Abstract
Pancreatic cystic lesions (PCLs) are being increasingly encountered in clinical practice, and sometimes, they can represent a diagnostic challenge. Recently, a through-the-needle micro forceps biopsy (MFB) device was introduced in the endosonography practice to facilitate EUS-guided sampling of PCLs. The aim was to perform a systematic review of studies evaluating the technical aspects, safety, and efficacy of the EUS-guided MFB for PCLs. A literature search was performed in three major databases, PubMed, Embase, and Web of Science in September 2019 using the search terms: “through-the-needle,” “biopsy forceps,” “microforceps,” “endoscopic ultrasound,” and “endosonography.” Case reports and case series with <10 patients were excluded from the analysis. Altogether nine studies reporting on 463 patients were included in our systematic review. The mean age of the patients was 68.3 years, with a slight female predominance (60.9%). Most of the cysts were located in the body/tail of the pancreas (61.2%), with an overall mean size of 33 mm. The technical success of EUS-guided MFB was reported in 98.5%. The tissue acquisition yield reported was 88.2%, and the diagnostic accuracy was 68.6%. Adverse events were reported in 9.7%. EUS-guided MFB is technically feasible, safe, and has a high diagnostic accuracy for PCLs.
Keywords: diagnostic accuracy, EUS, microbiopsy forceps, pancreatic cyst
INTRODUCTION
Pancreatic cystic lesions (PCLs) are being increasingly encountered in clinical practice due to the widespread use of high-resolution imaging. The prevalence of pancreatic cysts detected incidentally varies from 2% on computed tomography scans[1] to 9.3% on 3T magnetic resonance imagings of the abdomen.[2] The wide spectrum of PCLs includes benign and malignant lesions, and therefore, an accurate diagnosis is mandatory to decide the best management. In clinical practice, the challenge is to differentiate between nonneoplastic and neoplastic PCLs and to determine whether one should undergo either surveillance or surgery. This carries the risk of either sending a patient to unnecessary surgery or missing the opportunity to resect a potentially malignant lesion. Several guidelines have been published over the years, some of them favoring surgery, others being more prudent, and balancing towards surveillance.[3,4,5,6] Analysis of management decisions, according to these guidelines, has shown significant deficits.
Until recent years, EUS-FNA of PCLs with cyst fluid analysis was used for management decisions, but this has proven suboptimal performance due to scant cellularity and poor diagnostic accuracy of biomarkers.
In the last years, though, there has been a growing interest for tissue acquisition in PCLs, and diagnosis has evolved from cyst fluid sampling to histological criteria by means of needle-based confocal endomicroscopy, through-the-needle cystoscopy, and through-the-needle intracystic biopsy.[7] Among these techniques, cyst wall sampling using through-the-needle micro biopsy (TTNB) forceps has gained the most interest. The Moray micro forceps biopsy (MFB) device (US Endoscopy, Ohio, US) is a disposable tissue acquisition device that can be passed through a 19G needle and allows for tissue sampling of the cyst wall.
Our aim was to review the current data regarding TTNB in PCLs, with respect to feasibility, diagnostic accuracy, and safety.
METHODS
The literature search was performed in September 2019 in three major databases: PubMed, Embase, and Web of Science. The search terms used were: “endoscopic ultrasound”/“eus” and “biopsy forceps” or “microforceps.” No search restrictions were applied. We also searched abstracts from scientific meetings.
The initial search yielded 386 articles (Embase 110, PubMed 202, Web of Science 74).
After title and abstract screening and removing duplicates, 48 records remained for a detailed assessment of eligibility.
After excluding review, case reports, case series with <10 patients, articles without available full-text and articles reporting duplicate data, a total of 9 articles, comprising 463 patients (range: 27–114), were enrolled in the systematic review [Figure 1].
Figure 1.
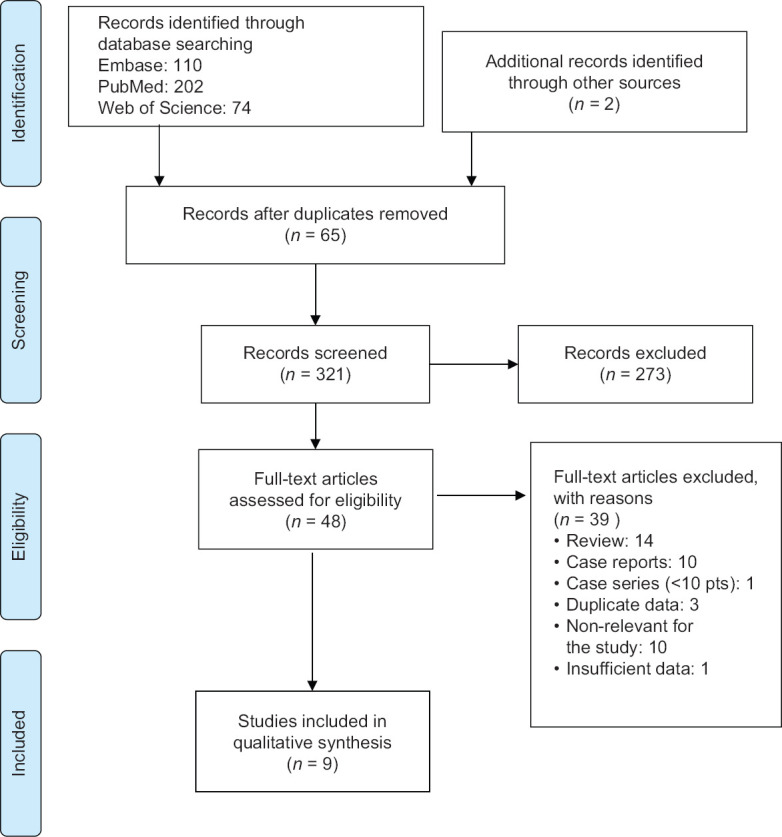
PRISMA 2009 flow diagram
Definitions of performance and outcome measures for the TTNB forceps were reviewed for all articles. Technical success was defined as the successful puncture of the lesion, mounting the forceps, and performing the biopsy. Tissue acquisition yield was reported as the ability to obtain at least one tissue sample. Diagnostic accuracy was set against the correct final diagnosis as defined by surgery or a multidisciplinary board based on cross section imaging, EUS characterization, cyst fluid markers and/or cytology.
Adverse events were reported according to the American Society for Gastrointestinal Endoscopy severity criteria, as defined in Cotton et al.[8]
RESULTS
Altogether nine studies[9,10,11,12,13,14,15,16,17] (9 retrospective, 1 prospective) reporting on 463 patients with 464 cysts (there was one patient with two lesions punctured in the study by Hrad[17]) were included in our systematic review. The mean age of the patients was 68.3 years, with a slight female predominance (60.9%). The size of the PCLs ranged from 11 to 88 mm (mean 33 mm) and most of them were located in the body-tail of the pancreas (61.2%) [Table 1].
Table 1.
Patient demographics and pancreatic cystic lesions characteristics
| Study design | Number patient | Age (mean) | Sex (% female) | Cyst size (mean mm) | Cyst location (head* + istmus/body + tail) | |
|---|---|---|---|---|---|---|
| Basar et al., 2018[12] | Retrospective | 42 | 69 | 23/42 | 28.2 | 16/26 (38.1/61.9%) |
| Barresi et al., 2018[13] | Retrospective | 56 | 57.5 | 39/56 | 38.6 | 27/29 (48.2/51.8%) |
| Zhang et al., 2018[14] | Retrospective | 48 | 69.6 | 25/48 | 31 | 15/33 (31.2/68.8%) |
| Mittal et al., 2018[15] | Retrospective | 27 | 65 | 16/27 | 37.8 | 14/13 (51.9/48.1%) |
| Kovacevic et al., 2018[16] | Retrospective | 31 | 69.9 | 15/31 | 33.5 | N/A |
| Hrad et al., 2018[17] | Retrospective | 37 | 71 | 27/37 | 30.6 | 13/25 (34.2/65.8%) |
| Yang et al., 2018[10] | Retrospective | 47 | 66.2 | 26/47 | 30.7 | 22/25 (46.8/53.2%) |
| Yang et al., 2019[11] | Prospective | 114 | 64.2 | 64/114 | 35 | 39/71 (34.2/62.3%) 4 not specified (3.5%) |
| Crinò et al., 2019[9] | Retrospective | 61 | 50.2 | 47/61 | 40.7 | 18/43 (29.5/70.5%) |
| Total | 463 | 68.3 | 282/463 (60.9%) | 33 | 164/265 (37.9/61.2%) |
*Including uncinate process. N/A: Not available
The most frequently used needle was Expect (Boston Scientific, Marlborough, Mass, USA), but punctures were also performed with EchoTip (Cook Medical, Bloomington, IN, USA) and SharkCore (Medtronic, Dublin, Ireland). All samplings were done using Moray MFB device (US Endoscopy, US).
Technical success of EUS-guided MFB device was reported in 456/463 cases (98.5%; 95% confidence interval [CI]: 89%–100%). The main reasons for technical difficulties were the inability to access the cyst, while the echoendoscope was fully flexed, difficulty to push the instrument through the needle, and difficulty to visualize the forceps on EUS scan. The tissue acquisition yield reported was 367/416 (88.2%; 95% CI: 79%–97%) [Table 2].
Table 2.
Feasibility, diagnostic accuracy and safety of through-the-needle microbiopsy
| Needle used | Tehnical succes | Tissue acquisition yield | Diagnostic accuracy | AE | AE (details) | |
|---|---|---|---|---|---|---|
| Basar et al., 2018[12] | 19G flex | 42/42 | 38/42 | 15/42 | 2 | Self-limited ICB -1 Mild abdominal pain - 1 |
| Barresi et al., 2018[13] | 19G EchoTip, \19G Expect and Expect flex and 19G SharkCore | 56/56 | 52/56 | 47/56 | 9 | Self-limited ICB - 6 Abdominal pain - 2 Both ICB and abdominal pain -1 |
| Zhang et al., 2018[14] | 19G FNA needle | 48/48 | N/A | 36/48 | 0 | - |
| Mittal et al., 2018[15] | 19G Expect or EchoTip Ultra | 27/27 | 24/27 | 21/27 | 0 | - |
| Kovacevic et al., 2018[16] | 19G Expect Flex | 27/31 | 22/31 | 12/22 | 3 | Mild AP - 1 Mild infection -2 |
| Hrad et al., 2018[17] | 19G needle | 38/38 | 35/38 | 35/38 | 2 | AP - 1 Atrial fibrillation - 1 |
| Yang et al., 2018[10] | 19G EchoTip Ultra | 46/47 | 40/47 | 26/47 | 2 | self-limited ICB - 1 AP - 1 |
| Yang et al., 2019[11] | 19G FNA needle | 111/114 | 95/114 | 75/114 | 13 | self-limited ICB - 7 AP - 6 |
| Crinò et al., 2019[9] | 19G expect | 61/61 | 61/61 | 45/61 | 14 | self-limited ICB -10 mild AP -2 peripancreatic bleed45ing - 1 transitory fever - 1 |
| Total | 456/463 (98.5%) | 367/416 (88.2%) | 312/455 (68.6%) | 45 (9.7%) | abdominal pain - 4 ICB - 26 peripancreatic bleeding - 1 AP - 11 Mild infection - 3 Atrial fibrillation - 1 |
AP: Acute pancreatitis; ICB: Intracystic bleed; AE: Adverse events; N/A: Not available; FNA: Fine needle aspiration
EUS-guided MFB was able to establish the correct final diagnosis in 312 PCLs out of 455 (diagnostic accuracy 68.6%; 95% CI, 61%–76%).
Forty-five patients (9.7%) having 46 adverse events were reported among the studies: abdominal pain-4, intracystic hemorrhage-26, peripancreatic bleeding-1, mild acute pancreatitis-11, postprocedural infection-3, and atrial fibrillation after procedure-1 case.
DISCUSSION
Over the time, diagnosis of PCLs evolved from EUS-based morphological assessment, to cyst fluid analysis by means of FNA (cellularity, amylase, glucose, carcinoembryonic antigen [CEA], CA 19-9, molecular markers) and recently by sampling the tissue wall.
EUS morphology alone has a modest diagnostic yield in distinguishing mucinous from nonmucinous pancreatic cysts, with variable diagnostic accuracy (48%–94%), sensitivity (36%–91%) and specificity (45%–81%).[5] Regarding cyst fluid analysis, CEA, with a cutoff value of 192 ng/mL, is considered the most accurate marker (52%–78% sensitivity; 63%–91% specificity) for differentiating nonmucinous from mucinous pancreatic cysts and is the preferred marker in low-volume aspirates.[5,18] Various studies showed that CEA values <5 ng/mL had 50% sensitivity and 95% specificity for serous cystadenoma (SCA) or pseudocyst diagnosis, while CEA >800 ng/mL had 48% sensitivity and 98% specificity in differentiating between mucinous and nonmucinous cysts.[19,20]
Cyst fluid amylase levels <250 U/L, although not useful in distinguishing between mucinous and nonmucinous cysts, may exclude pancreatic pseudocysts with a 44% sensitivity and a 98% specificity.[20,21] In addition, CEA >192 ng/mL values associating with amylase values below or above 250 U/L might guide the diagnosis towards mucinous cystic neoplasm (MCN) or intraductal papillary mucinous neoplasm.[19]
Cyst fluid glucose ≤50 mg/dL compared to CEA >192 ng/mL cutoff,[22] has been proven to be more sensitive (92% vs. 58%), more accurate (90% vs. 69%) but less specific (87% vs. 96%) in diagnosing mucinous pancreatic cysts. Similar results regarding sensitivity (88% vs. 50%) and diagnostic accuracy (94% vs. 89%) were also obtained for fluid glucose <41 mg/dL and CEA >184 ng/mL.[23] However, the measurement of both intracystic glucose and CEA might have significantly increased sensitivity in diagnosing mucinous cysts.[24]
The cytopathological examination of PLCs has a specificity higher than 93%.[25,26] and until now was the most accurate test for determining malignancy risk. Despite its potential to provide a definite diagnosis, it is less sensitive in diagnosing a malignant cyst (75%–78%)[12,25] and its use remains limited mostly because of low cellularity acquisition during EUS-FNA and gastrointestinal wall cell contamination, but also possibly due to the examiner's lack of experience.[12]
Considering this suboptimal performance of cyst fluid analysis in setting the correct management of PCLs, a need for better diagnostic tools has emerged. Thus, a growing interest has developed around the idea of targeting the cyst lining by the use of a microbiopsy forceps device. Several case reports and small case series have been reported.[27,28,29]
One of the anticipated issues regarding the MFB was the ability to perform the puncture, especially in positions known to be difficult even for conventional FNA, such as the transduodenal route. In this systematic review, a high rate of technical success was reported-98.5%, but these are results of expert centers. Technical failures were represented by punctures attempted with the echoendoscope in fully flexed position (by transduodenal route); there was one case with failed transgastric puncture but in fact it was reported as a failure because of transient hypoxia which leads to abortion of the procedure.
Regarding tissue acquisition yield, this was very high-88.2%, however, not all studies reported the number of biopsies performed per cyst. A lower rate of successful sampling of the epithelial lining was seen in tail-located cysts,[10,12] but this could be due to the higher frequency of MCNs and SCAs located in the tail and the fact that epithelial cyst denudation has been previously reported in these lesions.[30,31]
The diagnostic yield of the TTNB forceps was 68.6%. Six of the studies included in our review providing comparative information regarding diagnostic accuracy concluded that MFB is better than EUS-FNA in differentiating nonneoplastic from neoplastic PCLs and diagnosing the specific type of cyst.
Yang et al. were able to diagnose mucinous cysts in 34.3% of the cases by using TTNB alone, rather than by using biomarkers such as CEA >192 ng/mL alone (6.3%) or CEA >192 ng/mL and FNA cytology combination (9.4%).[11] Barresi et al. showed that TTNB diagnostic yield was significantly higher than needle cystic wall biopsy combined with fluid cytology analysis (83.9% vs. 41.6%).[13]
MFB also proved to be more accurate than EUS in diagnosing the specific type of cyst. MFB provided a specific diagnosis in 35.7%–50% of the cases compared to conventional analysis of cyst fluid 4.8%–18.8%.[12,14]
The impact of EUS-TTNB was clinically meaningful, as it provided a diagnosis of the specific type of cyst in significantly more cases than the conventional methods. A change in the presumed diagnosis/management was reported in 19.4%–26% of cases.[15,16]
Adverse events (AE) rate was highly variable among the reported studies-from 0% AE[14,15] 22.9%.[9] Intracystic bleeding was one of the most frequently reported AE. In most of the cases, bleeding was self-limiting and asymptomatic and did not require medical intervention. In some cases, bleeding was associated with abdominal pain caused by increased intracystic pressure.[9] Another potentially severe AE was acute pancreatitis, which was reported in 3%–7% of cases.[10,16]
There was significant heterogeneity of studies included in this systematic review. Not all studies reported on the morphology of the cysts-unilocular/septated, biopsy protocol, or mean number of biopsies per cyst [Supplementary Table 1], whether the biopsy site was targeted (from mural nodules or septations) or random from the wall. Furthermore, half of the studies were single center and sample sizes were small.
Supplementary Table 1.
Number of passes performed with the Moray forceps
| Author, year | Study methods on number of passes/cyst |
|---|---|
| Basar, 2018 | If the biopsy specimen seemed insufficient in size for histology, additional passes were made |
| Barresi, 2018 | The number of passes performed with the biopsy forceps was at the discretion of each endosonographer 1 biopsy-3 (5.3%), 2-7 (12.5%), 3-32 (57.1%), 4-11 (19.6%), 5-3 (5.3%) |
| Zhang, 2018 | More than one biopsy was obtained if initial biopsies appeared inadequate macroscopically |
| Mittal, 2018 | 3-4 passes were made with microforceps (or until 3-4 visible tissue fragments were obtained) |
| Kovacevic, 2018 | Median of 3 biopsies per patient (interquartile range 2-4) |
| Hrad, 2018 | Not mentioned |
| Yang, 2018 (retrospective) | Median number of passes of one (range 1-2). Two to three “bites” of microforceps biopsy specimens were obtained under EUS-guidance with each pass of the microforceps |
| Yang, 2019 (prospective) | Two to 3 bites were obtained from the cyst wall, septations, nodules, or adjacent solid components with each pass of the microforceps |
| Crino, 2019 | In the absence of intracystic bleeding, the procedure was repeated until 3 visible specimens were obtained |
Another significant limitation is that 8 out of 9 studies were retrospective. Also, pathologists were not blinded to follow-up of patients, and comparison with a surgical specimen was available in a limited number of cases. Molecular analysis of cyst fluid was also missing in most studies, which could have had an impact on the management decision, as it is known to improve the diagnostic yield of cytology alone.[32] Quality of specimens was not reported throughout all studies; this should be considered as an outcome measure in future trials.
Not least, the encouraging results of the studies included in our systematic review reflect the experience of high-volume centers with expert endosonographers and pathologists, which might not be replicated in a real-world setting.
Experience with this new technique is growing, and better insight about the indications and safety of MFB is expected from future studies. The cost of using TTNB forceps could be an issue given the expenses associated with EUS-FNA, but taking into account the change in management reported in up to 1 in 4 patients, it could prove to be cost-effective; this aspect should be certainly addressed in further studies.
CONCLUSION
EUS-guided MFB is technically feasible, safe, and has a high diagnostic accuracy for PCLs. The results of our systematic review should be interpreted with caution. Given the novelty of this technique, further ongoing studies are expected to offer a better understanding of the safety profile and diagnostic accuracy of EUS-MFB.
Supplementary Materials
Supplementary information is linked to the online version of the paper on the Endoscopic Ultrasound website.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Oliveira PB, Puchnick A, Szejnfeld J, et al. Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS One. 2015;10:e0121317. doi: 10.1371/journal.pone.0121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatol. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka M, Castillo CF, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatol. 2017;17:738–53. doi: 10.1016/j.pan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 5.European Study Group on Cystic Tumours of the Pancreas. European evidence. based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. doi: 10.1136/gutjnl-2018-316027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elta GH, Enestvedt BK, Sauer BG, et al. ACG Clinical Guideline: Diagnosis and management of pancreatic cysts. Am J Gastroenterol. 2018;113:464–79. doi: 10.1038/ajg.2018.14. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Sánchez MV, Napoléon B. New horizons in the endoscopic ultrasonography-based diagnosis of pancreatic cystic lesions. World J Gastroenterol. 2018;24:2853–66. doi: 10.3748/wjg.v24.i26.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Crinò SF, Bernardoni L, Brozzi L, et al. Association between macroscopically visible tissue samples and diagnostic accuracy of EUS-guided through-the-needle microforceps biopsy sampling of pancreatic cystic lesions. Gastrointest Endosc. 2019;90:933–43. doi: 10.1016/j.gie.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Yang D, Samarasena JB, Jamil LH, et al. Endoscopic ultrasound-guided through-the-needle microforceps biopsy in the evaluation of pancreatic cystic lesions: A multicenter study. Endosc Int Open. 2018;6:E1423–E30. doi: 10.1055/a-0770-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang D, Trindade AJ, Yachimski P, et al. Histologic analysis of endoscopic ultrasound-guided through the needle microforceps biopsies accurately identifies mucinous pancreas cysts. Clin Gastroenterol Hepatol. 2019;17:1587–96. doi: 10.1016/j.cgh.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Basar O, Yuksel O, Yang DJ, et al. Feasibility and safety of microforceps biopsy in the diagnosis of pancreatic cysts. Gastrointest Endosc. 2018;88:79–86. doi: 10.1016/j.gie.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 13.Barresi L, Crinò SF, Fabbri C, et al. Endoscopic ultrasound-through-the-needle biopsy in pancreatic cystic lesions: A multicenter study. Dig Endosc. 2018;30:760–70. doi: 10.1111/den.13197. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ML, Arpin RN, Brugge WR, et al. Moray micro forceps biopsy improves the diagnosis of specific pancreatic cysts. Cancer Cytopathol. 2018;126:414–20. doi: 10.1002/cncy.21988. [DOI] [PubMed] [Google Scholar]
- 15.Mittal C, Obuch JC, Hammad H, et al. Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video) Gastrointest Endosc. 2018;87:1263–9. doi: 10.1016/j.gie.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Kovacevic B, Klausen P, Hasselby JP, et al. A novel endoscopic ultrasound-guided through-the-needle microbiopsy procedure improves diagnosis of pancreatic cystic lesions. Endosc. 2018;50:1105–11. doi: 10.1055/a-0625-6440. [DOI] [PubMed] [Google Scholar]
- 17.Hrad V, Meidinger R, Peng HQ, et al. EUS-guided cyst wall biopsy is highly effective in definitive diagnosis of pancreatic cysts: 69. Am J Gastroenterol. 2018;113:S40–1. [Google Scholar]
- 18.Ngamruengphong S, Lennon AM. Analysis of pancreatic cyst fluid. Surg Pathol Clin. 2016;9:677–84. doi: 10.1016/j.path.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan JH, Gonda TA. The use of biomarkers in the risk stratification of cystic neoplasms. Gastrointest Endosc Clin N Am. 2018;28:549–68. doi: 10.1016/j.giec.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 20.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest Endosc. 2005;62:383–9. doi: 10.1016/s0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]
- 21.Al-Rashdan A, Schmidt CM, Al-Haddad M, et al. Fluid analysis prior to surgical resection of suspected mucinous pancreatic cysts.A single centre experience. J Gastrointest Oncol. 2011;2:208–14. doi: 10.3978/j.issn.2078-6891.2011.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carr RA, Yip-Schneider MT, Simpson RE, et al. Pancreatic cyst fluid glucose: Rapid, inexpensive, and accurate diagnosis of mucinous pancreatic cysts. Surgery. 2018;163:600–5. doi: 10.1016/j.surg.2017.09.051. [DOI] [PubMed] [Google Scholar]
- 23.Simons-Linares CR, Bhatt A, Jang S, et al. 157-The utility of intracystic glucose levels in differentiating mucinous from non-mucinous pancreatic cysts. Gastroenterol. 2018;154:S–43. doi: 10.1016/j.pan.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Figueiredo M, Zaarour A, Eisendrath P. Intracystic glucose and CEA measurement: A quick tool for diagnosing mucinous pancreatic cysts. Endoscopy. 2018;50:eP180V. [Google Scholar]
- 25.Scourtas A, Dudley JC, Brugge WR, et al. Preoperative characteristics and cytological features of 136 histologically confirmed pancreatic mucinous cystic neoplasms. Cancer Cytopathol. 2017;125:169–77. doi: 10.1002/cncy.21806. [DOI] [PubMed] [Google Scholar]
- 26.Thornton G, McPhail M, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 27.Shakhatreh MH, Naini SR, Brijbassie AA, et al. Use of a novel through-the-needle biopsy forceps in endoscopic ultrasound. Endosc Int Open. 2016;4:E439–42. doi: 10.1055/s-0042-101941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huelsen A, Cooper C, Saad N, et al. Endoscopic ultrasound-guided, through-the-needle forceps biopsy in the assessment of an incidental large pancreatic cystic lesion with prior inconclusive fine-needle aspiration. Endoscopy. 2017;49:E109–10. doi: 10.1055/s-0043-100217. [DOI] [PubMed] [Google Scholar]
- 29.Pham KD, Engjom T, Kollesete HG, et al. Diagnosis of a mucinous pancreatic cyst and resection of an intracystic nodule using a novel through-the-needle micro forceps. Endoscopy. 2016;48(Suppl 1):E125–6. doi: 10.1055/s-0042-105437. [DOI] [PubMed] [Google Scholar]
- 30.Collins BT. Serous cystadenoma of the pancreas with endoscopic ultrasound fine needle aspiration biopsy and surgical correlation. Acta Cytol. 2013;57:241–51. doi: 10.1159/000346911. [DOI] [PubMed] [Google Scholar]
- 31.Gómez V, Majumder S, Smyrk TC, et al. Pancreatic cyst epithelial denudation: A natural phenomenon in the absence of treatment. Gastrointestl Endosc. 2016;84:788–93. doi: 10.1016/j.gie.2016.03.1502. [DOI] [PubMed] [Google Scholar]
- 32.Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterol. 2015;149:1501–10. doi: 10.1053/j.gastro.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]


