Abstract
ERCP and EUS are complementary techniques in the management of biliary and pancreatic diseases. Combination of these two techniques can reach different levels of complexity with increasing rates of adverse events. In this article we propose a categorization of the relationship between EUS and ERCP based on whether EUS indicates, complements, facilitates or replaces ERCP. It has implications for the complexity of the technique, the training of the endoscopist and the necessary hospital resources. This classification can also be useful in planning endoscopist training and patient management.
Keywords: biliary drainage, celiac plexus neurolysis, collection drainage, endosonography, endoscopic retrograde cholangiopancreatography, pancreatic drainage
BACKGROUND
Since its inception in 1968,[1] ERCP has become the primary procedure for minimally invasive endoscopic treatment of biliary and pancreatic diseases. However, it has several limitations that have not been resolved.
The first limitation is the papillary cannulation rate. In patients with normal anatomy, bile duct cannulation should be achieved in at least 90% of cases.[2] It means that in 10% of patients, endoscopic treatment cannot be performed and therefore, a more aggressive and risky treatment such as percutaneous access or surgery must be chosen. In expert centers failure of cannulation may be significantly reduced and only reach 5%, but this is highly influenced by the type of patient in which ERCP is indicated and local expertise.
Another limitation is altered anatomy by previous surgery, especially after Roux-en-Y reconstruction, in which the papillary area is difficult to reach and cannulation rate may be lowered to 60% in expert centers.[3]
On the other hand, EUS was born in the 1980s to enhance the diagnostic yield in pancreatic pathology.[4] It is from the appearance of the linear echoendoscope, that allows the performance of EUS-guided puncture,[5] when it acquires a greater diagnostic capacity and therapeutic possibilities. In this way, different EUS-guided therapeutic procedures have been developed, especially in the biliary and pancreatic area such as choledochoduodenostomy,[6] pancreaticogastrostomy,[7] hepaticogastrostomy[8] and rendezvous.[9]
These procedures really constitute a new endoscopic technique, namely endosonographic cholangiopancreatography (ESCP).[10] The ESCP represents a change in the paradigm of endoscopic treatment of biliary and pancreatic pathology, previously assumed only by ERCP. In addition, it has resolved the limitations of ERCP in many cases. However, it is a complex technique that requires mastery in both EUS and ERCP and also an adequate multidisciplinary support, which means that it is not universally applicable in all centers.
Therefore, ERCP and EUS have forged a collaborative relationship so beneficial that it is unthinkable today to train an endoscopist in ERCP without also being trained in EUS. This relationship between both techniques has different stages of complexity, which should also determine the convenience to be developed in a hospital.
The objective of this article is to propose a classification of the relationship between EUS and ERCP according to its complexity and risks, and make a recommendation of its development in different kind of centers according to their characteristics and the experience of the endoscopists.
EUS AND ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY COLLABORATIVE STAGES
Four different collaborative levels between EUS and ERCP can be identified:
Stage I: EUS indicates endoscopic retrograde cholangiopancreatography
This first stage involves those situations in which EUS makes the definitive indication for ERCP. At this stage, EUS must be done first and determines whether ERCP is subsequently performed. So EUS is used to confirm the indication of ERCP.
The usual scenario is the intermediate risk of choledocholithiasis, defined as symptomatic biliary disease associated with cholestasis with or without bile duct dilation. In this situation, EUS or magnetic resonance cholangiopancreatography (MRCP) should be performed prior to ERCP to confirm the presence of choledocholithiasis.[11] When the pretest probability of choledocholithiasis is greater, EUS should be performed in the same endoscopic session as ERCP.[12]
In a meta-analysis of 5 comparative studies including 272 patients, the sensitivity and specificity for the diagnosis of choledocholithiasis reached 97% and 90% for EUS, and 87% and 92% for MRCP.[13] However, the diagnostic odds ratio (OR) was significantly higher for EUS (162.5 vs. 79, P = 0.008) due to the higher sensitivity of EUS compared with MRCP (P = 0.06), without differences in terms of specificity.
Moreover, a systematic review showed that performing EUS beforehand, avoided ERCP in 67% of these patients by proving the absence of choledocholithiasis.[14] This also resulted in a significant overall reduction in adverse events in the cohort of patients with previous EUS versus those who went directly to ERCP, 6,6% versus 19%, respectively. Specifically, a significant reduction in the incidence of post-ERCP pancreatitis was found, reaching 1.39% versus 7.85%, respectively.
In our opinion, this should be the stage by which the collaboration between both techniques should be started. It should be done in any hospital with both techniques available and by any endoscopist regardless of their experience.
Stage II: EUS complements endoscopic retrograde cholangiopancreatography
This stage refers to those conditions in which EUS and ERCP are indicated and complement each other. We can identify at least three of these situations in the routine clinical practice.
Firstly, the most common clinical scenario is jaundice secondary to a pancreatic or biliary neoplasm. In this situation, a pathological diagnosis achieved by performing EUS-guided tissue acquisiton (EUS-TA) is required, in addition to resolving jaundice by means of ERCP. EUS adds value to ERCP by achieving a pathological diagnosis for the cause of jaundice, which is resolved by ERCP.
Ideally, both examinations should be performed in a single endoscopic session. The outcomes of doing EUS and ERCP in a single session versus separately, were compared in a study showing there were no differences between the two groups in terms of total exploration time (93 ± 32.78 98.98 ± 38.17; P > 0.05), adverse events (5.12% vs. 15.2%, P > 0.05), diagnostic performance or therapeutic maneuvers.[15] A significant difference in the dose of propofol administered, which was lower in the tandem group (322.28 ± 250.54 mg 516.96 ± 289.06 mg; P = 0.001) was found. Similar conclusions were endorsed in studies carried out by other authors.[16,17,18]
Secondly, pancreatic collections associated with ductal pathology secondary to acute or chronic pancreatitis can also require complementary use of ERCP and EUS. This is especially indicated in patients with chronic pancreatitis in whom the finding of ductal pathology associated with pancreatic collections is common. In addition to the ERCP to solve the ductal lesions, EUS-guided collection drainage may be required since it has proved superiority over percutaneous and surgical treatment.[19,20,21]
Finally, another EUS-guided procedure that may complement ERCP is celiac plexus neurolysis (CPN). In patients with jaundice secondary to pancreatic cancer not amenable for surgical treatment, biliary drainage by means of ERCP associated with EUS-guided CPN for treatment of pain should be considered. In addition to jaundice resolution, pain control is achieved in approximately 73% of cases according to data from a meta-analysis including 119 patients.[22] It has been proved that the sooner CPN is performed, even at the time of diagnosis and staging by EUS, the better the response to this treatment.[23]
The level of complexity and risk of adverse events of the techniques included in this second stage, allow their application in all hospitals with EUS and ERCP after appropriate learning.
Stage III: EUS facilitates endoscopic retrograde cholangiopancreatography
EUS-guided procedures that allow the performance of an otherwise impossible ERCP, either because cannulation of the papilla cannot be achieved or because the papillary area is out of reach, are included in this stage. Therefore, EUS allows ERCP to be performed.
The simplest technique included in this stage is EUS-guided rendezvous. This technique is used when, despite being able to reach the papilla, papillary cannulation is not possible.
EUS-guided biliary rendezvous involves intra or extrahepatic bile duct puncture and subsequent antegrade transpapillary guidewire insertion into the duodenal lumen. Retrograde access to the biliary tree is then granted.
The current recommended technique by most authors includes 19G needle puncture of the extrahepatic bile duct from the duodenal bulb or second duodenal portion, with the endoscope in the short position, and passage of a 0.025-inch guidewire.[24]
The technical success of this procedure varies between 70% and 100%, with similar clinical success, and an adverse events rate about 15%.[25] It has not been clarified whether these adverse events are related to the biliary puncture and manipulation of the guidewire, or more likely to the papillary manipulation during ERCP.[26]
In EUS-guided pancreatic rendezvous, similar steps are followed by performing a transgastric puncture of the pancreatic duct, but the technical success rate is significantly lower,[27] especially in patients with chronic pancreatitis and ductal stenosis, a situation in which it is extremely difficult to achieve technical success. Clinical success is achieved in approximately 80% of patients in whom the technique is completed, and the adverse events rate is around 20%.[27]
In recent years, some technical advances have been described and aid to achieve technical success. Firstly, the use of a 3F caliber microcatheter that allows comfortable manipulation of the guidewire until transpapillary access to the duodenum is reached.[28] This microcatheter does not require transmural path dilation and supports passage of guidewires up to 0.025 inches caliber. It significantly improves the technical success of biliary and pancreatic rendezvous, reaching 80% in a series of 43 patients.[29] Improvement is greater for the pancreatic rendezvous, with 71% technical success.[29]
Secondly, the “hitch and ride” technique simplifies retrograde biliary cannulation during a rendezvous procedure.[30] Once a guidewire is transpapillary advanced under EUS and fluoroscopic control, a duodenoscope is inserted into the second duodenal portion. A cannula with a slit in its tip is hitched on the guidewire and advanced retrogradely into the bile duct. A preloaded guidewire is advanced through the cannula allowing transpapillary access.
Beside rendezvous, EUS-directed transgastric ERCP (EDGE) procedures performed in patients with gastric bypass, and EUS-guided gastroenterostomy procedures performed in patients with postsurgical altered anatomy, should be included in this stage.
The EDGE technique involves EUS-guided deployment of a lumen apposing metallic stent (LAMS) to get access into the remnant stomach in patients with gastric bypass with Roux-en-Y reconstruction.[31] The duodenum can then be reached through the stent to perform a standard ERCP. In a study comparing EDGE versus laparoscopy-assisted ERCP, no differences were seen in terms of technical success (96.5% vs. 100%), success rate of achieving therapeutic ERCP (96.5% vs. 97.7%), number of ERCP (1.2 vs. 1.02) needed to achieve clinical resolution and adverse events rates (24% vs. 19%).[32] The total procedure time (73 vs. 184 min) and length of hospital stay (0.8 vs. 2.65 days) was significantly shorter for EDGE procedure.
A similar technique has been described in patients with altered postsurgical anatomy connecting the gastric stump with the biliary afferent loop, completing an EUS-guided gastroenterostomy.[33] In the same way as EDGE technique, a standard ERCP is performed after reaching the papillary area through the LAMS.
The technical complexity of rendezvous, EDGE and gastroenterostomy is high and requires extensive experience in therapeutic EUS and ERCP. A multidisciplinary management of the complications is often required, so in our opinion these techniques should be performed exclusively in tertiary hospitals with interventional radiologists and biliopancreatic surgeons available.
Stage IV: EUS replaces endoscopic retrograde cholangiopancreatography
In the fourth stage, ERCP is not possible because of failure of papillary cannulation or inability to reach the papillary area. In this stage, EUS directly replaces ERCP so a transmural biliary or pancreatic drainage is performed. EUS-guided hepaticogastrostomy, choledochoduodenostomy and pancreaticogastrostomy should be included in this stage.
According to a systematic review and meta-analysis including 1192 patients, EUS-guided transmural biliary drainage leads to a 95.68% technical success, 90.32% clinical success and 24.41% adverse events rate.[34] In a more recent systematic review and meta-analysis including 1437 patients from 23 studies, a pooled rate of technical and clinical success of 91% (95% confidence interval [CI]: 87.7–94.2, I2 = 76.5) and 87% (95% CI: 82.3–90.6, I2 = 72.4), respectively, are shown.[35] The calculated pooled rate of re-intervention and adverse events were 6.5 (95% CI: 3.8–10.8, I2 = 69.3) and 17.9% (95% CI: 14.3–22.2, I2 = 69.1), respectively.
Therefore, these are very ressolutive but also complex techniques with a high rate of severe adverse events.
The outcomes of EUS-guided drainage techniques have been compared with percutaneous transhepatic biliary drainage (PTBD). Currently, we already have several comparative studies between the two techniques, at least three of them prospective and randomized. Firstly, Artifon et al. compared 13 patients treated with ESCP with 12 with PTBD, without finding significant differences in terms of technical success (100% vs. 100%, P > 0.05), clinical success (100% vs. 100%, P > 0.05) or adverse events (15.3% vs. 25%, P > 0.05).[36]
The second prospective randomized study compared 20 patients treated with ESCP with 21 with PTBD. There were also no differences in terms of technical success (95% vs. 85%, respectively, P > 0.05), but significant differences in terms of adverse events (35% vs. 60%, P < 0.05) were found, with 3 deaths related to these complications in each group.[37]
Finally, in a more recent study in which 34 patients were randomized to the ESCP group compared with 32 to the PTBD group, similar results were obtained. The technical success rate reached 94% versus 97% (P > 0.05), with a clinical success rate of 87% versus 87% (P > 0.05), and again a significant difference in the rate of adverse events in favor of the ESCP group, 9% versus 31%, respectively (P < 0.05).[38]
These three prospective and randomized studies, together with 7 other retrospective studies were included in a meta-analysis with a total of 483 patients.[39] No differences between both procedures were found in terms of technical success (OR, 1.78; 95% CI, 0.69–4.59; I2 Z 22%), but ESCP was associated with better clinical success (OR,.45; 95% CI, 0.23–0.89; I2 Z 0%), lower rate of adverse events (OR, 0.23; 95% CI, 0.12–0.47; I2 Z 57%), and less need for reintervention (OR, 0.13; 95% CI, 0.07–0.24; I2 Z 0%). No differences were found regarding hospital stay with a pooled standard mean difference of −0.48 (95% CI, −1.13 to 0.16), but ESCP was more cost-effective, with a pooled standard mean difference of −0.63 (95% CI, −1.06 to −0.20). The authors concluded that when ERCP fails to achieve biliary drainage, ESCP may be preferred over PTBD if adequate advanced endoscopy expertise and logistics are available.
According to the evidence obtained in these and other studies, the ESCP has been fully incorporated into the therapeutic algorithms recommended by different scientific societies ahead of PTBD.[40,41,42]
The good technical and clinical success rates of ESCP led to consider performing this technique even prior to ERCP in patients with distal malignant biliary obstruction. In a meta-analysis involving 10 studies with a total of 331 patients in the ESCP arm versus 425 in the ERCP arm, similar technical and clinical success rates were found for ESCP and ERCP (94.8% [294/310] and 93.8% [286/305] vs. 96.5% [386/400] and 95.7% [377/394], respectively), as well as the rate of adverse events (16.3% (54/331) vs. 18.3% (78/425), respectively).[43]
ESCP is a young technique still in evolution, with very few specifically designed devices available, while ERCP is an already standardized technique with indications and devices better defined. Therefore, in the coming years, as more specific material becomes available for ESCP, it is likely that ESCP definitively replace ERCP as the treatment of choice in specific clinical situations.
One of these situations has been well defined in a recent study in which 70 patients with distal biliary obstruction with ERCP failure were included.[44] EUS-guided choledochoduodenostomy was performed by releasing a lumen apposition metallic stent. The technical and clinical success rates were 98.6% and the adverse events rate was 1.6%, outcomes difficult to obtain using ERCP. A remarkable data of this study is that 6 out of the 10 participating endoscopists had little experience in ESCP (<20 EUS-guided biliary drainage procedures), but they achieved high technical success rates using the LAMS, especially when the common bile duct was >15 mm. In this specific situation the best drainage technique is likely to be EUS-guided choledochoduodenostomy even ahead of ERCP, although this must be confirmed in new, better-designed studies.
This would therefore be the final stage, in which EUS replaces ERCP by means of ESCP. In our opinin, these are complex techniques that must be performed only in tertiary hospitals by experienced personnel and with the support of interventional radiologists and biliopancreatic surgeons.
DISCUSSION
The relationship between EUS and ERCP has been collaborative from the beginning. This article proposes a categorization of this relationship based on the type of collaboration established between the two techniques and the complexity of the EUS procedures [Figure 1].
Figure 1.
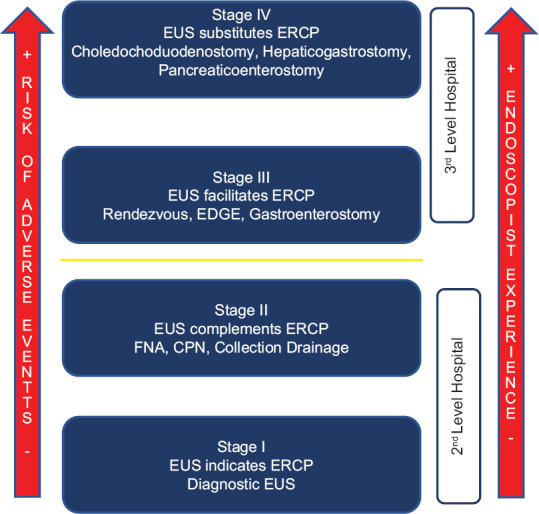
Categorization of EUS and endoscopic retrograde cholangiopancreatography collaborative stages according to the complexity of the procedure and the risk of adverse events
In the first stage, EUS only confirms the indication for ERCP. In the second stage, EUS complements ERCP by providing important clinical data or basic therapy. The role of EUS in these first two stages is basically diagnostic, and that is why it is considered that they are within the reach of second-level hospitals. CPN and drainage of pancreatic collections are included in the second stage, as they are therapeutic techniques that can be complementary to ERCP and are the simplest EUS-guided therapeutics.
The third stage involves a step forward in difficulty with techniques that help ERCP to achieve biliary or pancreatic drainage. This stage includes EUS-guided rendezvous, EDGE and EUS-guided gastrojejunostomy techniques. These procedures entail greater technical difficulty and involve a higher risk of complication, so in our opinion they should be limited to tertiary hospitals, with support of interventional radiology and specific surgery if needed.
Finally, the fourth stage includes those techniques in which EUS provides the ability to replace ERCP. Therefore, biliary or pancreatic drainage is achieved only by EUS and fluoroscopy guidance. These are the most complex techniques and entail the highest risk of adverse events.
As they involve correlative greater complexity and risk of adverse events, our proposal is that these stages should be ordinal. This means that to perform stage IV procedures, the endoscopist should have enough experience in stages I-III EUS procedures as well as in ERCP.
In our opinion, this classification could aid to plan better the teaching of these techniques, as well as to define which hospitals and endoscopists should be trained to perform techniques for each stage. Thus, in a hospital with the capacity to perform stage IV techniques, there could be stage I, II, III or IV endoscopists.
The teaching of techniques corresponding to all stages, and especially stages III and IV, is a subject of debate in the literature. It is estimated that 255 ERCP are necessary to achieve proficiency in routine biliary ERCP and 305 for more complex ERCP techniques.[45] Regarding EUS, 110 EUS-TA procedures were deemed necessary to achieve competence. To reach this number, at least 226 EUS procedures should have been performed. Oh et al. have described a plateau in the duration and adverse events rate for hepaticogastrostomy, included in stage IV, after the performance of 33 procedures.[46] For the EDGE technique, included in stage III, the requirements are lower, reaching a good level of efficiency after 9 procedures and a plateau lasting <40 min per procedure after 25–35 procedures.[47]
The level of technical complexity and the small number of patients candidates for stage IV procedures, makes the learning of these techniques very difficult, so learning models have been created that can be useful to shorten the learning curve.[48] That is another reason why performance of the techniques included in stages III and IV should be limited to a few number of hospitals and endoscopists.
The application of this classification could also help in the management of patients who are appropriate candidates to undergo EUS and ERCP, since many of them can be previously identified and can be referred to hospitals in which the stage necessary for their management has been reached. For instance, a jaundiced patient with Roux-en-Y reconstruction and nonsurgical malignancy will require a stage IV technique to achieve biliary drainage, so he should be transferred to a hospital with the capacity to perform stage IV procedures. Also, a patient with pancreatic head cancer and secondary jaundice will require ERCP and EUS-TA, that can be done in a single session performed in a second-level hospital without the need for a transfer.
Despite the described advantages, this classification also has several limitations. Firstly, it is a theoretical classification, and due to the low number of patients candidates for some of the EUS-guided techniques, it is not always possible to complete an adequate training in the techniques included in one stage to move to the procedures included in a higher stage. This is more evident with respect to the techniques included in stages III and IV. In this way, in clinical practice, an endoscopist may need to perform transmural biliary drainage without having much experience with rendezvous. Second, the technical difficulty of EDGE and EUS-guided gastrojejunostomy procedures, included in stage III, may seem even greater than that of a choledochoduodenostomy included in stage IV. However, the published articles describe better outcomes in terms of technical success and adverse effects rates than those described for biliary and pancreatic drainage. The aim and outcomes described for these procedures support their inclusion in stage III and not IV.
CONCLUSION
A categorization of the relationship between EUS and ERCP is proposed based on whether EUS indicates, complements, facilitates, or replaces ERCP. In our opinion, taking into account the complexity and outcomes of the EUS-guided and ERCP procedures, it may have implications to plan the endoscopists training, the hospital resources required and thus, patient management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We want to acknowledge Dr José Lariño from the Endoscopy Unit of Complejo Hospitalario Universitario de Santiago de Compostela for his corrections and suggestions for the draft.
REFERENCES
- 1.McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: A preliminary report. Ann Surg. 1968;167:752–6. doi: 10.1097/00000658-196805000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domagk D, Oppong KW, Aabakken L, et al. Performance measures for endoscopic retrograde cholangiopancreatography and endoscopic ultrasound: A European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. United European Gastroenterol J. 2018;6:1448–60. doi: 10.1177/2050640618808157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krutsri C, Kida M, Yamauchi H, et al. Current status of endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy. World J Gastroenterol. 2019;25:3313–33. doi: 10.3748/wjg.v25.i26.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiMagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet. 1980;1:629–31. doi: 10.1016/s0140-6736(80)91122-8. [DOI] [PubMed] [Google Scholar]
- 5.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 6.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 7.François E, Kahaleh M, Giovannini M, et al. EUS-guided pancreaticogastrostomy. Gastrointest Endosc. 2002;56:128–33. doi: 10.1067/mge.2002.125547. [DOI] [PubMed] [Google Scholar]
- 8.Burmester E, Niehaus J, Leineweber T, et al. EUS-cholangio-drainage of the bile duct: Report of 4 cases. Gastrointest Endosc. 2003;57:246–51. doi: 10.1067/mge.2003.85. [DOI] [PubMed] [Google Scholar]
- 9.Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100–7. doi: 10.1016/s0016-5107(03)02300-9. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Miranda M, de la Serna C, Diez-Redondo P, et al. Endosonography-guided cholangiopancreatography as a salvage drainage procedure for obstructed biliary and pancreatic ducts. World J Gastrointest Endosc. 2010;2:212–22. doi: 10.4253/wjge.v2.i6.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manes G, Paspatis G, Aabakken L, et al. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51:472–91. doi: 10.1055/a-0862-0346. [DOI] [PubMed] [Google Scholar]
- 12.Sonnenberg A, Enestvedt BK, Bakis G. Management of suspected choledocholithiasis: A decision analysis for choosing the optimal imaging modality. Dig Dis Sci. 2016;61:603–9. doi: 10.1007/s10620-015-3882-7. [DOI] [PubMed] [Google Scholar]
- 13.Meeralam Y, Al-Shammari K, Yaghoobi M. Diagnostic accuracy of EUS compared with MRCP in detecting choledocholithiasis: A meta-analysis of diagnostic test accuracy in head-to-head studies. Gastrointest Endosc. 2017;86:986–93. doi: 10.1016/j.gie.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Petrov MS, Savides TJ. Systematic review of endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis. Br J Surg. 2009;96:967–74. doi: 10.1002/bjs.6667. [DOI] [PubMed] [Google Scholar]
- 15.Vila JJ, Kutz M, Goñi S, et al. Endoscopic and anesthetic feasibility of EUS and ERCP combined in a single session versus two different sessions. World J Gastrointest Endosc. 2011;3:57. doi: 10.4253/wjge.v3.i3.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gornals JB, Moreno R, Castellote J, et al. Single-session endosonography and endoscopic retrograde cholangiopancreatography for biliopancreatic diseases is feasible, effective and cost beneficial. Dig Liver Dis. 2013;45:578–83. doi: 10.1016/j.dld.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Chu YL, Wang XF, Gao XZ, et al. Endoscopic ultrasonography in tandem with endoscopic retrograde cholangiopancreatography in the management of suspected distal obstructive jaundice. Eur J Gastroenterol Hepatol. 2013;25:455–9. doi: 10.1097/MEG.0b013e32835ca1d7. [DOI] [PubMed] [Google Scholar]
- 18.Kawakubo K, Kawakami H, Kuwatani M, et al. Safety and utility of single-session endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography for the evaluation of pancreatobiliary diseases. Gut Liver. 2014;8:329–32. doi: 10.5009/gnl.2014.8.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez-Munoz JE, Drewes AM, Lindkvist B, et al. Recommendations from the United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis. Pancreatology. 2018;18:847–54. doi: 10.1016/j.pan.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Khan MA, Hammad T, Khan Z, et al. Endoscopic versus percutaneous management for symptomatic pancreatic fluid collections: A systematic review and meta-analysis. Endosc Int Open. 2018;6:E474–3. doi: 10.1055/s-0044-102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varadarajulu S, Bang JY, Sutton BS, et al. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583–900. doi: 10.1053/j.gastro.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman M, Singh G, Das S, et al. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–34. doi: 10.1097/MCG.0b013e3181bb854d. [DOI] [PubMed] [Google Scholar]
- 23.Wyse JM, Carone M, Paquin SC, et al. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541–6. doi: 10.1200/JCO.2010.32.2750. [DOI] [PubMed] [Google Scholar]
- 24.Hindryckx P, Degroote H, Tate DJ, et al. Endoscopic ultrasound-guided drainage of the biliary system: Techniques, indications and future perspectives. World J Gastrointest Endosc. 2019;11:103–14. doi: 10.4253/wjge.v11.i2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulay BR, Lo SK. Endoscopic ultrasound–guided biliary drainage. Gastrointest Endosc Clin N Am. 2018;28:171–85. doi: 10.1016/j.giec.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Yamao K, Hara K, Mizuno N, et al. EUS-guided biliary drainage. Gut Liver. 2010;4:S67–75. doi: 10.5009/gnl.2010.4.S1.S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyberg A, Sharaiha RZ, Kedia P, et al. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: A multicenter international collaborative study. Gastrointest Endosc. 2017;85:164–9. doi: 10.1016/j.gie.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Vila J, Huertas C, Gonçalves B, et al. A novel method for endoscopic ultrasound-guided pancreatic rendezvous with a microcatheter. Endoscopy. 2015;47:E575–6. doi: 10.1055/s-0034-1393373. [DOI] [PubMed] [Google Scholar]
- 29.Oyón D, Carrascosa J, Elosua A, et al. Global impact of the use of microcatheter in EUS-guided pancreaticobiliary rendezvous in cases with inicial guidewire manipulation. Endoscopy. 2017;49:1129–94. doi:10.1055/s-0037-1607560. [Google Scholar]
- 30.Nakai Y, Isayama H, Matsubara S, et al. A novel “hitch-and-ride” deep biliary cannulation method during rendezvous endoscopic ultrasound-guided ERCP technique. Endoscopy. 2017;49:983–8. doi: 10.1055/s-0043-113444. [DOI] [PubMed] [Google Scholar]
- 31.Kedia P, Kumta NA, Widmer J, et al. Endoscopic ultrasound-directed transgastric ERCP (EDGE) for Roux-en-Y anatomy: A novel technique. Endoscopy. 2015;47:159–63. doi: 10.1055/s-0034-1390771. [DOI] [PubMed] [Google Scholar]
- 32.Kedia P, Tarnasky PR, Nieto J, et al. EUS-directed transgastric ERCP (EDGE) versus laparoscopy-assisted ERCP (LA-ERCP) for Roux-en-Y Gastric Bypass (RYGB) Anatomy: A multicenter early comparative experience of clinical outcomes. J Clin Gastroenterol. 2019;53:304–8. doi: 10.1097/MCG.0000000000001037. [DOI] [PubMed] [Google Scholar]
- 33.Martínez-Moreno B, Casellas JA, Aparicio Tormo JR. Endoscopic ultrasound-guided gastrojejunostomy-assisted ERCP in a Billroth II gastrectomy patient. Endoscopy. 2020;52:306–7. doi: 10.1055/a-1022-4453. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc. 2016;83:1218–27. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 35.Dhindsa BS, Mashiana HS, Dhaliwal A, et al. EUS-guided biliary drainage: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:101–9. doi: 10.4103/eus.eus_80_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artifon EL, Aparicio D, Paione JB, et al. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: Endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–74. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]
- 37.Giovannini M, Bories E, Napoleon B, et al. Multicenter randomized phase II study: Percutaneous biliary drainage vs EUS guided biliary drainage: Results of the intermediate analysis. Gastrointest Endosc. 2015;81:AB174. [Google Scholar]
- 38.Lee TH, Choi JH, Park DH, et al. Similar efficacies of endoscopic ultrasound–guided transmural and percutaneous drainage for malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2016;14:1011–9. doi: 10.1016/j.cgh.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 39.Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–14. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Liao WC, Angsuwatcharakon P, Isayama H, et al. International consensus recommendations for difficult biliary access. Gastrointest Endosc. 2017;85:295–304. doi: 10.1016/j.gie.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 41.Mukai S, Itoi T, Baron TH, et al. Indications and techniques of biliary drainage for acute cholangitis in updated Tokyo Guidelines 2018. J Hepatobiliary Pancreat Sci. 2017;24:537–49. doi: 10.1002/jhbp.496. [DOI] [PubMed] [Google Scholar]
- 42.Testoni PA, Mariani A, Aabakken L, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:657–83. doi: 10.1055/s-0042-108641. [DOI] [PubMed] [Google Scholar]
- 43.Han SY, Kim SO, So H. EUS-guided biliary drainage versus ERCP for first-line palliation of malignant distal biliary obstruction: A systematic review and meta-analysis. Sci Rep. 2019;9:16551. doi: 10.1038/s41598-019-52993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacques J, Privat J, Pinard F, et al. EUS-guided choledochoduodenostomy by use of electrocautery-enhanced lumen-apposing metal stents: A French multicenter study after implementation of the technique (with video) Gastrointest Endosc. 2020;92:134–41. doi: 10.1016/j.gie.2020.01.055. doi: 10.1016/j.gie.2020.01.055. [DOI] [PubMed] [Google Scholar]
- 45.Wani S, Han S, Simon V, et al. Setting minimum standards for training in EUS and ERCP: Results from a prospective multicenter study evaluating learning curves and competence among advanced endoscopy trainees. Gastrointest Endosc. 2019;89:1160–8. doi: 10.1016/j.gie.2019.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh D, Park DH, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017;10:42–53. doi: 10.1177/1756283X16671671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyberg A, Kedia P, Tawadros A, et al. EUS-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE): The first learning curve. J Clin Gastroenterol. 2020;54:569–72. doi: 10.1097/MCG.0000000000001326. [DOI] [PubMed] [Google Scholar]
- 48.Dhir V, Itoi T, Pausawasdi N, et al. Evaluation of a novel, hybrid model (Mumbai EUS II) for stepwise teaching and training in EUS-guided biliary drainage and rendezvous procedures. Endosc Int Open. 2017;5:E1087–E1095. doi: 10.1055/s-0043-118097. [DOI] [PMC free article] [PubMed] [Google Scholar]


