Abstract
Background:
Inflammatory cytokines, such as interleukin (IL)-1β, alter iron homeostasis and erythropoiesis, resulting in anemia, but whether inhibition of IL-1β can reverse these effects is unclear.
Objective:
To determine whether IL-1β inhibition with canakinumab reduces incident anemia and improves hemoglobin levels among those with prevalent anemia.
Design:
Exploratory analysis of a randomized controlled trial. (ClinicalTrials.gov: NCT01327846)
Setting:
Many clinical sites in 39 countries.
Participants:
8683 CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes Study) participants without anemia at trial entry and 1303 with prevalent anemia at trial entry.
Intervention:
Random assignment to receive placebo or canakinumab (50, 150, or 300 mg) subcutaneously once every 3 months.
Measurements:
Primary outcome was incident anemia (hemoglobin level <130 g/L in men or <120 g/L in women).
Results:
Anemia incidence increased with rising baseline levels of high-sensitivity C-reactive protein (hsCRP), and both hsCRP and IL-6 decreased among participants receiving canakinumab compared with the placebo group. During a median follow-up of 3.7 years, participants without baseline anemia who received canakinumab at any dosage had significantly less incident anemia than those who received placebo (hazard ratio, 0.84 [95% CI, 0.77 to 0.93]; P < 0.001). Compared with placebo, the greatest benefits of IL-1β inhibition on incident anemia were observed among participants with the most robust anti-inflammatory response, an effect corroborated in formal mediation analyses. Among those with baseline anemia, canakinumab increased mean hemoglobin levels by 11.3 g/L (P < 0.001) compared with placebo after 2 years of treatment. Canakinumab increased the risk for infection and was associated with mild cases of thrombocytopenia and neutropenia, none of which was grade 3 or higher.
Limitation:
CANTOS was not designed to assess the cause of anemia in individual trial participants.
Conclusion:
These exploratory analyses of randomized trial data provide proof of principle that inflammation inhibition, at least through the IL-1β/IL-6 signaling pathway, reduces the incidence of anemia and improves hemoglobin levels in patients with anemia.
Primary Funding Source:
Novartis Pharmaceuticals.
Inflammation contributes to the development of anemia in the setting of chronic disease or infection and may play a role in anemia onset in older adults. Several factors contribute to the anemia that accompanies chronic inflammation, and inflammatory cytokines, such as interleukin (IL)-1β and IL-6, participate in its pathogenesis (1–3). These cytokines cause anemia through myelosuppression, erythropoietin resistance, and decreased erythrocyte survival. In addition, IL-1β and IL-6 may promote hypoferremia, or decreased iron availability, by stimulating the expression of hepcidin. This iron regulatory mediator rises in iron overload and inflammatory states (4, 5). Hepcidin inhibits intestinal iron absorption and promotes sequestration of iron in macrophages, creating an iron-restricted state that impairs erythropoiesis (6, 7). Small studies of patients with rheumatologic disorders, such as refractory rheumatoid arthritis treated with tocilizumab–a monoclonal antibody that targets the IL-6 receptor–or systemic juvenile rheumatoid arthritis treated with the IL-1 receptor antagonist anakinra, showed improvement in anemia as well as clinical anemia-related symptoms (8, 9). These studies support the targeting of inflammatory cytokines to treat the anemia of chronic inflammation.
CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes Study), a multinational, double-blind, placebo-controlled trial, enrolled 10 061 participants with a history of myocardial infarction and high-sensitivity C-reactive protein (hsCRP) levels of 2 mg/L or greater despite receiving a full panel of guideline-directed secondary preventive care, including effective statin treatment. Trial participants randomly received placebo or 1 of 3 dosages (50, 150, or 300 mg) of the IL-1β inhibitor canakinumab, given subcutaneously once every 3 months. As reported elsewhere, during a median follow-up of 3.7 years, major cardiovascular event rates fell significantly in participants who received either the 150-or 300-mg canakinumab dosage, with the greatest magnitude of effect accruing among those with the most robust reductions in either their hsCRP or IL-6 level (10). Further analyses of CANTOS demonstrate that targeting the IL-1β innate immunity pathway beneficially affects several other systemic disorders associated with chronic inflammation, including lung cancer (11) and gout (12). CANTOS afforded the opportunity to explore in the context of a randomized placebo-controlled trial whether anti-inflammatory therapy targeting IL-1β might further slow the onset of incident anemia and improve hemoglobin levels among patients with prevalent anemia in a population at risk due, in part, to the presence of underlying low-grade inflammation.
Methods
CANTOS was conducted between 2011 and 2017. Eligible participants included those with a history of myocardial infarction at least 30 days before randomization and blood hsCRP concentrations of 2 mg/L or greater despite standard-of-care secondary preventive measures for cardiovascular disease. Exclusions included a history of uncontrolled hypertension or diabetes, history or evidence of active tuberculosis or high risk for HIV-related disease, chronic or recurrent infections, previous cancer other than basal cell skin carcinoma, a suspected or known immunocompromised state, and use of systemic anti-inflammatory treatments. The trial protocol was approved by institutional review boards or ethics committees in the 39 countries involved in the study. All participants provided written informed consent to participate, and the trial underwent surveillance by an independent data and safety monitoring committee.
As described in detail elsewhere (10), participants were randomly assigned through computer-generated code to receive standard care plus placebo or standard care plus canakinumab at a dosage of 50, 150, or 300 mg given subcutaneously once every 3 months. Randomization was stratified by time from the most recent myocardial infarction (30 to 180 days vs. longer). Of 10 061 participants randomly assigned, 9986 had both baseline and follow-up hemoglobin measures and were included in these analyses. At trial entry, 95% of participants in both the canakinumab and placebo groups were receiving antithrombotic or anticoagulant therapy. Regarding visit adherence after randomization among participants who remained alive and had not reached the end of the trial, 99.7% had a 12-month visit, 99.5% had a 24-month visit, 88.0% had a 36-month visit, 82.5% had a 48-month visit, and 60.2% had a 60-month visit. Baseline anemia, defined as a hemoglobin level less than 120 g/L for women or less than 130 g/L for men, was present in 414 women and 889 men. By contrast, 8683 CANTOS participants were free of baseline anemia. During follow-up, all participants had complete blood counts, lipid panels, hsCRP levels, and renal and hepatic function assessed at 3, 6, 9, 12, 18, 24, 30, 36, 42, 48, 54, and 60 months after randomization. Follow-up complete blood counts permitted assessment of incident anemia, defined prospectively as occurring on the date of first observation of a hemoglobin level lower than 130 g/L in a man or 120 g/L in a woman who had a normal hemoglobin level at trial enrollment.
Statistical Analysis
All analyses were done by using SAS, version 9.4 (SAS Institute), and followed the intention-to-treat principle, except for the causal mediation analyses.
Analyses Related to Incident Anemia
The distributions of baseline clinical characteristics that might contribute to anemia (such as age, renal function, hsCRP level, alcohol use, diabetes, hypertension, and heart failure) were compared between the placebo and active treatment groups by using X2 analysis for categorical variables. For continuous variables, Kruskal-Wallis testing was performed for multiple-group comparisons and Wilcoxon rank-sum testing was done for 2-group comparisons between the placebo and active treatment groups. Because time to first occurrence of anemia was an interval-censored variable determinable only at blood draws, we estimated relative hazard rates on the basis of the discrete analogue of the proportional hazards model proposed by Prentice and Gloeckler (13) with implementation described by Allison (14). To be specific, we used the complementary log-log transformation applied to logistic regression, with participants included at their repeated blood draws until the end of the study or the first sign of anemia. Plots of time to anemia used this interval-censored survival model. Relationships between inflammation and incident anemia were assessed across increasing tertiles of hsCRP levels at trial entry. Models adjusted for trial stratification variables and obtained estimated hazard ratios (HRs) and 95% CIs for incident anemia for each canakinumab dosage compared with placebo and for all canakinumab dosages combined. Additional subgroup analyses assessed factors associated with anemia and chronic inflammation, including age and kidney function. All P values are 2-sided, and all CIs were calculated at the 95% level.
Analyses Related to On-Treatment Inflammation Inhibition and Mediation Analysis
To parallel treatment response analyses that were prespecified in the CANTOS protocol for the trial’s primary cardiovascular end points (15, 16), we performed similar analyses to address whether the magnitude of anti-inflammatory response achieved by individual participants after a single dose of either placebo or canakinumab was related to incident anemia. This analysis divided the canakinumab-treated participants into 2 groups according to whether hsCRP levels fell below 2 mg/L at 3 months (robust responders) or were 2 mg/L or greater at 3 months (less robust responders). The 3-month time point corresponds to the trough after the first canakinumab dose, just before the second dose. We also used mediation analysis to control for baseline characteristics that might independently influence achieved levels of on-treatment markers of inflammation and to control for the effect of treatment. To be specific, we used Valeri and VanderWeele’s SAS macro (17) for causal mediation in the setting of survival analysis. Because mediation analysis is sensitive to distributional assumptions, we separately considered both a discrete and a continuous measure of achieved hsCRP levels. First, we took as mediator an indicator of whether a randomly assigned participant achieved a threshold 3-month hsCRP level below 2 mg/L. Alternatively, as a normalized, continuous measure of change in hsCRP, we took the natural log of achieved hsCRP level at 3 months minus the natural log of baseline hsCRP level. For both measures of mediation, potential baseline confounding variables were age in years; sex; natural log of baseline hsCRP level; and indicators of diabetes, heart failure, and hypertension at baseline. Because the outcome of anemia was not rare, we used the recommended Weibull accelerated failure time model to predict the time to incident anemia. Mediation analyses pooled the 3 active doses of canakinumab, and for each potential mediator, we included an interaction between the mediator and canakinumab treatment.
Analyses Related to Resolution of Anemia
Analyses of resolution of anemia used the same methods for interval-censored survival analysis applied to estimation of incident anemia. Included participants were those who had anemia at baseline, and they were followed at repeated blood draws until their first normal hemoglobin value (<120 g/L for women or <130 g/L for men) or until their last study blood draw. In addition, we used repeated-measures mixed models to estimate mean treatment effects on hemoglobin levels over time in participants who had anemia at baseline. The models assumed an unstructured covariance matrix and included fixed effects for treatment groups, time points, baseline hemoglobin level, time at baseline since index myocardial infarction, and interactions between treatment groups and time. Two models were fitted: a linear model of hemoglobin levels over time, which obtained a 3-degree-of-freedom test of interaction between treatment groups and time, and an unstructured model overtime, which obtained a 36-degree-of-freedom test of interaction between treatments over the 12 follow-up visits.
Role of the Funding Source
Novartis sponsored the investigator-driven CANTOS trial. The CANTOS protocol was designed by academic members of the study’s executive committee with input from physician and statistician employees of the sponsor. The sponsor was responsible for collecting the data. The first author, the corresponding author, and an academic statistician at Brigham and Women’s Hospital had full access to the trial databases, generated trial analyses, prepared the first draft of the manuscript, and made the decision to submit the manuscript for publication.
Results
CANTOS Participants Without Anemia at Baseline
The 8683 CANTOS participants without anemia at baseline randomly received either placebo (n = 2887) or canakinumab at 50 mg (n = 1892), 150 mg (n = 1973), or 300 mg (n = 1931) administered subcutaneously every 3 months. The groups were well matched for baseline clinical characteristics, including those predisposing to anemia, such as age, kidney function, and underlying inflammation as assessed by baseline hsCRP level (Table 1). Among participants without anemia at study entry, increasing baseline hsCRP levels were associated with increased rates of incident anemia during follow-up. Among those with hsCRP levels in the lowest (<3.15 mg/L), middle, and highest (>5.43 mg/L) tertiles at baseline, anemia incidence rates were 6.07, 7.09, and 8.69 per 100 person-years, respectively (P for trend across tertiles < 0.001). As reported previously, canakinumab treatment at all dosages significantly reduced hsCRP and IL-6 levels compared with placebo (10, 15, 16).
Table 1.
Baseline Clinical Characteristics of Participants Without Anemia at Trial Entry, According to Random Assignment to Placebo or Canakinumab
| Characteristic | Placebo (n = 2887) | Canakinumab | |||
|---|---|---|---|---|---|
| 50 mg (n = 1892) | 150 mg (n = 1973) | 300 mg (n = 1931) | All Doses (n = 5796) | ||
| Median age (IQR), y | 61.0 (54.0-67.0) | 61.0 (54.0-68.0) | 61.0 (54.0-67.0) | 61.0 (54.0-67.0) | 61.0 (54.0-67.0) |
| Female, n (%) | 721 (25.0) | 461 (24.4) | 490 (24.8) | 484 (25.1) | 1435 (24.8) |
| Median hemoglobin level (IQR), g/L | 145 (137-153) | 145 (137-153) | 145 (137-153) | 145 (137-153) | 145 (137-153) |
| Median BMI (IQR), kg/m2 | 29.9 (26.7-33.9) | 30.1 (26.8-34.0) | 29.8 (26.5-33.6) | 30.0 (26.7-33.8) | 30.0 (26.7-33.8) |
| Alcohol use (>1 drink per day), n (%) | 130 (4.51) | 73 (3.86) | 76 (3.85) | 84 (4.35) | 233 (4.02) |
| Hypertension, n (%) | 2256 (78.1) | 1512 (79.9) | 1557 (78.9) | 1531 (79.3) | 4600 (79.4) |
| Type 2 diabetes mellitus, n (%) | 1101 (38.1) | 718 (37.9) | 785 (39.8) | 722 (37.4) | 2225 (38.4) |
| Median hsCRP level (IQR), mg/L | 3.95 (2.70-6.50) | 4.10 (2.75-6.75) | 4.05 (2.75-6.65) | 4.05 (2.80-6.60) | 4.05 (2.75-6.65) |
| Median eGFR (IQR), mL/min/1.73 m2 | 80.0 (67.0-94.0) | 79.0 (65.5-93.0) | 80.0 (66.0-94.0) | 79.0 (66.0-93.0) | 79.0 (66.0-93.0) |
| Heart failure, n (%) | 591 (20.5) | 365 (19.3) | 390 (19.8) | 430 (22.3) | 1185 (20.4) |
BMI = body mass index; eGFR = estimated glomerular filtration rate; hsCRP = high-sensitivity C-reactive protein; IQR = interquartile range.
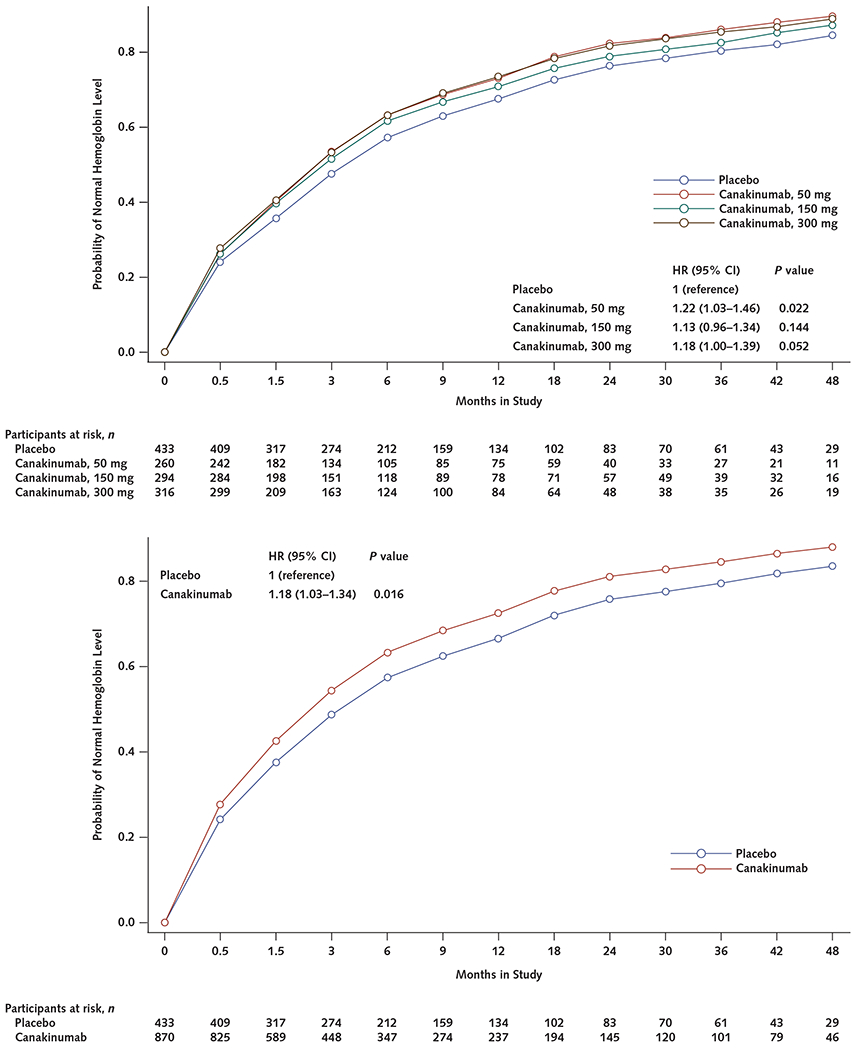
The risk for incident anemia during the median 3.7 years of follow-up was significantly lower in the combined canakinumab groups than the placebo group (HR, 0.84 [95% CI, 0.77 to 0.93]; P < 0.001) (Figure 1, top). Compared with placebo, HRs for incident anemia in each canakinumab group were 0.83 (CI, 0.73 to 0.94; P = 0.004) for the 50-mg group, 0.84 (CI, 0.75 to 0.95; P = 0.006) for the 150-mg group, and 0.85 (CI, 0.75 to 0.96; P = 0.008) for the 300-mg group (Table 2 and Figure 1, bottom).
Figure 1.
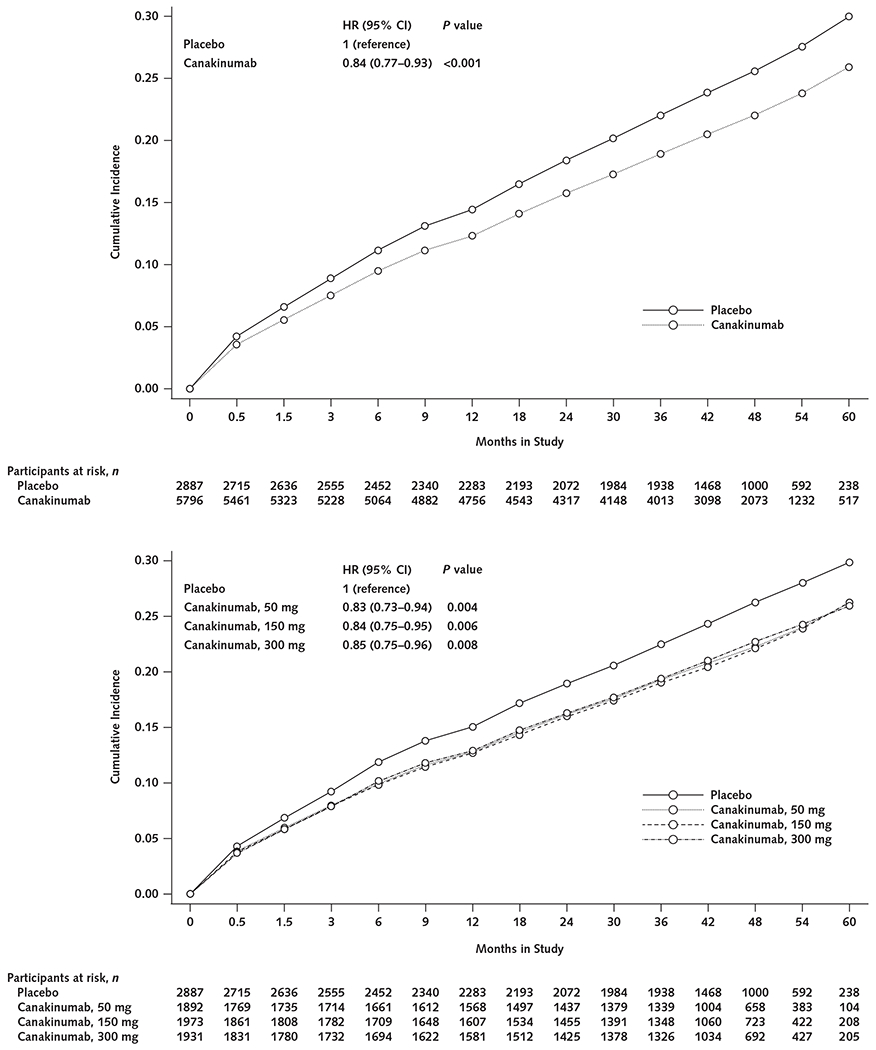
Cumulative incidence of anemia (hemoglobin level <130 g/L in men or <120 g/L in women) in the placebo group compared with all canakinumab dose groups combined (top) and with the groups receiving 50, 150, or 300 mg of canakinumab once every 3 months (bottom).
HR = hazard ratio.
Table 2.
Anemia Incidence Rates and HRs Across Treatment Groups, Overall and Within Subgroups
| Group | Placebo (n = 2887) | Canakinumab | |||
|---|---|---|---|---|---|
| 50 mg (n = 1892) | 150 mg (n = 1973) | 300 mg (n = 1931) | All Doses (n = 5796) | ||
| Total cohort | |||||
| Incidence rate (events, n) | 8.13 (690) | 6.63 (380) | 6.90 (412) | 6.94 (406) | 6.83 (1198) |
| HR (95% CI) | 1 (reference) | 0.83 (0.73-0.94) | 0.84 (0.75-0.95) | 0.85 (0.75-0.96) | 0.84 (0.77-0.93) |
| Age | |||||
| ≥65 y | |||||
| Incidence rate (events, n) | 12.27 (322) | 9.32 (180) | 9.12 (179) | 10.02 (188) | 9.48 (547) |
| HR(95% CI) | 1 (reference) | 0.79 (0.66-0.95) | 0.74 (0.62-0.89) | 0.80 (0.67-0.96) | 0.78 (0.68-0.89) |
| <65 y | |||||
| Incidence rate (events, n) | 6.28 (368) | 5.26 (200) | 5.82 (233) | 5.48 (218) | 5.52 (651) |
| HR(95% CI) | 1 (reference) | 0.85 (0.71-1.01) | 0.92 (0.78-1.08) | 0.87 (0.74-1.03) | 0.88 (0.78-1.00) |
| eGFR | |||||
| <60 mL/min/1.73 m2 | |||||
| Incidence rate (events, n) | 15.67 (176) | 12.51 (100) | 11.45 (93) | 12.93 (110) | 12.31 (303) |
| HR (95% CI) | 1 (reference) | 0.85 (0.66-1.08) | 0.74 (0.57-0.95) | 0.84 (0.66-1.06) | 0.80 (0.67-0.97) |
| ≥60 mL/min/1.73 m2 | |||||
| Incidence rate (events, n) | 6.99 (514) | 5.68 (280) | 6.19 (319) | 5.92 (296) | 5.93 (895) |
| HR(95% CI) | 1 (reference) | 0.82 (0.71-0.95) | 0.88 (0.76-1.01) | 0.84 (0.73-0.97) | 0.85 (0.76-0.95) |
| Type 2 diabetes | |||||
| Yes | |||||
| Incidence rate (events, n) | 11.30 (337) | 9.15 (189) | 8.68 (195) | 8.62 (180) | 8.82 (564) |
| HR (95% CI) | 1 (reference) | 0.83 (0.69-0.99) | 0.77 (0.65-0.92) | 0.76 (0.63-0.91) | 0.79 (0.69-0.90) |
| No | |||||
| Incidence rate (events, n) | 6.42 (353) | 5.21 (191) | 5.83 (217) | 6.00 (226) | 5.68 (634) |
| HR (95% CI) | 1 (reference) | 0.82 (0.69-0.98) | 0.90 (0.76-1.06) | 0.93 (0.79-1.10) | 0.89 (0.78-1.01) |
eGFR = estimated glomerular filtration rate; HR = hazard ratio.
No significant effect modification was observed for the effect of canakinumab versus placebo on incident anemia in analyses stratified by sex; age; presence of hypertension, diabetes, or heart failure; or baseline hsCRP levels (Figure 2).
Figure 2.
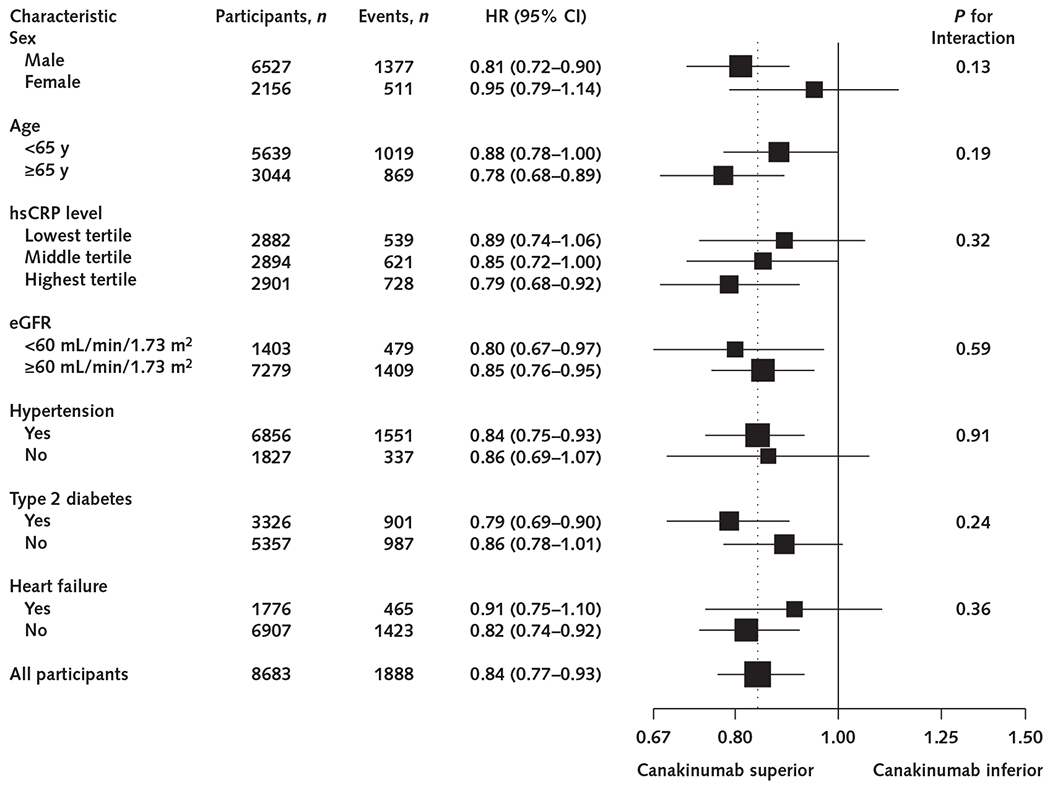
Efficacy of canakinumab versus placebo for incident anemia, according to subgroups based on baseline clinical characteristics.
Data are shown as HRs for all canakinumab groups (50, 150, and 300 mg) combined compared with the placebo group. The dotted line represents the overall effect of canakinumab versus placebo for the total cohort. eGFR = estimated glomerular filtration rate; HR = hazard ratio; hsCRP = high-sensitivity C-reactive protein.
As anticipated, participants aged 65 years and older had higher rates of incident anemia than those younger than 65 years. Among participants aged 65 and older, the HRs for incident anemia associated with canakinumab versus placebo were 0.79 (CI, 0.66 to 0.95; P = 0.012) for the 50-mg group, 0.74 (CI, 0.62 to 0.89; P = 0.001) for the 150-mg group, 0.80 (CI, 0.67 to 0.96; P = 0.017) for the 300-mg group, and 0.78 (CI, 0.68 to 0.89; P < 0.001) for all canakinumab groups combined. Canakinumab seemed to have smaller relative effects and absolute magnitude among participants younger than 65 years; in this subgroup, the HR for incident anemia associated with combined canakinumab groups versus placebo was 0.88 (CI, 0.78 to 1.00; P = 0.054) (Table 2 and Figure 3).
Figure 3.
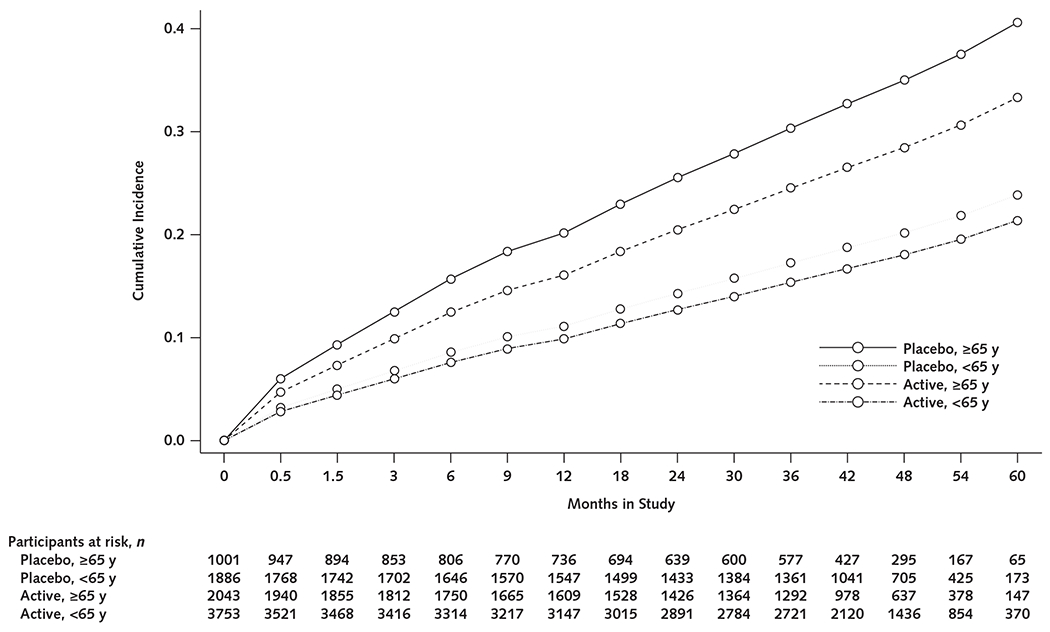
Cumulative incidence of anemia (hemoglobin level <130 g/L in men or <120 g/L in women) in participants treated with canakinumab (all doses) compared with placebo, stratified by age (≥65 vs. <65 years)
Also as anticipated, participants with an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2 had a higher incidence of anemia than those with an eGFR of 60 mL/min/1.73 m2 or greater. Risk reductions in incident anemia associated with canakinumab versus placebo were modestly greater for participants with an eGFR less than 60 mL/min/1.73 m2 (HR, 0.80 [CI, 0.67 to 0.97]; P = 0.020) than those with an eGFR of 60 mL/min/1.73 m2 or greater (HR, 0.85 [CI, 0.76 to 0.95]; P = 0.003) (Table 2).
Among participants with type 2 diabetes, the HRs for incident anemia associated with canakinumab versus placebo were 0.83 (CI, 0.69 to 0.99; P = 0.044) for the 50-mg group, 0.77 (CI, 0.65 to 0.92; P = 0.004) for the 150-mg group, 0.76 (CI, 0.63 to 0.91; P = 0.003) for the 300-mg group, and 0.79 (CI, 0.69 to 0.90; P = 0.001) for all canakinumab groups combined. Participants without type 2 diabetes did not have a statistically significant decrease in incident anemia associated with canakinumab treatment (HR for incident anemia associated with combined canakinumab groups vs. placebo, 0.89 [CI, 0.78 to 1.01]; P = 0.066) (Table 2).
Although canakinumab reduced incident anemia, IL-1β inhibition also was associated with mild thrombocytopenia and neutropenia, as reported previously (11). However, no increase was observed compared with placebo in episodes of thrombocytopenia or neutropenia of grade 3 or higher. Incidence rates for hemorrhage were also similar in the canakinumab and placebo groups. However, a small but statistically significant increase was seen in the risk for fatal infections that were not opportunistic in nature (10, 11). During the trial, 1 participant in the 300-mg canakinumab group received a transfusion in association with treatment for myelodysplastic syndrome.
Mediation of Anemia Risk Through Changes in hsCRP Level
When stratified by on-treatment hsCRP level achieved at 3 months, participants with the most robust anti-inflammatory response to canakinumab had the greatest reduction in incident anemia (Figure 4). For participants with an hsCRP level below 2 mg/L 3 months after initiating canakinumab treatment, the HR for anemia was 0.74 (CI, 0.66 to 0.83; P < 0.001) across all canakinumab groups versus the placebo group (Appendix Table 1, available at Annals.org). By contrast, among participants with an hsCRP level of 2 mg/L or greater at 3 months, the HR for anemia was 0.98 (CI, 0.88 to 1.09; P = 0.71) for the combined canakinumab groups versus the placebo group.
Figure 4.
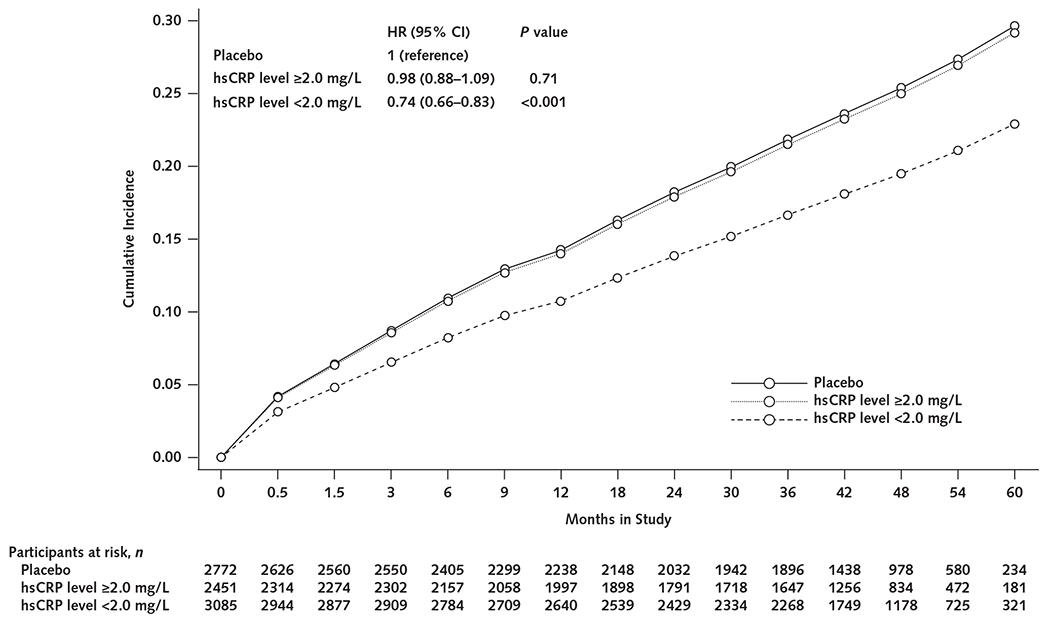
Cumulative incidence of anemia (hemoglobin level <130 g/L in men or <120 g/L in women) among all canakinumab group participants with an on-treatment hsCRP level of 2 mg/L or greater and those with a level less than 2 mg/L versus the placebo group.
HR = hazard ratio; hsCRP = high-sensitivity C-reactive protein.
Formal mediation analysis provided estimates of the causal effect of change in hsCRP, controlling for baseline variables that might independently influence postrandomization hsCRP levels (Table 3). Because the anemia outcome was common, survival analyses in this case used a Weibull accelerated failure time model, which yielded estimates of the relative mean survival time (that is, time free of anemia) in the active canakinumab versus the placebo group. Controlling for baseline covariates of age, sex, log(hsCRP), and comorbid conditions–heart failure, diabetes, and hypertension–the mean time free of anemia was 40% to 42% greater in the canakinumab than the placebo group (estimated total effects in Table 3, each P < 0.001). When we considered how this effect was mediated by whether a participant achieved a 3-month hsCRP threshold level below 2 mg/L, about 35% of this effect was mediated through this measure of inflammation. When we considered the more granular, continuous measure of change in hsCRP level at 3 months, the percentage mediated rose to 53%. Further, with this continuous measure, the natural direct effect of canakinumab on time to anemia (that is, the effect of canakinumab on anemia, controlling for the effect of canakinumab on hsCRP) was attenuated and no longer significant (estimated direct effect of canakinumab fixing hsCRP change, 1.18 [CI, 0.99 to 1.41]). We observed no significant interaction between canakinumab assignment and either measure of achieved hsCRP at 3 months on the time to anemia.
Table 3.
Mediation Analysis Estimating the Proportion of the Observed Canakinumab Effect on Anemia Resulting From Changes in hsCRP After Treatment, Adjusted for Confounders
| Effect | Relative Mean Survival* | 95% CI | P Value |
|---|---|---|---|
| Mediator: achieved 3-mo hsCRP level <2 mg/L (threshold effect) | |||
| Controlled direct† | 1.29 | 1.05-1.57 | 0.014 |
| Natural direct† | 1.27 | 1.07-1.51 | 0.006 |
| Natural indirect‡ | 1.11 | 1.03-1.20 | 0.007 |
| Total§ | 1.42 | 1.20-1.67 | <0.001 |
| Proportion mediated‖ | 0.35 | – | – |
| Mediator: log(hsCRP) at 3 mo minus log(hsCRP) at baseline (continuous effect) | |||
| Controlled direct† | 1.18 | 0.99-1.41 | 0.071 |
| Natural direct† | 1.18 | 0.998-1.41 | 0.053 |
| Natural indirect‡ | 1.18 | 1.10-1.26 | <0.001 |
| Total§ | 1.40 | 1.19-1.65 | <0.001 |
| Proportion mediated‖ | 0.53 | – | – |
hsCRP = high-sensitivity C-reactive protein.
Relative mean survival is estimated in a Weibull accelerated failure time model so that estimates above 1 indicate greater mean anemia-free survival. For example, in the threshold analysis (top), the total effect estimate of 1.42 indicates a 42% longer mean anemia-free survival associated with canakinumab vs. placebo.
Direct effects, both controlled and natural, measure the effect of canakinumab that is not mediated through its effect on hsCRP. Controlled direct effects fix the achieved level of hsCRP at 3 mo at a specific value, identical in treated and untreated participants, whereas natural direct effects consider the mediator effect fixed across the range of achieved hsCRP values at 3 mo observed in the placebo group.
Natural indirect effects compare the effect of canakinumab in 2 treated persons, 1 who achieves the hsCRP level expected with active treatment and the other with the expected hsCRP level seen in the placebo group.
Total effect estimates the relative mean anemia-free survival associated with canakinumab treatment vs. placebo, including both the direct effect of treatment and its indirect effect through the mediator, hsCRP change.
Proportion mediated estimates the proportion of the total canakinumab effect that acts through its effect on the mediator, change in hsCRP level. For example, 35% of the effect of canakinumab on anemia is estimated to be mediated by achievement of a threshold hsCRP level <2 mg/L at 3 mo (top), whereas 53% of the effect of canakinumab on anemia is estimated to be mediated by the continuous change in hsCRP level from baseline to 3 mo (bottom).
CANTOS Participants With Anemia at Baseline
The 1303 CANTOS participants with anemia at baseline also randomly received either placebo (n = 433) or canakinumab at 50 mg (n = 260), 150 mg (n = 294), or 300 mg (n = 316) administered subcutaneously every 3 months. Participants with anemia at baseline in the placebo and canakinumab groups were well matched for the baseline characteristics mentioned earlier. Compared with participants who did not have anemia at baseline, those who did were older, were more likely to be female, had a higher burden of comorbid illness (higher rates of hypertension and type 2 diabetes mellitus), had lower GFRs and higher hsCRP levels, and had mild anemia (mean hemoglobin level, 119 g/L) (Appendix Tables 2 and 3, available at Annals.org).
In the CANTOS subgroup with baseline anemia, participants allocated to the canakinumab group had significantly greater improvement in mean hemoglobin levels over time than those assigned to placebo. For example, among participants with baseline anemia, canakinumab increased mean hemoglobin levels by 11.3 g/L (P < 0.001) versus placebo after 2 years of treatment (Appendix Figure 1 and Appendix Table 4, available at Annals.org). In parallel, in the CANTOS subgroup with baseline anemia, the prevalence of anemia had decreased more among participants receiving canakinumab than those receiving placebo at 12 months (P = 0.005), 24 months (P = 0.018), and 36 months (P < 0.001) (Appendix Figure 2, available at Annals.org). The prevalence of anemia at 2 years among participants with baseline anemia was 49.2% for those in the placebo group and 41.3%, 42.0%, and 40.8% for those in the 50-, 150-, and 300-mg canakinumab groups, respectively.
Discussion
This secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial demonstrates that IL-1β inhibition with canakinumab reduces incident anemia and improves hemoglobin levels among patients with prevalent anemia. All canakinumab dosages seemed to produce this beneficial effect without a clear dose-response effect. The finding that the timing and magnitude of on-treatment hsCRP reduction with canakinumab correlate with the magnitude and timing of clinical benefit highlights the role of IL-1β signaling in anemia development.
Inflammatory cytokines, including those downstream in the inflammasome pathway, such as IL-1β and IL-6, contribute to anemia development by impairing iron homeostasis, proliferation and differentiation of erythroid progenitors, and erythropoietin response. Increased inflammatory signaling is an important facet of anemia of chronic disease, the second leading cause of anemia after iron deficiency (18). Chronic illnesses, such as chronic kidney disease and diabetes, are associated with chronic states of inflammation (18, 19). In addition, inflammation figures among the many concomitants and potential contributors of aging. In particular, enhanced activation of the NLRP3 (NOD-like receptor protein 3) inflammasome accompanies aging (20, 21). Older participants, those with impaired kidney function, and those with diabetes derived the greatest absolute benefit from canakinumab in this study, highlighting populations in which the NLRP3 inflammasome/IL-1β/IL-6 pathway may contribute to anemia.
Earlier analyses from CANTOS revealed that canakinumab treatment modifies several disease processes involving inflammasome signaling (22). The NLRP3 inflammasome, a multiprotein complex, senses cellular danger signals, including microbial stimuli as well as endogenous molecules, resulting in activation of caspase-1, an enzyme that processes pro-IL-1β to its active form. CANTOS showed a reduction in major adverse cardiovascular events with canakinumab treatment corresponding to reductions in hsCRP but not in atherogenic lipoproteins (10). Secondary analyses of CANTOS revealed that canakinumab treatment decreases incident lung cancer and lung cancer mortality, as well as rates of gout attacks, without lowering serum uric acid concentrations (11, 12). The current analyses extend the consequences of NLRP3 inflammasome pathway inhibition to the erythroid series.
We observed similar and significant 15% to 17% reductions in the hazard of anemia at all 3 active canakinumab dosages. This absence of an apparent dose-response effect according to randomization group was similar to what was seen for some other outcomes in CANTOS (such as gout [12]) but differed from the clear dose-response effect seen for incident lung cancer and cancer death (11). Yet, similar to previous analyses for atherosclerotic events (15, 16), the magnitude of inflammation inhibition achieved for individual participants, regardless of dosage, did track with the magnitude of benefit for anemia. To investigate this individual response issue further, we grouped all canakinumab dosages together for mediation analyses. The estimate that 53% of the effect of canakinumab was mediated through the difference between log(hsCRP) at 3 months and its baseline level clarifies the drug’s pathway of action. This percentage mediated likely underestimates the true magnitude of drug effect through the IL-1β pathway, both because of measurement error in hsCRP (23) and because hsCRP indirectly measures this pathway. Larger mediation percentages would likely be observed if direct measures of IL-1β itself were available.
CANTOS enrolled persons with previous myocardial infarction and an above-median hsCRP level despite treatment with statins and aspirin, as well as many participants with comorbid conditions, such as hypertension and diabetes mellitus. Thus, our study results may not be generalizable to populations without atherosclerotic disease or an elevated hsCRP level. Of interest, the prevalence of anemia in this study population, with a median age of 61 years, was elevated at 13% compared with other population-based estimates of anemia prevalence. The prevalence of anemia due to any cause is estimated to be 11.0% in men and 10.2% in women aged 65 years and older. The prevalence is lower in persons aged 50 to 64 years: 4.40% in men and 6.80% in women (24). In JUPITER (Justification for the Use of Statin in Prevention: An Intervention Trial Evaluating Rosuvastatin) (25), a study of 17 802 apparently healthy participants with an elevated hsCRP level, 4.50% had anemia at baseline (26). In contrast to JUPITER, the CANTOS participants were older, had a previous myocardial infarction, and had a higher burden of comorbid illnesses, likely contributing to the higher anemia rates.
A limitation of this study is that CANTOS was not designed to assess the cause of anemia in individual trial participants. Yet, prospective, close monitoring of complete blood counts from baseline to 48 months after randomization allowed for accurate assessment of incident anemia. Moreover, the randomized design of the trial ensures the equal distribution among treatment groups of potential known and unknown confounding factors associated with anemia, such as nutritional deficiencies, gastrointestinal bleeding, and alcohol use. Additional prospective evaluation might extend the present findings to other populations likely to benefit from IL-1β inhibition or other anti-inflammatory interventions. Although canakinumab was generally well tolerated in CANTOS, it was associated with a small increase in the risk for nonopportunistic fatal infections and an increase in thrombocytopenia and leukopenia. The latter effects were mild and below grade 3 in both the placebo and canakinumab groups.
In conclusion, in this randomized trial, targeted IL-1β inhibition reduced the incidence of anemia by 16%, with a pronounced effect in participants with the most robust anti-inflammatory response. These hypothesis-generating data highlight the role of IL-β/IL-6 pathway signaling in anemia onset in a large population with chronic inflammation and motivate the design of prospective confirmatory studies to identify populations that might benefit from anti-inflammatory therapies for anemia. Further interventional trials are necessary to establish efficacy and safety for IL-1β-targeting therapies in anemia of chronic inflammation.
Acknowledgments
Financial Support: This study was funded by Novartis Pharmaceuticals.
Disclosures: Ms. MacFadyen reports grants from Novartis during the conduct of the study. Dr. Glynn reports grants from Novartis during the conduct of the study and grants from AstraZeneca, Kowa, and Pfizer outside the submitted work. Dr. Thuren was an employee of and held stock in Novartis Pharmaceuticals during the conduct of the study. Dr. Libby reports grants from Novartis and DalCor Pharmaceuticals and has served as an unpaid consultant and/or advisory board member for Amgen, Kowa Pharmaceuticals, XBiotech, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Pfizer, Sanofi-Regeneron, Olatec Therapeutics, MedImmune, Corvidia Therapeutics, and IFM Therapeutics during the conduct of the study. Dr. Ridker reports grants and personal fees from Novartis during the conduct of the study and personal fees from Inflazome, Corvidia, and Civi Biopharma outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M19-2945.
Appendix Table 1.
Risk for Incident Anemia, Stratified by On-Treatment hsCRP Level at 3 Months
| On-Treatment hsCRP Level | Placebo (n = 2772) | Canakinumab | |||
|---|---|---|---|---|---|
| 50 mg | 150 mg | 300 mg | All Doses | ||
| < 2 mg/L | |||||
| Participants, n | – | 804 | 1056 | 1225 | 3085 |
| HR (95% CI) | 1 (reference) | 0.67 (0.56-0.81) | 0.78 (0.67-0.91) | 0.75 (0.65-0.87) | 0.74 (0.66-0.83) |
| P value | Reference | <0.001 | 0.002 | <0.001 | <0.001* |
| ≥2 mg/L | |||||
| Participants, n | – | 1012 | 827 | 612 | 2451 |
| HR (95% CI) | 1 (reference) | 0.98 (0.84-1.14) | 0.90 (0.76-1.06) | 1.07 (0.90-1.27) | 0.98 (0.88-1.09) |
| P value | Reference | 0.77 | 0.21 | 0.44 | 0.71* |
HR = hazard ratio; hsCRP = high-sensitivity C-reactive protein.
Compared with placebo.
Appendix Table 2.
Baseline Clinical Characteristics of Participants With Anemia at Study Entry Who Were Randomly Assigned to Receive Placebo or Canakinumab
| Characteristic | Placebo (n = 433) | Canakinumab | |||
|---|---|---|---|---|---|
| 50 mg (n = 260) | 150 mg (n = 294) | 300 mg (n = 316) | All Doses (n = 870) | ||
| Median age (IQR), y | 66.0 (58.0-73.0) | 65.0 (56.5-72.0) | 65.0 (57.0-72.0) | 65.0 (57.0-73.0) | 65.0 (57.0-72.0) |
| Female, n (%) | 138 (31.9) | 76 (29.2) | 81 (27.6) | 119 (37.7) | 276 (31.7) |
| Median hemoglobin level(IQR), g/L | 119 (112-126) | 119 (114-126) | 119 (113-126) | 118 (111-125) | 119 (113-126) |
| Median BMI (IQR), kg/m2 | 28.4 (25.3-33.3) | 29.1 (25.6-33.3) | 29.6 (26.2-34.4)* | 29.1 (24.8-34.2) | 29.3 (25.5-33.9) |
| Alcohol use (>1 drink per day), n (%) | 6 (1.39) | 11 (4.23)* | 5 (1.70) | 10 (3.16) | 26 (2.99) |
| Hypertension, n (%) | 368 (85.0) | 226 (86.9) | 243 (82.7) | 257 (81.3) | 726 (83.4) |
| Type 2 diabetes mellitus, n (%) | 221 (51.0) | 127 (48.8) | 165 (56.1) | 158 (50.0) | 450 (51.7) |
| Median hsCRP level (IQR), mg/L | 5.50 (3.10-10.4) | 5.65 (3.18-10.2) | 5.78 (3.40-10.7) | 5.78 (3.25-11.5) | 5.70 (3.25-10.7) |
| Median eGFR (IQR), mL/min/1.73 m2 | 69.0 (53.0-88.0) | 69.5 (52.0-85.5) | 71.0 (56.0-86.0) | 69.5 (51.5-89.0) | 70.0 (54.0-86.0) |
| Heart failure, n (%) | 129 (29.8) | 79 (30.4) | 84 (28.6) | 91 (28.8) | 254 (29.2) |
BMI = body mass index; eGFR = estimated glomerular filtration rate; hsCRP = high-sensitivity C-reactive protein; IQR = interquartile range.
P < 0.050 for the comparison of canakinumab with placebo.
Appendix Table 3.
Baseline Clinical Characteristics of Trial Participants With and Without Anemia at Study Entry
| Characteristic | No Anemia at Baseline (n = 8683) | Anemia at Baseline (n = 1303)* |
|---|---|---|
| Median age (IQR), y | 61.0 (54.0-67.0) | 65.0 (57.0-73.0) |
| Female, n (%) | 2156 (24.8) | 414 (31.8) |
| Median hemoglobin level(IQR), g/L | 145 (137-153) | 119 (113-126) |
| Median BMI (IQR), kg/m2 | 30.0 (26.7-33.8) | 29.0 (25.4-33.7) |
| Alcohol use (>1 drink per day), n (%) | 363 (4.18) | 32 (2.46) |
| Hypertension, n (%) | 6856 (79.0) | 1094 (84.0) |
| Type 2 diabetes mellitus, n (%) | 3326 (38.3) | 671 (51.5) |
| Median hsCRP level (IQR), mg/L | 4.05 (2.75-6.60) | 5.65 (3.20-10.6) |
| Median eGFR (IQR), mL/min/1.73 m2 | 79.0 (66.0-94.0) | 70.0 (54.0-87.0) |
| Heart failure, n (%) | 1776 (20.5) | 383 (29.4) |
BMI = body mass index; eGFR = estimated glomerular filtration rate; hsCRP = high-sensitivity C-reactive protein; IQR = interquartile range.
P < 0.050 for the comparison of participants with vs. those without anemia at baseline.
Appendix Figure 1.
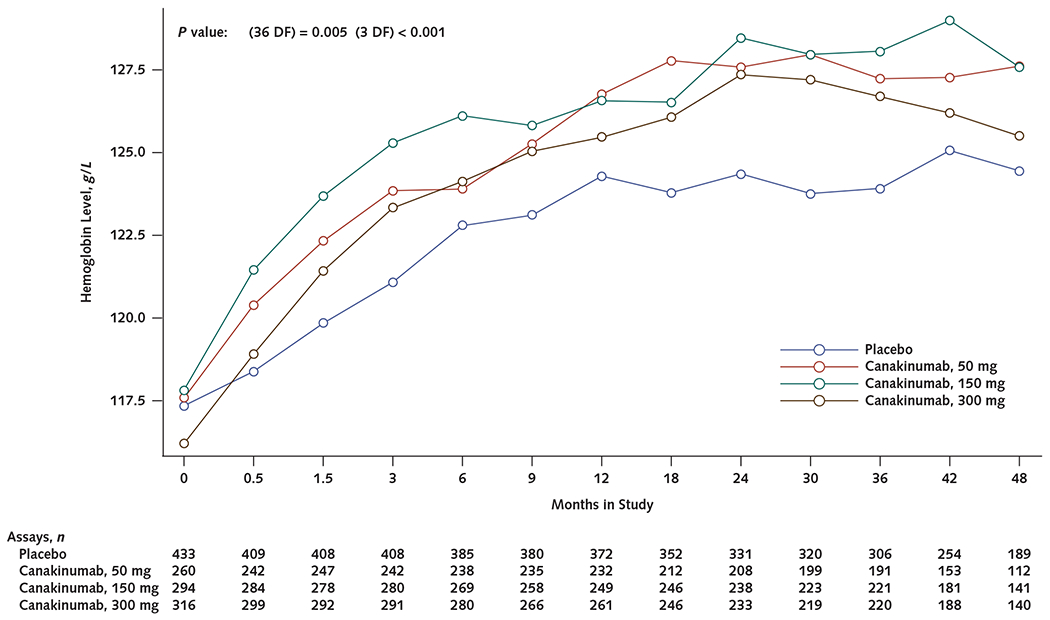
Mean hemoglobin levels over time in participants with anemia at baseline (<130 g/L in men or <120 g/L in women) who received canakinumab, 50, 150, or 300 mg every 3 months, compared with those who received placebo.
DF = degrees of freedom.
Appendix Table 4.
Mean Change in Hemoglobin Level in Participants With Baseline Anemia Who Received Placebo or Canakinumab
| Time Point | Placebo | Canakinumab | |||
|---|---|---|---|---|---|
| 50 mg | 150 mg | 300 mg | All Doses | ||
| 3 months | |||||
| Participants, n | 408 | 242 | 280 | 291 | 813 |
| Mean change in hemoglobin level (SD), g/L | 3.82 (11.02) | 6.45 (10.19) | 7.50 (11.36) | 7.14 (10.95) | 7.06 (10.8) |
| P value | Reference | <0.001 | <0.001 | <0.001 | <0.001 |
| 6 months | |||||
| Participants, n | 385 | 238 | 269 | 280 | 787 |
| Mean change in hemoglobin level (SD), g/L | 5.65 (12.89) | 6.63 (11.85) | 8.27 (12.43) | 8.05 (11.93) | 7.70 (12.08) |
| P value | Reference | 0.116 | 0.001 | 0.005 | 0.001 |
| 9 months | |||||
| Participants, n | 380 | 235 | 258 | 266 | 759 |
| Mean change in hemoglobin level (SD), g/L | 6.15 (13.75) | 7.97 (12.74) | 8.29 (13.53) | 8.96 (13.28) | 8.43 (13.19) |
| P value | Reference | 0.011 | 0.016 | 0.003 | 0.001 |
| 12 months | |||||
| Participants, n | 372 | 232 | 249 | 261 | 742 |
| Mean change in hemoglobin level (SD), g/L | 7.03 (13.55) | 9.49 (14.23) | 8.91 (13.20) | 9.76 (14.16) | 9.39 (13.85) |
| P value | Reference | 0.008 | 0.023 | 0.007 | 0.001 |
| 24 months | |||||
| Participants, n | 331 | 208 | 238 | 233 | 679 |
| Mean change in hemoglobin level (SD), g/L | 7.80 (14.73) | 10.80 (14.49) | 10.90 (14.60) | 12.20 (13.74) | 11.30 (14.27) |
| P value | Reference | 0.006 | 0.002 | <0.001 | <0.001 |
| 36 months | |||||
| Participants, n | 306 | 191 | 221 | 220 | 632 |
| Mean change in hemoglobin level (SD), g/L | 7.31 (14.80) | 10.37 (15.85) | 10.80 (13.83) | 11.73 (15.68) | 10.99 (15.10) |
| P value | Reference | 0.019 | 0.001 | 0.002 | <0.001 |
| 48 months | |||||
| Participants, n | 189 | 112 | 141 | 140 | 393 |
| Mean change in hemoglobin level (SD), g/L | 8.90 (15.53) | 11.90 (18.17) | 10.30 (14.10) | 10.00 (15.67) | 10.60 (15.88) |
| P value | Reference | 0.149 | 0.186 | 0.42 | 0.131 |
Appendix Figure 2.
Resolution of anemia over the course of the study among participants with anemia at baseline who received canakinumab, 50, 150, or 300 mg every 3 months, compared with the placebo group.
HR = hazard ratio.
Footnotes
Data Sharing Statement: The authors have indicated that they will not be sharing data. The CANTOS protocol is available from Dr. Ridker (pridker@bwh.harvard.edu).
References
- 1.Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest. 2004;113:1251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28:671–81, vi. doi: 10.1016/j.hoc.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penninx BW, Pahor M, Cesari M, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–24. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. [DOI] [PubMed] [Google Scholar]
- 7.Nemeth E, Valore EV, Territo M, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–3. [DOI] [PubMed] [Google Scholar]
- 8.Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–23. doi: 10.1136/ard.2008.092932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, MacFadyen JG, Thuren T, et al. ; CANTOS Trial Group. Effect of interleukin-1ß inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017; 390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X [DOI] [PubMed] [Google Scholar]
- 12.Solomon DH, Glynn RJ, MacFadyen JG, et al. Relationship of interleukin-1ß blockade with incident gout and serum uric acid levels: exploratory analysis of a randomized controlled trial. Ann Intern Med. 2018;169:535–542. doi: 10.7326/M18-1167 [DOI] [PubMed] [Google Scholar]
- 13.Prentice RL, Gloeckler LA. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978; 34:57–67. [PubMed] [Google Scholar]
- 14.Allison PD. Discrete-time methods for the analysis of event histories. In: Leinhardt S, ed. Sociological Methods and Research. San Francisco: Jossey-Bass; 1982:61–98. [Google Scholar]
- 15.Ridker PM, MacFadyen JG, Everett BM, et al. ; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. doi: 10.1016/S0140-6736(17)32814-3 [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39:3499–3507. doi: 10.1093/eurheartj/ehy310 [DOI] [PubMed] [Google Scholar]
- 17.Valeri L, VanderWeele TJ. SAS macro for causal mediation analysis with survival data [Letter]. Epidemiology. 2015;26:e23–4. doi: 10.1097/EDE.0000000000000253 [DOI] [PubMed] [Google Scholar]
- 18.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. [DOI] [PubMed] [Google Scholar]
- 20.Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY). 2012;4:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–12. doi: 10.1126/science.1201940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridker PM. Anticytokine agents: targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ Res. 2019;124: 437–450. doi: 10.1161/CIRCRESAHA.118.313129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high-sensitivity C-reactive protein after an initially elevated concentration: the JUPITER Study. Clin Chem. 2009;55:305–12. doi: 10.1373/clinchem.2008.120642 [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Danielson E, Fonseca FA, et al. ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 26.Brookhart MA, Solomon DH, Glynn RJ, et al. Effect of rosuvastatin on hemoglobin levels in patients with anemia and low-grade inflammation: a post hoc analysis of the JUPITER trial. J Clin Pharmacol. 2011;51:1483–7. doi: 10.1177/0091270010386607 [DOI] [PubMed] [Google Scholar]


