Abstract
Accurate and individualized breast cancer risk assessment can be used to guide personalized screening and prevention recommendations. Existing risk prediction models use genetic and nongenetic risk factors to provide an estimate of a woman’s breast cancer risk and/or the likelihood that she has a BRCA1 or BRCA2 mutation. Each model is best suited for specific clinical scenarios and may have limited applicability in certain types of patients. For example, the Breast Cancer Risk Assessment Tool, which identifies women who would benefit from chemoprevention, is readily accessible and user-friendly but cannot be used in women under 35 years of age or those with prior breast cancer or lobular carcinoma in situ. Emerging research on deep learning-based artificial intelligence (AI) models suggests that mammographic images contain risk indicators that could be used to strengthen existing risk prediction models. This article reviews breast cancer risk factors, describes the appropriate use, strengths, and limitations of each risk prediction model, and discusses the emerging role of AI for risk assessment.
Keywords: risk assessment, screening, breast cancer, mammography
Key Messages
Risk prediction models use genetic and nongenetic risk factors to estimate a woman’s breast cancer risk and/or the likelihood that she has a BRCA1 or BRCA2 mutation.
To use risk prediction models, clinicians and researchers must understand the populations to which each model is applicable and what each model predicts.
Emerging research on artificial intelligence-based models for risk assessment suggests that mammographic images include risk indicators beyond breast density.
Introduction
Breast cancer screening has shifted from a one-size-fits-all approach toward personalized strategies based on risk level and personal preference (1). Supplemental MRI screening is widely recommended for high-risk women (with an estimated lifetime risk of 20% or more) (2,3). In addition, burgeoning evidence supports the use of supplemental screening for intermediate-risk women (with an estimated lifetime risk of 15% to 20%) (4,5). Emerging technologies, such as abbreviated MRI and contrast-enhanced mammography, have been proposed as more cost-effective modalities for supplemental screening, particularly in intermediate-risk women (6,7).
As such, a critical component of comprehensive breast cancer screening programs is breast cancer risk assessment, which can identify those individuals who would benefit from early and supplemental methods of screening, genetic testing, and/or preventive therapies, in addition to helping the general population make educated decisions about screening (3,8,9). To this end, multiple risk prediction models have been created that estimate the probability of breast cancer incidence within a specified time period in an otherwise healthy woman and/or the likelihood that she has a BRCA1 or BRCA2 mutation (10). The specific risk factors, and their weightings, differ between models. In addition, because each was developed using a sample of women who met certain inclusion criteria, models may perform differently depending on the population. Emerging evidence suggests that traditional risk factors and mammographic images contain complementary information and that deep learning-based artificial intelligence (AI) models have the potential to strengthen existing epidemiology-based models (11).
In this article, breast cancer risk factors will be discussed. In addition, the strengths, weaknesses, and appropriate use of each of the risk prediction models will be reviewed. Risk prediction models include: the modified Gail model/Breast Cancer Risk Assessment Tool (BCRAT); the Breast Cancer Surveillance Consortium (BCSC) model; the Rosner–Colditz model; the Tyrer–Cuzick (International Breast Intervention Study (IBIS) model; the Claus model; the BRCAPRO model; the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA); and, the Myriad model. The emerging role of AI for personalized risk prediction will also be highlighted.
Risk Factors for Breast Cancer
Multiple breast cancer risk factors have been identified, as discussed below (12,13).
Reproductive and hormonal risk factors: Older age, older age at first live birth and at menopause, younger age at menarche, and nulliparity are associated with elevated breast cancer risk, all of which are related to prolonged exposure to endogenous estrogen (13,14). In addition, use of postmenopausal hormone therapy is a risk factor that is dependent on type and duration of use (13,15). Reproductive and hormonal factors are considered to be modest risk factors (with risk ratios ranging between 1.0 and 1.5) but, when multiple, have additive effects (12,16).
Breast density: Dense breast tissue is an independent risk factor for breast cancer, with many studies demonstrating an odds ratio of 4.0 or greater when comparing the most dense to least dense categories (17,18). Although increased breast density confers lower risk than some risk factors, it is more common among women and thus may account for a considerable proportion of population risk (19,20). The addition of breast density as a risk factor improves calibration and discrimination of various risk prediction models (21–25).
Genetic factors: Family history—in particular, an affected mother, sister, or male relative, early onset disease, and bilateral disease—is an established risk factor (13). Inheritance of high-risk genetic mutations, such as BRCA1 and BRCA2, account for some but not all of this risk (26). Common risk variants, mostly single-nucleotide variants (formerly single-nucleotide polymorphisms), can explain up to 18% of the familial risk of breast cancer and, when aggregated, can be incorporated into risk prediction models as a polygenic risk score (27,28).
Benign breast disease and prior biopsy: Proliferative disease with atypia is a known risk factor. Specifically, there exists a 6- to 10-fold increased risk of breast cancer in women with lobular carcinoma in situ and a 4- to 5-fold increased risk in women with atypical ductal hyperplasia (2,29). In addition, prior breast biopsy alone is a modest risk factor for breast cancer, with relative risk associated with histologic findings (ie, proliferative disease with atypia is of higher risk than proliferative disease without atypia, which is of higher risk than nonproliferative disease) (30).
Radiation exposure: Radiation exposure between the ages of 10 and 30 years (for example, in survivors of Hodgkin lymphoma) is a known risk factor (2,31).
Lifestyle factors: Obesity is associated with elevated breast cancer risk in postmenopausal women but is thought to have a protective effect in premenopausal women (13). In postmenopausal obese women, the aromatase enzyme in adipose tissue converts androgens to estrogen, thus increasing breast cancer risk (32). Premenopausal obese women, however, have lower levels of serum estradiol (33). Physical activity decreases breast cancer risk in a dose-dependent manner (34). High levels of alcohol intake are associated with elevated breast cancer risk (13).
Risk Assessment Algorithm
The American College of Radiology recommends that all women, especially women of Ashkenazi Jewish descent and Black women, undergo breast cancer risk evaluation by age 30 (3). Given the complexities of breast cancer risk assessment, women who may be at high risk would best be served by formal risk assessment performed by trained professionals (35). In some cases, the breast imaging radiologist may be the first healthcare provider to recognize that a woman would benefit from formal assessment (35,36). As such, breast imaging radiologists are encouraged to understand risk assessment, in addition to when a request for an examination (such as MRI) may or may not be indicated (35).
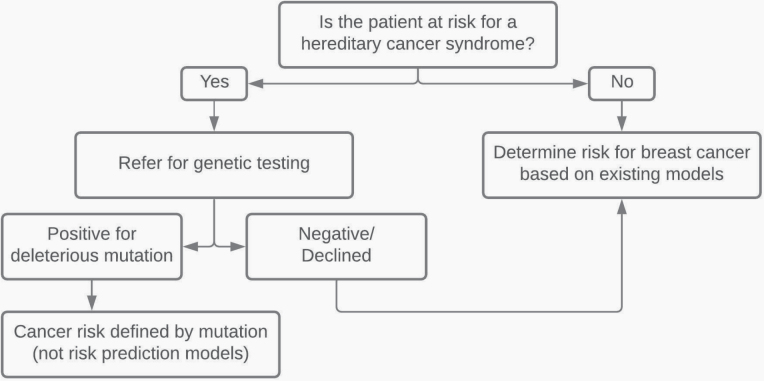
To begin the process of assessing a woman’s risk, it must first be determined whether she meets criteria for genetic testing, as risk prediction models that estimate breast cancer risk generally do not apply to women who harbor a genetic mutation (37). For example, BRCA mutations occur in approximately 1 in 300–500 women, accounting for 5%–10% of breast cancers; and, for these women, the mutation itself determines risk (ie, 45%–65% by 70 years of age) (38). If a woman does meet criteria for genetic testing, as discussed below, she would be directed to pursue genetic testing rather than risk assessment (37) (Figure 1). If she does not meet criteria for genetic testing, then risk assessment with existing models could be pursued.
Figure 1.
Risk assessment algorithm for breast cancer. Figure adapted from Barke and Freivogel (37), with permission.
Guidelines from the National Comprehensive Cancer Network are commonly used for determining eligibility for genetic counseling and testing (39). Examples of testing criteria for high-penetrance susceptibility genes include: a personal history of breast cancer diagnosed at or below the age of 45, at or below the age of 60 with triple-negative breast cancer, or at any age with Ashkenazi Jewish ancestry, or a family history of breast cancer in a first- or second-degree relative meeting any of the criteria listed above (39). Per the United States Preventive Service Task Force, the following tools accurately identify women who may have BRCA mutations: the 7-Question Family History Screening Tool, the Ontario Family History Assessment Tool, the Pedigree Assessment Tool, the Referral Screening Tool, the Manchester Scoring System, the Tyrer–Cuzick model, and brief versions of the BRCAPRO model (38).
Risk Prediction Models for Breast Cancer
Risk prediction models and appropriate clinical applications are summarized in Table 1 and Table 2. The risk prediction models are divided into regression models and genetic risk models. In regression models, risk calculations are based on the combination of a regression model (derived from cohort or case–control studies) with population-based incidence rates (derived from cancer registries) (40). In genetic risk models, segregation analysis is used to estimate the likelihood of harboring a genetic mutation based on family pedigree data, and then the penetration of that mutation is used to determine age-specific risk (40). Some models, such as the Tyrer–Cuzick model, incorporate both regression and genetic risk but are listed in the genetic risk section of this article.
Table 1.
Overview of Breast Cancer Risk Prediction Models
| Model | Population on Which Model Is Based | Risk Factors Included | Exclusion Criteria | Calibration (E/O)a | Discrimination (AUC) | Strengths | Weaknesses |
|---|---|---|---|---|---|---|---|
| Modified Gail/BCRAT (41–44) | Breast Cancer Detection Demonstration Project (White women in the United States) SEER Program (women in the United States) |
Age, age at menarche and at first live birth, number of previous breast biopsies, and number of first-degree female relatives with breast cancer. Race/ethnicity and history of atypia added to BCRAT. | Under 35 years of age, prior invasive breast cancer/DCIS/LCIS, prior mantle radiation, known genetic mutation (such as BRCA). | 0.69–1.12 | 0.58–0.74 | Readily available, simple to use, one of the only models available to assess eligibility for chemoprevention. | Cannot be used in patients with exclusion criteria, limited use in women of non-White ethnicity, considers only limited family history data. |
| BCSC (45–50) | BCSC (women in the United States) | Age, race/ethnicity, family history of breast cancer in a first-degree female relative, history of benign breast disease diagnoses, breast density. | Under 35 years of age, prior invasive breast cancer/DCIS, previous breast augmentation or mastectomy. | 0.94–1.04 | 0.61–0.67 | Readily available, simple to use. | Cannot be used in patients with exclusion criteria, considers only limited family history data, mammographic breast density must be known. |
| Rosner–Colditz (41,51–53) | Nurses’ Health Study (nurses in the United States) | Age at menarche, age at first birth and at each subsequent birth, and age at menopause. First-degree family history of breast cancer, benign breast disease, type of menopause, postmenopausal hormone use, body mass index, height, and alcohol consumption were added. | Prior breast cancer | 0.95–1.01 | 0.57–0.63 | Includes modifiable risk factors. | Modest discriminatory statistics, not readily accessible via website platform. |
| Tyrer–Cuzick/IBIS (23,40,54–58) | IBIS (women in Europe, Australia, and New Zealand) | Age at menarche, age at first live birth, age at menopause, parity, height, body mass index, atypical hyperplasia/LCIS, hormone replacement therapy, benign breast disease, family history of breast and ovarian cancer. Mammographic breast density and polygenic risk scores were added. | None | 0.95–1.03 | 0.71–0.75 | Combines genetic segregation model for familial risk and regression model for other risk factors, can be used in women younger than 35 years of age. | Requires detailed family history, computer program needed. |
| Claus (10,58,59) | Cancer and Steroid Hormone Study (White women in the United States) | Extensive family history, including ovarian cancer, age at diagnoses, and paternal history. | No family history of breast or ovarian cancer | 0.56–2.25 | 0.72–0.75 | Includes ovarian cancer data and paternal family history. | Does not include nonhereditary risk factors, calculations vary between published tables and software package. |
| BRCAPRO (55,58,60–63) | SEER Program (women in the United States) | Extensive family history that includes first- and second-degree relatives with breast and ovarian cancer. Race/ethnicity and tumor markers were added. | None | 0.59–1.16 | 0.68–0.82 | Best for high-risk women, includes both affected and unaffected relatives. | Assumes breast and ovarian cancers are due to BRCA mutations, considers only first- and second-degree relatives, does not include nonhereditary risk factors. |
| Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (55,58,64–66) |
Anglian Breast Cancer Study (women registered in the East Anglian Cancer Registry) Multiple case families (families in the UK) |
Extensive family history and nongenetic risk factors, such as hormonal factors. Tumor pathology and breast density were added. | None | 0.98–1.05 | 0.70–0.79 | Best for high-risk women, family history not limited to particular relatives or degrees. | Requires detailed family history, dedicated software needed. |
| Myriad II (62,67–73) | Patients who underwent gene sequence analyses by Myriad Genetic Laboratories | Personal history of breast cancer, Ashkenazi Jewish descent, family history of a first- or second-degree relative with breast cancer diagnosed before the age of 50 or ovarian cancer at any age. | No personal or family history of breast or ovarian cancer | 0.52–0.86 | 0.71–0.77 | Best for high-risk women based on family history. | Incorporates risk from up to two relatives, attributes the same degree of risk to all breast cancers diagnosed before age 50. |
Abbreviations: AUC, area under the receiver operating characteristic curve; BCRAT, Breast Cancer Risk Assessment Tool; BCSC, Breast Cancer Surveillance Consortium; DCIS, ductal carcinoma in situ; E/O, expected/observed; IBIS, International Breast Intervention Study; LCIS, lobular carcinoma in situ; SEER Program, Surveillance, Epidemiology, and End Results Program.
aExpected number of breast cancers based on the cumulative disease-specific hazards (and, in some cases, cumulative incidence), divided by the actual observed number of women with breast cancer.
Table 2.
Clinical Applications of Risk Prediction Modelsa for Breast Cancer
| Risk Factors | Appropriate Models | Not Appropriate |
|---|---|---|
| Known or suspected genetic mutation | Tyrer–Cuzick, BRCAPRO, BOADICEA, Myriad II | Gail/BCRAT, BCSC, Claus |
| Prior history of lobular carcinoma in situ | Tyrer–Cuzick | Gail/BCRAT, Claus |
| No known family history | Gail/BCRAT, BCSC, Tyrer–Cuzick | Claus, BRCAPRO, BOADICEA, Myriad II |
| 3 or more relatives with breast cancer | Tyrer–Cuzick | Gail/BCRAT, BCSC, Claus |
| Under 35 years old | Tyrer-Cuzick, Claus, BRCAPRO, BOADICEA | Gail/BCRAT, BCSC |
| History of mantle radiation | None (NCCN guidelines) | All |
Abbreviations: BCRAT, Breast Cancer Risk Assessment Tool; BCSC, Breast Cancer Surveillance Consortium; BOADICEA, Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm; NCCN, National Comprehensive Cancer Network.
aOf note, none of the risk prediction models can be used in women with a history of mantle radiation.
Interpretation of Statistics
Metrics describing risk prediction model performance include expected/observed (E/O) statistics that are used to measure calibration and concordance (C) statistics that are used to measure discrimination and often reported as the area under the receiver operating characteristic curve (AUC) (41). The key measure for estimating population risk is calibration, or E/O statistics, which has implications for public policy, while the key measure for estimating individual risk is discrimination, or C statistics (40).
With regard to calibration, the “E” in E/O statistics refers to the expected number of breast cancers based on the cumulative disease-specific hazards and, in some cases, cumulative incidence (model probability of disease) (40). The “O” refers to the actual observed number of women with breast cancer. The perfect model would have calibration (expressed as E/O) of 1. A ratio below 1 indicates underestimation of breast cancer incidence and above 1 indicates overestimation.
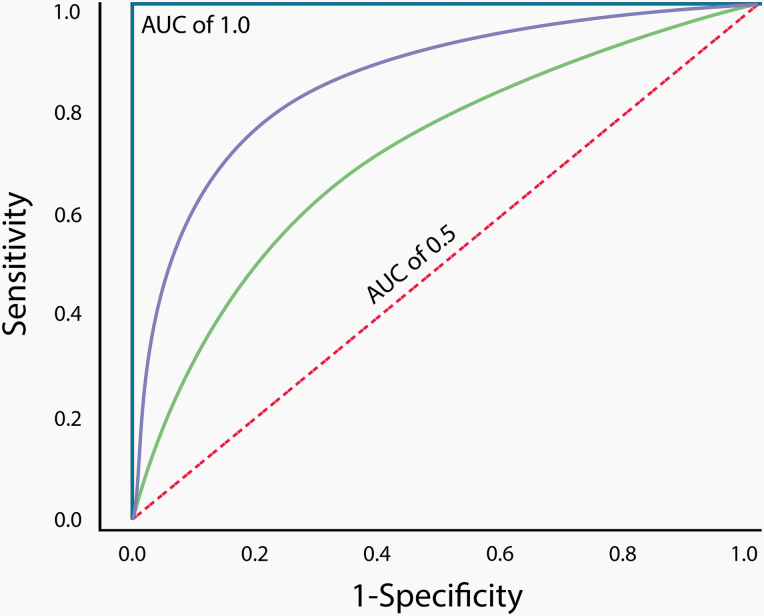
The C statistic measures the discrimination performance of the model (ie, its ability to distinguish between individuals who develop breast cancer and those who do not) and corresponds to the AUC (41). The perfect model would have an AUC of 1. A ratio of 0.5 amounts to chance occurrence, and generally an AUC above 0.7 is considered clinically acceptable (74) (Figure 2). The process of validation refers to application of the model to an independent patient population that was not used to develop the model (41).
Figure 2.
Receiver operating characteristic curve. A perfect classifier with an area under the curve (AUC) of 1.0 is represented by the solid blue line, and a random classifier with an AUC of 0.5 is represented by the dashed red line. The classifier represented by the solid purple line has better discriminatory accuracy than the classifier represented by the solid green line, since its AUC is closer to 1.0.
Regression Models
Modified Gail Model/Breast Cancer Risk Assessment Tool
The risk prediction model commonly known as the Gail model was developed in 1989 by Gail et al for women without prior breast cancer (42). The model has undergone several modifications over the years and is now available through the National Cancer Institute website as the BCRAT (43,44). The model is simple in design and can be used in various settings, including primary care. The first version, model 1, was composed of five questions focusing on reproductive and hormonal risk factors: age, age at menarche and at first live birth, number of previous breast biopsies, and number of first-degree female relatives with breast cancer (42). The risk factors and relative risks were based on case–control data from the Breast Cancer Detection Demonstration Project (BCDDP), a United States screening study conducted from 1973–1980, and the analysis used for the Gail model included data only from White women (42).
The second version, model 2, was developed in 1999 by the National Surgical Adjuvant Breast and Bowel Project investigators, in part, to determine eligibility criteria for the Breast Cancer Prevention Trial (43). This modified version uses incidence rates from the Surveillance, Epidemiology, and End Results (SEER) Program rather than data from the BCDDP and estimates only invasive cancer risk rather than invasive and in situ risk (43). Since then, the model, better known as the BCRAT, has undergone further modifications to include breast cancer incidence rates for African American and other non-White women and personal history of atypia as one of the risk factors (44). Other risk factors explored include breast density and weight (21).
The most recent version of the BCRAT provides estimates for five-year invasive cancer risk and lifetime invasive cancer risk for women who are at least 35 years of age (44). In particular, the risk calculator is used to identify individuals with a five-year risk of at least 1.67% who would benefit from chemoprevention (75). Multiple validation studies have been performed, some of which have found that the BCRAT tends to underestimate risk in certain populations (41,55,75,76). For example, the BCRAT underestimates risk in individuals with a strong family history of breast or other cancer, as it only includes first-degree female relatives with breast cancer and does not consider onset ages, paternal family history, or family history of other cancers (75). To address concern regarding risk underestimation in African American women, the Women’s Contraceptive and Reproductive Experiences (CARE) study data were used to create the CARE model, which is based on the BCRAT, to estimate invasive cancer risk in African American women (77).
Breast Cancer Surveillance Consortium Model
The BCSC model has a similar appearance to the BCRAT but also includes breast density. Earlier work on the Gail model using breast density as a continuous variable showed improved discrimination for invasive cancer risk in White women, and Tice et al demonstrated that the addition of Breast Imaging Reporting and Data System (BI-RADS)–based breast density improved calibration (21,45). The risk factors included in the BCSC model are similar to that of the Gail model/BCRAT and include age, race/ethnicity, family history of breast cancer in a first-degree female relative, and history of a breast biopsy with benign breast disease, in addition to BI-RADS breast density (46). The addition of other risk factors has been explored, such as polygenic risk scores and two sequential BI-RADS density measures (eg, breast density in 2007 and 2008) rather than one (47,78,79).
The BCSC model provides estimates for five- and 10-year invasive cancer risks for women who are at least 35 years of age without prior breast cancer, mastectomy, or breast augmentation (46). In the BCSC study population, the model slightly underestimated risk in younger women aged 40 to 44, Asian women, and Hispanic women (45). Subsequently, it was validated in cohorts from the Mayo Mammography Health Study and Metro Chicago Breast Cancer Registry (47,48). In the cohort of women in Chicago, 26% of whom were non-Hispanic Black, the BCSC model was well calibrated, with an E/O of 0.94, but it underestimated invasive cancer risk in younger women and in those with non-dense breasts (48). Discriminatory accuracy was 0.63, similar to that calculated in other validation studies (47,49,50).
Rosner–Colditz Model
The Rosner–Colditz model was based on the Pike model of breast tissue age, which was described in 1983 (51,80). The Pike model proposes that breast tissue age largely depends on estrogen and progesterone levels. Specifically, first full-term pregnancy at an early age is associated with reduced breast cancer risk, due to terminal differentiation of the mammary gland (which makes it less susceptible to carcinogens), while subsequent pregnancies are associated with transient increases in risk, due to the growth-enhancing effects of estrogens on premalignant cells (81). Following menopause, hormone levels depend on the peripheral conversion of androgens into estrogen by fat metabolism.
Using Nurses’ Health Study data, Rosner and Colditz extended the Pike model by incorporating the following features into their risk prediction model: age at menarche, age at first birth and at each subsequent birth, and age at menopause (51). In 2000, the following risk factors were included: first-degree family history of breast cancer, benign breast disease, type of menopause, postmenopausal hormone use, body mass index (BMI), height, and alcohol consumption (82). The addition of those risk factors was shown to improve the model’s AUC from 0.57 to 0.63 (52). Other risk factors identified in the Nurses’ Health Study, such as breastfeeding, vegetable intake, physical activity, and breast density, were subsequently incorporated, which improved discriminatory statistics (83).
The Rosner–Colditz model predicts invasive cancer risk in women up to 70 years of age without prior breast cancer (67). It was validated based on California Teachers Study data, at which time it was revamped with newer data from the Nurses’ Health Study, and performed similarly when applied to the California Teachers Study data (AUC of 0.59) and to the newer Nurses’ Health Study data (AUC of 0.60) (53). It performed best in women aged 47 to 69 when estimating five-year risk. The Rosner–Colditz model highlighted the effects of modifiable lifestyle risk factors on breast cancer incidence and placed less importance on chronologic age; however, its clinical use is limited due to its modest discriminatory statistics and lack of availability through a website platform.
Genetic Risk Models
Tyrer–Cuzick (IBIS) Model
Developed in 2004, the Tyrer–Cuzick model, or IBIS model, is among the most well-known and widely used tools (56). It is based on data from the IBIS conducted in the UK and combines a genetic segregation model for familial risk and a regression model for other risk factors (40). The genetic segregation model assumes a two-locus genetic model, with one locus for BRCA1 or BRCA2 and the other locus for an unknown, low penetrance gene (67). Risk factors considered in the model include: age at menarche, age at first live birth, age at menopause, parity, height, BMI, atypical hyperplasia/lobular carcinoma in situ, hormone replacement therapy, benign breast disease, family history of breast and ovarian cancer in first- and second-degree relatives, and age at diagnoses (40). The latest additions are breast density and polygenic risk scores (22–25,40). A UK study demonstrated that polygenic risk scores based on a large number of single-nucleotide variants lead to improved risk stratification when combined with Tyrer–Cuzick risk and breast density (84).
The computer program for the Tyrer–Cuzick model displays a chart that shows a woman’s breast cancer risk until 85 years of age, in addition to 10-year and lifetime risks (37). It also calculates the likelihood of having a BRCA mutation or a hypothetical autosomal dominant gene mutation assumed to have a low penetrance but a high frequency in the population (37,57). The risk estimates can be used to identify women who would benefit from chemoprevention and/or MRI screening (2,8). The Tyrer–Cuzick model includes a multitude of genetic and nongenetic risk factors, can be used in women younger than 35, and requires use of a specific computer program (57). It demonstrates good calibration and discrimination when used in high-risk populations, and recent evidence suggests that models with multigenerational family history, such as Tyrer–Cuzick, better estimate risk even for women with below-average or average breast cancer risk (55,67).
Claus Model
Developed in 1991, the Claus model is based on data from the Cancer and Steroid Hormone Study, which was conducted by the Centers for Disease Control (85,86). The study population was composed of 4730 White women aged 20 to 54 years with breast cancer and 4688 matched controls, registered between 1980 and 1982 at eight SEER centers (85,86). The original purpose of the model was to calculate familial breast cancer risk in women with a known family history of the disease. The model thus focused on family history of breast cancer (including age at diagnoses and including paternal history) and subsequently also incorporated family history of ovarian cancer (87). It does not include nongenetic risk factors.
The Claus model predicts risk of invasive cancer and ductal carcinoma in situ in women without a genetic mutation and can be used to identify women who would qualify for supplemental screening with MRI (2,37). Based on the assumption that breast cancer is transmitted as an autosomal dominant trait, the Claus model was the first model based on familial cancer history with a single dominant hereditary genetic mutation as a cause (85–87). The results of the authors’ studies laid a foundation for the existence of a heritable, germline mutation as a cause of breast cancer in women with family history, as their studies predated the identification of the BRCA1 and BRCA2 mutations. Following the discovery of the BRCA genes and their link to ovarian cancer, the model was revised and has now been integrated into a pedigree drawing software (Cyrillic) (88). It calculates the likelihood of carrying a genetic mutation and the cumulative risk of developing breast cancer. However, discrepancies exist between published tables and the extended Claus model available through the software package, possibly because the tables make no adjustments for unaffected relatives (10). Overall, the Claus model does not perform as well as other genetic risk prediction models (58,59).
BRCAPRO Model
Developed in 1997, the BRCAPRO model is a genetic risk prediction model that uses Bayes’ theorem to estimate the likelihood of carrying a BRCA mutation in patients with a family history of breast and/or ovarian cancer (60,61). Whereas the Claus model predated the discovery of the BRCA mutations and assumed a genetic cause for familial breast cancer, the BRCAPRO model estimates the likelihood of carrying a BRCA mutation based on a Mendelian inheritance pattern and relies on published data about gene penetrance and prevalence (60). Initially, the original model assumed two-allele and autosomal dominant inheritance of BRCA1 genes, with the expectation that the BRCA2 gene would be incorporated later with further evidence, and regarded other genes as sporadic. As such, consideration for BRCA2 was later added and is included in the current version (89,90). Since the first version, other factors such as race/ethnicity and tumor markers (eg, estrogen receptor and progesterone receptor status) have been added, which improved its performance, and the current model consistently shows good discrimination between carriers and noncarriers (55,58,62,63,90).
The BRCAPRO model can be used to determine whether a patient would benefit from genetic testing (38). In addition, lifetime risk above 20%–25%, as calculated by the BRCAPRO model, can be used to determine eligibility for supplemental MRI screening (2). The software program for the BRCAPRO model is freely available as part of the CancerGene genetic counseling package, the open-source R package BayesMendel, and as a web-based interface (91–93). Although the BRCAPRO model incorporates affected and unaffected relatives, its limitations include the following: it incorporates only first- and second-degree relatives, it considers only breast and ovarian cancer, it does not account for genes other than BRCA1 and BRCA2, and it does not include nongenetic risk factors (10,37,89).
Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm
The BOADICEA is a genetic risk model that is based on breast cancer genetic susceptibility being attributed to the effects of the BRCA1 and BRCA2 mutations and the assumption that residual clustering within families is due to the multiplicative effects of many genes (polygenic component) (64,65). The specific pathogenic mutations it now incorporates are BRCA1, BRCA2, PALB2, CHEK2, and ATM (94). The BOADICEA was developed in 2002 with data from the Anglian Breast Cancer Study (which was later renamed SEARCH and included women with breast cancer diagnosed before the age of 55 who were registered in the East Anglian Cancer Registry) and from multiple case families in the UK (which included families with two or more breast cancer cases, one of which was diagnosed before the age of 50) (64,65). It was then updated using data from the UK National Case Control Study, the Manchester Study, and pooled pedigree data from 22 studies (65,95–97). Similar to the Tyrer–Cuzick model, the BOADICEA incorporates nongenetic risk factors; more recently, tumor pathology and breast density were added (66,98).
The BOADICEA predicts the probability of carrying a BRCA1 or BRCA2 mutation (or the proposed polygenic component) and also predicts breast cancer and ovarian cancer risk (65). The model is available online, through which family history can be entered beyond second-degree relatives (66). Unlike other genetic risk models, family history is not limited to particular relatives or degrees (67). Compared to other models, the BOADICEA performs well with good calibration and discrimination (58,67).
Myriad Model
The Myriad model is based on gene sequencing analyses performed by Myriad Genetic Laboratories (Salt Lake City, UT). Created in 1997, Myriad I (or the Shattuck-Eidens model) is an empirical model that was developed with 798 unrelated individuals from the United States and Europe thought to be at high risk of a BRCA1 mutation (68). Myriad I estimates the risk of harboring a BRCA1 mutation based on the following risk factors: personal history of unilateral/bilateral breast cancer or ovarian cancer, patient age at first diagnosis of cancer, Ashkenazi Jewish descent, and number of relatives with breast or ovarian cancer (68).
The current empirical model, Myriad II by Frank et al, was developed in 1998 based on women with breast cancer diagnosed before the age of 50 or ovarian cancer diagnosed at any age and at least one first- or second-degree relative with either breast or ovarian cancer (69). The model was then refined and tested in 2002 with the results of 10 000 gene sequence analyses, which were performed to identify deleterious BRCA1 or BRCA2 mutations and three specific Ashkenazi Jewish founder mutations (69,70). The current model is based on the following risk factors: personal history of breast cancer, Ashkenazi Jewish descent, and family history of a first- or second-degree relative with breast cancer diagnosed before the age of 50 or ovarian cancer diagnosed at any age (71). The mutation prevalence tables are separated according to Ashkenazi Jewish ancestry, as this population has higher rates of BRCA1 and BRCA2 mutations attributed to three founder mutations (70). Myriad II is used in high-risk women based on family history but is only able to include risk from up to two relatives and attributes the same level of risk to all breast cancers diagnosed before age 50 (eg, breast cancer diagnosed in the 20s is treated similarly to cancer diagnosed in the 40s) (67).
AI for Risk Assessment
Breast density has recently been added into risk prediction models, but density alone is not likely to reflect all of the data contained within mammographic images. Emerging research on deep learning models for breast cancer risk assessment suggests that mammographic images contain risk indicators beyond breast density that may not be perceptible by humans (99,100). For example, investigators in Sweden trained a deep learning model to estimate breast cancer risk and then tested it on 2283 women, 278 of whom were diagnosed with breast cancer (99). The model output was a score from 0 to 1, reflecting the likelihood of developing breast cancer based on a single mammographic image, and the scores from all four of each woman’s mammographic images were averaged to estimate individual risk. The investigators’ model, which achieved an AUC of 0.65, outperformed other models based on age and breast density and also had a lower false-negative rate rather than the best-performing density model.
In a recent study, three models were created to estimate five-year breast cancer risk: a risk factor–only logistic regression model, a deep learning model based on mammographic images, and a hybrid model that combined the logistic regression and the image-based deep learning model (100). These three models were compared to the Tyrer–Cuzick model. The hybrid model achieved the highest AUC (0.70), while the Tyrer–Cuzick model had the lowest (0.62). The image-only model also had a higher AUC than the Tyrer–Cuzick model. These results demonstrate that mammographic images and traditional risk factors have information that is complementary, as illustrated by the improvement in AUC of the hybrid model over the other models. This deep learning model could strengthen existing models based on traditional risk factors, but further research is warranted to validate it across breast imaging practices and mammography vendors. Future research will also shed light on the imaging features and patterns that are being used by the deep learning models to predict risk.
Conclusion
Risk prediction models use genetic and nongenetic risk factors to estimate a woman’s breast cancer risk and/or the likelihood that she has a BRCA1 or BRCA2 mutation. Each model uses different risk factors and is best suited to provide risk estimates in certain patient populations. Emerging research on AI-based models for breast cancer risk assessment suggests that mammographic images have risk indicators beyond breast density that could be used to strengthen existing models, but further research is warranted to validate such models across breast imaging practices and mammography vendors.
Funding
This work is supported in part by the National Cancer Institute under the National Institutes of Health (K08CA241365). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Statement
M.B. is a consultant for Lunit (a medical AI software company) and an expert panelist for 2nd.MD (a digital health company). There are no other conflicts of interest.
References
- 1. Onega T, Beaber EF, Sprague BL, et al. Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level. Cancer 2014;120(19):2955–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saslow D, Boetes C, Burke W, et al. ; American Cancer Society Breast Cancer Advisory Group . American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 3. Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol 2018;15(3):408–414. [DOI] [PubMed] [Google Scholar]
- 4. Bakker MF, de Lange SV, Pijnappel RM, et al. ; DENSE Trial Study Group . Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 2019;381(22):2091–2102. [DOI] [PubMed] [Google Scholar]
- 5. Wang L, Strigel RM. Supplemental screening for patients at intermediate and high risk for breast cancer. Radiol Clin North Am 2021;59(1):67–83. [DOI] [PubMed] [Google Scholar]
- 6. Chhor CM, Mercado CL. Abbreviated MRI protocols: wave of the future for breast cancer screening. AJR Am J Roentgenol 2017;208(2):284–289. [DOI] [PubMed] [Google Scholar]
- 7. Sorin V, Yagil Y, Yosepovich A, et al. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. AJR Am J Roentgenol 2018;211(5):W267–W274. [DOI] [PubMed] [Google Scholar]
- 8. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2013;31(23):2942–2962. [DOI] [PubMed] [Google Scholar]
- 9. Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164(4):268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst 2010;102(10):680–691. [DOI] [PubMed] [Google Scholar]
- 11. Bahl M.Harnessing the power of deep learning to assess breast cancer risk. Radiology 2020;294(2):273–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Ann Intern Med 2012;156(9):635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ban KA, Godellas CV. Epidemiology of breast cancer. Surg Oncol Clin N Am 2014;23(3):409–422. [DOI] [PubMed] [Google Scholar]
- 14. Evans DG, Howell A. Breast cancer risk-assessment models. Breast Cancer Res 2007;9(5):213. doi: 10.1186/bcr1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chlebowski RT, Kuller LH, Prentice RL, et al. ; WHI Investigators . Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med 2009;360(6):573–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singletary SE. Rating the risk factors for breast cancer. Ann Surg 2003;237(4):474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology 2004;230(1):29–41. [DOI] [PubMed] [Google Scholar]
- 18. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15(6):1159–1169. [DOI] [PubMed] [Google Scholar]
- 19. Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium . Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol 2017;3(9):1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee CI, Chen LE, Elmore JG. Risk-based breast cancer screening: implications of breast density. Med Clin North Am 2017;101(4):725–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst 2006;98(17):1215–1226. [DOI] [PubMed] [Google Scholar]
- 22. Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015;17(1):147. doi: 10.1186/s13058-015-0653-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brentnall AR, Cuzick J, Buist DSM, Bowles EJA. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol 2018;4(9):e180174. doi: 10.1001/jamaoncol.2018.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brentnall AR, Cohn WF, Knaus WA, Yaffe MJ, Cuzick J, Harvey JA. A case-control study to add volumetric or clinical mammographic density into the Tyrer-Cuzick breast cancer risk model. J Breast Imag 2019;1(2):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vilmun BM, Vejborg I, Lynge E, et al. Impact of adding breast density to breast cancer risk models: a systematic review. Eur J Radiol 2020;127:109019. doi: 10.1016/j.ejrad.2020.109019 [DOI] [PubMed] [Google Scholar]
- 26. Stratton MR, Rahman N. The emerging landscape of breast cancer susceptibility. Nat Genet 2008;40(1):17–22. [DOI] [PubMed] [Google Scholar]
- 27. Michailidou K, Lindström S, Dennis J, et al. ; NBCS Collaborators; ABCTB Investigators; ConFab/AOCS Investigators . Association analysis identifies 65 new breast cancer risk loci. Nature 2017;551(7678):92–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open 2020;3(7):e208501. doi: 10.1001/jamanetworkopen.2020.8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985;312(3):146–151. [DOI] [PubMed] [Google Scholar]
- 30. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005;353(3):229–237. [DOI] [PubMed] [Google Scholar]
- 31. Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst 2005;97(19):1428–1437. [DOI] [PubMed] [Google Scholar]
- 32. Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst 1995;87(3):190–197. [DOI] [PubMed] [Google Scholar]
- 33. Potischman N, Swanson CA, Siiteri P, Hoover RN. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst 1996;88(11):756–758. [DOI] [PubMed] [Google Scholar]
- 34. McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. ; 2018 Physical Activity Guidelines Advisory Committee . Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc 2019;51(6):1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol 2010;7(1):18–27. [DOI] [PubMed] [Google Scholar]
- 36. Narayan AK, Mercaldo SF, Gupta YP, Warner ET, Lehman CD, Miles RC. Potential of using mammography screening appointments to identify high-risk women: cross sectional survey results from the national health interview survey [published online ahead of print November 12, 2020]. Breast Cancer Res Treat 2020. doi: 10.1007/s10549-020-06002-9. [DOI] [PubMed] [Google Scholar]
- 37. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am 2017;55(3):457–474. [DOI] [PubMed] [Google Scholar]
- 38. Owens DK, Davidson KW, Krist AH, et al. ; US Preventive Services Task Force . Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA 2019;322(7):652–665. [DOI] [PubMed] [Google Scholar]
- 39. Daly MB, Pilarski R, Yurgelun MB, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Canc Netw 2020;18(4):380–391. [DOI] [PubMed] [Google Scholar]
- 40. Brentnall AR, Cuzick J. Risk models for breast cancer and their validation. Stat Sci 2020;35(1):14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meads C, Ahmed I, Riley RD. A systematic review of breast cancer incidence risk prediction models with meta-analysis of their performance. Breast Cancer Res Treat 2012;132(2):365–377. [DOI] [PubMed] [Google Scholar]
- 42. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81(24):1879–1886. [DOI] [PubMed] [Google Scholar]
- 43. Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst 1999;91(18):1541–1548. [DOI] [PubMed] [Google Scholar]
- 44. National Cancer Institute (NCI). The Breast Cancer Risk Assessment Tool. Available at: https://bcrisktool.cancer.gov. Accessed November 2, 2020.
- 45. Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med 2008;148(5):337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Breast Cancer Surveillance Consortium (BCSC). BCSC risk calculator. Available at: https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm. Accessed November 2, 2020.
- 47. Vachon CM, Pankratz VS, Scott CG, et al. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst 2015;107(5):dju397. doi: 10.1093/jnci/dju397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tice JA, Bissell MCS, Miglioretti DL, et al. Validation of the breast cancer surveillance consortium model of breast cancer risk. Breast Cancer Res Treat 2019;175(2):519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol 2015;33(28):3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kerlikowske K, Ma L, Scott CG, et al. Combining quantitative and qualitative breast density measures to assess breast cancer risk. Breast Cancer Res 2017;19(1):97. doi: 10.1186/s13058-017-0887-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rosner B, Colditz GA. Nurses’ health study: log-incidence mathematical model of breast cancer incidence. J Natl Cancer Inst 1996;88(6):359–364. [DOI] [PubMed] [Google Scholar]
- 52. Rockhill B, Byrne C, Rosner B, Louie MM, Colditz G. Breast cancer risk prediction with a log-incidence model: evaluation of accuracy. J Clin Epidemiol 2003;56(9):856–861. [DOI] [PubMed] [Google Scholar]
- 53. Rosner BA, Colditz GA, Hankinson SE, Sullivan-Halley J, Lacey JV Jr, Bernstein L. Validation of Rosner-Colditz breast cancer incidence model using an independent data set, the California Teachers Study. Breast Cancer Res Treat 2013;142(1):187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cuzick J, Brentnall AR, Segal C, et al. Impact of a panel of 88 single nucleotide polymorphisms on the risk of breast cancer in high-risk women: results from two randomized Tamoxifen prevention trials. J Clin Oncol 2017;35(7):743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Terry MB, Liao Y, Whittemore AS, et al. 10-year performance of four models of breast cancer risk: a validation study. Lancet Oncol 2019;20(4):504–517. [DOI] [PubMed] [Google Scholar]
- 56. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004;23(7):1111–1130. [DOI] [PubMed] [Google Scholar]
- 57. Ready K, Litton JK, Arun BK. Clinical application of breast cancer risk assessment models. Future Oncol 2010;6(3):355–365. [DOI] [PubMed] [Google Scholar]
- 58. Fischer C, Kuchenbäcker K, Engel C, et al. ; German Consortium for Hereditary Breast and Ovarian Cancer . Evaluating the performance of the breast cancer genetic risk models BOADICEA, IBIS, BRCAPRO and Claus for predicting BRCA1/2 mutation carrier probabilities: a study based on 7352 families from the German Hereditary Breast and Ovarian Cancer Consortium. J Med Genet 2013;50(6):360–367. [DOI] [PubMed] [Google Scholar]
- 59. Amir E, Evans DG, Shenton A, et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet 2003;40(11): 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Berry DA, Parmigiani G, Sanchez J, Schildkraut J, Winer E. Probability of carrying a mutation of breast-ovarian cancer gene BRCA1 based on family history. J Natl Cancer Inst 1997;89(3):227–238. [DOI] [PubMed] [Google Scholar]
- 61. Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet 1998;62(1):145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parmigiani G, Chen S, Iversen ES Jr, et al. Validity of models for predicting BRCA1 and BRCA2 mutations. Ann Intern Med 2007;147(7):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Biswas S, Tankhiwale N, Blackford A, et al. Assessing the added value of breast tumor markers in genetic risk prediction model BRCAPRO. Breast Cancer Res Treat 2012;133(1):347–355. [DOI] [PubMed] [Google Scholar]
- 64. Antoniou AC, Pharoah PD, McMullan G, et al. A comprehensive model for familial breast cancer incorporating BRCA1, BRCA2 and other genes. Br J Cancer 2002;86(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer 2008;98(8): 1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee AJ, Cunningham AP, Kuchenbaecker KB, Mavaddat N, Easton DF, Antoniou AC; Consortium of Investigators of Modifiers of BRCA1/2; Breast Cancer Association Consortium . BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer 2014;110(2):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cintolo-Gonzalez JA, Braun D, Blackford AL, et al. Breast cancer risk models: a comprehensive overview of existing models, validation, and clinical applications. Breast Cancer Res Treat 2017;164(2):263–284. [DOI] [PubMed] [Google Scholar]
- 68. Shattuck-Eidens D, Oliphant A, McClure M, et al. BRCA1 sequence analysis in women at high risk for susceptibility mutations. Risk factor analysis and implications for genetic testing. JAMA 1997;278(15):1242–1250. [PubMed] [Google Scholar]
- 69. Frank TS, Manley SA, Olopade OI, et al. Sequence analysis of BRCA1 and BRCA2: correlation of mutations with family history and ovarian cancer risk. J Clin Oncol 1998;16(7):2417–2425. [DOI] [PubMed] [Google Scholar]
- 70. Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol 2002;20(6):1480–1490. [DOI] [PubMed] [Google Scholar]
- 71. Myriad. Hereditary breast cancer. Available at: https://myriad.com/patients-families/disease-info/breast-cancer. Accessed November 2, 2020.
- 72. Lindor NM, Lindor RA, Apicella C, et al. Predicting BRCA1 and BRCA2 gene mutation carriers: comparison of LAMBDA, BRCAPRO, Myriad II, and modified Couch models. Fam Cancer 2007;6(4):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Antoniou AC, Hardy R, Walker L, et al. Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet 2008;45(7):425–431. [DOI] [PubMed] [Google Scholar]
- 74. Hosmer DW, Lemeshow S.. Applied Logistic Regression. 2nd ed. New York: John Wiley and Sons, Inc.; 2000. [Google Scholar]
- 75. NCCN Clinical Practice Guidelines in Oncology. Breast cancer risk reduction, version 1.2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf. Accessed November 2, 2020.
- 76. Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst 2001;93(5):358–366. [DOI] [PubMed] [Google Scholar]
- 77. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst 2007;99(23):1782–1792. [DOI] [PubMed] [Google Scholar]
- 78. Kerlikowske K, Gard CC, Sprague BL, Tice JA, Miglioretti DL, Breast Cancer Surveillance Consortium . One versus two breast density measures to predict 5- and 10-year breast cancer risk. Cancer Epidemiol Biomarkers Prev 2015;24(6):889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shieh Y, Hu D, Ma L, et al. Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res Treat 2016;159(3):513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature 1983;303(5920):767–770. [DOI] [PubMed] [Google Scholar]
- 81. Russo J, Moral R, Balogh GA, Mailo D, Russo IH. The protective role of pregnancy in breast cancer. Breast Cancer Res 2005;7(3):131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol 2000;152(10):950–964. [DOI] [PubMed] [Google Scholar]
- 83. Rice MS, Tworoger SS, Hankinson SE, et al. Breast cancer risk prediction: an update to the Rosner-Colditz breast cancer incidence model. Breast Cancer Res Treat 2017;166(1):227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brentnall AR, van Veen EM, Harkness EF, et al. A case-control evaluation of 143 single nucleotide polymorphisms for breast cancer risk stratification with classical factors and mammographic density. Int J Cancer 2020;146(8):2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Claus EB, Risch N, Thompson WD. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet 1991;48(2):232–242. [PMC free article] [PubMed] [Google Scholar]
- 86. Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994;73(3):643–651. [DOI] [PubMed] [Google Scholar]
- 87. Claus EB, Risch N, Thompson WD. The calculation of breast cancer risk for women with a first degree family history of ovarian cancer. Breast Cancer Res Treat 1993;28(2):115–120. [DOI] [PubMed] [Google Scholar]
- 88. Tischkowitz M, Wheeler D, France E, et al. A comparison of methods currently used in clinical practice to estimate familial breast cancer risks. Ann Oncol 2000;11(4):451–454. [DOI] [PubMed] [Google Scholar]
- 89. Berry DA, Iversen ES Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol 2002;20(11):2701–2712. [DOI] [PubMed] [Google Scholar]
- 90. Mazzola E, Blackford A, Parmigiani G, Biswas S. Recent enhancements to the genetic risk prediction model BRCAPRO. Cancer Inform 2015;14(Suppl 2):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen S, Wang W, Broman KW, Katki HA, Parmigiani G. BayesMendel: an R environment for Mendelian risk prediction. Stat Appl Genet Mol Biol 2004;3:Article21. doi: 10.2202/1544-6115.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chipman J, Drohan B, Blackford A, Parmigiani G, Hughes K, Bosinoff P. Providing access to risk prediction tools via the HL7 XML-formatted risk web service. Breast Cancer Res Treat 2013;140(1):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. UT Southwestern Medical Center at Dallas and The BayesMendel Group. CancerGene. Available at: http://www4.utsouthwestern.edu/breasthealth/cagene/. Accessed November 2, 2020.
- 94. Lee AJ, Cunningham AP, Tischkowitz M, et al. Incorporating truncating variants in PALB2, CHEK2, and ATM into the BOADICEA breast cancer risk model. Genet Med 2016;18(12):1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Peto J, Collins N, Barfoot R, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 1999;91(11):943–949. [DOI] [PubMed] [Google Scholar]
- 96. Antoniou AC, Pharoah PD, McMullan G, Day NE, Ponder BA, Easton D. Evidence for further breast cancer susceptibility genes in addition to BRCA1 and BRCA2 in a population-based study. Genet Epidemiol 2001;21(1):1–18. [DOI] [PubMed] [Google Scholar]
- 97. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003;72(5):1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee A, Mavaddat N, Wilcox AN, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med 2019;21(8):1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dembrower K, Liu Y, Azizpour H, et al. Comparison of a deep learning risk score and standard mammographic density score for breast cancer risk prediction. Radiology 2020;294(2):265–272. [DOI] [PubMed] [Google Scholar]
- 100. Yala A, Lehman C, Schuster T, Portnoi T, Barzilay R. A deep learning mammography-based model for improved breast cancer risk prediction. Radiology 2019;292(1):60–66. [DOI] [PubMed] [Google Scholar]