventilation-free survival and was well tolerated in patients with ARDS associated with COVID-19.
Introduction
The clinical course of coronavirus disease 2019 (COVID-19) varies from mild to severe cases, with a minority of patients evolving to critical conditions (1). The most common complication of COVID-19–related pneumonia is acute respiratory distress syndrome (ARDS), a condition of respiratory failure with acute onset, bilateral lung opacities, and hypoxemia. This condition is characterized by dramatic inflammatory features evoking similarities with other clinical scenarios such as septic shock, cytokine-release syndrome, and macrophage activation syndrome (MAS) (2).
Mortality in patients with ARDS associated with severe COVID-19 was reported to be between 50 and 65% early in the pandemic (3). Although therapy is not standardized, there is no doubt regarding the beneficial role of noninvasive ventilation in severe cases and critical COVID-19–related pneumonia, starting with continuous positive airway pressure (CPAP) using the lowest effective pressures (e.g., 5–10 cm H2O). Nevertheless, critical cases often warrant intubation and invasive mechanical ventilation (4).
The initial understanding of the pathological pathway of the disease led investigators to hypothesize that IL blockade may control the cytokine-release storm resembling MAS. A potential clinical therapeutic option was consequently found in the IL-1 receptor antagonist, anakinra (5).
Anakinra blocks the activity of the proinflammatory cytokines IL-1α and IL-1β and is commonly used in the treatment of rheumatologic diseases (i.e., adult-onset Still disease, systemic-onset juvenile idiopathic arthritis, and familial Mediterranean fever). Anakinra has also been reported to reduce mortality in pediatric and adult patients with secondary hemophagocytic lymphohistiocytosis or MAS triggered by a virus (6). A further possible role for this drug has also been suggested in the treatment of sepsis when the clinical course assumes the features of MAS (7).
Whereas early clinical experiences highlighted the potential role of anakinra in the treatment of COVID-19 (8–14), subsequent observational studies evaluated its impact in the setting of hyperinflammation or in cases of symptoms indicative of worsening respiratory function (15, 16).
As the results of these studies were highly encouraging, we aimed to confirm the clinical role of anakinra in the early survival of patients with COVID-19–related severe or critical ARDS and to evaluate its impact on long-term outcomes and possible adverse events.
Materials and Methods
Patients and treatment
We conducted a retrospective, observational analysis of all patients with a diagnosis of COVID-19 ARDS who were admitted to our institution, the Azienda Socio Sanitaria Territoriale of Lecco (Alessandro Manzoni Hospital and San Leopoldo Mandic Hospital), between March 5th to April 15th, 2020, and had received off-label treatment with anakinra. We also evaluated a cohort of control patients with the same clinical diagnosis during the same time interval but who did not receive any immunomodulatory treatment, with a 1:1 match performed according to age, sex, oxygen support at baseline, and Charlson comorbidity index.
Inclusion criteria were the identification of SARS-CoV-2 on pharyngeal swab specimen by real-time RT-PCR assay, evidence of bilateral pneumonia either at chest radiography or computed tomography scan of the thorax, altered levels of serum inflammatory biomarkers [C-reactive protein (CRP) ≥ 10.0 mg/dl, or ferritin ≥ 900 ng/ml, or both, given the evidence that ARDS in COVID-19–infected patients is caused by an overstimulation of the immune system (17)] and a deterioration in respiratory function. All patients had a ratio of partial pressure of oxygen in arterial blood to fractional concentration of oxygen of <250 mm Hg in inspired air and required ventilatory support, either with CPAP or orotracheal intubation, to achieve a positive end-expiratory pressure of at least 8 cm H2O. Patients who were not on positive pressure ventilation support and those who received remdesivir, corticosteroids, or any immunomodulatory treatment, with the exception of anakinra, were excluded from the study.
All patients were treated according to local evidence-based indications in use at our institution during the study period, including the use of lopinavir/ritonavir 400/100 mg twice and 200 mg of hydroxychloroquine twice by mouth (18). Patients received a first-line antibiotic therapy (i.e., 500 mg of azithromycin by mouth daily and 2 g of ceftriaxone i.v. daily), and all patients were treated with 4000 IU of enoxaparin daily as standard but with dosage adjusted based on thrombotic risk and d-dimer levels (19). Anakinra was administered for 7 d at the dosage of 100 mg four times a day s.c., if managed in a regular ward, or 200 mg three times daily i.v., if managed in the intensive care unit, because intensive care unit patients were more frequently affected by anasarca and s.c. edema (20).
When possible, according to their state of consciousness, all patients were informed of the possible effects and adverse events, and gave written informed consent for the off-label use of antivirals used for the treatment of COVID-19 and for the off-label use of anakinra when it was prescribed; clinical data were recorded using a dedicated electronic case report form.
Biochemical parameters and oxygen support were evaluated over time from baseline, which was considered as anakinra prescription (treatment group) or oxygen support introduction (control group); biochemical follow-up was conducted for 2 wk from baseline, according to standard COVID-19 protocols at our institution.
Data were extracted from patients’ medical records for the period of the COVID-19 infection prior to and during hospitalization, and for at least 3 mo after hospital admission, to evaluate time-related survival.
Statistical analysis
Parametric tests (t test and ANOVA), nonparametric tests (Mann–Whitney and Kruskal–Wallis), and the Pearson χ2 test (or Fisher exact test, when necessary) were used to compare normally distributed, nonnormally distributed continuous, and categorical variables of patients, respectively. The primary end point was 28-d survival, evaluated by χ2 test and logistic regression models, correcting for factors involved in possible increases in mortality rates (21): baseline CRP, d-dimer, ferritin, obesity, hypertension, diabetes, previous cardiac or pulmonary comorbidities, and time from disease onset. Two multivariate models were tested to evaluate the role of anakinra. In model A, we tested all correlates that were significantly associated with survival in the univariate model; in model B, we tested all correlates that were differently distributed at baseline in the two study groups, to correct for possible sampling confounders.
Kaplan–Meier curves and Cox regression models were used to compare overall survival and ventilation-free survival. Between-group differences were assessed using the log-rank test.
Results
Study population
In total, 56 patients treated with anakinra and 56 matched controls were included in our analysis. Demographic, anamnestic, and clinical characteristics for the overall population and both treatment groups are shown in Table I. Most patients were male (77.7%), the median age of the group was 67 y, and 78.6% of the patients received CPAP as their main oxygen support. Median symptom duration before hospital admission was 7 d (interquartile range, 5–10 d), and the median Charlson comorbidity index was 3 (interquartile range, 2–4). Among all 112 patients, hypertension was reported in 59 patients (52.7%), ischemic heart disease was reported in 20 patients (17.9%), chronic obstructive pulmonary disease or asthma was reported in 8 patients (7.1%), and diabetes mellitus was reported in 19 patients (17.0%); 49 patients (43.8%) were obese (body mass index > 25 kg/m2), and 11 patients (9.8%) had cancer. No significant between-treatment group differences were identified for age, sex, CPAP as respiratory support, or Charlson comorbidity index.
Table I. Baseline demographic and clinical characteristics.
| Overall Population (n = 112) | Anakinra + SOC (n = 56) | SOC (n = 56) | p Valuea | |
|---|---|---|---|---|
| Demographics | ||||
| Sex, male | 87 (77.7) | 41 (73.2) | 46 (82.1) | 0.364 |
| Age | 67 (59–62) | 65 (58–71) | 68 (60–73) | 0.265 |
| White | 110 (98.2) | 55 (98.2) | 55 (98.2) | 1.000 |
| Comorbidities | ||||
| CCI | 3 (2–4) | 3 (2–3) | 3 (2–4) | 0.110 |
| Hypertension | 59 (52.7) | 27 (48.2) | 32 (57.1) | 0.449 |
| Ischemic heart disease | 20 (17.9) | 6 (10.7) | 14 (25.0) | 0.084 |
| COPD | 8 (7.1) | 5 (8.9) | 3 (5.4) | 0.714 |
| Diabetes mellitus | 19 (17.0) | 6 (10.7) | 13 (23.2) | 0.131 |
| Obesity/overweight (BMI >25 kg/m2) | 49 (43.8) | 28 (50) | 21 (37.5) | 0.253 |
| Cancer | 11 (9.8) | 5 (8.9) | 6 (10.7) | 1.000 |
| Chronic kidney disease | 8 (7.1) | 3 (5.4) | 5 (8.9) | 0.714 |
| Tobacco smoke | 9 (8.0) | 6 (10.7) | 3 (5.4) | 0.483 |
| Clinical conditions | ||||
| P/F | 133 (110–196) | 134 (106–200) | 132 (111–183) | 0.956 |
| Patients with CPAP | 88 (78.6) | 42 (75.0) | 46 (82.1) | 0.823 |
| Symptom duration before hospitalization, days | 7 (5–10) | 7 (5–10) | 7 (4–11) | 0.799 |
| Laboratory values | ||||
| CRP, mg/dl | 17.5 (11.0–24.9) | 17.9 (11.8–30.7) | 15.6 (9.4–21.1) | 0.021 |
| Lymphocytes, cells/mm3 | 689 (486–966) | 594 (454–1090) | 798 (533–904) | 0.238 |
| D-dimer, ng/ml | 3096 (1194–14,871) | 4216 (1375–17,302) | 1331 (815–5530) | 0.025 |
| Ferritin, ng/ml | 1620 (918–2988) | 1852 (1090–3044) | 1105 (597–2730) | 0.155 |
| Lactate dehydrogenase, U/L | 423 (336–557) | 421 (328–577) | 423 (343–548) | 0.884 |
| Alanine aminotransferase, U/l | 50 (38–81) | 49 (38–81) | 50 (38–79) | 0.910 |
| Creatinine, mg/dl | 0.94 (0.67–1.13) | 0.94 (0.68–0.18) | 0.90 (0.60–1.02) | 0.593 |
| Troponin I, ng/l | 15 (15–87) | 15 (15–126) | 15 (15–87) | 0.811 |
Data are median value (interquartile range) or n (%), respectively for continuous and categorical variables.
Mann–Whitney nonparametric test and Pearson χ2 test were used, respectively, to compare continuous and categorical variables of patients in the two groups.
BMI, body mass index; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; P/F, partial pressure of oxygen in arterial blood to fractional concentration of oxygen.
Among baseline laboratory values, patients treated with anakinra had significantly higher median CRP (p = 0.021) and d-dimer (p = 0.025) levels compared with patients who received the standard of care (SOC) only (Table I).
Outcome
For the overall population, survival at day 28 was obtained in 69 patients (61.6%; Table II). Treatment with anakinra was associated with a significantly higher proportion of patients achieving 28-d survival compared with those receiving SOC treatment (75.0 versus 48.2%, p = 0.007; Table II). This finding was confirmed by logistic regression modeling, with univariate analysis identifying anakinra usage (odds ratio [OR], 3.2; 95% confidence interval [CI], 1.47–7.17) and baseline ratio of partial pressure of oxygen in arterial blood to fractional concentration of oxygen (OR per 10 mm Hg higher, 1.21; 95% CI, 1.08–1.36) as significant predictors of survival (Table III). In contrast, predictors of a lower probability of survival at day 28 were identified as preexisting hypertension (OR, 0.37; 95% CI, 0.17–0.82), ischemic heart disease (OR, 0.35; 95% CI, 0.13–0.95), and higher age (OR per 1 y older, 0.87; 95% CI, 0.82–0.93). Baseline levels of d-dimer, ferritin, obesity, diabetes, previous pulmonary comorbidities, and time from disease onset were not associated with survival at day 28.
Table II. Treatment outcomes.
| Overall Population (n = 112) | Anakinra + SOCa (n = 56) | SOC (n = 56) | p Valueb | |
|---|---|---|---|---|
| 28-d survival | 69 (61.6) | 42 (75.0) | 27 (48.2) | 0.007 |
| 28-d survival in orotracheal-intubated patients | 18/24 (75.0) | 11/14 (78.6) | 7/10 (70) | 0.634 |
| 28-d survival in CPAP-treated patients | 51/88 (58.0) | 31/42 (73.8) | 20/46 (43.5) | 0.008 |
| Major infective events | 24 (21.4) | 15 (26.8) | 9 (16.1) | 0.250 |
| 90-d survival | 63 (56.3) | 37 (66.1) | 26 (46.4) | 0.036 |
| 90-d ventilation-free survival | 41/88 (46.6) | 24/42 (58.5) | 17/46 (41.5) | 0.093 |
| Need of invasive ventilation | 17/88 (19.3) | 9/42 (21.4) | 8/46 (17.4) | 0.835 |
| Duration of recovery in survivors, days | 34 (18–48) | 35 (18–49) | 34 (18–48) | 0.950 |
Data are median value (interquartile range) or n (%), respectively, for continuous and categorical variables.
SOC included lopinavir/ritonavir (400/100 mg twice), hydroxychloroquine (200 mg twice by mouth), first-line antibiotic therapy (i.e., 500 mg of azithromycin daily by mouth and 2 g of ceftriaxone daily i.v.), and enoxaparin (4000 IU daily as standard but dosage adjusted based on thrombotic risk and d-dimer levels).
Mann–Whitney nonparametric test and Pearson χ2 test were used, respectively, to compare continuous and categorical variables of patients in the two groups.
Table III. Logistic regression models evaluating 28-d survival.
| Univariate |
Multivariate (Model A)a |
Multivariate (Model B)b |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Gender, males versus females | 0.67 | 0.27–1.79 | — | — | — | — |
| Oxygen support, CPAP versus intubation | 0.46 | 0.17–1.30 | — | — | — | — |
| Age, per 1 y more | 0.87 | 0.82–0.93 | 0.88 | 0.81–0.95 | — | — |
| P/F, per 10 mm Hg higher | 1.21 | 1.08–1.36 | 1.20 | 1.05–1.37 | — | — |
| CCI, per 1 point higher | 0.46 | 0.32–0.64 | 0.55 | 0.31–0.98 | — | — |
| Hypertension | 0.37 | 0.17–0.82 | 0.98 | 0.32–3.16 | — | — |
| Ischemic heart disease | 0.35 | 0.13–0.95 | 0.67 | 1.16–2.79 | — | — |
| COPD | 1.042 | 0.24–4.60 | — | — | — | — |
| Diabetes mellitus | 0.94 | 0.54–1.73 | — | — | — | — |
| Obesity/overweight (BMI >25 kg/m2) | 2.13 | 0.96–4.71 | — | — | — | — |
| Anakinra use | 3.20 | 1.47–7.17 | 2.46 | 0.87–7.00 | 5.61 | 1.75–10.01 |
| CRP, per mg/dl higher | 0.97 | 0.93–1.02 | — | — | 0.99 | 0.94–1.04 |
| D-dimer, per 100 ng/ml higher | 0.98 | 0.96–1.01 | — | — | 0.99 | 0.97–1.01 |
| Lymphocytes, per 1000 cells/mm3 higher | 0.48 | 0.18–1.29 | — | — | — | — |
| Ferritin, per 100 ng/ml higher | 1.03 | 0.98–1-08 | — | — | — | — |
| Symptom duration before hospitalization, per 1 d higher | 1.05 | 0.96–1.14 | — | — | — | — |
Model A included all correlates that were significantly associated with survival in the univariate model.
Model B included all the correlates that were differently distributed at baseline in the two study groups (i.e., CRP and d-dimer).
BMI, body mass index; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; P/F, partial pressure of oxygen in arterial blood to fractional concentration of oxygen.
Multivariate modeling did not support the use of anakinra for improved 28-d survival when corrected for all significant predictors of survival identified in the univariate analysis (model A: OR, 2.46; 95% CI, 0.87–7.00). On the contrary, anakinra maintained a significant role as predictor of 28-d survival when tested against all correlates that were differently distributed at baseline in the two study groups (i.e., CRP and d-dimer) (model B: OR, 5.61; 95% CI, 1.75–10.01; Table III).
Among surviving subjects, the median duration of days spent in hospital was similar between study groups (35 versus 34 d for patients treated with anakinra versus SOC, respectively; Table II).
When stratified by CPAP support at baseline, significantly more anakinra-treated patients than those receiving SOC treatment were alive at day 28 (p = 0.008); no significant between-treatment group differences in 28-d survival were identified in intubated patients (Table II).
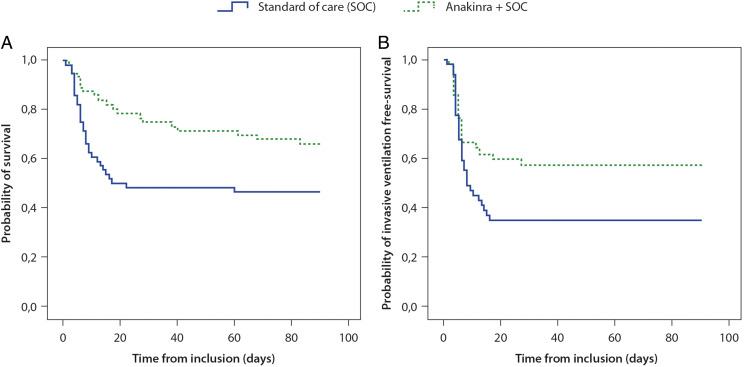
A lower cumulative risk of death was observed among patients receiving anakinra compared with those who did not receive immunomodulatory therapy (p = 0.027) when the follow-up period was continued to 3 mo after baseline (Fig. 1A). Cox regression modeling confirmed the use of anakinra as a predictor of a lower risk of death over time (hazard ratio [HR], 0.50; 95% CI, 0.28–0.89). Other factors associated with this outcome were age (HR per 1 y older, 1.09; 95% CI, 1.05–1.14), baseline ratio of partial pressure of oxygen in arterial blood to fractional concentration of oxygen (HR per 10 mm Hg higher, 0.95; 95% CI, 0.92–0.98), preexisting hypertension (HR, 2.79; 95% CI, 1.49–5.19), and ischemic heart disease (HR, 1.95; 95% CI, 1.01–3.75) in the univariate model. Treatment with anakinra was not confirmed as a significant predictor of better survival in the multivariate analysis when correcting for all other significant predictors obtained in the univariate analysis (OR, 0.94; 95% CI, 0.44–2.15).
FIGURE 1.
Kaplan–Meier cumulative estimates of probability of survival (A) or invasive mechanical ventilation-free survival (B) in patients receiving anakinra compared with those receiving SOC only. Between group differences were assessed using the log-rank test.
When only patients on CPAP respiratory support at baseline were considered, the number of patients requiring invasive ventilation during the study period was not statistically different between-treatment groups (21.4 versus 17.4% for patients treated with anakinra versus SOC, p value NS; Table II), whereas anakinra use was associated with a higher cumulative invasive ventilation-free survival (p = 0.048; Fig. 1B).
A marked reduction in inflammatory biomarkers was observed from baseline to day 7 in patients treated with anakinra versus the control group when comparing variations in CRP and ferritin. No significant differences in the variation of lactate dehydrogenase or d-dimer levels were observed between the two treatment groups during the same time interval, whereas lymphocyte counts increased significantly in patients receiving anakinra compared with SOC (Table IV).
Table IV. Median change in inflammatory biomarkers from baseline to day 7.
| Biomarker | Anakinra + SOC | SOC | p Value |
|---|---|---|---|
| CRP (mg/dl) | −10.3 (−20.2 to −4.2) | −3.5 (−9.5 to −2.2) | <0.001 |
| Ferritin (mg/dl) | −613 (−1430 to −15) | +132 (−341 to +438) | 0.018 |
| Lactate dehydrogenase (mg/dl) | −185 (−205 to −36) | −58 (−89 to −12) | NS |
| D-dimer (ng/ml) | −894 (−2386 to +553) | −611 (−1561 to +547) | NS |
| Lymphocytes cells (cells per microliter) | +300 (+114 to +501) | +26 (−150 to +189) | 0.049 |
Data are median value (interquartile range). NS, not significant.
When considering variations from baseline to day 14, both CRP (–11.9 versus –8.7 mg/dl, p = 0.028) and ferritin (–849 versus –78 ng/ml, p = 0.004) levels decreased in patients treated with anakinra more than in subjects treated with the SOC, whereas lymphocyte counts increased in both groups without a significant difference (median increase 549 versus 210 cells per microliter, p = 0.029).
Adverse events
Infectious-related adverse events were identified in 15 patients treated with anakinra (26.8%) and in nine patients in the control group (16.1%), with no significant differences between-treatment groups. Among those receiving anakinra, major infectious events identified were nine bloodstream infections (three Enterococcus faecalis, two Staphylococcus epidermidis, one Staphylococcus aureus, one Escherichia coli, one Candida albicans, one Candida parapsilosis), two E. faecalis–complicated urinary tract infections, and four pneumonia infections (two Pseudomonas aeruginosa ventilator -associated pneumonia, one Stenotrophomonas maltophilia, one Aspergillus fumigatus). Four of the patients who developed serious infections during treatment with anakinra died before day 28, with no significant difference in survival compared with the remaining subjects receiving anakinra (26.7 versus 24.4%, p = NS). Among patients receiving SOC only, reported infectious-related events were four bloodstream infection events (two Enterobacter aerogenes, one S. epidermidis, one C. albicans), four pneumonia infections (one P. aeruginosa ventilator-associated pneumonia, one Serratia marcescens ventilator-associated pneumonia, one Streptococcus pneumoniae, one S. maltophilia), and one complicated urinary tract infection (E. faecalis).
No local adverse events or reactivation of hepatitis B or Mycobacterium tuberculosis infection were observed among subjects receiving anakinra. Furthermore, no newly reported symptoms (e.g., gastrointestinal symptoms or headache) or alterations in hematologic or biochemistry parameters (e.g., neutropenia or thrombocytopenia) were observed in relation to the use of anakinra.
Discussion
As knowledge of the pathology of COVID-19, including the role of proinflammatory cytokines, has evolved, anticytokine therapies have emerged as theoretically viable treatments (22, 23). Because of their potent anti-inflammatory effects, agents that block IL-1 and IL-6 have been the focus of attention in this regard. To date, evidence has mainly come from case reports/series and retrospective cohort studies, although randomized controlled trials are ongoing and data from them are beginning to emerge. As an established IL-1 antagonist, anakinra has been investigated in a number of studies, with encouraging results (8–16). Interestingly, the IL-6R antagonist, tocilizumab, although reducing the risk of mechanical ventilation in patients hospitalized with COVID-19, has shown inconsistent effects on short-term mortality (24).
Our present study provides additional evidence of the effect of anakinra in ARDS associated with COVID-19. Previous observational cohorts evaluated subjects with severe or critical COVID-19 requiring various oxygen support and compared anakinra-treated individuals with historical cohorts (15, 16). Of note, previous cohort studies evaluated outcomes at 21 d from anakinra introduction (15, 16). We chose to conduct a retrospective analysis of subjects receiving anakinra on major oxygen support (mainly CPAP), with a minority of patients on invasive ventilation support, evaluating both early and delayed outcomes of this immunomodulatory treatment. We chose an end point of 28-d survival, whereas 21-d survival has been used in previous studies of anakinra in COVID-19–related respiratory distress [e.g., Cavalli et al. (15)]. This is because severe COVID-19 has a long-term course and, therefore, we feel that information on longer-term outcomes (e.g., 28 d) will be useful for informing current management of the disease.
Our study has shown that the role of anakinra is more evident in patients treated with CPAP than in those who had received orotracheal intubation at baseline. Although no significant impact of anakinra was observed in intubated patients, this may be because of the low number of patients requiring this form of oxygen support and included in this study. Nevertheless, the high rate of success on anakinra treatment observed in this setting suggests that intubated patients could be challenged with anticytokine strategies with a certain success rate.
Different from previous studies, we excluded remdesivir-treated patients from our study to reduce possible confounding effects, despite considering the association of remdesivir, heparin, and an immunomodulatory treatment the most interesting treatment option in severe COVID-19, as previously described (25). To our knowledge, remdesivir is the only available antiviral treatment shown to be effective in the treatment of COVID-19 to date (26), but it was not used extensively at our institution at the time of this study because of a drug shortage.
Notably, there are differences in the dosage and administration of anakinra in our patients. In our experience, i.v. infusion was preferred in intensive care unit patients and reserved for those with possible anasarca and s.c. edema or when s.c. administration was relatively contraindicated, for example in cases of severe thrombocytopenia or hemorrhagic complications, as described previously (20).
On this basis, our experience supports the effort to perform additional studies to evaluate the use of anakinra as a possible part of the treatment to control ARDS in patients with COVID-19, as it was associated with a reduced 28-d mortality and improved overall survival and invasive ventilation-free survival when follow-up was extended to 3 mo after treatment introduction. The better clinical outcomes reported in this study were supported by the observation of more relevant improvement of inflammatory biomarkers (CRP and ferritin) in patients receiving anakinra, as has also been observed in previous studies (15, 16). Baseline CRP levels were higher and decreased to a greater extent by 14 d in patients who received anakinra compared with controls. Previous studies have indicated that lung involvement and clinical deterioration in patients with COVID-19 are associated with high levels of inflammatory markers, including CRP (27). Thus, the patients in our study were possibly at high risk of adverse outcomes, and despite this, anakinra improved their 28-d survival.
The role of anakinra in patients with COVID-19–related severe or critical ARDS may be based on blockade of the IL-1/IL-6 axis, which may be associated with inflammation control and possible relief of the ARDS trigger and the so-called cytokine storm associated with COVID-19 (5). Nonetheless, other possible mechanisms have been evoked to explain the pathological pathway of COVID-19, which may not affect the expression of IL-1, preventing any possible role of IL-1 blockade in the treatment of severe and critical COVID-19. Some evidence exists that most of the clinical pattern may be sustained by immune paralysis because of a decrease of HLA-DR isotype on CD14 monocytes, with subsequent overproduction of TNF-α and IL-6 (28).
Although our findings confirm the possible efficacy of this strategy, whichever the underlying pathological mechanism may be, we believe a more precise comprehension of specific pathological patterns of disease progression and their clinical correlates is imperative. Moreover, particular effort should be dedicated to the possible implications of specific biomarkers in the prediction of therapeutic success, to achieve a more specific pathogenic-targeted intervention. In particular, hyperinflammation and respiratory impairment should be better defined by specific characteristics, predicting the possible role of IL blockade, to create clinical decision tools, such as the hemophagocytosis score used in other clinical settings (29). Nonetheless, further evaluations of such scores are needed to avoid the implication of parameters such as pancytopenia, hypercoagulation, acute kidney injury, and hepatobiliary dysfunction, which are much more unusual in COVID-19 than in secondary hemophagocytic lymphohistiocytosis.
In addition to the small sample size (n = 56 per treatment group), another possible limitation of our study is the lack of data about IL concentrations at baseline and on treatment. Indeed, the concentration of IL-6 was not quickly obtainable at that time at our institution, despite its current implication in clinical studies about COVID-19 treatment. Nevertheless, previous evaluations in the field of MAS are skeptical about the ability of cytokine concentrations measured in peripheral blood to predict the outcome of optimal immunomodulatory therapies, as pathogenic phenomena may be limited to specific organs with corresponding differences between local concentrations, which might be more informative than cytokinaemia (20).
Moreover, we acknowledge the different distribution of baseline comorbidities between the two groups of patients. As an example, diabetes and ischemic heart diseases were more frequent in the SOC-alone group, whereas obesity and chronic obstructive pulmonary disease were more frequent in the anakinra group. Nevertheless, these differences were not statistically significant, and the heterogeneity in distribution of specific comorbidities was as expected and was unavoidable, given the observational nature of the study. A logistic regression model was included in the design of the study to correct for possible confounders, and, indeed, a lack of significant association between anakinra usage and outcome improvement was noted when a statistical correction was made for confounders. Nonetheless, the multivariate analysis was possibly limited more by the sample size of the study than the lack of efficacy of this treatment option. Indeed, we had attempted to minimize possible bias in our analysis by matching of baseline epidemiologic and clinical characteristics, thus excluding major differences between the two groups. Limiting the correlates evaluated in the multivariate analysis to those showing different patterns at baseline (i.e., CRP and d-dimer), treatment with anakinra was associated with better 28-d survival (OR, 5.61; 95% CI, 1.75–10.01).
Of note, we also observed what appeared to be a similar probability of septic adverse events in the two study groups, with no relapse of previous infectious conditions induced by the immunomodulatory function of anakinra, although the study was not powered to fully investigate this association. This finding is in line with the good tolerability of the drug when prescribed in other clinical conditions and over longer periods of treatment, such as use in rheumatological conditions (5). Despite this, we believe that special attention should be paid to possible infective reactivations or bacterial sepsis. Although we did not observe an increased incidence of such events in anakinra-treated patients, we believe it may be useful to determine previous exposure to M. tuberculosis or hepatitis B virus or colonization with multidrug-resistant bacteria in patients in whom anakinra is being considered. In this context, the pharmacologic profile of the drug is favorable because its short half-life, despite necessitating several injections, allows for the rapid interruption of treatment in the case of adverse events associated with a potential immune suppression, differently from what is observed for more long-acting agents, including tocilizumab (30).
In conclusion, further observational evaluations, including higher numbers of patients, are warranted to overcome the role of possible confounders, however its good tolerability and short half-life highlight the potential of anakinra as an excellent candidate for future clinical trials in COVID-19 ARDS.
Acknowledgments
Editorial assistance was provided by Melanie Gatt, an independent medical writer, on behalf of Springer Healthcare; by Alma Orts-Sebastian (PhD) of Springer Healthcare Communications, prior to submission; and by Tracy Harrison and Kate Palmer of Springer Healthcare Communications, after submission.
Portions of this work were presented at the European Society of Clinical Microbiology and Infectious Diseases Conference on Coronavirus Disease, which took place online, September 23–25, 2020.
- ARDS
- acute respiratory distress syndrome
- CI
- confidence interval
- COVID-19
- coronavirus disease 2019
- CPAP
- continuous positive airway pressure
- CRP
- C-reactive protein
- HR
- hazard ratio
- MAS
- macrophage activation syndrome
- OR
- odds ratio
- SOC
- standard of care.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Huang, C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colafrancesco, S., Alessandri C., Conti F., Priori R.. 2020. COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome? Autoimmun. Rev. 19: 102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatraju, P. K., Ghassemieh B. J., Nichols M., Kim R., Jerome K. R., Nalla A. K., Greninger A. L., Pipavath S., Wurfel M. M., Evans L., et al. 2020. Covid-19 in critically ill patients in the Seattle region - case series. N. Engl. J. Med. 382: 2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ñamendys-Silva, S. A. 2020. Respiratory support for patients with COVID-19 infection. Lancet Respir. Med. 8: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopalco, G., Rigante D., Giannini M., Galeazzi M., Lapadula G., Iannone F., Cantarini L.. 2016. Safety profile of anakinra in the management of rheumatologic, metabolic and autoinflammatory disorders. Clin. Exp. Rheumatol. 34: 531–538. [PubMed] [Google Scholar]
- 6.Monteagudo, L. A., Boothby A., Gertner E.. 2020. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. 2: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shakoory, B., Carcillo J. A., Chatham W. W., Amdur R. L., Zhao H., Dinarello C. A., Cron R. Q., Opal S. M.. 2016. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 44: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filocamo, G., Mangioni D., Tagliabue P., Aliberti S., Costantino G., Minoia F., Bandera A.. 2020. Use of anakinra in severe COVID-19: a case report. Int. J. Infect. Dis. 96: 607–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pontali, E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F., Angelelli A., Caorsi R., Feasi M., Calautti F., et al. 2020. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J. Allergy Clin. Immunol. 146: 213–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González-García, A., García-Sánchez I., Lopes V., Moreno-Arrones O. M., Tortosa-Cabañas M., Elías-Sáenz I., Hernández-Rodríguez J.. 2020. Successful treatment of severe COVID-19 with subcutaneous anakinra as a sole treatment. Rheumatology (Oxford) 59: 2171–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day, J. W., Fox T. A., Halsey R., Carpenter B., Kottaridis P. D.. 2020. Interleukin-1 blockade with anakinra in acute leukaemia patients with severe COVID-19 pneumonia appears safe and may result in clinical improvement. Br. J. Haematol. 190: e80–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aouba, A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N., Justet A.. 2020. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann. Rheum. Dis. 79: 1381–1382. [DOI] [PubMed] [Google Scholar]
- 13.Iglesias-Julián, E., López-Veloso M., de-la-Torre-Ferrera N., Barraza-Vengoechea J. C., Delgado-López P. D., Colazo-Burlato M., Ubeira-Iglesias M., Montero-Baladía M., Lorenzo-Martín A., Minguito-de-la-Iglesia J., et al. 2020. High dose subcutaneous Anakinra to treat acute respiratory distress syndrome secondary to cytokine storm syndrome among severely ill COVID-19 patients. J. Autoimmun. 115: 102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro-Millán, I., Sattui S. E., Lakhanpal A., Zisa D., Siegel C. H., Crow M. K.. 2020. Use of anakinra to prevent mechanical ventilation in severe COVID-19: a case series. Arthritis Rheumatol. 72: 1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavalli, G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C., Castiglioni B., Tassan Din C., Boffini N., et al. 2020. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2: e325–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huet, T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I., Sacco E., Naccache J. M., Bézie Y., Laplanche S., et al. 2020. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2: e393–e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, S. H., Zhao Y. S., Zhou D. X., Zhou F. C., Xu F.. 2020. Coronavirus disease 2019 (COVID-19): cytokine storms, hyper-inflammatory phenotypes, and acute respiratory distress syndrome. Genes Dis. 7: 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lombardy Section Italian Society Infectious And Tropical Diseases . 2020. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 28: 143–152. [PubMed] [Google Scholar]
- 19.Tang, N., Bai H., Chen X., Gong J., Li D., Sun Z.. 2020. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 18: 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta, P., Cron R. Q., Hartwell J., Manson J. J., Tattersall R. S.. 2020. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2: e358–e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou, F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roshanravan, N., Seif F., Ostadrahimi A., Pouraghaei M., Ghaffari S.. 2020. Targeting cytokine storm to manage patients with COVID-19: a mini-review. Arch. Med. Res. 51: 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ucciferri, C., Vecchiet J., Falasca K.. 2020. Role of monoclonal antibody drugs in the treatment of COVID-19. World J. Clin. Cases 8: 4280–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tleyjeh, I. M., Kashour Z., Damlaj M., Riaz M., Tlayjeh H., Altannir M., Altannir Y., Al-Tannir M., Tleyjeh R., Hassett L., Kashour T.. 2020. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin. Microbiol. Infect. DOI: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franzetti, M., Pozzetti U., Carugati M., Pandolfo A., Molteni C., Faccioli P., Castaldo G., Longoni E., Ormas V., Iemoli E., Piconi S.. 2020. Interleukin-1 receptor antagonist anakinra in association with remdesivir in severe COVID-19: a case report. Int. J. Infect. Dis. 97: 215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grein, J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M. L., Lescure F. X., et al. 2020. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 382: 2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malik, P., Patel U., Mehta D., Patel N., Kelkar R., Akrmah M., Gabrilove J. L., Sacks H.. 2020. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid. Based Med. DOI: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giamarellos-Bourboulis, E. J., Netea M. G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M. E., Katsaounou P., et al. 2020. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27: 992–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimopoulos, G., de Mast Q., Markou N., Theodorakopoulou M., Komnos A., Mouktaroudi M., Netea M. G., Spyridopoulos T., Verheggen R. J., Hoogerwerf J., et al. 2020. Favorable anakinra responses in severe covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe 28: 117–123.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell, L., Chen C., Bhagat S. S., Parker R. A., Östör A. J.. 2011. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford) 50: 552–562. [DOI] [PubMed] [Google Scholar]