Abstract
Background and Objectives:
Balloon enteroscopy-assisted ERCP (BE-ERCP) has become the first-line therapy for biliopancreatic anastomotic strictures. However, it is not always successful, and salvage methods have not been established. This study aimed to evaluate the outcomes of EUS-guided transanastomotic drainage using a forward-viewing (FV) echoendoscope.
Patients and Methods:
Of eight cases wherein BE-ERCP treatment failed due to severe or complete benign anastomotic stricture, seven cases underwent EUS-guided choledochojejunostomy, and EUS-guided pancreaticojejunostomy was applied in one case after intubating an FV echoendoscope into the anastomotic site.
Results:
The success rate of reaching the target site was 100% (8/8) for patients after modified Child resection. The median time to reach the anastomosis was 5 min (range: 3–17 min), and the technical success rate for drainage was 75% (6/8). The median total procedure time was 33.5 min (range: 22–45 min) for six successful cases. Cautery dilatation catheters were necessary to dilate the puncture site in all cases, and no early complications were observed. During the follow-up period (median: 13.3 months [range: 6.5–60.3]), recurrence of the stricture occurred in one case, and a stent-free status was achieved after 6–12 months of stent placement in five cases.
Conclusions:
EUS-guided transanastomotic drainage using an FV echoendoscope is a feasible and safe rescue technique for the management of benign severe biliopancreatic anastomotic strictures.
Keywords: anastomotic stricture, benign stricture, EUS-guided transanastomotic drainage, surgically altered anatomy
INTRODUCTION
The management of biliopancreatic anastomotic strictures in patients with surgically altered anastomosis (SAA) is challenging. Recently, balloon enteroscopy-assisted ERCP (BE-ERCP) has become the first-line therapy in patients with SAA.[1,2,3] However, BE-ERCP is not always successful, and salvage methods have not been established. Recently, transgastric or transenteric EUS-guided interventions are widely performed because they are relatively less invasive and provide easy access to the target. However, it is desirable to select an original drainage route aiming for a stent-free status, especially in the case of benign anastomotic stricture. There are several rescue options, such as percutaneous transhepatic biliary drainage (PTBD) and sequential rendezvous technique; however, it is impossible to pass through a guidewire where severe anastomotic stricture exists. This study aimed to evaluate the feasibility and safety of EUS -guided transanastomotic drainage using a forward-viewing (FV) echoendoscope in patients with SAA.
PATIENTS AND METHODS
Patients
All consecutive patients who underwent BE-ERCP due to suspected anastomotic stricture between January 2008 and September 2019 were retrospectively retrieved from an endoscopic database of Kitasato University Hospital. Among 141 cases with suspected bilioenteric stricture, EUS-guided choledochojejunostomy (EUS-CJS) was applied in seven cases. Among six cases with suspected pancreatoenteric stricture, EUS-guided pancreaticojejunostomy (EUS -PJS) was applied in one case. All patients provided written informed consent before the procedure.
Procedures
A short-type single-balloon enteroscope (SIF-Q260, SIF-H290S; Olympus Medical Systems, Tokyo, Japan) was used for the initial BE-ERCP. In the event that the enteroscope could not reach the anastomotic site, PTBD or EUS-guided transgastric or transenteric drainage was considered an alternative method of drainage. If a 0.025” guidewire could pass through the anastomosis, a mechanical dilatation catheter, such as a tapered tip catheter or balloon dilatation catheter, was applied; additionally, a cautery dilatation catheter was applied when it was extremely difficult to pass through the stricture. In cases where it was impossible to pass through the anastomotic stricture with a guidewire, EUS-guided transenteric drainage from the anastomotic site was applied with a FV echoendoscope (TGF-U260J; Olympus Medical Systems). After intubating an FV echoendoscope to the anastomosis, detecting the dilated bile or pancreatic duct under echo imaging, it was punctured using a 19G needle (EZ shot3; Olympus Medical Systems) to place a guidewire into the duct. Subsequently, the puncture site was dilated with a cautery dilatation catheter (6-Fr Cysto-Gastro-Set; Endo-flex, Voerde, Germany, or Fine025; Medico's Hirata, Osaka, Japan), and a plastic or metallic stent was placed to maintain the fistula [Figures 1 and 2].
Figure 1.
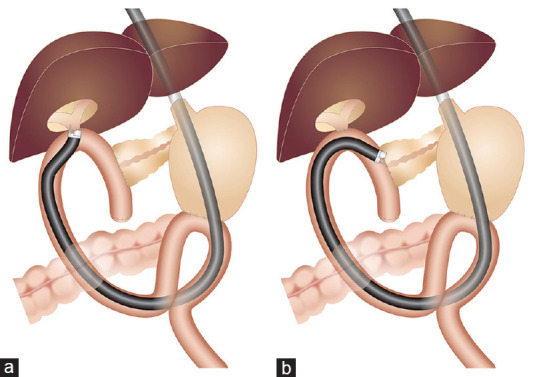
Schema of EUS-guided transanastomotic drainage using a forward-viewing echoendoscope in patients with modified Child resection and Braun anastomosis. (a) EUS -guided choledochojejunostomy for severe anastomotic stricture. (b) EUS-guided pancreaticojejunostomy for severe anastomotic stricture
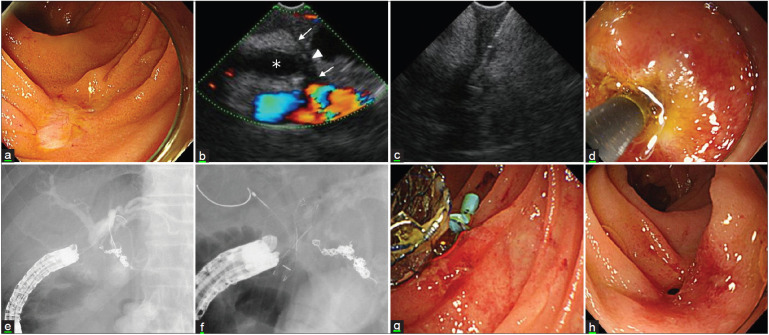
Figure 2A.
EUS-guided choledochojejunostomy for severe anastomotic stricture. (a) Endoscopic image shows a complete stricture of choledochojejunostomy. (b) EUS image shows jejunal muscle layer (arrow), scar (arrowhead), dilated bile duct (asterisk), and blood flow signals. (c) Puncture an anastomosis with a 19G needle. (d) Dilate puncture site with a cautery dilator. (e) Fluoroscopic image shows right and left intrahepatic bile ducts. (f) Deployed fully covered metallic stent and plastic stent in each bile duct to prevent obstruction. (g) The endoscopic image shows two stents placed at the anastomosis. (h) Endoscopic image shows recanalized anastomosis after stent removal
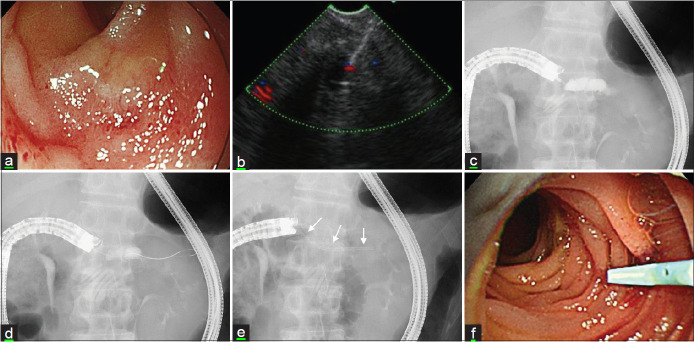
Figure 2B.
EUS-guided pancreaticojejunostomy for severe anastomotic stricture. (a) The endoscopic image illustrates the complete stricture of pancreaticojejunostomy. (b) EUS image shows a 19G needle passed into a dilated main pancreatic duct. (c) The fluoroscopic image shows the injection of the contrast medium into the main pancreatic duct. (d) Advancement of a guidewire into the main pancreatic duct. (e) Placement of a 7-Fr pancreatic stent (allow) after dilatation with a cautery dilator. (f) The endoscopic image depicts a plastic stent at the anastomosis
Outcome measurements
We evaluated the technical success and adverse events associated with EUS-guided transanastomotic drainage and the outcomes during follow-up.
RESULTS
The number of cases of suspected bilioenteric stricture was 141, and of these, the anastomotic site could not be reached in three cases. Of the remaining 138 patients in whom the anastomosis could be reached, no stricture was observed in 18 cases. Of the remaining 120 cases, the anastomosis was successfully treated in 112. The stricture was treated using a mechanical dilatation catheter in 85 cases, and a cautery dilatation catheter was needed in 13 cases. Malignant strictures were detected in 14 cases, and all were treated with placement of biliary stents. In the remaining eight cases, it was impossible to pass the guidewire through the anastomosis due to severe or complete stricture. One case underwent PTBD rendezvous, and EUS-CJS was applied in seven cases (5.0%) (7/141) [Figure 3a]. The number of cases with suspected pancreatoenteric stricture was six, and in all cases, it was possible to reach the anastomosis; additionally, treatment of the stricture was successfully performed using a mechanical dilatation catheter in one case, and no dilatation was needed in two cases. EUS-guided pancreatic duct rendezvous treatment was performed in two cases, and EUS-PJS was applied in one case (16.7%) (1/6) [Figure 3b].
Figure 3.
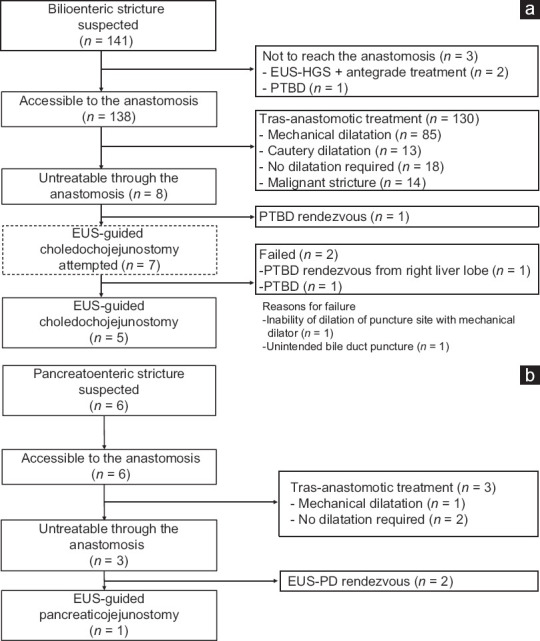
(a) Flowchart of treatment for patients suspected of having bilioenteric stricture. (b) Flowchart of treatment for patients suspected of having pancreatoenteric stricture. EUS-HGS: EUSguided hepaticojejunostomy; PTBD: Percutaneous transhepatic biliary drainage; EUS-PD: EUS-guided pancreatic duct drainage
In total, eight EUS-guided transanastomotic drainage procedures were performed. These patients comprised six men and two women, with a median age of 69 years (range: 51–78 years). All cases had undergone pylorus-preserving pancreaticoduodenectomy with modified Child reconstruction, and the median period between the surgery and EUS-guided treatment was 11.7 months (range: 4.4–61.8 months). The original disease leading to surgery was pancreatic carcinoma in six cases, pancreatic neuroendocrine neoplasm in one case, and adenoma of the papilla of Vater in one case [Table 1]. In total, eight EUS-guided transanastomotic drainage procedures were applied. The success rate for reaching the target site in the afferent limb with an FV echoendoscope was 100% (8/8). The median time to reach the anastomosis was 5 min (range: 3–17 min), and the technical success rate was 75% (6/8). The reasons for the unsuccessful procedures were failure to dilate the puncture site with a mechanical dilator in one case and unintended bile duct puncture in another case. The median procedure time was 33.5 min (range: 22–45 min) for the six successful cases. A cautery dilatation catheter was necessary in all cases; a plastic stent (PS) was placed in four cases, and a covered self-expandable metallic stent (cSEMS) was placed in two cases. No early (≤30 days after procedure) complications were observed. During the follow-up period (median: 13.3 months [range: 6.5–60.3]), recurrence occurred in one case (16.7%; 1/6), and a cSEMS was placed to maintain the fistula. A stent-free status was achieved after 6–12 months of stent placement in the remaining five cases [Tables 2 and 3].
Table 1.
Patients characteristics
| Sex, male/female | 6/2 |
| Median age (years) (range) | 69 (51-78) |
| Type of reconstruction n (%) | |
| Modified Child reconstruction with Braun anastomosis | 8 (100) |
| Reasons for surgery n (%) | |
| Pancreatic carcinoma | 6 (75) |
| Pancreatic neuroendocrine neoplasm | 1 (12.5) |
| Adenoma of the papilla Vater | 1 (12.5) |
| Indication for treatment n (%) | |
| Cholangitis due to choledochojejunostomy stricture | 7 (87.5) |
| Pancreatitis due to pancreaticojejunostomy stricture | 1 (12.5) |
| Period after surgery (months) (range) | 11.7 (4.4-61.8) |
PPPD: Pylorus-preserving pancreaticoduodenectomy
Table 2.
Outcomes and complications
| Success rate reaching anastomosis, % | 100 (8/8) |
| Median time reaching anastomosis, min (range) | 5 (3-17) |
| Technical success rate, % | 75 (6/8) |
| Clinical success rate, % * | 100 (6/6) |
| Median total procedure time, min * | 33.5 (22-45) |
| Complications * | |
| Early complications (≤30 days), % | 0 (0) |
| Late complications (>30 days), % | |
| Recurrence of stricture | 16.7 (1/6) |
| Follow up period, months (range) * | 13.3 (6.5-60.3) |
*Except for two unsuccessful cases
Table 3.
Characteristics and outcomes in individual cases
| Case | Sex/age | Disease | Type of reconstruction | Period after surgery (month) | Type of stricture | Drainage method | Time to reach anastomosis (min) | Total procedure time (min) | Dilatation device | First indwelling stent | Current State | Complication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/74 | PC | Child with Braun | 13.9 | SBBS | EUS-CJS | 17 | 45 | 6Fr cautery dilator | 10mm cSEMS | Stent free | No |
| 2 | M/51 | PC | Child with Braun | 37 | SBBS | EUS-CJS | 3 | 43 | 6Fr cautery dilator | 7Fr PS | Stent free | No |
| 3 | M/78 | PC | Child with Braun | 5.9 | SBBS | EUS-CJS | 5 | 24 | 6Fr cautery dilator | 8.5Fr PS | cSEMS is in place after recurrence | Recurrence |
| 4 | M/47 | Adenoma of the PV | Child with Braun | 5.4 | SBBS | EUS-CJS | 3 | 23 | Tapered tip cautery dilator | 7Fr PS | Stent free | No |
| 5 | M/78 | NEN | Child with Braun | 61.8 | SBPS | EUS-PJS | 6 | 22 | 6Fr cautery dilator | 7Fr PS | Stent free | No |
| 6 | F/70 | PC | Child with Braun | 9.4 | SBBS | EUS-CJS | 5 | 45 | 6Fr cautery dilator | 10mm cSEMS | Stent free | No |
PC: Pancreatic carcinoma; NEN: Neuroendocrine neoplasm; PV: Papillae Vater; EUS-CJS: EUS-guided choledochojejunostomy; EUS-PJS: EUS-guided pancreaticojejunostomy; SBBS: Severe benign biliary stricture; SBPS: Severe benign pancreatic duct stricture; PS: Plastic stent; cSMES: Covered self expandable metallic stent
DISCUSSION
Symptomatic biliopancreatic anastomotic stricture is one of the most problematic adverse events in patients with SAA. The incidence of anastomotic stricture after hepaticojejunostomy reportedly ranges between 4% and 11.9%.[4] Recently, BE-ERCP has made it possible to approach the surgically reconstructed intestine, and strictures can be treated endoscopically in a less invasive way.[1,2,3] Simultaneously, EUS-guided drainage is becoming established as feasible biliary and pancreatic drainage procedures. They are less invasive than PTBD and are less time-consuming than BE-ERCP treatment, respectively. Khashab et al.[5] reported the outcomes of EUS-guided biliary drainage (EUS-BD) and BE-ERCP in 98 patients with SAA; it was demonstrated that EUS-BD could be performed with a high success rate (88% vs. 59.1%, P = 0.03) within a short period (55 min vs. 95 min, P < 0.0001); however, adverse events occurred more commonly in the EUS-BD group (20% vs. 4%, P = 0.01). Chen et al.[6] reported the results of a retrospective study on EUS-guided pancreatic duct drainage (EUS-PD) and BE-ERCP in 66 patients who underwent Whipple surgery; the procedural success rate was revealed to be far superior in the EUS-PD group (92.5% vs. 20%, P < 0.001); however, adverse events occurred more commonly in the EUS-PD group (35% vs. 2.9%, P < 0.001). Although EUS-guided transgastric or transenteric treatment has a higher success rate, data from high-volume centers revealed many complications associated with this procedure that should be resolved in the future.
When limited to benign anastomotic strictures, treatment at the stricture site is desirable to achieve long-term patency. As indicated by the results, the balloon enteroscope reached the anastomotic site in 97.9% (138/141) of cases, and transanastomotic treatment was successful in 94.2% (130/138) of bilioenteric stricture cases [Figure 3]. On the contrary, it was successful in only 50% (3/6) of pancreatoenteric stricture cases [Figure 1]. The low success rate for pancreatoenteric stricture with BE-ERCP could be explained by the difficulty in identifying the pinhole of the anastomosis and performing coaxial cannulation using a tangential approach.
Difficult cases with severe scars caused by inflammation, ischemia, and neoadjuvant radiation chemotherapy are occasionally encountered. In these cases, the stricture is too tight to pass with a mechanical dilatation catheter, although a guidewire could pass. As previously reported,[7] a wire-guided diathermic dilator is effective to overcome such situations. Percutaneous transhepatic drainage and sequential percutaneous transhepatic cholangioscopy-assisted procedures[8] as well as EUS-guided hepaticoenterostomy for antegrade therapy[9] have been reported as alternative therapies. However, the aforementioned techniques are ineffective for complete strictures. There are some case reports of EUS-guided transanastomotic drainage using an oblique-viewing (OV) echoendoscope;[10,11] however, intubating the OV echoendoscope in the afferent limb is extremely challenging, and the risk of perforation is high.
Lately, single case reports involving EUS-guided transanastomotic drainage with FV echoendoscopy have been reported by previous studies (including a case from our institution).[12,13,14,15,16,17] The appropriate indication for applying FV echoendoscopy is benign severe or complete anastomotic stricture after Whipple or Child resection. The significant advantages of FV echoendoscopy are as follows: the ease of manipulation throughout the gastrointestinal tract due to FV, the shorter length of the hard tip, and the wider angulation range compared to OV echoendoscopy. EUS-guided transanastomotic drainage with FV echoendoscopy has the following advantages over other options: (1) scope insertion up to the anastomosis can be feasible and safe, unlike OV echoendoscope; (2) the puncture point can be safely determined under echo guidance; (3) no bile or pancreatic juice leakage occurs because of a procedure through the scar area, leading to fewer complications; and (4) the fistula can be a permanent physiological drainage route after balloon dilatation or stent removal.
In this study, the FV echoendoscope could reach the anastomosis in a short time in all attempted cases. The reasons for the failure of treatment in two cases were as follows: in one case, the procedure was abandoned because only the unintended peripheral bile duct could be imaged after contrast. In another case, we could not pass through a puncture site because a cautery dilatation catheter could not be used due to nearby major vessels. In both cases, preoperative radiochemotherapy was performed; therefore, severe fibrosis may have caused poor echo view in the first case and the difficulty in performing mechanical dilatation in the second case. One of the six cases experienced a recurrence of stricture 11 months after balloon dilatation treatment. The remaining five cases became stent free after 12 months of PS placement or 6 months of nonflared cSEMS placement. Lately, maintaining the placement of nonflared cSEMS for 6 months has been reported safe and effective for refractory benign choledochojejunal anastomotic strictures[18] and equally for establishing a permanent fistula between the common bile duct and the duodenum.[19] Therefore, nonflared cSEMS is currently being placed for 6 months to maintain anastomosis, as in those cases. Notably, no treatment-related complications were observed, probably due to the absence of bile or pancreatic juice leakage.
The limitations of this study include the single-center setting, small sample size, noncomparative design, short follow-up period, and the limited availability of the echoendoscope. However, this technique showed promising results for the severe anastomotic stricture site.
CONCLUSION
EUS-guided transanastomotic drainage using an FV echoendoscope is a feasible, effective, and safe rescue technique for the management of severe biliopancreatic anastomotic strictures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Yamauchi H, Kida M, Imaizumi H, et al. Innovations and techniques for balloon-enteroscope-assisted endoscopic retrograde cholangiopancreaticography in patients with altered gastrointestinal anatomy. World J Gastroenterol. 2015;21:6460–9. doi: 10.3748/wjg.v21.i21.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwai T, Kida M, Yamauchi H, et al. Short-type and conventional single-balloon enteroscopes for endoscopic retrograde cholangiopancreaticography in patients with surgically altered anatomy: Single-center experience. Dig Endosc. 2014;26:156–63. doi: 10.1111/den.12258. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi H, Kida M, Miyata E, et al. Endoscopic balloon dilation for benign bilioenteric stricture: Outcomes and factors affecting recurrence. Dig Dis Sci. 2019;64:3557–67. doi: 10.1007/s10620-019-05811-3. [DOI] [PubMed] [Google Scholar]
- 4.Dimou FM, Adhikari D, Mehta HB, et al. Incidence of hepaticojejunostomy stricture after hepaticojejunostomy. Surgery. 2016;160:691–8. doi: 10.1016/j.surg.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khashab MA, El Zein MH, Sharzehi K, et al. EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: an international comparative study. Endosc Int Open. 2016;4:E1322–7. doi: 10.1055/s-0042-110790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YI, Levy MJ, Moreels TG, et al. An international multicenter study comparing EUS-guided pancreatic duct drainage with enteroscopy-assisted endoscopic retrograde pancreaticography after Whipple surgery. Gastrointest Endosc. 2017;85:170–7. doi: 10.1016/j.gie.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Miyata E, Yamauchi H, Kida M, et al. Successful endoscopic dilation of severe bilioenteric strictures with a wire-guided diathermic dilator and short-type single-balloon enteroscope. Endoscopy. 2015;47:E94–5. doi: 10.1055/s-0034-1391240. [DOI] [PubMed] [Google Scholar]
- 8.Shimatani M, Takaoka M, Ikeura T, et al. Rendezvous technique: double-balloon endoscopy and SpyGlass direct visualization system in a patient with severe stenosis of a choledochojejunal anastomosis. Endoscopy. 2014;46:E275–6. doi: 10.1055/s-0034-1365785. [DOI] [PubMed] [Google Scholar]
- 9.James TW, Fan YC, Baron TH. EUS-guided hepaticoenterostomy as a portal to allow definitive antegrade treatment of benign biliary diseases in patients with surgically altered anatomy. Gastrointest Endosc. 2018;88:547–54. doi: 10.1016/j.gie.2018.04.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuyama M, Aoyama H, Kyoden Y. Endoscopic ultrasound-guided choledochojejunostomy: Novel method to treat a severely stenotic choledochojejunal anastomosis. Dig Endosc. 2016;28:221. doi: 10.1111/den.12572. [DOI] [PubMed] [Google Scholar]
- 11.Itoi T, Ikeuchi N, Tonozuka R, et al. EUS-guided choledochojejunostomy with a lumen-apposing metal stent in a post-Whipple patient. Gastrointest Endosc. 2015;81:1259–60. doi: 10.1016/j.gie.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Kida M, Yamauchi H, Okuwaki K, et al. Endoscopic ultrasound-guided choledochojejunostomy with a forward-viewing echoendoscope for severe benign bilioenteric stricture in a patient with Child's resection. Endoscopy. 2015;47:E303–4. doi: 10.1055/s-0034-1392208. [DOI] [PubMed] [Google Scholar]
- 13.Nakaji S, Hirata N, Shiratori T, et al. Endoscopic ultrasound-guided pancreaticojejunostomy with a forward-viewing echoendoscope as a treatment for stenotic pancreaticojejunal anastomosis. Endoscopy. 2015;47:E41–2. doi: 10.1055/s-0034-1391245. [DOI] [PubMed] [Google Scholar]
- 14.Hodo Y, Shirota Y, Suda T, et al. Successful EUS-guided retrograde pancreatic duct stent placement for refractory pancreaticojejunostomy stricture after pancreaticoduodenectomy with a forward-viewing echoendoscope. Video GIE. 2018;3:196–8. doi: 10.1016/j.vgie.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishiwatari H, Sato J, Kaneko J. Hepaticojejunostomy for the right hepatic bile duct using a forward-viewing echoendoscope in a patient after pancreaticoduodenectomy. Dig Endosc. 2019;31:e82–3. doi: 10.1111/den.13390. [DOI] [PubMed] [Google Scholar]
- 16.Kin T, Hayashi T, Katanuma A. Endoscopic ultrasound-guided fistulation between bile duct and afferent limb for treatment of complete choledochojejunal obstruction using forward-viewing echoendoscope. Dig Endosc. 2019;31:e97–8. doi: 10.1111/den.13460. [DOI] [PubMed] [Google Scholar]
- 17.Kuwatani M, Kato S, Sakamoto N. Direct recanalization of pancreaticogastrostomy obstruction with a forward-viewing echoendoscope. Dig Endosc. 2019;31:e18–9. doi: 10.1111/den.13284. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi H, Tadehara M, Kida M. Temporary non-flared fully covered self-expandable metal stent placement for refractory benign choledochojejunal anastomotic stricture. Dig Endosc. 2018;30:541–2. doi: 10.1111/den.13069. [DOI] [PubMed] [Google Scholar]
- 19.Iwai T, Kida M, Yamauchi H, et al. Long-lasting patent fistula after EUS-guided choledochoduodenostomy in a patient with refractory benign biliary stricture. Video GIE. 2018;3:193–5. doi: 10.1016/j.vgie.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]