Abstract
Background
Cholangiocarcinoma (CCA) is a malignancy that originates from bile duct cells. The incidence and mortality of CCA are very high especially in Southeast Asian countries. Moreover, most CCA patients have a very poor outcome. Presently, there are still no effective treatment regimens for CCA. The resistance to several standard chemotherapy drugs occurs frequently; thus, searching for a novel effective treatment for CCA is urgently needed.
Methods
In this study, comprehensive bioinformatics analyses for identification of novel target genes for CCA therapy based on three microarray gene expression profiles (GSE26566, GSE32225 and GSE76297) from the Gene Expression Omnibus (GEO) database were performed. Based on differentially expressed genes (DEGs), gene ontology and pathway enrichment analyses were performed. Protein-protein interactions (PPI) and hub gene identifications were analyzed using STRING and Cytoscape software. Then, the expression of candidate genes from bioinformatics analysis was measured in CCA cell lines using real time PCR. Finally, the anti-tumor activity of specific inhibitor against candidate genes were investigated in CCA cell lines cultured under 2-dimensional and 3-dimensional cell culture models.
Results
The three microarray datasets exhibited an intersection consisting of 226 DEGs (124 up-regulated and 102 down-regulated genes) in CCA. DEGs were significantly enriched in cell cycle, hemostasis and metabolism pathways according to Reactome pathway analysis. In addition, 20 potential hub genes in CCA were identified using the protein-protein interaction (PPI) network and sub-PPI network analysis. Subsequently, CDC20 was identified as a potential novel targeted drug for CCA based on a drug prioritizing program. In addition, the anti-tumor activity of a potential CDC20 inhibitor, namely dinaciclib, was investigated in CCA cell lines. Dinaciclib demonstrated huge anti-tumor activity better than gemcitabine, the standard chemotherapeutic drug for CCA.
Conclusion
Using integrated bioinformatics analysis, CDC20 was identified as a novel candidate therapeutic target for CCA.
Keywords: Bioinformatic analysis, Cholangiocarcinoma, GEO, TCGA, Spheroid, In silico, CDC20, Dinaciclib
Introduction
Nowadays, bioinformatics approaches have been widely used for analysis and management of data from several sources. The Gene Expression Omnibus (GEO) database and The Cancer Genomics Atlas (TCGA) offer tools for screening and discovery of novel molecular pathways, promising biomarkers, genetic alterations, prognosis and novel effective target molecules for several cancers. By this approach, several potential key genes, biomarkers and pathways involved with gastric cancer (Yan et al., 2018); lung adenocarcinoma (Guo, Ma & Zhou, 2019) and myelodysplastic syndrome (Le, 2019) have been identified.
Cholangiocarcinoma is a fatal cancer arising from malignant transformation of cholangiocytes. CCA represents 3% of gastrointestinal cancers worldwide. The incidence and mortality of CCA, however, are very high in the Eastern world, especially in Southeast Asian countries (Banales et al., 2016). The prognosis of CCA patients is very poor, with a very low 5-year survival (Ramírez-Merino, 2013). The gold standard treatment for CCA is the surgery with R0 resection. Unfortunately, approximately 3% of CCA patients could be offered with R0 resections (Luvira et al., 2016; Adeva et al., 2019). Most CCA patients usually come with an advanced stage, in which the tumor has already spread to the secondary sites or other organs (Blechacz, 2017). Currently, many chemotherapy drugs have been used and reported for CCA treatment such as gemcitabine, 5-Fluorouracil (5-FU), cisplatin, sorafenib, capecitabine plus cisplatin and oxaliplatin/cetuximab (Keating & Santoro, 2009; Adeva et al., 2019; Chen et al., 2015; Ben-Josef et al., 2015). The widely used one is gemcitabine with an approximate survival time of 21.5 months (Murakami et al., 2009). 5-FU also provided a poor overall response rate with 0–40% and a median survival about 2–12 months (Thongprasert, 2005). Resistance to this wide range of chemotherapeutic drugs and high damage to adjacent tissues often occurs in CCA patients. Thus, searching for novel target molecules or alternative approaches for an effective treatment for CCA is urgently needed. Using the bioinformatics approaches may accelerate the discovery of the potential molecules that might be a new target for CCA treatment.
In this study, we performed an in-silico analysis for screening and identification of potent novel targets for CCA treatments using three microarray datasets retrieved from the GEO database. The differential gene expression analysis was performed for the identification of differentially expressed genes (DEGs) between CCA and normal tissues. Then, Gene Ontology (GO) and Reactome pathways were analyzed. The protein-protein interaction network (PPI) and sub-PPI network analyses of common DEGs were constructed to identify the key genes associated with CCA. Subsequently, prediction and prioritizing of candidate novel anti-cancer drugs was performed. Finally, the cytotoxicity of the candidate drug target on viability of CCA cell lines in 2- and 3-dimensional (2D, 3D) cell culture models were validated to prove the concept.
Materials & Methods
Cell culture
Human CCA cell lines derived from CCA patient tissues that included KKU-213A (poorly differentiated squamous cell carcinoma), KKU-213B (well differentiated squamous carcinoma) (Sripa et al., 2020), KKU-100 (poorly differentiated adenocarcinoma) (Sripa et al., 2005), and KKU-452 (poorly differentiated adenocarcinoma) (Saensa-ard et al., 2017) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)(Gibco life technologies Corporation, Grand Island, NY) with 25 mM glucose supplemented with 1% antimycotic-antibiotic and 10% heat-inactivated fetal bovine serum at 37 °C, 5% CO2.
For the 3D cell culture, the adherent CCA cells were trypsinized and seeded into a 96 well ultra-low attachment plates (Corning, Incorporated, Kennebunk, Maine) at the optimal starting cell numbers. CCA cells were then cultured at 37 °C, 5% CO2 for 24–72 h for spheroid formation. Spheroid morphology was observed under an inverted light microscope. Spheroid integrity were analyzed by ImageJ NIH software (https://imagej.nih.gov/ij/download.html).
Microarray data sources
Three gene expression datasets of CCA and normal tissues were retrieved from the GEO database (https://www.ncbi.nlm.nih.gov/geo/) including GSE26566, GSE32225 and GSE76297. GSE26566 based on GPL6104 Illumina Humanref-8 v2.0 expression bead chip platforms, GSE32225 based on GPL8432 Illumina Humanref-8 WG-DASL v3.0 platforms and GSE76297 based on GPL17586 [HTA-2_0] Affymetrix Human Transcriptome Array 2.0 [transcript (gene) version].
Differentially expressed gene (DEGs) analysis
The DEGs between cancer and normal tissues were analyzed using the GEO2R online tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/). Genes that met the cutoff criteria, adjusted P-value <0.05 and —logFC—≥ 0.5, were considered DEGs. A volcano plot was performed for data visualization using RStudio (https://rstudio.com/). Moreover, Venn diagrams were plotted (http://bioinformatics.psb.ugent.be/webtools/Venn/) for elucidating common DEGs among three microarray datasets.
Gene ontology, pathway enrichment, protein-protein interaction (PPI) and hub gene identification analysis
The common DEGs were inputted into STRING software version 11.0 (https://string-db.org/) for analyzing the interactions among various proteins to constitute a PPI network, gene ontology and pathway enrichment. Gene functions were classified into cellular components (CC), cellular processes (CP), molecular function (MF) and the Reactome pathway. GO results were visualized as a bubble plot by RStudio software. Screening and identification of hub genes were conducted with cytohubba (https://apps.cytoscape.org/apps/cytohubba) (Chin et al., 2014) of the Cytoscape (https://cytoscape.org/) plug-in MCC model.
Prediction and prioritizing of novel candidate anti-cancer drugs
After hub gene identifications, the next step was further prioritizing and predicting a novel anti-cancer drug based on up-regulated hub genes using PanDrugs software (https://www.pandrugs.org) (Piñeiro Yáñez et al., 2018). Prioritization criteria for the selection of a novel anti-tumor drug was that the candidate drug had to have been used in clinical trials or is already approved for clinical use.
RNA extraction and real-time PCR
Cells were collected and total RNA samples were extracted from CCA cell lines using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Van Allen Way, California.) according to the manufacturer’s protocol. RNA (2 µg) was reverse transcribed to cDNA. The system conditions for real-time PCR contained (10 µl): 5 µl for 2X SYBR Green I Master LightCycler®480 SYBR green I master (Roche Diagnostics GmbH, Mannheim, Germany), 2 µl of cDNA (25 ng/µl), 2 µl of forward and reverse primers and 1 µl DD H2O PCR grade. The primers for CDC20 sequences were as follows: forward, 5′-CGGGTAGCAGAACACCATGT-3′reverse, 5′-ACTGGCCAAATGTCGTCCAT-3′. The PCR conditions were 95° for 5 min, followed by 50 cycles at 95° for 10 s and 62° for 10 s.
Western blot analysis
Cells were lysed with RIPA buffer (Thermo Fisher Scientific, Rockford, Illinois) containing Proteinase Inhibitor Cocktail (NACALAI TESQUE, INC., Kyoto, JAPAN). The lysates were shaken at 4 °C overnight. Protein content was determined using BCA assay kit (Thermo Fisher Scientific, Rockford, Illinois). Protein lysates were separated in a 12% (for actin) or 15% (for CDC20) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on a polyvinylidene fluoride or polyvinylidene difluoride (PVDF) membrane (Merck KGaA, Darmstadt, Germany) using Towbin transfer buffer in a wet tank transfer blotting system (Bio-Rad Laboratories (Shanghai) Co., Ltd., Shanghai, China). After blocking with 5% skimmed milk for 1 h at room temperature (RT), membranes were incubated with each primary antibody, CDC20 (Cell Signaling Technology, Tokyo, Japan), or actin (Santa Cruz Biotechnology, Inc., Dallas, Texas) at 4 °C, overnight. Blots were then incubated with anti-rabbit IgG, HRP-linked Antibody (Cell Signaling Technology, Tokyo, Japan) or anti-mouse IgG, HRP-linked Antibody (Cell Signaling), at RT for 1 h. The western blot bands were detected using an ImageQuant LAS 4000 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden).
The cytotoxicity test in two-dimensional (2D) and three-dimensional (3D) cell culture models
For the 2D cell culture model, CCA cell lines were seeded into 96-well plates (1,500 cells/well) overnight (16 h) and then treated with various concentrations of gemcitabine (Sigma-Aldrich, St. Louis, Missouri) or dinaciclib (Abcam plc., Cambridge, UK) for 72 h. After incubation, cell viability was measured by ATPlite 1step (PerkinElmer, inc, Waltham, Massachusetts) for luminescence detection of ATP from cultured cells under the 2D model using SpectraMax L Microplate Reader (Molecular Devices, St, San Jose, California).
For the 3D cell culture model, 2,500 cells/well of CCA cell lines were seeded into 96 well ultra-low attachment flat-bottom plates (Corning, Incorporated, Kennebunk, Maine), and incubated for 24 to 78 h depending on cell type. The formed spheroids were then exposed to gemcitabine or dinaciclib for 72 h. Cell viability was measured by the ATPlite3D kit (PerkinElmer, inc, Waltham, Massachusetts) using SpectraMax L Microplate Reader. Cells cultured in DMSO; 0.01% for 2D-culture and 0.4% for 3-D culture, were used as a vehicle control in all drug testing experiments. Three separated experiments with triplicate assays were performed.
Statistical analysis
Statistical analyses were conducted using Graphpad Prism software version 7.03 (GraphPad Software, Inc., San Diego, California). All experiments were performed in three independent experiments. The cytotoxicity results are expressed as mean ± SD. Statistical significance was determined by Students’ t test and P < 0.05 was noted as statistical significance.
Results
Identification of common DEGs
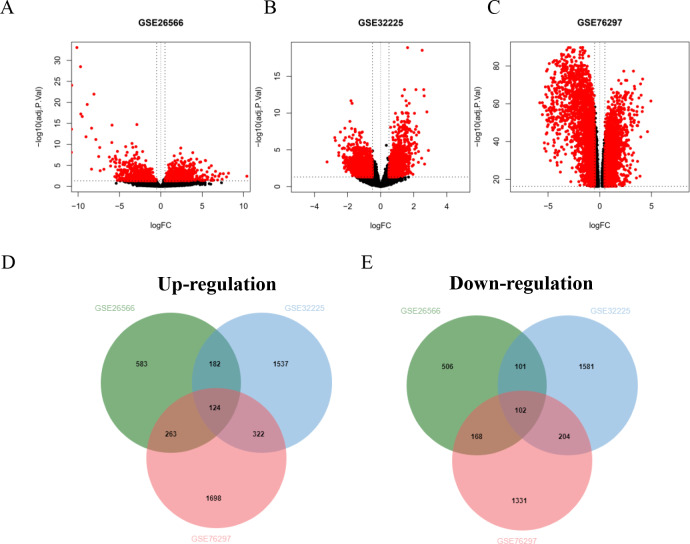
Four GSE datasets of CCA were retrieved form GEO including GSE26566, GSE32225, GSE76297 and GSE89749. Of these, GSE89749 which comprised of a small number of normal cases and showed the abnormality of gene distribution was excluded from the study. The GSE26566 contained 104 cancer and 59 normal cases, while GSE32225 comprised of 149 cancer and 6 normal cases. The CCA cases that formed both datasets were non-Opisthorchis viverrini (OV)-associated CCA. GSE76297 datasets consisted of 91 OV-associated CCA and 92 surrounding liver tissues. The volcano plot elucidated DEGs of CCA and normal tissues (Figs. 1A–1C). Based on the selection criteria of an adjusted P value <0.05 and absolute log fold-changes ≥ 0.5, a total of 2,029, 4,153 and 4,212 DEGs were identified from GSE26566, GSE32225 and GSE76297. 1,152 and 877 genes were up- and down-regulated in the GSE26566 dataset. 2,165 and 1,988 genes were up- and down-regulated in GSE32225. 2,407 upregulated and 1,805 down-regulated genes were identified in GSE76297 dataset. Finally, Venn diagram analysis revealed that there are 226 common DEGs in the three microarray datasets, in which 124 were up-regulated and 102 down-regulated genes (Figs. 1D and 1E).
Figure 1. GEO dataset of differentially expressed genes (DEGs) from cholangiocarcinoma (CCA) patients.
(A–C) Volcano plot of DEGs in each GEO dataset. Red represents the genes that were significantly up- or down-regulated in CCA samples. Black dots represent the genes that were not significantly up- or down- regulated in CCA samples. The dotted vertical lines indicate the significant threshold filters. Venn diagrams illustrate the number of common (D) up- and (E) down- regulated DEGs shared by the three GEO datasets.
Gene ontology and pathway enrichment analysis
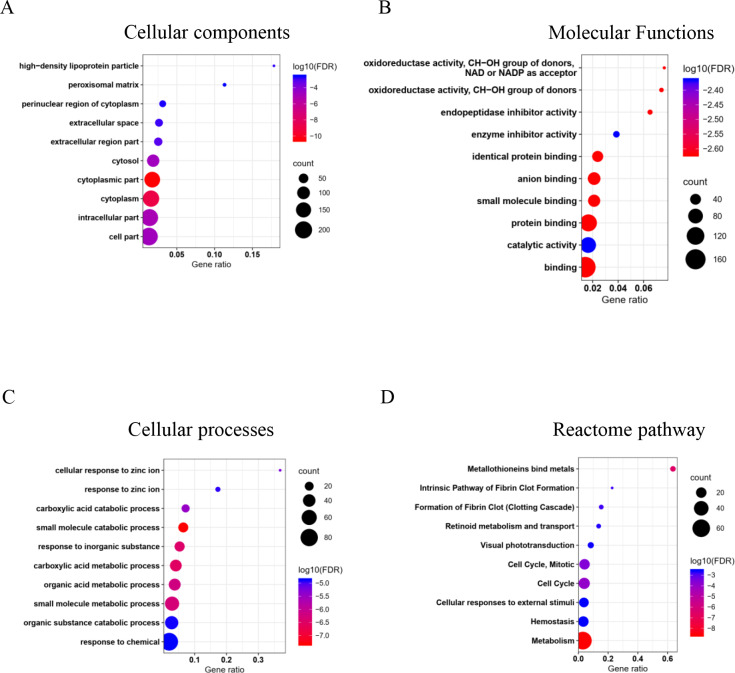
The GO and pathway enrichment analysis of common DEGs elucidated in cellular component (CC) terms (Fig. 2A), common DEGs were mainly enriched in cytoplasm, extracellular space, high-density lipoprotein particles and in the peroxisomal matrix. The common DEGs were also involved with several molecular functions (MF) (Fig. 2B) such as oxidoreductase activity, endopeptidase inhibitor activity and enzyme inhibitor activity. In cellular process terms (Fig. 2C), the results showed that common DEGs were significantly enriched in carboxylic acid catabolic processes, whereas small molecule metabolic processes, were responsive to inorganic substances and organic acid metabolic processes. Moreover, the Reactome pathway enrichment analysis indicated that common DEGs were mainly enriched in metabolism, metallothionein bound metals and cell cycles, especially mitosis (Fig. 2D).
Figure 2. Top 10 gene ontology (GO) and reactome pathway enrichment analyses of 226 common DEGs identified from three GEO datasets.
The common DEGs are categorized by GO and pathway enrichment analysis into (A) cellular components, (B) molecular function, (C) cellular processes, and (D) reactome pathways. The x-axis demonstrates gene ratio. The pseudo color from red to blue represents the false discovery rate, from the lowest to the highest, respectively. The circle sizes indicate the gene count.
PPI of common DEGs and hub gene identifications
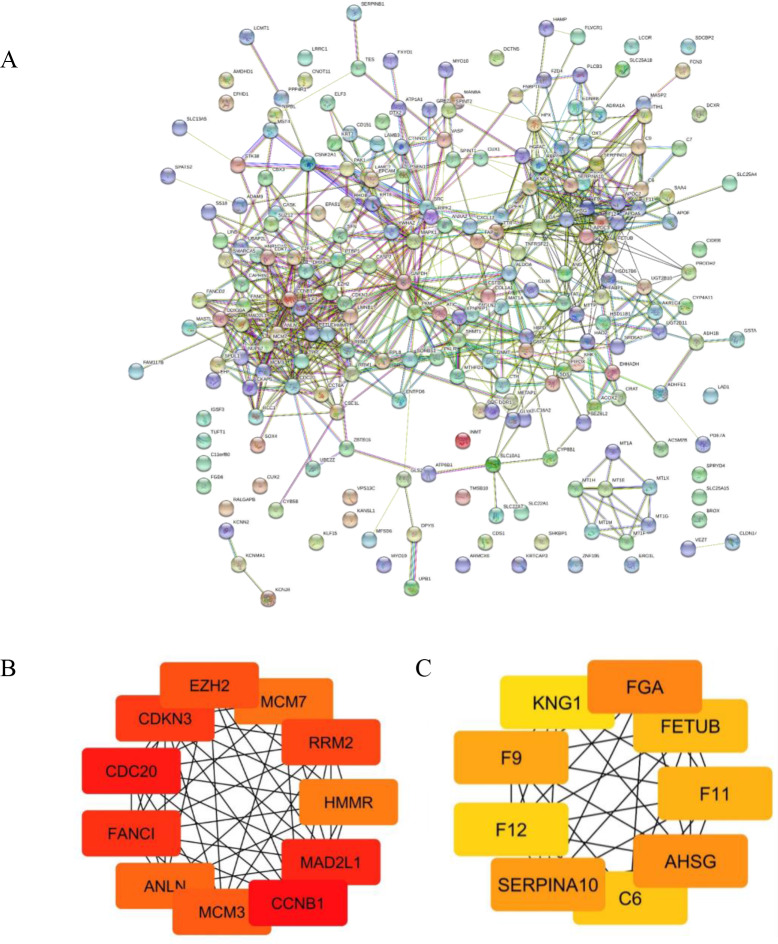
The PPIs of 226 common DEGs were constructed using STRING software as shown in (Fig. 3A). The hub genes based on the PPI network were further prioritized (Figs. 3B and 3C). The results categorized the top 20 hub genes automatically into two groups. The up-regulated hub genes from common DEGs, including CCNB1, CDC20, MAD2L1, FANCI, CDKN3, RRM2, EZH2, MCM3, ANLN, MCM7 and HMMR were ranked from the degree of connectivity with other proteins (binding scores) (Table 1). Down-regulated hub genes included FGA, AHSG, SERPINA10, F9, F11, FETUB, C6, F12 and KNG1. Node colors are represented for the degree of connectivity. The pseudocolor scale from red to yellow represents the gene ranking from 1 to 20. The dark red color represents the highest degree while an orange color stands for the intermediate degree and yellow color is the lowest degree. Most of the up-regulated hub genes were involved with cell division and cell cycle control, nucleotide metabolism and cell movement.
Figure 3. Protein-protein interaction (PPI) network of common DEGs and Module analysis.
(A) STRING protein-protein interaction network of 226 common DEGs identified from three GEO datasets. (B–C) Subnetwork of top 20 hub genes from protein-protein interaction network using Cytoscape software. Node color reflects degree of connectivity. The pseudocolor scale from red to yellow represents the top nine hub genes rank from 1–20. Red color represents highest degree, and orange color represents intermedia degree, and yellow color represents lowest degree.
Table 1. Functional roles of top 20 hub genes in CCA based on transcriptomic data.
| Rank | Name | Hub score | Expression | Function |
|---|---|---|---|---|
| 1 | CCNB1 | 138571 | Up | The protein encoded by this gene is a regulatory protein involved in mitosis. |
| 2 | CDC20 | 138182 | Up | CDC20 appears to act as a regulatory protein interacting with several other proteins at multiple points in the cell cycle. |
| 3 | MAD2L1 | 134648 | Up | MAD2L1 is a component of the mitotic spindle assembly checkpoint that prevents the onset of anaphase until all chromosomes are properly aligned at the metaphase plate. |
| 4 | FANCI | 131160 | Up | Fanconi anemia is a genetically heterogeneous recessive disorder characterized by cytogenetic instability, hypersensitivity to DNA crosslinking agents, increased chromosomal breakage, and defective DNA repair. |
| 5 | CDKN3 | 130518 | Up | The gene was identified as a cyclin-dependent kinase inhibitor, and has been shown to interact with, and dephosphorylate CDK2 kinase, thus prevent the activation of CDK2 kinase. |
| 6 | RRM2 | 129534 | Up | This gene encodes one of two non-identical subunits for ribonucleotide reductase. |
| 7 | EZH2 | 123630 | Up | This gene encodes a member of the Polycomb-group involved in maintaining the transcriptional repressive state of genes over successive cell generations. |
| 8 | MCM3 | 89514 | Up | Involved in the initiation of eukaryotic genome replication. |
| 9 | ANLN | 87121 | Up | This gene encodes an actin-binding protein that plays a role in cell growth and migration, and in cytokinesis. |
| 10 | MCM7 | 49994 | Up | The protein encoded by this gene is one of the highly conserved mini-chromosome maintenance proteins (MCM) that are essential for the initiation of eukaryotic genome replication. |
| 11 | HMMR | 46968 | Up | The protein encoded by this gene is involved in cell motility. |
| 12 | FGA | 25564 | Down | This gene encodes the alpha subunit of the coagulation factor fibrinogen. |
| 13 | AHSG | 25254 | Down | It is involved in several processes, including endocytosis, brain development, and the formation of bone tissue. Defects in this gene are a cause of susceptibility to leanness. |
| 14 | SERPINA10 | 23448 | Down | It inhibits the activity of coagulation factors Xa and XIa in the presence of protein Z, calcium and phospholipid. |
| 15 | F9 | 23304 | Down | This gene encodes vitamin K-dependent coagulation factor IX that circulates in the blood as an inactive zymogen. |
| 16 | F11 | 21072 | Down | This gene encodes coagulation factor XI of the blood coagulation cascade. |
| 17 | FETUB | 17683 | Down | The protein encoded by this gene is a member of the fetuin family, part of the cystatin superfamily of cysteine protease inhibitors. |
| 18 | C6 | 15364 | Down | This gene encodes a component of the complement cascade. |
| 19 | F12 | 11646 | Down | This gene encodes coagulation factor XII which circulates in blood as a zymogen. |
| 20 | KNG1 | 6769 | Down | This gene uses alternative splicing to generate two different proteins- high molecular weight kininogen (HMWK) and low molecular weight kininogen (LMWK). HMWK is essential for blood coagulation and assembly of the kallikrein-kinin system. |
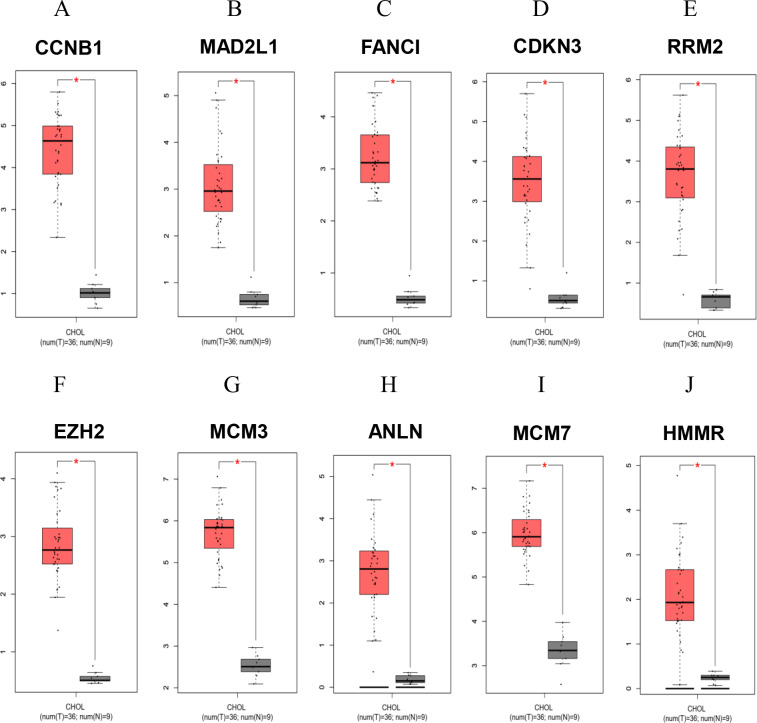
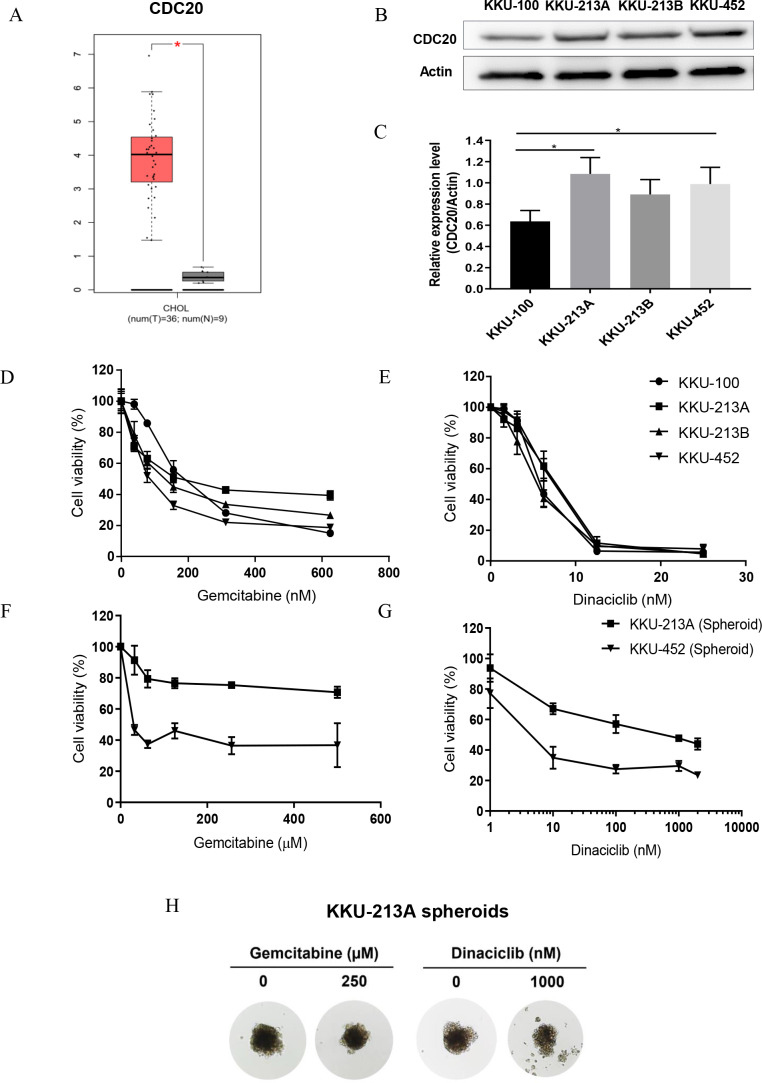
To confirm the reliability of the analyzed results, the expression levels of all up-regulated hub genes using different datasets and different databases were further examined. RNA sequencing profiles were retrieved from The Cancer Genome Atlas (TCGA, https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga). Box plots were plotted for the elucidation expression level of the hub genes in patient CCA tissues compared with the normal tissues (Figs. 4 and 5A). The results elucidated strong evidence to support the analysis that all the identified hub genes were highly expressed in CCA when compared with normal controls even though the analysis was performed on different databases and different platforms.
Figure 4. Significantly expressed 10 genes in CCA and normal bile duct epithelium tissues.
Box plots analyses compared the expression levels of the specified genes in patient CCA tissues (red) and normal adjacent tissues (grey). The data were analyzed from The Cancer Genome Atlas (TCGA) databases using GEPIA (Gene Expression Profiling Interactive Analysis), a web-based tool. *P < 0.05.
Figure 5. Anti-tumor activities of dinaciclib and gemcitabine in CCA cell lines.
(A) The expression levels of CDC20 mRNA in patient CCA tissues (red) and normal counterparts (grey) retrieved from TCGA. (B) Representative western blots of CDC20 in four CCA cell lines and (C) the semi-quantitative analysis. Anti-tumor activities of dinaciclib and gemcitabine in CCA cell lines were determined in (D–E) 2D and (F–G) 3D cell culture model. (H) The representative multicellular tumor spheroids of KKU-213A after 72 h incubation with or without the indicated drugs. *P < 0.05.
Prioritizing and prediction of a novel targeted drug for CCA
Novel candidate drugs targeted for CCA were based on 11 up-regulated hub genes using PanDrugs, a bioinformatics platform, to prioritize anticancer drug treatments. PanDrugs not only provided drug status descriptions but it also predicted the possible drug response and the interaction between drugs. The drug prediction and prioritizing in Table 2 shows several potential novel drugs for 6 of the 11 upregulated hub genes (Fig. 3B). CDC20 was the candidate that fit to the selection criteria (Fig. S1) as CDC20 plays significant roles in cell cycle and mitosis, and was the high-scoring hub gene (Fig. 3B). There are several candidate drugs suggested for CDC20 (Table 2). Dinaciclib, however, was selected to be the drug of choice for CDC20 in this study as it has never been reported for CCA, and the safety and efficacy has been reported in the clinical trial phase III (Ghia et al., 2015; Sharp & Corp, 2017).
Table 2. The potentially druggable targets and prioritized drugs identified for CCA treatment using PanDrugs software.
| Gene(s) | Show Drug Name | Status Description | Therapy | Drug response | Best Interaction |
|---|---|---|---|---|---|
| RRM2 | CLADRIBINE | Approved for blood cancer | CHEMOTHERAPY | SENSITIVITY | direct-target |
| RRM2 | CLOFARABINE | Approved for blood cancer | CHEMOTHERAPY | SENSITIVITY | biomarker |
| RRM2 | HYDROXYUREA | Approved for blood cancer | CHEMOTHERAPY | SENSITIVITY | biomarker |
| EZH2 | DABRAFENIB | Approved for skin cancer | TARGETED_THERAPY | SENSITIVITY | biomarker |
| RRM2 | FLUDARABINE | Approved for blood cancer | CHEMOTHERAPY | SENSITIVITY | biomarker |
| RRM2 | FLUDARABINE PHOSPHATE | Approved for blood cancer | CHEMOTHERAPY | SENSITIVITY | biomarker |
| CDC20—CDKN3 | AT7519 | Clinical Trials | – | SENSITIVITY | pathway-member |
| RRM2 | MOTEXAFIN GADOLINIUM | Clinical Trials | – | SENSITIVITY | direct-target |
| RRM2 | IMEXON | Clinical Trials | – | SENSITIVITY | direct-target |
| CDC20 | DINACICLIB | Clinical Trials | – | SENSITIVITY | pathway-member |
| CDC20 | FLAVOPIRIDOL | Clinical Trials | – | SENSITIVITY | pathway-member |
| CDC20 | BAY1000394 | Clinical Trials | – | SENSITIVITY | pathway-member |
| CCNB1—EZH2 | SELUMETINIB | Clinical Trials | – | BOTH? | biomarker |
| EZH2 | 1032350 − 13 − 2 | Clinical Trials | – | SENSITIVITY | biomarker |
| RRM2 | TRIAPINE | Clinical Trials | – | SENSITIVITY | biomarker |
| CDKN3 | 602306 − 29 − 6 | Clinical Trials | – | SENSITIVITY | biomarker |
| CDKN3 | PHA-793887 | Clinical Trials | – | SENSITIVITY | biomarker |
| CDKN3 | RONICICLIB | Clinical Trials | – | SENSITIVITY | biomarker |
| HMMR | HYALURONIC ACID | Clinical Trials | – | SENSITIVITY | direct-target |
| CDC20 | ALSTERPAULLONE | Experimental | – | SENSITIVITY | pathway-member |
| CDC20 | HYMENIALDISINE | Experimental | – | SENSITIVITY | pathway-member |
| CDC20 | INDIRUBIN-3′-MONOXIME | Experimental | – | SENSITIVITY | pathway-member |
| CDC20 | OLOMOUCINE | Experimental | – | SENSITIVITY | pathway-member |
| CDC20 | SU9516 | Experimental | – | SENSITIVITY | pathway-member |
| EZH2 | A-395 | Experimental | – | SENSITIVITY | biomarker |
| EZH2 | GSK126 | Experimental | – | SENSITIVITY | biomarker |
| RRM2 | TEZACITABINE | Experimental | – | SENSITIVITY | biomarker |
| FANCI | LY2183240 | Experimental | – | SENSITIVITY | biomarker |
| FANCI | SB 225002 | Experimental | – | SENSITIVITY | biomarker |
CDC20 is a candidate novel target for CCA
After the in-silico analysis, a series of experiments for investigating the antitumor activity of dinaciclib in CCA cell lines under 2D and 3D cell culture models was performed. CDC20 mRNA and protein were investigated in four CCA cell lines, KKU-100, KKU-213A, KKU-213B and KKU-452, using real-time PCR and western blot analysis. Expression of CDC20 in patient CCA tissues was significantly higher than those of normal counterpart (Fig. 5A), and all CCA cell lines differentially expressed CDC20 mRNA (Fig. S2 and File S1) and protein (Figs. 5B and 5C). The anti-tumor activity of dinaciclib in CCA cell lines was compared with that of gemcitabine, the standard chemotherapeutic agent for CCA treatment. In the 2D cell culture model (Figs. 5D and 5E), dinaciclib elucidated very effective anti-tumor activity against CCA cell lines at approximately 40 times of the 50% inhibitory concentrations (IC50) lower than gemcitabine (Table 3). In the 3D cell culture model (Figs. 5F and 5G), KKU213A and KKU-452 spheroids were treated with various concentrations of dinaciclib or gemcitabine for 72 h. The results demonstrated that dinaciclib had highly effective antitumor activity better than a thousand times gemcitabine in both CCA cell lines (Table 3). The IC50 of KKU-213A and KKU-452 spheroids to dinaciclib were 514.8 nM and 4.7 nM, whereas the IC50 of gemcitabine against KKU-213A spheroid cannot be calculated because it was higher than 500 ×103 nM, the maximum tested drug concentration. A similar trend was observed for KKU-452 spheroids with gemcitabine IC50 is 25.78 ×103 nM. The KKU-213 spheroid morphology at 72 h exposure time with both drugs and vehicle were demonstrated in Fig. 5H. It was then concluded that the CCA cells were more sensitive to dinaciclib than gemcitabine.
Table 3. Comparison the anti-tumor activity of dinaciclib and gemcitabine in CCA cell lines cultured under 2D and 3D cell culture models.
| 2D cell culture models | IC50 (nM) | |
|---|---|---|
| CCA cell lines | Gemcitabine | Dinaciclib |
| KKU-100 | 193 ± 16 | 5.82 ± 0.42 |
| KKU-213A | 241 ± 13 | 7.37 ± 0.37 |
| KKU-213B | 146 ± 30 | 5.43 ± 0.42 |
| KKU-452 | 87 ± 3 | 6.93 ± 0.38 |
| 3D cell culture models | IC50 (nM) | |
| CCA cell lines | Gemcitabine | Dinaciclib |
| KKU-213A | >500 ×103 | 514.8 ± 139 |
| KKU-452 | 25.78 ± 8.36 ×103 | 4.70 ± 1.20 |
Discussion
The identification of the molecular mechanisms of cancer cells is crucial for diagnosis and therapy of cancer patients. Various high throughput screening (HTS) techniques, cDNA microarray and RNA sequencing, are widely used to explore DEGs involved in carcinogenesis and progression which has provided valuable information for clinical applications (Lowe et al., 2017). A huge amount of corresponding data from a cDNA microarray and RNA sequencing is stored in several public databases such as ENCODE (https://www.encodeproject.org/), TCGA, ICGC (https://dcc.icgc.org/) and GEO. Integration of multiple HTS datasets (cDNA microarray and RNA sequencing) is considered a better approach of enhancing the reliability of results than an individual HTS dataset (Yan et al., 2018; Le, 2019; Huang et al., 2019).
In the present study, an in-silico analysis using several bioinformatics approaches for screening and identification novel molecular targets for CCA treatment was performed using three microarray datasets of patient CCA tissues. The analyses revealed several novel targets for CCA treatment. Of these, CDC20, a regulatory protein involves in multi-cell cycle checkpoints was identified as a novel target molecule for CCA treatment and dinaciclib, a pan CDK inhibitor, was suggested as a compatible drug for CDC20. The suggestion from the in-silico analyses was proved by the in vitro cytotoxic experiments of dinaciclib in comparison with Gemcitabine in human CCA cell lines.
Three microarray datasets from CCA patients including GSE26566, GSE32225 and GSE76297 were retrieved from the GEO database and used in this study. From GEO2R and Venn diagram analysis, a total of 226 common DEGs including 124 up-regulated and 102 down-regulated genes in CCA tissues were revealed. GO and pathway enrichment analysis revealed that common DEGs were enriched in metabolism, and cell cycles especially in the mitosis phase. As is well known, one of the hallmarks of cancers is the alteration of metabolism in which cancer cells predominantly use the glycolytic pathway as the main energy source rather than tricarboxylic acid cycle (TCA) even in an adequate oxygen condition, known as “Aerobic glycolysis” or the “Warburg effect” (Warburg, 1956; Schwartz, Supuran & Alfarouk, 2017). The understanding of the cancer metabolism also provides an opportunity for development specific novel targets for cancer diagnosis and treatment. At present, several alterations of glycolytic related molecules have been reported in CCA such as glucose transporters (GLUT) hexokinase (HK) II (Paudyal et al., 2008; Thamrongwaranggoon et al., 2017), and Tumor M2-pyruvate kinase (PKM2) (Cuenco et al., 2018). Moreover, the alteration in expression levels of other molecules such, c-MET, RAS/BRAF, EGFR/ERBB2 which are involved downstream, affect cancer cell proliferation and development. (Terada, Nakanuma & Sirica, 1998; Miyamoto et al., 2011; Churi et al., 2014; O’Dell et al., 2012; Leone et al., 2006; Ito et al., 2001).
The PIP network and module analysis in the currently study demonstrated the top 20 high-scoring hub genes in CCA. Several of these identified hub genes, for instance, CCNB1, CDC20, MAD2L1, were involved in mitosis and cell cycle control (Table 1) (Percy et al., 2000; Chi et al., 2019; Zhang et al., 2019; Wang et al., 2015). The computational analysis for prioritizing and predicting of novel targeted drugs based on the 11 up-regulated hub genes by PanDrugs suggested several new targets and drug of choices. Among these, CDC20 is the most interested target as: firstly, it was upregulated in patient CCA tissues compared with the normal counterpart tissues (Fig. 5A). Secondly, it plays an important role in chromosome segregation (Kapanidou, Curtis & Bolanos-Garcia, 2017) during metaphase-anaphase transition (Fujimitsu, Grimaldi & Yamano, 2016; Wang et al., 2015) and also other cellular processes, for example suppressing apoptosis (Harley et al., 2010; Wan et al., 2014). Thirdly, expression of CDC20 have been demonstrated to be involved with poor patient outcomes in several human malignancies. For instance, the high expression of the CDC20 gene were associated with poor prognosis and outcome of breast cancer patients (Karra et al., 2014). Overexpression of CDC20 in tumor tissues was related with poor differentiation and a lower 5-year recurrence-free survival rate of pancreatic cancer patients (Li et al., 2003; Chang et al., 2012), and a short survival of colorectal cancer (Wu et al., 2013). Moreover, knockdown of CDC20 gene inhibited cell growth and induced the G2/M arrest in cell cycle of lung cancer cells (Kidokoro et al., 2008). Lastly, several agents affected CDC20 as the pathway-member are approved for clinical trials or under experiments (Table 2).
Dinaciclib treatment effectively suppressed tumor growth in CCA cell lines cultured under 2D and 3D cell culture models (Figs. 5D–5G). In similar in-vitro studies, dinaciclib elucidated antitumor activity against cultured cells in 2D models at low nanomolar levels such as, 12, 8, 17 and 14 nM IC50 for prostate, breast, colon, and ovarian cancer cell lines (Parry et al., 2010). Interestingly, the tumor-suppressing effect of dinaciclib was better than gemcitabine, the current standard treatment drugs for CCA. The 4 CCA cell lines tested showed different response to gemcitabine as indicated by a wide IC50 range of 87–241 nM, whereas all cell lines showed a similar sensitivity to dinaciclib with a narrow IC50 range of 5.4–6.9 nM. Cancers seem to be more sensitive to dinaciclib than gemcitabine as the similar observations were also reported in several pancreatic cancer cell lines (Khan et al., 2020) and hepatocellular carcinoma (Shao et al., 2019; Hassan et al., 2019). The different response of cancer cells to gemcitabine and dinaciclib may be due to the different drug targets and actions. Gemcitabine targets the DNA synthesis and induces cell death, whereas dinaciclib acts on several CDKs in cell cycle and hence targets several points of the cell cycle at the same time. Moreover, as the 4 CCA cell lines were established from different subtypes of CCA, using dinaciclib as the treatment of choice for CCA may give more advantage than gemcitabine in such the way that dinaciclib exhibited a high anti-tumor activity independently of CCA subtypes.
Dinaciclib is the novel generation of the potent multi-CDKs inhibitor which can suppress CDK1, CDK2, CDK5 and CDK9 (Parry et al., 2010) with the IC50 in a low nanomolar range (1–4 nM). The effective anti-tumor activity of Dinaciclib have been elucidated both in vitro and in vivo in various types of cancers (Rello-Varona et al., 2019; Chen et al., 2016). Enhancing G2/M phase arrest and induction of apoptosis by dinaciclib have been demonstrated in the in vitro and xenografted mouse model in thyroid cancer (Lin et al., 2017) and the triple negative breast cancer (Rajput et al., 2016). In addition, dinaciclib was shown to trigger abnormal mitotic division (anaphase catastrophe) in lung cancer cells through, CDK1 and CDK2 suppression (Danilov et al., 2016). Moreover, dinaciclib has been used in clinical trials of several cancers, such as chronic lymphocytic leukemia (Flynn et al., 2015; Gojo et al., 2013) and breast cancer (Mita et al., 2014). The tolerant side effects such as transient gastrointestinal toxicities, liver function tests abnormalities, fatigue, and hypotension have been reported (Gojo et al., 2013).
A similar but different approach has been performed using a dataset from GEO (GSE26566) to find the potential candidate treatment agents for CCA without validation (Chujan et al., 2018). Recently, Ye et al. (2020) has identified the key genes associated with the progression of intrahepatic CCA using three GEO dataset including GSE107943, GSE119336 and GSE26566. The present study provided several target candidates different from those reported in the previous studies. This may be due to the different objectives, different workflow of the analysis and different GEO dataset used. Combining various bioinformatics methods using several gene expression datasets may reduce the bias and strengthen the analysis outcome. The experimental evidence in vitro and in vivo must be conducted to confirm the analysis outcome before translating to the clinical practice.
Taken together, the current study provided several novel draggable-target molecules for CCA treatment from computational analysis. The reliability of in-silico results for biological investigation was confirmed in CCA cell lines. The compatible results between computational and biological investigations demonstrated very interesting and strong evidence that all identified hub genes, especially CDC20 was highly expressed in CCA tissues and may be used as an effective novel target for CCA treatment.
Conclusions
In the present study, the aim was to identify a novel molecular target for CCA treatment using bioinformatics analysis based on three transcriptomic datasets available in the GEO database. The identified hub genes were mostly involved with metabolism, mitosis and cell cycle control which indicated that, CCA was highly effective in these processes. CDC20 was suggested by bioinformatic analysis as a potential novel target for CCA. Not only using in-silico analysis, the expression levels of CDC20 in CCA cell lines as well as the investigation of the anti-tumor activity of novel potential targeted drugs against CCA cells were compared with the standard chemotherapy, gemcitabine. This novel drug has a stronger antitumor activity than gemcitabine.
Supplemental Information
Funding Statement
This research was supported by the Program Management Unit for Human Resources & Institutional Development, Research and Innovation [630000050061-4] and Mekong Health Science Research Institute (MeHSRI), Khon Kaen University for Wunchana Seubwai. P. Sungwan and W. Seubwai are scholars of Research Fund for Supporting Lecturer to Admit High Potential Student to Study and Research on His Expert Program (601H109) from Graduate School, Khon Kaen University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Prin Sungwan and Wunchana Seubwai conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Worachart Lert-itthiporn analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Atit Silsirivanit and Sopit Wongkham conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Nathakan Klinhom-on performed the experiments, prepared figures and/or tables, and approved the final draft.
Seiji Okada conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Raw measurements are available in the Supplemental Files.
References
- Adeva et al. (2019).Adeva J, Sangro B, Salati M, Edeline J, La Casta A, Bittoni A, Berardi R, Bruix J, Valle JW. Medical treatment for cholangiocarcinoma. Liver International. 2019;39:123–142. doi: 10.1111/liv.14100. [DOI] [PubMed] [Google Scholar]
- Banales et al. (2016).Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RIR, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJG, Alvaro D. Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nature Reviews Gastroenterology & Hepatology. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- Ben-Josef et al. (2015).Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, Thomas CR, Alberts SR, Dawson LA, Micetich KC, Thomas MB, Siegel AB, Blanke CD. SWOG S0809: A Phase II Intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. Journal of Clinical Oncology. 2015;33:2617–2622. doi: 10.1200/JCO.2014.60.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechacz (2017).Blechacz B. Cholangiocarcinoma: current knowledge and new developments. Gut and Liver. 2017;11:13–26. doi: 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang et al. (2012).Chang DZ, Ma Y, Ji B, Liu Y, Hwu P, Abbruzzese JL, Logsdon C, Wang H. Increased CDC20 expression is associated with pancreatic ductal adenocarcinoma differentiation and progression. Journal of Hematology & Oncology. 2012;5 doi: 10.1186/1756-8722-5-15. Article 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2015).Chen JS, Hsu C, Chiang NJ, Tsai CS, Tsou HH, Huang SF, Bai LY, Chang IC, Shiah HS, Ho CL, Yen CJ, Lee KD, Chiu CF, Rau KM, Yu MS, Yang Y, Hsieh RK, Chang JY, Shan YS, Chao Y, Chen LT, Shen W-C, Hsu H-C, Hsu C-H, Shen Y-C, Wang T-E, Li C-P, Chen M-H, Kao W-Y, Chang P-Y, Wu C-C, Teng C-L, Lu C-H, Lin S-J, Wang B-W, Chen Y-Y, Chin Y-H, Chung T-R, Yu W-L, Lee M-H, Lin L-F, Lin P-C, Wu Y-L, Wang H-L, Lu L-J, Chen S-Y, Wu C-C, Wei T-C. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Annals of Oncology. 2015;26:943–949. doi: 10.1093/annonc/mdv035. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2016).Chen Z, Wang Z, Pang JC, Yu Y, Bieerkehazhi S, Lu J, Hu T, Zhao Y, Xu X, Zhang H, Yi JS, Liu S, Yang J. Multiple CDK inhibitor dinaciclib suppresses neuroblastoma growth via inhibiting CDK2 and CDK9 activity. Scientific Reports. 2016;6:29090. doi: 10.1038/srep29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi et al. (2019).Chi J (Jack), Li H, Zhou Z, Izquierdo-Ferrer J, Xue Y, Wavelet CM, Schiltz GE, Zhang B, Cristofanilli M, Lu X, Bahar I, Wan Y. A novel strategy to block mitotic progression for targeted therapy. EBioMedicine. 2019;49:40–54. doi: 10.1016/j.ebiom.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin et al. (2014).Chin C-H, Chen S-H, Wu H-H, Ho C-W, Ko M-T, Lin C-Y. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Systems Biology. 2014;8:S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chujan et al. (2018).Chujan S, Suriyo T, Ungtrakul T, Pomyen Y, Satayavivad J. Potential candidate treatment agents for targeting of cholangiocarcinoma identified by gene expression profile analysis. Biomedical Reports. 2018;9:42–52. doi: 10.3892/br.2018.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churi et al. (2014).Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, Zuo M, Zinner R, Hong D, Meric-Bernstam F, Janku F, Crane CH, Mishra L, Vauthey J-N, Wolff RA, Mills G, Javle M. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLOS ONE. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenco et al. (2018).Cuenco J, Wehnert N, Blyuss O, Kazarian A, Whitwell HJ, Menon U, Dawnay A, Manns MP, Pereira SP, Timms JF. Identification of a serum biomarker panel for the differential diagnosis of cholangiocarcinoma and primary sclerosing cholangitis. Oncotarget. 2018;9:17430–17442. doi: 10.18632/oncotarget.24732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov et al. (2016).Danilov AV, Hu S, Orr B, Godek K, Mustachio LM, Sekula D, Liu X, Kawakami M, Johnson FM, Compton DA, Freemantle SJ, Dmitrovsky E. Dinaciclib induces anaphase catastrophe in lung cancer cells via inhibition of cyclin dependent kinases 1 and 2. Molecular Cancer Therapeutics. 2016;15:2758–2766. doi: 10.1158/1535-7163.MCT-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn et al. (2015).Flynn J, Jones J, Johnson AJ, Andritsos L, Maddocks K, Jaglowski S, Hessler J, Grever MR, Im E, Zhou H, Zhu Y, Zhang D, Small K, Bannerji R, Byrd JC. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia. 2015;29:1524–1529. doi: 10.1038/leu.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimitsu, Grimaldi & Yamano (2016).Fujimitsu K, Grimaldi M, Yamano H. Cyclin-dependent kinase 1-dependent activation of APC/C ubiquitin ligase. Science. 2016;352:1121–1124. doi: 10.1126/science.aad3925. [DOI] [PubMed] [Google Scholar]
- Ghia et al. (2015).Ghia P, Scarfo L, Pathiraja K, Derosier M, Small K, Patton N. A phase 3 study to evaluate the efficacy and safety of dinaciclib compared to ofatumumab in patients with refractory chronic lymphocytic leukemia. Blood. 2015;126:4171–4171. doi: 10.1182/blood.V126.23.4171.4171. [DOI] [PubMed] [Google Scholar]
- Gojo et al. (2013).Gojo I, Sadowska M, Walker A, Feldman EJ, Iyer SP, Baer MR, Sausville EA, Lapidus RG, Zhang D, Zhu Y, Jou Y-M, Poon J, Small K, Bannerji R. Clinical and laboratory studies of the novel cyclin-dependent kinase inhibitor dinaciclib (SCH 727965) in acute leukemias. Cancer Chemotherapy and Pharmacology. 2013;72:897–908. doi: 10.1007/s00280-013-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Ma & Zhou (2019).Guo T, Ma H, Zhou Y. Bioinformatics analysis of microarray data to identify the candidate biomarkers of lung adenocarcinoma. PeerJ. 2019;7:e7313. doi: 10.7717/peerj.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley et al. (2010).Harley ME, Allan LA, Sanderson HS, Clarke PR. Phosphorylation of Mcl-1 by CDK1–cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest. The EMBO Journal. 2010;29:2407–2420. doi: 10.1038/emboj.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan et al. (2019).Hassan T, Jinho P, Hytham HG, Masters AR, Abdel-Aleem JA, Abdelrahman SI, Abdelrahman AA, Lyle LT, Yeo Y. Development of liposomal gemcitabine with high drug loading capacity. Molecular Pharmaceutics. 2019;16:2858–2871. doi: 10.1021/acs.molpharmaceut.8b01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2019).Huang H, Jiang X, Hao M, Shan M, Qiu Y, Hu G, Wang Q, Yu Z, Meng L, Zou Y. Identification of biomarkers in macrophages of atherosclerosis by microarray analysis. Lipids in Health and Disease. 2019;18 doi: 10.1186/s12944-019-1056-x. Article 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito et al. (2001).Ito Y, Ito Y, Takeda T, Sasaki Y, Sakon M, Yamada T, Ishiguro S, Imaoka S, Tsujimoto M, Higashiyama S, Monden M, Matsuura N. Expression and clinical significance of the erbB family in intrahepatic cholangiocellular carcinoma. Pathology - Research and Practice. 2001;197:95–101. doi: 10.1078/0344-0338-00016. [DOI] [PubMed] [Google Scholar]
- Kapanidou, Curtis & Bolanos-Garcia (2017).Kapanidou M, Curtis NL, Bolanos-Garcia VM. Cdc20: at the crossroads between chromosome segregation and mitotic exit. Trends in Biochemical Sciences. 2017;42:193–205. doi: 10.1016/j.tibs.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Karra et al. (2014).Karra H, Repo H, Ahonen I, Löyttyniemi E, Pitkänen R, Lintunen M, Kuopio T, Söderström M, Kronqvist Cdc20 and securin overexpression predict short-term breast cancer survival. British Journal of Cancer. 2014;110:2905–2913. doi: 10.1038/bjc.2014.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating & Santoro (2009).Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- Khan et al. (2020).Khan T, Seddon A, Dalgleish A, Khelwatty S, Ioannou N, Mudan S, Modjtahedi H. Synergistic activity of agents targeting growth factor receptors, CDKs and downstream signaling molecules in a panel of pancreatic cancer cell lines and the identification of antagonistic combinations: Implications for future clinical trials in pancreatic cancer. Oncology Reports. 2020;44:2581–2594. doi: 10.3892/or.2020.7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro et al. (2008).Kidokoro T, Tanikawa C, Furukawa Y, Katagiri T, Nakamura Y, Matsuda K. CDC20, a potential cancer therapeutic target, is negatively regulated by p53. Oncogene. 2008;27:1562–1571. doi: 10.1038/sj.onc.1210799. [DOI] [PubMed] [Google Scholar]
- Le (2019).Le Y. Screening and identification of key candidate genes and pathways in myelodysplastic syndrome by bioinformatic analysis. PeerJ. 2019;7:e8162. doi: 10.7717/peerj.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone et al. (2006).Leone F, Cavalloni G, Pignochino Y, Sarotto I, Ferraris R, Piacibello W, Venesio T, Capussotti L, Risio M, Aglietta M. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clinical Cancer Research. 2006;12:1680–1685. doi: 10.1158/1078-0432.CCR-05-1692. [DOI] [PubMed] [Google Scholar]
- Li et al. (2003).Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, Friess H, Sen S. Overexpression of oncogenic STK15/BTAK/Aurora a kinase in human pancreatic cancer. Clinical Cancer Research. 2003;9:991–997. [PubMed] [Google Scholar]
- Lin et al. (2017).Lin S-F, Lin J-D, Hsueh C, Chou T-C, Wong RJ. A cyclin-dependent kinase inhibitor, dinaciclib in preclinical treatment models of thyroid cancer. PLOS ONE. 2017;12:e0172315. doi: 10.1371/journal.pone.0172315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe et al. (2017).Lowe R, Shirley N, Bleackley M, Dolan S, Shafee T. Transcriptomics technologies. PLOS Computational Biology. 2017;13:e1005457. doi: 10.1371/journal.pcbi.1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luvira et al. (2016).Luvira V, Nilprapha K, Bhudhisawasdi V, Pugkhem A, Chamadol N, Kamsa-ard S. Cholangiocarcinoma patient outcome in Northeastern Thailand: single-center prospective study. Asian Pacific Journal of Cancer Prevention. 2016;17:401–406. doi: 10.7314/APJCP.2016.17.1.401. [DOI] [PubMed] [Google Scholar]
- Mita et al. (2014).Mita MM, Joy AA, Mita A, Sankhala K, Jou Y-M, Zhang D, Statkevich P, Zhu Y, Yao S-L, Small K, Bannerji R, Shapiro CL. Randomized phase II trial of the cyclin- dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clinical Breast Cancer. 2014;14:169–176. doi: 10.1016/j.clbc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Miyamoto et al. (2011).Miyamoto M, Ojima H, Iwasaki M, Shimizu H, Kokubu A, Hiraoka N, Kosuge T, Yoshikawa D, Kono T, Furukawa H, Shibata T. Prognostic significance of overexpression of c- Met oncoprotein in cholangiocarcinoma. British Journal of Cancer. 2011;105:131–138. doi: 10.1038/bjc.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami et al. (2009).Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakamura H, Nakashima A, Sueda T. Gemcitabine-based adjuvant chemotherapy improves survival after aggressive surgery for hilar cholangiocarcinoma. Journal of Gastrointestinal Surgery. 2009;13:1470–1479. doi: 10.1007/s11605-009-0900-0. [DOI] [PubMed] [Google Scholar]
- O’Dell et al. (2012).O’Dell MR, Huang J-L, Whitney-Miller CL, Deshpande V, Rothberg P, Grose V, Rossi RM, Zhu AX, Land H, Bardeesy N, Hezel AF. KrasG12D and p53 mutation cause primary intra-hepatic cholangiocarcinoma. Cancer Research. 2012;72:1557–1567. doi: 10.1158/0008-5472.CAN-11-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry et al. (2010).Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, Seghezzi W, Paruch K, Dwyer MP, Doll R, Nomeir A, Windsor W, Fischmann T, Wang Y, Oft M, Chen T, Kirschmeier P, Lees EM. Dinaciclib (SCH 727965), a novel and potent cyclin- dependent kinase inhibitor. Molecular Cancer Therapeutics. 2010;9:2344–2353. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- Paudyal et al. (2008).Paudyal B, Oriuchi N, Paudyal P, Higuchi T, Nakajima T, Endo K. Expression of glucose transporters and hexokinase II in cholangiocellular carcinoma compared using [ 18 F]-2-fluro-2-deoxy-d-glucose positron emission tomography. Cancer Science. 2008;99:260–266. doi: 10.1111/j.1349-7006.2007.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy et al. (2000).Percy MJ, Myrie KA, Neeley CK, Azim JN, Ethier SP, Petty EM. Expression and mutational analyses of the human MAD2L1 gene in breast cancer cells. Genes Chromosomes Cancer. 2000;29:356–362. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1044>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Piñeiro Yáñez et al. (2018).Piñeiro Yáñez E, Reboiro-Jato M, Gómez-López G, Perales-Patón J, Troulé K, Rodríguez JM, Tejero H, Shimamura T, López-Casas PP, Carretero J, Valencia A, Hidalgo M, Glez-Peña D, Al-Shahrour F. PanDrugs: a novel method to prioritize anticancer drug treatments according to individual genomic data. Genome Medicine. 2018;10 doi: 10.1186/s13073-018-0546-1. Article 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput et al. (2016).Rajput S, Khera N, Guo Z, Hoog J, Li S, Ma CX. Inhibition of cyclin dependent kinase 9 by dinaciclib suppresses cyclin B1 expression and tumor growth in triple negative breast cancer. Oncotarget. 2016;7:56864–56875. doi: 10.18632/oncotarget.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Merino (2013).Ramírez-Merino N. Chemotherapy for cholangiocarcinoma: an update. World Journal of Gastrointestinal Oncology. 2013;5:171–176. doi: 10.4251/wjgo.v5.i7.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rello-Varona et al. (2019).Rello-Varona S, Fuentes-Guirado M, López-Alemany R, Contreras-Pérez A, Mulet-Margalef N, García-Monclús S, Tirado OM, Muro XGarcíadel. Bcl-xL inhibition enhances Dinaciclib-induced cell death in soft-tissue sarcomas. Scientific Reports. 2019;9:3816. doi: 10.1038/s41598-019-40106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saensa-ard et al. (2017).Saensa-ard S, Leuangwattanawanit S, Senggunprai L, Namwat N, Kongpetch S, Chamgramol Y, Loilome W, Khansaard W, Jusakul A, Prawan A, Pairojkul C, Khantikeo N, Yongvanit P, Kukongviriyapan V. Establishment of cholangiocarcinoma cell lines from patients in the endemic area of liver fluke infection in Thailand. Tumor Biology. 2017;39:101042831772592. doi: 10.1177/1010428317725925. [DOI] [PubMed] [Google Scholar]
- Schwartz, Supuran & Alfarouk (2017).Schwartz L, Supuran CT, Alfarouk KO. The warburg effect and the hallmarks of cancer. Anti-Cancer Agents in Medicinal Chemistry. 2017;17:164–170. doi: 10.2174/1871520616666161031143301. [DOI] [PubMed] [Google Scholar]
- Shao et al. (2019).Shao Y-Y, Li Y-S, Hsu H-W, Lin H, Wang H-Y, Wo RR, Cheng A-L, Hsu C-H. Potent activity of composite cyclin dependent kinase inhibition against hepatocellular carcinoma. Cancer. 2019;11 doi: 10.3390/cancers11101433. Article 1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp & Corp (2017).Sharp M, Corp D. A phase 3 study to evaluate the efficacy and safety of dinaciclib or ofatumumab in subjects with refractory chronic lymphocytic leukemia. clinicaltrials.gov 2017 [Google Scholar]
- Sripa et al. (2005).Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C, Miwa M. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100) World Journal of Gastroenterology. 2005;11:3392. doi: 10.3748/wjg.v11.i22.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa et al. (2020).Sripa B, Seubwai W, Vaeteewoottacharn K, Sawanyawisuth K, Silsirivanit A, Kaewkong W, Muisuk K, Dana P, Phoomak C, Lert-itthiporn W, Luvira V, Pairojkul C, Teh BT, Wongkham S, Okada S, Chamgramol Y. Functional and genetic characterization of three cell lines derived from a single tumor of an Opisthorchis viverrini-associated cholangiocarcinoma patient. Human Cell. 2020;33:695–708. doi: 10.1007/s13577-020-00334-w. [DOI] [PubMed] [Google Scholar]
- Terada, Nakanuma & Sirica (1998).Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Human Pathology. 1998;29:175–180. doi: 10.1016/S0046-8177(98)90229-5. [DOI] [PubMed] [Google Scholar]
- Thamrongwaranggoon et al. (2017).Thamrongwaranggoon U, Seubwai W, Phoomak C, Sangkhamanon S, Cha’on U, Boonmars T, Wongkham S. Targeting hexokinase II as a possible therapy for cholangiocarcinoma. Biochemical and Biophysical Research Communications. 2017;484:409–415. doi: 10.1016/j.bbrc.2017.01.139. [DOI] [PubMed] [Google Scholar]
- Thongprasert (2005).Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Annals of Oncology. 2005;16:ii93–ii96. doi: 10.1093/annonc/mdi712. [DOI] [PubMed] [Google Scholar]
- Wan et al. (2014).Wan L, Tan M, Yang J, Inuzuka H, Dai X, Wu T, Liu J, Shaik S, Chen G, Deng J, Malumbres M, Letai A, Kirschner MW, Sun Y, Wei W. APCCdc20 suppresses apoptosis through targeting bim for ubiquitination and destruction. Developmental Cell. 2014;29:377–391. doi: 10.1016/j.devcel.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2015).Wang L, Zhang J, Wan L, Zhou X, Wang Z, Wei W. Targeting Cdc20 as a novel cancer therapeutic strategy. Pharmacology & Therapeutics. 2015;151:141–151. doi: 10.1016/j.pharmthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg (1956).Warburg O. On the origin of cancer cells. Science, New Series. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2013).Wu W, Hu K, Wang D, Zeng Z, Zhang D, Chen D, Bai L, Xu R. CDC20 overexpression predicts a poor prognosis for patients with colorectal cancer. Journal of Translational Medicine. 2013;11 doi: 10.1186/1479-5876-11-142. Article 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. (2018).Yan P, He Y, Xie K, Kong S, Zhao W. In silico analyses for potential key genes associated with gastric cancer. PeerJ. 2018;6:e6092. doi: 10.7717/peerj.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye et al. (2020).Ye Z, Zeng Z, Wang D, Lei S, Shen Y, Chen Z. Identification of key genes associated with the progression of intrahepatic cholangiocarcinoma using weighted gene co-expression network analysis. Oncology Letters. 2020;20:483–494. doi: 10.3892/ol.2020.11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2019).Zhang H, Zhang X, Li X, Meng W-B, Bai Z-T, Rui S-Z, Wang Z-F, Zhou W-C, Jin X-D. Effect of CCNB1 silencing on cell cycle, senescence, and apoptosis through the p53 signaling pathway in pancreatic cancer: ZHANG others, . Journal of Cellular Physiology. 2019;234:619–631. doi: 10.1002/jcp.26816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Raw measurements are available in the Supplemental Files.