Abstract
Myasthenia gravis (gMG) is a critical autoimmune disease, which has a serious impact on the life and survival of patients. Ocular Myasthenia Gravis (oMG) is often the initial manifestation of MG and has the potential to progress to gMG. However, to date no distinct mechanism has been found to clarify the pathogenesis of conversion from oMG to gMG. Recent studies have shown that the development and clinical progression of MG is closely associated with the abnormal function of follicular helper T (Tfh) cells. Thus, this article reviews the recently achieved research progress on the involvement of Tfh cells in MG immunopathogenesis and focuses on the role of Tfh cells and related-factors (IL-21, CXCL13, CXCR5, bcl-6 etc.) in germinal center formation and antibody production in MG immune response.
Keywords: Generalized myasthenia gravis, ocular myasthenia gravis, follicular helper T cells, germinal centers, pathogenesis
INTRODUCTION
Myasthenia gravis (MG) is a typical autoimmune disorder generally caused by anti-acetylcholine receptor (anti-AChR) antibodies and is characterized by progressive weakness and exhaustibility of muscles without atrophy. Ocular myasthenia gravis (oMG) is one of the clinical subtypes of MG. The symptoms of oMG are limited to the extraocular muscles, such as diplopia, strabismus and blepharoptosis. However prior reports indicate that more than 50% of oMG patients will convert to generalized myasthenia gravis (gMG), which may cause generalized weakness of muscles, even those involved in respiratory functions. Besides 90% of oMG patients converting to gMG do so within 2 years of disease onset (1). gMG and oMG are caused by auto-antibodies that are immunoreactive with muscle cell membrane proteins. These antibodies emerge through a B cell-mediated and T cell-dependent immune response, which also involves the complement system.
Antibodies form as a result of interaction of B cells and CD4+ T cells. Upon antigen encounter, B cells get activated and subsequently migrate to the lymphatic follicle region, forming germinal centers (GCs) and differentiating into plasma cells that produce autoantibodies (2). AChR-Ab is highly specific to MG and frequently found in both oMG (50%) and gMG (85–90%), and the mechanism of inducing the autoimmune response to AChR is unknown (3). Follicular helper T cells (Tfh), accepted as a new CD4+ T cell subset, were first proposed in tonsils by Schaerli and Breitfeld in 2000 (4). These distinct CD4+ T cells play a role in assisting B cell humoral immunity by expressing chemokine receptor CXCR5 and migrating into peripheral lymphoid follicles, which are also essential for both the production of antibodies and the maintenance of the GC. Current studies have indicated that GC may be the important site to produce pathogenic autoantibodies in autoimmune disease.
Recent studies have shown that the occurrence and development of a variety of autoimmune diseases are closely correlated with the imbalance of the expression of Tfh cells. The frequencies of Tfh cells and levels of Tfh-related factors in MG patients are significantly increased. Moreover, they are positively correlated with serum levels of AChR-Ab (5, 6). However, whether and how Tfh cells participate in MG remains unclear. Here we will review the studies focused on the relationship among Tfh, oMG and gMG in order to explore mechanisms of action of Tfh cells in MG. The studies about Tfh cells will putatively further elaborate on the immunopathogenesis of MG.
TFH CELLS AND THEIR FUNCTIONS IN GC RESPONSES AND ANTIBODY FORMATION
It has been well known that Tfh cell is a CD4 + T cell subset that mainly exists in B cell follicles of secondary lymphoid tissues. They are characterized by the expression of chemokine receptor CXCR5, costimulatory receptors PD-1, B cell lymphoma 6 (Bcl-6), ICOS, IL-21 and low levels of CCR7 and CXCL13 (7, 8). Significantly, CXCR5 can drive Tfh cells to home to B cell follicles where CXCL13 (the ligand for CXCR5) is expressed (9). Therefore, Tfh cells play an important role in the maturation of B cells in follicles, the differentiation of plasma cells and the formation of GC, as well as the recombination and homotypic transformation of antibodies (7, 10). GC, as the special structure formed by the proliferation, activation and maturation of B cell follicles in secondary lymphoid tissues following antigen stimulation, is also an important site for the formation of pathogenic antibodies in autoimmune diseases.
Figure 1.
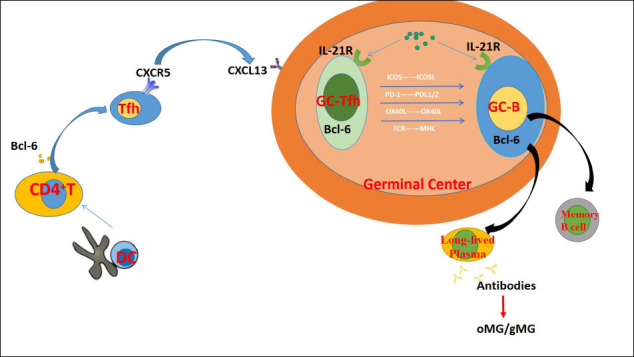
The mechanisms that Tfh cells are involved in MG pathogenesis.
The key role of Tfh cells is to help the formation of GC, and to interact with B cells in GC (11). The structure of GC is divided into two parts. While dark zone is composed of the rapid proliferating B cells, the light zone is made up of Tfh cells, follicular dendritic cells (FDC) and slowly proliferating B cells. Right after the antigenic stimulus, slowly proliferating B cells gain the ability to bind antigens presented by FDC and pass the antigen signals to Tfh cells in the light zone. Tfh cells then provide survival and proliferation signals to proliferating B cells through CD40L, T cells receptor (TCR), ICOS, IL-2 and SAP (12). Upon receiving signals, GC-B cells migrate to dark zones for the processes of proliferation, somatic cell hypermutation and B cells receptor (BCR) production, then return to the light zone and interact with Tfh cells. Tfh cells will select B cells with higher antigen affinity for further mutation and transformation, stimulate them to differentiate into memory B cells or plasma cells and help them to exit from GC (13, 14). B cells with low antigen affinity fail to receive the survival signals from Tfh cells and undergo apoptosis (15). Without Tfh cells, GC would not develop and long-lived plasma cells and antibodies would not be produced (12).
THE RELATIONSHIP AMONG TFH CELLS, GMG AND OMG
The Role of Tfh in MG
Recent studies have found that the frequency of CD4+CXCR5+PD-1 hiICOShi T cells in the peripheral blood of MG patients was higher compared to healthy subjects (16). In addition, they were positively correlated with the level of serum anti-AChR-Ab levels (16) and there was a positive association between the percentage of Tfh cells and the severity of MG [6,7]. Moreover, the frequency of Tfh cells was reduced in MG patients after treatment (16). MG is an autoimmune disease considered to be mediated by B cells. These results demonstrate the important role of Tfh cells in the pathogenesis of oMG/gMG, since these cells display an auxiliary function of promoting B cells to form plasma cells and inducing the secretion of a large number of antigen-specific antibodies with high affinity. In addition, the interactions between B cells and T cells require the involvement of cytokines, chemokines and costimulatory molecules associated with Tfh cells, such as CXCR5, Bcl-6, PD-1, ICOS and IL-21 (17). The levels of circulating antibodies may change with the varied levels of Tfh-related cytokines. CXCL13 and its receptor CXCR5 are important molecules produced by Tfh cells and are essential to form lymphoid follicles. It was found that the level of serum CXCL13 was increased in MG patients (18) and positively correlated with disease severity and the frequency of Tfh cells (5). IL-21, the main cytokine for Tfh cells to perform their effector functions, was discovered to be highly expressed in oMG and gMG and positively related to the percentage of Tfh cells in MG patients as well (5). Its absence could lead to decreased production of antibodies (19–21). These studies suggest that Tfh may promote the development of MG by acting on B cells that secrete antibodies through related cytokines.
Besides, it has been demonstrated that the frequency of CD4+ CXCR5+PD-1+ Tfh cells and Tfh-associated molecules, such as Bcl-6 and IL-21, were significantly increased during the pathogenesis of experimental autoimmune myasthenia Gravis (EAMG) induced in mice (22). EAMG, a conventional model of MG with similar clinical features and immunopathological mechanisms, has regularly been used to simulate and explore the pathogenesis and therapy of MG. In addition, RNA interference targeting Bcl-6 in EAMG can provide significant improvement in the clinical severity and reduce the frequency of Tfh cells, the expression of Bcl-6 and IL-21, and the levels of anti-AChR antibodies (22, 23). On the other hand, some studies have shown that while circulating Tfh cells are significantly more abundant in gMG patients without thymic abnormalities than those in healthy subjects, while they are not elevated in oMG patients (24). This finding indicates that Tfh cells may participate in the pathogenesis of MG, contribute to the clinical progression of MG and thus may be a marker of disease activity.
Role of Tfh-Related Factors
The maturation of Tfh cells begins with DC-induced antigen in the T-cell region around the lymphoid follicles, and continues to have homologous interactions between Tfh and B cells at the T-B cell boundary of the follicles. These events lead to the induction of transcription factor Bcl-6, which controls the T cell lineage’s commitment to Tfh’s fate. These early Tfh-B cell interactions require the expression of surface receptors ICOS, OX40 and CD40-ligands, as well as cytokines IL-4 and IL-21, and have shown effects on Tfh’s fate commitment and viability and B cell entry into GC response. Tfh cells have various associated-factors such as CXCR5, Bcl6, ICOS, PD-1, CXCL13, IL-21 and these factors have a certain correlation in the pathogenesis of oMG, which will be described in the following sections.
CXCR5 and CXCL13 expresssion on Tfh: Naive CD4+T cells can differentiate into various subsets of T helper (Th) cells such as Th1, Th2, Th17 and Tfh cells, which are characterized by the expression of unique factors and cytokines. Tfh cells are characterized by a high expression level of CXCR5, which is different from other subsets of Th cells that loose the expression of CXCR5 with differentiation (25). CXCR5 is expressed on the surface of Tfh cells and B cells and constitutes the molecular basis for the migration of Tfh cells and B cells to lymphoid follicles, where chemokine CXCL13 acts as the ligand for CXCR5 (18). CXCL13 is mainly expressed by antigen-presenting cells. Tfh cells and B cells can both migrate to the same location under the attraction of CXCL13 and thus may easily interact. Tfh cells can both produce high levels of CXCR5 and low levels of CXCL13, which is conducive to the migration of Tfh cells into follicles and to the auxiliary function of B cell activation and other GC reactions (26).
Studies have reported that CXCL13 is highly expressed in MG peripheral blood cells and levels of CXCL13 are associated with clinical features of MG patients (27, 28). Therefore, different expressions of CXCL13 in patients with MG might be of great significance for prediction of the progress of MG.
Transcription factor of Tfh: Bcl-6: Differentiation of Tfh cells begins with the interaction of naive CD4+ T cells with antigen-presenting DCs in lymphoid follicles or T-cell regions. CD4+ T cells acquire the expression of CXCR5 and Bcl-6 by DC priming, which induces the formation of early Tfh cells. Then these early Tfh cells differentiate into GC-Tfh cells by migrating to the T-cell boundary and interacting with homologous B cells (29). This differentiation process leads to the induction of the transcription factor Bcl-6, which is mainly expressed in B cells and Tfh cells. This differentiation process is controlled by signals provided by signal transduction and transcription activator 3 (STAT3) ultimately activating cytokines, such as interleukin 21 (IL-21), which further increases the induction of Bcl-6. It is a characteristic transcription factor of Tfh cells and plays an auxiliary role in the migration of Tfh cells into GC. Bcl-6 induction is a necessary and sufficient condition for differentiation of Tfh cells in vivo. Sustained Bcl-6 expression is essential for the development and persistence of Tfh cells. It promotes and modulates the expression of Tfh cell-related factors, such as CXCR5, ICOS, PD-1 and CXCL13 (8, 29). So, lack of expression of Bcl-6 would abort differentiation of Tfh cells and effective stimulation of B cells for antibody production. Not surprisingly, Bcl-6 deficiency in CD4+ T cells was reported to result in impaired Tfh cell development and impaired GC reactions (8, 29). Additional experiments showed that Bcl-6 knockout mice fail to form GC in vivo, which confirms the above-mentioned statement (30).
Bcl-6 was also reported to be essential for the differentiation and maturation of B cells, since it can inhibit GC B cell differentiation into plasma cells or memory cells by inhibiting the transcriptional activation of B lymphocyte-induced mature protein 1 (Blimp-1) (31–33). Bcl-6 and Blimp-1 are two antagonistic transcription factors in the differentiation of Tfh cells and B cells. Contrary to Bcl-6, Blimp-1 inhibits the IL-2 induced differentiation of Tfh cells, mainly by inhibiting the expression of Bcl-6 (29).
IL-21 production: IL-21 is secreted mainly by Tfh cells and also by Th17 cells at lower amounts. IL-21 is also the key cytokine for the growth, development and effector functions of Tfh cells. The lack of IL-21 seriously affects the differentiation of Tfh cells. IL-21 knockout mice exhibit low expression levels of Tfh cells and the loss of GC (25). IL-21 mainly acts by binding to IL-21R widely expressed on the surface of B cells, some NK cells and T cells (34). In an autocrine fashion, IL-21 produced by Tfh cells not only promotes self-differentiation, but also promotes the expression of Bcl-6 and CXCR5 by Tfh cells.
At the same time, the high level of IL-21 secreted by Tfh cells is also an important factor for Tfh cells themselves to stimulate the survival of B cells and promote the proliferation and differentiation of B cells in GC (8). In the early stages of GC formation and B cell maturation, the homologous Tfh-B cell interactions require the increased expression of cytokines IL-21 and IL-4 to enable B cells to present specific antigens to Tfh cells at the T cell-B cell boundary. In addition, while contributing to GC responses, B cells need to present antigens to Tfh cells from FDC by continuously moving between LZ and DZ. This process also requires the participation of IL-21.
IL-21 also plays a complex and critical role in the development and maturation of B cells and the immune response mediated by B cells (35). On one hand, IL-21 can significantly promote the CD40L-induced proliferation of B cells (36, 37). On the other hand, IL-21 may inhibit IgM and IL-4-induced proliferation of B cells and may induce the apoptosis of antigen-specific B lymphocytes to maintain the homeostasis of B lymphocyte (38). In addition, previous studies have shown that exogenous use of IL-21 increases the proportion of memory B cells (39).
IL-21 can also regulate B cell antibody class switching, which is essential for B cells to establish a long-term immune response. IL-21 mainly promotes CD40-induced B cell proliferation and differentiation, resulting in IgG1 and IgG3 antibody production (40, 41). Lack of IL-21R and IL-4 causes a distinct IgG response impairment (42). In IL-21 knockout mice, the production of antigen-specific IgG is decreased, further indicating the important role of this cytokine in the class-switching function of B cells (25). As for MG patients, the production of AChR antibody is the key reason for the imbalance of immune environment. AChR antibodies are mainly of the IgG1 and IgG3 isotypes, suggesting that IL-21-mediated class switching may play a key role in the pathogenesis of MG. In oMG and gMG studies, the serum levels of IL-21, Tfh cells, B cells and plasma cells were found to be increased (5, 24), and IL-21R mRNA levels of peripheral blood mononuclear cells (PBMC) were higher than those of normal controls (43). Besides in oMG and gMG patients, the expression of IL-21R on B cells was increased and was positively correlated with the levels of AChR-IgG, IgG1 and IgG3 subtypes (43). Moreover, inhibition of the IL-21R with rIL-21-fc significantly inhibited antibody secretion (24).
These results indicate an important role of IL-21 and its receptor in the pathogenesis of MG. IL-21 may promote the pathogenesis and progression of MG by inducing AChR-Ab production through activation of Tfh cells and induction of antibody switching. Therefore, the symptoms of MG patients can putatively be alleviated by drugs antagonizing IL-21 in future treatment trials.
PD-1 and ICOS expression on Tfh: ICOS is a member of CD28 family and plays an important role in T cell dependent antibody response. Unlike other CD28 members, ICOS can only be expressed on activated T cells. . ICOS is highly expressed on the surface of Tfh cells in light zone region of GC, and its ligand (ICOSL) is expressed on the surface of B cells. The interaction between ICOS and ICOSL can induce Tfh cells to produce more cytokines, such as IL-2, IL-4, IL-10 and IL-21. Blocking ICOS significantly reduces the expression of IL-21, as well as the frequencies of Tfh cells and by this way causes the dysfunction of GC. ICOS deficiency results in GC dysfunction and a severe reduction of serum antibodies and Tfh and memory B cells both in peripheral blood and GC (44). In contrast, overexpression of ICOS increases the number of Tfh cells in follicles and yields in a more robust GC reaction (45).
Another member of the CD28 family, programmed death receptor 1 (PD-1), is an important negative immunoregulatory factor. It is mainly expressed on the surface of activated T lymphocytes, B lymphocytes and macrophages, and its ligands (PD-L1/PD-L2) are mainly expressed on the surface of B cells. PD-1 binds and interacts with its ligands PD-L1 or PD-L2 with different affinities. Negative signals from PD-1 regulate the proliferation and differentiation of lymphocyte cells and the secretion of cytokines, weaken the immune response of T cells and affect the release of specific antibodies.
Same as ICOS, co-stimulator PD-1 also plays an important role in the formation of GC and the production of antibody-secreting cells through Tfh-mediated mechanisms. The interaction between PD-1 and PD-L1/PD-L2 can be enhanced by repeated T-B cell interactions, which in turn may inhibit the differentiation of Tfh cells. However, studies have shown that although the number of Tfh cells increases in the absence of PD-1, the number of long-lived plasma cells and specific antibodies decreases (46). The possible mechanism is that the lack of PD-1 signaling causes Tfh cells to stagnate in the G0/G1 phase, reduces the ability of Tfh cells to synthesize important cytokines such as IL-21 and IL-4, and eventually inhibits B cell differentiation and antibody production (46).
Blocking the binding of PD-1 to PD-L1/L2 may lead to the decrease of Tfh cell activity, the activation of antigen-specific B cells and the production of antibodies against pathogenic antigens, and yet again may induce the occurrence of various autoimmune diseases such as MG (47). PD-1 expression levels of T cells were significantly higher in MG patients than those of the control group (18). Severe oMG symptoms, such as blepharoptosis, diplopia and ocular dyskinesia, may occur in patients receiving targeted immunosuppressive therapy of PD-1/PD-L1, and gradually develop into gMG (48). The exact mechanisms by which inhibition of PD1/PD-L1 interaction cause MG need to be further analyzed.
CONCLUSION
In recent years, a significant progress has been made in understanding Tfh cells and their significance in GC B cells response. It is clear that the Tfh cell is an important tool for producing antibodies with high affinity. In MG, most of the progress in the Tfh field has been achieved in AChR-antibody positive patients. MuSK antibody positive MG patients exhibit equally high levels of IL-21 levels as AChR antibody patients (49). Moreover, in a recent treatment trial tacrolimus has been shown to ameliorate MG symptoms through inhibition of Tfh cells among other factors (50). Overall, these studies suggest that Tfh cells have utmost importance in other antibody subtypes of MG, as well. It might be possible to effectively control MG at the initial stages and prevent its progression through manipulation of the Tfh cell and its microenvironment. Therefore, it is necessary to thoroughly understand the factors affecting Tfh-B cell interactions.
Conflict of Interest Statement
We confirm that no conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the manuscript has not been published previously, and not under consideration for publication elsewhere, in whole or in part.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept- NW; Design- ET; Supervision- YC; Resource- LY; Materials- YW; Data Collection and/ or Processing- JR; Analysis and/or Interpretation- HH; Literature Search- SL; Writing- LS; Critical Reviews- XW.
Conflict of Interest: No.
Financial Disclosure: 1. National Natural Science Foundation of China (No. 81760179, 81360151); 2. Key research plan of Jiangxi Provincial Department of Science and Technology (No.20192BBG70042); 3. Key projects of Jiangxi Youth Science Fund(No.20202ACBL216008); 4. Natural Science Foundation of Jiangxi Province (No. 20171BAB205046); 5. Technology and Science Foundation of Jiangxi Province (No. 20141BBG70027); 6. Health Development Planning Commission Science Foundation of Jiangxi Province (No. 20185118); 7. Jiangxi Province Education Department Key Foundation (No. GJJ160033).
REFERENCES
- 1.Nagia L, Lemos J, Abusamra K, Cornblath WT, Eggenberger ER. Prognosis of Ocular Myasthenia Gravis:Retrospective Multicenter Analysis. Ophthalmology. 2015;122:1517–1521. doi: 10.1016/j.ophtha.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26:1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- 4.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo C, Li Y, Liu W, Feng H, Wang H, Huang X, Qiu L, Ouyang J. Expansion of circulating counterparts of follicular helper T cells in patients with myasthenia gravis. J Neuroimmunol. 2013;256:55–61. doi: 10.1016/j.jneuroim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Zhang M, Ye Y, Ma S, Fan L, Li Z. High frequencies of circulating Tfh-Th17 cells in myasthenia gravis patients. Neurol Sci. 2017;38:1599–1608. doi: 10.1007/s10072-017-3009-3. [DOI] [PubMed] [Google Scholar]
- 7.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao DA. T Cells That Help B Cells in Chronically Inflamed Tissues. Front Immunol. 2018;9:1924. doi: 10.3389/fimmu.2018.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Crotty S. A brief history of T cell help to B cells. Nat Rev Immunol. 2015;15:185–189. doi: 10.1038/nri3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesquita Jr D, Cruvinel WM, Resende LS, Mesquita FV, Silva NP, Camara NO, Andrade LE. Follicular helper T cell in immunity and autoimmunity. Braz J Med Biol Res. 2016;49 doi: 10.1590/1414-431X20165209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott RK, Lee JH, Menis S, Skog P, Rossi M, Ota T, Kulp DW, Bhullar D, Kalyuzhniy O, Havenar-Daughton C, Schief WR, Nemazee D, Crotty S. Precursor Frequency and Affinity Determine B Cell Competitive Fitness in Germinal Centers, Tested with Germline-Targeting HIV Vaccine Immunogens. Immunity. 2018;48:133–146. e6. doi: 10.1016/j.immuni.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwickert TA, Victora GD, Fooksman DR, Kamphorst, AO, Mugnier MR, Gitlin AD, Dustin ML, Nussenzweig MC. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med. 2011;208:1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akkaya M, Traba J, Roesler AS, Miozzo P, Akkaya B, Theall BP, Sohn H, Pena M, Smelkinson M, Kabat J, Dahlstrom E, Dorward DW, Skinner J, Sack MN, Pierce SK. Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat Immunol. 2018;19:871–884. doi: 10.1038/s41590-018-0156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito R, Onodera H, Tago H, Suzuki Y, Shimizu M, Matsumura Y, Kondo T, Itoyama Y. Altered expression of chemokine receptor CXCR5 on T cells of myasthenia gravis patients. J Neuroimmunol. 2005;170:172–178. doi: 10.1016/j.jneuroim.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular Helper T Cells. Annu Rev Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 18.Shiao Y, Lee C, Hsu Y, Huang SF, Lin CY, Li LH, Fann CS, Tsai CY, Tsai SF, Chiu HC. Ectopic and high CXCL13 chemokine expression in myasthenia gravis with thymic lymphoid hyperplasia. J Neuroimmunol. 2010;221:101–106. doi: 10.1016/j.jneuroim.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Alahgholi-Hajibehzad M, Durmuş H, Aysal F, Gulsen-Parman Y, Oflazer P, Deymeer F, Saruhan-Direskeneli G. The effect of interleukin (IL)-21 and CD4+CD25++T cells on cytokine production of CD4+responder T cells in patients with myasthenia gravis:IL-21 and Treg effects on cytokines. Clin Exp Immunol. 2017;190:201–207. doi: 10.1111/cei.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hocaoglu M, Durmus H, Ozkan B, Yentur SP, Dogan O, Parman Y, Deymeer F, Saruhan-Direskeneli G. Increased costimulatory molecule expression of thymic and peripheral B cells and a sensitivity to IL-21 in myasthenia gravis. J Neuroimmunol. 2018;323:36–42. doi: 10.1016/j.jneuroim.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Rauniyar VK, Yin WF, Hu B, Ouyang S, Xiao B, Yang H. Serum IL-21 levels decrease with glucocorticoid treatment in myasthenia gravis. Neurol Sci. 2014;35:29–34. doi: 10.1007/s10072-013-1460-3. [DOI] [PubMed] [Google Scholar]
- 22.Xin N, Fu L, Shao Z, Guo M, Zhang X, Zhang Y, Dou C, Zheng S, Shen X, Yao Y, Wang J, Wang J, Cui G, Liu Y, Geng D, Xiao C, Zhang Z, Dong R. RNA interference targeting Bcl-6 ameliorates experimental autoimmune myasthenia gravis in mice. Mol Cell Neurosci. 2014;58:85–94. doi: 10.1016/j.mcn.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Yi W, Gupta S, Ricker E, Manni M, Jessberger R, Chinenov Y, Molina H, Pernis AB. The mTORC1–4E-BP-eIF4E axis controls de novo Bcl6 protein synthesis in T cells and systemic autoimmunity. Nat Commun. 2017;8(1):254. doi: 10.1038/s41467-017-00348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang CJ, Gong Y, Zhu W, Qi Y, Yang CS, Fu Y, Chang G, Li Y, Shi S, Wood K, Ladha S, Shi FD, Liu Q, Yan Y. Augmentation of Circulating Follicular Helper T Cells and Their Impact on Autoreactive B Cells in Myasthenia Gravis. J Immunol. 2016;197:2610–2617. doi: 10.4049/jimmunol.1500725. [DOI] [PubMed] [Google Scholar]
- 25.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Zou L, Liu Y. T follicular helper cells, T follicular regulatory cells and autoimmunity. Int Immunol. 2016;28:173–179. doi: 10.1093/intimm/dxv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meraouna A, Cizeron-Clairac G, Panse RL, Bismuth J, Truffault F, Tallaksen C, Berrih-Aknin S. The chemokine CXCL13 is a key molecule in autoimmune myasthenia gravis. Blood. 2006;108:432–440. doi: 10.1182/blood-2005-06-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, Guo J, Li H, Zhou Y, Tian F, Gong L, Wang X, Li Z, Zhang W. Expression of immune molecules CD25 and CXCL13 correlated with clinical severity of myasthenia gravis. J Mol Neurosci. 2013;50:317–323. doi: 10.1007/s12031-013-9976-9. [DOI] [PubMed] [Google Scholar]
- 29.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol. 2013;13:412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 30.Polo JM, Dell'Oso T, Ranuncolo SM, Cerchietti L Beck D, Da Silva GF, Prive GG, Licht JD, Melnick A. Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat Med. 2004;10:1329–1335. doi: 10.1038/nm1134. [DOI] [PubMed] [Google Scholar]
- 31.Finotto S. B lymphocyte-induced maturation protein 1(Blimp-1), a negative regulator of TH9 development, orchestrates the resolution of airway inflammation in patients with allergic asthma. J Allergy Clin Immunol. 2019;143:937–939. doi: 10.1016/j.jaci.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 32.Reljic R, Wagner SD, Peakman LJ, Fearon DT. Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J Exp Med. 2000;192:1841–1848. doi: 10.1084/jem.192.12.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 34.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 35.Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 37.Ettinger R, Sims GP, Fairhurst AM, Robbins R, Da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 38.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaccari M, Franchini G. T Cell Subsets in the Germinal Center:Lessons from the Macaque Model. Front Immunol. 2018;9:348. doi: 10.3389/fimmu.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boumendjel A, Tawk L, Malefijt RW, Boulay V, Yssel H, Pene J. IL-27 induces the production of IgG1 by human B cells. Eur Cytokine Netw. 2006;17:281–289. [PubMed] [Google Scholar]
- 41.Pene J, Gauchat JF, Lecart S, Drouet E, Guglielmi P, Boulay V, Delwail A, Foster D, Lecron JC, Yssel H. Cutting edge:IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172:5154–5157. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- 42.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC Rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 43.Hu B, Tian X, Huang H, Jian A, Ouyang S, Yin W, Duan W, Yang H. Expression of IL-21 in the peripheral blood of myasthenia gravis patients and its correlation with anti-AChR-Ab class switch. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:958–963. doi: 10.3969/j.issn.1672-7347.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier M, Welcher AA, Peter HH, Warnatz K. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 45.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, Copley, RR, Bell JI, Cornall RJ, Goodnow CC. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 46.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamani MR, Aslani S, Salmaninejad A, Javan MR, Rezaei N. PD-1/PD-L and autoimmunity:A growing relationship. Cell Immunol. 2016;310:27–41. doi: 10.1016/j.cellimm.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, Schmidgen MI, Gutzmer R, Utikal JS, Goppner D, Hassel JC, Meier F, Tietze JK, Forschner A, Weishaupt C, Leverkus M, Wahl R, Dietrich U, Garbe C, Kirchberger MC, Eigentler T, Berking C, Gesierich A, Krackhardt AM, Schadendorf D, Schuler G, Dummer R, Heinzerling LM. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–225. doi: 10.1016/j.ejca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Yilmaz V, Oflazer P, Aysal F, Durmus H, Poulas K, Yentur SP, Gulsen-Parman Y, Tzartos S, Marx A, Tuzun E, Deymeer F, Saruhan-Direskeneli G. Differential Cytokine Changes in Patients with Myasthenia Gravis with Antibodies against AChR and MuSK. PloS One. 2015;10:e123546. doi: 10.1371/journal.pone.0123546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Guptill JT, Russo MA, Massey JM, Juel VC, Hobson–Webb LD, Howard JF, Chopra M, Liu W, Yi JS. Tacrolimus inhibits Th1 and Th17 responses in MuSK–antibody positive myasthenia gravis patients. Exp Neurol. 2019;312:43–50. doi: 10.1016/j.expneurol.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


